Quantifying the Effect of COD to TN Ratio, DO Concentration and Temperature on Filamentous Microorganisms’ Population and Trans-Membrane Pressure (TMP) in Membrane Bio-Reactors (MBR)
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot-Scale MBR Operation and Examined Parameters
- The influent COD to TN ratio (10:1 and 5:1), which was adjusted by using different concentrations of the employed synthetic wastewater components.
- The DO concentration (2.5 ± 0.5 and 0.5 ± 0.3 mg/L) in the filaments’ tank, which operated as a “regulator” for adjusting the population of filamentous microorganisms. On the contrary, DO concentration was constant in the denitrification tank (<0.1 mg/L) and in the aeration tank of the bioreactor (2.5 ± 0.5 mg/L).
- Mixed liquor temperature followed the seasonal temperature change and, consequently, temperature was also investigated as a system parameter.
2.2. Analytical Techniques and Protocols
2.2.1. Estimation of Filaments’ Population with the Determination of Filament Index (FI)
2.2.2. Measurement of SMPc Concentration
2.2.3. Estimation of Sludge Filterability with the TTF Method
2.2.4. Estimation of Sludge Settleability with the Determination of SVI (mL/g MLSS)
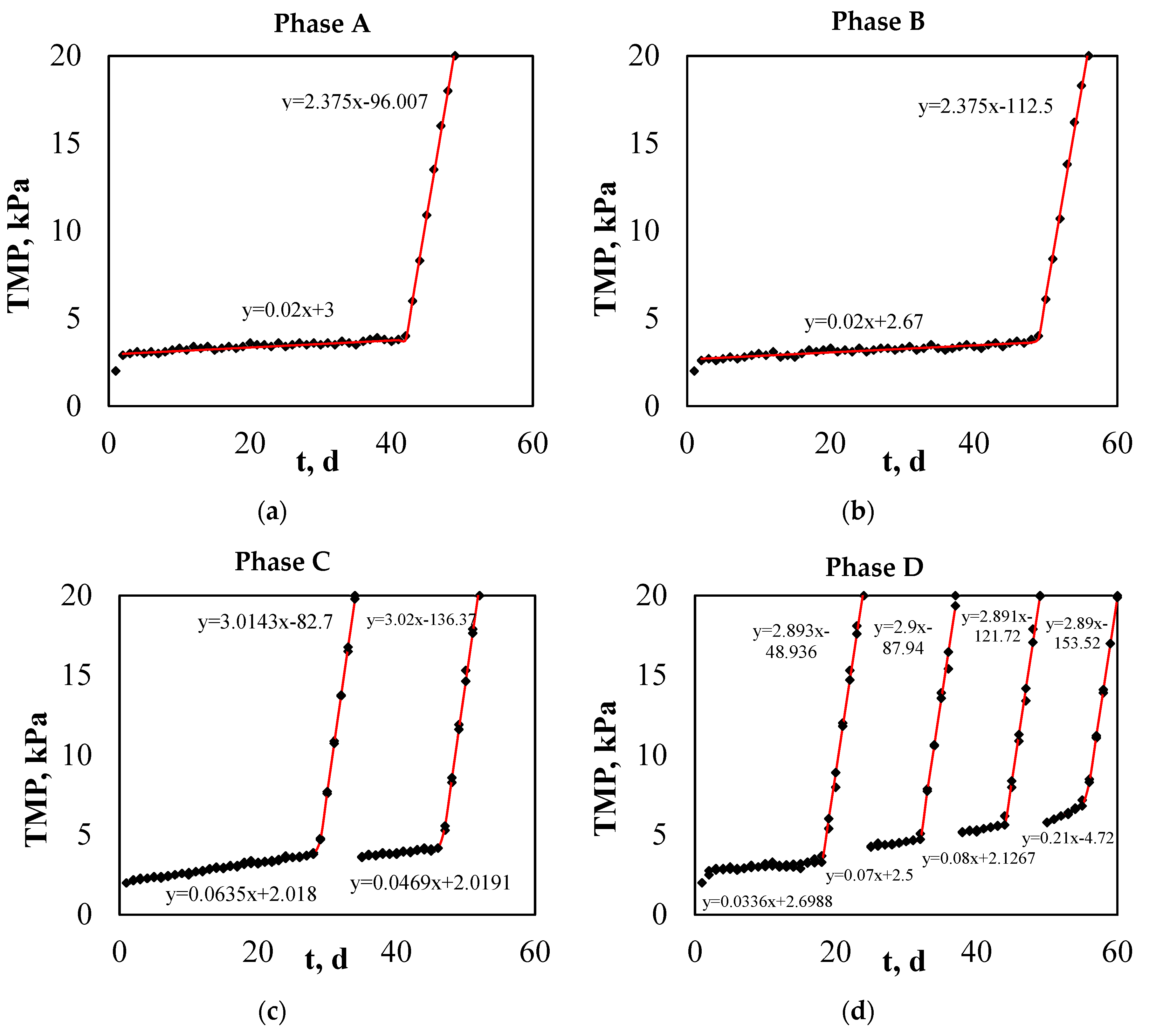
2.2.5. TMP Fitting
- (a)
- Cake blocking, which occurs when particles accumulate on the surface of a membrane in a permeable cake of increasing thickness that adds resistance to flow.
- (b)
- Complete blocking, which assumes that particles seal off the membrane pores and prevent flow.
- (c)
- Standard blocking, which occurs when particles accumulate inside the membranes on the walls of straight cylindrical pores. As particles are increasingly deposited, the pores become constricted and membrane permeability decreases.
- (d)
- Intermediate blocking, which is similar to complete blocking, but assumes that some particles seal off the membrane pores while the rest accumulate on top of other deposited particles.
- (a)
- Stage 1 (or conditioning fouling), when the initial adsorption of substances (usually organics, such as EPS and SMP) takes place immediately after the immersion of the membrane in the biomass.
- (b)
- Stage 2 (or long-term fouling), which is characterized by further adsorption and deposition of organics, colloids and bio-flocs on the membrane surface.
- (c)
- Stage 3 (or TMP jump), when TMP suddenly rises until it reaches the maximum limit (according to the membrane specifications). During this stage, there are regions of the membrane which are more fouled than others and are characterized by low permeability. As a result, permeability is promoted in less fouled areas, exceeding a critical flux in these locations. Under such conditions, fouling rate increases rapidly and almost exponentially with the flux [3].
3. Results and Discussion
3.1. Influence of COD to TN Ratio and Temperature on Filaments’ Growth
3.2. Effect of Filament Index (FI) and Temperature on TMP and SMPc
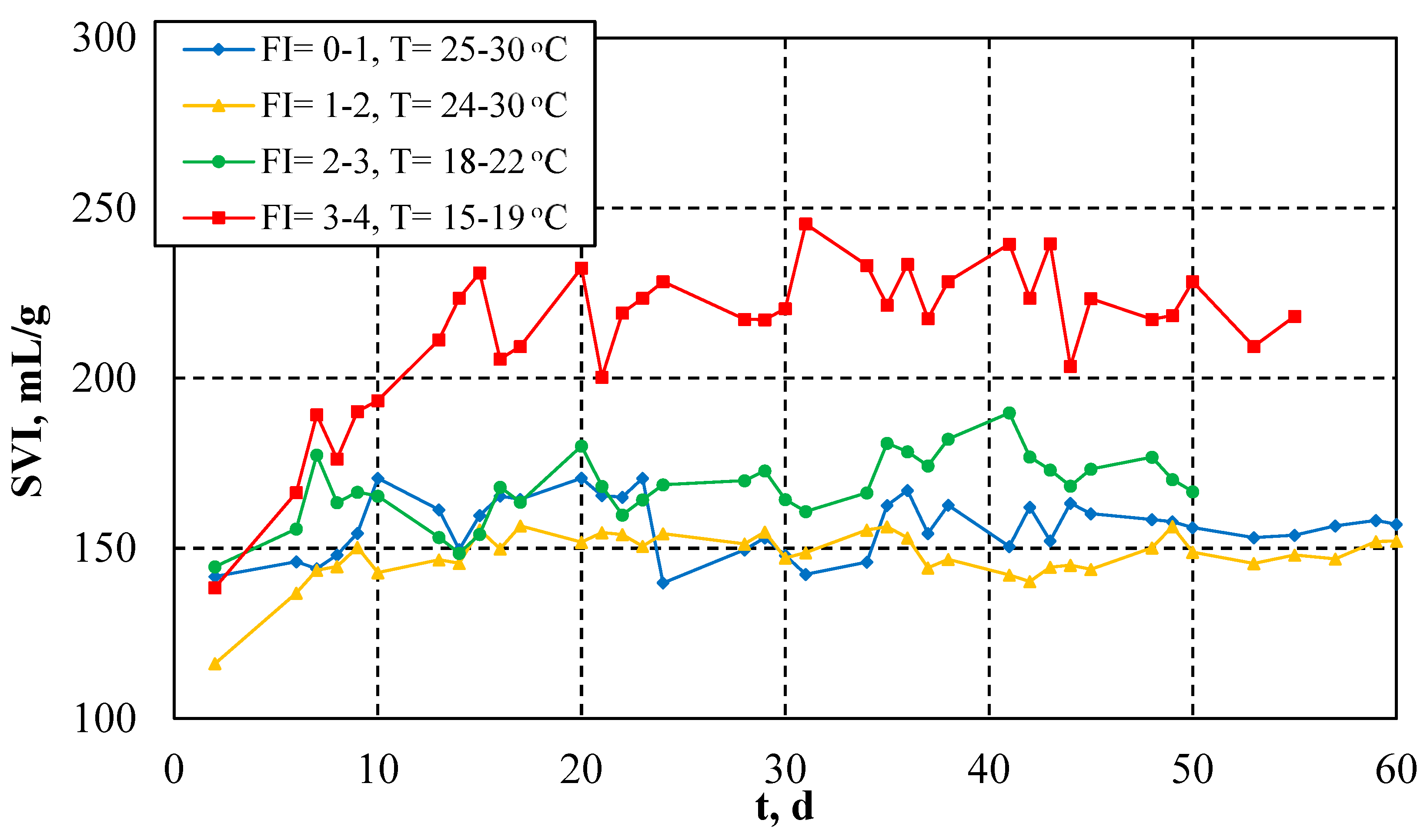
3.3. Effect of Filament Index (FI) and Temperature on Sludge Filterability and Settleability
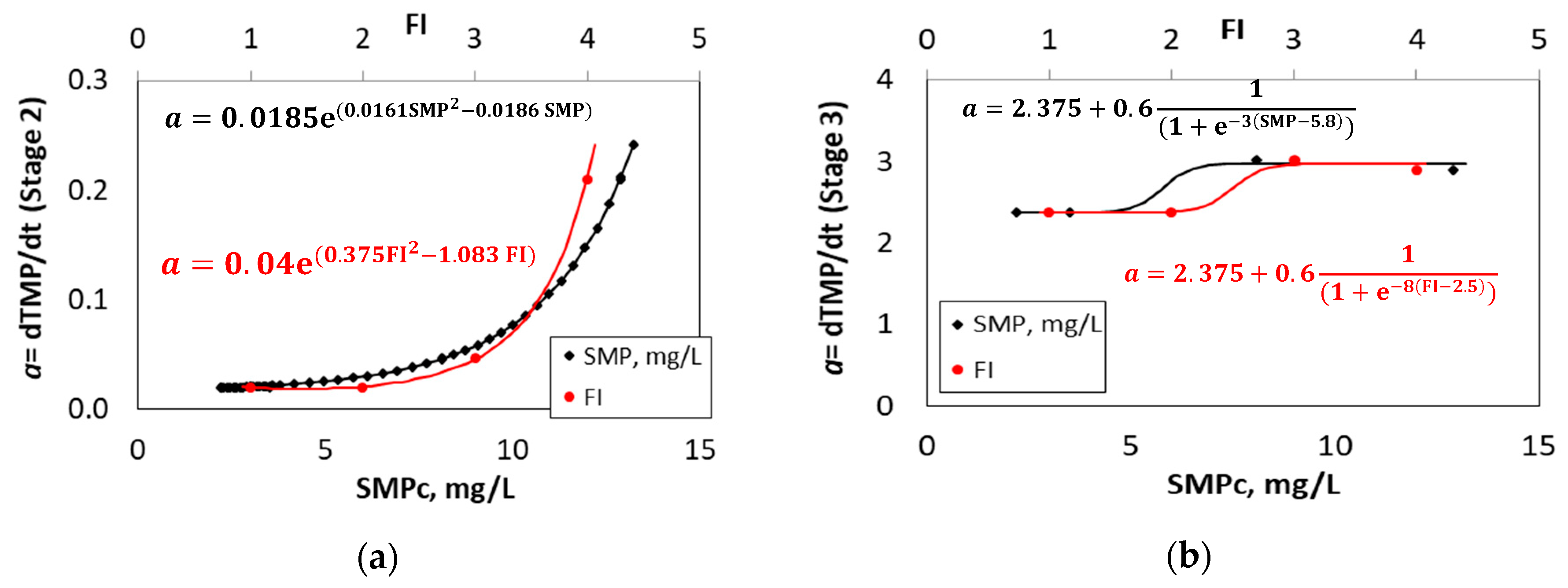
3.4. TMP Fitting Results and Development of a Novel Mathematical Equation
3.5. Effluent Quality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Description of the Eikelboom Method for the Estimation of Filaments’ Population
Appendix B
Description of the Phenol-Sulfuric Acid Method for the Determination of SMPc
References
- Yoon, S.-H. Membrane Bioreactor Processes: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gkotsis, P.K.; Zouboulis, A.I.; Mitrakas, M.M. Using Additives for Fouling Control in a Lab-Scale MBR.; Comparing the Anti-Fouling Potential of Coagulants, PAC and Bio-Film Carriers. Membranes 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Judd, S. The MBR book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed.; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Gkotsis, P.K.; Mitrakas, M.M.; Tolkou, A.K.; Zouboulis, A.I. Batch and continuous dosing of conventional and composite coagulation agents for fouling control in a pilot-scale MBR. Chem. Eng. J. 2017, 311, 255–264. [Google Scholar] [CrossRef]
- Gkotsis, P.K.; Banti, D.C.; Peleka, E.N.; Zouboulis, A.I.; Samaras, P.E. Fouling issues in membrane bioreactors (MBRs) for wastewater treatment: Major mechanisms, prevention and control strategies. Processes 2014, 2, 795–866. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Gkotsis, P.K.; Zouboulis, A.I. Biomass Characteristics and Their Effect on Membrane Bioreactor Fouling. Molecules 2019, 24, 2867. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Xie, K.; Sellamuthu, B. Role of adding dried sludge micropowder in aerobic granular sludge reactor with extended filamentous bacteria. Bioresour. Technol. Rep. 2019, 5, 51–58. [Google Scholar] [CrossRef]
- Li, W.-M.; Liao, X.-W.; Guo, J.-S.; Zhang, Y.-X.; Chen, Y.-P.; Fan, F.; Yan, P. New insights into filamentous sludge bulking: The potential role of extracellular polymeric substances in sludge bulking in the activated sludge process. Chemosphere 2020, 248, 126012. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, H.; Yang, F.; Li, Y.; Xiao, J.; Zhang, X. Effect of filamentous bacteria on membrane fouling in submerged membrane bioreactor. J. Membr. Sci. 2006, 272, 161–168. [Google Scholar] [CrossRef]
- Meng, F.; Yang, F.; Xiao, J.; Zhang, J.; Gong, Z. A new insight into membrane fouling mechanism during membrane filtration of bulking and normal sludge suspension. J. Membr. Sci. 2006, 285, 159–165. [Google Scholar] [CrossRef]
- Meng, F.; Yang, F. Fouling mechanisms of deflocculated sludge, normal sludge, and bulking sludge in membrane bioreactor. J. Membr. Sci. 2007, 305, 48–56. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Ohandja, D.G.; Yang, F.; Wong, F.S.; Chua, H.C. Impact of filamentous bacteria on properties of activated sludge and membrane-fouling rate in a submerged MBR. Sep. Purif. Technol. 2008, 59, 238–243. [Google Scholar] [CrossRef]
- Ma, C.; Jin, R.C.; Yang, G.F.; Yu, J.J.; Xing, B.S.; Zhang, Q.Q. Impacts of transient salinity shock loads on Anammox process performance. Bioresour. Technol. 2012, 112, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Liu, Q.S.; Liu, Y. Microscopic observation of aerobic granulation in sequential aerobic sludge blanket reactor. J. Appl. Microbiol. 2001, 91, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, P.; Wang, Q.; Wu, Z.; Zhou, Q.; Yang, D. Effective control of membrane fouling by filamentous bacteria in a submerged membrane bioreactor. Chem. Eng. J. 2010, 158, 608–615. [Google Scholar] [CrossRef]
- Hao, L.; Liss, S.N.; Liao, B.Q. Influence of COD: N ratio on sludge properties and their role in membrane fouling of a submerged membrane bioreactor. Water Res. 2016, 89, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Banti, D.C.; Karayannakidis, P.D.; Samaras, P.; Mitrakas, M.G. An innovative bioreactor set-up that reduces membrane fouling by adjusting the filamentous bacterial population. J. Membr. Sci. 2017, 542, 430–438. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Meng, X.; Fan, J.; Xia, S. New insight into the effects of Ca(II) on cake layer structure in submerged membrane bioreactors. Biofouling 2014, 30, 571–578. [Google Scholar] [CrossRef]
- Eikelboom, D.H. Process Control of Activated Sludge Plants by Microscopic Investigation, 1st ed.; IWA Publishing: Zutphen, The Netherlands, 2000. [Google Scholar]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- De la Torre, T.; Lesjean, B.; Drews, A.; Kraume, M. Monitoring of transparent exopolymer particles (TEP) in a membrane bioreactor (MBR) and correlation with other fouling indicators. Water Sci. Technol. 2008, 58, 1903–1909. [Google Scholar] [CrossRef]
- Rosenberger, S.; Kraume, M. Filterability of activated sludge in membrane bioreactors. Desalination 2002, 151, 195–200. [Google Scholar] [CrossRef]
- Hermia, J. Constant pressure blocking filtration laws—Application to power-law non-Newtonian fluids. Trans Istit. Chem. Eng. 1982, 60, 183–187. [Google Scholar]
- Hlavacek, M.; Bouchet, F. Constant flowrate blocking laws and an example of their application to deadend microfiltration of protein solutions. J. Membr. Sci. 1993, 82, 285–295. [Google Scholar] [CrossRef]
- Bolton, G.; LaCasse, D.; Kuriyel, R. Combined models of membrane fouling: Development and application to microfiltration and ultrafiltration of biological fluids. J. Membr. Sci. 2005, 277, 75–84. [Google Scholar] [CrossRef]
- Pajdak-Stós, A.; Fiałkowska, E. The influence of temperature on the effectiveness of filamentous bacteria removal from activated sludge by rotifers. Water Environ. Res. 2012, 84, 619–625. [Google Scholar] [CrossRef]
- Knoop, S.; Kunst, S. Influence of temperature and sludge loading on activated sludge settling, especially on Microthrix parvicella. Water Sci. Technol. 1998, 37, 27–35. [Google Scholar] [CrossRef]
- Krzeminski, P.; Iglesias-Obelleiro, A.; Madebo, G.; Garrido, J.M.; van dr Graaf, J.H.J.M.; van Lier, J.B. Impact of temperature on raw wastewater composition and activated sludge filterability in full-scale MBR systems for municipal sewage treatment. J. Membr. Sci. 2012, 423–424, 348–361. [Google Scholar] [CrossRef]
- Ma, C.; Yu, S.; Shi, W.; Heijman, S.G.J.; Rietveld, L.C. Effect of different temperatures on performance and membrane fouling in high concentration PAC-MBR system treating micro-polluted surface water. Bioresour. Technol. 2013, 141, 19–24. [Google Scholar] [CrossRef]
- Ma, Z.; Wen, X.; Zhao, F.; Xia, Y.; Huang, X.; Waite, D.; Guan, J. Effect of temperature variation on membrane fouling and microbial community structure in membrane bioreactor. Bioresour. Technol. 2013, 133, 462–468. [Google Scholar] [CrossRef]
- Van den Brink, P.; Satpradit, O.-A.; van Bentem, A.; Zwijnenburg, A.; Temmink, H.; van Loosdrecht, M. Effect of temperature shocks on membrane fouling in membrane bioreactors. Water Res. 2011, 45, 4491–4500. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, F.; Zhou, F.; Xue, Y. Control of COD/N ratio for nutrient removal in a modified membrane bioreactor (MBR) treating high strength wastewater. Bioresour. Technol. 2009, 100, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Liao, B.Q. Effect of organic matter to nitrogen ratio on membrane bioreactor performance. Environ. Technol. 2015, 36, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Ekama, G.A.; Capodici, M.; Cosenza, A.; Di Trapani, D.; Ødegaard, H. Moving bed membrane bioreactors for carbon and nutrient removal: The effect of C/N variation. Biochem. Eng. J. 2017, 125, 31–40. [Google Scholar] [CrossRef]


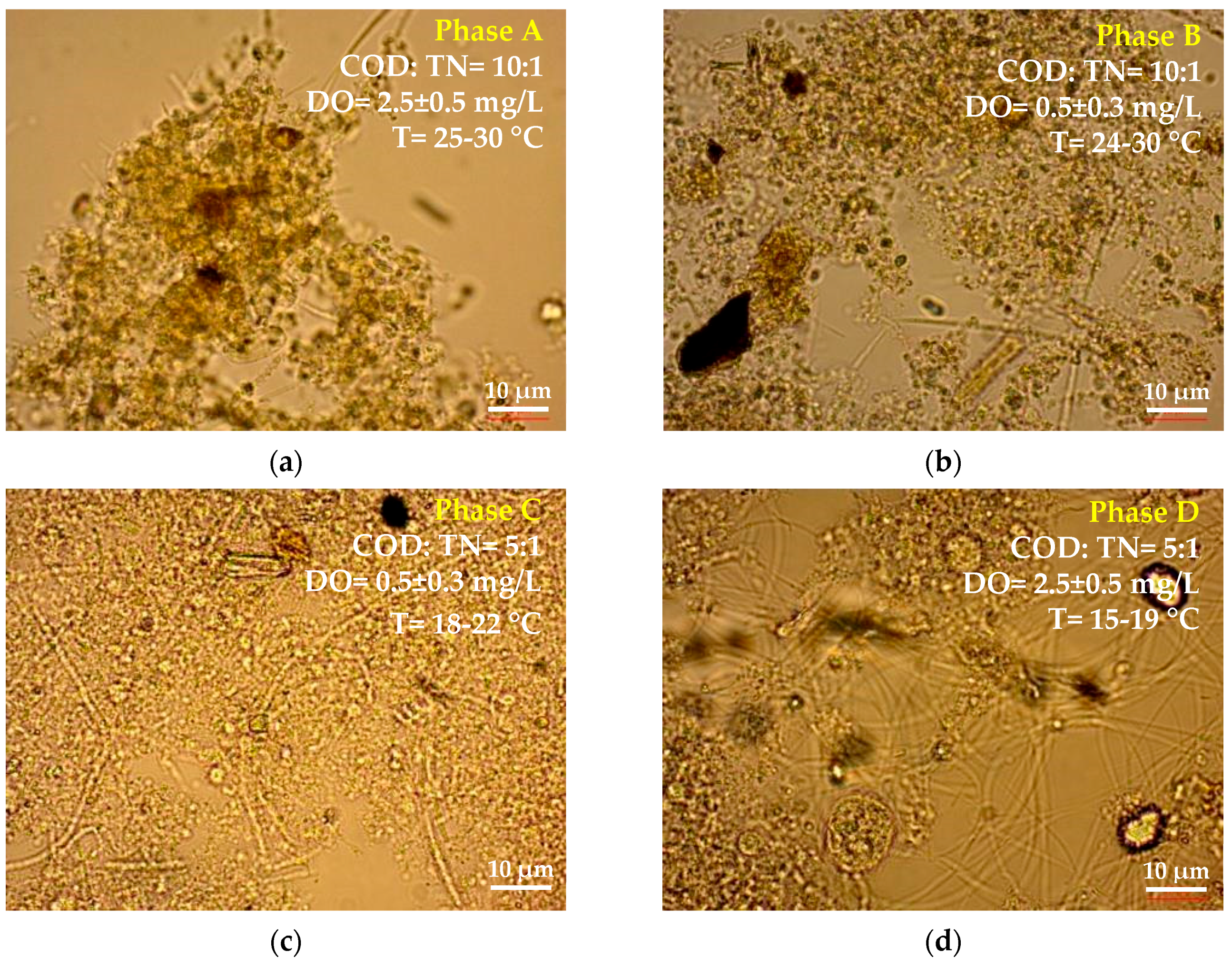



| Phase | COD to TN Ratio | DO Concentration 1, mg/L | T, °C |
|---|---|---|---|
| A | ~10:1 COD = 587.1 ± 77.1 mg/L TN = 58.6 ± 6.6 mg/L | 2.5 ± 0.5 | 25–30 |
| B | ~10:1 COD = 587.1 ± 77.1 mg/L TN = 58.6 ± 6.6 mg/L | 0.5 ± 0.3 | 24–30 |
| C | ~5:1 COD = 267.9 ± 34.3 mg/L TN = 52.5 ± 5.7 mg/L | 0.5 ± 0.3 | 18–22 |
| D | ~5:1 COD = 267.9 ± 34.3 mg/L TN = 52.5 ± 5.7 mg/L | 2.5 ± 0.5 | 15–19 |
| Basic Models—Hlavacek and Bouchet [26] | |
| Model | Equation |
| Cake blocking | TMP = TMP0 + kc2 TMP0t |
| Complete blocking | |
| Standard blocking | |
| Intermediate blocking | ln = ln + ki2 J0 t |
| Combined models—Bolton et al. [27] | |
| Model | Equation |
| Cake-intermediate blocking | |
| Cake-complete blocking | |
| Complete-standard blocking | |
| Intermediate-standard blocking | |
| Cake-standard blocking | |
| Sampling Site | Phase A (FI = 0–1) | |||
| COD, mg/L | TN, mg/L | NH4+-N, mg/L | NO3−-N, mg/L | |
| MBR inlet | 587.06 | 58.57 | 42.77 | 1.02 |
| DEN. tank | 21.45 | 18.93 | 11.32 | 2.13 |
| FIL. tank | 17.24 | 17.39 | 0.16 | 7.54 |
| MBR outlet | 16.21 | 13.81 | 0.11 | 12.22 |
| Sampling Site | Phase B (FI = 1–2) | |||
| COD, mg/L | TN, mg/L | NH4+-N, mg/L | NO3−-N, mg/L | |
| MBR inlet | 587.06 | 58.57 | 42.77 | 1.02 |
| DEN. tank | 24.67 | 16.46 | 12.84 | 1.83 |
| FIL. tank | 17.83 | 13.85 | 0.18 | 5.56 |
| MBR outlet | 15.44 | 12.39 | 0.10 | 11.10 |
| Sampling Site | Phase C (FI = 2–3) | |||
| COD, mg/L | TN, mg/L | NH4+-N, mg/L | NO3−-N, mg/L | |
| MBR inlet | 267.93 | 52.50 | 36.73 | 1.28 |
| DEN. tank | 23.40 | 25.43 | 17.65 | 5.03 |
| FIL. tank | 20.30 | 22.65 | 2.03 | 8.35 |
| MBR outlet | 19.73 | 18.79 | 0.54 | 15.39 |
| Sampling Site | Phase D (FI = 3–4) | |||
| COD, mg/L | TN, mg/L | NH4+-N, mg/L | NO3−-N, mg/L | |
| MBR inlet | 267.93 | 52.50 | 36.73 | 1.28 |
| DEN. tank | 29.35 | 30.45 | 20.10 | 6.76 |
| FIL. tank | 24.76 | 26.34 | 3.74 | 12.35 |
| MBR outlet | 22.60 | 21.70 | 0.76 | 16.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkotsis, P.; Lemonidis, G.; Mitrakas, M.; Pentedimos, A.; Kostoglou, M.; Zouboulis, A. Quantifying the Effect of COD to TN Ratio, DO Concentration and Temperature on Filamentous Microorganisms’ Population and Trans-Membrane Pressure (TMP) in Membrane Bio-Reactors (MBR). Processes 2020, 8, 1514. https://doi.org/10.3390/pr8111514
Gkotsis P, Lemonidis G, Mitrakas M, Pentedimos A, Kostoglou M, Zouboulis A. Quantifying the Effect of COD to TN Ratio, DO Concentration and Temperature on Filamentous Microorganisms’ Population and Trans-Membrane Pressure (TMP) in Membrane Bio-Reactors (MBR). Processes. 2020; 8(11):1514. https://doi.org/10.3390/pr8111514
Chicago/Turabian StyleGkotsis, Petros, Giannis Lemonidis, Manassis Mitrakas, Alexandros Pentedimos, Margaritis Kostoglou, and Anastasios Zouboulis. 2020. "Quantifying the Effect of COD to TN Ratio, DO Concentration and Temperature on Filamentous Microorganisms’ Population and Trans-Membrane Pressure (TMP) in Membrane Bio-Reactors (MBR)" Processes 8, no. 11: 1514. https://doi.org/10.3390/pr8111514
APA StyleGkotsis, P., Lemonidis, G., Mitrakas, M., Pentedimos, A., Kostoglou, M., & Zouboulis, A. (2020). Quantifying the Effect of COD to TN Ratio, DO Concentration and Temperature on Filamentous Microorganisms’ Population and Trans-Membrane Pressure (TMP) in Membrane Bio-Reactors (MBR). Processes, 8(11), 1514. https://doi.org/10.3390/pr8111514








