Drying Kinetics, Grinding Characteristics, and Physicochemical Properties of Broccoli Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Moisture Content and Water Activity
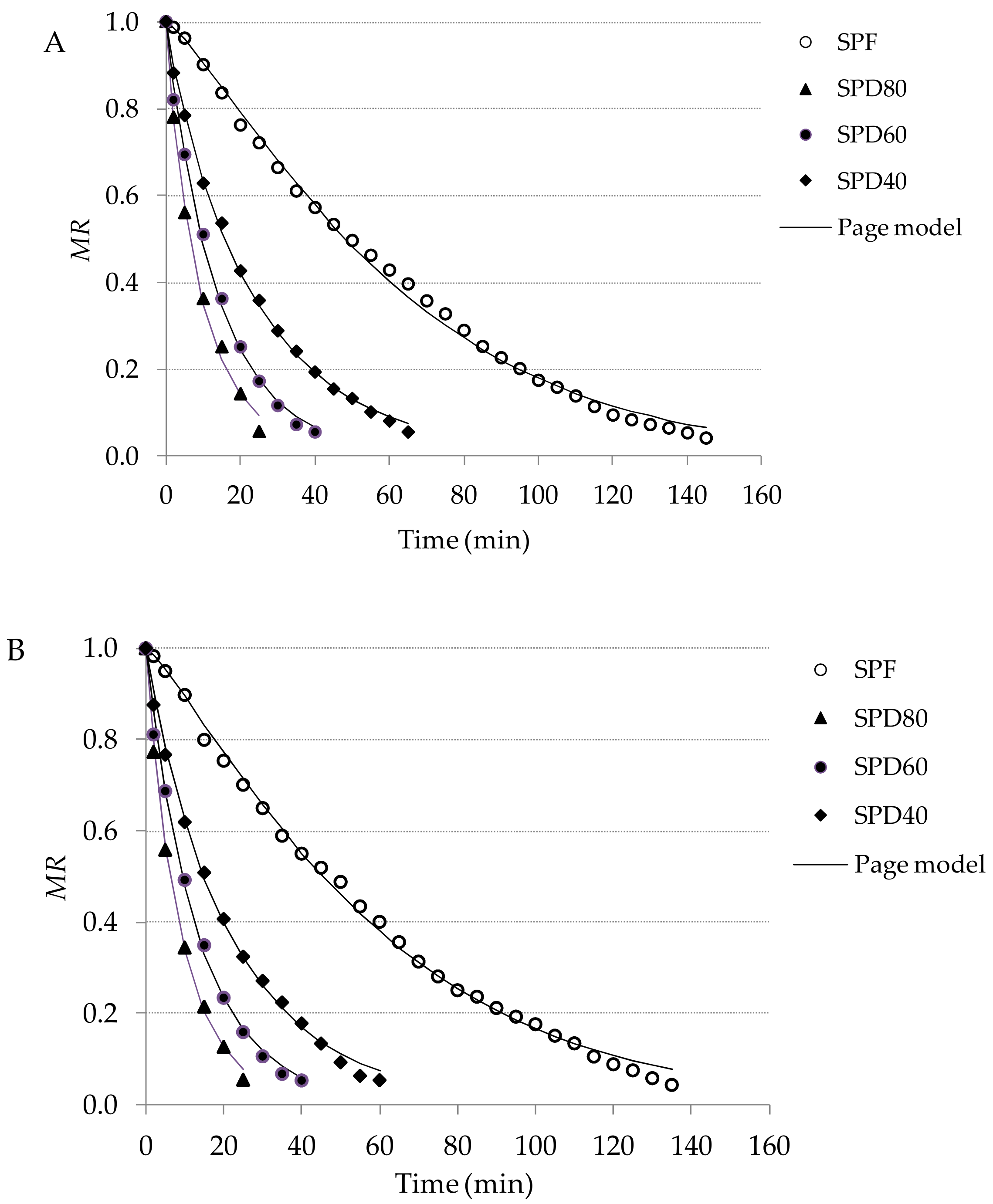
2.3. Drying Process
2.4. Grinding Process
2.5. Color Evaluation
2.6. Total Phenolics Content (TPC) and Antioxidant Activity (AA)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Moisture Content and Water Activity
3.2. Drying Results
3.3. Grinding Results
3.4. Color
3.5. TPC and AC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Šamec, D.; Pavlović, I.; Radojčić Redovniković, I.; Salopek-Sondi, B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five brassicaceae sprouts. Food Chem. 2018, 269, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xu, X.; Liu, Y.; Xie, L.; Pan, S. Effect of se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem. 2016, 190, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Steevens, J.; Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P.A. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int. J. Cancer 2011, 129, 2681–2693. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.K.; Kim, J.R.; Ahn, Y.J.; Shibamoto, T. Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2010, 58, 6672–6677. [Google Scholar] [CrossRef]
- Paśko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasińska, J.; Żmudzki, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S. Comparative study of predominant phytochemical compounds and proapoptotic potential of broccoli sprouts and florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef]
- Ding, J.; Yang, T.; Feng, H.; Dong, M.; Slavin, M.; Xiong, S.; Zhao, S. Enhancing contents of γ-aminobutyric acid (GABA) and other micronutrients in dehulled rice during germination under normoxic and hypoxic conditions. J. Agric. Food Chem. 2016, 64, 1094–1102. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Nowak, R.; Świeca, M.; Olech, M.; Pietrzak, W. Influence of sprouting and elicitation on phenolic acids profile and antioxidant activity of wheat seedlings. J. Cereal Sci. 2016, 70, 221–228. [Google Scholar] [CrossRef]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B. 2008, 9, 165–191. [Google Scholar] [CrossRef]
- Grewal, A.; Jood, S. Effect of processing treatments on nutritional and antinutritional contents of green gram. J. Food Biochem. 2006, 30, 535–546. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sugier, D. Improvement of nutraceutical value of broccoli sprouts by natural elicitors. Acta Sci. Pol. Hortoru. 2013, 12, 129–140. [Google Scholar]
- Vale, A.P.; Santos, J.; Melia, N.; Peixoto, V.; Brito, N.V.; Beatriz, M.; Oliveira, P.P. Phytochemical composition and antimicrobial properties of four varieties of brassica oleracea sprouts. Food Control 2015, 55, 248–256. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef]
- Hassini, I.; Martinez-Ballesta, M.C.; Boughanmi, N.; Moreno, D.A.; Carvajal, M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci. Hortic. Amst. 2017, 226, 141–151. [Google Scholar] [CrossRef]
- Bello, C.; Maldini, M.; Baima, S.; Scaccini, C.; Natella, F. Glucoraphanin and sulforaphane evolution during juice preparation from broccoli sprouts. Food Chem. 2018, 268, 249–256. [Google Scholar] [CrossRef]
- Liang, H.; Wei, Y.; Li, R.; Cheng, L.; Yuan, Q.; Zheng, F. Intensifying sulforaphane formation in broccoli sprouts by using other cruciferous sprouts additions. Food Sci. Biotechnol. 2018, 27, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Murashima, M.; Watanabe, S.; Zhuo, X.G.; Uehara, M.; Kurashige, A. Phase 1 study of multiple biomarkers for metabolism and oxidative stress after one-week intake of broccoli sprouts. Biofactors 2004, 22, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Marton, M.; Mandoki, Z.S.; Csapo-Kiss, Z.S.; Csapo, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae Aliment. 2010, 3, 81–117. [Google Scholar]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Valdenegro, M.; Almonacid, S.; Henríquez, C.; Lutz, M.; Fuentes, L.; Simpson, R. The effects of drying processes on organoleptic characteristics and the health quality of food ingredients obtained from goldenberry fruits (Physalis peruviana). Open Access Sci. Rep. 2013, 2, 2. [Google Scholar]
- Mattioli, S.; Dal Bosco, A.; Castellini, C.; Falcinelli, B.; Sileoni, V.; Marconi, O.; Mancinelli, A.C.; Cotozzolo, E.; Benincasa, P. Effect of heat- and freeze-drying treatments on phytochemical content and fatty acid profile of alfalfa and flax sprouts. J. Sci. Food Agric. 2019, 99, 4029–4035. [Google Scholar] [CrossRef]
- Złotek, U.; Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Nowak, R.; Martinez, E. Influence of drying temperature on phenolic acids composition and antioxidant activity of sprouts and leaves of white and red quinoa. J. Chem. 2019. [Google Scholar] [CrossRef]
- Awolu, O.O.; Manohar, B. Mechanical characteristics and grinding studies of mango seed kernel. Eng. Agric. Environ. Food 2018, 11, 256–261. [Google Scholar] [CrossRef]
- Wu, S.C.; Wu, S.H.; Chau, C.F. Improvement of the hypocholesterolemic activities of two common fruit fibers by micronization processing. J. Agric. Food Chem. 2009, 57, 5610–5614. [Google Scholar] [CrossRef] [PubMed]
- Platat, C.; Hillary, S.; Tomas-Barberan, F.A.; Martinez-Blazquez, J.A.; Al-Meqbali, F.; Souka, U.; Al-Hammadi, S.; Ibrahim, W. Urine metabolites and antioxidant effect after oral intake of date (phoenix dactylifera L.) seeds-based products (powder, bread and extract) by human. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jin, X.; Chen, X.D. Investigation of the effects of mechanical treatments on cellular structure integrity and vitamin C extractability of broccoli (Brassica oleracea L. var. Italica) by LF-NMR. Food Funct. 2018, 9, 2942–2950. [Google Scholar] [CrossRef]
- Yu, L.; Gao, B.; Li, Y.; Wang, T.T.Y.; Luo, Y.; Wang, J.; Yu, L. Home food preparation techniques impacted the availability of natural antioxidants and bioactivities in kale and broccoli. Food Funct. 2018, 9, 585–593. [Google Scholar] [CrossRef]
- Świeca, M.; Surdyka, M.; Gawlik-Dziki, U.; Złotek, U.; Baraniak, B. Antioxidant potential of fresh and stored lentil sprouts affected by elicitation with temperature stresses. Int. J. Food Sci. Tech. Technol. 2013, 49, 1811–1817. [Google Scholar] [CrossRef]
- Dimov, Z.; Suprianto, E.; Hermann, F.; Möllers, C. Genetic variation for seed hull and fibre content in a collection of european winter oilseed rape material (Brassica napus L.) and development of NIRS calibrations. Plant Breed. 2012, 131, 361–368. [Google Scholar] [CrossRef]
- Kowalski, S.J. Intensification of drying processes due to ultrasound enhancement. Chem. Process Eng. 2018, 39, 251–262. [Google Scholar]
- Rudy, S.; Dziki, D.; Krzykowski, A.; Gawlik-Dziki, U.; Polak, R.; Różyło, R.; Kulig, R. Influence of pre-treatments and freeze-drying temperature on the process kinetics and selected physico-chemical properties of cranberries (Vaccinium macrocarpon ait.). LWT Food Sci. Technol. 2015, 63, 497–503. [Google Scholar] [CrossRef]
- Sarimeseli, A. Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energ. Convers. Manag. 2011, 52, 1449–1453. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U.; Różyło, R.; Miś, A. Drying and grinding characteristics of four-day-germinated and crushed wheat: A novel approach for producing sprouted flour. Cereal Chem. 2015, 92, 312–319. [Google Scholar] [CrossRef]
- Dziki, D.; Polak, R.; Rudy, S.; Krzykowski, A.; Gawlik-Dziki, U.; Rózyło, R.; Miś, A.; Combrzyński, M. Simulation of the process kinetics and analysis of physicochemical properties in the freeze drying of kale. Int. Agrophys. 2018, 32, 49–56. [Google Scholar] [CrossRef]
- Adiletta, G.; Russo, P.; Senadeera, W.; Di Matteo, M. Drying characteristics and quality of grape under physical pretreatment. J. Food Eng. 2016, 172, 9–18. [Google Scholar] [CrossRef]
- Adiletta, G.; Wijerathne, C.; Senadeera, W.; Russo, P.; Crescitelli, A.; Matteo, M.D. Dehydration and rehydration characteristics of pretreated pumpkin slices. Ital. J. Food Sci. 2018, 30, 684–706. [Google Scholar]
- Doymaz, I. Drying kinetics of white mulberry. J. Food Eng. 2003, 61, 341–346. [Google Scholar] [CrossRef]
- Dziki, D.; Miś, A.; Gładyszewska, B.; Laskowski, J.; Kwiatkowski, S.; Gawlik-Dziki, U. Physicochemical and grinding characteristics of dragonhead seeds. Int. Agrophs. 2013, 27, 403–408. [Google Scholar] [CrossRef][Green Version]
- Dziki, D. Effect of preliminary grinding of the wheat grain on the pulverizing process. J. Food Eng. 2011, 104, 585–591. [Google Scholar] [CrossRef]
- Sokołowski, M. Energy consumed in grinding—A new idea of a general law of comminution—New tests stands and testing results. Récents Prog. Génie Procédés 1996, 10, 221–226. [Google Scholar]
- Alibas, I. Microwave, vacuum, and air-drying characteristics of collard leaves. Drying Technol. 2009, 27, 1266–1273. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Sęczyk, Ł.; Różyło, R.; Szymanowska, U. Bread enriched with Chenopodium quinoa leaves powder—The procedures for assessing the fortification efficiency. LWT Food Sci. Technol. 2015, 62, 1226–1234. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstics acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Guo, J.T.; Lee, H.L.; Chiang, S.H.; Lin, H.I.; Chang, C.Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction—Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Preziuso, M.; Panfili, G.; Cipriano, L.; Messia, M.C. Stabilization of sourdough starter by spray drying technique: New breadmaking perspective. LWT Food Sci. Technol. 2019, 99, 468–475. [Google Scholar] [CrossRef]
- Rahman, M.S. Food stability beyond water activity and glass transtion: Macro-micro region concept in the state diagram. Int. J. Food Prop. 2009, 12, 726–740. [Google Scholar] [CrossRef]
- Antal, T.; Tarek, M.; Tarek-Tilistyák, J.; Kerekes, B. Comparative effects of three different drying methods on drying kinetics and quality of jerusalem artichoke (Helianthus tuberosus L.). J. Food Process. Pres. 2017, 41, e12971. [Google Scholar] [CrossRef]
- Izli, N.; Izli, G.; Taskin, O. Impact of different drying methods on the drying kinetics, color, total phenolic content and antioxidant capacity of pineapple. CYTA J. Food 2018, 16, 213–221. [Google Scholar] [CrossRef]
- Różyło, R.; Rudy, S.; Krzykowski, A.; Dziki, D. Novel application of freeze-dried amaranth sourdough in gluten-free bread production. J. Food Process Eng. 2015, 38, 135–143. [Google Scholar] [CrossRef]
- Neethirajan, S.; Jayas, D.S.; White, N.D.G. Detection of sprouted wheat kernels using soft X-ray image analysis. J. Food Eng. 2007, 81, 509–513. [Google Scholar] [CrossRef]
- Dziki, D.; Laskowski, J. Study to analyze the influence of sprouting of the wheat grain on the grinding process. J. Food Eng. 2010, 96, 562–567. [Google Scholar] [CrossRef]
- Abasi, S.; Minaei, S. Effect of drying temperature on mechanical properties of dried corn. Drying Technol. 2014, 32, 774–780. [Google Scholar] [CrossRef]
- Valdivia-López, M.Á.; Tecante, A. Chia (Salvia hispanica): A review of native mexican seed and its nutritional and functional properties. Adv. Food Nutr. Res. 2015, 75, 53–75. [Google Scholar] [PubMed]
- Chunthaworn, S.; Achariyaviriya, S.; Achariyaviriya, A.; Namsanguan, K. Color kinetics of longan flesh drying at high temperature. Pap. Presented Procedia Eng. 2012, 32, 104–111. [Google Scholar] [CrossRef]
- Orak, H.H.; Aktas, T.; Yagar, H.; Isbilir, S.S.; Ekinci, N.; Sahin, F.H. Effects of hot air and freeze drying methods on antioxidant activity, colour and some nutritional characteristics of strawberry tree (arbutus unedo L) fruit. Food Sci. Technol. Int. 2012, 18, 391–402. [Google Scholar] [CrossRef]
- Albanese, D.; Cinquanta, L.; Cuccurullo, G.; Di Matteo, M. Effects of microwave and hot- air-drying methods on color β-carotene and radical scavenging activity of apricots. Int. J. Food Sci. Tech. 2013, 48, 1327–1333. [Google Scholar] [CrossRef]
- Falcinelli, B.; Maranghi, S.; Paoletti, A.; Marconi, O.; Rosati, A.; Famiani, F.; Benincasa, P. Sprouting olive (Olea europaea L.) seeds as a source of antioxidants from residual whole stones. Sci. Hortic. Amst. 2018, 240, 558–560. [Google Scholar] [CrossRef]
- Gan, R.Y.; Lui, W.Y.; Chan, C.L.; Corke, H. Hot air drying induces browning and enhances phenolic content and antioxidant capacity in mung bean (Vigna radiata L.) sprouts. J. Food Process. Pres. 2016, 41. [Google Scholar] [CrossRef]
| Germination Time (Days) | Drying Method | k (min−1) | n | R2 | RSME |
|---|---|---|---|---|---|
| 3 | SPD40 | 0.0519 | 0.9368 | 0.999 | 0.00698 |
| SPD60 | 0.0822 | 0.9584 | 0.997 | 0.01609 | |
| SPD80 | 0.1309 | 0.9215 | 0.998 | 0.02083 | |
| SPF | 0.0070 | 1.2053 | 0.997 | 0.01540 | |
| 6 | SPD40 | 0.0520 | 0.9367 | 0.999 | 0.01495 |
| SPD60 | 0.0773 | 0.9667 | 0.997 | 0.01687 | |
| SPD80 | 0.9001 | 0.1310 | 0.997 | 0.01262 | |
| SPF | 0.0055 | 1.2469 | 0.997 | 0.01687 |
| Range of Class, mm | Seeds | SPD40 | SPD60 | SPD80 | SPF |
|---|---|---|---|---|---|
| After 3 Days of Germination | |||||
| >0.8 | 8.2 ± 0.21 fD | 7.1 ± 0.18 fC | 6.9 ± 0.15 bC | 5.8 ± 0.11 fB | 4.9 ± 0.08 aA |
| 0.6–0.8 | 21.9 ± 0.53 eC | 8.8 ± 0.26 eA | 8.0 ± 0.45 eA | 8.8 ± 0.36 eA | 8.5 ± 0.87 eB |
| 0.4–0.6 | 35.8 ± 0.79 dA | 39.6 ± 1.12 dB | 42.1 ± 1.18 dC | 43.2 ± 1.36 dC | 43.6 ± 1.02 dB |
| 0.2–0.4 | 28.9 ± 0.63 cA | 35.1 ± 0.99 cD | 33.3 ± 1.32 cC | 32.6 ± 1.18 cC | 31.6 ± 0.65 cB |
| 0.1–0.2 | 4.1 ± 0.11 bB | 7.2 ± 0.11 bC | 6.9 ± 0.13 bCD | 7.4 ± 0.08 bA | 7.3 ± 0.21 bD |
| <0.1 | 1.1 ± 0.08 aA | 2.2 ± 0.05 aB | 2.8 ± 0.11 aC | 2.2 ± 0.07 aB | 4.1 ± 0.19 bD |
| After 6 days of germination | |||||
| >0.8 | 8.2 ± 0.21 fD | 6.7 ± 0.26 fC | 6.6 ± 0.31 bC | 5.4 ± 0.23 fB | 4.3 ± 0.18 aA |
| 0.6–0.8 | 21.9 ± 0.53 eC | 8.7 ± 0.33 eA | 8.2 ± 0.38 eA | 8.9 ± 0.31 eA | 8.3 ± 0.65 eB |
| 0.4–0.6 | 35.8 ± 0.79 dA | 41.3 ± 1.26 dB | 41.6 ± 1.72 dC | 42.1 ± 1.88 dC | 43.5 ± 1.13 dB |
| 0.2–0.4 | 28.9 ± 0.63 cA | 33.2 ± 1.28 cD | 33.5 ± 1.32 cD | 33.8 ± 1.18 cD | 31.2 ± 0.82 cB |
| 0.1–0.2 | 4.1 ± 0.11 bB | 6.6 ± 0.23 bC | 6.6 ± 0.18 bCD | 7.2 ± 0.14 bA | 6.9 ± 0.19 bD |
| <0.1 | 1.1 ± 0.08 aA | 2.5 ± 0.07 aB | 3.5 ± 0.12 aC | 2.6 ± 0.11 aB | 4.8 ± 0.23 bD |
| Sample | Time of Germination (Days) | dp (mm) | Er (kJ kg−1) | Ef (m2 kJ−1) | Ks (kJ kg−1 mm0.5) |
|---|---|---|---|---|---|
| Seeds | - | 0.508 ± 0.026 b | 12.2 ± 0.63 d | 0.74 ± 0.042 a | 17.7 ± 0.92 e |
| SPD40 | 3 | 0.457 ± 0.011 b | 10.5 ± 0.57 c | 0.98 ± 0.051 b | 13.5 ± 0.56 d |
| 6 | 0.452 ± 0.008 b | 10.1 ± 0.51 c | 1.03 ± 0.055 bc | 12.8 ± 0.84 cd | |
| SPD60 | 3 | 0.449 ± 0.013 ab | 9.8 ± 0.46 c | 1.05 ± 0.017 bc | 12.6 ± 0.78 c |
| 6 | 0.447 ± 0.007 ab | 9.5 ± 0.35 bc | 1.09 ± 0.056 bc | 12.2 ± 0.61 bc | |
| SPD80 | 3 | 0.449 ± 0.023 ab | 9.5 ± 0.34 b | 1.08 ± 0.062 bc | 12.2 ± 0.43 bc |
| 6 | 0.445 ± 0.027 ab | 9.4 ± 0.44 b | 1.10 ± 0.068 bc | 12.0 ± 0.72 bc | |
| SPF | 3 | 0.434 ± 0.013 a | 9.1 ± 0.42 ab | 1.16 ± 0.049 c | 11.4 ± 0.35 b |
| 6 | 0.430 ± 0.012 a | 8.5 ± 0.38 a | 1.26 ± 0.073 d | 10.5 ± 0.37 a |
| Sample | Germination Time (Days) | L* (mm) | a* | b* | ΔE |
|---|---|---|---|---|---|
| Seeds | - | 59.9 ± 1.28 b | 6.7 ± 0.23 d | 30.9 ± 1.22 dc | - |
| SPD40 | 3 | 44.6 ± 1.16 a | 7.8 ± 0.31 e | 29.1 ± 1.17 bc | 15.4 ± 0.37 c |
| 6 | 43.1 ± 1.52 a | 7.3 ± 0.25 e | 28.2 ± 1.31 ab | 17.1 ± 0.46 d | |
| SPD60 | 3 | 44.2 ± 1.23 a | 4.8 ± 0.18 c | 27.8 ± 1.28 a | 16.4 ± 0.58 d |
| 6 | 43.2 ± 0.94 a | 7.4 ± 0.37 e | 28.4 ± 1.09 ab | 16.7 ± 0.64 d | |
| SPD80 | 3 | 44.8 ± 0.88 a | 7.6 ± 0.32 e | 28.7 ± 1.23 ab | 15.3 ± 0.45 c |
| 6 | 45.5 ± 0.96 a | 8.6 ± 0.41 f | 31.1 ± 1.52 cd | 14.8 ± 0.46 c | |
| SPF | 3 | 62.3 ± 1.27 c | 1.5 ± 0.12 a | 29.6 ± 1.19 bc | 5.9 ± 0.38 b |
| 6 | 60.9 ± 1.33 bc | 2.6 ± 0.22 b | 32.2 ± 1.07 d | 4.4 ± 0.31 a |
| Sample | Germination Time (Days) | TPC (mg GAE/g DM) | ABTS EC50 (mg DM/mL) | CHEL EC50 (mg DM/mL) | RED (mg DM/mL) |
|---|---|---|---|---|---|
| Seeds | - | 7.6 ± 0.26 a | 164.3 ± 6.8 h | 76.3 ± 3.7 e | 53.2 ± 2.1 e |
| Fresh sprouts | 3 | 12.6 ± 044 e | 111.2 ± 4.3 c | 48.8 ± 2.6 b | 28.4 ± 1.2 a |
| 6 | 13.5 ± 0.39 fg | 99.7 ± 4.2 a | 56.7 ± 2.8 c | 30.6 ± 2.5 ab | |
| SPD40 | 3 | 11.8 ± 0.36 cd | 125.8 ± 6.2 e | 65.6 ± 4.2 d | 30.1 ± 1.6 a |
| 6 | 14.2 ± 0.28 g | 119.6 ± 5.7 cd | 72.3 ± 4.1 e | 32.8 ± 1.5 cd | |
| SPD60 | 3 | 11.4 ± 0.42 c | 132.3 ± 5.8 f | 88.4 ± 4.8 f | 31.7 ± 1.8 bc |
| 6 | 12.5 ± 0.18 e | 117.8 ± 4.5 cd | 91.4 ± 4.5 f | 32.6 ± 1.1 bc | |
| SPD80 | 3 | 10.4 ± 0.12 b | 150.9 ± 6.7 g | 112.5 ± 5.3 g | 33.2 ± 1.3 bc |
| 6 | 13.1 ± 0.21 f | 146.7 ± 6.3 g | 132.8 ± 4.2 h | 35.6 ± 1.6 d | |
| SPF | 3 | 12.2 ± 0.32 de | 121.2 ± 3.2 de | 42.1 ± 1.7 a | 30.5 ± 0.7 a |
| 6 | 14.7 ± 0.17 h | 104.2 ± 5.2 b | 57.3 ± 2.3 c | 31.2 ± 1.9 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziki, D.; Habza-Kowalska, E.; Gawlik-Dziki, U.; Miś, A.; Różyło, R.; Krzysiak, Z.; Hassoon, W.H. Drying Kinetics, Grinding Characteristics, and Physicochemical Properties of Broccoli Sprouts. Processes 2020, 8, 97. https://doi.org/10.3390/pr8010097
Dziki D, Habza-Kowalska E, Gawlik-Dziki U, Miś A, Różyło R, Krzysiak Z, Hassoon WH. Drying Kinetics, Grinding Characteristics, and Physicochemical Properties of Broccoli Sprouts. Processes. 2020; 8(1):97. https://doi.org/10.3390/pr8010097
Chicago/Turabian StyleDziki, Dariusz, Ewa Habza-Kowalska, Urszula Gawlik-Dziki, Antoni Miś, Renata Różyło, Zbigniew Krzysiak, and Waleed H. Hassoon. 2020. "Drying Kinetics, Grinding Characteristics, and Physicochemical Properties of Broccoli Sprouts" Processes 8, no. 1: 97. https://doi.org/10.3390/pr8010097
APA StyleDziki, D., Habza-Kowalska, E., Gawlik-Dziki, U., Miś, A., Różyło, R., Krzysiak, Z., & Hassoon, W. H. (2020). Drying Kinetics, Grinding Characteristics, and Physicochemical Properties of Broccoli Sprouts. Processes, 8(1), 97. https://doi.org/10.3390/pr8010097







