Synergistic Effect on the Non-Oxygenated Fraction of Bio-Oil in Thermal Co-Pyrolysis of Biomass and Polypropylene at Low Heating Rate
Abstract
1. Introduction
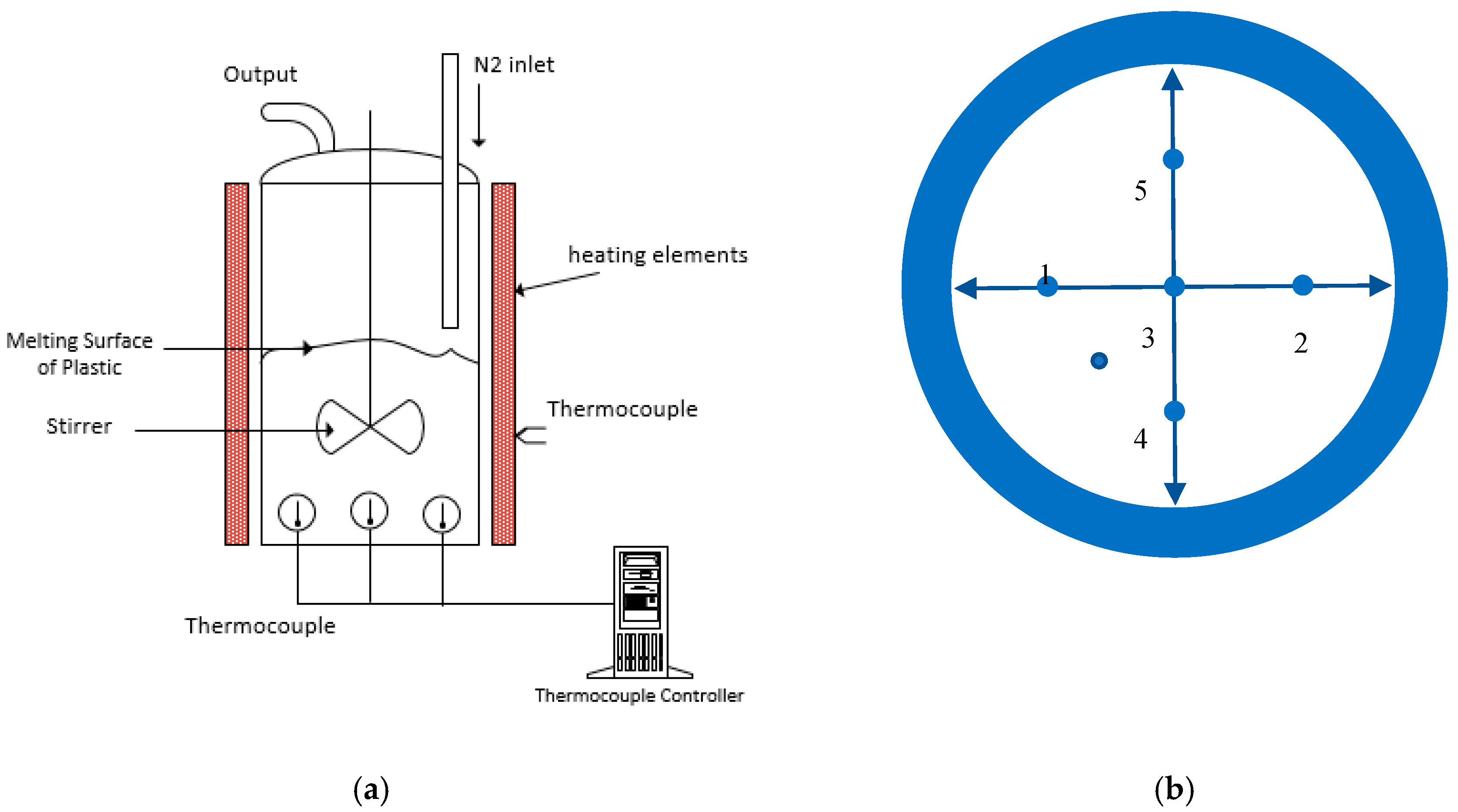
2. Material and Method
3. Results and Discussion
3.1. Non-Oxygenated Fraction in Bio-Oil
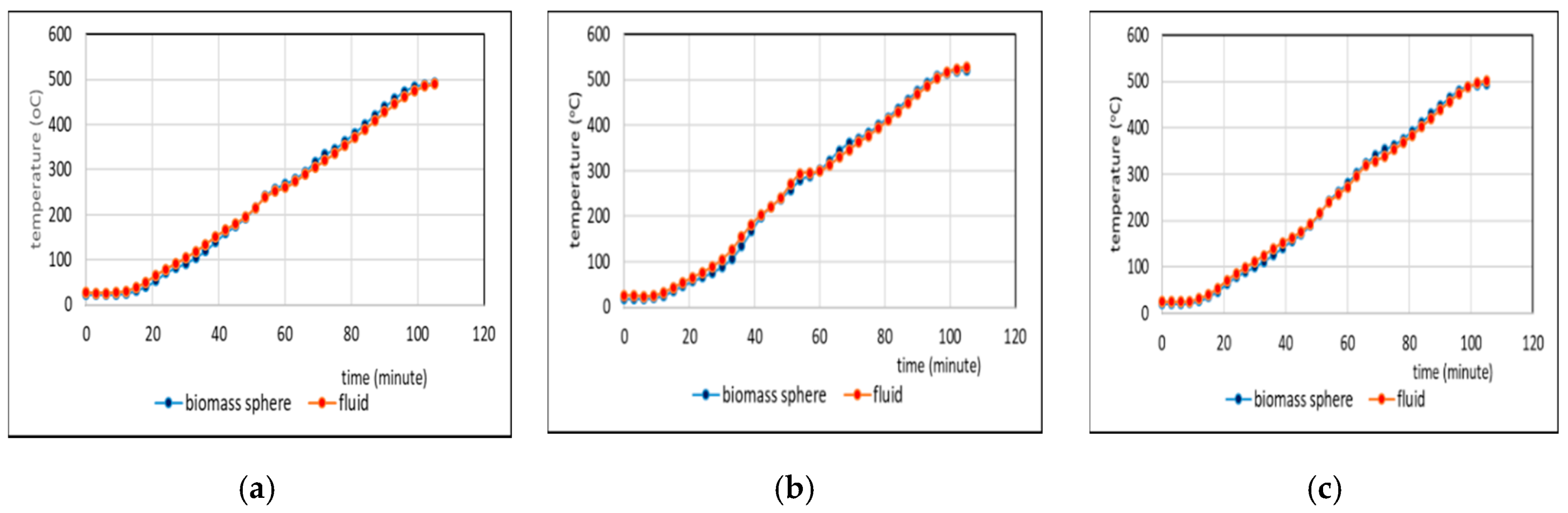
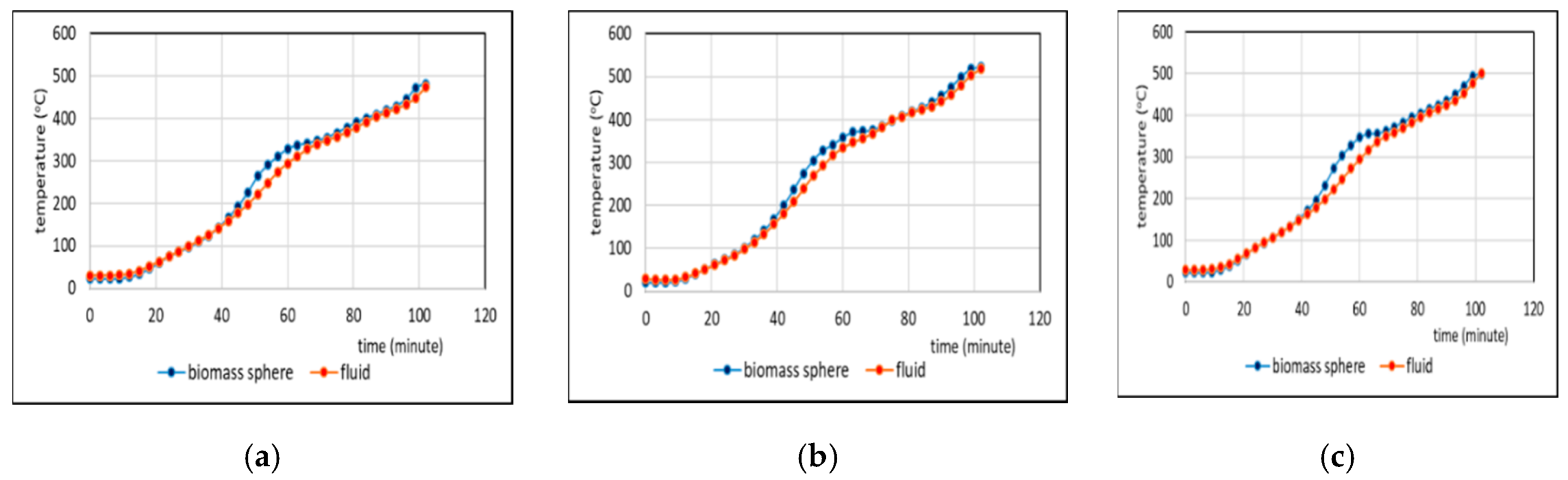
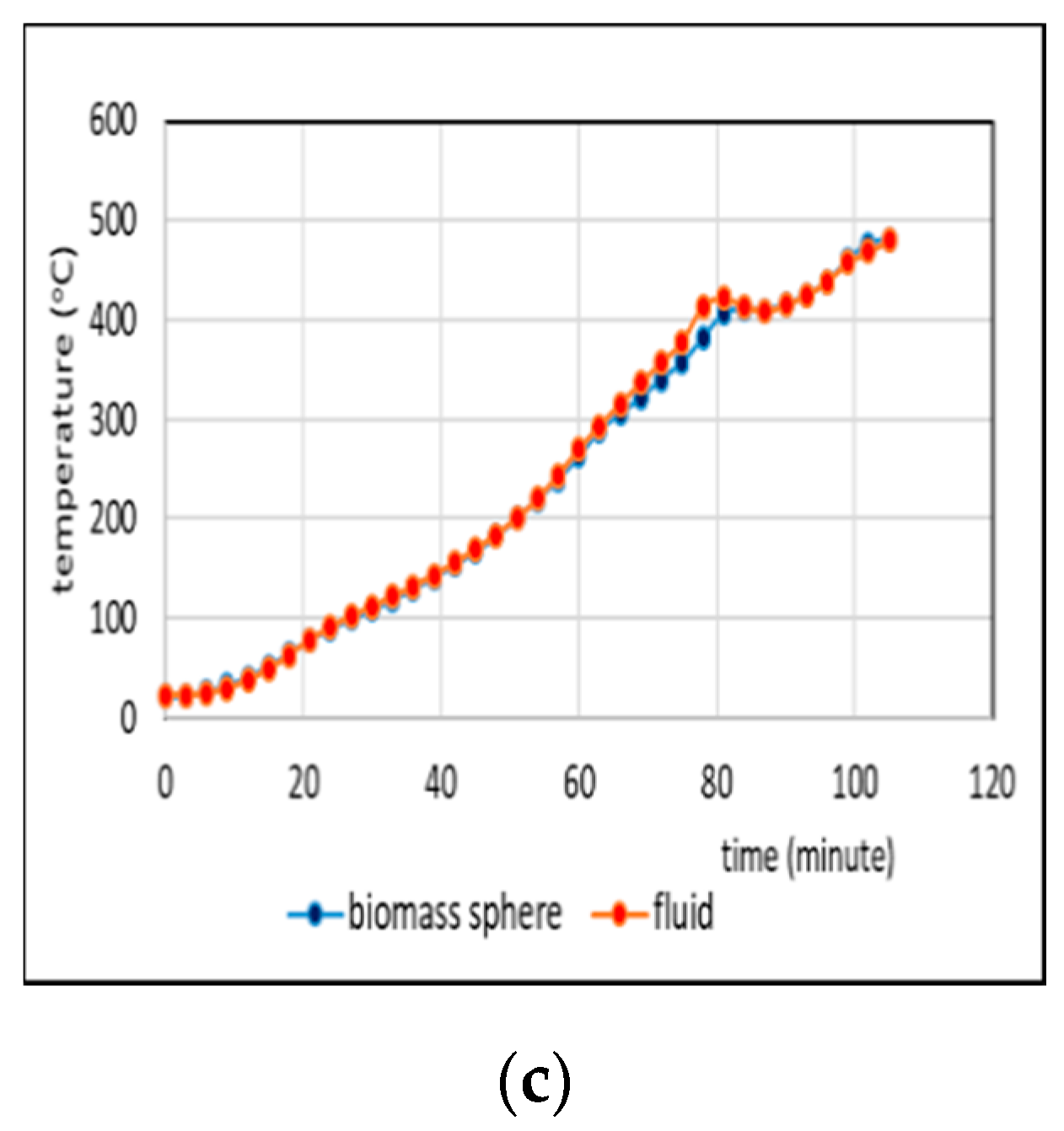
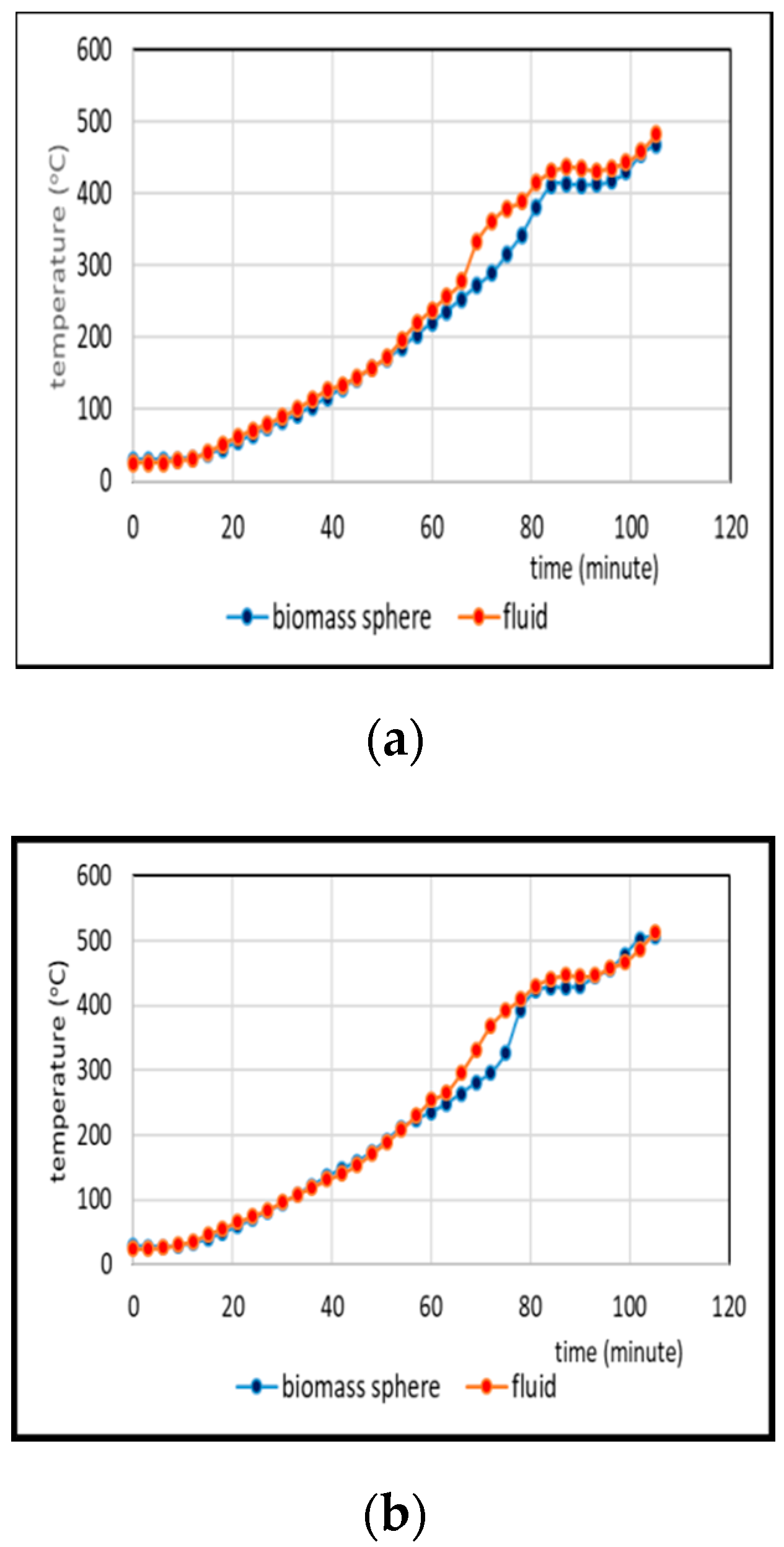
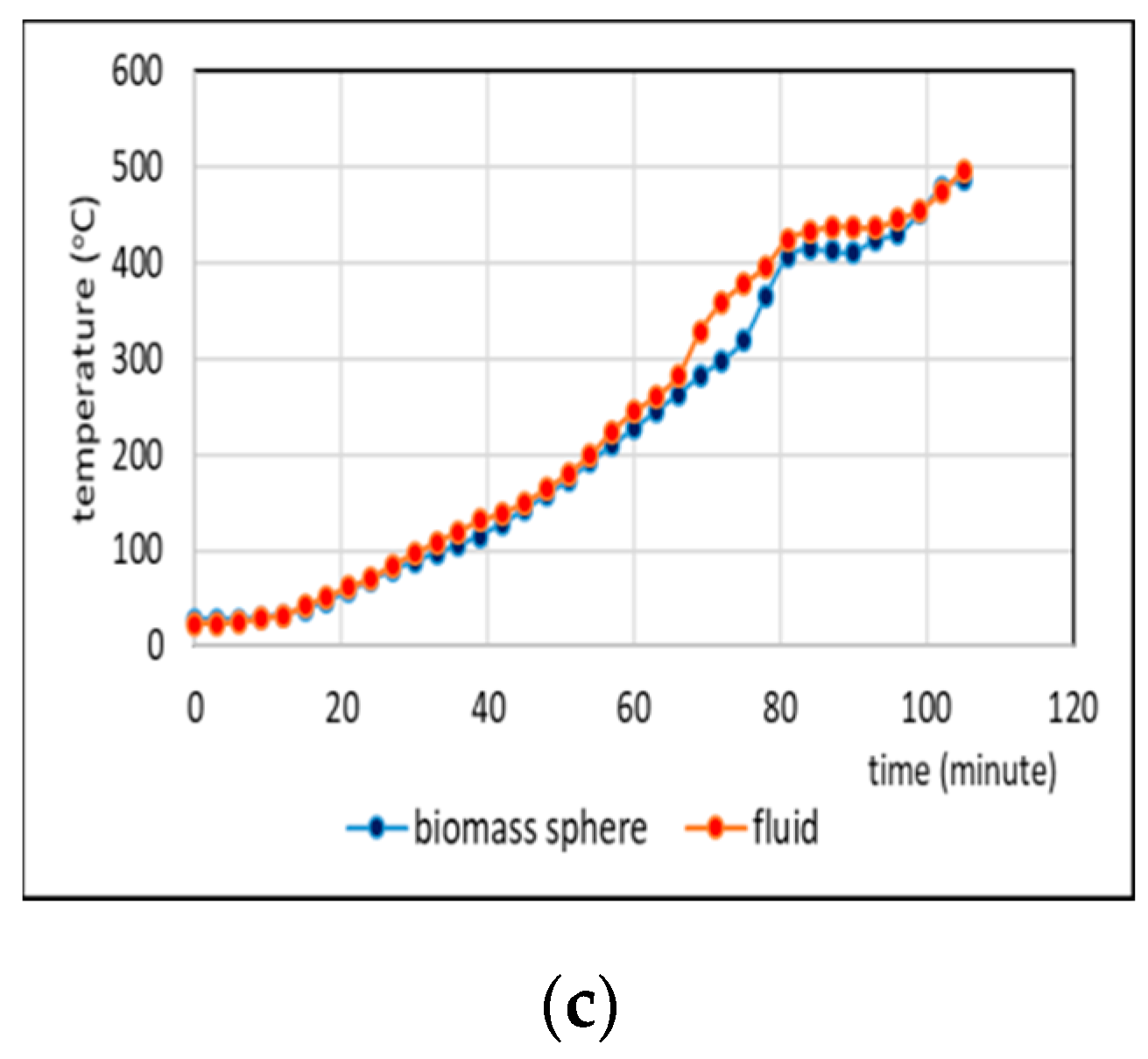
3.2. Comparison of Temperatures of Biomass Spheres and Pyrolysis Fluid
3.2.1. Co-Pyrolysis Using Feeds Containing 0 and 25% Weight of PP
3.2.2. Co-Pyrolysis Using Feeds Containing 50%, and 75% Weight of PP in Regime 2
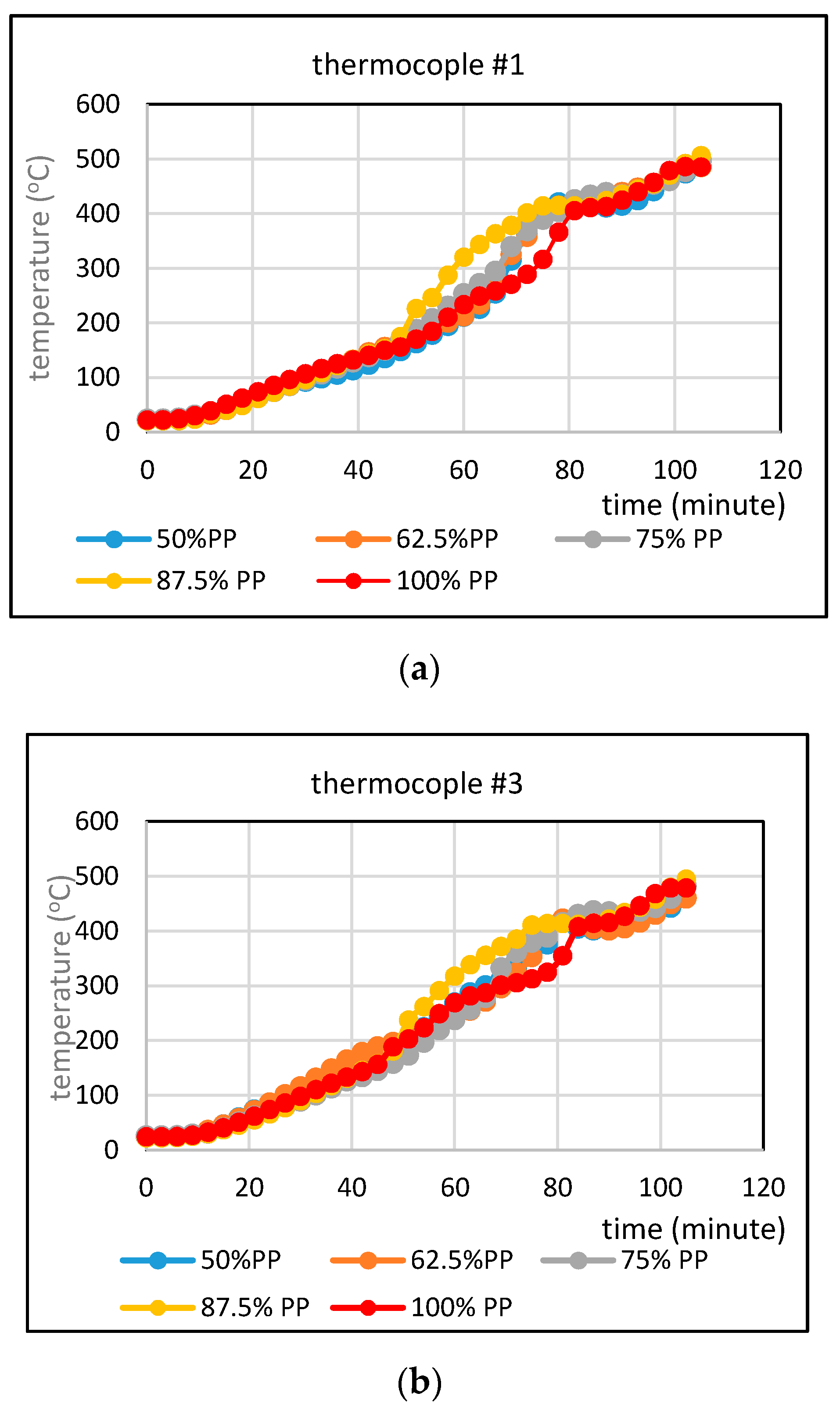
3.3. Comparison of Temperatures of Pyrolysis Fluid at Different Feed Compositions in Regime 2
3.4. H-NMR of Non-Oxygenated Fraction of Bio-Oil and Wax
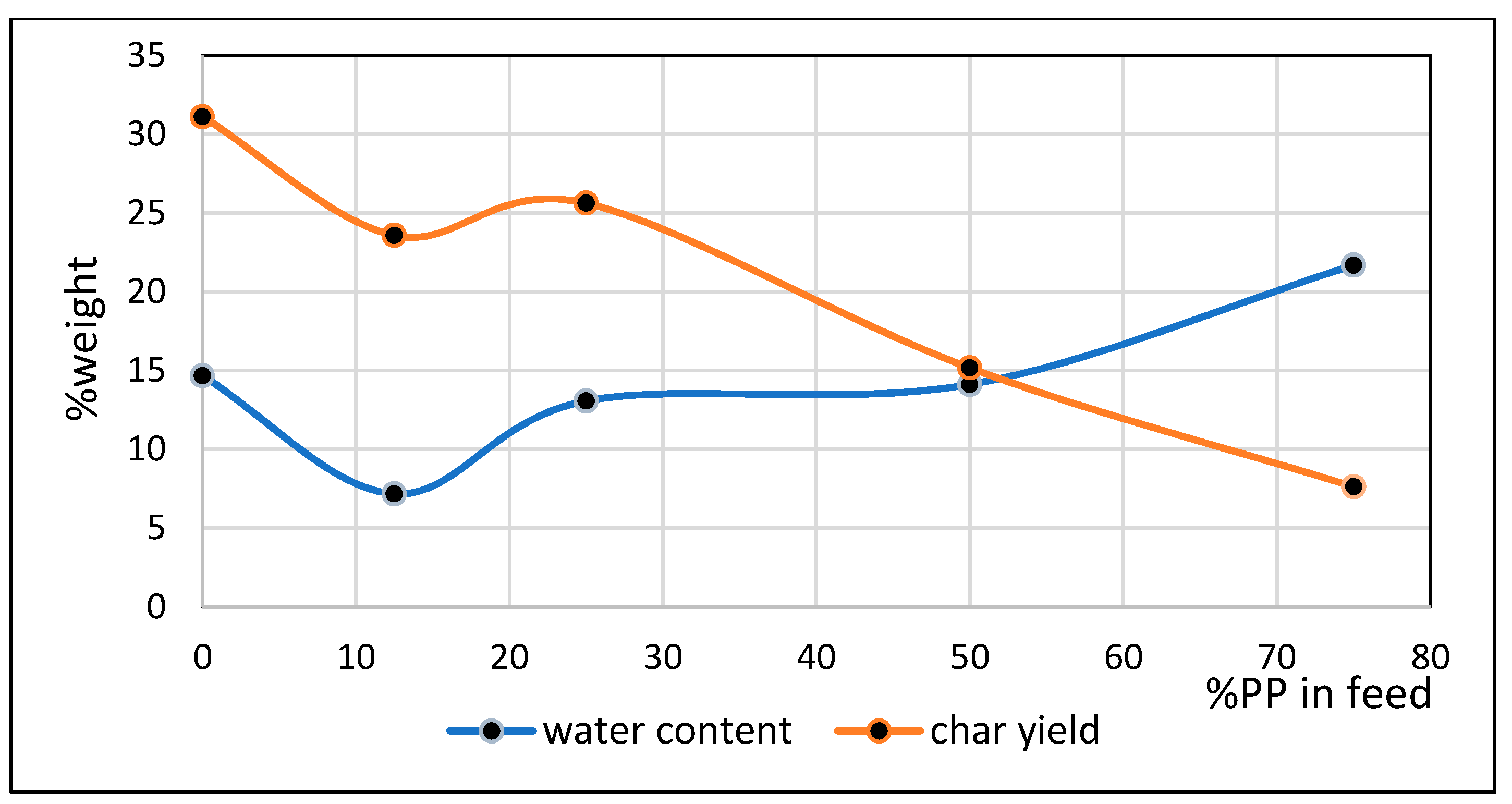
3.5. Water Content in Oxygenated Fraction of Bio-Oil
3.6. Chemical Properties of the Non-Polar Fraction of Bio-Oil and Diesel Fuel
4. Conclusions
- (1)
- Comparison of temperatures of pyrolysis fluid and biomass spheres indicates that in regime 1 convective and radiative heat transfers sparingly occurred and synergistic effect on the yield of non-oxygenated phase increased with increasing convective heat transfer at increasing %PP in feed, while in regime 2, convective heat transfer was predominant with the synergistic effect reducing at increasing %PP in feed. In the later regime the reduction of synergistic effect occurred at increasing %PP composition in the feed due to more disappearance of H radicals to form water. The optimum PP composition in the feed to reach maximum synergistic effect was 50%.
- (2)
- In co-pyrolysis, non-oxygenated phase portion in the reactor leading to the wax formation acted as donor of methyl and hydrogen radicals in the removal of oxygen in the synergistic effect. Maximum donation occurred at optimum PP composition in the feed.
- (3)
- The non-oxygenated fractions of bio-oil contained mostly methyl groups comprising about 53% by mole fraction irrespective of the PP composition employed in the co-pyrolysis. By comparison, commercial diesel contains mostly methylenes comprising about 59% by mole fraction. Non-oxygenated fractions exhibited branching indices about 50% higher than that of commercial diesel.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Khor, K.H.; Lim, K.O.; Zainal, Z.A. Characterization of bio-oil: A by-product from slow pyrolysis of oil palm empty fruit bunches. Am. J. Appl. Sci. 2009, 6, 1647–1652. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An Overview on catalytic hydrodeoxygenation of pyrolysis oil and its model compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. A Review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Bahrami, A.; Soltani, N.; Pech-Canul, M.I.; Gutiérrez, C.A. Development of metal-matrix composites from industrial/agricultural waste materials and their derivatives. Crit. Rev. Environ. Sci. Technol. 2016, 46, 143–208. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; Poduval, D.G.; Degirmenci, V.; Ligthart, D.; Chen, W.B.; Mauge, F.; Rigutto, M.S.; van Veen, J.A.R. Acidity characterization of amorphous silica-alumina. J. Phys. Chem. C 2012, 116, 21416–21429. [Google Scholar] [CrossRef]
- Crepeau, G.; Montouillout, V.; Vimont, A.; Mariey, L.; Cseri, L.; Mauge, F. Nature, structure and strength of the acidic sites of amorphous silica alumina: An IR and NMR study. J. Phys. Chem. B 2006, 110, 15172–15185. [Google Scholar] [CrossRef]
- Supramono, D.; Setiadi, H.; Nasikin, M. Phase separation of bio-oil produced by co-pyrolysis of corn cobs and polypropylene. IOP Conf. Ser. Earth Environ. Sci. 2017, 93, 012072. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Polycyclic aromatic hydrocarbon formation from the pyrolysis/gasification of lignin at different reaction conditions. Energy Fuels 2014, 28, 6371–6379. [Google Scholar] [CrossRef]
- Ben, H.X.; Ragauskas, A.J. Comparison for the compositions of fast and slow pyrolysis oils by NMR characterization. Bioresour. Technol. 2013, 147, 577–584. [Google Scholar] [CrossRef]
- Vollmer, D. Thermodynamics and phase-separation kinetics of microemulsions in thermal behavior of dispersed systems. Surfactant Sci. Ser. 2000, 93, 23. [Google Scholar]
- Lamorgese, A.G.; Mauri, R. Effect of viscosity ratio on structure evolution during mixing/demixing of regular binary mixtures. Chem. Eng. Trans. 2017, 57, 1225–1230. [Google Scholar]
- Brebu, M.; Ucar, S.; Vasile, C.; Yanik, J. Co-pyrolysis of pine cone with synthetic polymers. Fuel 2010, 89, 1911–1918. [Google Scholar] [CrossRef]
- Onal, E.; Uzun, B.B.; Putun, A.E. Bio-oil production via co-pyrolysis of almond shell as biomass and high density polyethylene. Energy Convers. Manag. 2014, 78, 704–710. [Google Scholar] [CrossRef]
- Abnisa, F. Study on Pyrolysis of Oil Palm Solid Wastes and Co-Pyrolysis of Palm Shell with Plastic and Waste Tyre. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2015. [Google Scholar]
- Bhattacharya, P.; Steele, P.H.; Hassan, E.B.M.; Mitchell, B.; Ingram, L.; Pittman, C.U., Jr. Wood/plastic copyrolysis in an auger reactor: Chemical and physical analysis of the products. Fuel 2009, 88, 1251–1260. [Google Scholar] [CrossRef]
- Rotliwala, Y.C.; Shah, J.A.; Dhingra, N.; Parekh, D.B.; Parikh, P.A. Plastics/biomass copyrolysis in an auger reactor: Physico-chemical analysis of the products. In Proceedings of the 7th International Symposium on Feedstock Recycling of Polymeric Materials (7th ISFR), New Delhi, India, 23–26 October 2013. [Google Scholar]
- Martinez, J.D.; Veses, A.; Mastral, A.M.; Murillo, R.; Navarro, M.V.; Puy, N. Copyrolysis of biomass with waste tyres: Upgrading of liquid bio-fuel. Fuel Process. Technol. 2014, 119, 263–271. [Google Scholar] [CrossRef]
- Xue, Y.; Kelkar, A.; Bai, X. Catalytic co-pyrolysis of biomass and polyethylene in a tandem micropyrolyzer. Fuel 2016, 166, 227–236. [Google Scholar] [CrossRef]
- Ojha, D.K.; Vinu, R. Fast co-pyrolysis of cellulose and polypropylene using Py-GC/MS and Py-FT-IR. R. Soc. Chem. Adv. 2016, 5, 66861–66870. [Google Scholar] [CrossRef]
- Fei, J.; Zhang, J.; Wang, F.; Wang, J. Synergistic Effects on co-pyrolysis of lignite and high-sulfur swelling coal. J. Anal. Appl. Pyrolysis 2012, 95, 61–67. [Google Scholar] [CrossRef]
- Yang, H.; Appari, S.; Kudo, S.; Hayashi, J.; Kumagai, S.; Norinaga, K. Chemical structures and primary pyrolysis characteristics of lignins obtained from different preparation methods. J. Jpn. Inst. Energy 2014, 93, 986–994. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Zhou, L.; Zhang, G.; Zhang, L.; Klinger, D.; Meyer, B. Effects of contact conditions between particles and volatiles during co-pyrolysis of brown coal and wheat straw in a thermos-gravimetric analyser and fixed-bed reactor. Processes 2019, 7, 179. [Google Scholar] [CrossRef]
- Xue, Y. Thermochemical Conversion of Organic and Plastic Waste Materials through Pyrolysis. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2017. [Google Scholar]
- Eriksson, I.; Haglind, I.; Lidbrandt, O.; Salmen, L. Fiber swelling favoured by lignin softening. Wood Sci. Technol. 1991, 25, 135–144. [Google Scholar] [CrossRef]
- Dufour, A.; Castro-Díaz, M.; Marchal, P.; Brosse, P.; Olcese, R.; Bouroukba, M.; Snape, C. In situ analysis of biomass pyrolysis by high temperature rheology in relations with 1H NMR. Energy Fuels 2012, 26, 6432–6441. [Google Scholar] [CrossRef]
- Serio, M.A.; Charpenay, S.; Bassilakis, R.; Solomon, P.R. Measurement and modeling of lignin pyrolysis. Biomass Bioenergy 1994, 7, 107–124. [Google Scholar] [CrossRef]
- Sato, S.; Oka, K.; Murakami, A. Heat transfer behavior of melting polymers in laminar flow field. Polym. Eng. Sci. 2004, 44, 423–432. [Google Scholar] [CrossRef]
- Supramono, D.; Nabil, M.A.; Nasikin, M. Effect of feed composition of co-pyrolysis of corncobs-polypropylene plastic on mass interaction between biomass particles and plastics. IOP Conf. Ser. Earth Environ. Sci. 2017, 105, 012049. [Google Scholar] [CrossRef]
- Zonta, F.; Soldati, A. Effect of temperature dependent fluid properties on heat transfer in turbulent mixed convection. J. Heat Transf. 2014, 136, 022501. [Google Scholar] [CrossRef]
- Baliga, V.; Sharma, R.; Miser, D.; McGrath, T.; Hajaligol, M.J. Physical characterization of pyrolyzed tobacco and tobacco components. Anal. Appl. Pyrolysis 2003, 66, 191–215. [Google Scholar] [CrossRef]
- Jarvis, M.W.; Haas, T.J.; Donohoe, B.S.; Daily, J.W.; Gaston, K.R.; Frederick, W.J.; Nimlos, M.R. Elucidation of biomass pyrolysis products using a laminar entrained flow reactor and char particle imaging. Energy Fuels 2011, 25, 324–336. [Google Scholar] [CrossRef]
- Yan, G.; Jing, X.; Wen, H.; Xiang, S. Thermal cracking of virgin and waste plastics of PP and LDPE in a semibatch reactor under atmospheric pressure. Energy Fuels 2015, 29, 2289. [Google Scholar] [CrossRef]
- Mazur, R.D. Where Does the Oxygen Go? Pathways and Partitioning in Autothermal Pyrolysis. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2017. [Google Scholar]
- Hayashi, J.; Nakahara, T.; Kusakabe, K.; Morooka, S. Pyrolysis of polypropylene in the presence of oxygen. Fuel Process. Technol. 1998, 55, 265–275. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Kiang, J.K.Y. Pyrolysis and oxidative pyrolysis of polypropylene. In Stabilization and Degradation of Polymers; Advances in Chemistry; Allara, D., Ed.; American Chemical Society: Washington, DC, USA, 1978. [Google Scholar]
- Bruch, C.; Peters, B.; Nussbaumer, T. Modelling wood combustion under fixed bed conditions. Fuel 2003, 82, 729–738. [Google Scholar] [CrossRef]
- Bandara, J.C.; Eikeland, M.S.; Moldestad, B.M.E. Analyzing the effects of particle density, size and size distribution for minimum fluidization velocity with Eulerian-Lagrangian CFD simulation. In Proceedings of the 58th Conference on Simulation and Modelling, Reykjavik, Iceland, 25–27 September 2017. [Google Scholar]
- Tsilingiris, P.T. Comparative evaluation of the infrared transmission of polymer films. Energy Convers. Manag. 2003, 44, 2839–2856. [Google Scholar] [CrossRef]
- Baldwin, R.M.; Magrini-Bair, K.A.; Nimlos, M.R.; Pepiot, P.; Donohoe, B.S.; Hensley, J.E.; Phillips, S.D. Current research on thermochemical conversion of biomass at the national renewable energy laboratory. Appl. Catal. B Environ. 2012, 115, 320–329. [Google Scholar] [CrossRef]
- Brzozowska-Stanuch, A.; Rabiej, S.; Fabia, J.; Nowak, J. Changes in thermal properties of isotactic polypropylene with different additives during aging process. Polimery 2014, 59, 302–307. [Google Scholar] [CrossRef]
- Supramono, D.; Nasikin, M. Improving bio-oil quality through co-pyrolysis of corn cobs and polypropylene in a stirred tank reactor. Int. J. Technol. 2016, 11, 1381–1391. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Scharler, R. Modelling heat of reaction in biomass pyrolysis with detailed reaction schemes. Fuel 2017. [Google Scholar] [CrossRef]
- Soják, L.; Kubineca, R.; Jurdákováa, H.; Hájekováb, E.; Bajus, M. GC-MS of polyethylene and polypropylene thermal cracking products. Pet. Coal 2006, 48, 1–14. [Google Scholar]
- Jouhara, H.; Ahmada, D.; van den Boogaerta, I.; Katsoua, E.; Simonsa, S.; Spencerb, N. Pyrolysis of domestic based feedstock at temperatures up to 300 °C. Ther. Sci. Eng. Prog. 2018, 5, 117–143. [Google Scholar] [CrossRef]
- Attinger, D.; Frankiewicz, C.; Betz, A.R.; Schutzius, T.M.; Ganguly, R.; Das, A.; Kim, C.J.; Megaridis, C.M. Surface engineering for phase change heat transfer: A Review. MRS Energy Sustain. 2014, 1, 1. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Lin, F.; Waters, C.L.; Mallinson, R.G.; Lobban, L.L.; Bartley, L.E. Relationships between biomass composition and liquid products formed via pyrolysis. Front. Energy Res. 2015, 3, 45. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, C.S. Recent efforts to prevent undesirable reactions from fractionation to depolymerisation of lignin: Toward maximizing the value from lignin. Front. Energy Res. 2018, 6, 92. [Google Scholar] [CrossRef]
- Paulsen, A.D. Primary and Secondary Reactions of Cellulose Melt Pyrolysis. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2014. [Google Scholar]
- Feng, Y.; Liu, L.; Wang, J.T.; Zhao, S.W.; Guo, Q.X. Homolytic C-H and N-H bond dissociation energies of strained organic compounds. J. Org. Chem. 2004, 69, 3129–3138. [Google Scholar] [CrossRef]
- Kruse, T.M.; Wong, H.W.; Broadbelt, L.J. Mechanistic modeling of polymer pyrolysis: Polypropylene. Macromolecules 2003, 36, 9594–9607. [Google Scholar] [CrossRef]
- Gunawardena, D.A. Deoxygenation of Biomass Oxygenates to Hydrocarbon Fuels Via Methane Intervention. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2014. [Google Scholar]
- Antal, M.J., Jr.; Gronli, M. The Art, science and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Scheirs, J.; Camino, G.; Tumiatti, W. Overview of water evolution during the thermal degradation of cellulose. Eur. Polym. J. 2007, 37, 933–942. [Google Scholar] [CrossRef]
- Sharypov, V.I.; Beregovtsova, N.G.; Kuznetsov, B.N.; Membrado, L.; Cebolla, V.L.; Marin, N.; Weber, J.V. Co-pyrolysis of wood biomass and synthetic polymers mixtures. Part III: Characterisation of heavy products. J. Anal. Appl. Pyrolysis 2003, 67, 325–340. [Google Scholar] [CrossRef]
- Vinu, R.; Broadbelt, L.J. Unraveling reaction pathways and specifying reaction kinetics for complex systems. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 29–54. [Google Scholar] [CrossRef]
- Ojha, D.K.; Shukla, S.; Sachin, R.S.; Vinu, R. Understanding the interactions between cellulose and polypropylene during fast co-pyrolysis via experiments and DFT calculations. Chem. Eng. Trans. 2016, 50, 67–72. [Google Scholar]
- Zhou, X.; Nolte, M.W.; Mayes, H.B.; Shanks, B.H.; Broadbelt, I.J. Experimental and mechanistic modeling of fast pyrolysis of neat glucose-based carbohydrates. 1. Experiments and development of a detailed mechanistic model. Ind. Eng. Chem. Res. 2014, 53, 13274–13289. [Google Scholar] [CrossRef]
- Yoshiga, A.; Otaguro, H.; Parra, D.F.; Lima, L.F.C.P.; Lugao, A.B. Controlled degradation and crosslinking of polypropylene induced by gamma radiation and acetylene. Polym. Bull. 2009, 63, 397–409. [Google Scholar] [CrossRef]
- Hellier, P.; Ladommatos, N.; Allan, R.; Rogerson, J. The importance of double bond position and cis-trans isomerisation in diesel combustion and emissions. Fuel 2013, 105, 477–489. [Google Scholar] [CrossRef]




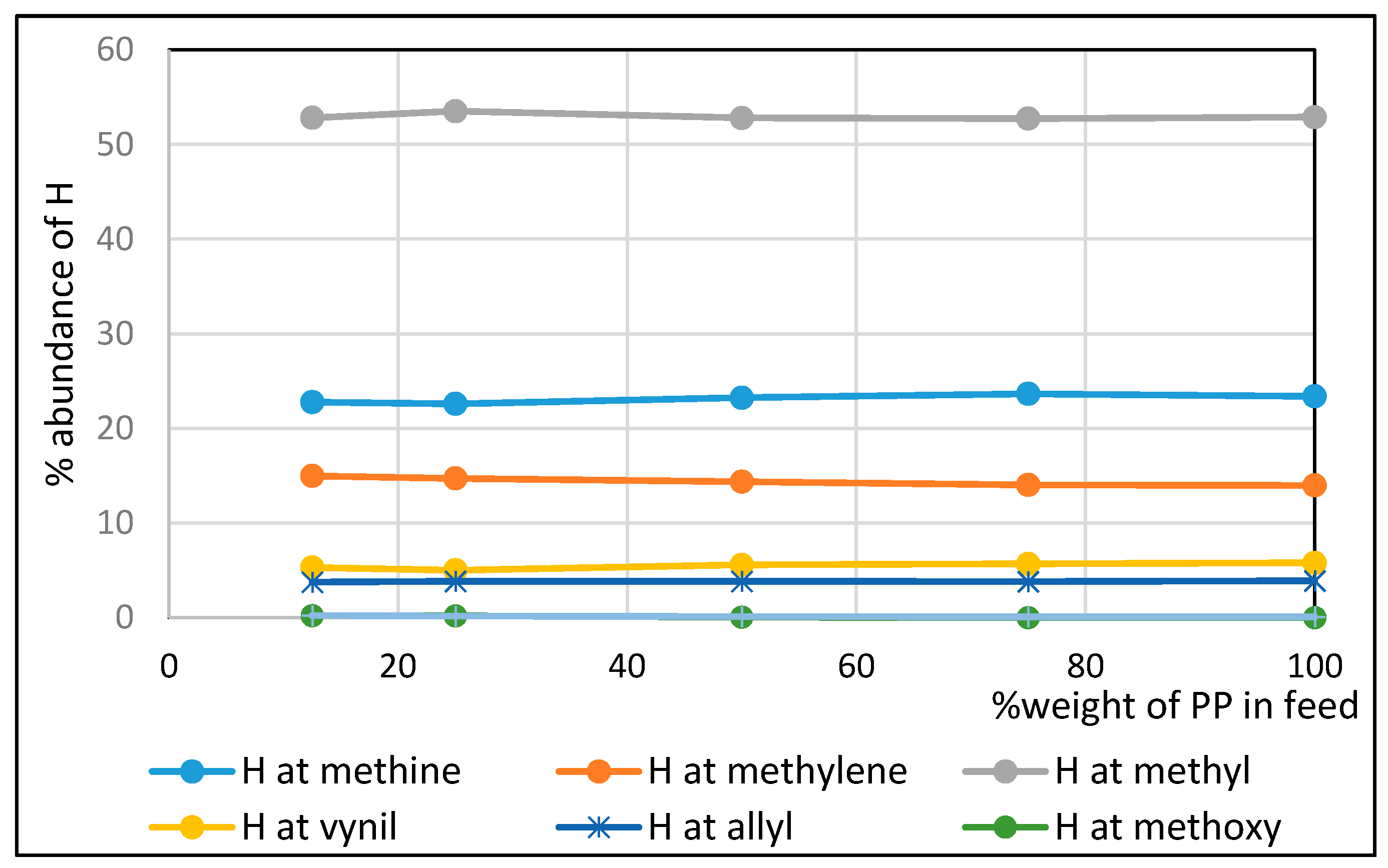
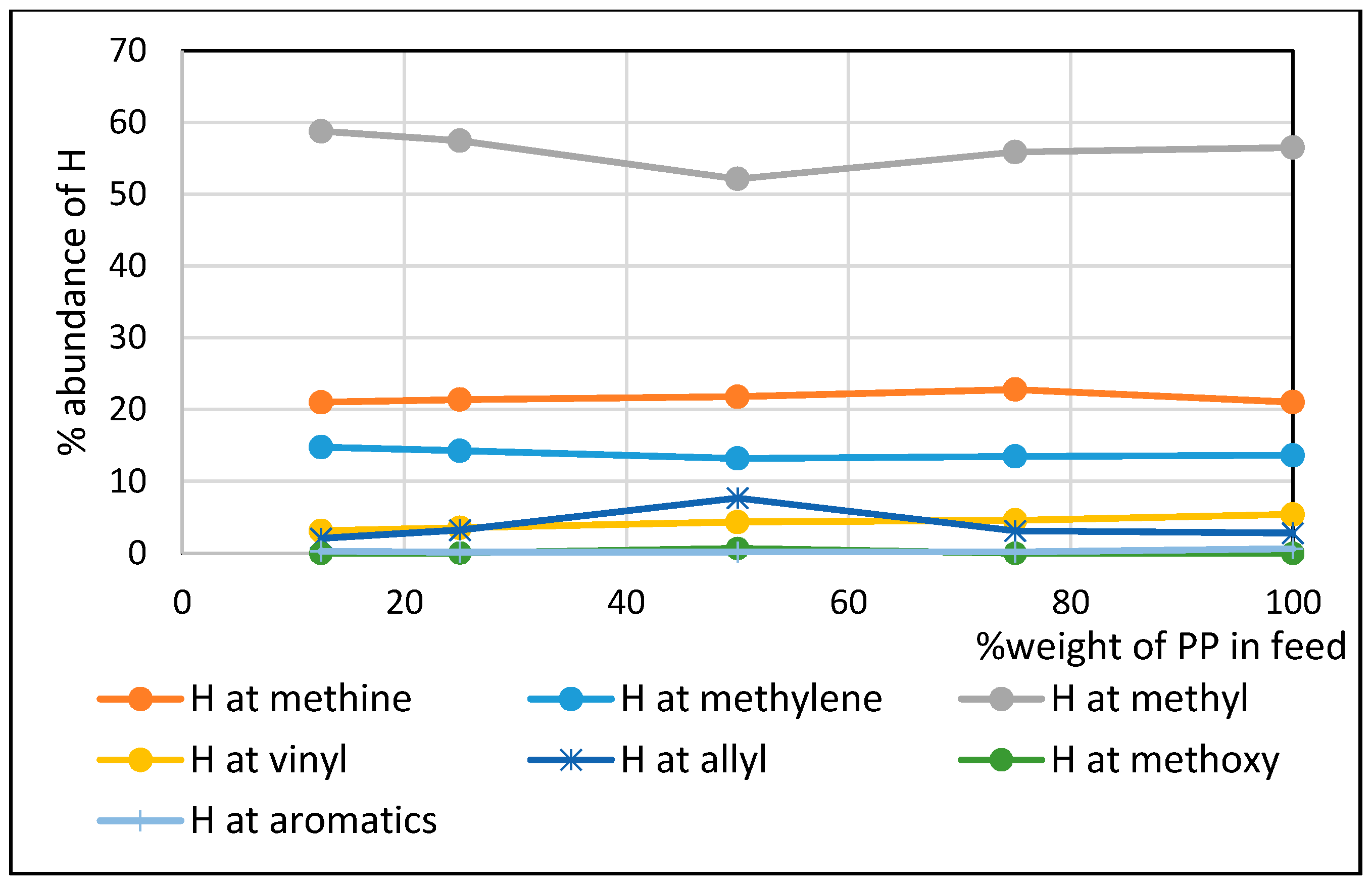
| 25%PP in Feed | 50%PP in Feed | 75%PP in Feed | 100%PP in Feed | Diesel Fuel | |
|---|---|---|---|---|---|
| %H at methyl | 53.61 | 52.87 | 52.79 | 52.93 | 34.90 |
| %H at methylene | 14.73 | 14.39 | 14.04 | 13.99 | 58.61 |
| %H at methine | 22.63 | 23.26 | 23.67 | 23.40 | 0 |
| %H at allylic C | 3.84 | 3.85 | 3.82 | 3.81 | 0 |
| % H at vinylic C | 5.01 | 5.58 | 5.68 | 5.87 | 0 |
| % H at aromatic ring | 0 | 0 | 0 | 0 | 1.93 |
| %H at benzylic C | 0 | 0 | 0 | 0 | 4.56 |
| Branching Index (BI) | 0.60 | 0.57 | 0.57 | 0.58 | 0.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supramono, D.; Sitorus, A.F.; Nasikin, M. Synergistic Effect on the Non-Oxygenated Fraction of Bio-Oil in Thermal Co-Pyrolysis of Biomass and Polypropylene at Low Heating Rate. Processes 2020, 8, 57. https://doi.org/10.3390/pr8010057
Supramono D, Sitorus AF, Nasikin M. Synergistic Effect on the Non-Oxygenated Fraction of Bio-Oil in Thermal Co-Pyrolysis of Biomass and Polypropylene at Low Heating Rate. Processes. 2020; 8(1):57. https://doi.org/10.3390/pr8010057
Chicago/Turabian StyleSupramono, Dijan, Adithya Fernando Sitorus, and Mohammad Nasikin. 2020. "Synergistic Effect on the Non-Oxygenated Fraction of Bio-Oil in Thermal Co-Pyrolysis of Biomass and Polypropylene at Low Heating Rate" Processes 8, no. 1: 57. https://doi.org/10.3390/pr8010057
APA StyleSupramono, D., Sitorus, A. F., & Nasikin, M. (2020). Synergistic Effect on the Non-Oxygenated Fraction of Bio-Oil in Thermal Co-Pyrolysis of Biomass and Polypropylene at Low Heating Rate. Processes, 8(1), 57. https://doi.org/10.3390/pr8010057





