Lanthanum Effect on Ni/Al2O3 as a Catalyst Applied in Steam Reforming of Glycerol for Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Characterization
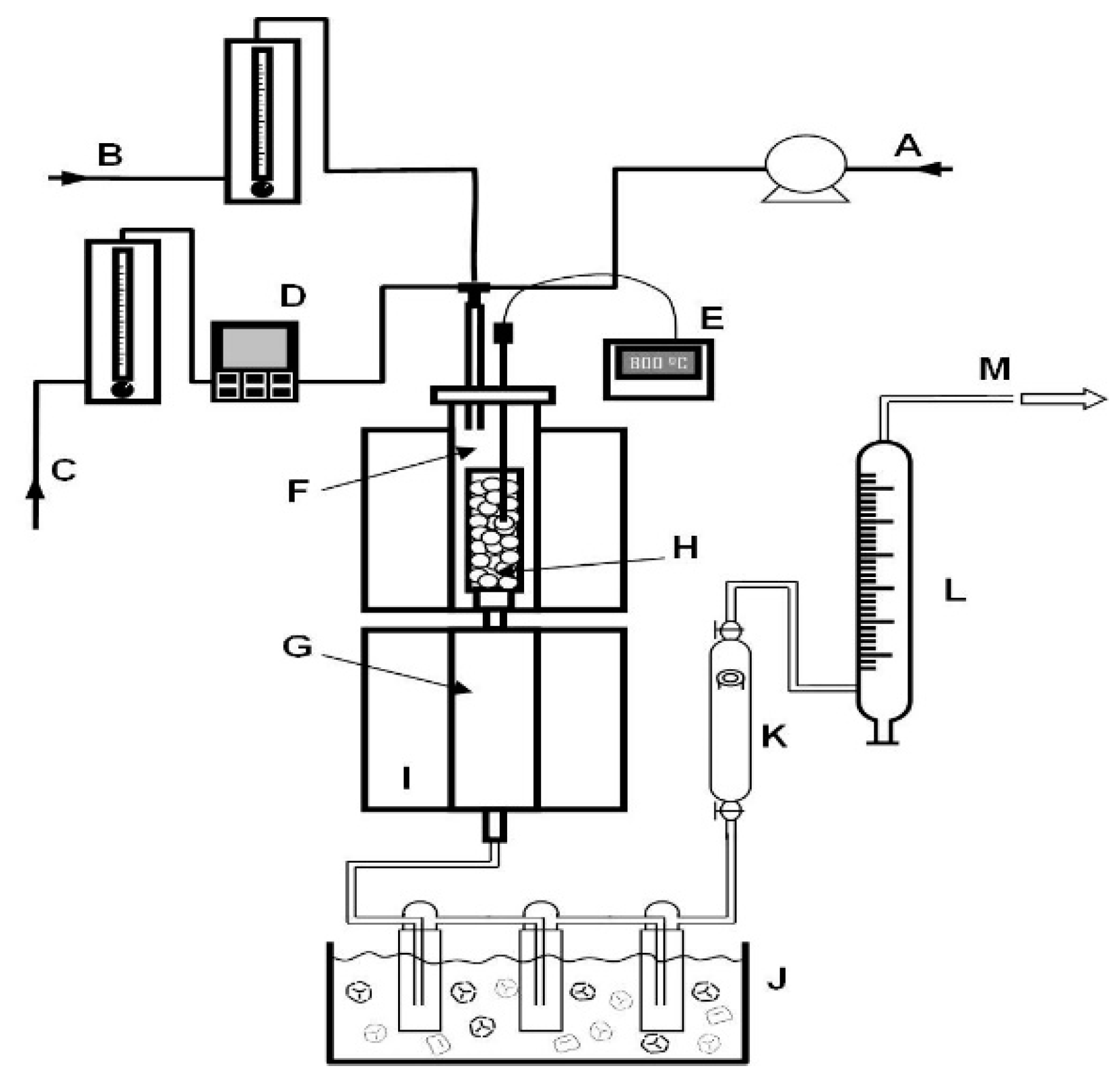
2.4. Catalyst Performance Testing
2.5. Data Analysis
3. Results and Discussion
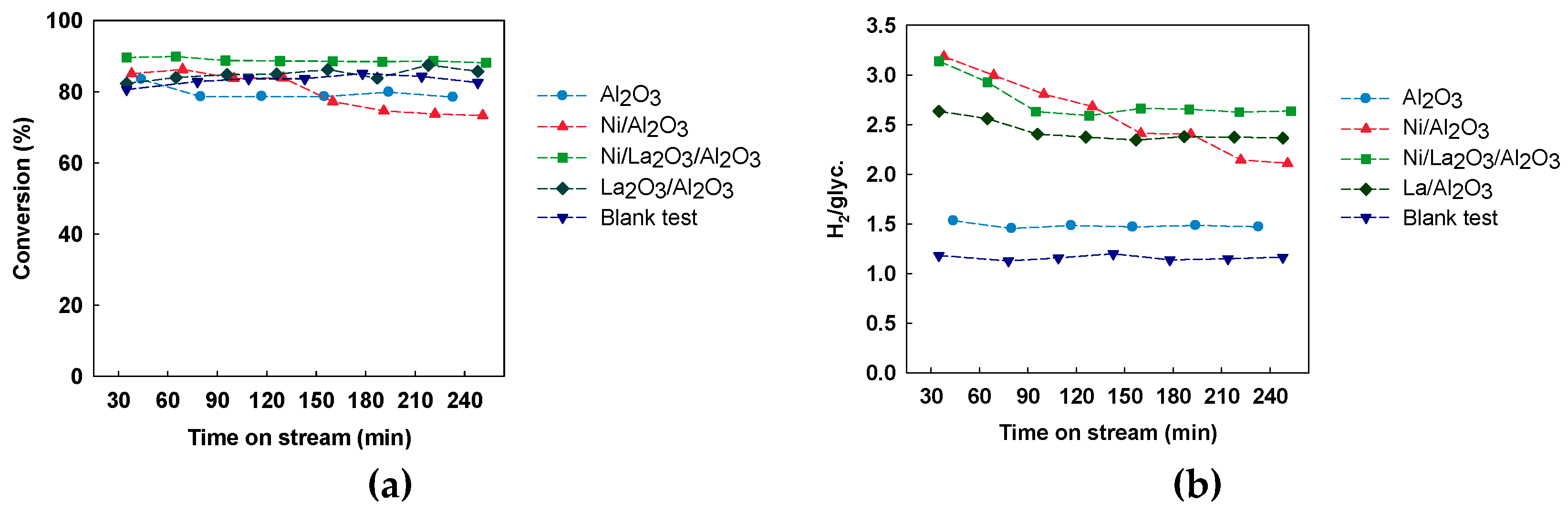
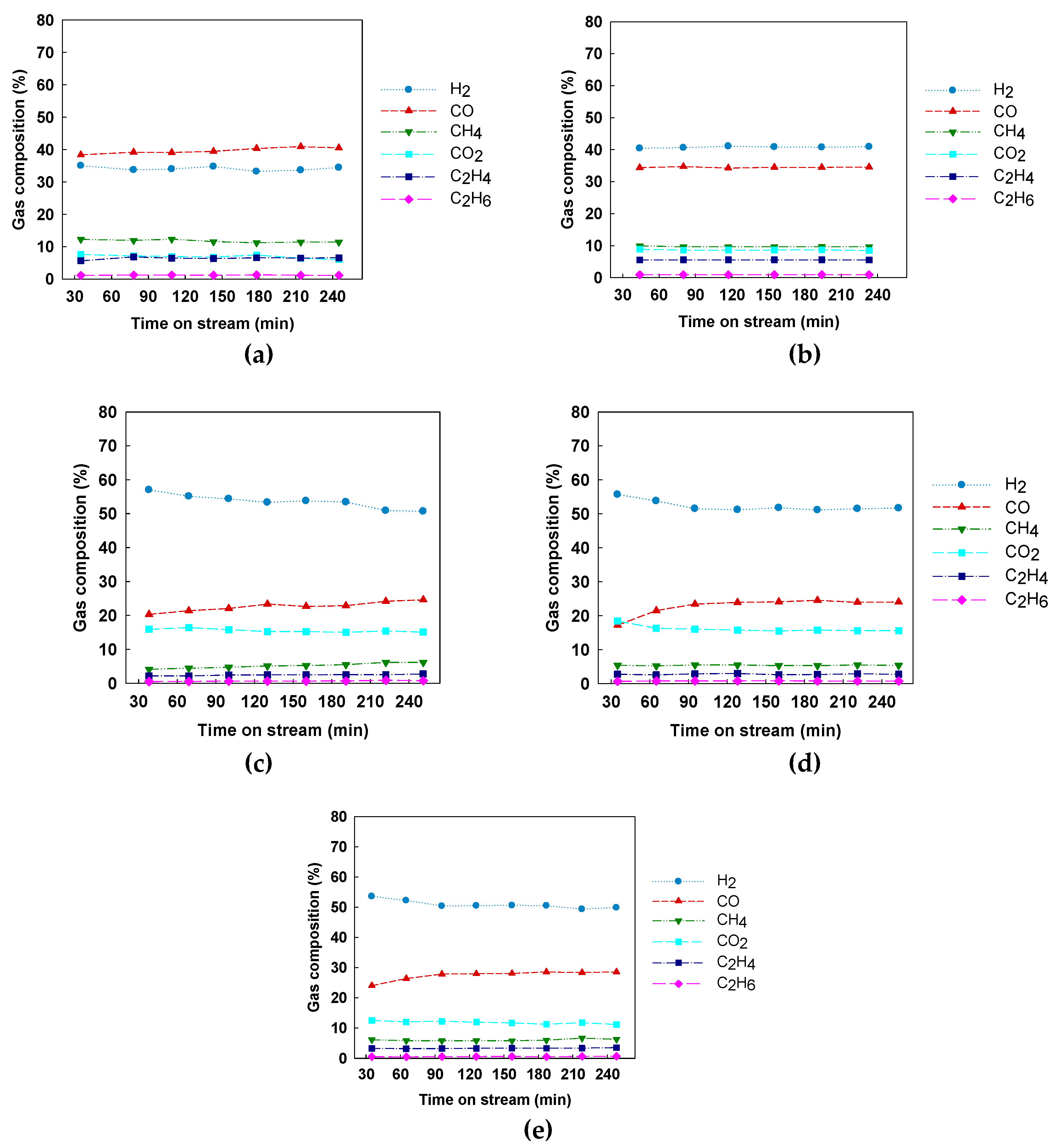
3.1. Catalytic Activity
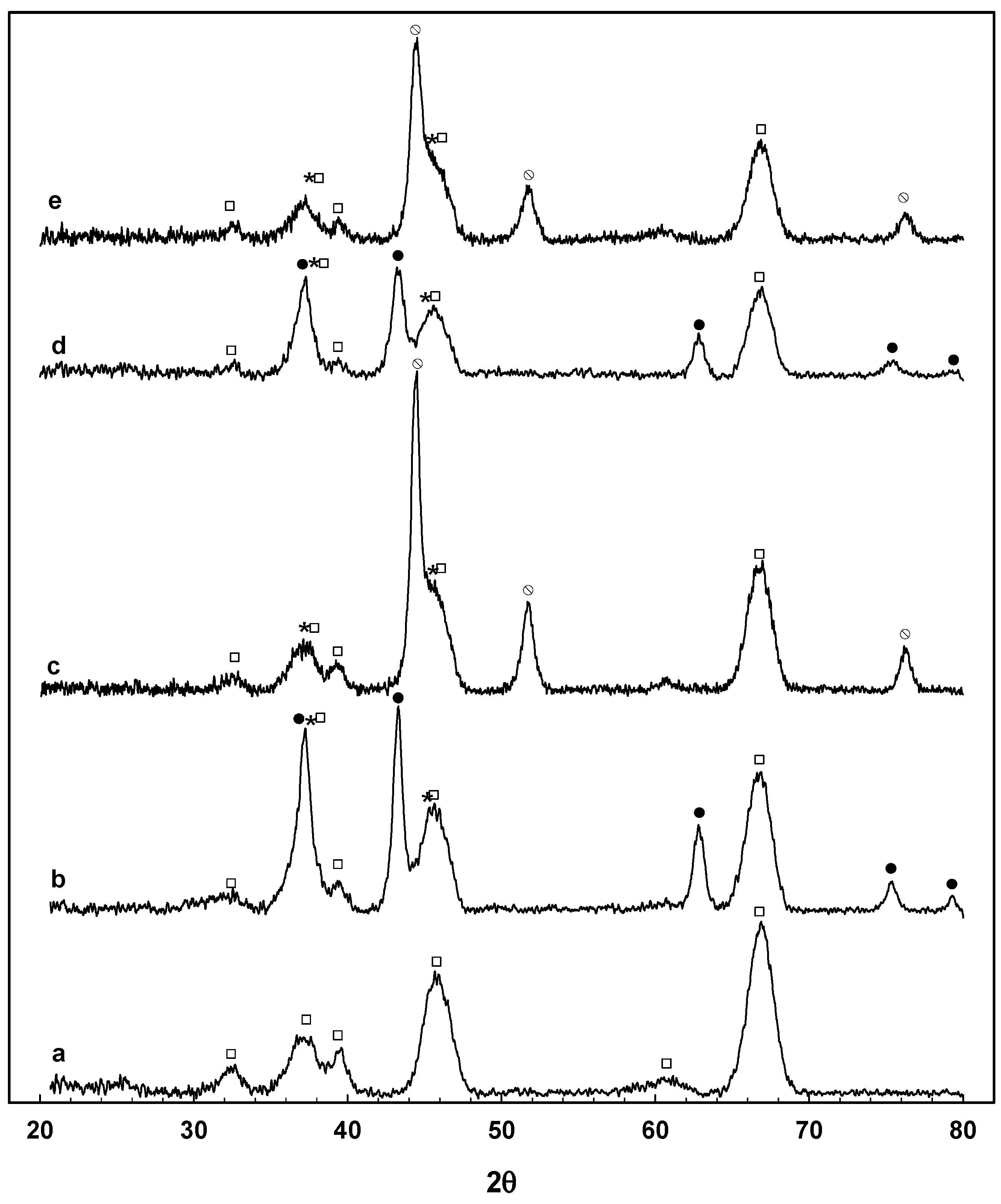
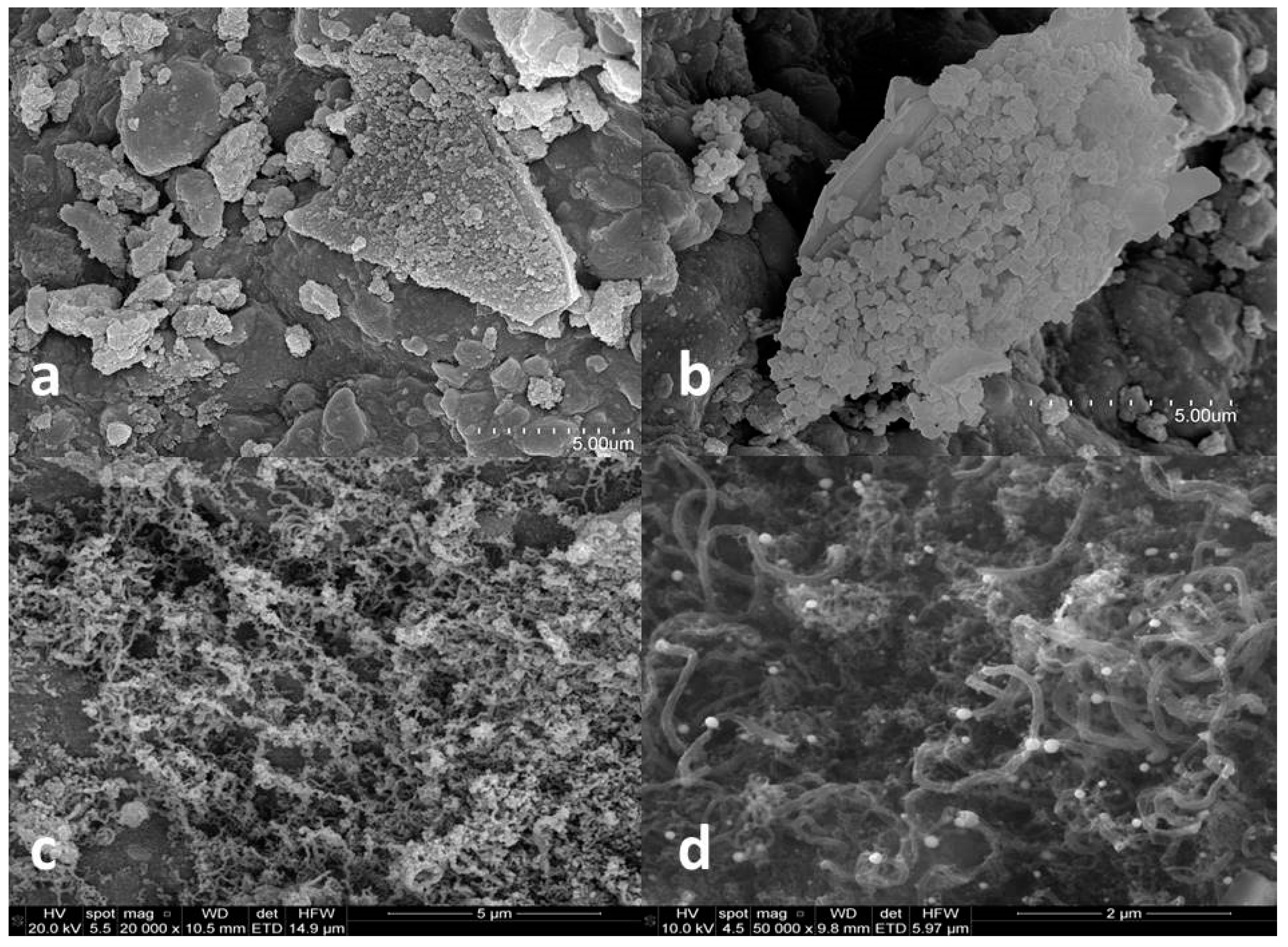
3.2. Catalyst Characterization
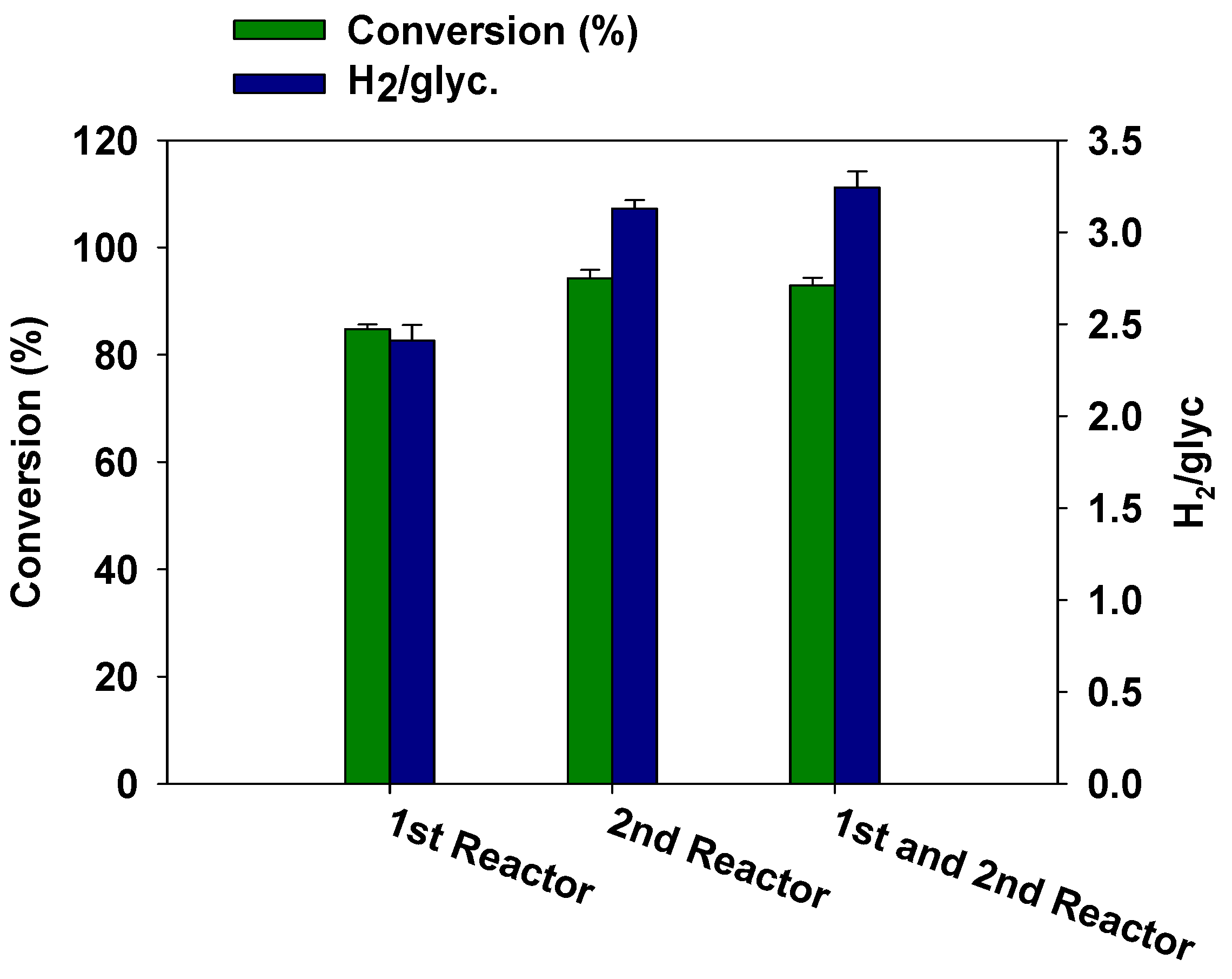
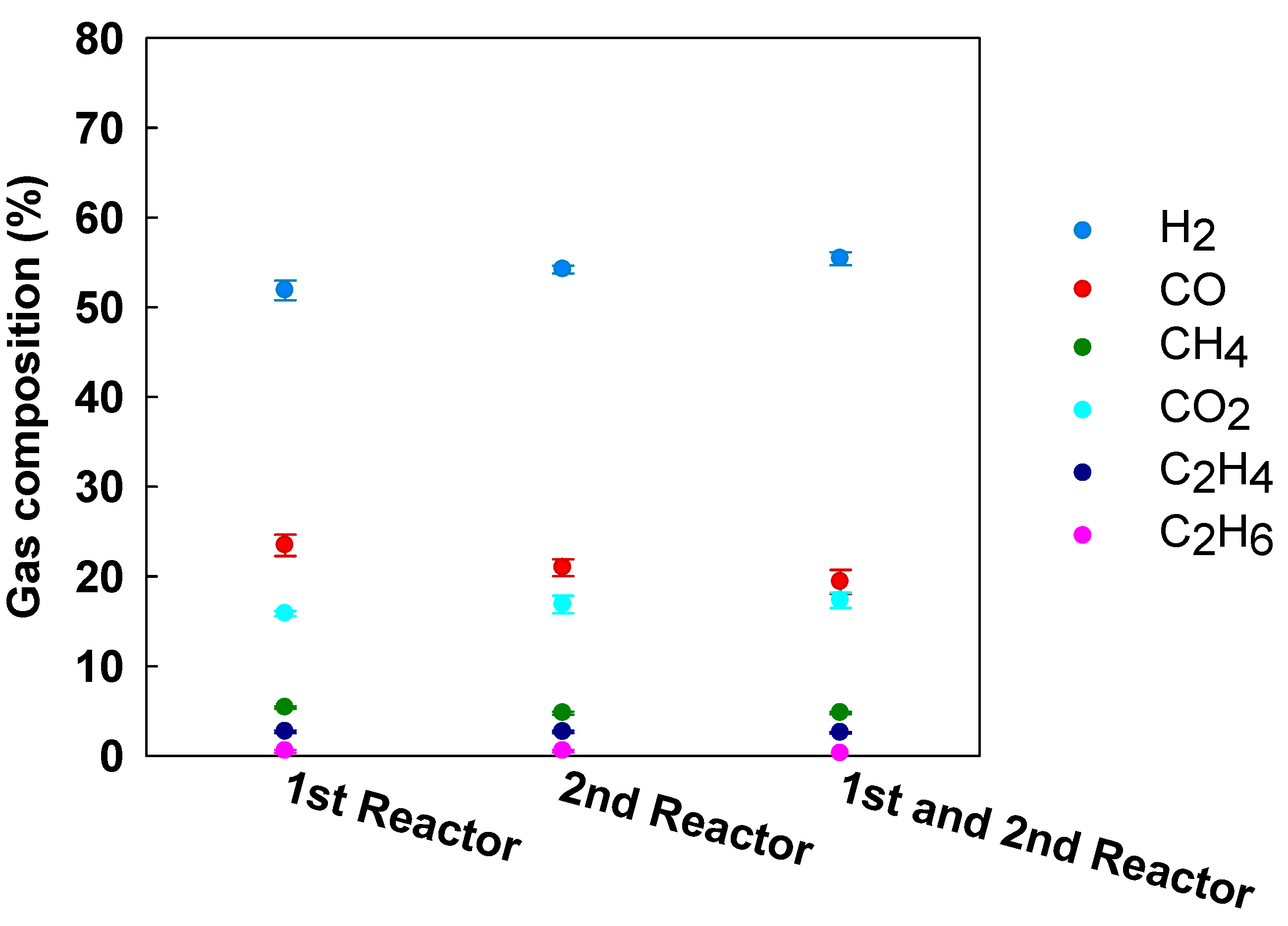
3.3. Effect of Catalyst Arrangement
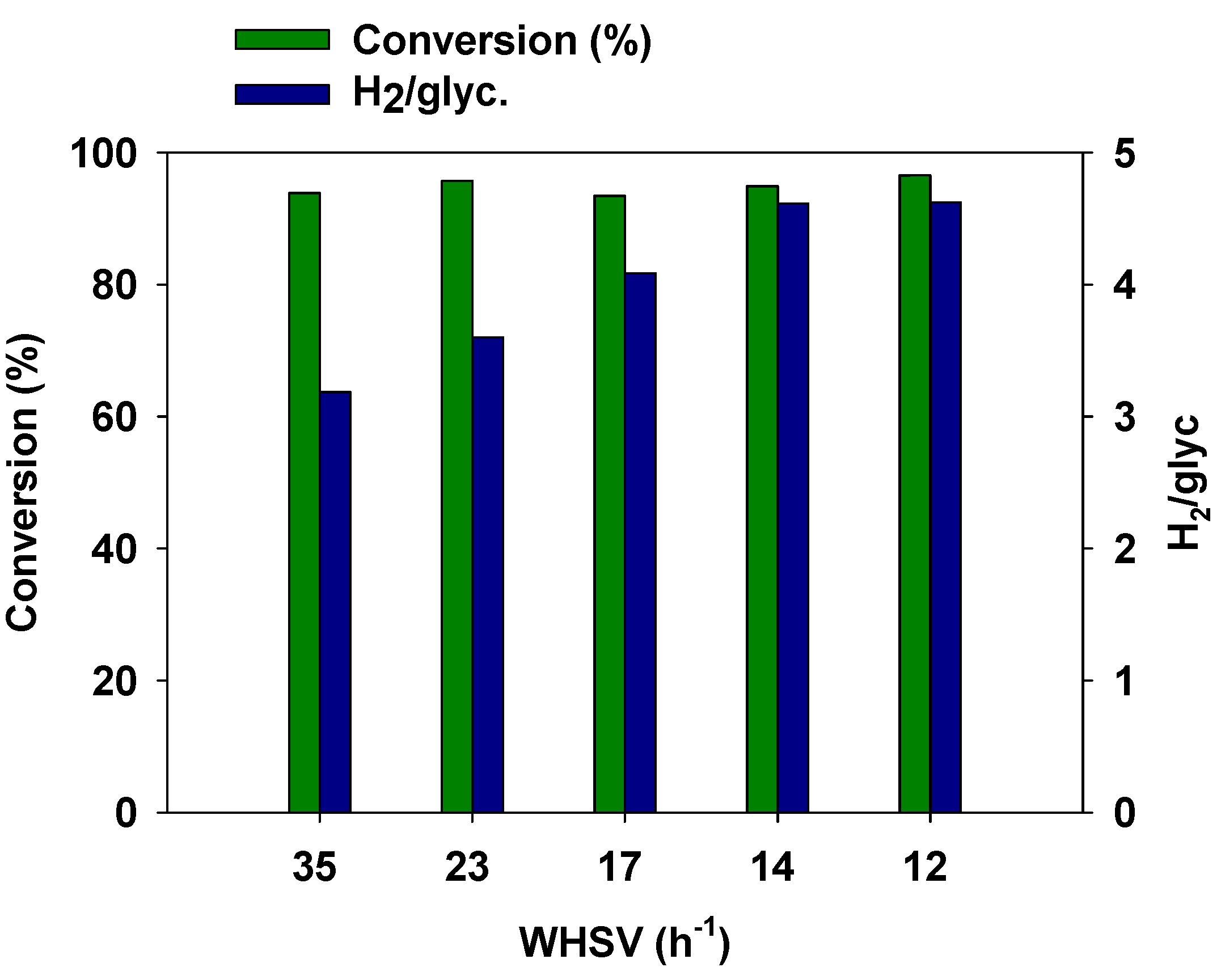
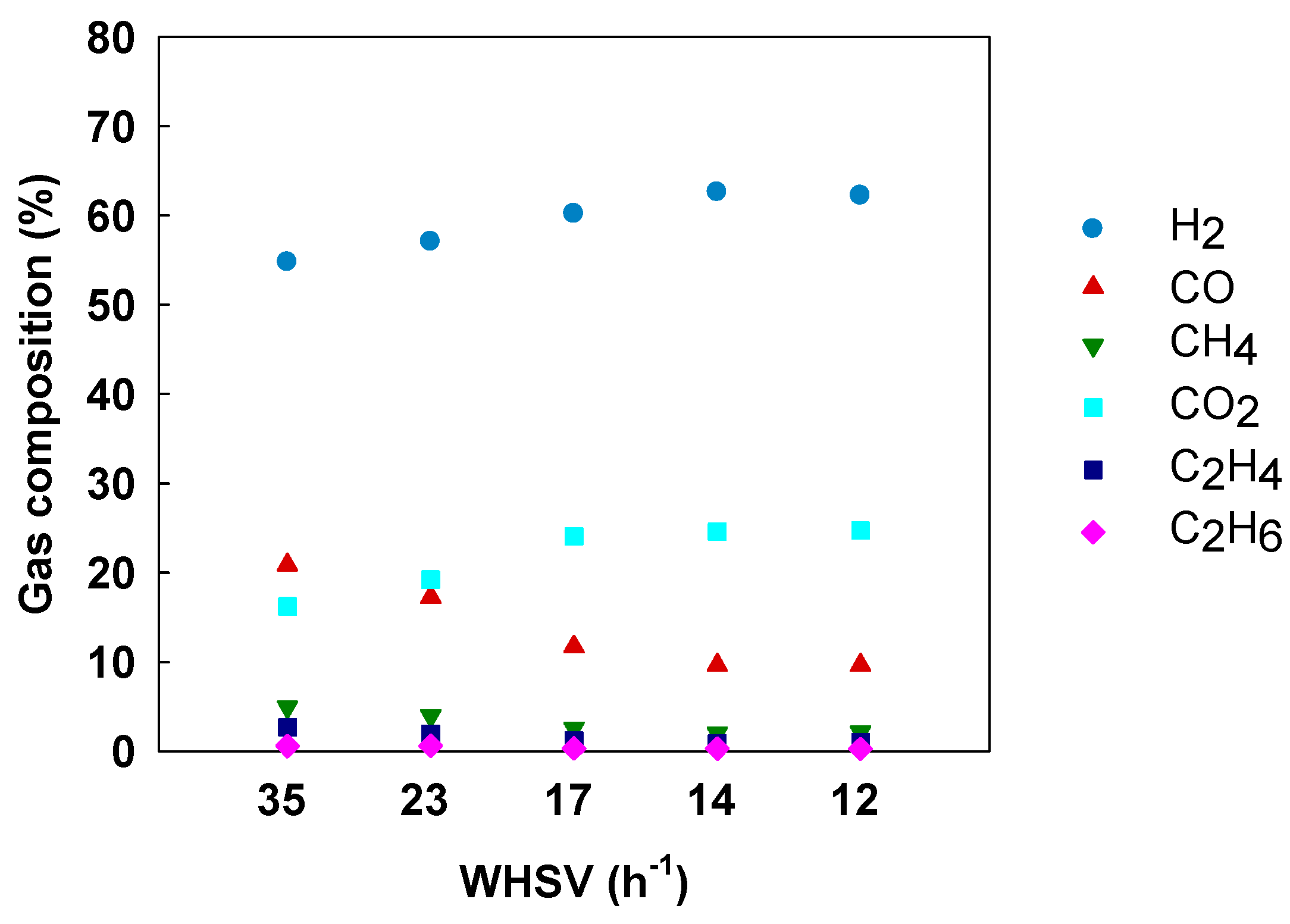
3.4. Effect of Space Velocity
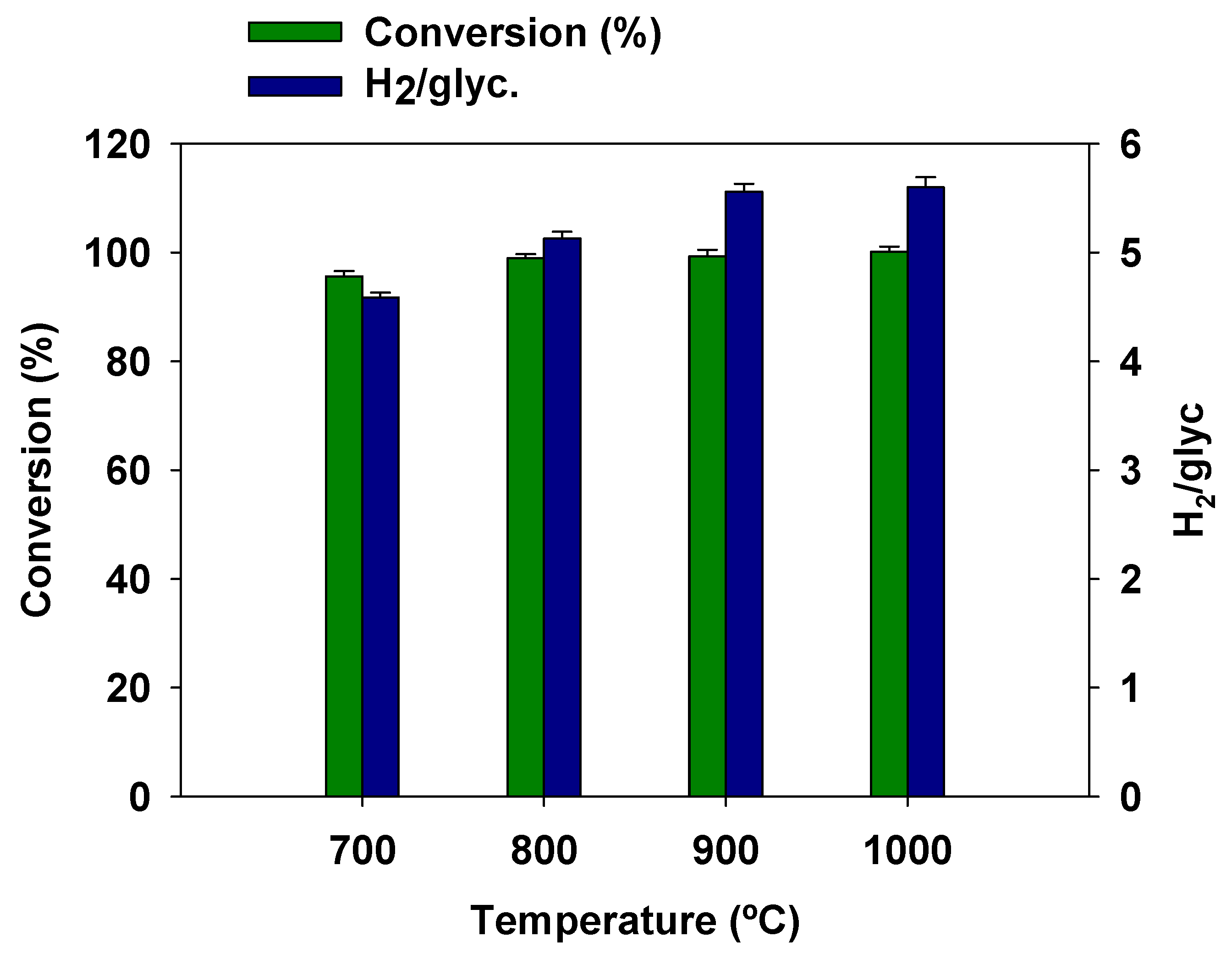
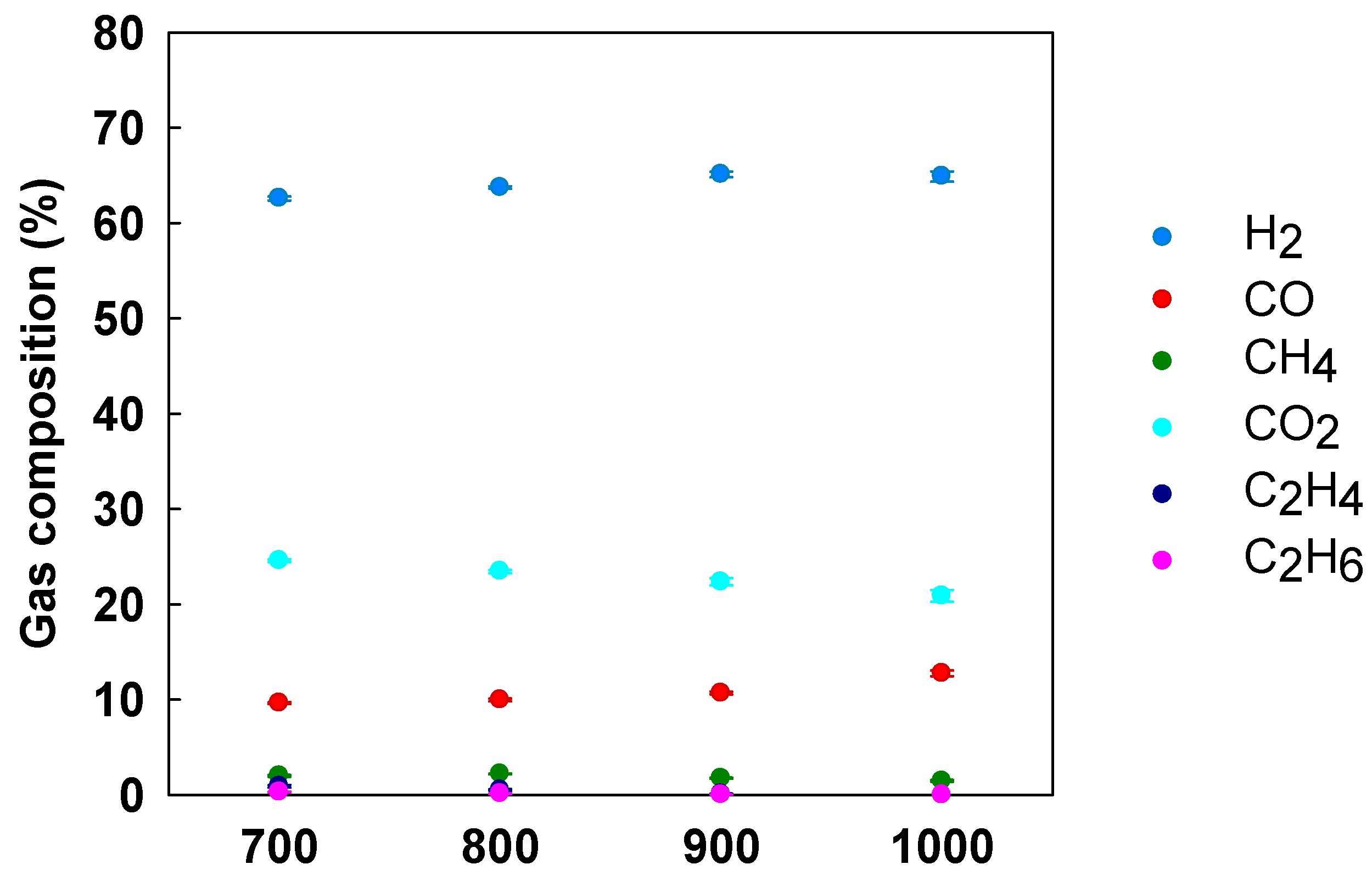
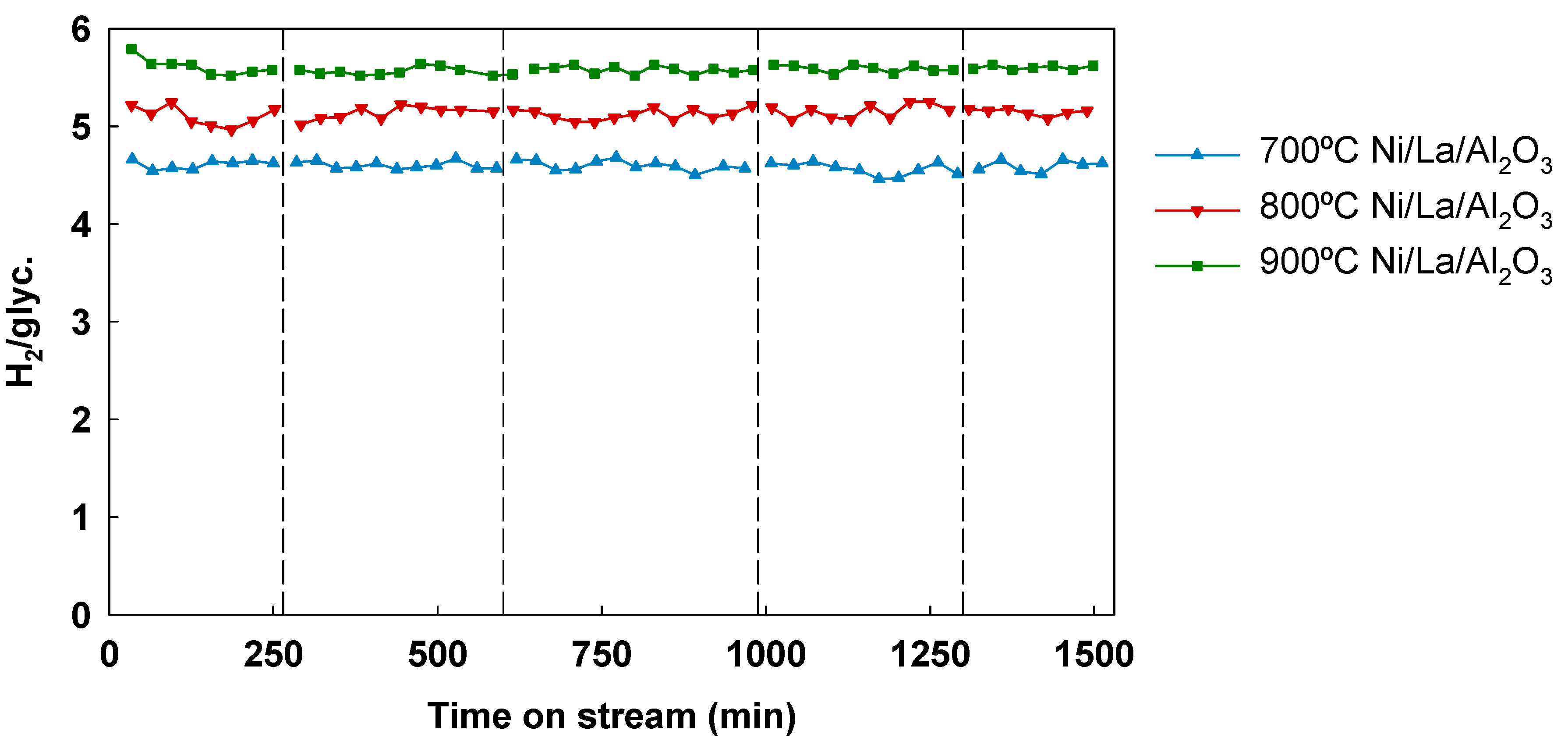
3.5. Effect of Temperature
3.6. Energy Use of the Product
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Fernández, J.; Hernández, J.J.; Calle-Asensio, A.; Ramos, A.; Barba, J. Selection of blends of diesel fuel and advanced biofuels based on their physical and thermochemical properties. Energies 2019, 12, 2034. [Google Scholar] [CrossRef]
- White, R.; Navarro-Pineda, F.S.; Cockerill, T.; Dupont, V.; Sacramento Rivero, J.C. Direct methanation of glycerol to bio-synthetic natural gas at a biodiesel refinery. Energies 2019, 12, 678. [Google Scholar] [CrossRef]
- Avasthi, K.S.; Reddy, R.N.; Patel, S. Challenges in the production of hydrogen from glycerol—A biodiesel byproduct via steam reforming process. Procedia Eng. 2013, 51, 423–429. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Leoneti, A.B.; Aragão-Leoneti, V.; de Oliveira, S.V.W.B. Glycerol as a by-product of biodiesel production in Brazil: Alternatives for the use of unrefined glycerol. Renew. Energ. 2012, 45, 138–145. [Google Scholar] [CrossRef]
- Susmozas, A.; Iribarren, D.; Dufour, J. Assessing the life-cycle performance of hydrogen production via biofuel reforming in Europe. Resources 2015, 4, 398–411. [Google Scholar] [CrossRef]
- Charisiou, N.; Siakavelas, G.I.; Dou, B.; Sebastian, V.; Hinder, S.; Baker, M.A.; Polychronopoulou, K.; Goula, M. Nickel supported on AlCeO3 as a highly selective and stable catalyst for hydrogen production via de glycerol steam reforming reaction. Catalysts 2019, 9, 411. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Rempel, G.L.; Ng, F.T.T. The promoting effect of Ni on glycerol hydrogenolysis to 1,2-propanediol with in situ hydrogen from methanol steam reforming using a Cu/ZnO/Al2O3 catalyst. Catalyst 2019, 9, 412. [Google Scholar] [CrossRef]
- Lin, Y.C. Catalytic valorization of glycerol to hydrogen and syngas. Int. J. Hydrogen Energy 2013, 38, 2678–2700. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.D.; Haryanto, A. Hydrogen production from glycerol: An update. Energy Convers. Manag. 2009, 50, 2600–2604. [Google Scholar] [CrossRef]
- Shao, S.; Shi, A.-W.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Hydrogen production from steam reforming of glycerol over Ni/CeZrO catalysts. Fuel Process Technol. 2014, 125, 1–7. [Google Scholar] [CrossRef]
- Sánchez, N.; Encinar, J.M.; González, J.F. Sorption enhanced steam reforming of glycerol: Use of La-modified Ni/Al2O3 as catalyst. Ind. Eng. Chem. Res. 2016, 55, 3736–3741. [Google Scholar] [CrossRef]
- Hashaikeh, R.; Butler, I.S.; Kozinski, J.A. Selective promotion of catalytic reactions during biomass gasification to hydrogen. Energy Fuel 2006, 20, 2743–2747. [Google Scholar] [CrossRef]
- Dauenhauer, P.J.; Salge, J.R.; Schmidt, L.D. Renewable hydrogen by autothermal steam reforming of volatile carbohydrates. J. Catal. 2006, 244, 238–247. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef]
- Barati, M.; Babatabar, M.; Tavasoli, A.; Dalai, A.K.; Das, U. Hydrogen production via supercritical water gasification of bagasse using unpromoted and zinc promoted Ru/γ-Al2O3nanocatalysts. Fuel Process Technol. 2014, 123, 140–148. [Google Scholar] [CrossRef]
- Byrd, A.J.; Pant, K.K.; Gupta, R.B. Hydrogen production from glycerol by reforming in supercritical water over Ru/Al2O3 catalyst. Fuel 2008, 87, 2956–2960. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.; Haryanto, A. Production of hydrogen by steam reforming of glycerin over alumina-supported metal catalysts. Catal. Today 2007, 129, 355–364. [Google Scholar] [CrossRef]
- Barbarias, I.; Artetxe, M.; Lopez, G.; Arregi, A.; Santamaria, L.; Bilbao, J.; Olazar, M. Catalyst performance in the HDPE pyrolysis-reforming under reaction-regeneration cycles. Catalysts 2019, 9, 414. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; García-Moreno, L. Hydrogen production by glycerol steam reforming over SBA-15-supported nickel catalysts: Effect of alkaline earth promoters on activity and stability. Catal. Today 2014, 227, 198–206. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L.; Güemez, M.B.; Navarro, R.M.; Sánchez-Sánchez, M.C.; Fierro, J.L.G. Influence of La2O3 modified support and Ni and Pt active phases on glycerol steam reforming to produce hydrogen. Catal. Commun. 2009, 10, 1275–1278. [Google Scholar] [CrossRef]
- Pompeo, F.; Santori, G.F.; Nichio, N.N. Hydrogen production by glycerol steam reforming with Pt/SiO2 and Ni/SiO2 catalysts. Catal. Today 2011, 172, 183–188. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, X.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production from steam reforming of ethanol and glycerol over ceria-supported metal catalysts. Int. J. Hydrogen Energy 2007, 32, 2367–2373. [Google Scholar] [CrossRef]
- Pompeo, F.; Santori, G.; Nichio, N.N. Hydrogen and/or syngas from steam reforming of glycerol. Study of platinum catalysts. Int. J. Hydrogen Energy 2010, 35, 8912–8920. [Google Scholar] [CrossRef]
- Valliyappan, T.; Ferdous, D.; Bakhshi, N.N.; Dalai, A.K. Production of hydrogen and syngas via steam gasification of glycerol in a fixed-bed reactor. Top Catal. 2008, 49, 59–67. [Google Scholar] [CrossRef]
- Soares, R.R.; Simonetti, D.A.; Dumesic, J.A. Glycerol as a Source for Fuels and Chemicals by Low-Temperature Catalytic Processing. Angew. Chem. Int. Ed. 2006, 45, 3982–3985. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L.; Güemez, M.B.; Navarro, R.M.; Sánchez-Sánchez, M.D.; Fierro, J.L.G. Hydrogen production from glycerol over nickel catalysts supported on Al2O3 modified by Mg, Zr, Ce or La. Top Catal. 2008, 49, 46–58. [Google Scholar] [CrossRef]
- Montini, T.; Singh, R.; Das, P.; Lorenzut, B.; Bertero, N.; Riello, P.; Benedetti, A.; Giambastiani, G.; Bianchini, C.; Zinoviev, S.; et al. Renewable H2 from glycerol steam reforming: Effect of La2O3 and CeO2 addition to Pt/Al2O3 catalysts. ChemSusChem 2010, 3, 619–628. [Google Scholar] [CrossRef]
- Montero, C.; Remiro, A.; Benito, P.L.; Bilbao, J.; Gayubo, A.G. Optimumoperatingconditions in ethanolsteamreformingover a Ni/La2O3-αAl2O3 catalyst in a fluidizedbed reactor. Fuel Process. Technol. 2018, 169, 207–216. [Google Scholar] [CrossRef]
- Carrero, A.; Vizcaíno, A.J.; Calles, J.A.; García-Moreno, L.J. Hydrogenproductionthroughglycerolsteamreformingusing Co catalystssupportedon SBA-15 dopedwith Zr, Ce and La. Energy Chem. 2017, 26, 42–48. [Google Scholar] [CrossRef]
- Pu, J.; Luo, Y.; Wang, N.; Bao, H.; Wang, X.; Qian, E.W. Ceria-promoted Ni-Al2O3 core-chell catalyst for steam reforming of acetic acid with enhanced activity and coke resistance. Int. J. Hydrogen Energy 2018, 43, 3142–3153. [Google Scholar] [CrossRef]
- Fatsikostas, A.N.; Verykios, X.E. Reaction network of steam reforming of ethanol over Ni-based catalysts. J. Catal. 2004, 225, 439–452. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Ethanol steam reforming over Ni/La–Al2O3 catalysts: Influence of lanthanum loading. Catal. Today 2007, 129, 336–345. [Google Scholar] [CrossRef]
- Valle, B.; Remiro, A.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Catalysts of Ni/α-Al2O3 and Ni/La2O3-αAl2O3 for hydrogen production by steam reforming of bio-oil aqueous fraction with pyrolytic lignin retention. Int. J. Hydrogen Energy 2013, 38, 1307–1318. [Google Scholar] [CrossRef]
- Mazumder, J.; de Lasa, H.I. Ni catalysts for steam gasification of biomass: Effect of La2O3 loading. Catal. Today 2014, 237, 100–110. [Google Scholar] [CrossRef]
- Boudjeloud, M.; Boulahouache, A.; Rabia, C.; Salhi, N. La-doped supported Ni catalysts for steam reforming of methane. Int. J. Hydrogen Energy 2019, 44, 9906–9913. [Google Scholar] [CrossRef]
- Menezes, J.P.S.Q.; Manfro, R.L.; Souza, M.M.V.M. Hydrogen production from glycerol steam reforming over nickel catalysts supported on alumina and niobia: Deactivation process, effect of reaction conditions and kinetic modeling. Int. J. Hydrogen Energy 2018, 43, 15064–15082. [Google Scholar] [CrossRef]
- Manfro, R.L.; Souza, M.M.V.M. Production of Renewable Hydrogen by Glycerol Steam Reforming Using Ni–Cu–Mg–Al Mixed Oxides Obtained from Hydrotalcite-like Compounds. Catal. Lett. 2014, 144, 867–877. [Google Scholar]
- Bettman, M.; Chase, R.E.; Otto, K.; Weber, W.H. Dispersion studies on the system La2O3γ-Al2O3. J. Catal. 1989, 117, 447–454. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Luo, N.-J.; Wang, J.-A.; Xiao, T.-C.; Cao, F.-H.; Fang, D.-Y. Influence of nitrogen on the catalytic behaviour of Pt/γ-Al2O3 catalyst in glycerol reforming process. Catal. Today 2011, 166, 123–128. [Google Scholar] [CrossRef]
- Takenaka, S.; Ogihara, H.; Yamanaka, I.; Otsuka, K. Decomposition of methane over supported-Ni catalysts: Effects of the supports on the catalytic lifetime. Appl. Catal. A Gen. 2001, 217, 101–110. [Google Scholar] [CrossRef]
- Sanchez, E.A.; Comelli, R.A. Hydrogen by glycerol steam reforming on a nickel–alumina catalyst: Deactivation processes and regeneration. Int. J. Hydrogen Energy 2012, 37, 14740–14746. [Google Scholar] [CrossRef]
- Khaodee, W.; Wongsakulphasatch, S.; Kiatkittipong, W.; Arpornwichanop, A.; Laosiripojana, N.; Assabumrungrat, S. Selection of appropriate primary fuel for hydrogen production for different fuel cell types: Comparison between decomposition and steam reforming. Int. J. Hydrogen Energy 2011, 36, 7696–7706. [Google Scholar] [CrossRef]
- Muraki, H.; Fujitani, Y. Steam reforming of n-heptane using a Rh/MgAl: I. Support and kinetics2O4 catalyst. Appl. Catal. 1989, 47, 75–84. [Google Scholar] [CrossRef]
- Sánchez, E.A.; D’Angelo, M.A.; Comelli, R.A. Hydrogen production from glycerol on Ni/Al2O3 catalyst. Int. J. Hydrogen Energy 2010, 35, 5902–5907. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lim, H.; Woo, H.C. Catalytic activity and characterizations of Ni/K2TixOy–Al2O3 catalyst for steam methane reforming. Int. J. Hydrogen Energy 2014, 39, 17645–17655. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Blay, V.; Álvarez, A.; Domínguez, M.I.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A. Intensifying glycerol steam reforming on a monolith catalyst: A reaction kinetic model. Chem. Eng. J. 2016, 306, 933–941. [Google Scholar] [CrossRef]
- Salehi, E.; Azad, F.S.; Harding, T.; Abedi, J. Production of hydrogen by steam reforming of bio-oil over Ni/Al2O3 catalysts: Effect of addition of promoter and preparation procedure. Fuel Process Technol. 2011, 92, 2203–2210. [Google Scholar] [CrossRef]
- Zamzuri, N.H.; Mat, R.; Amin, N.A.S.; Telebian-Kiakalaieh, A. Hydrogen production from catalytic steam reforming of glycerol over various supported nickel catalysts. Int. J. Hydrogen Energy 2017, 42, 9087–9098. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Siakavelas, G.; Tzounis, L.; Kousi, K.; Baker, M.A.; Hinder, S.J.; Sebastian, V.; Polychronopoulou, K.; Goula, M.A. Glycerol steam reforming for hydrogen production over nickel supported on alumina, zirconia and silica catalysts. Top. Catal. 2017, 60, 1226–1250. [Google Scholar] [CrossRef]
- Montero, C.; Remiro, A.; Arandia, A.; Benito, P.L.; Bilbao, J.; Gayubo, A.G. Reproducible performance of a Ni/La2O3-aAl2O3 catalyst in ethanol steam reforming under reaction-regeneration cycles. Fuel Process Technol. 2016, 152, 215–222. [Google Scholar] [CrossRef]
| Al2O3 | Ni/Al2O3 | Ni/La2O3/Al2O3 | |||
|---|---|---|---|---|---|
| Fresh | Used | Fresh | Used | ||
| SBET (m2·g−1) | 159 | 135 | 123 | 205 | 140 |
| Carbonaceous deposits (%) | 4.81 | 2.49 | |||
| Ni/Al2O3 | Ni/La2O3/Al2O3 | |
|---|---|---|
| Ni 2p | 9.94 | 7.35 |
| La 3d5/2 | – | 0.96 |
| Al 2s | 32.06 | 35.38 |
| O 1s | 50.04 | 50.66 |
| C 1s | 7.96 | 5.65 |
| Ni/La/O/Al ratio | 0.31/0/1.56/1 | 0.21/0.03/1.43/1 |
| (0.13/0/1.63/1) | (0.13/0.02/1.66/1) |
| Energy Production (MJ·kgglyc.−1) | |
|---|---|
| Blank (WHSV = 35 h−1) | 14.48 |
| Al2O3 (WHSV = 35 h−1) | 14.23 |
| Ni/Al2O3 (WHSV = 35 h−1) | 13.06 |
| Ni/La2O3/Al2O3 (WHSV = 35 h−1) | 15.70 |
| La2O3/Al2O3 (WHSV = 35 h−1) | 15.64 |
| Ni/La2O3/Al2O3 (WHSV = 14 h−1) | 17.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, N.; Encinar, J.M.; Nogales, S.; González, J.F. Lanthanum Effect on Ni/Al2O3 as a Catalyst Applied in Steam Reforming of Glycerol for Hydrogen Production. Processes 2019, 7, 449. https://doi.org/10.3390/pr7070449
Sánchez N, Encinar JM, Nogales S, González JF. Lanthanum Effect on Ni/Al2O3 as a Catalyst Applied in Steam Reforming of Glycerol for Hydrogen Production. Processes. 2019; 7(7):449. https://doi.org/10.3390/pr7070449
Chicago/Turabian StyleSánchez, Nuria, José María Encinar, Sergio Nogales, and Juan Félix González. 2019. "Lanthanum Effect on Ni/Al2O3 as a Catalyst Applied in Steam Reforming of Glycerol for Hydrogen Production" Processes 7, no. 7: 449. https://doi.org/10.3390/pr7070449
APA StyleSánchez, N., Encinar, J. M., Nogales, S., & González, J. F. (2019). Lanthanum Effect on Ni/Al2O3 as a Catalyst Applied in Steam Reforming of Glycerol for Hydrogen Production. Processes, 7(7), 449. https://doi.org/10.3390/pr7070449








