Modeling and Simulation of the Absorption of CO2 and NO2 from a Gas Mixture in a Membrane Contactor
Abstract
1. Introduction
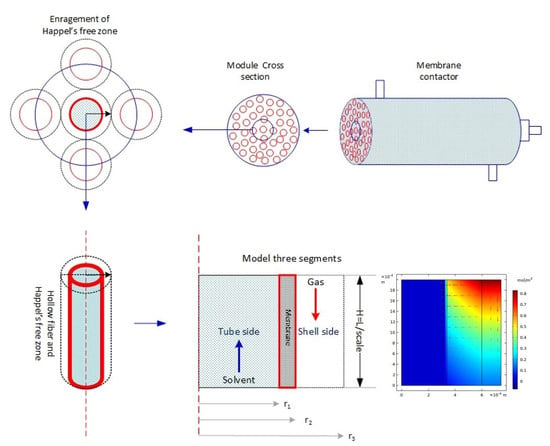
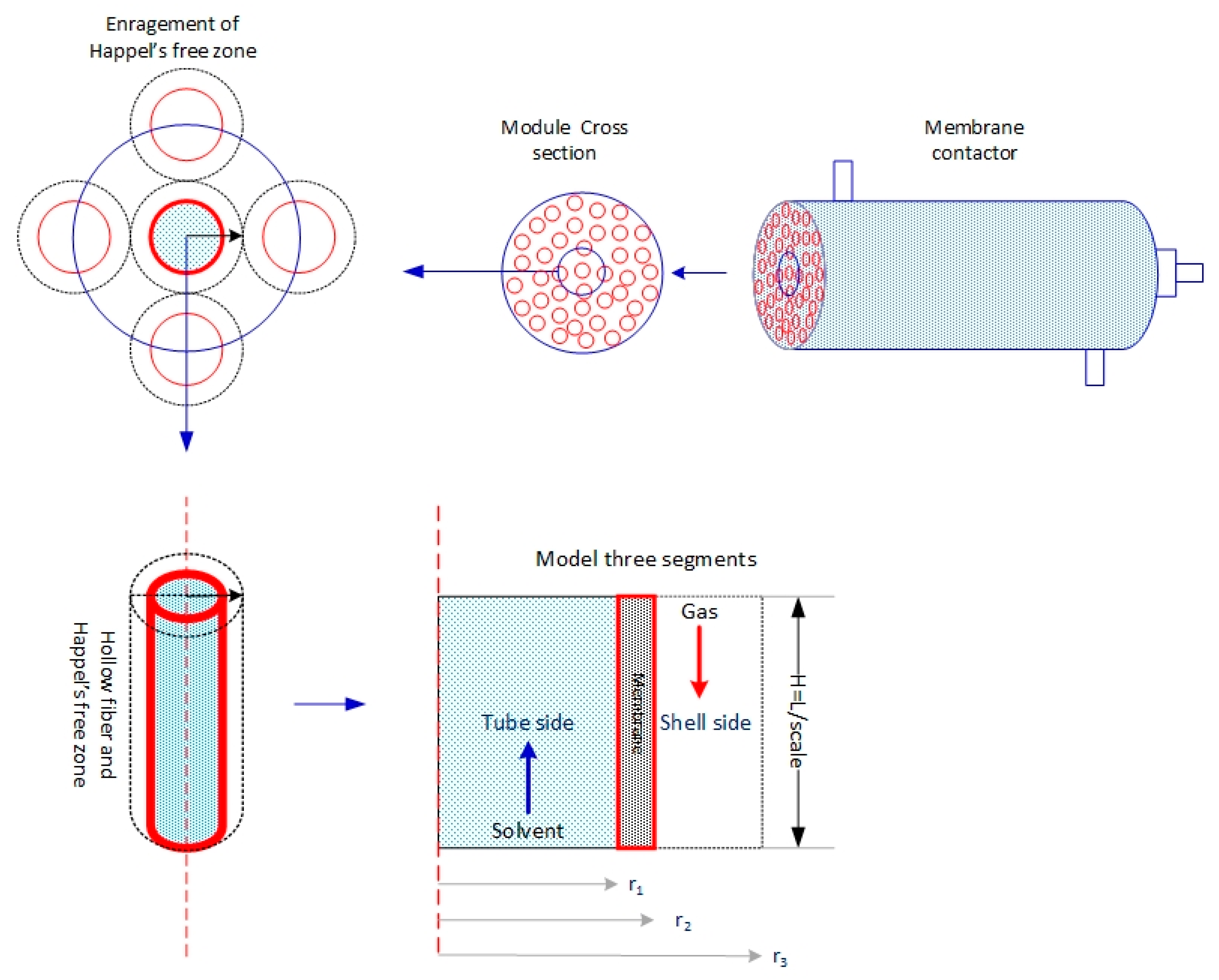
2. Model Development
- The process is at isothermal and steady state conditions;
- Gas phase is an ideal gas, and the liquid phase is incompressible and Newtonian;
- Solubility is based on Henry’s law at the liquid-gas interface.
2.1. Tube Side
2.2. Membrane Side
2.3. Shell Side
3. Numerical Solution
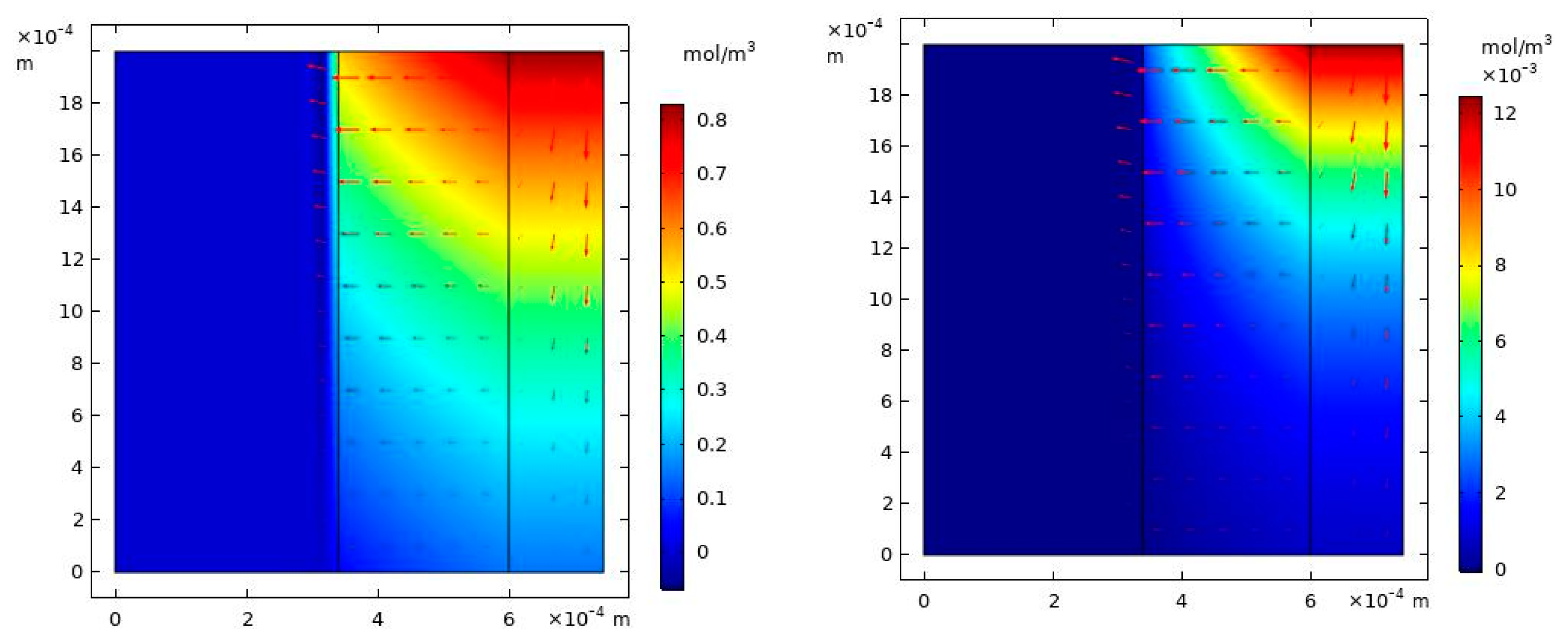
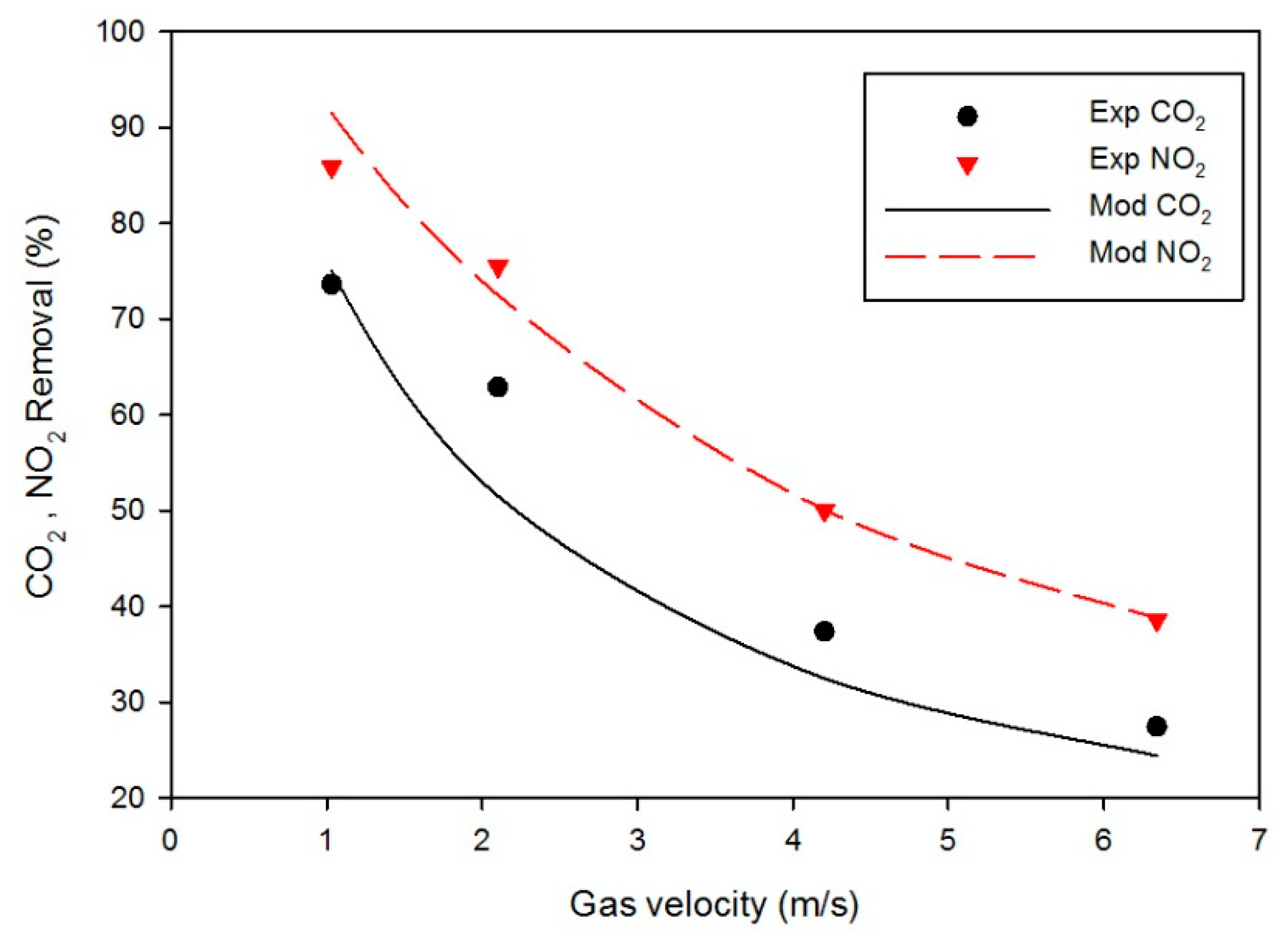
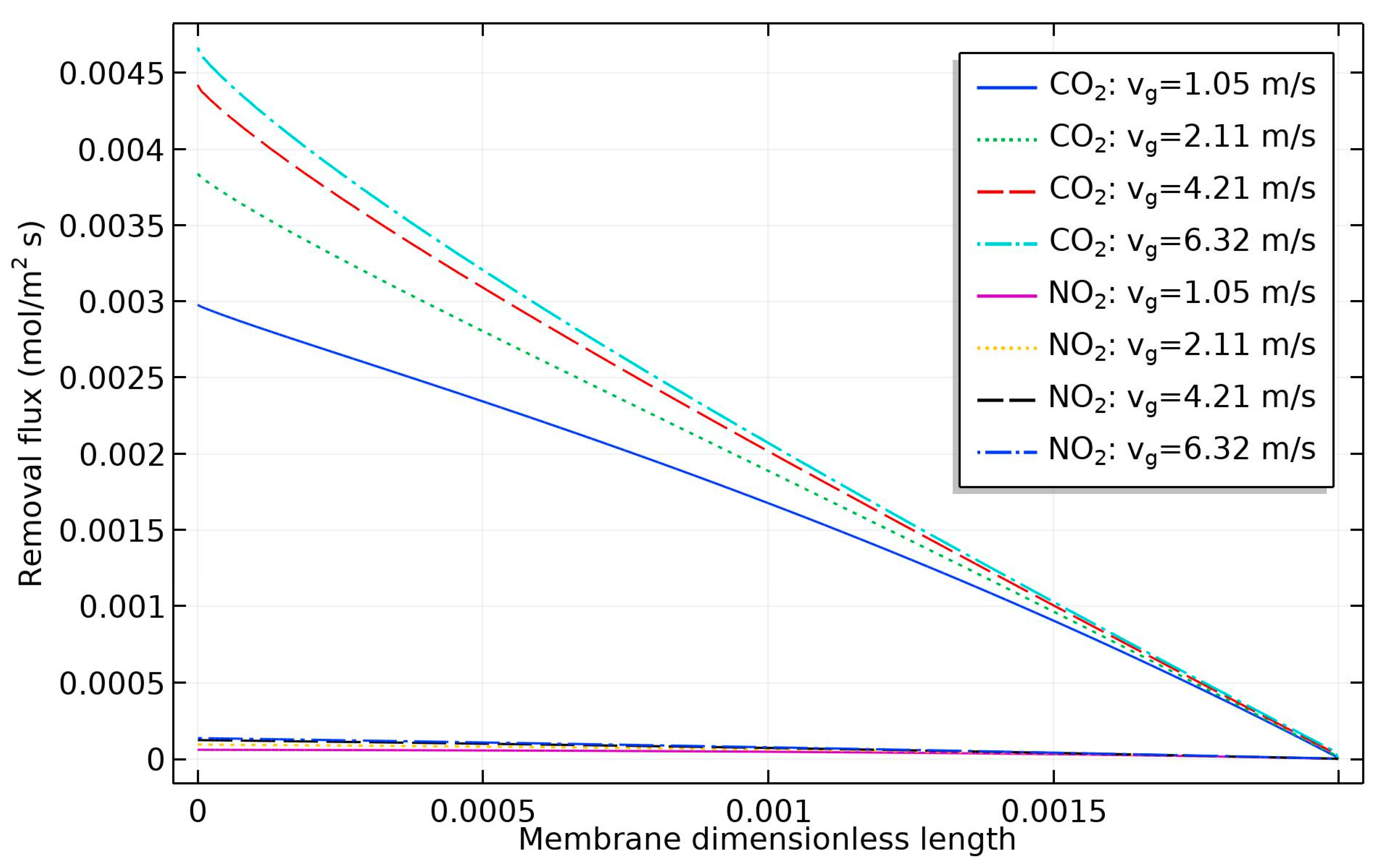
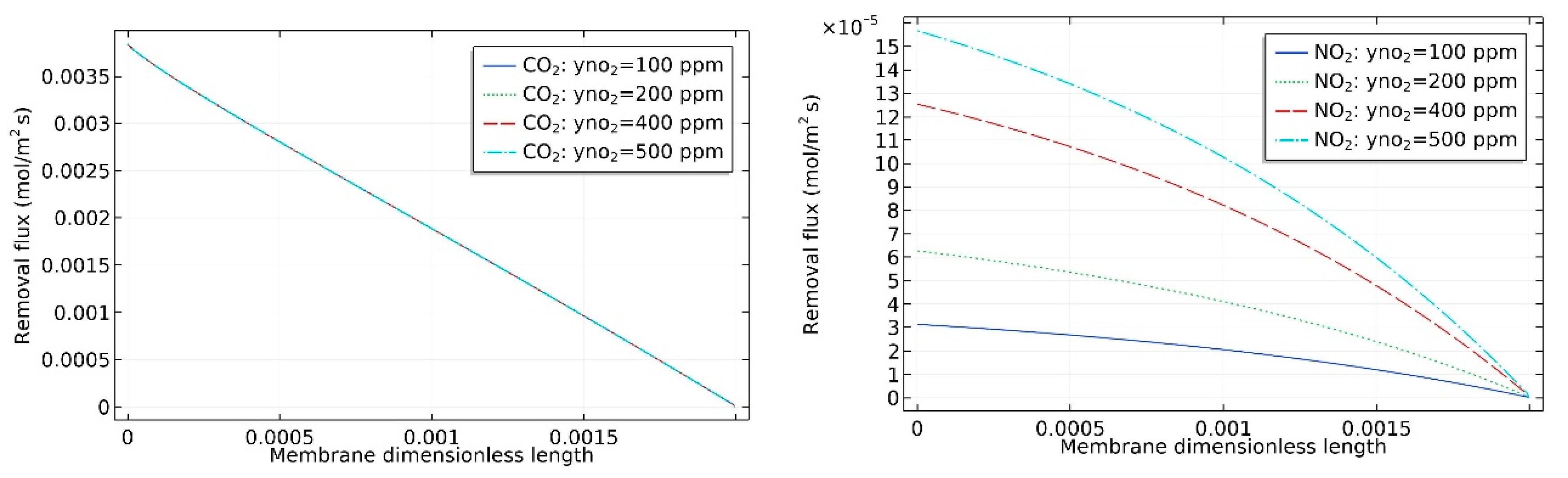
4. Results and Discussion
5. Conclusions
Conflicts of Interest
References
- Sun, B.; Sheng, M.; Gao, W.; Zhang, L.; Arowo, M.; Liang, Y.; Shao, L.; Chu, G.W.; Zou, H.; Chen, J.F. Absorption of nitrogen oxides into sodium hydroxide solution in a rotating packed bed with preoxidation by ozone. Energy Fuels 2017, 31, 11019–11025. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jung, W.S.; Choi, Y.R.; Oh, J.S.; Jang, S.D.; Son, Y.G.; Cho, M.H.; Namkung, W.; Koh, D.J.; Mok, Y.S.; et al. Application of pulsed corona induced plasma chemical process to an industrial incinerator. Env. Sci. Technol. 2003, 37, 2563–2567. [Google Scholar] [CrossRef]
- Golkhar, A.; Keshavarz, P.; Mowla, D. Investigation of CO2 removal by silica and CNT nanofluids in microporous hollow fiber membrane contactors. J. Memb. Sci. 2013, 433, 17–24. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Gas. Purification, 5th ed.; Gulf Publishing Company: Houston, TX, USA, 1997. [Google Scholar]
- Holstvoogd, R.D.; Van Swaaij, W.P.M. The influence of adsorption capacity on enhanced gas absorption in activated carbon slurries. Chem. Eng. Sci. 1990, 45, 151–162. [Google Scholar] [CrossRef]
- Yildirim, Ö.; Kiss, A.A.; Hüser, N.; Leßmann, K.; Kenig, E.Y. Reactive absorption in chemical process industry: A review on current activities. Chem. Eng. J. 2012, 213, 371–391. [Google Scholar] [CrossRef]
- Qi, Z.; Cussler, E.L. Microporous hollow fibers for gas absorption: I. Mass transfer in the liquid. J. Memb. Sci. 1985, 23, 321–332. [Google Scholar] [CrossRef]
- Qi, Z.; Cussler, E.L. Microporous hollow fibers for gas absorption: II. Mass transfer across the membrane. J. Memb. Sci. 1985, 23, 333–345. [Google Scholar] [CrossRef]
- Ghasem, N.; Al-Marzouqi, M. Modeling and experimental study of carbon dioxide absorption in a flat sheet membrane contactor. J. Membr. Sci. Res. 2017, 3, 57–63. [Google Scholar]
- Ghasem, N.; Al-Marzouqi, M.; Al-Marzouqi, R.; Dowaidar, A.; Vialatte, M. Removal of CO2 from gas mixture using hollow fiber membrane contactors fabricated from PVDF/Triacetin/Glycerol cast solution. In Proceedings of the European Conference of Chemical Engineering, ECCE’10, European Conference of Civil Engineering, ECCIE’10, European Conference of Mechanical Engineering, ECME’10, European Conference of Control, ECC’10, Tenerife, Spain, 30 November–2 December 2010. [Google Scholar]
- Darabi, M.; Rahimi, M.; Molaei Dehkordi, A. Gas absorption enhancement in hollow fiber membrane contactors using nanofluids: Modeling and simulation. Chem. Eng. Process. Process. Intensif. 2017, 119, 7–15. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. Rsc Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Lv, Y.; Yu, X.; Tu, S.T.; Yan, J.; Dahlquist, E. Experimental studies on simultaneous removal of CO2 and SO2 in a polypropylene hollow fiber membrane contactor. Appl. Energy 2012, 97, 283–288. [Google Scholar] [CrossRef]
- Gabelman, A.; Hwang, S.-T. Hollow fiber membrane contactors. J. Memb. Sci. 1999, 159, 61–106. [Google Scholar] [CrossRef]
- Ansaripour, M.; Haghshenasfard, M.; Moheb, A. Experimental and Numerical Investigation of CO2 Absorption Using Nanofluids in a Hollow-Fiber Membrane Contactor. Chem. Eng. Technol. 2018, 41, 367–378. [Google Scholar] [CrossRef]
- Zhang, Z.E.; Yan, Y.F.; Zhang, L.; Ju, S.X. Hollow fiber membrane contactor absorption of CO2 from the flue gas: Review and perspective. Glob. Nest J. 2014, 16, 354–373. [Google Scholar]
- Sohrabi, M.R.; Marjani, A.; Moradi, S.; Davallo, M.; Shirazian, S. Mathematical modeling and numerical simulation of CO2 transport through hollow-fiber membranes. Appl. Math. Model. 2011, 35, 174–188. [Google Scholar] [CrossRef]
- Mavroudi, M.; Kaldis, S.P.; Sakellaropoulos, G.P. A study of mass transfer resistance in membrane gas-liquid contacting processes. J. Memb. Sci. 2006, 272, 103–115. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Darabi, M.; Soroush, E.; Mesbah, M. CO2 absorption enhancement by water-based nanofluids of CNT and SiO2 using hollow-fiber membrane contactor. Sep. Purif. Technol. 2019, 210, 920–926. [Google Scholar] [CrossRef]
- Li, J.L.; Chen, B.H. Review of CO2 absorption using chemical solvents in hollow fiber membrane contactors. Sep. Purif. Technol. 2005, 41, 109–122. [Google Scholar] [CrossRef]
- Park, H.H.; Deshwal, B.R.; Jo, H.D.; Choi, W.K.; Kim, I.W.; Lee, H.K. Absorption of nitrogen dioxide by PVDF hollow fiber membranes in a G-L contactor. Desalination 2009, 243, 52–64. [Google Scholar] [CrossRef]
- Ghasem, N.; Al-Marzouqi, M.; Duidar, A. Effect of PVDF concentration on the morphology and performance of hollow fiber membrane employed as gas-liquid membrane contactor for CO2 absorption. Sep. Purif. Technol. 2012, 98, 174–185. [Google Scholar] [CrossRef]
- Ghobadi, J.; Ramirez, D.; Khoramfar, S.; Jerman, R.; Crane, M.; Hobbs, K. Simultaneous absorption of carbon dioxide and nitrogen dioxide from simulated flue gas stream using gas-liquid membrane contacting system. Int. J. Greenh. Gas. Control. 2018, 77, 37–45. [Google Scholar] [CrossRef]
- Ghobadi, J.; Ramirez, D.; Jerman, R.; Crane, M.; Khoramfar, S. CO2 separation performance of different diameter polytetrafluoroethylene hollow fiber membranes using gas-liquid membrane contacting system. J. Memb. Sci. 2018, 549, 75–83. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y.; Zhang, L.; Ju, S. Numerical simulation and analysis of CO2 removal in a polypropylene hollow fiber membrane contactor. Int. J. Chem. Eng. 2014, 2014. [Google Scholar] [CrossRef]
- Rahmatmand, B.; Keshavarz, P.; Ayatollahi, S. Study of Absorption Enhancement of CO2 by SiO2, Al2O3, CNT, and Fe3O4 Nanoparticles in Water and Amine Solutions. J. Chem. Eng. Data 2016, 61, 1378–1387. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Hosseinzadeh, M.; Vatani, A.; Mohammadi, T. Mathematical modeling for the simultaneous absorption of CO2 and SO2 using MEA in hollow fiber membrane contactors. Chem. Eng. Process. Process. Intensif. 2017, 111, 35–45. [Google Scholar] [CrossRef]
- Nii, S.; Takeuchi, H. Removal of CO2 and/or SO2 from gas streams by a membrane absorption method. Gas. Sep. Purif. 1994, 8, 107–114. [Google Scholar] [CrossRef]
- Mavroudi, M.; Kaldis, S.P.; Sakellaropoulos, G.P. Reduction of CO2emissions by a membrane contacting process. Fuel 2003, 82, 2153–2159. [Google Scholar] [CrossRef]
- Hedayat, M.; Soltanieh, M.; Mousavi, S.A. Simultaneous separation of H2S and CO2 from natural gas by hollow fiber membrane contactor using mixture of alkanolamines. J. Memb. Sci. 2011, 377, 191–197. [Google Scholar] [CrossRef]
- Mohammaddoost, H.; Azari, A.; Ansarpour, M.; Osfouri, S. Experimental investigation of CO2 removal from N2 by metal oxide nanofluids in a hollow fiber membrane contactor. Int. J. Greenh. Gas. Control. 2018, 69, 60–71. [Google Scholar] [CrossRef]
- Dai, Z.; Usman, M.; Hillestad, M.; Deng, L. Modelling of a tubular membrane contactor for pre-combustion CO2 capture using ionic liquids: Influence of the membrane configuration, absorbent properties and operation parameters. Green Energy Env. 2016, 1, 266–275. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, J.; Chen, F.; Li, H.; Zhang, W.; Qi, W. Progress in enhancement of CO2 absorption by nanofluids: A mini review of mechanisms and current status. Renew. Energy 2018, 118, 527–535. [Google Scholar] [CrossRef]
- Hajilary, N.; Rezakazemi, M. CFD modeling of CO2 capture by water-based nanofluids using hollow fiber membrane contactor. Int. J. Greenh. Gas. Control. 2018, 77, 88–95. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Ji, G.; Gu, X. CFD simulation of hollow fiber supported NaA zeolite membrane modules. Sep. Purif. Technol. 2019, 213, 1–10. [Google Scholar] [CrossRef]
- Khan, M.J.H.; Hussain, M.A.; Mansourpour, Z.; Mostoufi, N.; Ghasem, N.M.; Abdullah, E.C. CFD simulation of fluidized bed reactors for polyolefin production—A review. J. Ind. Eng. Chem. 2014, 20, 3919–3946. [Google Scholar] [CrossRef]
- Ghasem, N.; Al-Marzouqi, M.; Abdul Rahim, N. Modeling of CO2 absorption in a membrane contactor considering solvent evaporation. Sep. Purif. Technol. 2013, 110, 1–10. [Google Scholar] [CrossRef]
- Rosli, A.; Shoparwe, N.F.F.; Ahmad, A.L.L.; Low, S.C.; Lim, J.K.K. Dynamic modelling and experimental validation of CO2 removal using hydrophobic membrane contactor with different types of absorbent. Sep. Purif. Technol. 2019, 219, 230–240. [Google Scholar] [CrossRef]
- Günther, J.; Schmitz, P.; Albasi, C.; Lafforgue, C. A numerical approach to study the impact of packing density on fluid flow distribution in hollow fiber module. J. Memb. Sci. 2010, 348, 277–286. [Google Scholar] [CrossRef]
- Happel, J. Viscous flow relative to arrays of cylinders. Aiche J. 1959, 5, 174–177. [Google Scholar] [CrossRef]
- Villeneuve, K.; Albarracin Zaidiza, D.; Roizard, D.; Rode, S.; Nagy, E.; Feczkó, T.; Koroknai, B. Enhancement of oxygen mass transfer rate in the presence of nanosized particles. Chem. Eng. Sci. 2007, 62, 7391–7398. [Google Scholar]
- Zanfir, M.; Gavriilidis, A.; Wille, C.; Hessel, V. Carbon dioxide absorption in a falling film microstructured reactor: Experiments and modeling. Ind. Eng. Chem. Res. 2005, 44, 1742–1751. [Google Scholar] [CrossRef]
- Versteeg, G.F.; van Swaal, W.P.M. Solubility and diffusivity of acid gases (CO2, N2O) in aqueous alkanolamine solutions. J. Chem. Eng. Data 1988, 33, 29–34. [Google Scholar] [CrossRef]


| Property | Value |
|---|---|
| Inner fiber radius (mm) | 0.34 |
| Outer fiber radius (mm) | 0.60 |
| Number of fibers | 590 |
| Module outer radius (mm) | 25.4 |
| Module effective length (mm) | 200 |
| Physical Property | Value | Reference |
|---|---|---|
| Diffusivity of CO2 in shell, | 1.855 × 10−5 m2/s | [9] |
| Diffusivity of CO2 in tube, | 1.92 × 10−9 m2/s | [19] |
| Diffusivity of CO2 in membrane, | × ε/τ | [37] |
| Diffusivity of NO2 in shell, | 1.54 × 10−5 m2/s | [43] |
| Diffusivity of NO2 in tube, | 1.4 × 10−9 m2/s | [43] |
| Diffusivity of solvent in tube, Ds,t | 0.5 × | Estimated |
| Diffusivity of NO2 in membrane, | × ε/τ | [37] |
| Porosity, ε | 0.52 | [23] |
| Tortuosity, τ | (2−ε)2/ε | [9] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasem, N. Modeling and Simulation of the Absorption of CO2 and NO2 from a Gas Mixture in a Membrane Contactor. Processes 2019, 7, 441. https://doi.org/10.3390/pr7070441
Ghasem N. Modeling and Simulation of the Absorption of CO2 and NO2 from a Gas Mixture in a Membrane Contactor. Processes. 2019; 7(7):441. https://doi.org/10.3390/pr7070441
Chicago/Turabian StyleGhasem, Nayef. 2019. "Modeling and Simulation of the Absorption of CO2 and NO2 from a Gas Mixture in a Membrane Contactor" Processes 7, no. 7: 441. https://doi.org/10.3390/pr7070441
APA StyleGhasem, N. (2019). Modeling and Simulation of the Absorption of CO2 and NO2 from a Gas Mixture in a Membrane Contactor. Processes, 7(7), 441. https://doi.org/10.3390/pr7070441