The Effect of Various Nanofluids on Absorption Intensification of CO2/SO2 in a Single-Bubble Column
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.2.1. Nanofluid Preparation Instruments
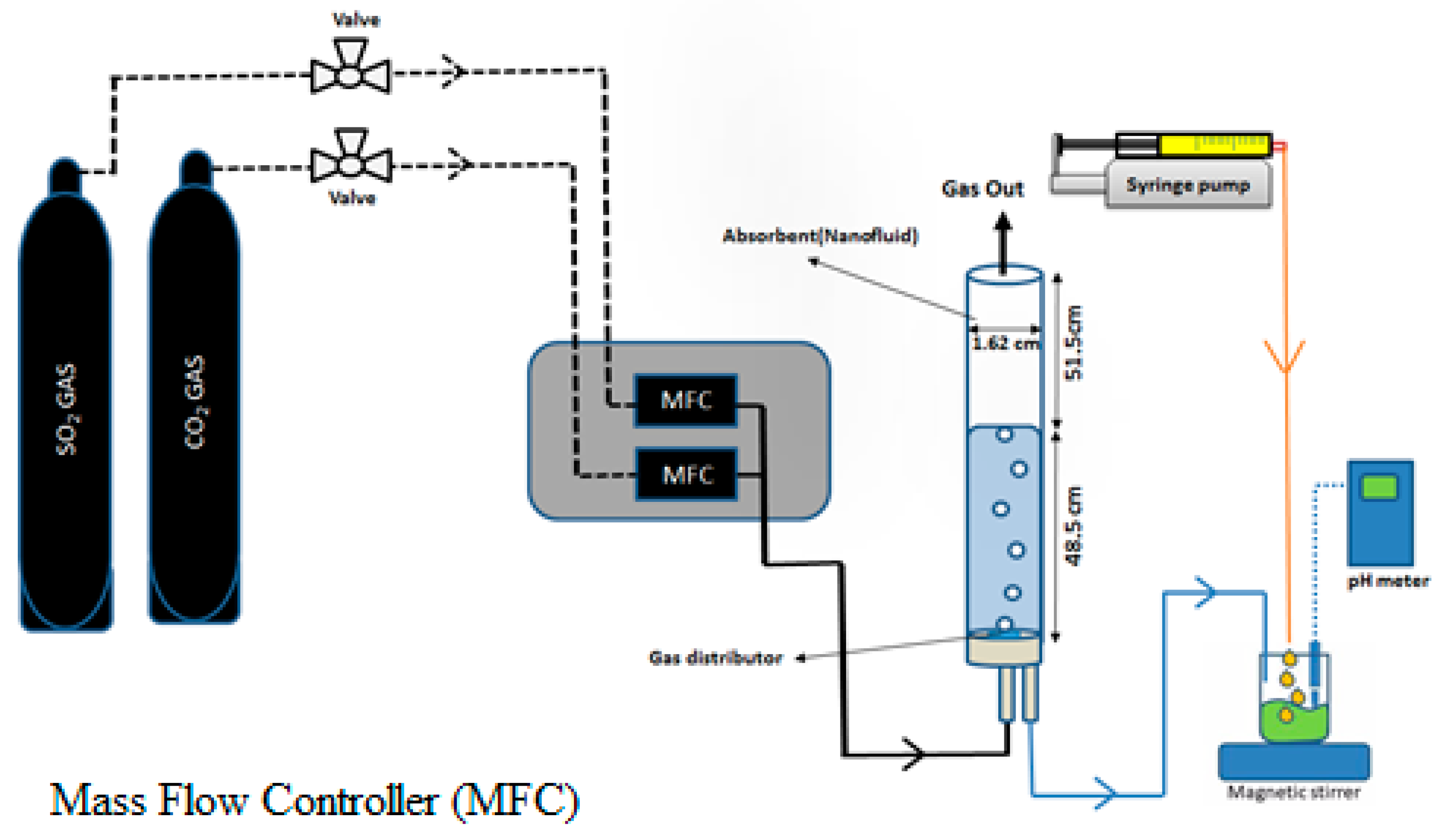
2.2.2. Experimental Set-Up
2.3. Methods
2.3.1. Nanofluid Preparation Procedure
2.3.2. Experimental Procedure
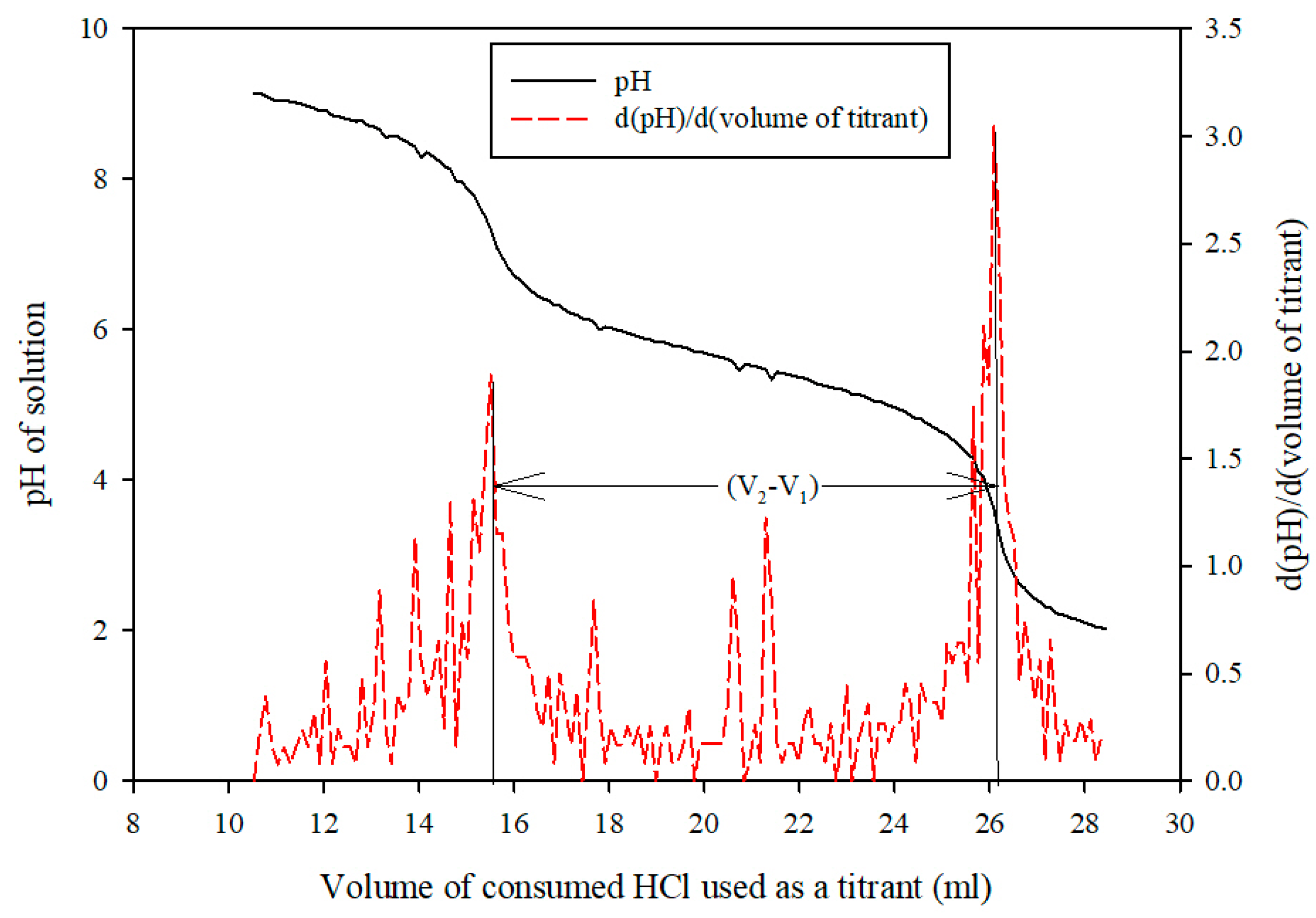
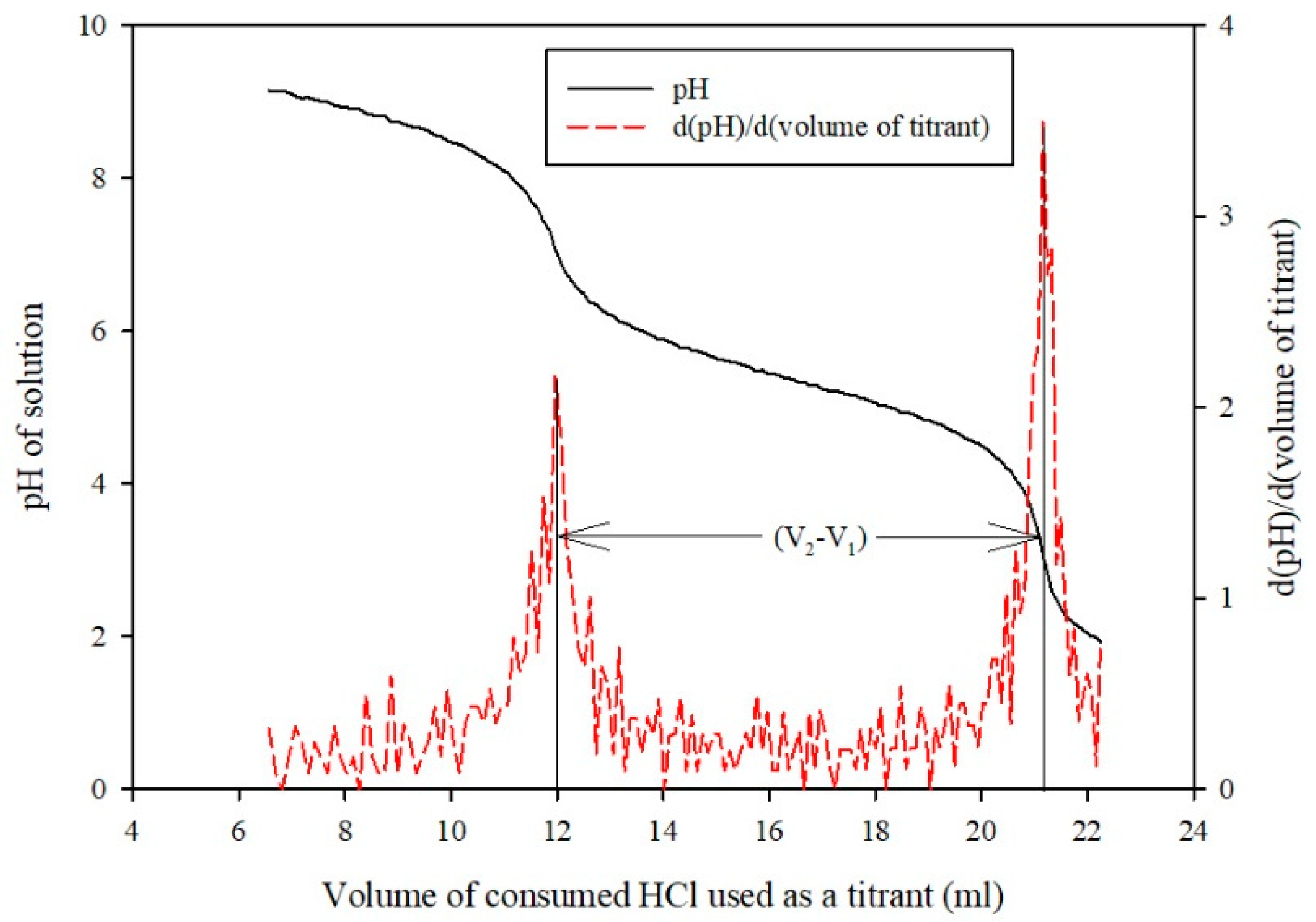
Sample Analysis Procedure
Measurement of Mass Transfer Parameters
Uncertainty Analysis
3. Results and Discussion
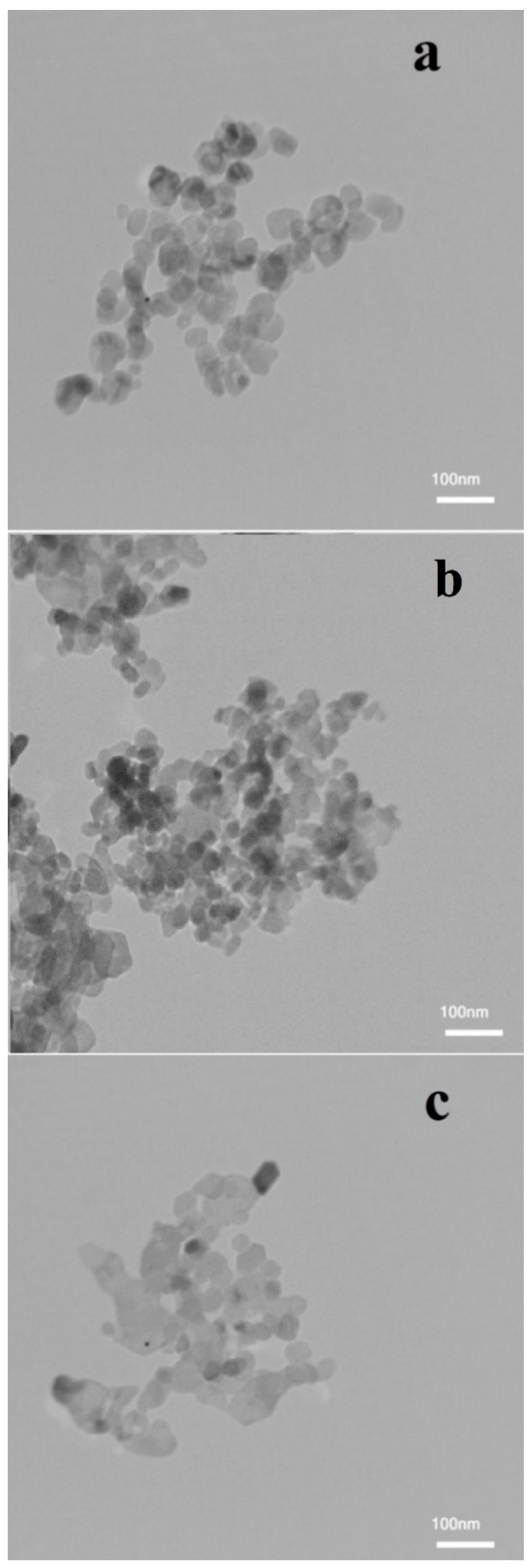
3.1. Nanofluid Characterization
3.2. Absorption
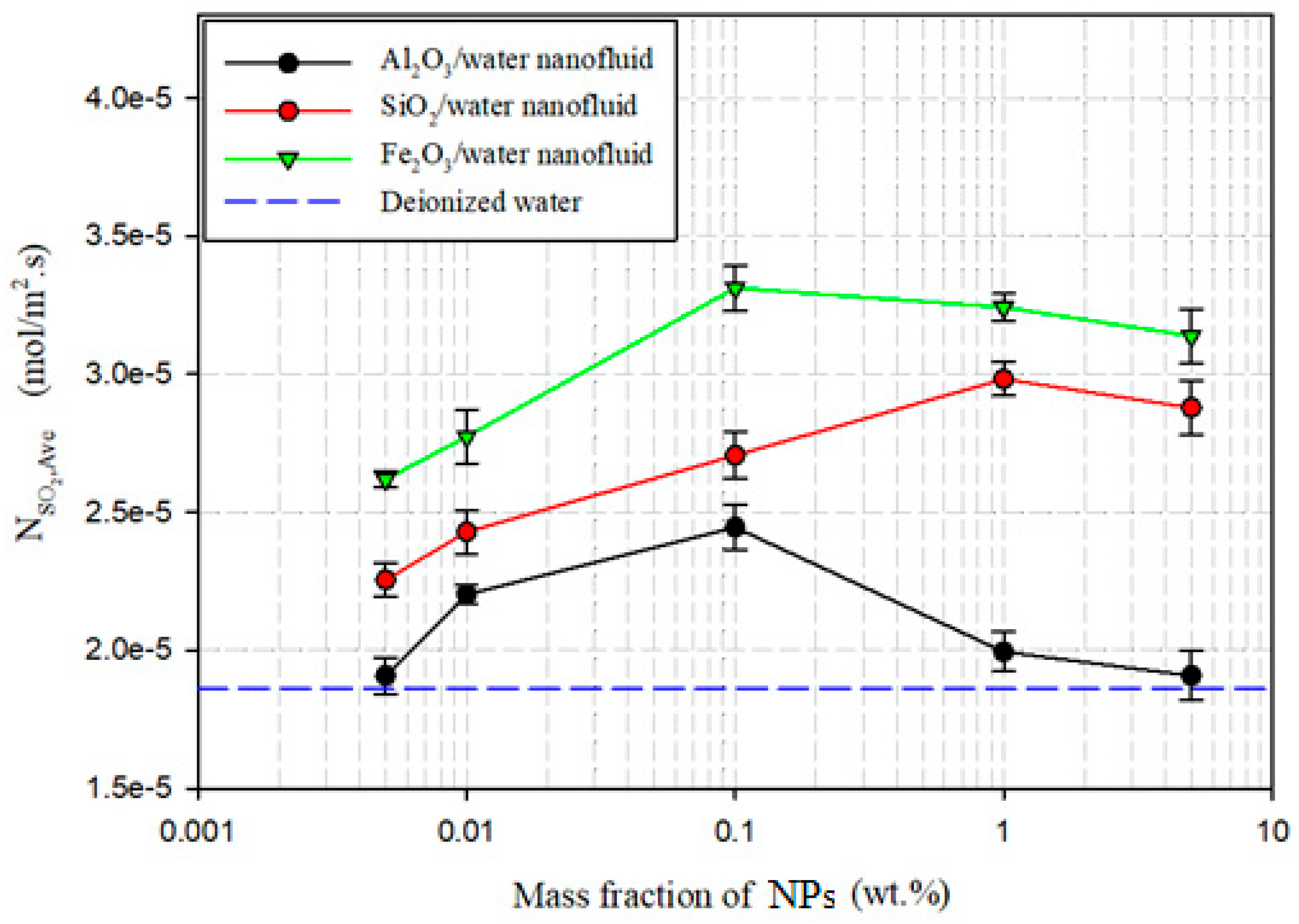
3.2.1. Maximum Absorption
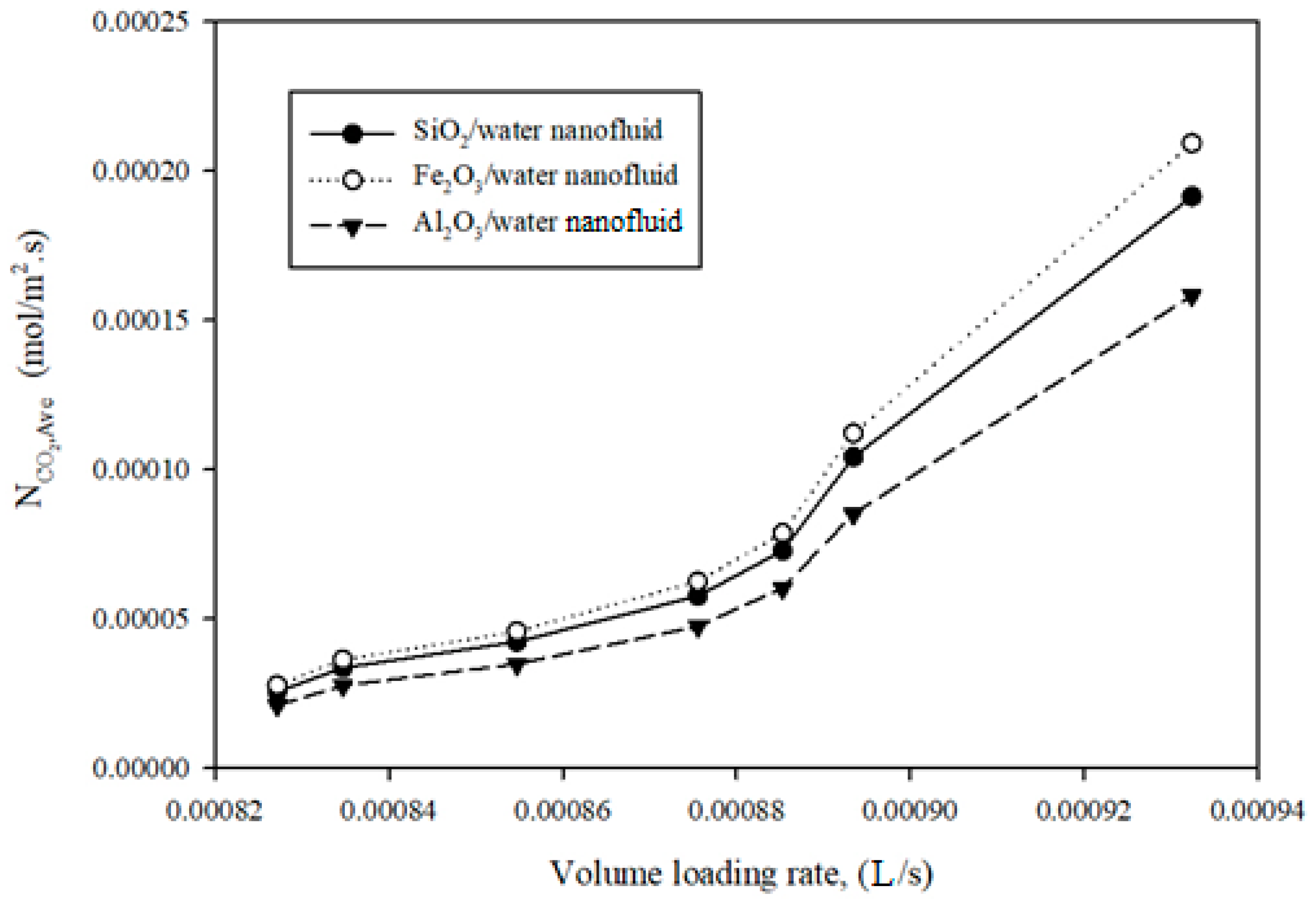
3.2.2. Probing of Mass Transfer Rate
3.2.3. Mass Transfer Coefficient
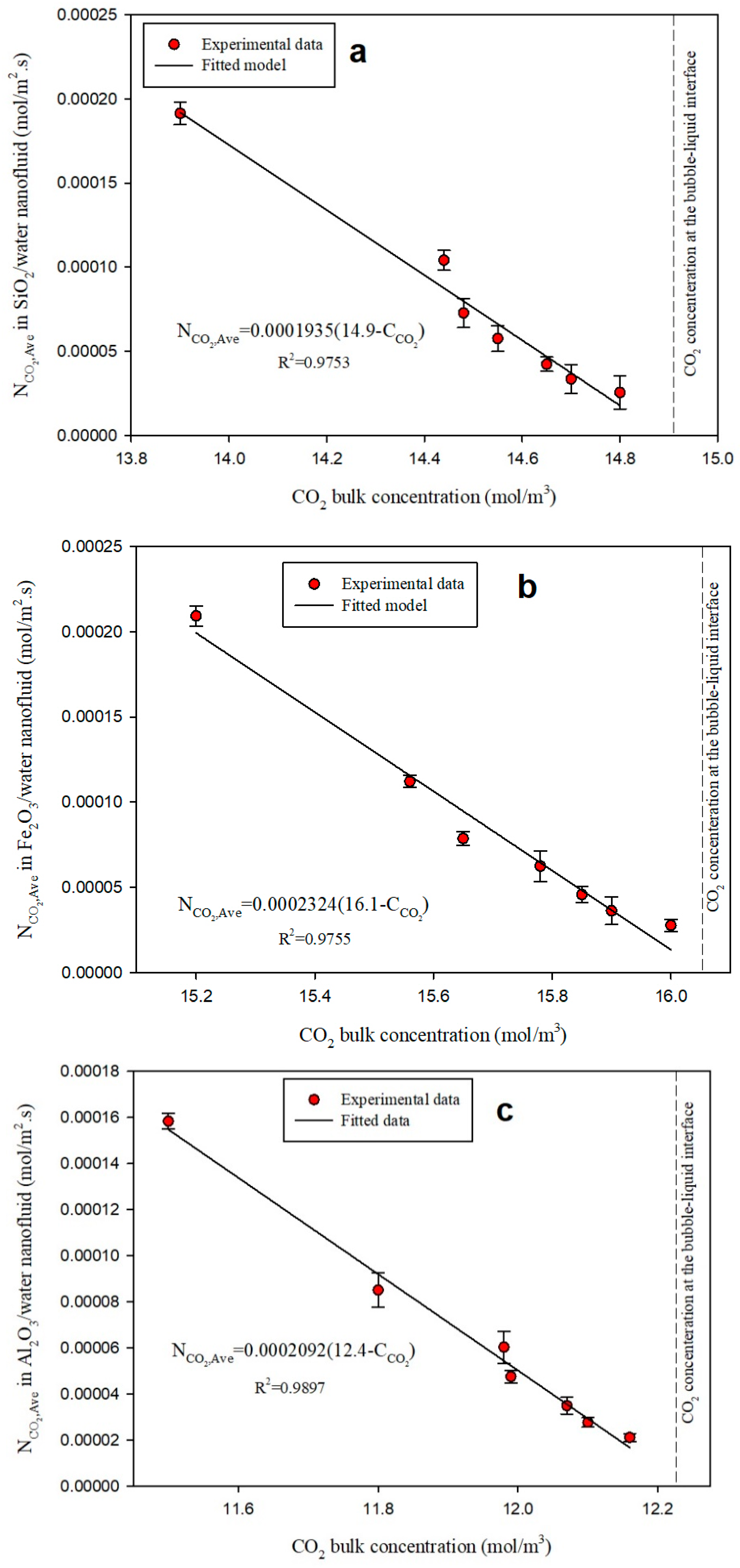
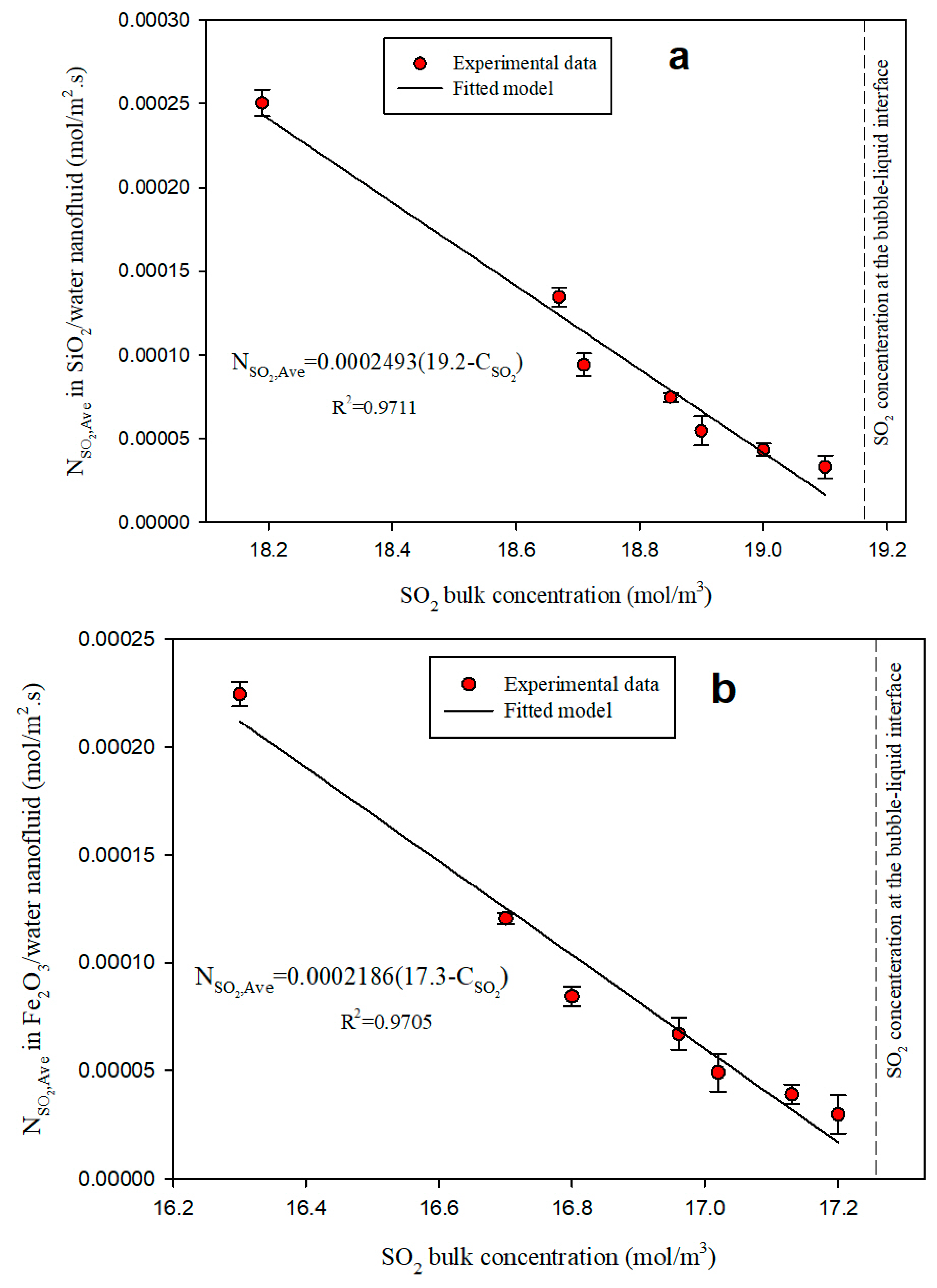
3.3. Diffusivity Coefficient
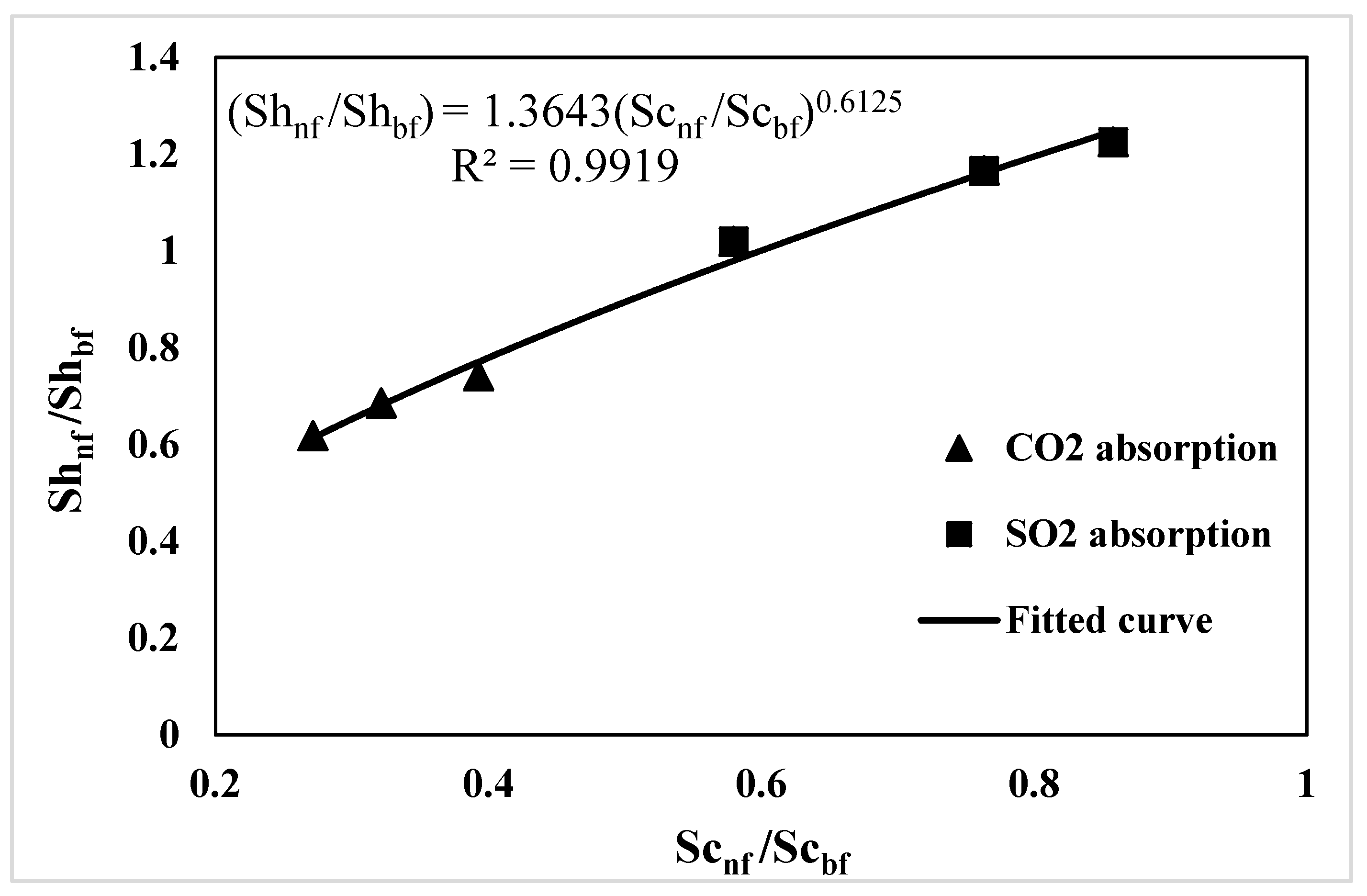
3.4. Correlation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Molar flux (mol/m2 s) | |
| Gas concentration at liquid bulk (mol/m3) | |
| The observed gas concentration at gas-liquid interface (mol/m3) | |
| Volume of nanofluid in the single bubble absorber (m3) | |
| Number of bubbles | |
| Average rising time for one bubble through the column (s) | |
| Average radius of bubbles (m) | |
| Mass transfer coefficient in liquid phase (m/s) | |
| Diffusion coefficient (m2/s) | |
| Diffusion layer thickness (mm) | |
| Renewal surface factor (1/s) | |
| Reb | Reynolds number (Ubdb/ʋnf) |
| Sc | Schmidt number (ʋnf/Dnf) |
| Sh | Sherwood number (kLdb/Dnf) |
| Diameter of bubbles raising through nanofluid (m) | |
| Volume fraction (%) | |
| w | Mass fraction (%) |
| Density (kg/m3) | |
| Kinematic viscosity (m2/s) | |
| Constant value for calculation of Brownian momentum transfer | |
| Reff | Relative absorption rate (Nnf/Nbf) |
| M | HCl molarity (mol/lit) |
| Constant value as a function of nanoparticles density, temperature, volume fraction, mean diameter, heat capacity, and Boltzmann constant. | |
| Mo | Momentum that can be transferred by means of nanoparticle random motion |
| Subscript | |
| Nanofluid | |
| Basefluid | |
| Nanoparticles | |
| B | Bubble |
References
- Nii, S.; Takeuchi, H. Removal of CO2 and/or SO2 from gas streams by a membrane absorption method. Gas Sep. Purif. 1994, 8, 107–114. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Esfahany, M.N.; Esmaeili-Faraj, S.H. Investigation of the Effects of Nanoparticle Size on CO2 Absorption by Silica-Water Nanofluid. Sep. Purif. Technol. 2017. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Pahlevaninezhad, M.; Abdollahi, A.; Davoodi, S.M. Investigation of the effect of magnetic field on mass transfer parameters of CO2 absorption using Fe3O4-water nanofluid. AIChE J. 2017, 63, 2176–2186. [Google Scholar] [CrossRef]
- Esmaeili-Faraj, S.H.; Nasr Esfahany, M.; Jafari-Asl, M.; Etesami, N. Hydrogen sulfide bubble absorption enhancement in water-based nanofluids. Ind. Eng. Chem. Res. 2014, 53, 16851–16858. [Google Scholar] [CrossRef]
- Esmaeili-Faraj, S.H.; Nasr Esfahany, M. Absorption of hydrogen sulfide and carbon dioxide in water based nanofluids. Ind. Eng. Chem. Res. 2016, 55, 4682–4690. [Google Scholar] [CrossRef]
- Kim, J.-K.; Jung, J.Y.; Kang, Y.T. Absorption performance enhancement by nano-particles and chemical surfactants in binary nanofluids. Int. J. Refrig. 2007, 30, 50–57. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Richard, M.A.; Bénard, P.; Chahine, R. Gas adsorption process in activated carbon over a wide temperature range above the critical point. Part 1: Modified Dubinin-Astakhov model. Adsorption 2009, 15, 43–51. [Google Scholar] [CrossRef]
- Whitman, W.G. The two film theory of gas absorption. Int. J. Heat Mass Transf. 1962, 5, 429–433. [Google Scholar] [CrossRef]
- Ashrafmansouri, S.S.; Nasr Esfahany, M. Mass transfer into/from nanofluid drops in a spray liquid-liquid extraction column. AIChE J. 2016, 62, 852–860. [Google Scholar] [CrossRef]
- Ashrafmansouri, S.S.; Esfahany, M.N. Mass transfer in nanofluids: A review. Int. J. Therm. Sci. 2014, 82, 84–99. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Esfahany, M.N. Experimental investigation of the effect of nanoparticle size on thermal conductivity of in-situ prepared silica–ethanol nanofluid. Int. Commun. Heat Mass Transf. 2016, 77, 148–154. [Google Scholar] [CrossRef]
- Chol, S. Enhancing thermal conductivity of fluids with nanoparticles. ASME-Publ.-Fed 1995, 231, 99–106. [Google Scholar]
- Krishnamurthy, S.; Bhattacharya, P.; Phelan, P.; Prasher, R. Enhanced mass transport in nanofluids. Nano Lett. 2006, 6, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Jung, J.Y.; Kim, J.H.; Kim, M.-G.; Kashiwagi, T.; Kang, Y.T. The effect of chemical surfactants on the absorption performance during NH3/H2O bubble absorption process. Int. J. Refrig. 2006, 29, 170–177. [Google Scholar] [CrossRef]
- Ma, X.; Su, F.; Chen, J.; Zhang, Y. Heat and mass transfer enhancement of the bubble absorption for a binary nanofluid. J. Mech. Sci. Technol. 2007, 21, 1813. [Google Scholar] [CrossRef]
- Zhou, M.; Cai, W.F.; Xu, C.J. A new way of enhancing transport process–The hybrid process accompanied by ultrafine particles. Korean J. Chem. Eng. 2003, 20, 347–353. [Google Scholar] [CrossRef]
- Kang, Y.T.; Lee, J.-K.; Kim, B.-C. Absorption heat transfer enhancement in binary nanofluids. In International Congress of Refrigeration; Curran Associates, Inc.: Beijing, China, 2007. [Google Scholar]
- Yang, L.; Du, K.; Niu, X.F.; Cheng, B.; Jiang, Y.F. Experimental study on enhancement of ammonia–water falling film absorption by adding nano-particles. Int. J. Refrig. 2011, 34, 640–647. [Google Scholar] [CrossRef]
- Attari, H.; Derakhshanfard, F.; Darvanjooghi, M.H.K. Effect of temperature and mass fraction on viscosity of crude oil-based nanofluids containing oxide nanoparticles. Int. Commun. Heat Mass Transf. 2017, 82, 103–113. [Google Scholar] [CrossRef]
- Heris, S.Z.; Etemad, S.G.; Esfahany, M.N. Experimental investigation of oxide nanofluids laminar flow convective heat transfer. Int. Commun. Heat Mass Transf. 2006, 33, 529–535. [Google Scholar] [CrossRef]
- Kang, Y.T.; Kim, H.J.; Lee, K.I. Heat and mass transfer enhancement of binary nanofluids for H2O/LiBr falling film absorption process. Int. J. Refrig. 2008, 31, 850–856. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Mujumdar, A.S. Heat transfer characteristics of nanofluids: A review. Int. J. Therm. Sci. 2007, 46, 1–19. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Lee, J.W.; Kang, Y.T. CO2 absorption characteristics of nanoparticle suspensions in methanol. J. Mech. Sci. Technol. 2012, 26, 2285–2290. [Google Scholar] [CrossRef]
- Andrade, Â.L.; Fabris, J.D.; Ardisson, J.D.; Valente, M.A.; Ferreira, J.M. Effect of tetramethylammonium hydroxide on nucleation, surface modification and growth of magnetic nanoparticles. J. Nanomater. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Andrade, Â.L.; Souza, D.M.; Pereira, M.C.; Fabris, J.D.; Domingues, R.Z. pH effect on the synthesis of magnetite nanoparticles by the chemical reduction-precipitation method. Quim. Nova 2010, 33, 524–527. [Google Scholar] [CrossRef] [Green Version]
- Davoodi, S.M.; Sadeghi, M.; Naghsh, M.; Moheb, A. Olefin–paraffin separation performance of polyimide Matrimid®/silica nanocomposite membranes. RSC Adv. 2016, 6, 23746–23759. [Google Scholar] [CrossRef]
- Terraglio, F.P.; Manganelli, R.M. The absorption of atmospheric sulfur dioxide by water solutions. J. Air Pollut. Control Assoc. 1967, 17, 403–406. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, J.; Yang, W.; Jin, Y. Gas–liquid mass transfer in slurry bubble systems: I. Mathematical modeling based on a single bubble mechanism. Chem. Eng. J. 2003, 96, 23–27. [Google Scholar] [CrossRef]
- Skoog, D.A.; West, D.M. Fundamentals of Analytical Chemistry; Thomson Brooks/Cole: Pacific Grove, CA, USA, 2004. [Google Scholar]
- Teng, T.-P.; Hung, Y.-H.; Teng, T.-C.; Mo, H.-E.; Hsu, H.-G. The effect of alumina/water nanofluid particle size on thermal conductivity. Appl. Therm. Eng. 2010, 30, 2213–2218. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Koltypin, Y.; Felner, I.; Gedanken, A. Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Mater. Sci. Eng. A 2000, 286, 101–105. [Google Scholar] [CrossRef]
- áO’Brien, R.W. Electroacoustic studies of moderately concentrated colloidal suspensions. Faraday Discuss. Chem. Soc. 1990, 90, 301–312. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010, 7, 1–37. [Google Scholar] [CrossRef]
- Kim, W.-G.; Kang, H.U.; Jung, K.-M.; Kim, S.H. Synthesis of silica nanofluid and application to CO2 absorption. Sep. Sci. Technol. 2008, 43, 3036–3055. [Google Scholar] [CrossRef]
- Koo, J.; Kleinstreuer, C. Impact analysis of nanoparticle motion mechanisms on the thermal conductivity of nanofluids. Int. Commun. Heat Mass Transf. 2005, 32, 1111–1118. [Google Scholar] [CrossRef]
- Purser, G.H. Lewis structures are models for predicting molecular structure, not electronic structure. J. Chem. Educ. 1999, 76, 1013. [Google Scholar] [CrossRef]
- Vasconcelos, J.M.; Orvalho, S.P.; Alves, S.S. Gas–liquid mass transfer to single bubbles: Effect of surface contamination. AIChE J. 2002, 48, 1145–1154. [Google Scholar] [CrossRef]
- Calderbank, P.; Lochiel, A. Mass transfer coefficients, velocities and shapes of carbon dioxide bubbles in free rise through distilled water. Chem. Eng. Sci. 1964, 19, 485–503. [Google Scholar] [CrossRef]
- Mishra, P.C.; Mukherjee, S.; Nayak, S.K.; Panda, A. A brief review on viscosity of nanofluids. Int. Nano Lett. 2014, 4, 109–120. [Google Scholar] [CrossRef] [Green Version]




| Properties | SiO2 NPs | Al2O3 NPs | Fe2O3 NPs |
|---|---|---|---|
| Molecular weight (g/mol) | 60.08 | 101.96 | 159.69 |
| Density (g/cm3) | 2.196 | 3.980 | 5.242 |
| Melting point (°C) | 1713 | 2054 | 1539 |
| Appearance | White solid powder | White solid powder | Red-brown solid powder |
| Absorbent | SO2 Absorption | CO2 Absorption | ||||
|---|---|---|---|---|---|---|
| Water (bf) | 1.871 × 10−5 | 1.000 | 1.566 × 10−5 | 1.000 | ||
| SiO2/water | 1.0 | 2.983 × 10−5 | 1.594 | 0.01 | 2.774 × 10−5 | 1.771 |
| Al2O3/water | 0.1 | 2.445 × 10−5 | 1.307 | 0.1 | 2.098 × 10−5 | 1.340 |
| Fe2O3/water | 0.1 | 3.312 × 10−5 | 1.770 | 1.0 | 2.566 × 10−5 | 1.638 |
| Gas | Absorbent | ||
|---|---|---|---|
| CO2 | Water (BF) | 1.030 | 1.00 |
| Water/SiO2 NF | 1.935 | 1.88 | |
| Water/Fe2O3 NF | 2.324 | 2.03 | |
| Water/Al2O3 NF | 2.092 | 2.17 | |
| SO2 | Water (BF) | 1.450 | 1.00 |
| Water/SiO2 NF | 2.493 | 1.71 | |
| Water/Fe2O3 NF | 2.186 | 1.42 | |
| Water/Al2O3 NF | 2.063 | 1.50 |
| Gas | Absorbent | D, (m2/s) | Sc | Reb | Sh. | |
|---|---|---|---|---|---|---|
| CO2 | Water/SiO2 | 5.38 × 10−9 | 8.899 × 10−7 | 165 | 1298 | 234 |
| Water/Fe2O3 | 7.76 × 10−9 | 8.864 × 10−7 | 114 | 1303 | 195 | |
| Water/Al2O3 | 6.28 × 10−9 | 8.451 × 10−7 | 135 | 1367 | 217 | |
| Deionized water | 2.12 × 10−9 | 8.900 × 10−7 | 420 | 1298 | 316 | |
| SO2 | Water/SiO2 | 8.89 × 10−9 | 8.706 × 10−7 | 98 | 1327 | 182 |
| Water/Fe2O3 | 6.85 × 10−9 | 8.864 × 10−7 | 129 | 1303 | 207 | |
| Water/Al2O3 | 6.12 × 10−9 | 8.852 × 10−7 | 145 | 1305 | 219 | |
| Deionized water | 5.27 × 10−9 | 8.900 × 10−7 | 169 | 1298 | 179 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamian, S.; Mowla, D.; Esmaeilzadeh, F. The Effect of Various Nanofluids on Absorption Intensification of CO2/SO2 in a Single-Bubble Column. Processes 2019, 7, 393. https://doi.org/10.3390/pr7070393
Karamian S, Mowla D, Esmaeilzadeh F. The Effect of Various Nanofluids on Absorption Intensification of CO2/SO2 in a Single-Bubble Column. Processes. 2019; 7(7):393. https://doi.org/10.3390/pr7070393
Chicago/Turabian StyleKaramian, Soroush, Dariush Mowla, and Feridun Esmaeilzadeh. 2019. "The Effect of Various Nanofluids on Absorption Intensification of CO2/SO2 in a Single-Bubble Column" Processes 7, no. 7: 393. https://doi.org/10.3390/pr7070393
APA StyleKaramian, S., Mowla, D., & Esmaeilzadeh, F. (2019). The Effect of Various Nanofluids on Absorption Intensification of CO2/SO2 in a Single-Bubble Column. Processes, 7(7), 393. https://doi.org/10.3390/pr7070393




