Enhancement Effect of Ordered Hierarchical Pore Configuration on SO2 Adsorption and Desorption Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Coal-Based Activated Carbon
2.2. Synthesis of Ordered Mesoporous Carbon and Hierarchical Carbon
2.3. Characterizations
2.4. SO2 Adsorption Measurements
2.5. Water-Washing Regeneration
3. Results
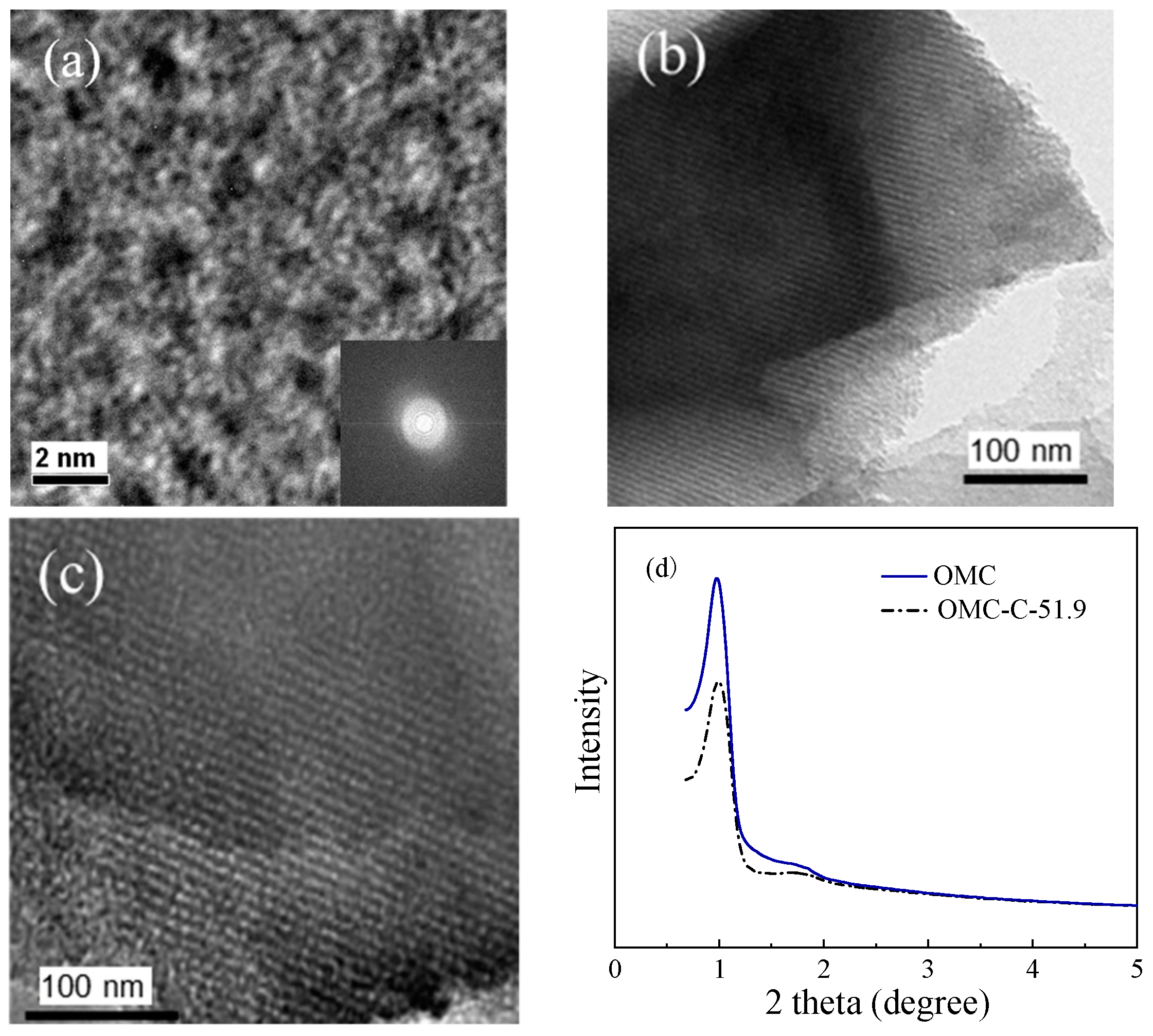
3.1. Structural Characterization of Two Different Carbon Adsorbents
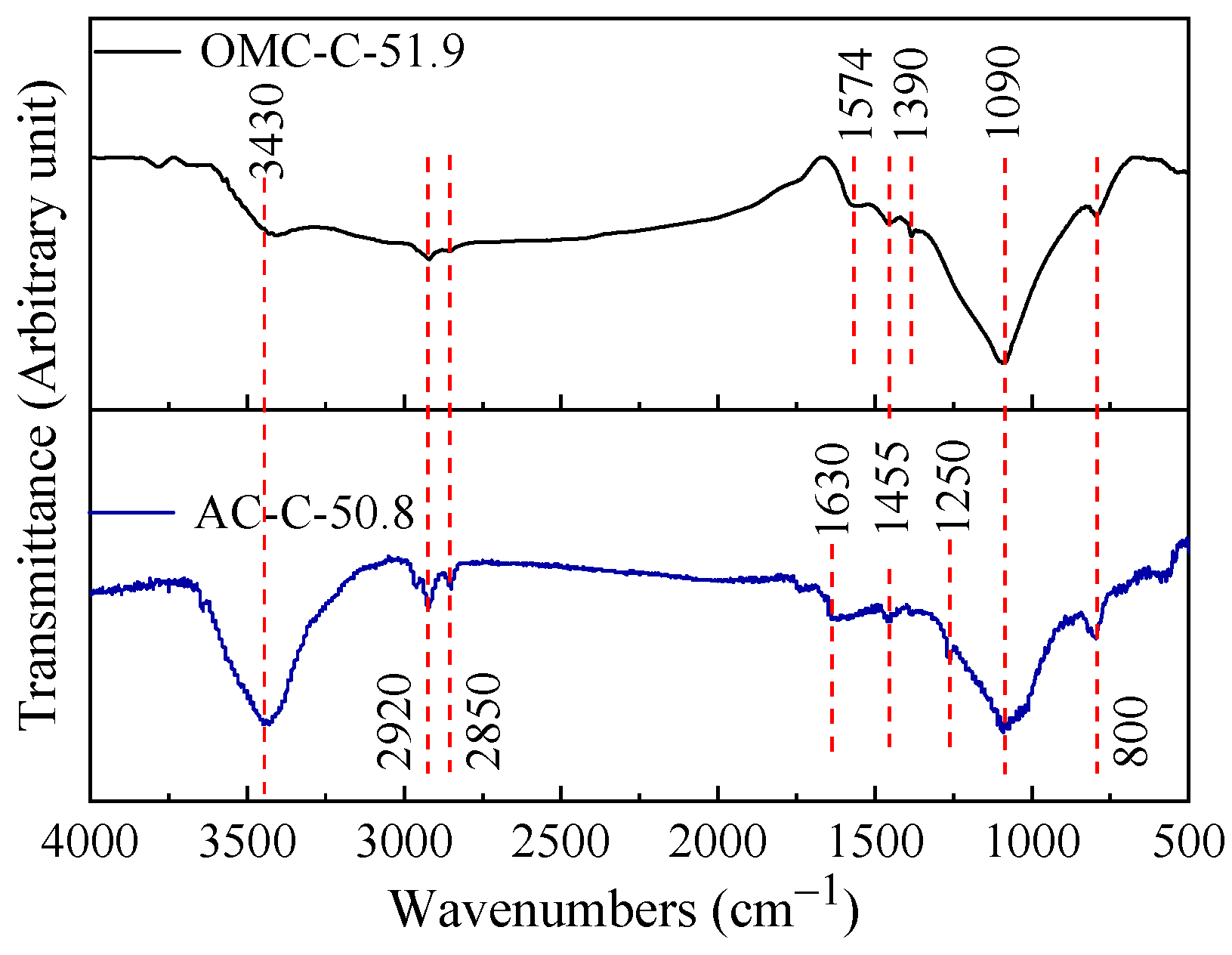
3.2. Chemical Composition of Two Different Carbon Adsorbents
3.3. SO2 Removal Performance of Adsorbents
3.4. Regeneration and Cycling Stability Performance of Adsorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, L.; Yang, K.; Zhao, Q.P.; Wang, H.Y.; Cui, Q. Characterization and mechanisms of H2S and SO2 adsorption by activated carbon. Energy Fuels 2015, 29, 6678–6685. [Google Scholar] [CrossRef]
- Grzyb, B.; Albiniak, A.; Broniek, E.; Furdin, G.; Marêché, J.F.; Bégin, D. SO2 adsorptive properties of activated carbons prepared from polyacrylonitrile and its blends with coal-tar pitch. Microporous Mesoporous Mater. 2009, 118, 163–168. [Google Scholar] [CrossRef]
- Liu, C.F.; Shih, S.M. Effects of flue gas components on the reaction of Ca(OH)2 with SO2. Ind. Eng. Chem. Res. 2006, 45, 8765–8769. [Google Scholar] [CrossRef]
- Lisovskii, A.; Semiat, R.; Aharoni, C. Adsorption of sulfur dioxide by active carbon treated by nitric acid: I. Effect of the treatment on adsorption of SO2 and extractability of the acid formed. Carbon 1997, 35, 1639–1643. [Google Scholar] [CrossRef]
- Qu, Y.F.; Guo, J.X.; Chu, Y.H.; Sun, M.C.; Yin, H.Q. The influence of Mn species on the SO2 removal of Mn-based activated carbon catalysts. Appl. Surf. Sci. 2013, 282, 425–431. [Google Scholar] [CrossRef]
- Li, J.; Kobayashi, N.; Hu, Y. The activated coke preparation for SO2 adsorption by using flue gas from coal power plant. Chem. Eng. Process. 2008, 47, 118–127. [Google Scholar] [CrossRef]
- Sun, F.; Gao, J.H.; Liu, X.; Tang, X.F.; Wu, S.H. A systematic investigation of SO2 removal dynamics by coal-based activated cokes: The synergic enhancement effect of hierarchical pore configuration and gas components. Appl. Surf. Sci. 2015, 357, 1895–1901. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, X.; Jiang, W.J.; Wang, P.; Jin, Y. Cyclic regeneration of pyrolusite modified activated coke by blending method for flue gas desulfurization. Energy Fuels 2017, 31, 4556–4564. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Li, C.H.; Li, Y.X. SO2 removal from flue gas by activated semicokes-1. The preparation of catalysts and determination of operating conditions. Carbon 2003, 41, 2217–2223. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, L.L.; Zhang, Y.L.; Liang, J.; Wang, J.; Zhang, Z.; Wang, X. Activated semi-coke in SO2 removal from flue gas: Selection of activation methodology and desulfurization mechanism study. Energy Fuels 2013, 27, 3080–3089. [Google Scholar] [CrossRef]
- Mochida, I.; Korai, Y.; Shirahama, M. Removal of SOx and NOx over activated carbon fibers. Carbon 2000, 38, 227–239. [Google Scholar] [CrossRef]
- Jastrzab, K. Properties of activated cokes used for flue gas treatment in industrial waste incineration plants. Fuel Process. Technol. 2012, 101, 16–22. [Google Scholar] [CrossRef]
- Ren, S.; Hou, Y.; Tian, S.; Wu, W.; Liu, W. Deactivation and regeneration of an ionic liquid during desulfurization of simulated flue gas. Ind. Eng. Chem. Res. 2012, 51, 3425–3429. [Google Scholar] [CrossRef]
- Ren, S.; Hou, Y.; Wu, W.; Jin, M. Oxidation of SO2 absorbed by an ionic liquid during desulfurization of simulated flue gases. Ind. Eng. Chem. Res. 2011, 50, 998–1002. [Google Scholar] [CrossRef]
- Davini, P. Flue gas desulphurization by activated carbon fibers obtained from polyacrylonitrile by-product. Carbon 2003, 41, 277–284. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Cazorla-Amoros, D.; Linares-Solano, A. Temperature programmed desorption study on the mechanism of SO2 oxidation by activated carbon and activated carbon fibres. Carbon 2001, 39, 231–242. [Google Scholar] [CrossRef]
- Karatepe, N.; Orbak, İ.; Yavuz, R.; Özyuğuran, A. Sulfur dioxide adsorption by activated carbons having different textural and chemical properties. Fuel 2008, 87, 3207–3215. [Google Scholar] [CrossRef]
- Wang, Z.M.; Kaneko, K. Effect of pore width on micropore filling mechanism of SO2 in carbon micropores. J. Phys. Chem. B 1998, 102, 2863–2868. [Google Scholar] [CrossRef]
- Mangun, C.L.; DeBarr, J.A.; Economy, J. Adsorption of sulfur dioxide on ammonia-treated activated carbon fibers. Carbon 2001, 39, 1689–1696. [Google Scholar] [CrossRef]
- Bagreev, A.; Bashkova, S.; Bandosz, T.J. Adsorption of SO2 on activated carbons: The effect of nitrogen functionality and pore sizes. Langmuir 2002, 18, 1257–1264. [Google Scholar] [CrossRef]
- Bashkova, S.; Armstrong, T.R.; Schwartz, V. Selective catalytic oxidation of hydrogen sulfide on activated carbons impregnated with sodium hydroxide. Energy Fuels 2009, 23, 1674–1682. [Google Scholar] [CrossRef]
- Bollini, P.; Didas, S.A.; Jones, C.W. Amine-oxide hybrid materials for acid gas separations. J. Mater. Chem. 2011, 21, 15100–15120. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous titanium dioxide nanofibers with a significantly enhanced photocatalytic activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Importance of small micropores in CO2 capture by phenolic resin-based activated carbon. J. Mater. Chem. A 2012, 1, 112–116. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.W.; Chuang, S.S.C.; Jana, S.C. Fabrication of hierarchical V2O5 nanorods on TiO2 nanofibers and their enhanced photocatalytic activity under visible light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M.M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Pei, T.; Sun, F.; Gao, J.H.; Wang, L.J.; Pi, X.X.; Qie, Z.P.; Zhao, G.B. Introducing catalytic gasification into chemical activation for the conversion of natural coal into hierarchically porous carbons with broadened pore size for enhanced supercapacitive utilization. RSC Adv. 2018, 8, 37880–37889. [Google Scholar] [CrossRef]
- Davini, P. SO2 adsorption by activated carbons with various burnoffs obtained from a bituminous coal. Carbon 2001, 39, 1387–1393. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Gao, J.H.; Li, Y.; Sun, F.; Gao, J.M.; Wu, S.H.; Qin, Y.K. Preparation of activated carbons for SO2 adsorption by CO2 and steam activation. J. Taiwan Inst. Chem. E. 2012, 43, 112–119. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Q.Y.; Liu, Z.Y.; Huang, Z.G.; Guo, Y.X.; Yang, J.L. Roles of lattice oxygen in V2O5 and activated coke in SO2 removal over coke-supported V2O5 catalysts. Appl. Catal. B Environ. 2008, 82, 114–119. [Google Scholar] [CrossRef]
- Pietrzak, R. XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 2009, 88, 1871–1877. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lee, J.S.; Zhu, Q.; Liu, J.; Wang, Y.; Dai, S. Ammonia-treated ordered mesoporous carbons as catalytic materials for oxygen reduction reaction. Chem. Mater. 2010, 22, 2178–2180. [Google Scholar] [CrossRef]
- Sun, F.; Gao, J.H.; Zhu, Y.W.; Chen, G.Q.; Wu, S.H.; Qin, Y.K. Adsorption of SO2 by typical carbonaceous material: A comparative study of carbon nanotubes and activated carbons. Adsorption 2013, 19, 959–966. [Google Scholar] [CrossRef]
- To, J.W.F.; He, J.J.; Mei, J.G.; Haghpanah, R.; Chen, Z.; Kurosawa, T.; Chen, S.; Bae, W.; Pan, L.J.; Jeffrey, B.-H.T.; et al. Hierarchical N-doped carbon as CO2 adsorbent with high CO2 selectivity from rationally designed polypyrrole precursor. J. Am. Chem. Soc. 2016, 138, 1001–1009. [Google Scholar] [CrossRef]
- Jia, Y.F.; Xiao, B.; Thomas, K.M. Adsorption of metal ions on nitrogen surface functional groups in activated carbons. Langmuir 2002, 18, 470–478. [Google Scholar] [CrossRef]
- Biniak, S.; Szymanski, G.; Siedlewski, J. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorla-Amorós, D.; Lecea, S.M.D.; Linares-Solano, A. Factors controling the SO2 removal by porous carbons: Relevance of the SO2 oxidation step. Carbon 2000, 38, 335–344. [Google Scholar] [CrossRef]
- Lizzio, A.A.; DeBarr, J.A. Mechanism of SO2 removal by carbon. Energy Fuels 1997, 11, 284–291. [Google Scholar] [CrossRef]
- Pi, X.X.; Sun, F.; Gao, J.H.; Zhu, Y.W.; Wang, L.J.; Qu, Z.B.; Liu, H.; Zhao, G.B. Microwave irradiation induced high-efficiency regeneration for desulfurized activated coke: A comparative study with conventional thermal regeneration. Energy Fuels 2017, 31, 9693–9702. [Google Scholar] [CrossRef]
- Ania, C.O.; Parra, J.B.; Menéndez, J.A.; Pis, J.J. Effect of microwave and conventional regeneration on the microporous and mesoporous network and on the adsorptive capacity of activated carbons. Microporous Mesoporous Mater. 2005, 85, 7–15. [Google Scholar] [CrossRef]
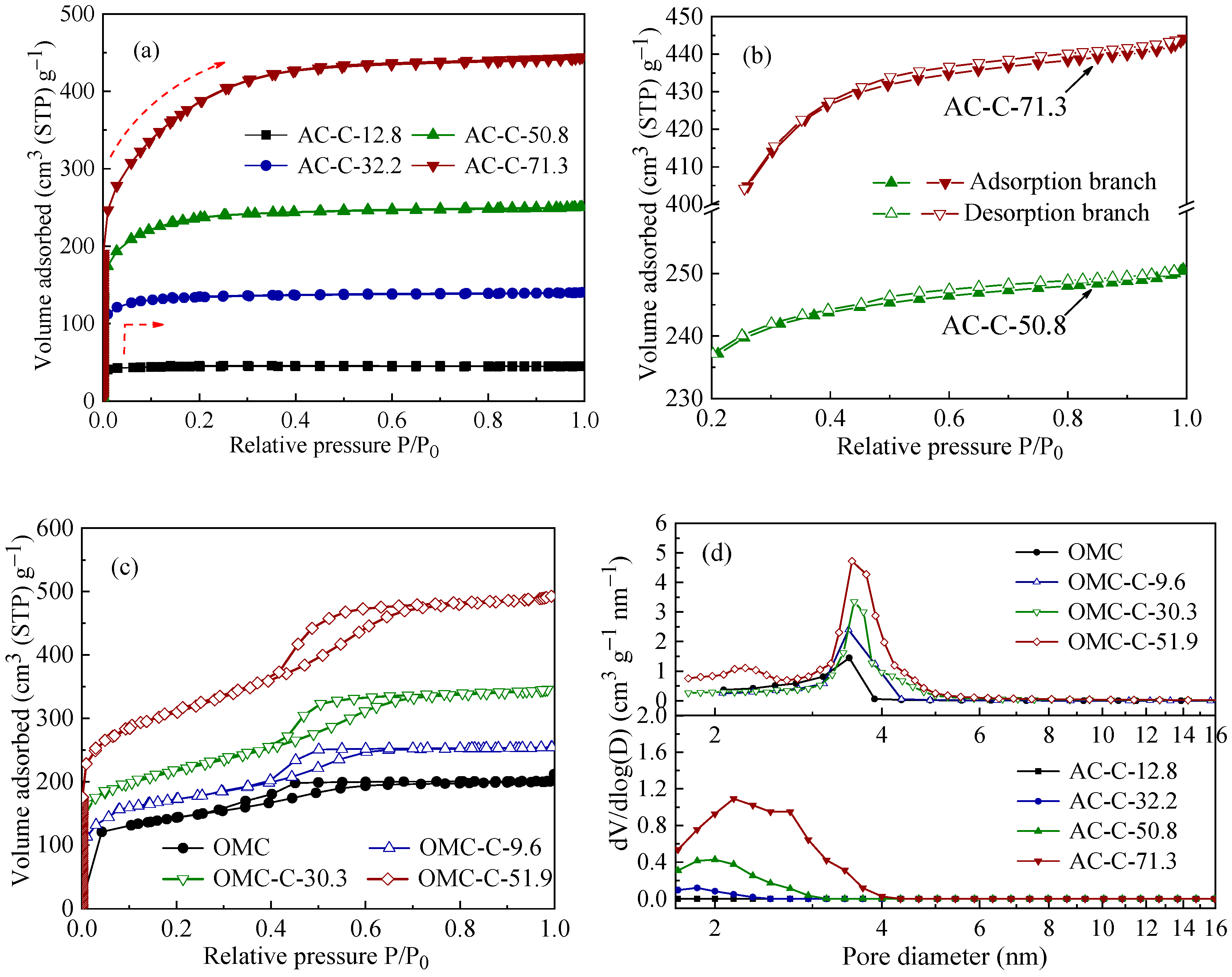
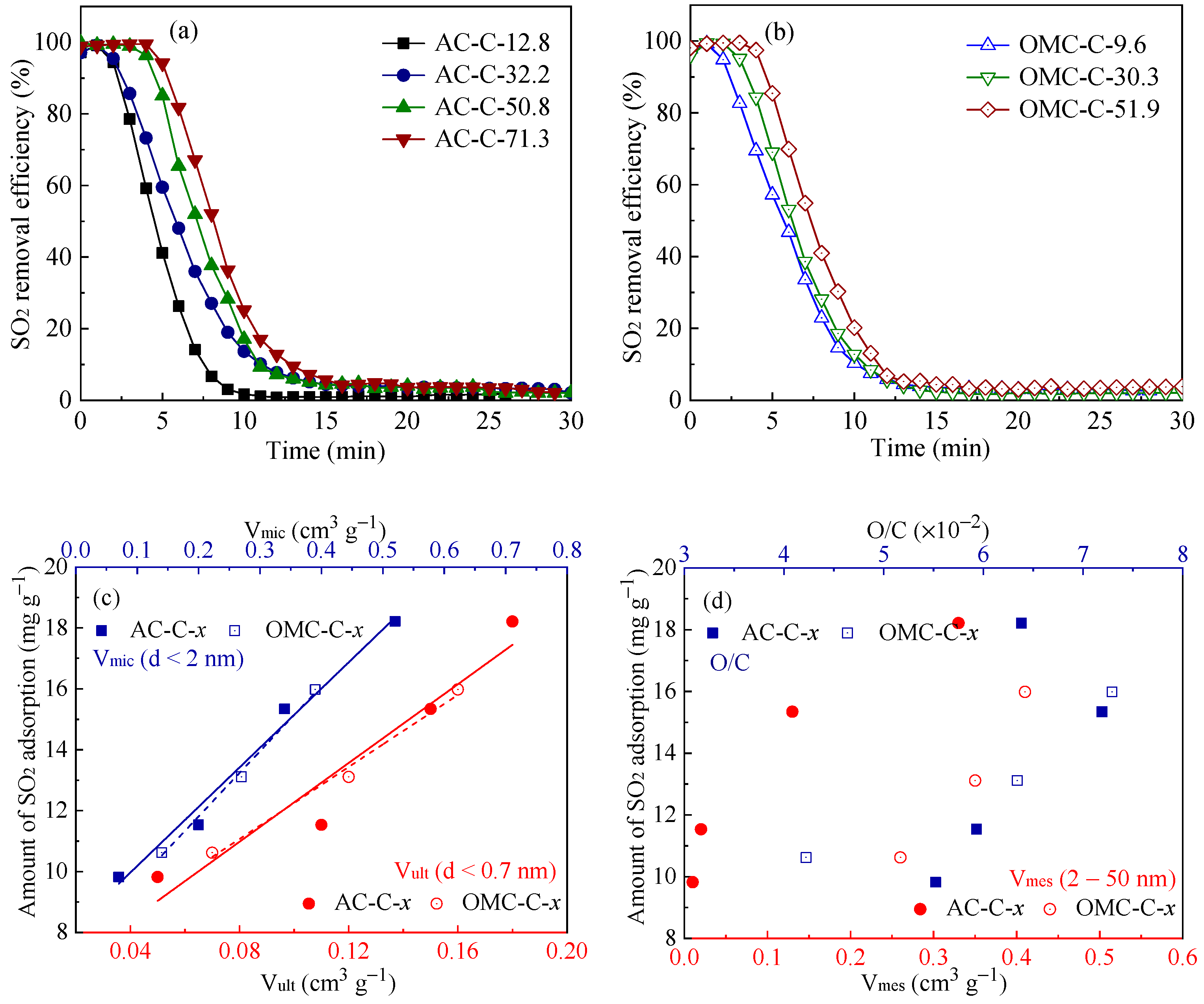
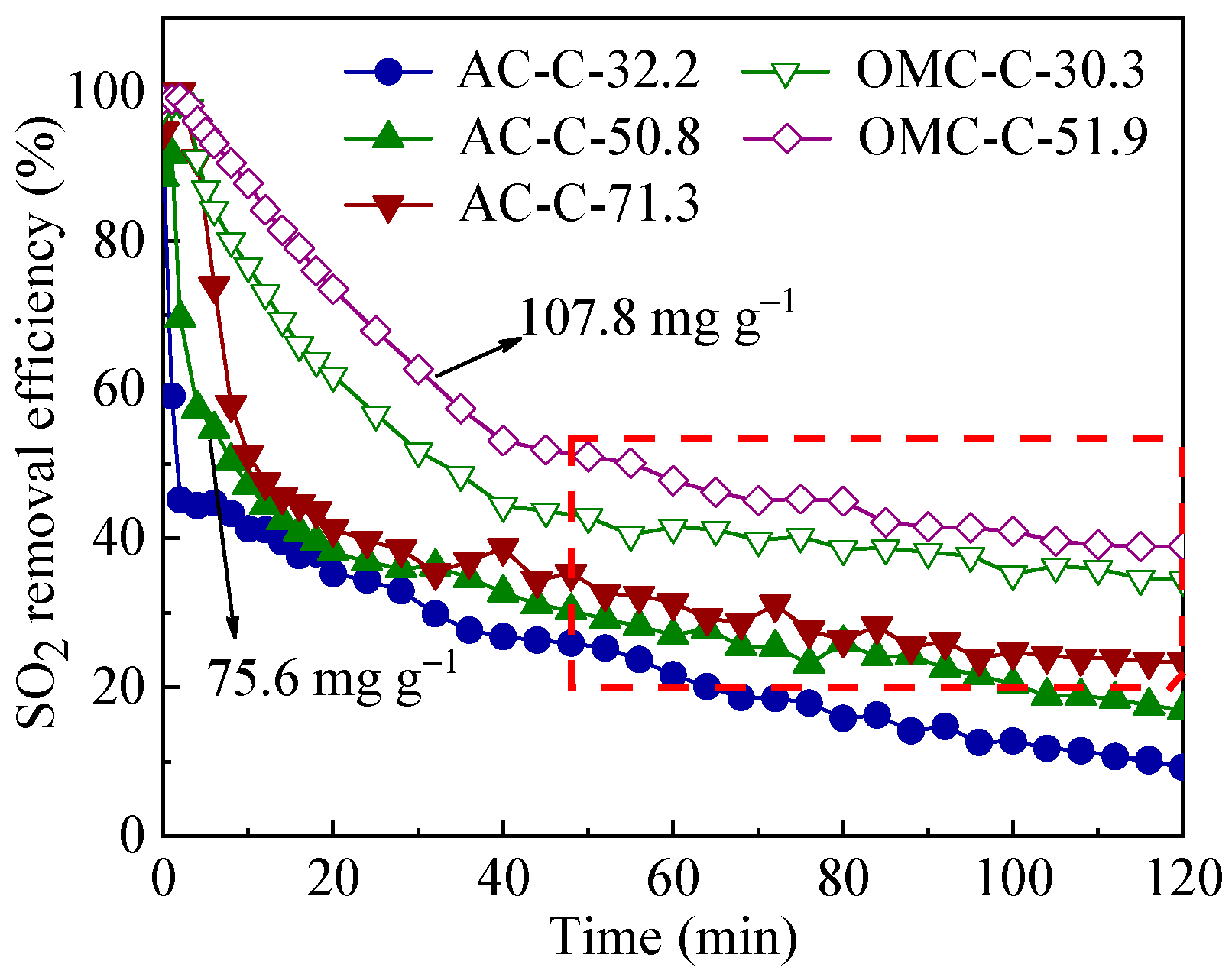
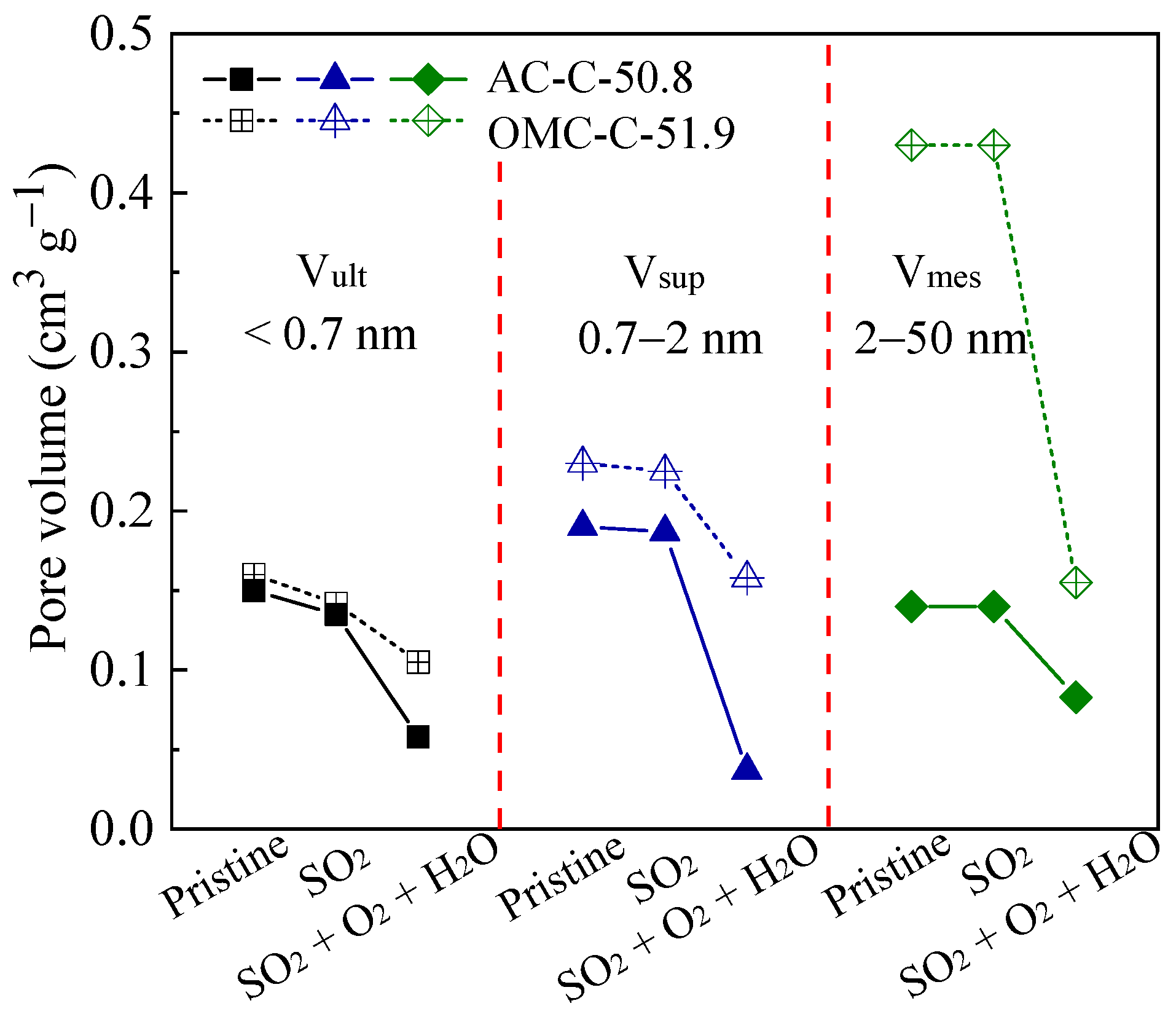
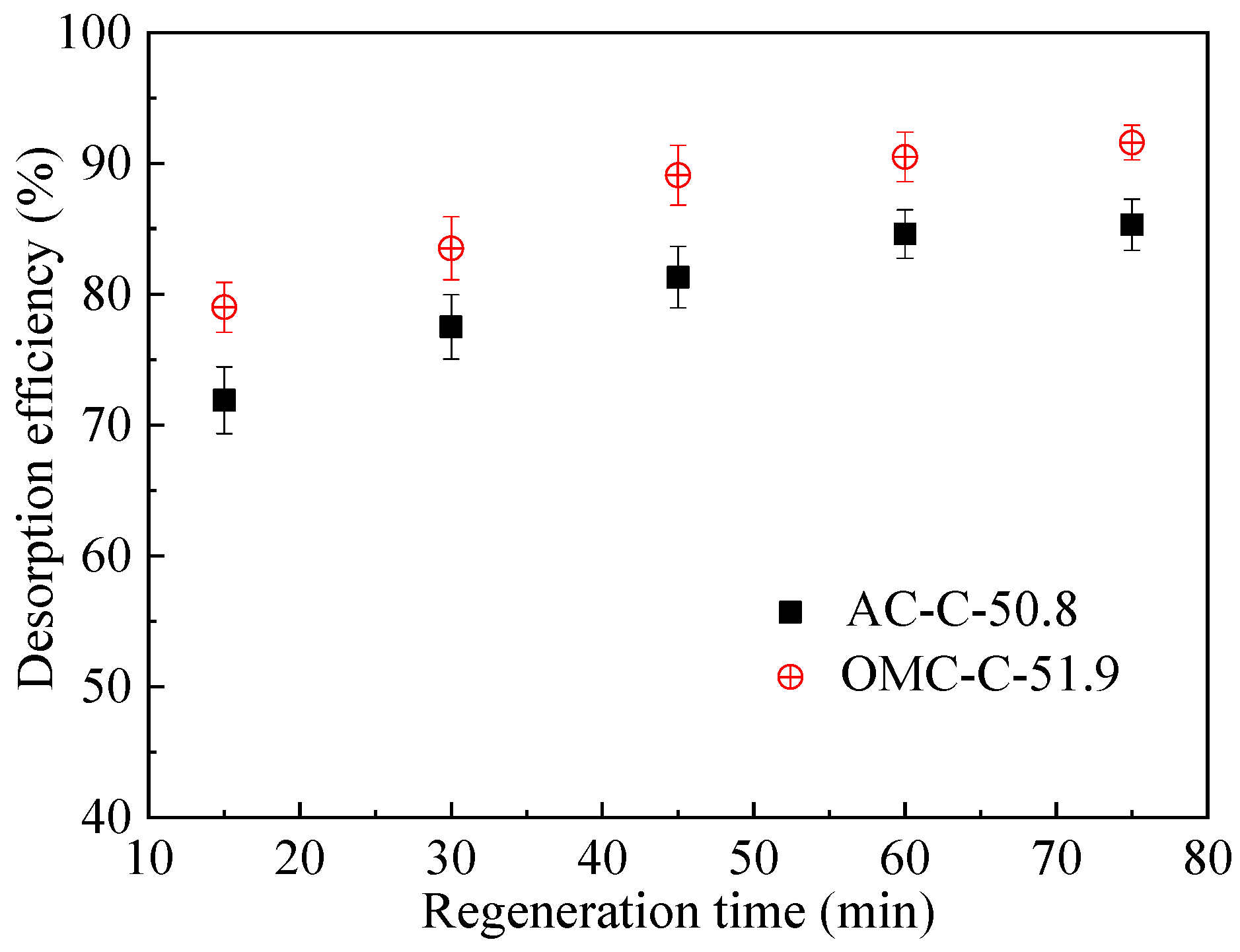
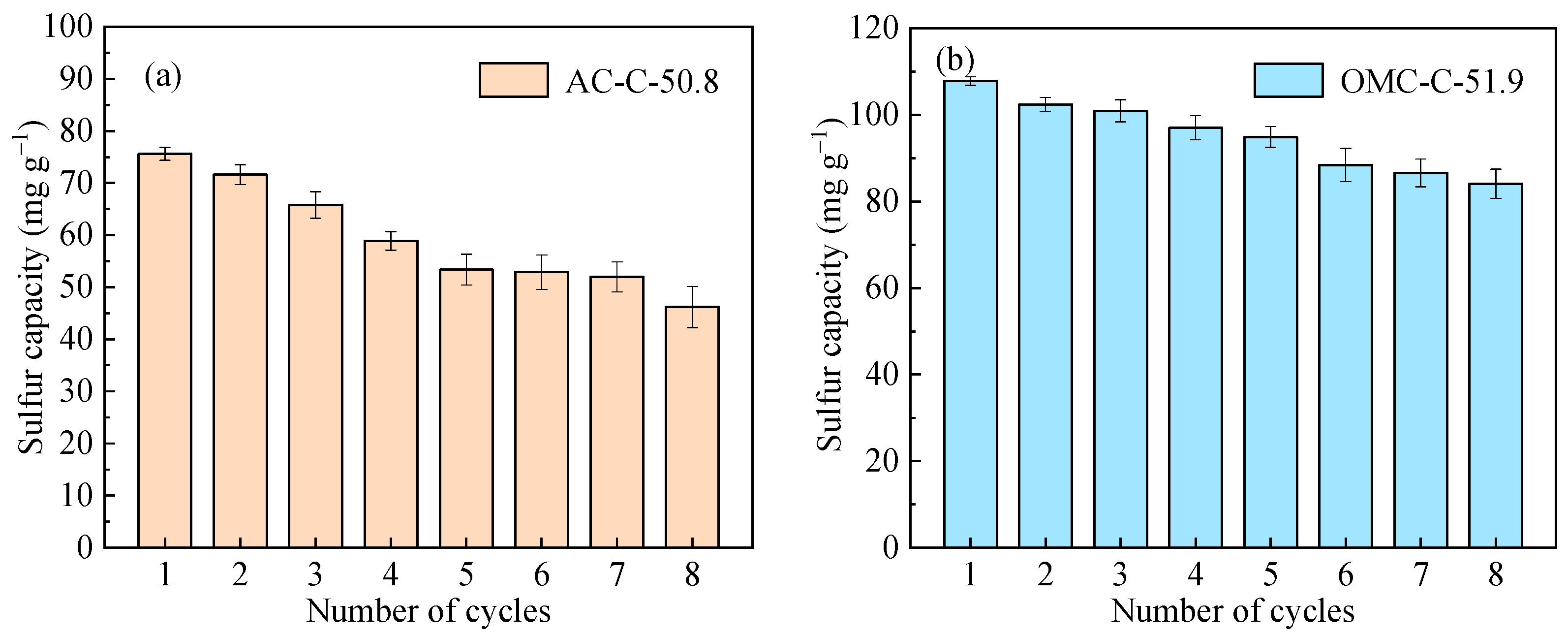
| Sample | SBET 1 (m2 g−1) | Vult 2 (cm3 g−1) | Vmic 3 (cm3 g−1) | Vmes 4 (cm3 g−1) | Dmes 5 (nm) | Elemental Analysis (wt.%) | ||
|---|---|---|---|---|---|---|---|---|
| C | O | O/C × 10−2 | ||||||
| AC-C-x | ||||||||
| AC-C-12.8 | 149(138) | 0.05 | 0.07 | 0.01 | 2.75 | 86.6 | 4.78 | 5.52 |
| AC-C-32.2 | 449(373) | 0.11 | 0.20 | 0.02 | 3.08 | 85.6 | 5.08 | 5.93 |
| AC-C-50.8 | 809(537) | 0.15 | 0.34 | 0.14 | 3.12 | 83.5 | 6.01 | 7.19 |
| AC-C-50.8 after 8 cycles 6 | 364(188) | 0.05 | 0.12 | 0.13 | 3.29 | – | – | – |
| AC-C-71.3 | 1379(404) | 0.18 | 0.52 | 0.33 | 3.31 | 80.8 | 5.16 | 6.38 |
| OMC-C-x | ||||||||
| OMC | 475(205) | – | – | 0.21 | 3.17 | 95.1 | 3.4 | 3.58 |
| OMC-C-9.6 | 576(287) | 0.07 | 0.14 | 0.26 | 3.27 | 94.7 | 4.0 | 4.22 |
| OMC-C-30.3 | 824(439) | 0.12 | 0.27 | 0.35 | 3.44 | 93.1 | 5.9 | 6.34 |
| OMC-C-51.9 | 1056(508) | 0.16 | 0.39 | 0.43 | 3.51 | 91.9 | 6.7 | 7.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Miao, Y.; Li, H. Enhancement Effect of Ordered Hierarchical Pore Configuration on SO2 Adsorption and Desorption Process. Processes 2019, 7, 173. https://doi.org/10.3390/pr7030173
Zhu Y, Miao Y, Li H. Enhancement Effect of Ordered Hierarchical Pore Configuration on SO2 Adsorption and Desorption Process. Processes. 2019; 7(3):173. https://doi.org/10.3390/pr7030173
Chicago/Turabian StyleZhu, Yuwen, Yanfang Miao, and Haoyu Li. 2019. "Enhancement Effect of Ordered Hierarchical Pore Configuration on SO2 Adsorption and Desorption Process" Processes 7, no. 3: 173. https://doi.org/10.3390/pr7030173
APA StyleZhu, Y., Miao, Y., & Li, H. (2019). Enhancement Effect of Ordered Hierarchical Pore Configuration on SO2 Adsorption and Desorption Process. Processes, 7(3), 173. https://doi.org/10.3390/pr7030173





