Model for the Patterns of Salt-Spray-Induced Chloride Corrosion in Concretes under Coupling Action of Cyclic Loading and Salt Spray Corrosion
Abstract
:1. Introduction
2. Experimental Research on Chloride Transport in Concrete
2.1. Materials
2.2. Test Methods
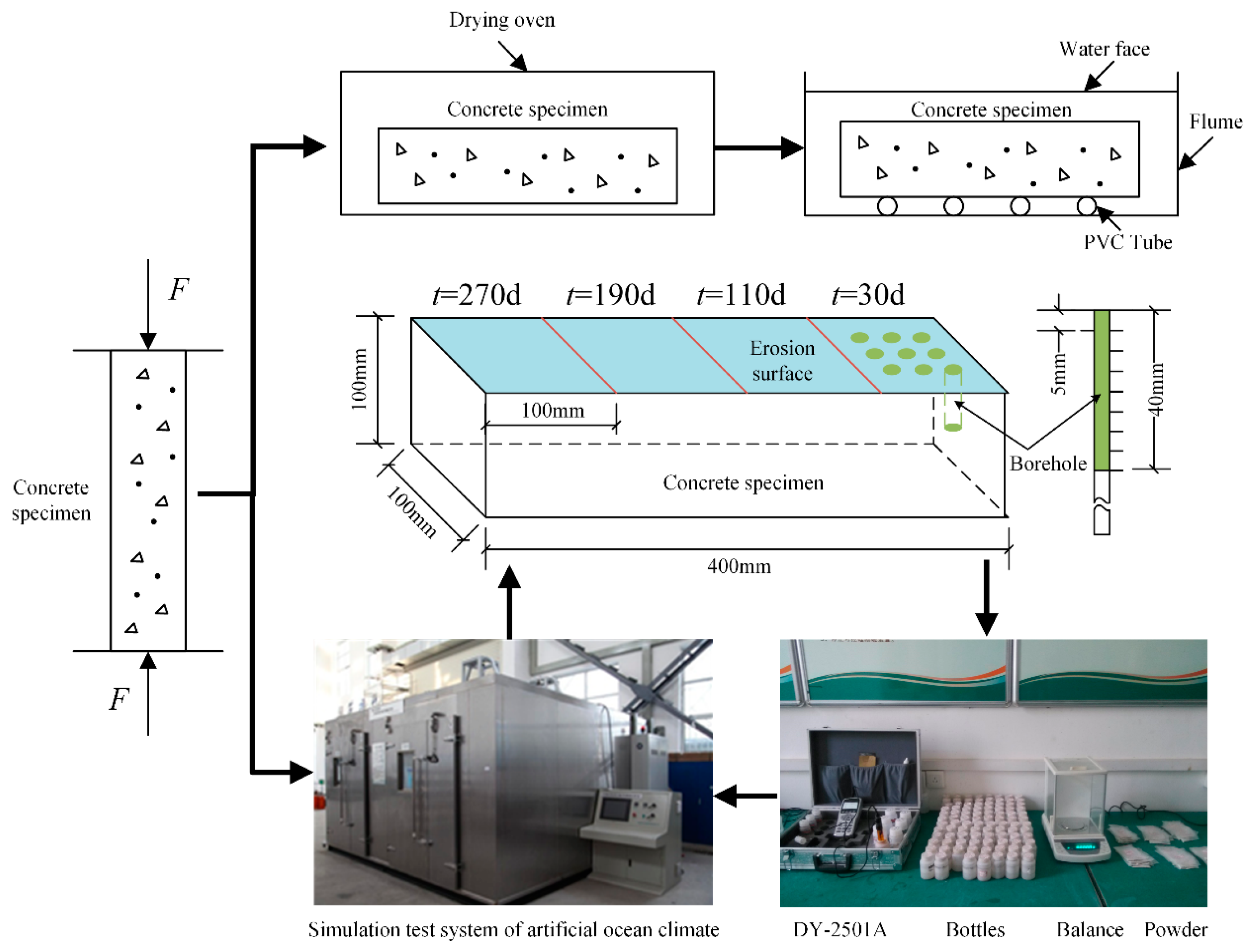
- Uniaxial compression tests were performed on the concrete samples that had been cured for 28 days to measure their strength ().
- Three cyclic loading protocols (i.e., 50%, 65%, and 80% ) were used to obtain concrete samples with three different porosities and were numbered as #1, #2, and #3, respectively. The detailed experimental steps of applying the cyclic loads are as follows: firstly, each specimen was loaded at the loading rate of 5 kN/s using force control, until the load reached the specified load setting value (here, the set value was 50%, 65%, or 80% of the compressive strength limit (c)), and then the load was maintained for 120 s. After that, each specimen was unloaded at the unloading rate of 5 kN/s, until the load value was 0 kN, and then the completely unloaded state was maintained for 120 s. This procedure was repeated for 5 cycles in total, according to the above loading and unloading operations. Finally, the sample was completely unloaded (load was 0 kN).
- After cyclic loading, the samples with different porosities were placed in a drying oven and dried at 40 °C for 48 h. Subsequently, the samples were weighed, and the initial weights were recorded. Subsequently, the samples were soaked in water for 48 h. The weights of the samples at saturation were measured, and their porosities were calculated (Table 3).
- One of the 100 × 400 mm surfaces of the samples was used as the corrosion surface, whereas the other sides were sealed using epoxy resins. Subsequently, the concrete samples were placed in an artificial marine climate simulation laboratory for salt spray corrosion tests. The relative humidity was 75–80%, and the duration of the salt spray was 12 h, after which the samples were dried for another 12 h—this cycle was continuously repeated for the duration of the test. The concentration of the NaCl solution used in this test was 5%.
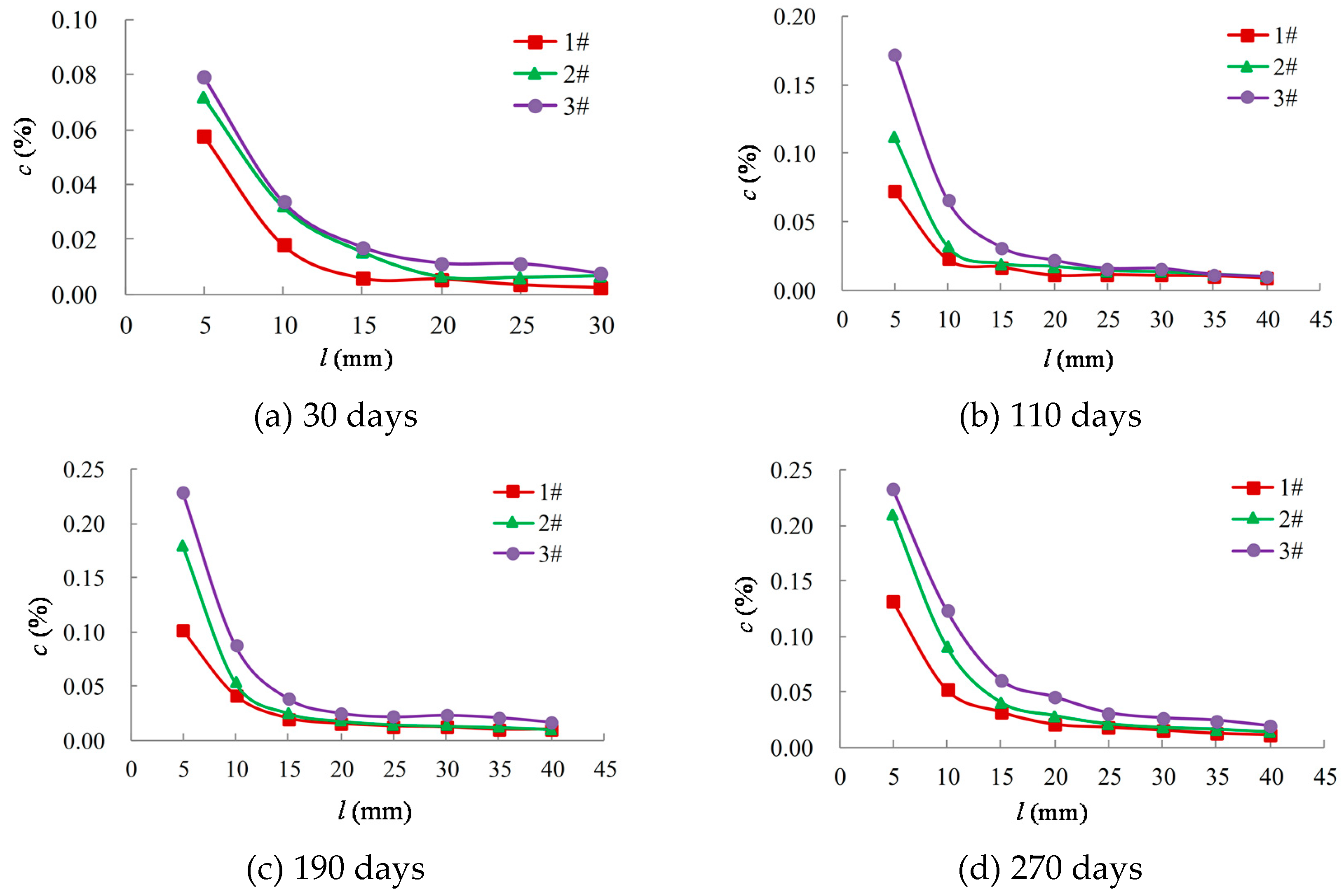
- When the corrosion time reached 30, 110, 190, and 270 days, concrete powders were obtained from the samples at depths of 5, 10, 15, 20, 25, 30, 35, and 40 mm, which were sealed in plastic bags. Subsequently, 4 g of the concrete powder was mixed with 40 g of distilled water in a reagent bottle. The bottle was shaken for 30 min in a solution shaker before being allowed to settle for 24 h.
- The concentration of the chloride ions was measured using a DY-2501A Chloride Meter.
- After testing, the holes in the concrete samples were filled with silicon sealant, and the samples were returned to the laboratory for further corrosion until the subsequent time point, and subsequently, step (6) was repeated. This was continued until the end of the experiment.
2.3. Results and Discussion
3. Model for Coupled Water–Gas–Chloride Transport
3.1. Basic Assumption
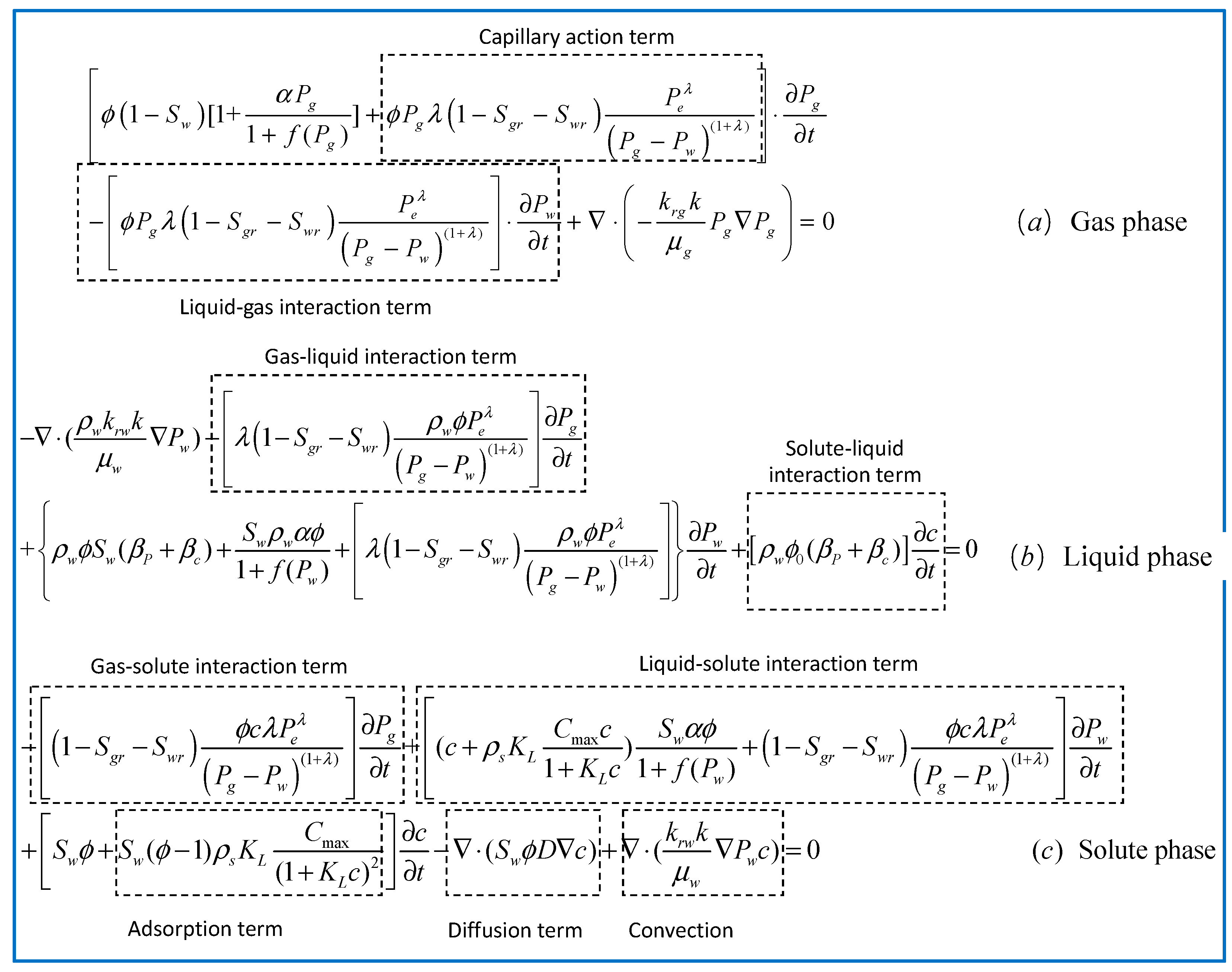
3.2. Gas Flow Model
3.3. Flow Model for the Liquid Phase
3.4. Convection–Diffusion Models for Chlorides
3.5. Model for Coupled Water–Gas–Chloride Transport
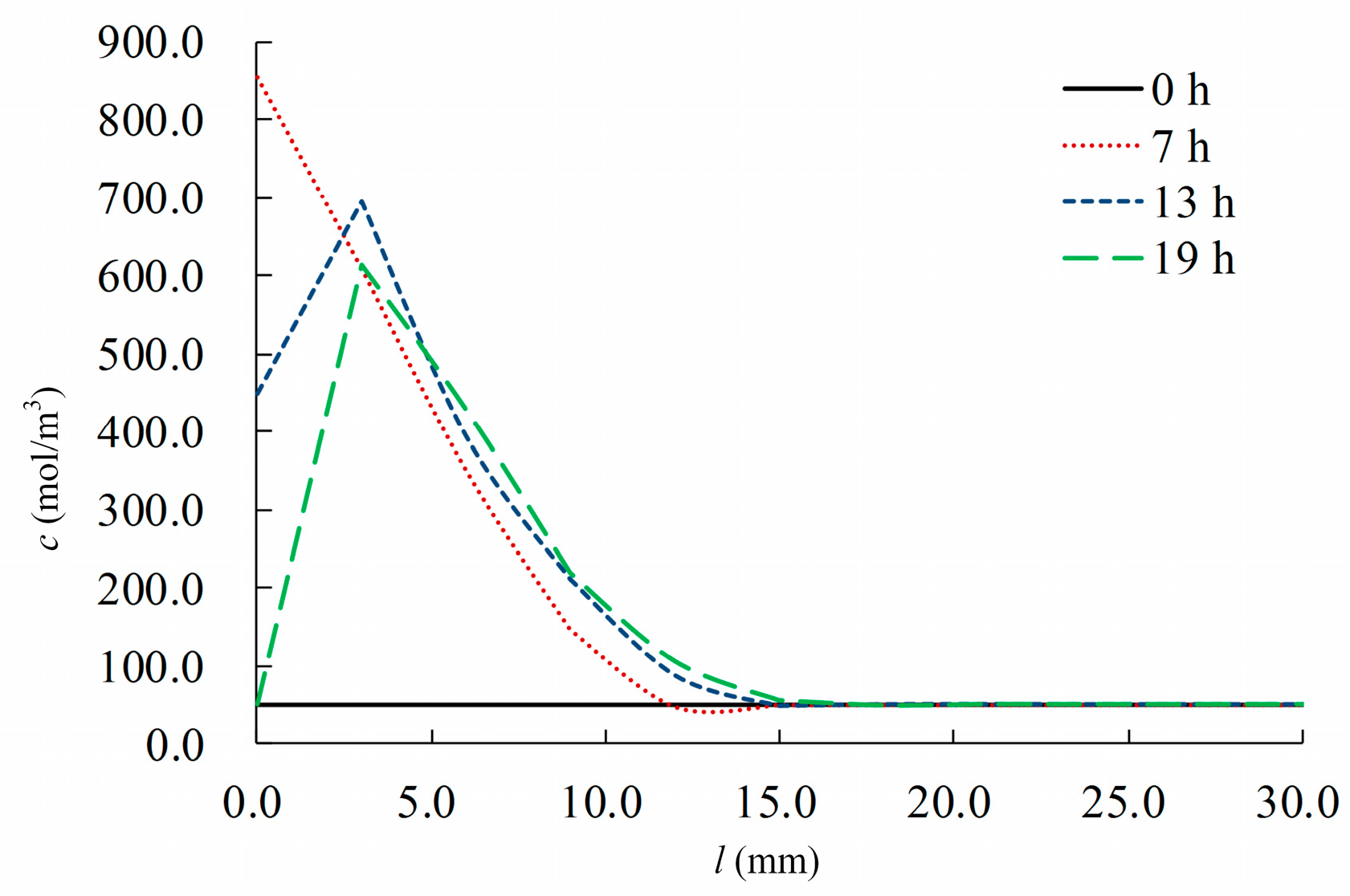
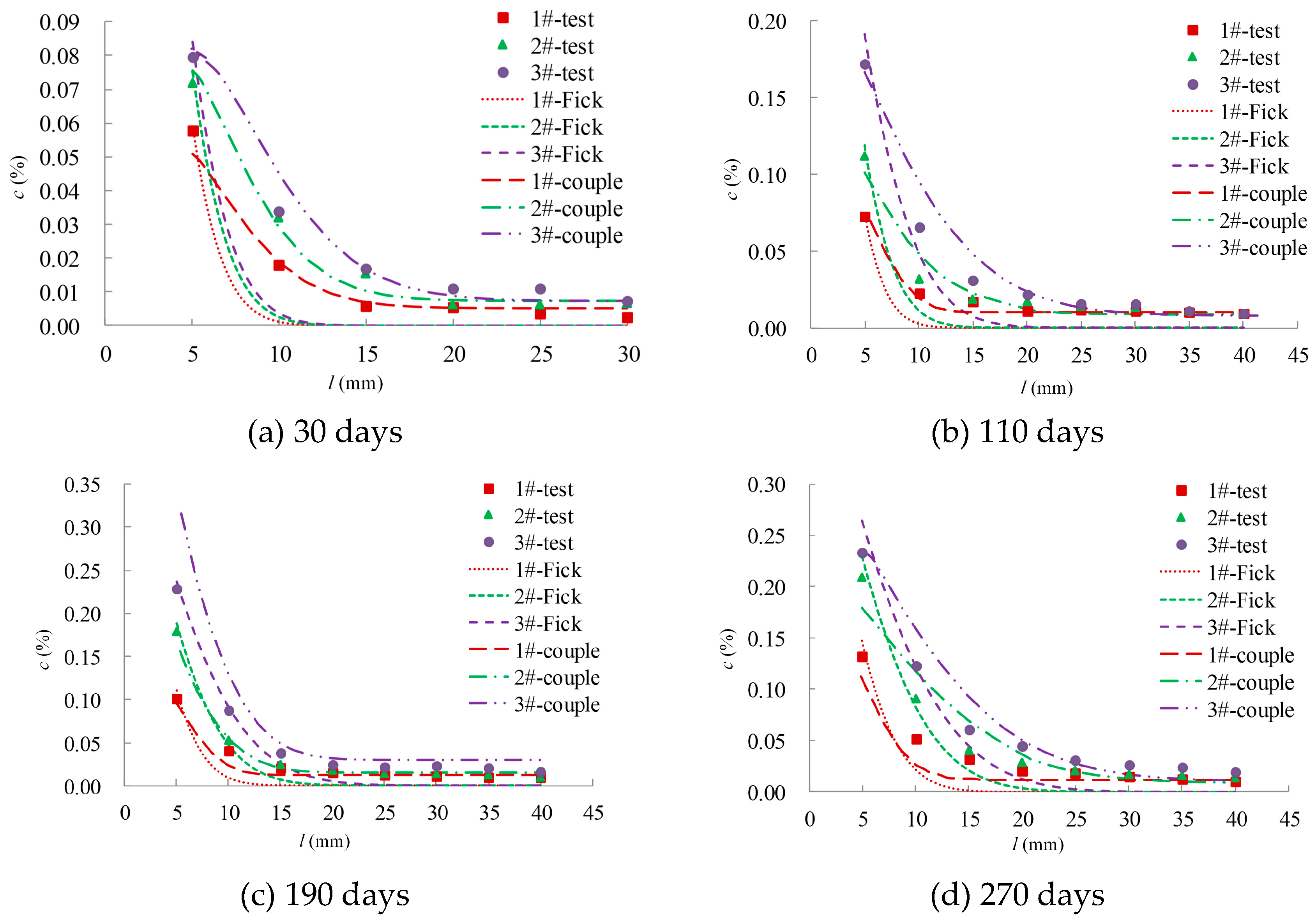
4. Model Calculations and Validation
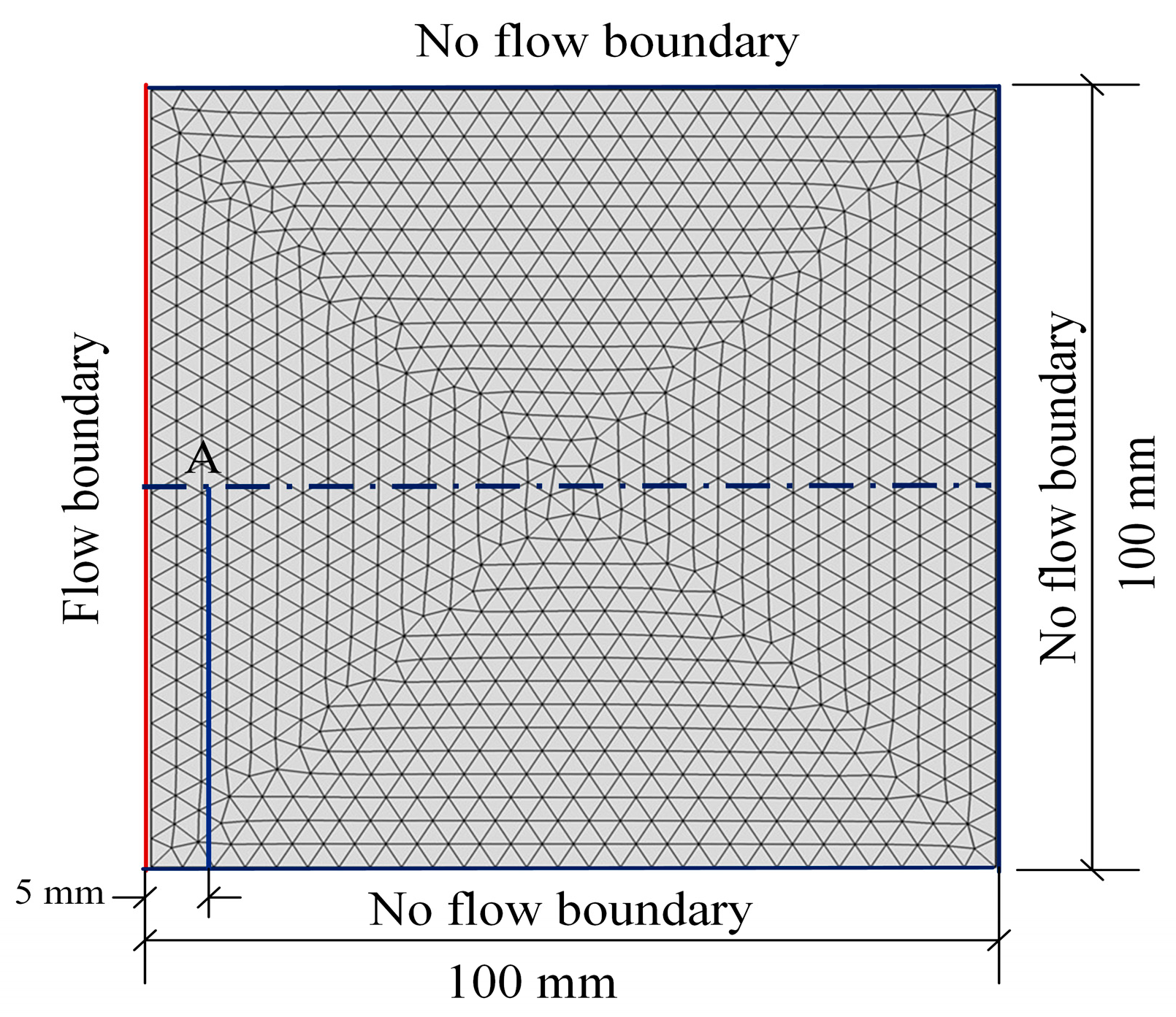
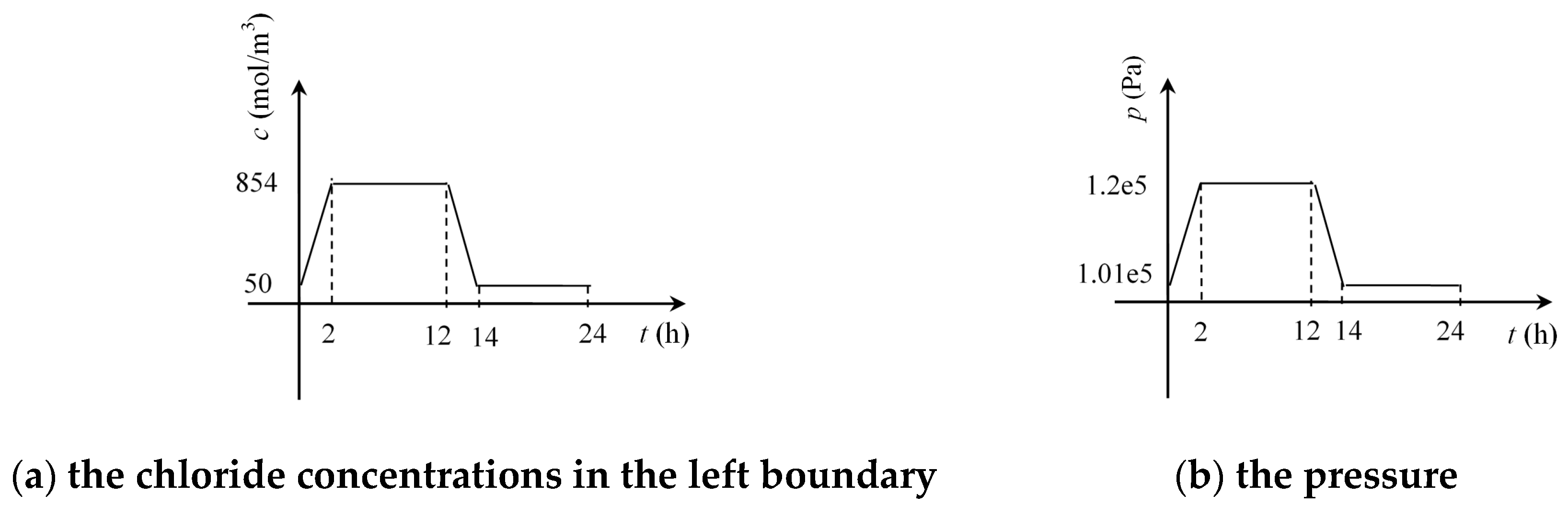
4.1. Calculation Scheme
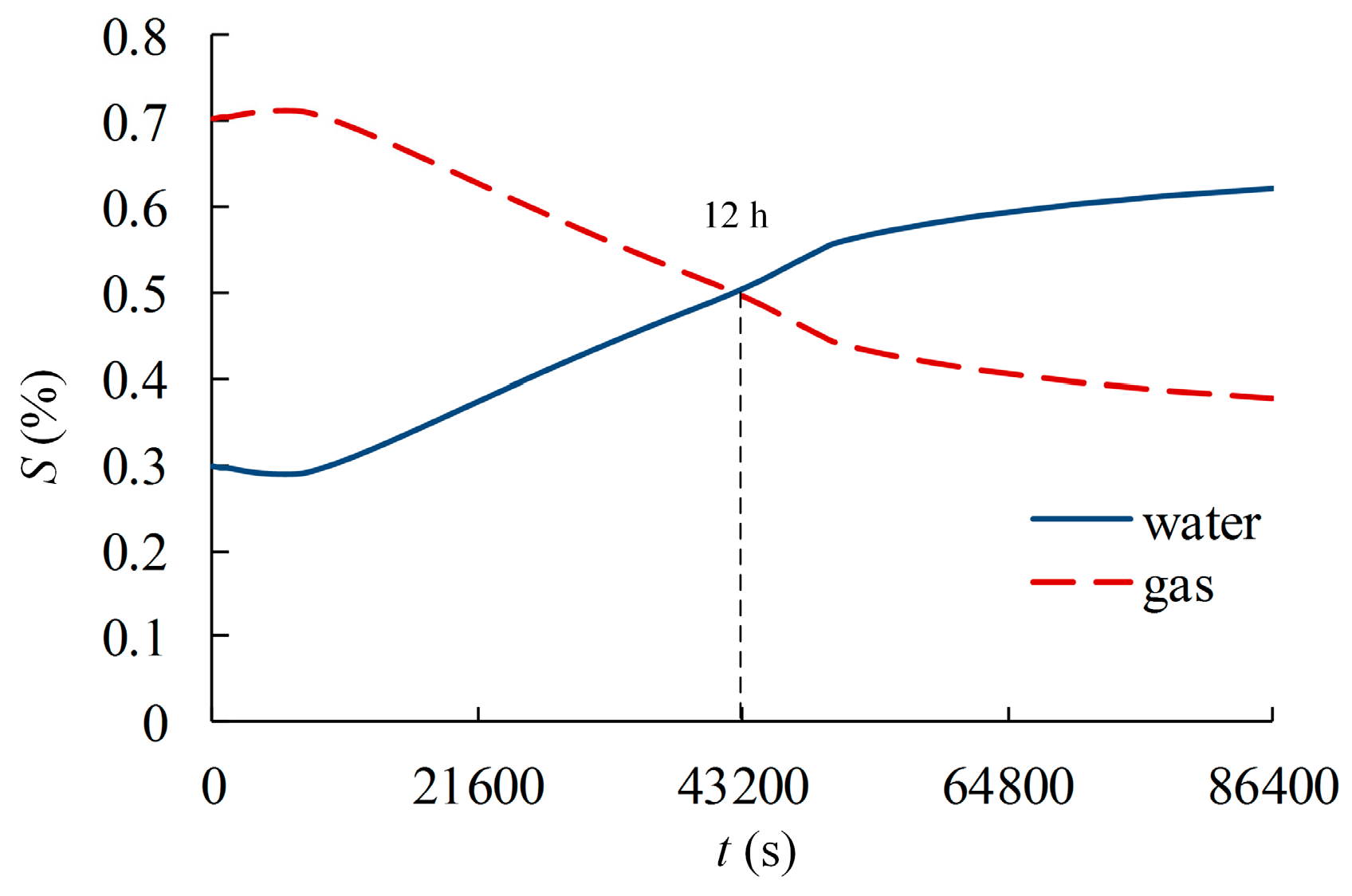
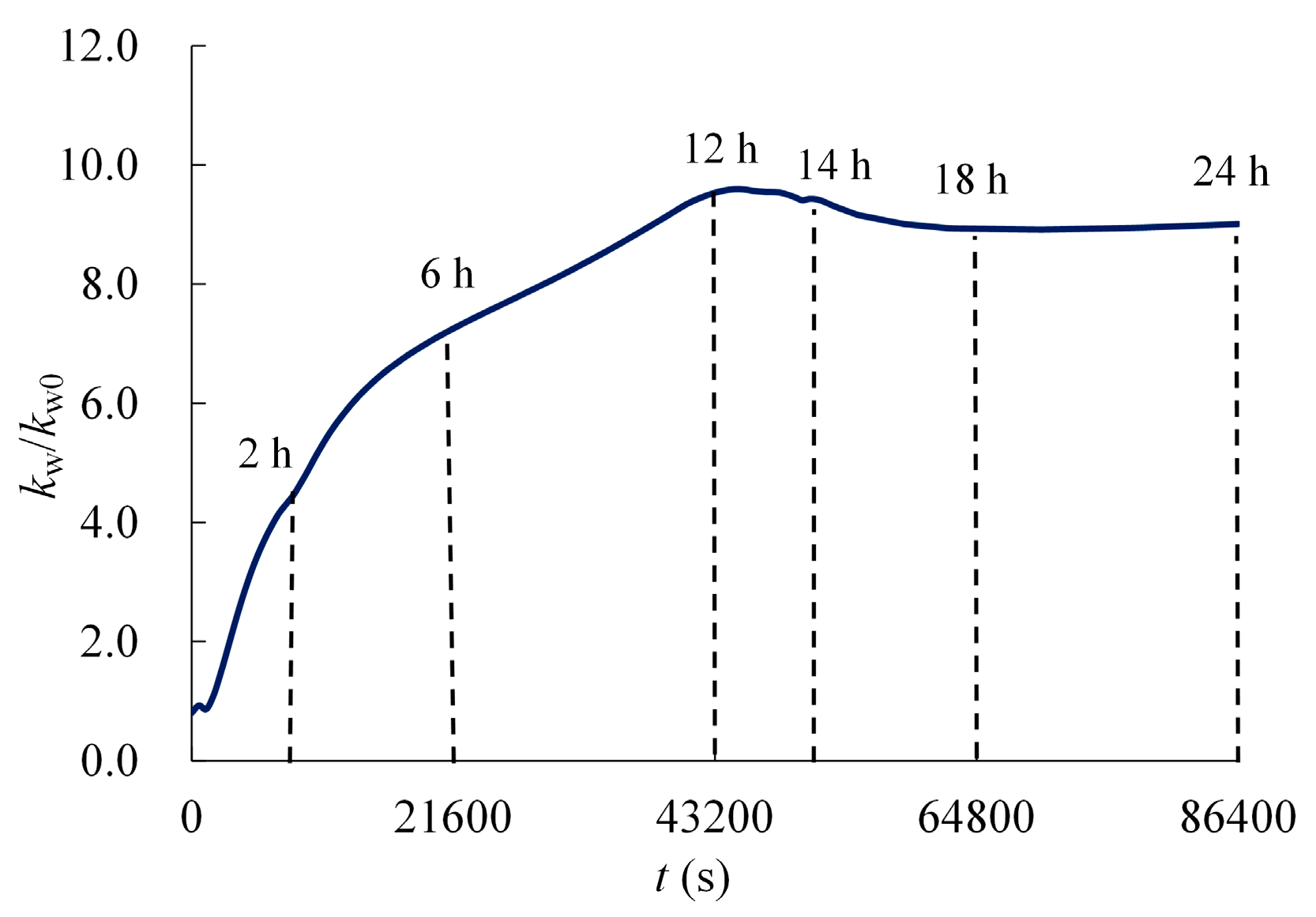
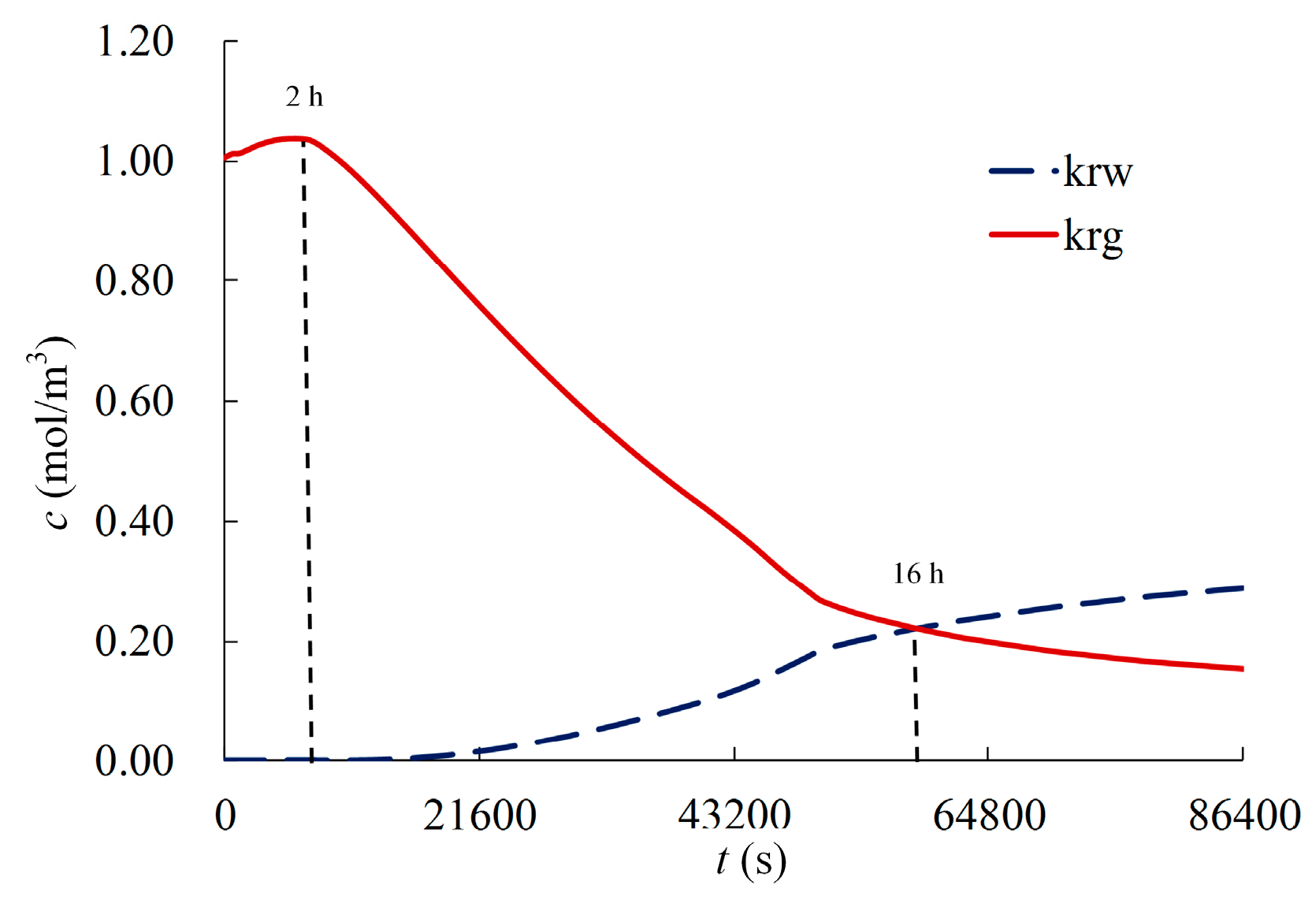
4.2. Results and Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lv, J.F.; Tab, Z.J.; Ba, H.J. Micro-structure and performance analysis of marine concrete after extended exposure to marine environment. J. China Univ. Min. Technol. 2010, 39, 530–531. [Google Scholar]
- Rita, B.F. Electrochemical sensors for monitoring the corrosion conditions of reinforced concrete structures: A review. Appl. Sci. 2017, 7, 1157. [Google Scholar]
- Geng, J.; Easterbrook, D.; Liu, Q.F.; Li, L.Y. Effect of carbonation on release of bound chlorides in chloride contaminated concrete. Mag. Concr. Res. 2015, 68, 353–363. [Google Scholar] [CrossRef]
- Yu, B.; Ning, C.; Li, B. Probabilistic durability assessment of concrete structures in marine environments: Reliability and sensitivity analysis. China Ocean Eng. 2017, 31, 63–73. [Google Scholar] [CrossRef]
- Dai, H.J.; Wang, F.C.; Wu, Y.H.; Nan, F. Durability of concrete used for oil-platform in tidal zone. J. Building Mater. 2000, 3, 135–141. [Google Scholar]
- Li, B.; Yin, H.G.; Mao, X.B.; Li, Y.; Zhang, L.Y.; Liu, R.X.; Qiu, P.T. Macroscopic and microscopic fracture features of concrete used in coal mine under chlorine salt erosion. Int. J. Min. Sci. Technol. 2016, 26, 455–459. [Google Scholar] [CrossRef]
- Jin, W.L.; Zhang, Y.; Lu, Z.Y. Mechanism and mathematic modeling of chloride permeation in concrete under unsaturated state. J. Chin. Ceram. Soc. 2008, 36, 1362–1369. [Google Scholar]
- Jin, W.L.; Jin, L.B.; Yan, Y.D.; Yao, C.J. Field inspection on chloride ion-intrusion effect of seawater in dry-wet cycling zone of concrete structures. J. Hydraul. Eng. 2009, 40, 364–365. [Google Scholar]
- Zhang, Q.Z.; Gu, X.L.; Zhang, W.P.; Huang, Q.H. Model on capillary pressure saturation relationship for concrete. J. Tong Ji Univ. Nat. Sci. 2012, 40, 1753–1755. [Google Scholar]
- Pan, Z.C.; Chen, A.R. Numerical simulation of chloride transportation process in unsaturated concrete. J. Tong Ji Univ. Nat. Sci. 2011, 39, 314–315. [Google Scholar]
- Yan, Y.D.; Jin, W.L.; Wang, H.L.; Lu, C.H. Chloride transport in cracked concrete under wet-dry cycling condition. J. Tong Ji Univ. Nat. Sci. 2013, 44, 2060–2062. [Google Scholar]
- Li, C.Q.; Li, K.F. Chloride ion transport in cover concrete under drying-wetting cycles: Theory, experiment and modeling. J. Chin. Ceram. Soc. 2010, 38, 581–582. [Google Scholar]
- Li, C.Q.; Li, K.F. Moisture transport in cover concrete under drying-wetting cycles: Theory, experiment and modeling. J. Chin. Ceram. Soc. 2010, 38, 1151–1153. [Google Scholar]
- Polder, B.P.; Peelen, W.H.A. Characterisation of chloride transport and reinforcement corrosion in concrete under cyclic wetting and drying by electrical resistivity. Cem. Concr. Compos. 2002, 24, 427–435. [Google Scholar] [CrossRef]
- Thiery, M. Modelisation du sechage des betons: Analyse des differents modes de transfert hydrique. Revue Européenne de Génie Civil 2007, 11, 541–578. [Google Scholar] [CrossRef]
- Ma, D.; Cai, X.; Li, Q.; Duan, H.Y. In-situ and numerical investigation of groundwater inrush hazard from grouted karst collapse pillar in longwall mining. Water 2018, 10, 1187. [Google Scholar] [CrossRef]
- Li, K.F.; Li, C.Q.; Chen, Z.Y. Influential depth of moisture transport in concrete subject to drying-wetting cycles. Cem. Concr. Compos. 2009, 31, 693–698. [Google Scholar] [CrossRef]
- Ma, D.; Duan, H.Y.; Liu, J.F.; Li, X.B.; Zhou, Z.L. The role of gangue on the mitigation of mining-induced hazards and environmental pollution: An experimental investigation. Sci. Total Environ. 2019. [Google Scholar] [CrossRef]
- Zanden, A.; Taher, A.; Arends, T. Modelling of water and chloride transport in concrete during yearly wetting/drying cycles. Constr. Build. Mater. 2015, 81, 120–123. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Chen, C.; Gao, X.J.; Jin, W.L. Seasonal variation of surface chloride ion content and chloride diffusion coefficient in a concrete doc. Adv. Struct. Eng. 2013, 16, 395–403. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Cai, X.; Ma, D.; Du, X.; Chen, L.; Wang, H.; Zang, H. Water saturation effects on dynamic fracture behavior of sandstone. Int. J. Rock Mech. Min. Sci. 2019, 114, 46–61. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, J.Z. Mesoscale simulation of chloride diffusion in concrete subjected to flexural loading. Adv. Struct. Eng. 2014, 17, 561–571. [Google Scholar] [CrossRef]
- Bourbatache, K.; Millet, O.; Aït-Mokhtar, A. Ionic transfer in charged porous media. Periodic homogenization and parametric study on 2D microstructures. Int. J. Heat Mass Transf. 2012, 55, 5979–5991. [Google Scholar] [CrossRef]
- Ma, D.; Duan, H.Y.; Cai, X.; Li, Z.; Li, Q.; Zhang, Q. A global optimization-based method for the prediction of water inrush hazard from mining floor. Water 2018, 10, 1618. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.S.; Elsworth, D. Dual Poroelastic Response of coal Seam to CO2 Injection. Int. J. Greenh. Gas Control 2010, 4, 668–678. [Google Scholar] [CrossRef]
- Ma, D.; Rezania, M.; Yu, H.-S.; Bai, H.-B. Variations of hydraulic properties of granular sandstones during water inrush: Effect of small particle migration. Eng. Geol. 2017, 217, 61–70. [Google Scholar] [CrossRef]
- Chilingar, G.V. Relationship between porosity, permeability, and grain size distribution of sands and sandstones. Dev. Sedimentol. 1964, 1, 71–74. [Google Scholar]
- Zhou, Z.; Cai, X.; Ma, D.; Chen, L.; Wang, S.; Tan, L. Dynamic tensile properties of sandstone subjected to wetting and drying cycles. Constr. Build. Mater. 2018, 182, 215–232. [Google Scholar] [CrossRef]
- Ma, D.; Cai, X.; Zhou, Z.L.; Li, X.B. Experimental investigation on hydraulic properties of granular sandstone and mudstone mixtures. Geofluids 2018, 2018, 9216578. [Google Scholar] [CrossRef]
- Ge, Y.F. Fine Numerical Simulation and Experimental Study of Chloride Corrosion of Self-Compacting Concrete; School of Civil Engineering Southeast University: Nanjing, China, 2017; pp. 3–4. [Google Scholar]
| Water–Cement Ratio | Admixture | Content (%) | Cement (kg/m3) | Fly Ash (kg/m3) | Mineral Powder (kg/m3) | Sand (kg/m3) | Stone (kg/m3) | Water (kg/m3) |
|---|---|---|---|---|---|---|---|---|
| 0.32 | F + G | 15 + 35 | 226 | 68 | 158 | 740 | 1112 | 145 |
| Sample NO. | 1# | 2# | 3# |
|---|---|---|---|
| NO./piece | 3 | 3 | 3 |
| Sample NO. | Porosity ϕ (%) | |||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Mean | |
| 1 | 3.92 | 3.22 | 3.30 | 3.49 |
| 2 | 3.90 | 4.21 | 3.38 | 3.83 |
| 3 | 4.73 | 4.13 | 5.26 | 4.71 |
| Sample NO. | Chloride Diffusion Coefficient (D) (×10−12 m2/s) | ||||
|---|---|---|---|---|---|
| t = 30 d | t = 110 d | t = 190 d | t = 270 d | Mean | |
| 1 | 5.243 | 1.592 | 1.126 | 0.979 | 2.235 |
| 2 | 5.804 | 2.089 | 2.046 | 1.957 | 2.974 |
| 3 | 6.123 | 3.314 | 3.066 | 2.667 | 3.793 |
| Scheme | Parameter | |
|---|---|---|
| ϕ0 % | D0 (×10−12 m2/s) | |
| Scheme 1 | 3.49 | 2.235 |
| Scheme 2 | 3.83 | 2.974 |
| Scheme 3 | 4.71 | 3.793 |
| Parameter | Value |
|---|---|
| Initial porosity ϕ0/% | ϕ0 |
| Pore deformation coefficient α/(1/Pa) | 1e-6 |
| Pressure compressibility βp /(1/Pa) | 5e-8 |
| Concentration compressibility βc/ (m3/mol) | 1e-5 |
| Water permeability kw/(m2) | 1e-15 |
| Langmuir adsorption constant KL/(m3/mol) | 0.001 |
| Langmuir maximum adsorbing capacity Cmax/(mol/m3) | 100 |
| Initial concentration C0/(mol/L) | 50 |
| Solution density ρw/(kg/m3) | 1025 |
| Gas density ρg/(kg/m3) | 1.23 |
| Concrete density ρc/(kg/m3) | 2450 |
| Chloride diffusion coefficient D0/(m2/s) | D0 |
| Liquid dynamic viscosity uw/(Pa·s) | 0.00101 |
| Gas dynamic viscosity ug/(Pa·s) | 0.00085 |
| Liquid initial pressure P0/(Pa) | 1.01e5 |
| Liquid initial saturation Swr/% | 30 |
| Gas initial saturation Sgr/% | 70 |
| Concrete permeability K0/(m2) | 1e-14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Yin, H.; Zhang, L.; Li, B.; Mao, X. Model for the Patterns of Salt-Spray-Induced Chloride Corrosion in Concretes under Coupling Action of Cyclic Loading and Salt Spray Corrosion. Processes 2019, 7, 84. https://doi.org/10.3390/pr7020084
Liu R, Yin H, Zhang L, Li B, Mao X. Model for the Patterns of Salt-Spray-Induced Chloride Corrosion in Concretes under Coupling Action of Cyclic Loading and Salt Spray Corrosion. Processes. 2019; 7(2):84. https://doi.org/10.3390/pr7020084
Chicago/Turabian StyleLiu, Ruixue, Huiguang Yin, Lianying Zhang, Bing Li, and Xianbiao Mao. 2019. "Model for the Patterns of Salt-Spray-Induced Chloride Corrosion in Concretes under Coupling Action of Cyclic Loading and Salt Spray Corrosion" Processes 7, no. 2: 84. https://doi.org/10.3390/pr7020084
APA StyleLiu, R., Yin, H., Zhang, L., Li, B., & Mao, X. (2019). Model for the Patterns of Salt-Spray-Induced Chloride Corrosion in Concretes under Coupling Action of Cyclic Loading and Salt Spray Corrosion. Processes, 7(2), 84. https://doi.org/10.3390/pr7020084





