Filtration Performances of Different Polysaccharides in Microfiltration Process
Abstract
:1. Introduction
2. Materials and Methods
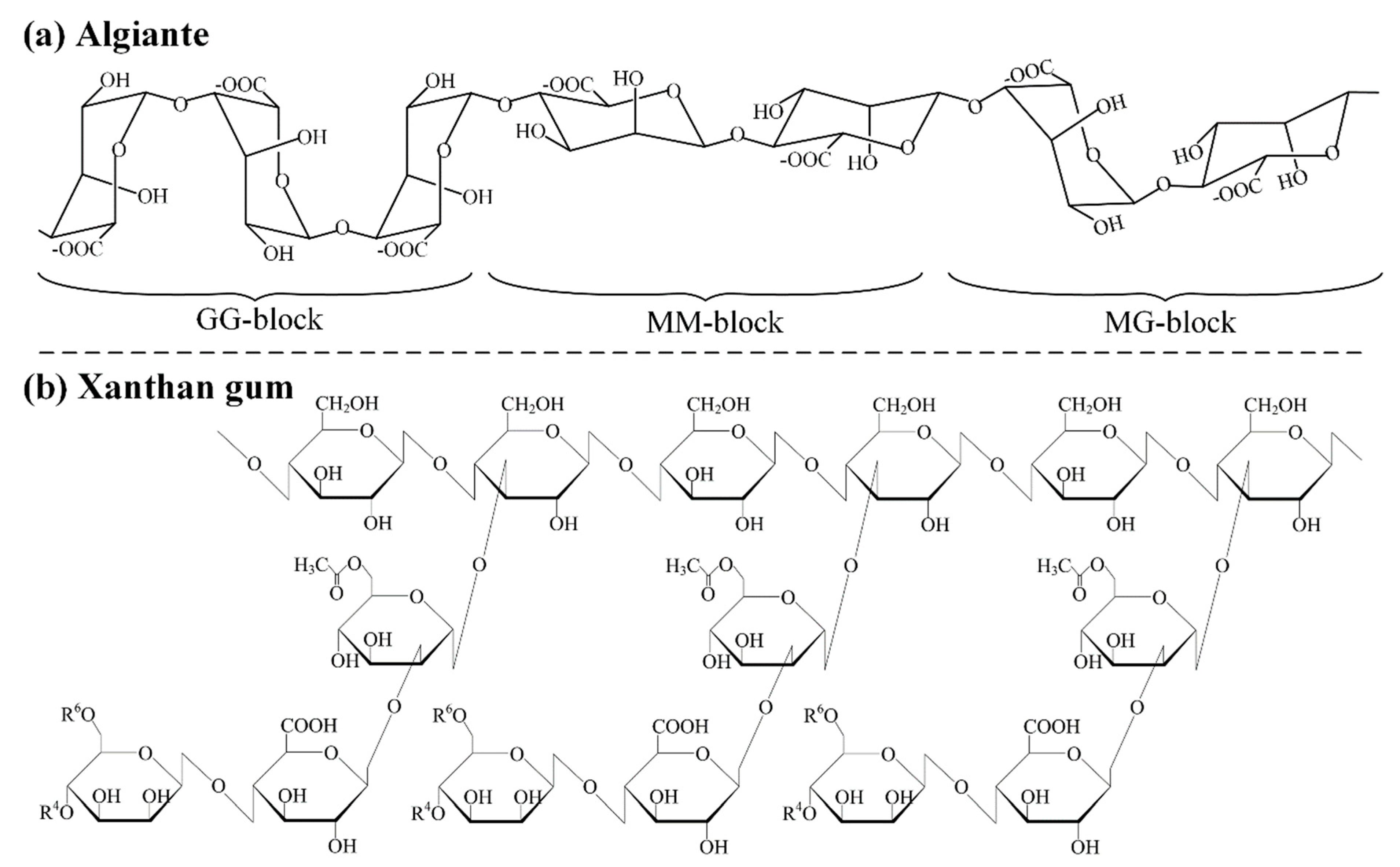
2.1. Alginate and Xanthan Gum
2.2. Dead End Filtration Setup
2.3. Preparation of Feed Solutions
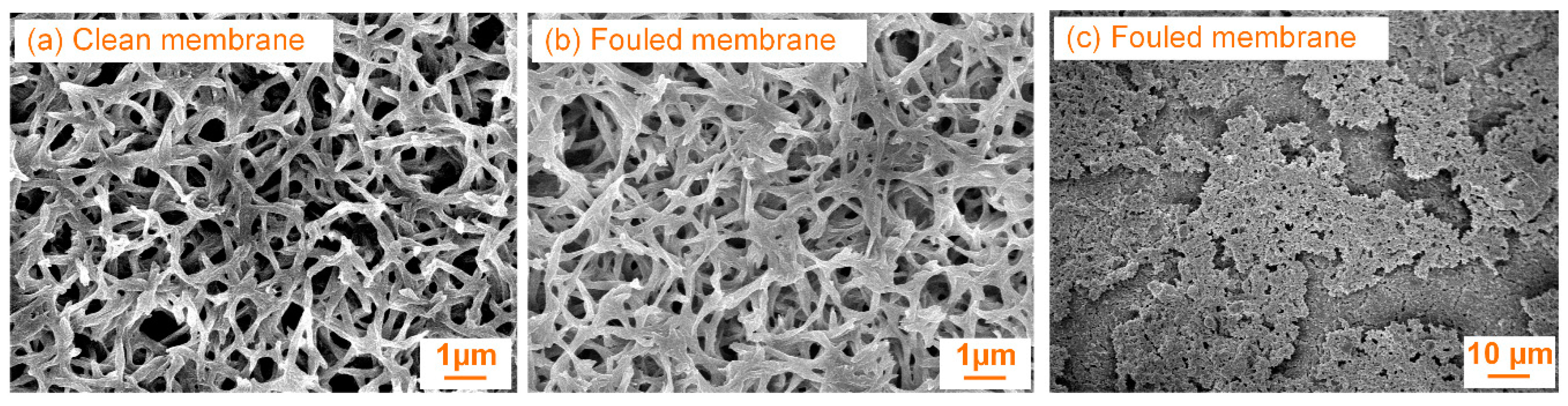
2.4. Field Emission Scanning Electron Microscopy Observation of Fouled Membrane
3. Results and Discussion
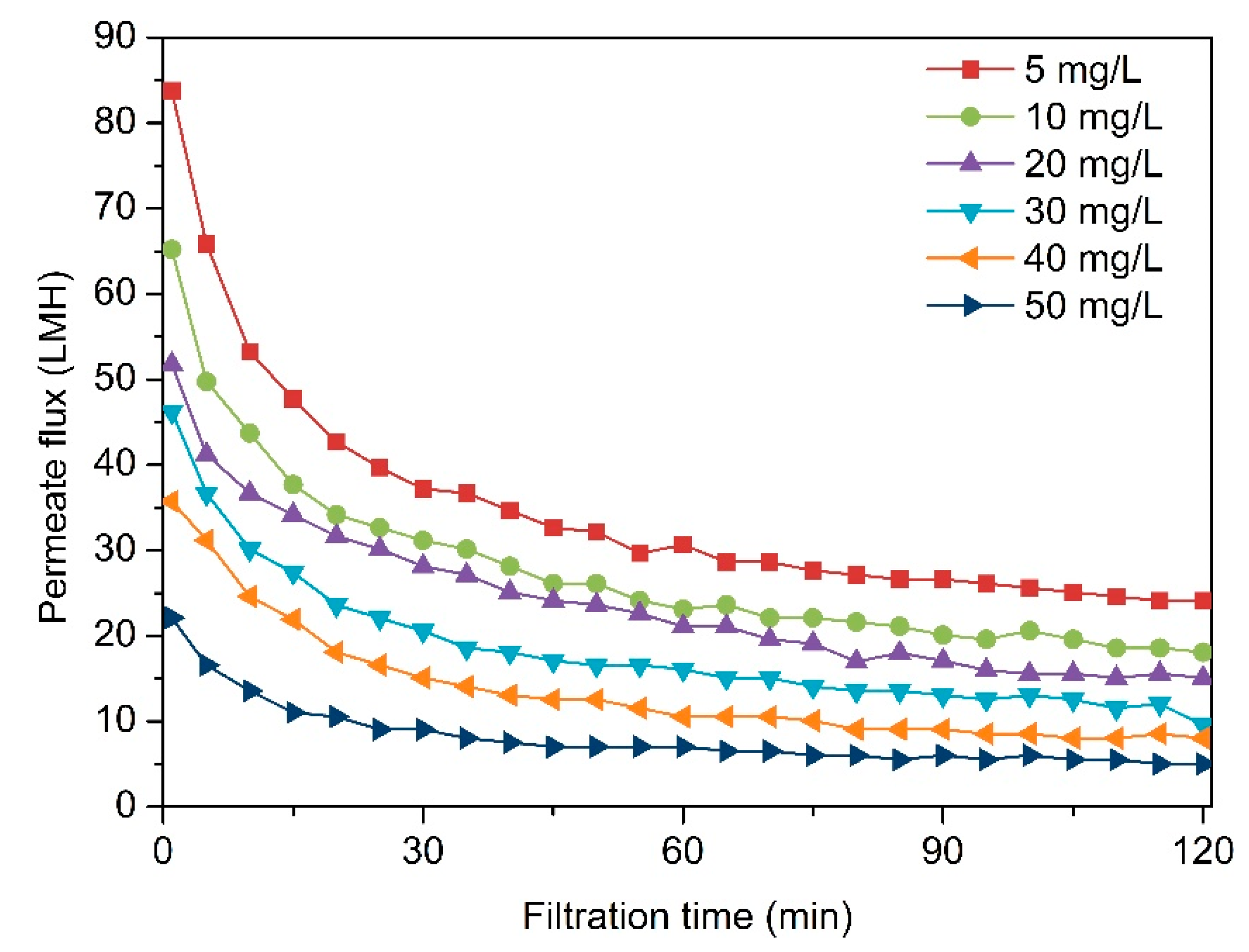
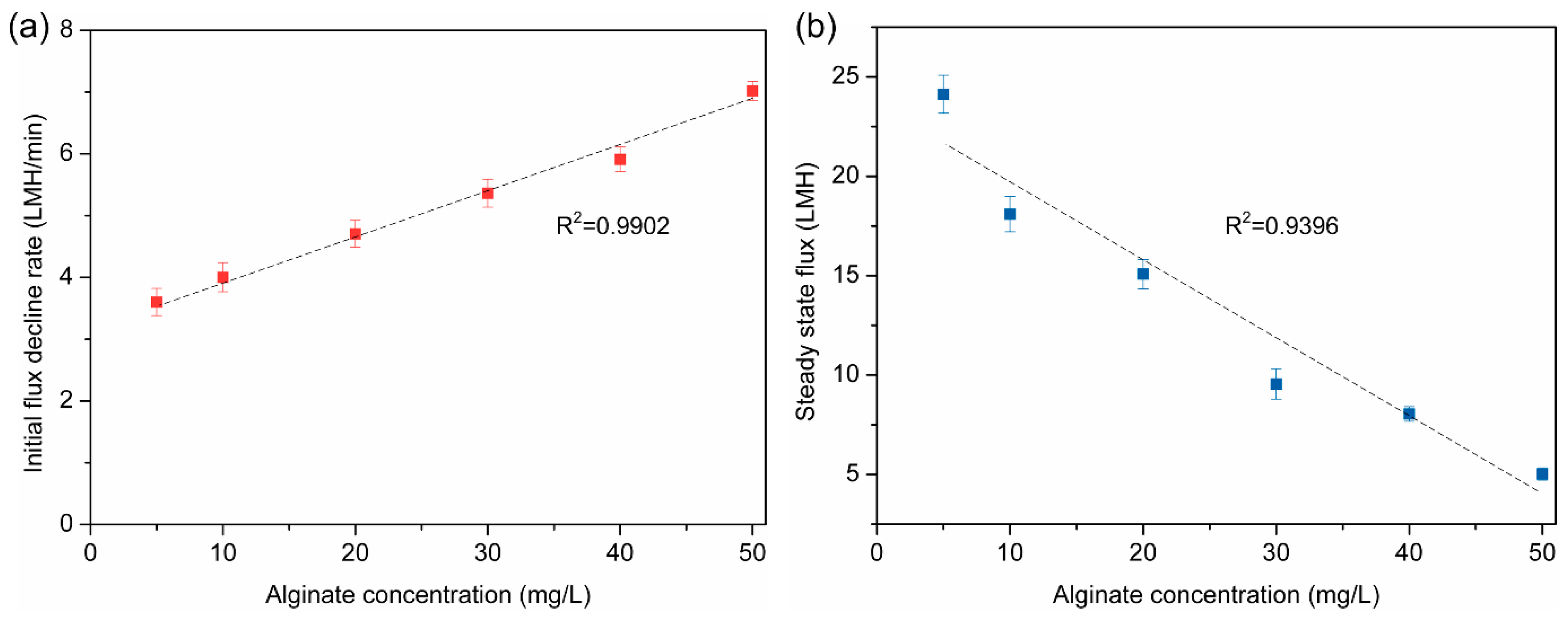
3.1. Effect of Alginate Concentration on Membrane Fouling
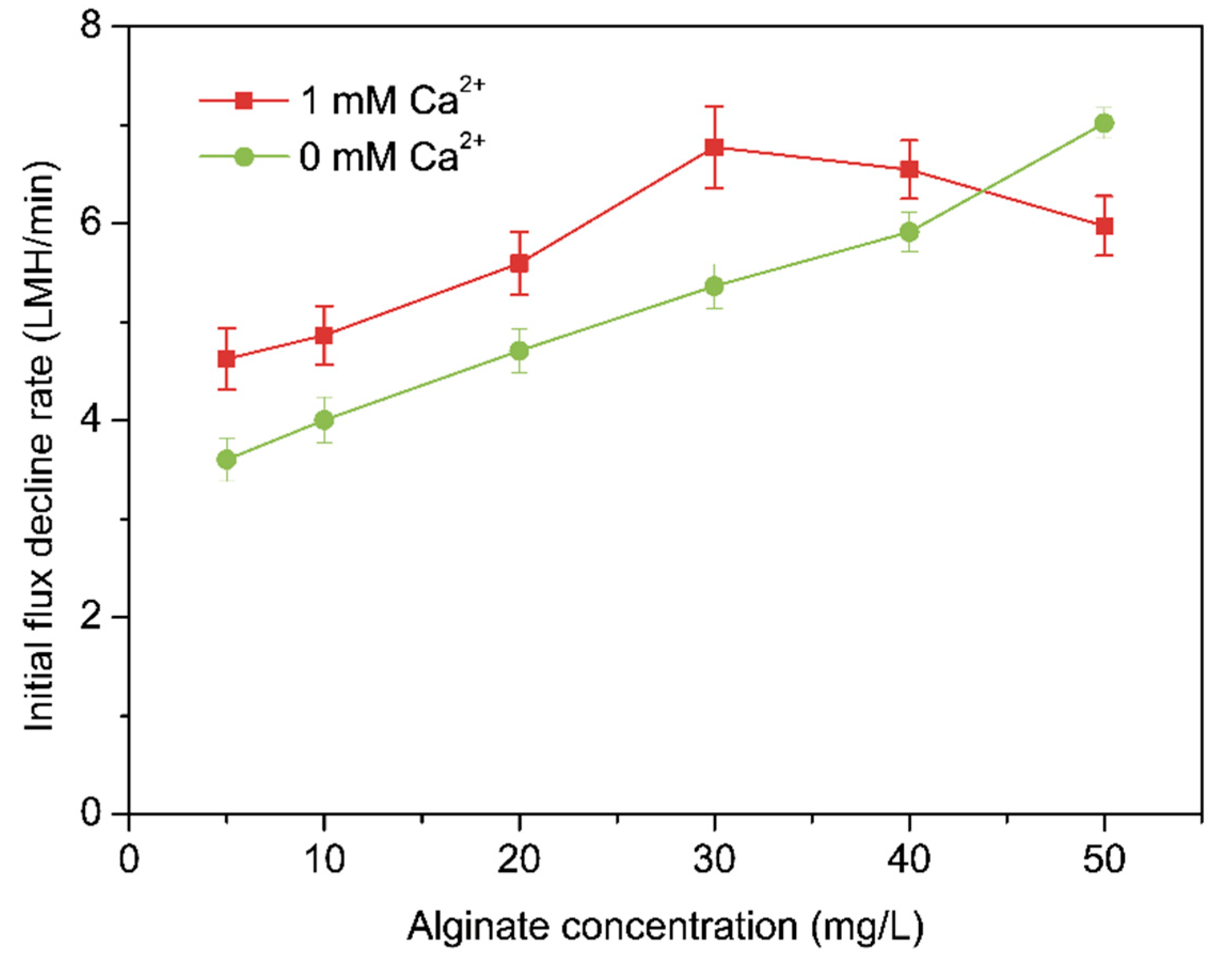
3.2. Effects of Calcium Ion on Alginate Fouling
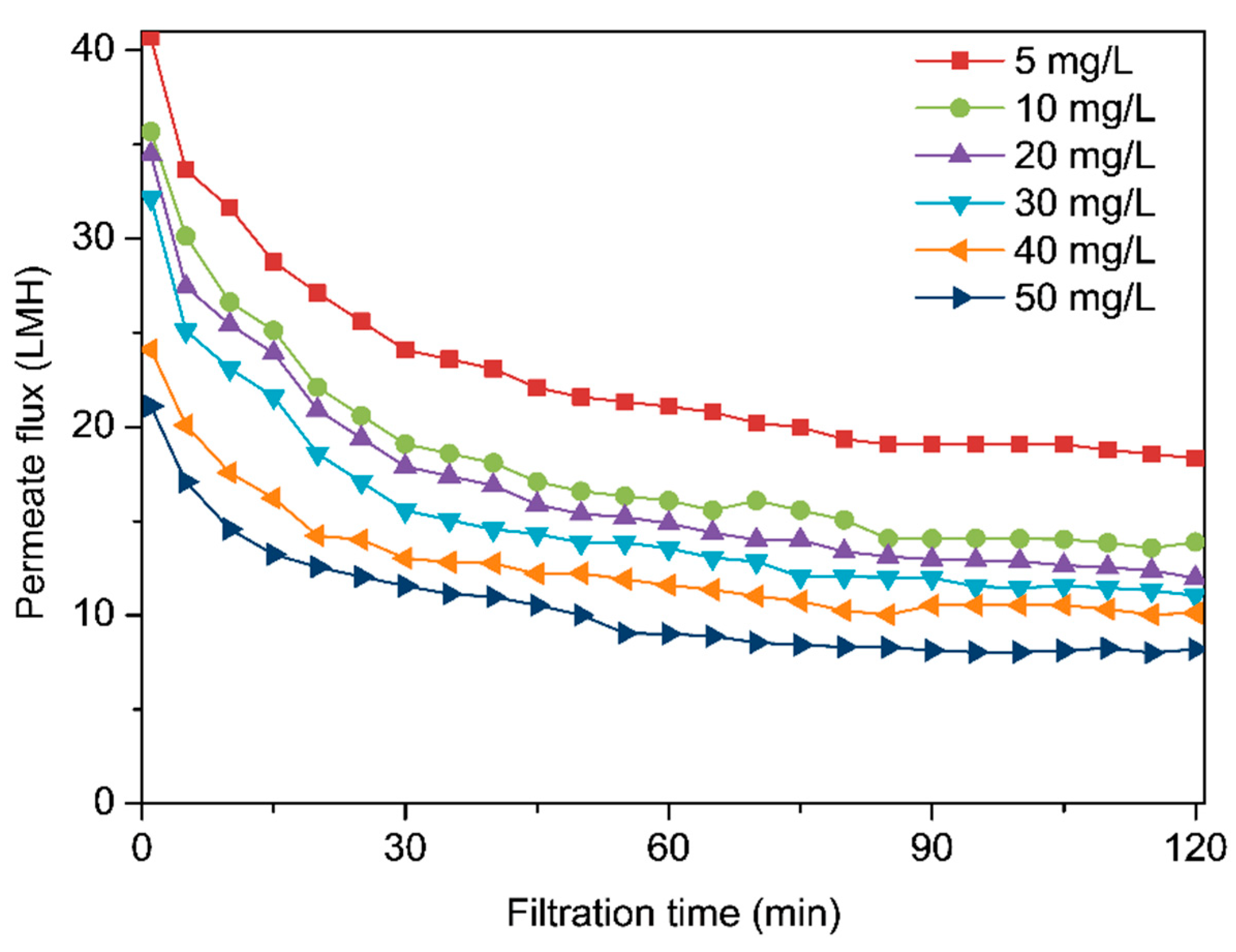
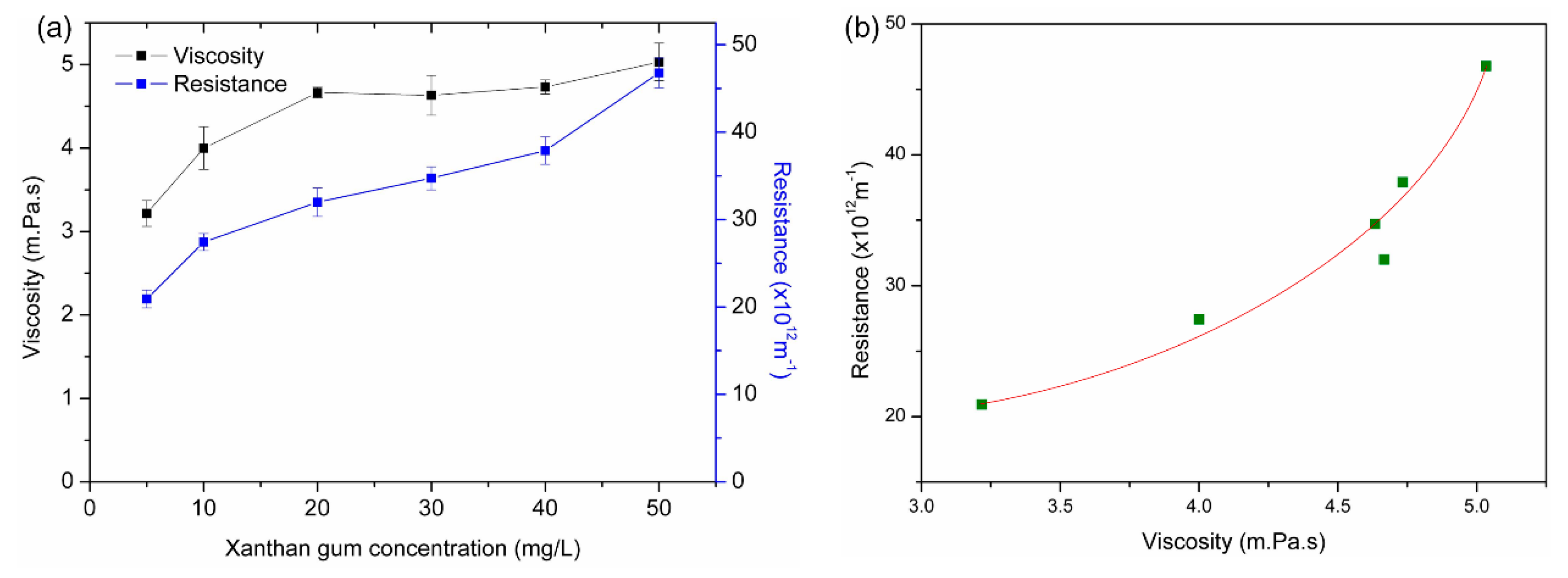
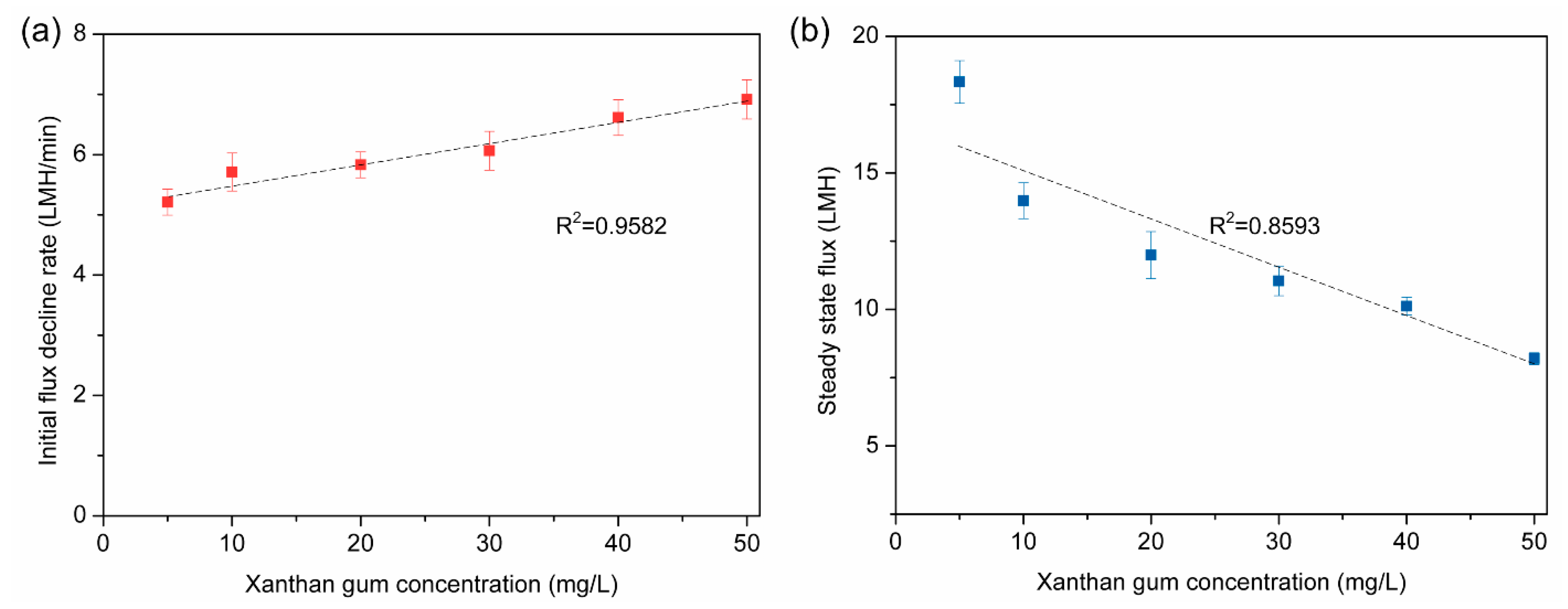
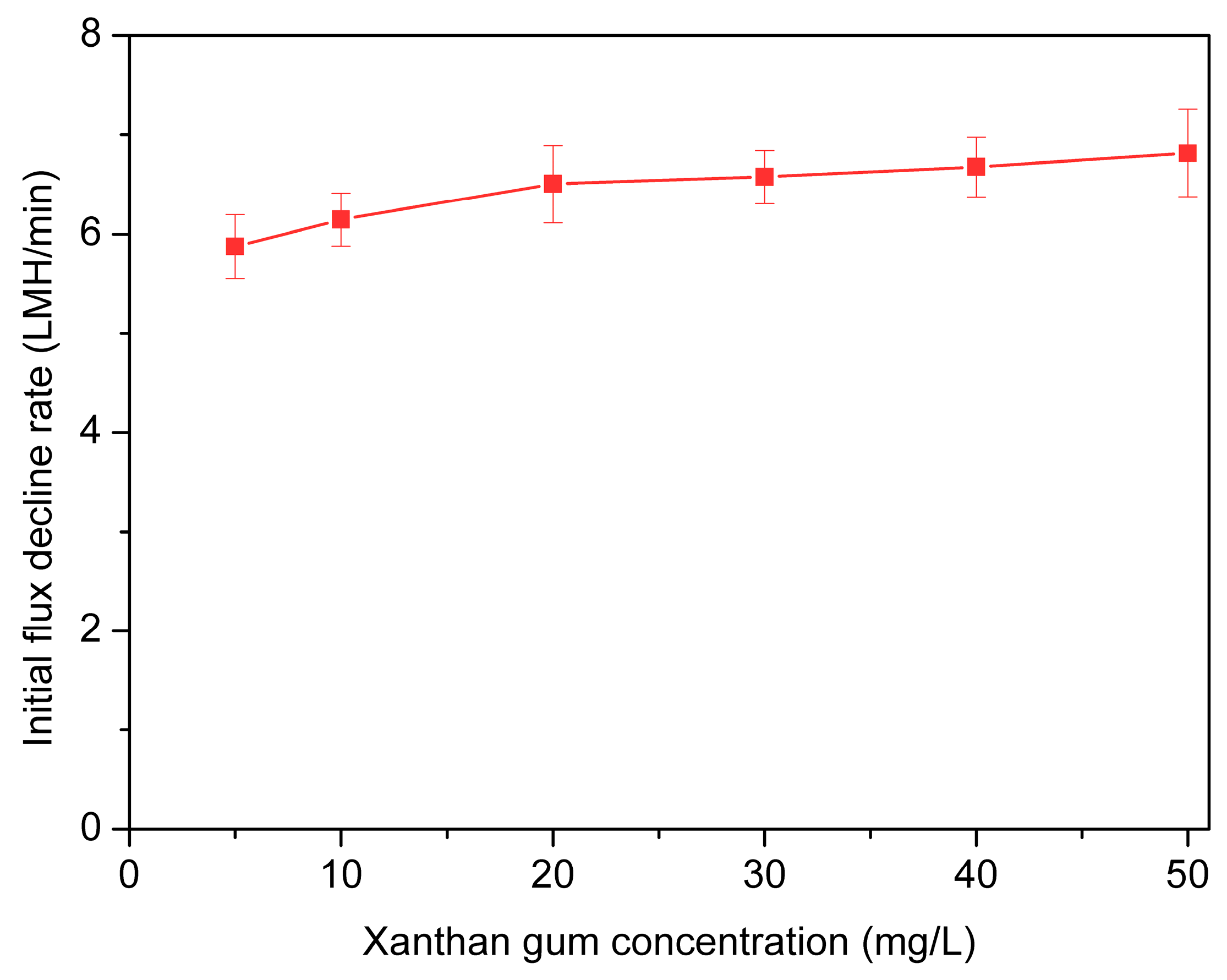
3.3. Effects of Concentrations of Xanthan Gum on Membrane Fouling
3.4. Effects of Calcium Ion on Xanthan Gum Fouling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water Treatment Using Metallic Iron: A Tutorial Review. Processes 2019, 7, 622. [Google Scholar] [CrossRef]
- Yin, H.; Qiu, P.; Qian, Y.; Kong, Z.; Zheng, X.; Tang, Z.; Guo, H. Textile Wastewater Treatment for Water Reuse: A Case Study. Processes 2019, 7, 34. [Google Scholar] [CrossRef]
- Qu, C.; Lu, S.; Liang, D.; Chen, S.; Xiang, Y.; Zhang, S. Simultaneous electro-oxidation and in situ electro-peroxone process for the degradation of refractory organics in wastewater. J. Hazard. Mater. 2019, 364, 468–474. [Google Scholar] [CrossRef]
- Qu, C.; Soomro, G.S.; Ren, N.; Liang, D.W.; Lu, S.-F.; Xiang, Y.; Zhang, S.-J. Enhanced electro-oxidation/peroxone (in situ) process with a Ti-based nickel-antimony doped tin oxide anode for phenol degradation. J. Hazard. Mater. 2019. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Wagh, P.; Zhang, X.; Blood, R.; Kekenes-Huskey, P.M.; Rajapaksha, P.; Wei, Y.; Escobar, I.C. Increasing Salt Rejection of Polybenzimidazole Nanofiltration Membranes via the Addition of Immobilized and Aligned Aquaporins. Processes 2019, 7, 76. [Google Scholar] [CrossRef]
- Bis, M.; Montusiewicz, A.; Piotrowicz, A.; Łagód, G. Modeling of Wastewater Treatment Processes in Membrane Bioreactors Compared to Conventional Activated Sludge Systems. Processes 2019, 7, 285. [Google Scholar] [CrossRef]
- Tran, T.; Nguyen, T.B.; Ho, H.L.; Le, D.A.; Lam, T.D.; Nguyen, D.C.; Hoang, A.T.; Do, T.S.; Hoang, L.; Nguyen, T.D.; et al. Integration of Membrane Bioreactor and Nanofiltration for the Treatment Process of Real Hospital Wastewater in Ho Chi Minh City, Vietnam. Processes 2019, 7, 123. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Daigger, G.T.; Lozier, J.C.; Crawford, G.V. Water reuse applications using membrane technology. Proc. Water Environ. Federation. 2006, 2006, 2625–2633. [Google Scholar] [CrossRef]
- Wang, J.; Cahyadi, A.; Wu, B.; Pee, W.; Fane, A.G.; Chew, J.W. The roles of particles in enhancing membrane filtration: A review. J. Membr. Sci. 2019. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, F. Membrane fouling in aerobic granular sludge (AGS)-membrane bioreactor (MBR): Effect of AGS size. Water Res. 2019, 157, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Yang, Z.; Guo, H.; Wen, J.J.; Nghiem, L.D.; Cornelissen, E. Potable Water Reuse through Advanced Membrane Technology. Environ. Sci. Technol. 2018, 52, 10215–10223. [Google Scholar] [CrossRef] [PubMed]
- Martin Vincent, N.; Tong, J.; Yu, D.; Zhang, J.; Wei, Y. Membrane Fouling Characteristics of a Side-Stream Tubular Anaerobic Membrane Bioreactor (AnMBR) Treating Domestic Wastewater. Processes 2018, 6, 50. [Google Scholar] [CrossRef]
- Utoro, P.A.R.; Sukoyo, A.; Sandra, S.; Izza, N.; Dewi, S.R.; Wibisono, Y. High-Throughput Microfiltration Membranes with Natural Biofouling Reducer Agent for Food Processing. Processes 2018, 7, 1. [Google Scholar] [CrossRef]
- Gkotsis, P.K.; Banti, D.C.; Peleka, E.N.; Zouboulis, A.I.; Samaras, P.E. Fouling Issues in Membrane Bioreactors (MBRs) for Wastewater Treatment: Major Mechanisms, Prevention and Control Strategies. Processes 2014, 2, 795–866. [Google Scholar] [CrossRef]
- Said, S.A.; Emtir, M.; Mujtaba, I.M. Flexible Design and Operation of Multi-Stage Flash (MSF) Desalination Process Subject to Variable Fouling and Variable Freshwater Demand. Processes 2013, 1, 279–295. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Y. Transparent exopolymer particles (TEP)-associated membrane fouling at different Na+ concentrations. Water Res. 2017, 111, 52–58. [Google Scholar] [CrossRef]
- Bagheri, M.; Mirbagheri, S.A. Critical review of fouling mitigation strategies in membrane bioreactors treating water and wastewater. Bioresour. Technol. 2018, 258, 318–334. [Google Scholar] [CrossRef]
- Al-Amoudi, A.S. Factors affecting natural organic matter (NOM) and scaling fouling in NF membranes: A review. Desalination 2010, 259, 1–10. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Amy, G. Fundamental understanding of organic matter fouling of membranes. Desalination 2008, 231, 44–51. [Google Scholar] [CrossRef]
- Meng, S.; Fan, W.; Li, X.; Liu, Y.; Liang, D.; Liu, X. Intermolecular interactions of polysaccharides in membrane fouling during microfiltration. Water Res. 2018, 143, 38–46. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Meng, F.; Zhou, Z.; Ni, B.-J.; Zheng, X.; Huang, G.; Jia, X.; Li, S.; Xiong, Y.; Kraume, M. Characterization of the size-fractionated biomacromolecules: Tracking their role and fate in a membrane bioreactor. Water Res. 2011, 45, 4661–4671. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Liu, Y. Alginate block fractions and their effects on membrane fouling. Water Res. 2013, 47, 6618–6627. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wang, R.; Zhang, M.; Meng, X.; Liu, H.; Wang, L. Insights into the Fouling Propensities of Natural Derived Alginate Blocks during the Microfiltration Process. Processes 2019, 7, 858. [Google Scholar] [CrossRef]
- Mayer, M.; Braun, R.; Fuchs, W. Comparison of various aeration devices for air sparging in crossflow membrane filtration. J. Membr. Sci. 2006, 277, 258–269. [Google Scholar] [CrossRef]
- Nataraj, S.; Schomäcker, R.; Kraume, M.; Mishra, I.M.; Drews, A. Analyses of polysaccharide fouling mechanisms during crossflow membrane filtration. J. Membr. Sci. 2008, 308, 152–161. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Tang, C.Y. Nanofiltration Membrane Fouling by Oppositely Charged Macromolecules: Investigation on Flux Behavior, Foulant Mass Deposition, and Solute Rejection. Environ. Sci. Technol. 2011, 45, 8941–8947. [Google Scholar] [CrossRef]
- Meng, S.; Winters, H.; Liu, Y. Ultrafiltration behaviors of alginate blocks at various calcium concentrations. Water Res. 2015, 83, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from algae. Biopolym. Online Biol. Chem. Biotechnol. Appl. 2005, 6. [Google Scholar]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Susanto, H.; Arafat, H.; Janssen, E.M.L.; Ulbricht, M. Ultrafiltration of polysaccharide–protein mixtures: Elucidation of fouling mechanisms and fouling control by membrane surface modification. Sep. Purif. Technol. 2008, 63, 558–565. [Google Scholar] [CrossRef]
- Jin, X.; Huang, X.; Hoek, E.M. Role of specific ion interactions in seawater RO membrane fouling by alginic acid. Environ. Sci. Technol. 2009, 43, 3580–3587. [Google Scholar] [CrossRef]
- Wang, R.; Liang, D.; Liu, X.; Fan, W.; Meng, S.; Cai, W. Effect of magnesium ion on polysaccharide fouling. Chem. Eng. J. 2020, 379, 122351. [Google Scholar] [CrossRef]
- Miao, R.; Li, X.; Wu, Y.; Wang, P.; Wang, L.; Wu, G.; Wang, J.; Lv, Y.; Liu, T. A comparison of the roles of Ca2+ and Mg2+ on membrane fouling with humic acid: Are there any differences or similarities? J. Membr. Sci. 2018, 545, 81–87. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, H.; Shen, L.; Liao, B.-Q.; Wu, X.; Li, R. Effect of calcium ions on fouling properties of alginate solution and its mechanisms. J. Membr. Sci. 2017, 525, 320–329. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; Schneider, S.; Wessling, M. On the rejection and reversibility of fouling in ultrafiltration as assessed by hydraulic impedance spectroscopy. J. Membr. Sci. 2018, 564, 532–542. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; Schneider, S.; Yüce, S.; Wessling, M. Interplay between physical cleaning, membrane pore size and fluid rheology during the evolution of fouling in membrane bioreactors. Water Res. 2018, 147, 393–402. [Google Scholar] [CrossRef]
- Pritchard, M.; Howell, J.A.; Field, R.W. The ultrafiltration of viscous fluids. J. Membr. Sci. 1995, 102, 223–235. [Google Scholar] [CrossRef]
- Dário, A.F.; Hortêncio, L.M.A.; Sierakowski, M.R.; Neto, J.C.Q.; Petri, D.F.S. The effect of calcium salts on the viscosity and adsorption behavior of xanthan. Carbohydr. Polym. 2011, 84, 669–676. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, S.; Liu, H.; Zhao, Q.; Shen, N.; Zhang, M. Filtration Performances of Different Polysaccharides in Microfiltration Process. Processes 2019, 7, 897. https://doi.org/10.3390/pr7120897
Meng S, Liu H, Zhao Q, Shen N, Zhang M. Filtration Performances of Different Polysaccharides in Microfiltration Process. Processes. 2019; 7(12):897. https://doi.org/10.3390/pr7120897
Chicago/Turabian StyleMeng, Shujuan, Hongju Liu, Qian Zhao, Nan Shen, and Minmin Zhang. 2019. "Filtration Performances of Different Polysaccharides in Microfiltration Process" Processes 7, no. 12: 897. https://doi.org/10.3390/pr7120897
APA StyleMeng, S., Liu, H., Zhao, Q., Shen, N., & Zhang, M. (2019). Filtration Performances of Different Polysaccharides in Microfiltration Process. Processes, 7(12), 897. https://doi.org/10.3390/pr7120897





