Copper Adsorption by Magnetized Pine-Needle Biochar
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Adsorption Experiments
3. Results and Discussion
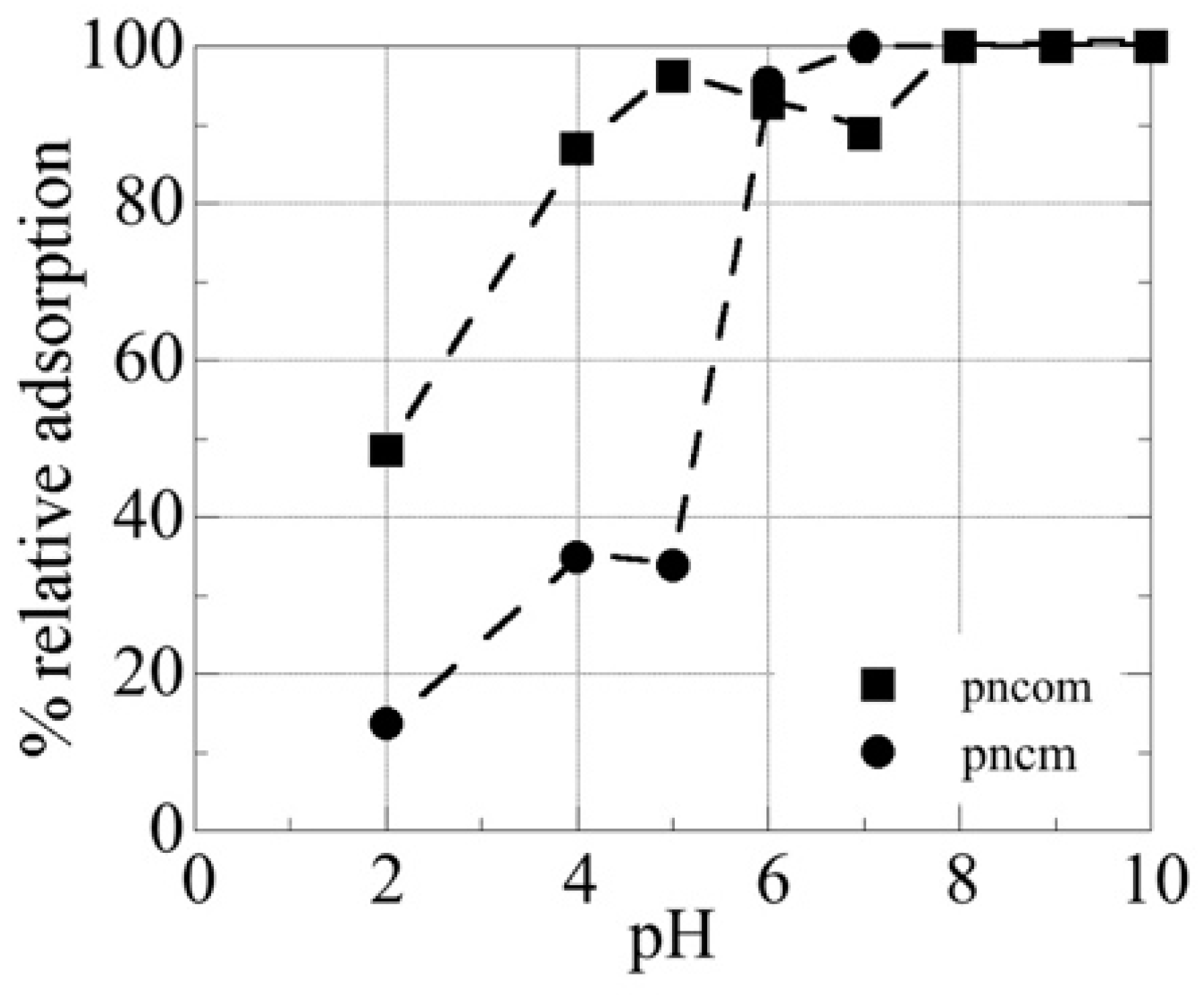
3.1. Effect of pH
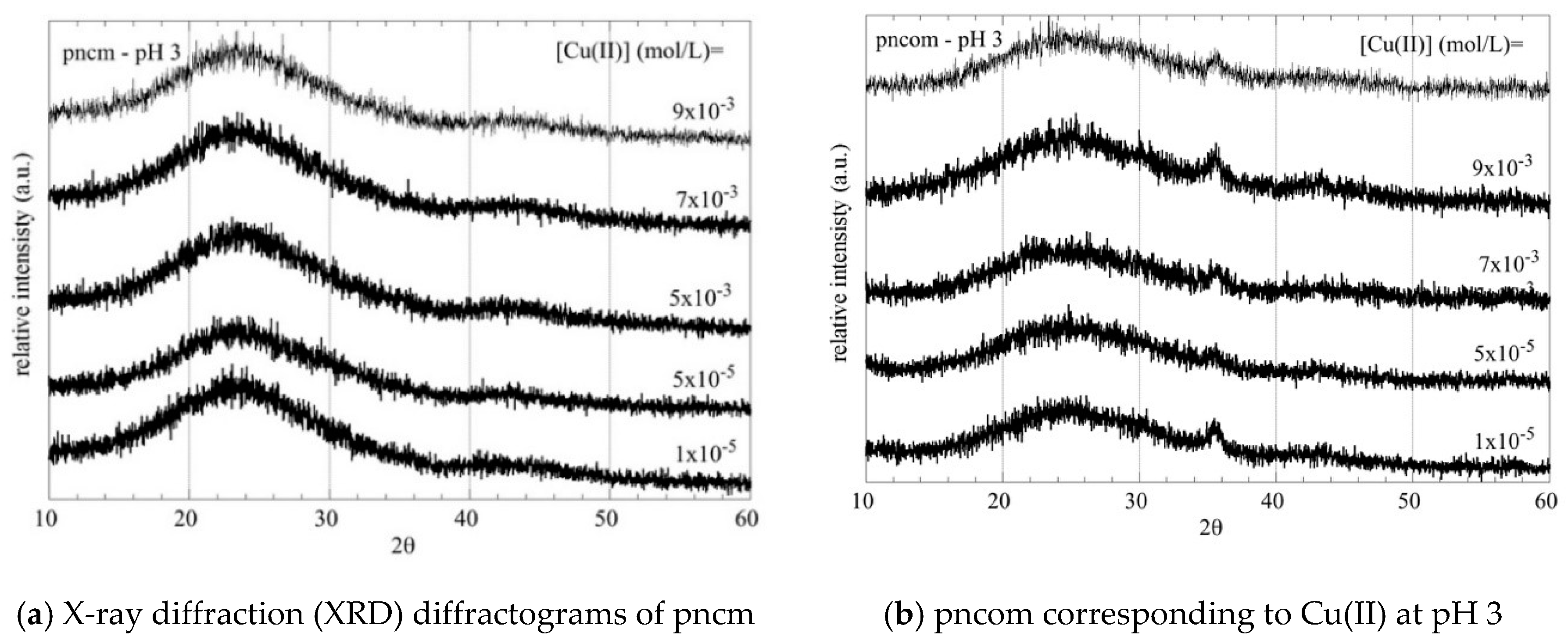
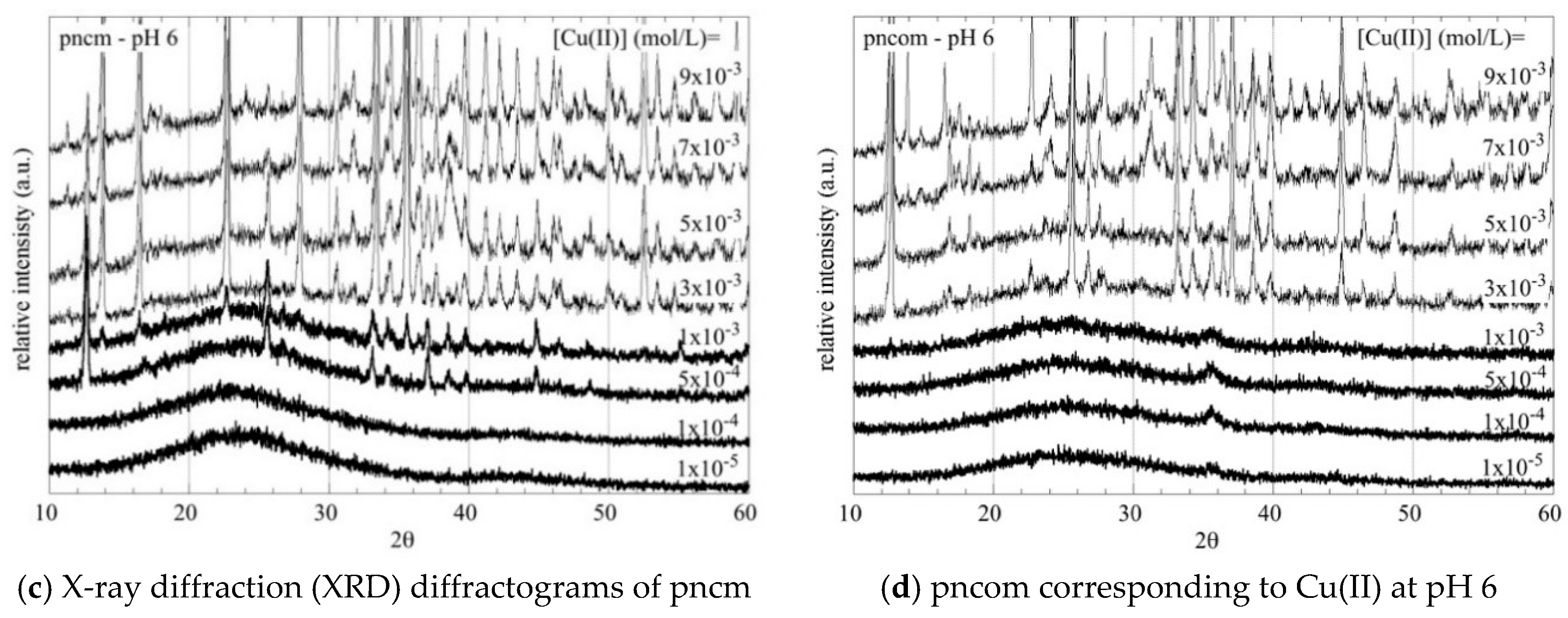
3.2. XRD Measurements
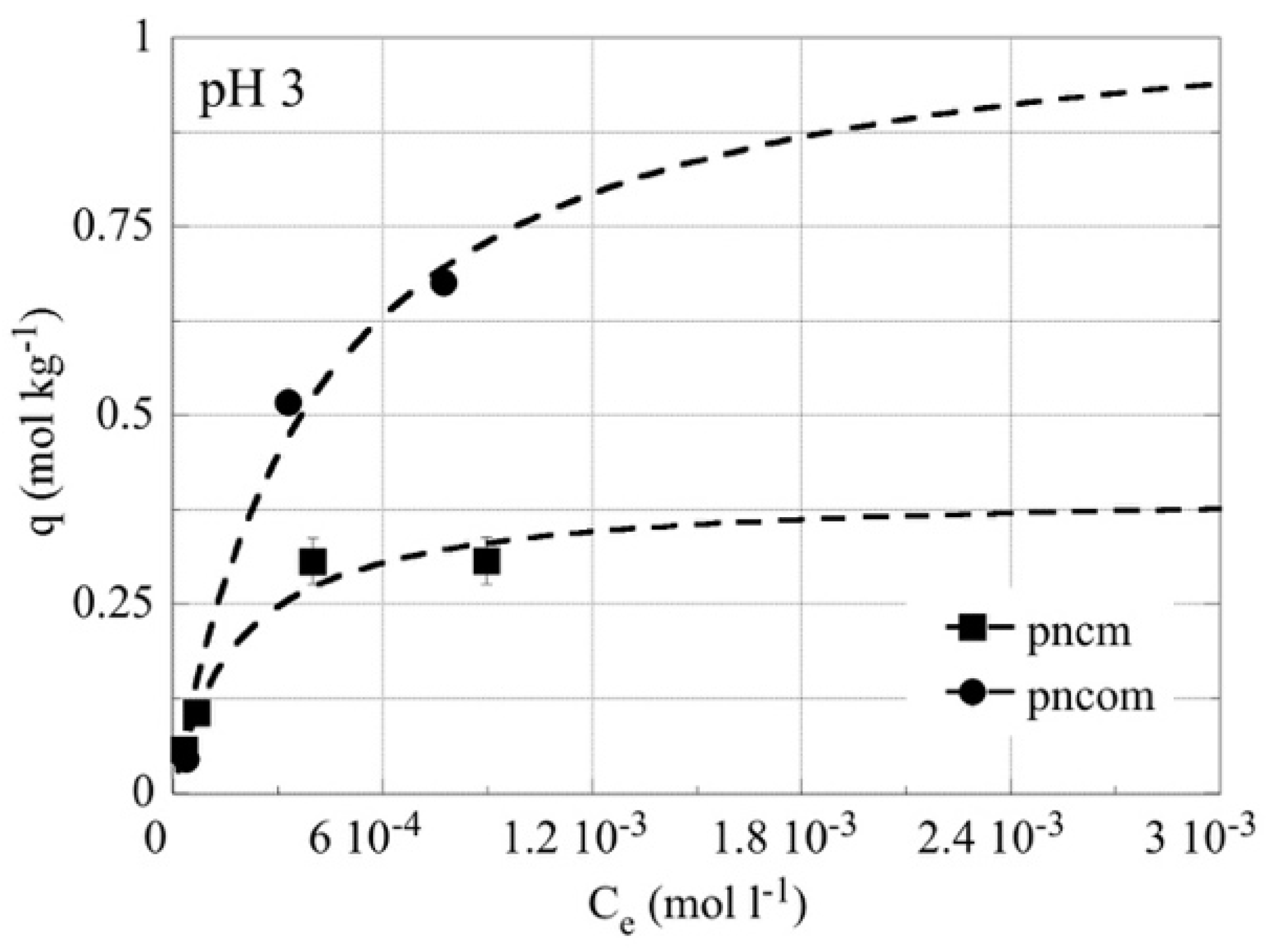
3.3. Adsorption Data
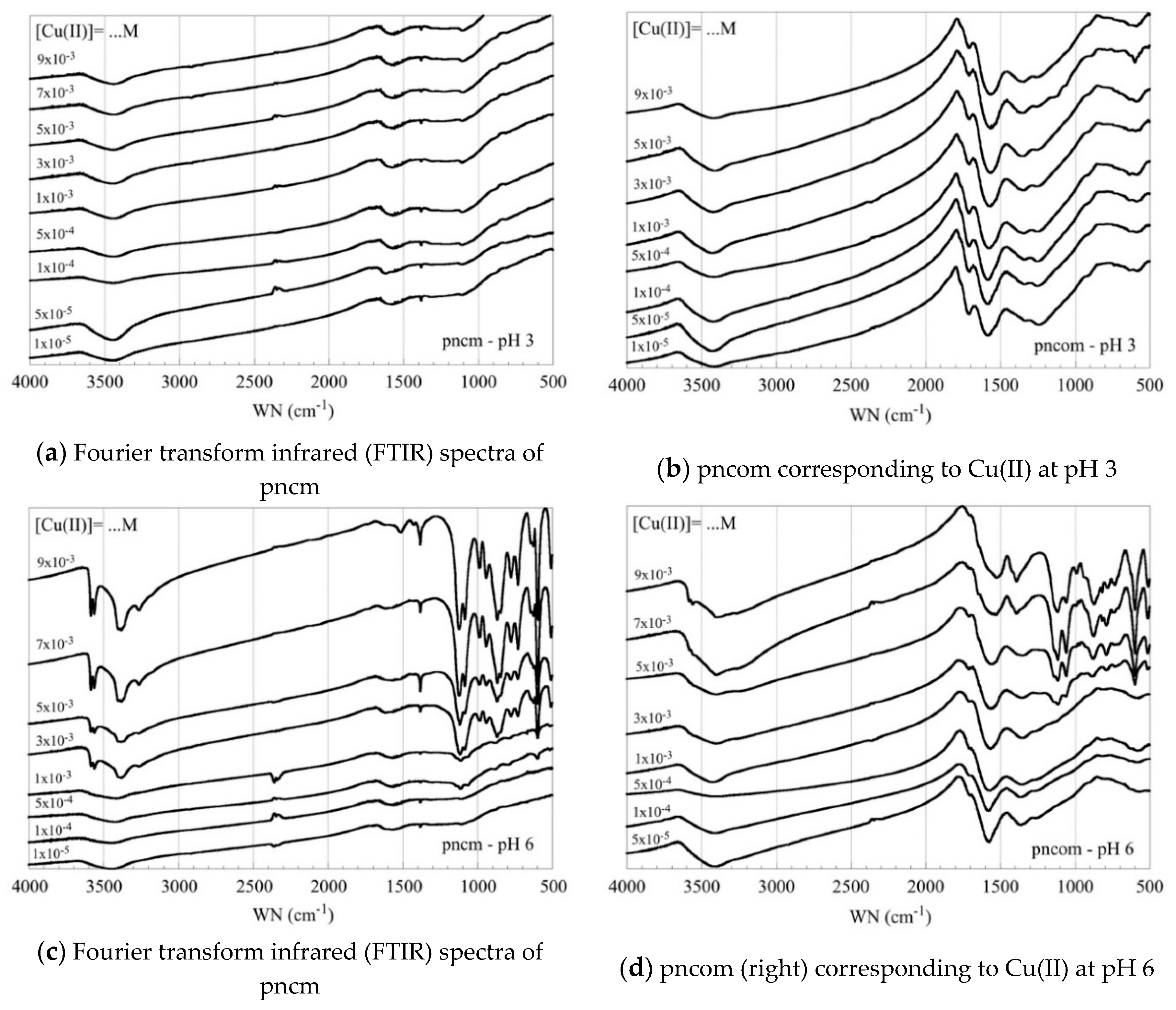
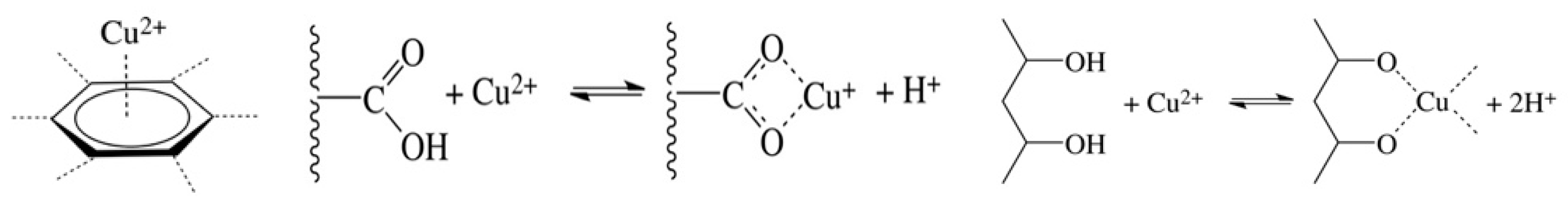
3.4. FTIR Measurements
3.5. Kinetic Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process—A review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Hadjittofi, L.; Prodromou, M.; Pashalidis, I. Activated biochar derived from cactus fibres–preparation, characterization and application on Cu (II) removal from aqueous solutions. Bioresour. Technol. 2014, 159, 460–464. [Google Scholar] [CrossRef]
- Liatsou, I.; Constantinou, P.; Pashalidis, I. Copper binding by activated biochar fibres derived from Luffa Cylindrica. Water AirSoil Pollut. 2017, 228, 255. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu (II) and Cd (II) from aqueous solutions by ferromanganese binary oxide–biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Hadjiyiannis, P.; Pashalidis, I. Copper (II) adsorption by Opuntia ficus-indica biochar fiber-MnO2 composites. Desalin. Water Treat. 2019, 159, 60–65. [Google Scholar] [CrossRef]
- Philippou, K.; Savva, I.; Pashalidis, I. Uranium(VI) binding by pine needles prior and after chemical modification. J. Radioanal. Nucl. Chem. 2018, 318, 2205–2211. [Google Scholar] [CrossRef]
- Liatsou, I.; Pashalidis, I.; Dosche, C. Cu(II) adsorption on 2-thiouracil-modified Luffa Cylindrica biochar fibres from artificial and real samples, and competition reactions with U(VI). J. Hazard. Mater. 2019. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.; Summers, R.S.; Cook, S.M. Environmental comparison of biochar and activated carbon for tertiary wastewater treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef]
- Nair, V.D.; Nair, P.K.R.; Dari, B.; Freitas, A.M.; Chatterjee, N.; Pinheiro, F.M. Biochar in the Agroecosystem-Climate-Change-Sustainability Nexus. Front. Plant Sci. 2017, 8, 2051. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Balasubramanian, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crops Prod. 2019, 128, 405–423. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Pashalidis, I. Uranium sorption from aqueous solutions by activated biochar fibres investigated by FTIR spectroscopy and batch experiments. J. Radioanal. Nuclear Chem. 2015, 304, 897–904. [Google Scholar] [CrossRef]
- Liatsou, I.; Pashalidis, I.; Oezaslan, M.; Dosche, C. Surface characterization of oxidized biochar fibers derived from Luffa Cylindrica and lanthanide binding. J. Environ. Chem. Eng. 2017, 5, 4069–4074. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J. Use of three types of magnetic biochar in the removal of copper (II) ions from wastewaters. Sep. Sci. Technol. 2018, 53, 1045–1057. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Fabris, J.D.; Garg, V.; Sapag, K.; Lago, R.M. Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon 2002, 40, 2177–2183. [Google Scholar] [CrossRef]
- Milonjić, S.; Kopečni, M.; Ilić, Z. The point of zero charge and adsorption properties of natural magnetite. J. Radioanal. Chem. 1983, 78, 15–24. [Google Scholar] [CrossRef]
- Majumder, S.; Sardar, M.; Satpati, B.; Kumar, S.; Banerjee, S. Magnetization Enhancement of Fe3O4 by Attaching onto Graphene Oxide: An Interfacial Effect. J. Phys. Chem. C 2018, 122, 21356–21365. [Google Scholar] [CrossRef]
- Devamani, R.H.P.; Alagar, M. Synthesis and characterisation of copper II hydroxide nano particles. Nano Biomed. Eng. 2013, 5, 116–120. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Nyamunda, B.C.; Chivhanga, T.; Guyo, U.; Chigondo, F. Removal of Zn (II) and Cu (II) Ions from Industrial Wastewaters Using Magnetic Biochar Derived from Water Hyacinth. J. Eng. 2019. [Google Scholar] [CrossRef]
- Son, E.B.; Poo, K.M.; Chang, J.S.; Chae, K.J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Yang, B.; Wang, H.; Xu, S.; Cao, Y. Effective removal of copper (II) and cadmium (II) by adsorbent prepared from chitosan-modified magnetic biochar. J. Residuals Sci. Technol 2016, 13, 197–205. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Liu, S.; Jiang, L.; Tan, X.; Zeng, G.; Li, M.; Liu, S.; Tian, S.; Fang, Y. Activated magnetic biochar by one-step synthesis: Enhanced adsorption and coadsorption for 17β-estradiol and copper. Sci. Total Environ. 2018, 639, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations; John Wiley & Sons: New York, NY, USA, 1976; Volume 81, pp. 245–246. [Google Scholar]
- Philippou, K.; Anastopoulos, I.; Dosche, C.; Pashalidis, I. Synthesis and characterization of a novel Fe3O4-loaded oxidized biochar from pine needles and its application for uranium removal. Kinetic, thermodynamic, and mechanistic analysis. J. Environ. Manag. 2019, 252, 109677. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetensk. Handingarl 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef]
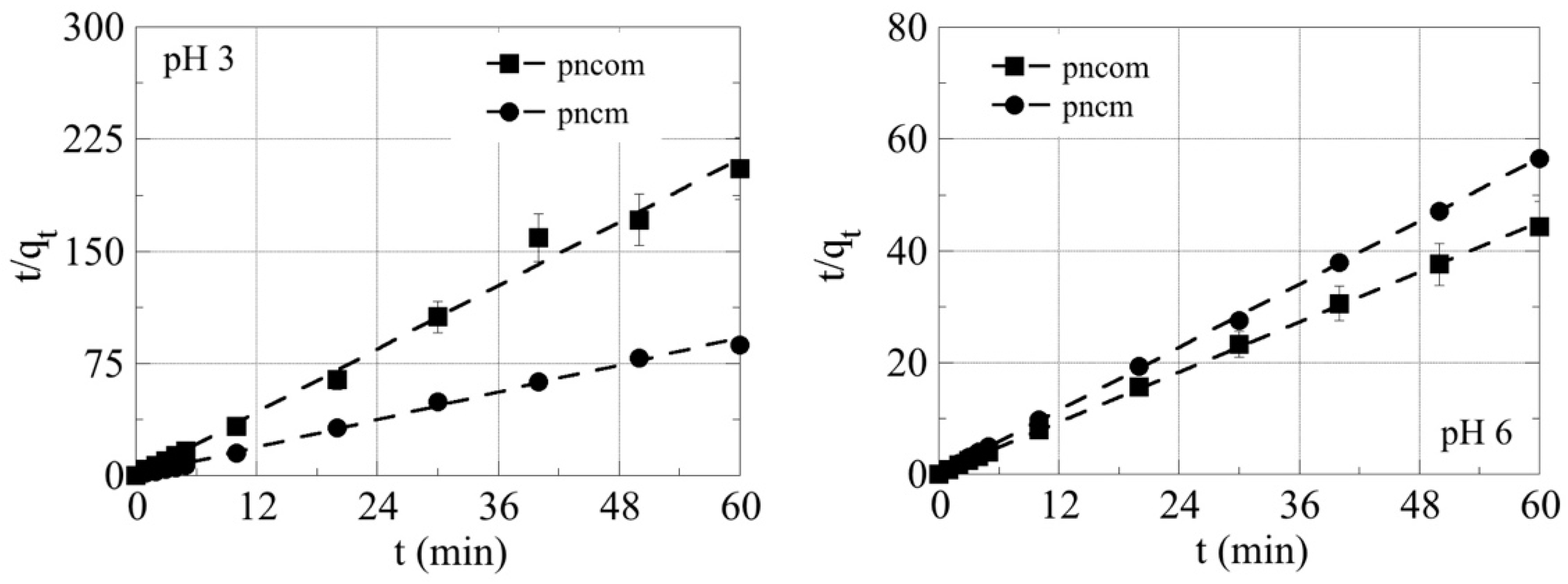
| Pseudo-First-Order | Pseudo-Second-Order | |||||||
|---|---|---|---|---|---|---|---|---|
| pncm | pncοm | pncm | pncom | |||||
| pH | k1 (min−1) | R | k1 (min−1) | R | k2 (g·mg−1·min−1) | R | k2 (g·mg−1·min−1) | R |
| 3 | 0.199 | 0.583 | 0.002 | 0.187 | 1.525 | 0.998 | 3.525 | 0.996 |
| 6 | 0.001 | 0.126 | 0.001 | 0.992 | 0.939 | 0.999 | 0.746 | 0.999 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolaou, E.; Philippou, K.; Anastopoulos, I.; Pashalidis, I. Copper Adsorption by Magnetized Pine-Needle Biochar. Processes 2019, 7, 903. https://doi.org/10.3390/pr7120903
Nicolaou E, Philippou K, Anastopoulos I, Pashalidis I. Copper Adsorption by Magnetized Pine-Needle Biochar. Processes. 2019; 7(12):903. https://doi.org/10.3390/pr7120903
Chicago/Turabian StyleNicolaou, Eleni, Katerina Philippou, Ioannis Anastopoulos, and Ioannis Pashalidis. 2019. "Copper Adsorption by Magnetized Pine-Needle Biochar" Processes 7, no. 12: 903. https://doi.org/10.3390/pr7120903
APA StyleNicolaou, E., Philippou, K., Anastopoulos, I., & Pashalidis, I. (2019). Copper Adsorption by Magnetized Pine-Needle Biochar. Processes, 7(12), 903. https://doi.org/10.3390/pr7120903







