Kinetics of Luteolin Extraction from Peanut Shells and Reseda luteola for Potential Applications as a Biofunctional Ingredient
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Chemicals and Reagents
2.3. Exhaustive Extraction of Reseda luteola and Peanut Shells
2.4. Kinetic Study of Luteolin Extraction from Reseda luteola
2.5. Analyses and Treatments of the Extracts
2.5.1. Determination of Total Solid Yield (TSY)
2.5.2. Determination of Total Phenolic Content (TPC)
2.5.3. Determination of Antiradical Capacity (AC)
2.5.4. Determination of Flavonoids by HPLC-DAD Analyses
2.5.5. Acid Hydrolysis of Flavonoid Glycosides for the Determination of the Respective Aglycones
2.6. Statistical Analysis
3. Results and Discussion
3.1. Comparative Study of the Exhaustive Extractions of Reseda luteola and Peanut Shells
3.2. Kinetic Study of Reseda luteola Aerial Parts Extraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Çetinkaya, M.; Baran, Y. Therapeutic Potential of Luteolin on Cancer. Vaccines 2023, 11, 554. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.-H.; Wang, R. Quantitative Structure-Activity Relationship Analysis of Phenolic Antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Liu, W.; Zhang, C.; Xi, Y.; Zhou, Y.; Li, H.; Liu, X. Structure–Activity Relationships and Changes in the Inhibition of Xanthine Oxidase by Polyphenols: A Review. Foods 2024, 13, 2365. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Wölfle, U.; Schempp, C.M. Anti-Carcinogenic Effects of the Flavonoid Luteolin. Molecules 2008, 13, 2628–2651. [Google Scholar] [CrossRef] [PubMed]
- Tuorkey, M.J. Molecular Targets of Luteolin in Cancer. Eur. J. Cancer Prev. 2016, 25, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Liu, A.-L.; Liu, B.; Qin, H.-L.; Lee, S.; Wang, Y.-T.; Du, G.-H. Anti-Influenza Virus Activities of Flavonoids from the Medicinal Plant Elsholtzia rugulosa. Planta Med. 2008, 74, 847–851. [Google Scholar] [CrossRef]
- Xie, Y.-Z.; Peng, C.-W.; Su, Z.-Q.; Huang, H.-T.; Liu, X.-H.; Zhan, S.-F.; Huang, X.-F. A Practical Strategy for Exploring the Pharmacological Mechanism of Luteolin Against COVID-19/Asthma Comorbidity: Findings of System Pharmacology and Bioinformatics Analysis. Front. Immunol. 2022, 12, 769011. [Google Scholar] [CrossRef]
- Liao, P.H.; Hung, L.M.; Chen, Y.H.; Kuan, Y.H.; Zhang, F.B.; Lin, R.H.; Shih, H.C.; Tsai, S.K.; Huang, S.S. Cardioprotective Effects of Luteolin during Ischemia-Reperfusion Injury in Rats. Circ. J. 2011, 75, 443–450. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N. Food-Based Phytochemical Luteolin, Their Derivatives, Sources, and Medicinal Benefits. Int. J. Agric. Life Sci. 2017, 3, 195–207. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID Syndrome-Associated Brain Fog and Chemofog: Luteolin to the Rescue. BioFactors 2021, 47, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Bangar, P.S.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A Flavone with Myriads of Bioactivities and Food Applications. Food Biosci. 2023, 52, 102366. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary luteolin: A narrative review of Focusing on Its Pharmakokinetic Properies and Effects on Glycolipid Metabolism. J. Agric. Food Chem. 2021, 69, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, A.; Ito, J.; Parida, I.S.; Syoji, N.; Fujii, T.; Takahashi, H.; Nakagawa, K. Improving water dispersibility and bioavailability of luteolin using microemulsion system. Sci. Rep. 2022, 12, 11949. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhao, Y.; He, M.; Feng, N.P. Nanostructured lipid carriers versus microemulsions for delivery of the poorly water-soluble drug luteolin. Int. J. Pharm. 2014, 476, 169–177. [Google Scholar] [CrossRef]
- Mod Razif, M.R.F.; Chan, S.Y.; Chew, Y.-L.; Hassan, M.; Ahmad Hisham, S.; Abdul Rahman, S.; Mai, C.-W.; Teo, M.Y.M.; Kee, P.E.; Khoo, K.S.; et al. Recent Developments in Luteolin-Loaded Nanoformulations for Enhanced Anti-Carcinogenic Activities: Insights from In Vitro and In Vivo Studies. Sci 2024, 6, 68. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, S.; Li, L.; Liu, Y.; Wang, D.; Liu, G. Enhancement of Oral Bioavailability and Anti-Colitis Effect of Luteolin-Loaded Polymer Micelles with RA-SS-mPEG as Carrier. Drug Dev. Ind. Pharm. 2023, 49, 17–29. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Wu, X.; Bhagwat, S. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2018. Available online: http://www.ars.usda.gov/nutrientdata/flav (accessed on 26 June 2025).
- Abdallah, M.S.; de Wit, H.C.D. The Resedaceae: A Taxonomical Revision of the Family (Final Instalment); Mededelingen Landbouwhogeschool Wageningen: Wageningen, The Netherlands, 1978; pp. 259–262. Available online: https://edepot.wur.nl/287583 (accessed on 22 July 2025).
- Cristea, D. Identification and Quantitative HPLC Analysis of the Main Flavonoids Present in Weld (Reseda luteola L.). Dye. Pigm. 2003, 57, 267–272. [Google Scholar] [CrossRef]
- Moiteiro, C.; Gaspar, H.; Rodrigues, A.I.; Lopes, J.F.; Carnide, V. HPLC Quantification of Dye Flavonoids in Reseda luteola L. from Portugal. J. Sep. Sci. 2008, 31, 3683–3687. [Google Scholar] [CrossRef]
- Angelini, L.G.; Bertoli, A.; Rolandelli, S.; Pistelli, L. Agronomic Potential of Reseda luteola L. as New Crop for Natural Dyes in Textiles Production. Ind. Crops Prod. 2003, 17, 199–207. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanic Garden and Botanical Museum Berlin-Dahlem & Hellenic Botanical Society: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Botanical Summary Report. European Food Safety Authority. Available online: https://www.efsa.europa.eu/en/microstrategy/botanical-summary-report (accessed on 29 July 2025).
- Wölfle, U.; Simon-Haarhaus, B.; Merfort, I.; Schempp, C.M. Reseda luteola L. extract displays antiproliferative and pro-apoptotic activities that are related to its major flavonoids. Phytother. Res. 2010, 24, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Chen, Z.; Deng, Q.; Zhu, S.; Wang, G. The Roles of Luteolin in Peanut Shell Extract-Mediated Protection of Erythrocytes against Hypoxanthine-Xanthine Oxidase-Induced Toxicity. Food Biosci. 2021, 39, 100826. [Google Scholar] [CrossRef]
- Daigle, D.J.; Conkerton, E.J.; Sanders, T.H.; Mixon, A.C. Peanut Hull Flavonoids: Their Relationship with Peanut Maturity. J. Agric. Food Chem. 1988, 36, 1179–1181. [Google Scholar] [CrossRef]
- Kotzamanidis, S.T.; Stavropoulos, N.; Ipsilandis, C.G. Classification and Evaluation of Greek Groundnut (Arachis hypogaea L.) Using 17 Main Agronomic and Quality Traits. Pak. J. Biol. Sci. 2006, 9, 1021–1027. [Google Scholar] [CrossRef]
- Meirinhos, J.; Silva, B.M.; Valentão, P.; Seabra, R.M.; Pereira, J.A.; Dias, A.; Andrade, P.B.; Ferreres, F. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat. Prod. Res. 2005, 19, 189–195. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef]
- Burger, P.; Monchot, A.; Bagarri, O.; Chiffolleau, P.; Azoulay, S.; Fernandez, X.; Michel, T. Whitening Agents from Reseda luteola L. and Their Chemical Characterization Using Combination of CPC, UPLC-HRMS and NMR. Cosmetics 2017, 4, 51. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, L.; Si, X.; Li, L.; Sheng, Z. Microwave-Assisted Extraction of Luteolin from Peanut Shells Using Natural Deep Eutectic Solvents and Its Molecular Mechanism. Ind. Crops Prod. 2025, 225, 120578. [Google Scholar] [CrossRef]
- Papageorgiou, C.; Dermesonlouoglou, E.; Tsimogiannis, D.; Taoukis, P. Enrichment of Bakery Products with Antioxidant and Dietary Fiber Ingredients Obtained from Spent Coffee Ground. Appl. Sci. 2024, 14, 6863. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Merken, H.M.; Beecher, G.R. Liquid chromatographic method for the separation and quantification of prominent flavonoid aglycones. J. Chromatogr. A 2000, 897, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kouri, G.; Tsimogiannis, D.; Bardouki, H.; Oreopoulou, V. Extraction and Analysis of Antioxidant Components from Origanum dictamnus. Innov. Food Sci. Emerg. Technol. 2007, 8, 155–162. [Google Scholar] [CrossRef]
- Imran, A.; Humiyion, M.; Arshad, M.U.; Saeed, F.; Arshad, M.S.; Afzaal, M.; Al Jbawi, E. Extraction, Amino Acid Estimation, and Characterization of Bioactive Constituents from Peanut Shell through Eco-Innovative Techniques for Food Application. Int. J. Food Prop. 2022, 25, 2055–2065. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D. Antioxidant Activity of Methanolic Extracts of Peanut Hulls from Various Cultivars. J. Am. Oil Chem. Soc. 1995, 72, 1065–1067. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Goussias, G.; Tsimogiannis, D.; Oreopoulou, V. Hydro-alcoholic extraction kinetics of phenolics from oregano: Optimization of the extraction parameters. Food Bioprod. Process. 2020, 123, 378–389. [Google Scholar] [CrossRef]
- Psarrou, I.; Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction Kinetics of Phenolic Antioxidants from the Hydro Distillation Residues of Rosemary and Effect of Pretreatment and Extraction Parameters. Molecules 2020, 25, 4520. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Alexandraki, A.-C.; Tsimogiannis, D.; Oreopoulou, V. Semi-Batch Extraction of Phenolic Compounds from Rosmarinus officinalis: Kinetic Study and Dimensionless Modeling. J. Food Eng. 2024, 370, 111962. [Google Scholar] [CrossRef]
- Fu, Y.J.; Liu, W.; Zu, Y.G.; Tong, M.H.; Li, S.M.; Yan, M.M.; Efferth, T.; Luo, H. Enzyme Assisted Extraction of Luteolin and Apigenin from Pigeonpea (Cajanus cajan (L.) Millsp.) Leaves. Food Chem. 2008, 111, 508–512. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.; Rahaman, A.; Aadil, R.M.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Abidin, L.; Mujeeb, M.; Mir, S.R.; Khan, S.A.; Ahmad, A. Comparative Assessment of Extraction Methods and Quantitative Estimation of Luteolin in the Leaves of Vitex negundo Linn. by HPLC. Asian Pac. J. Trop. Med. 2014, 7, S289–S293. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Optimization of Oleuropein and Luteolin-7-O-Glucoside Extraction from Olive Leaves by Ultrasound-Assisted Technology. Energies 2019, 12, 2486. [Google Scholar] [CrossRef]
- Hussain, A.I.; Chatha, S.A.S.; Noor, S.; Arshad, M.; Rathore, H.A. Effect of Extraction Techniques and Solvent Systems on the Extraction of Antioxidant Components from Peanut (Arachis hypogaea L.) Hulls. Food Anal. Methods 2012, 5, 890–896. [Google Scholar] [CrossRef]
- Hou, M.; Hu, W.; Wang, A.; Xiu, Z.; Shi, Y.; Hao, K.; Sun, X.; Cao, D.; Lu, R.; Sun, J. Ultrasound-Assisted Extraction of Total Flavonoids from Pteris cretica L.: Process Optimization, HPLC Analysis, and Evaluation of Antioxidant Activity. Antioxidants 2019, 8, 425. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Zu, Y.; Zhao, X. Microwave Assisted Simultaneous Extraction of Luteolin and Apigenin from Tree Peony Pod and Evaluation of its Antioxidant Activity. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of Oleuropein and Luteolin-7-O-Glucoside from Olive Leaves: Optimization of Technique and Operating Conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef]
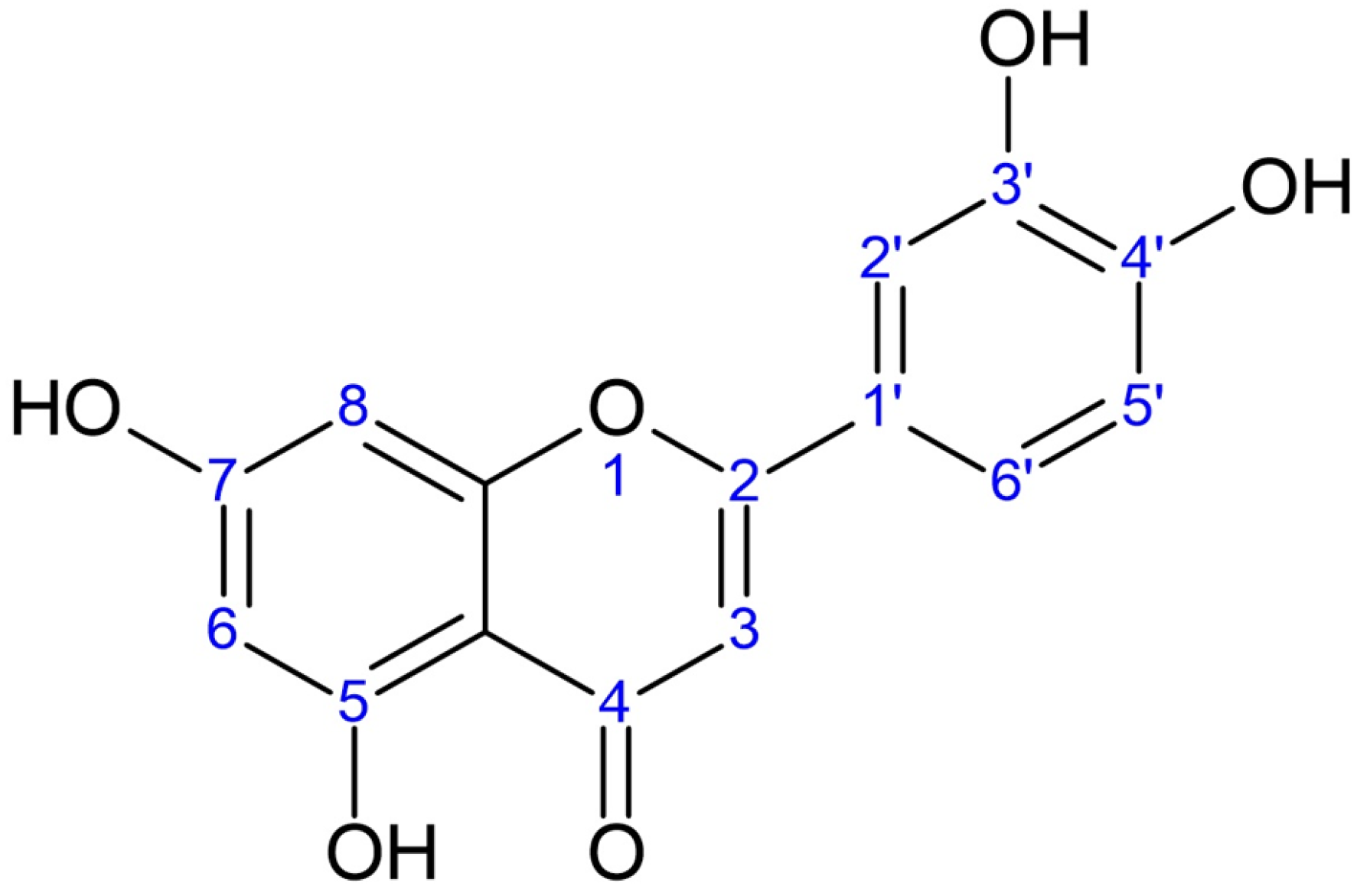

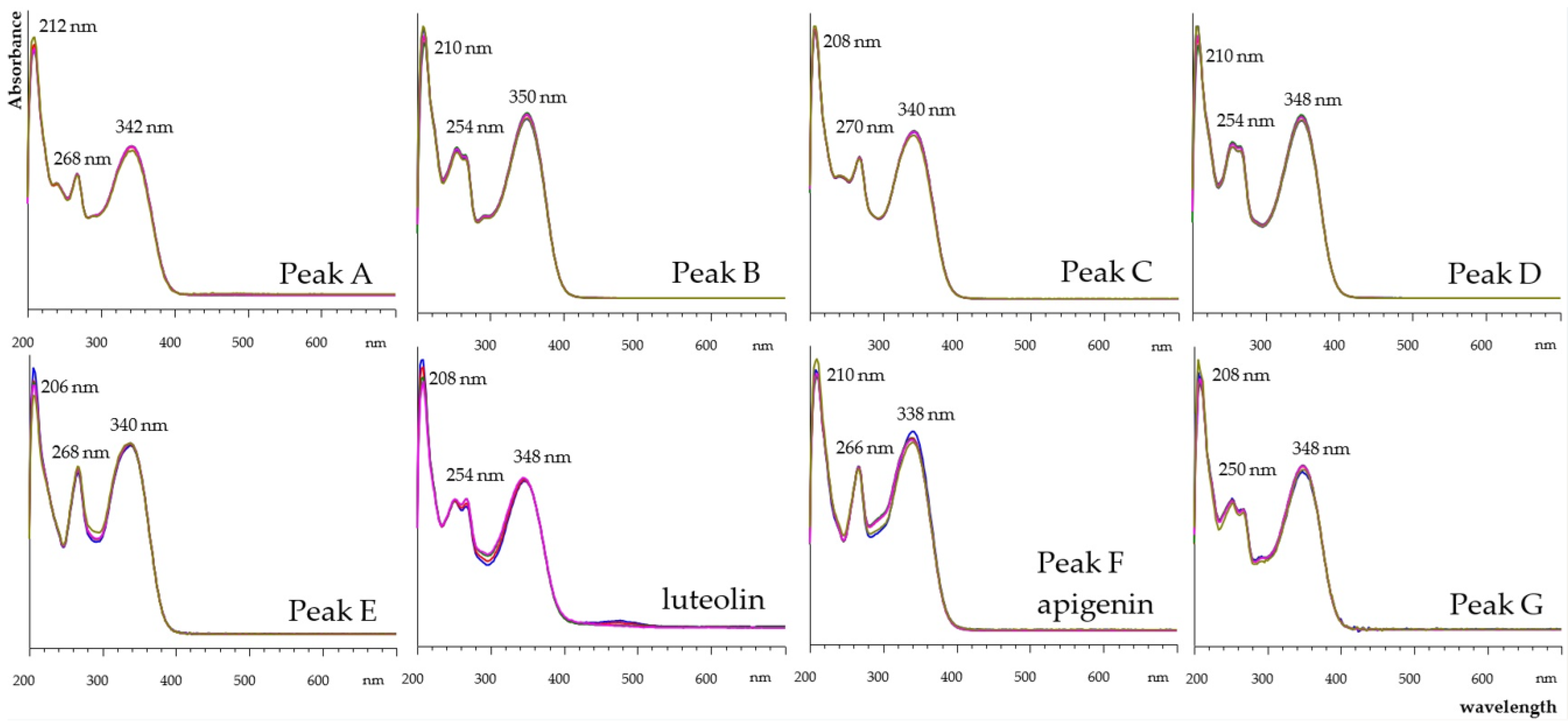
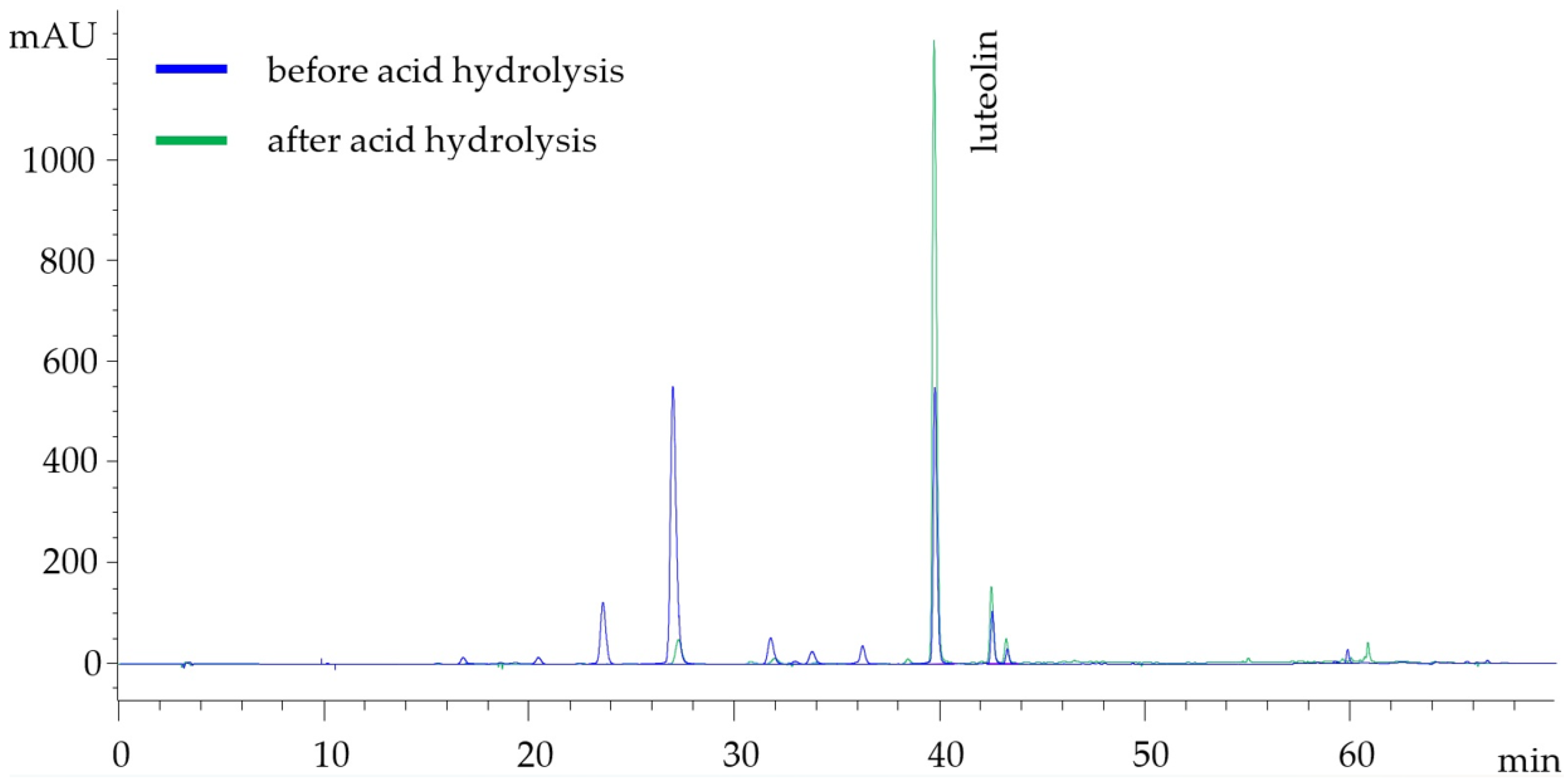

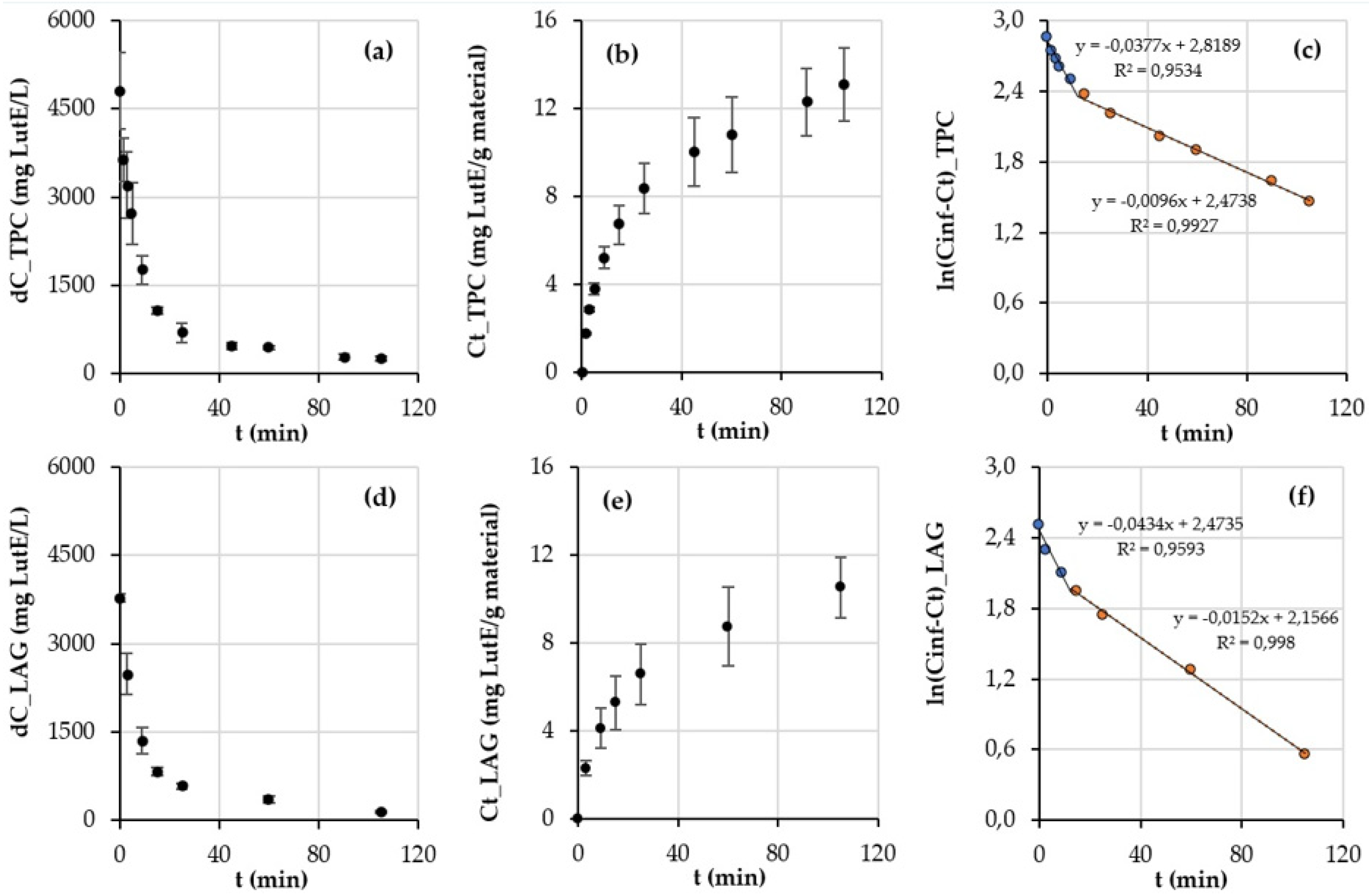
| Time (min) | %A (Water + 0.2% TFA) | %B (Methanol + 0.2% TFA) | %C (Acetonitrile + 0.2% TFA) |
|---|---|---|---|
| 0 | 90 | 6 | 4 |
| 5 | 85 | 9 | 6 |
| 30 | 71 | 17.4 | 11.6 |
| 60 | 0 | 85 | 15 |
| Parameters Measured | Reseda luteola Methanolic Extract (1:30 Solid-to-Liquid Ratio) | Peanut Hull Methanolic Extract (1:30 Solid-to-Liquid Ratio) |
|---|---|---|
| TSY | ||
| (mg solids/g material) | 210 a ± 18 | 137 b ± 37 |
| TPC | ||
| (mg GAE/g material) | 16 a ± 3 | 4.6 b ± 0.6 |
| (mg LutE/g material) | 20 a ± 3 | 5.7 b ± 0.8 |
| AC | ||
| (mg TE/g material) | 29 a ± 4 | 4.6 b ± 0.1 |
| (mg LutE/g material) | 22 a ± 3 | 3.2 b ± 0.1 |
| Selectivity of extraction (Folin) | ||
| (mg GAE/g dried extract) | 77 a ± 12 | 36 b ± 11 |
| (mg LutE/g dried extract) | 98 a ± 21 | 44 b ± 14 |
| Selectivity of extraction (DPPH) | ||
| (mg TE/g dried extract) | 148 a ± 34 | 35 b ± 10 |
| (mg LutE/g dried extract) | 114 a ± 22 | 24 b ± 7 |
| Parameters Measured | Reseda luteola Methanolic Extract (1:30 Solid-to-Liquid Ratio) | Peanut Shell Methanolic Extract (1:30 Solid-to-Liquid Ratio) |
|---|---|---|
| Total Flavonoid Content (mg LutE/g material) | 18 a ± 3 | 1.7 b ± 0.5 |
| Total Luteolin Aglycone and Glycoside Content (LAG) (mg LutE/g material) | 14 a ± 3 | 1.5 b ± 0.5 |
| Selectivity of LAG extraction (HPLC) (mg LutE/g dried extract) | 69 a ± 8 | 25 b ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Episkopou, E.; Tsimogiannis, D.; Giannakourou, M.; Taoukis, P. Kinetics of Luteolin Extraction from Peanut Shells and Reseda luteola for Potential Applications as a Biofunctional Ingredient. Processes 2025, 13, 3009. https://doi.org/10.3390/pr13093009
Episkopou E, Tsimogiannis D, Giannakourou M, Taoukis P. Kinetics of Luteolin Extraction from Peanut Shells and Reseda luteola for Potential Applications as a Biofunctional Ingredient. Processes. 2025; 13(9):3009. https://doi.org/10.3390/pr13093009
Chicago/Turabian StyleEpiskopou, Efstratios, Dimitrios Tsimogiannis, Maria Giannakourou, and Petros Taoukis. 2025. "Kinetics of Luteolin Extraction from Peanut Shells and Reseda luteola for Potential Applications as a Biofunctional Ingredient" Processes 13, no. 9: 3009. https://doi.org/10.3390/pr13093009
APA StyleEpiskopou, E., Tsimogiannis, D., Giannakourou, M., & Taoukis, P. (2025). Kinetics of Luteolin Extraction from Peanut Shells and Reseda luteola for Potential Applications as a Biofunctional Ingredient. Processes, 13(9), 3009. https://doi.org/10.3390/pr13093009







