Production and Evaluation of Green Soybean (Glycine max L.) Powder Fortified with Encapsulated Crude Procyanidin Extract Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Encapsulated Procyanidin Extract Powder
2.3. Storage Stability Test of Green Soybeans Extract Powder
2.4. Drum Drying
2.5. Spray Drying
2.6. Preparation of Instant Green Soybean Powder Fortified with Encapsulated Procyanidin Extract Powder
2.7. Chemical Analysis
2.7.1. Total Phenolic Contents
2.7.2. Total Flavonoid Contents
2.7.3. Determination of Procyanidin
2.7.4. Water Activity and Moisture Content
2.8. Antioxidant Analysis
2.8.1. DPPH Radical Scavenging Capacity Assay
2.8.2. Ferric-Reducing Antioxidant Power Assay
2.9. Flavonoid Identification and Quantification Through HPLC
2.10. Physical Properties Analysis
2.10.1. Color Determination
2.10.2. Creaming Stability
2.10.3. Water Solubility
2.10.4. Hygroscopicity
2.11. Proximate Composition Analysis of GSP
2.12. Sensory Evaluation
Ethical Guidelines
2.13. Statistical Analysis
3. Results and Discussion
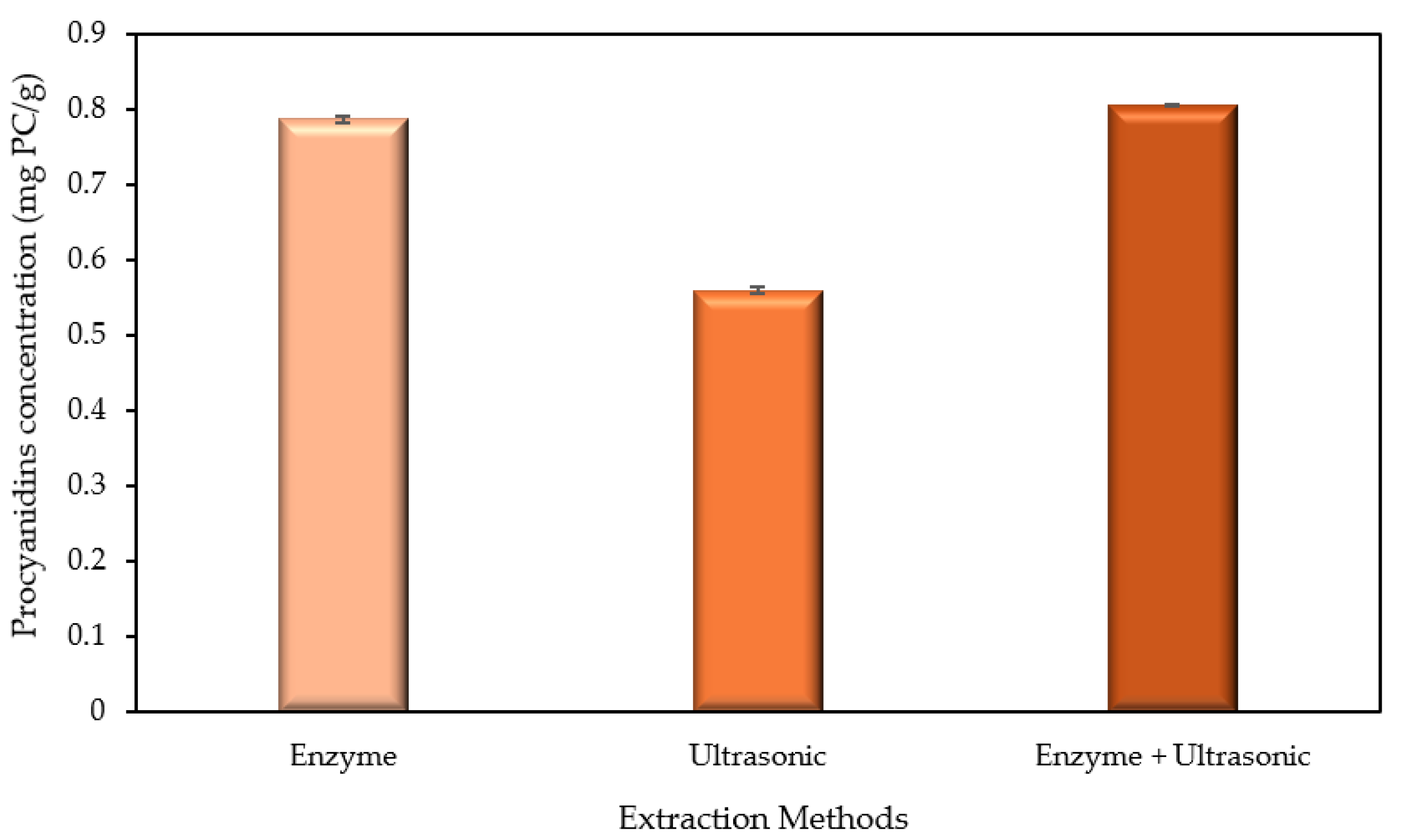
3.1. Extraction of Procyanidins from Green Soybean Seed
3.2. Assessment of Encapsulated Crude Procyanidin Extract During Storage at Different Temperatures and Time
3.3. Effect of Drying Methods on Bioactive Compounds and Antioxidant Activity from GSP
3.4. Development of Antioxidant-Rich Green Soybean Powder Fortified with ECPE
3.5. Physical Properties of GSP After Fortified with ECPE Powder
3.6. Nutritional Composition of GSP Fortified with ECPE Powder
3.7. Sensory Analysis of Fortified GSP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| aw | water activity |
| CAE | catechin equivalents |
| CI | creaming index |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl |
| ECPE | encapsulated crude procyanidin extract |
| EUAE | enzymatic hydrolysis followed by ultrasonic-assisted extraction |
| FRAP | ferric-reducing antioxidant power |
| GAE | gallic acid equivalents |
| GSP | green soybean powder |
| HPLC | high-performance liquid chromatography |
| PC | procyanidin |
| SD | standard deviation |
| TFC | total flavonoid content |
| TPC | total phenolic content |
| TPTZ | 2,4,6-Tris(2-pyridyl)-1,3,5-triazine |
| UAE | ultrasonic-assisted extraction |
| UV | ultraviolet radiation |
References
- Islam, M.; Zhang, M.; Fan, D. Ultrasonically Enhanced Low-Temperature Microwave-Assisted Vacuum Frying of Edamame: Effects on Dehydration Kinetics and Improved Quality Attributes. Dry. Technol. 2019, 37, 2087–2104. [Google Scholar] [CrossRef]
- Nurkistin, D.; Tamtomo, D.G.; Wiboworini, B. Hypolipidemic Effects of Modified Edamame Tempeh Flour on Lipid Profile Levels in Dyslipidemia Rats. Amerta Nutr. 2022, 6, 422–431. [Google Scholar] [CrossRef]
- An, N.-N.; Sun, W.-H.; Li, B.-Z.; Wang, Y.; Shang, N.; Lv, W.-Q.; Li, D.; Wang, L.-J. Effect of Different Drying Techniques on Drying Kinetics, Nutritional Components, Antioxidant Capacity, Physical Properties and Microstructure of Edamame. Food Chem. 2022, 373, 131412. [Google Scholar] [CrossRef] [PubMed]
- Galaz, P.; Valdenegro, M.; Ramírez, C.; Nuñez, H.; Almonacid, S.; Simpson, R. Effect of Drum Drying Temperature on Drying Kinetic and Polyphenol Contents in Pomegranate Peel. J. Food Eng. 2017, 208, 19–27. [Google Scholar] [CrossRef]
- Bordón, M.G.; Alasino, N.P.X.; Villanueva-Lazo, Á.; Carrera-Sánchez, C.; Pedroche-Jiménez, J.; Millán-Linares, M.d.C.; Ribotta, P.D.; Martínez, M.L. Scale-Up and Optimization of the Spray Drying Conditions for the Development of Functional Microparticles Based on Chia Oil. Food Bioprod. Process. 2021, 130, 48–67. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Appeldoorn, M.M. Dietary A-and B-Type Procyanidins Characterization and Biofunctional Potential of an Abundant and Diverse Group of Phenolics. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Leksawasdi, N.; Taesuwan, S.; Prommajak, T.; Techapun, C.; Khonchaisri, R.; Sittilop, N.; Halee, A.; Jantanasakulwong, K.; Phongthai, S.; Nunta, R.; et al. Ultrasonic Extraction of Bioactive Compounds from Green Soybean Pods and Application in Green Soybean Milk Antioxidants Fortification. Foods 2022, 11, 588. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.R.; Botrel, D.A.; Fernandes, R.V.D.B.; Borges, S.V. Encapsulation as a Tool for Bioprocessing of Functional Foods. Curr. Opin. Food Sci. 2017, 13, 31–37. [Google Scholar] [CrossRef]
- Vázquez-Núñez, M.d.l.Á.; Aguilar-Zárate, M.; Gómez-García, R.; Reyes-Luna, C.; Aguilar-Zárate, P.; Michel, M.R. The Specific Encapsulation of Procyanidins from Litchi Peel and Coffee Pulp Extracts via Spray-Drying Using Green Polymers. Polymers 2023, 15, 3823. [Google Scholar] [CrossRef]
- Poozesh, S.; Bilgili, E. Scale-Up of Pharmaceutical Spray Drying Using Scale-Up Rules: A Review. Int. J. Pharm. 2019, 562, 271–292. [Google Scholar] [CrossRef]
- Khonchaisri, R.; Sumonsiri, N.; Prommajak, T.; Rachtanapun, P.; Leksawasdi, N.; Techapun, C.; Taesuwan, S.; Halee, A.; Nunta, R.; Khemacheewakul, J. Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract. Foods 2022, 11, 1775. [Google Scholar] [CrossRef]
- Murkovic, M. Phenolic Compounds: Occurrence, Classes, and Analysis. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 346–351. [Google Scholar] [CrossRef]
- Pohntadavit, K.; Duangmano, S.; Osiriphan, M.; Leksawasdi, N.; Techapun, C.; Sumonsiri, N.; Sommano, S.R.; Rachtanapun, P.; Nunta, R.; Khemacheewakul, J. Tyrosinase Inhibitory Activity of Crude Procyanidin Extract from Green Soybean Seed and the Stability of Bioactive Compounds in an Anti-Aging Skin Care Formulation. Cosmetics 2024, 11, 178. [Google Scholar] [CrossRef]
- Bano, S.; Sommano, S.R.; Leksawasdi, N.; Taesuwan, S.; Rachtanapun, P.; Techapun, C.; Sumonsiri, N.; Khemacheewakul, J. Innovative Cold Plasma Pretreatment and Enzyme-Assisted Extraction of Genistein from Edamame and Storage Stability of Dried Extract Powder. Foods 2025, 14, 2118. [Google Scholar] [CrossRef]
- Navarro-Flores, M.J.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Ayora-Talavera, T.d.R.; Abud-Archila, M. Spray Drying Encapsulation of a Native Plant Extract Rich in Phenolic Compounds with Combinations of Maltodextrin and Non-Conventional Wall Materials. J. Food Sci. Technol. 2020, 57, 4111. [Google Scholar] [CrossRef] [PubMed]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action. J. AOAC Int. 2018, 101, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid. Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- Li, H.Z.; Tan, Y.L.; Zhang, Z.J.; Xia, Y.Y.; Li, X.J.; Cui, L.X.; Chen, T. Optimization of Ultrasound-Assisted Extraction of Procyanidins from Perilla Seed Hull and Their Antioxidant Activities In Vitro. Food Sci. Technol. 2019, 39, 378–387. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, D.W.; Zhang, Z. Methods for Measuring Water Activity (Aw) of Foods and Its Applications to Moisture Sorption Isotherm Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 1052–1058. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of Antioxidant Capacity of 13 Plant Extracts by Three Different Methods: Cluster Analyses Applied for Selection of the Natural Extracts with Higher Antioxidant Capacity to Replace Synthetic Antioxidant in Lamb Burgers. J. Food Sci. Technol. 2016, 53, 451. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Kumar, S. A Validated High-Performance Liquid Chromatography Method for Determination of Tannin-Related Marker Constituents Gallic Acid, Corilagin, Chebulagic Acid, Ellagic Acid and Chebulinic Acid in Four Terminalia Species from India. J. Chromatogr. Sci. 2015, 53, 625–632. [Google Scholar] [CrossRef]
- Ma, K.K.; Grossmann, L.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional and Physical Properties of Commercial Pulse Proteins Compared to Soy Derived Protein. Future Foods 2022, 6, 100155. [Google Scholar] [CrossRef]
- Plazzotta, S.; Moretton, M.; Calligaris, S.; Manzocco, L. Physical, Chemical, and Techno-Functional Properties of Soy Okara Powders Obtained by High Pressure Homogenization and Alkaline-Acid Recovery. Food Bioprod. Process. 2021, 128, 95–101. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Yuan, Y.; Peng, X.; Zhang, Q.; Zhang, S.; Xie, C.; Zhang, X.; Yan, S.; Xu, J.; et al. Effect of Spray-Drying and Freeze-Drying on the Properties of Soybean Hydrolysates. J. Chem. 2020, 2020, 9201457. [Google Scholar] [CrossRef]
- Chow, Y.N.; Ishak, W.R.W. Effects of Young Corn Ear Addition on Nutritional Composition and Acceptability of Conventional Cake. Mal. J. Nutr. 2014, 20, 93–99. [Google Scholar]
- Zhu, Z.; Li, S.; He, J.; Thirumdas, R.; Montesano, D.; Barba, F.J. Enzyme-Assisted Extraction of Polyphenol from Edible Lotus (Nelumbo nucifera) Rhizome Knot: Ultra-Filtration Performance and HPLC-MS2 Profile. Food Res. Int. 2018, 111, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, Y.; Wu, L.; Weng, L.; Qiu, H.; Zhong, W.; Meng, F. Advances in Extraction Protocols, Degradation Methods, and Bioactivities of Proanthocyanidins. Molecules 2024, 29, 2179. [Google Scholar] [CrossRef]
- Wang, K.Y. Research on the Extraction Technology of Acai Berry Proanthocyanidins and Its Anti-Exercise Fatigue. Act. J. Food Saf. Qual. 2022, 13, 2981. [Google Scholar]
- Pavlović, A.N.; Mrmošanin, J.M.; Krstić, J.N.; Mitić, S.S.; Tošić, S.B.; Mitić, M.N.; Arsić, B.B.; Micić, R.J. Effect of Storage Temperature on the Decay of Catechins and Procyanidins in Dark Chocolate. Czech J. Food Sci. 2017, 35, 360–366. [Google Scholar] [CrossRef]
- Berendsen, R.; Güell, C.; Ferrando, M. A Procyanidin-Rich Extract Encapsulated in Water-in-Oil-in-Water Emulsions Produced by Premix Membrane Emulsification. Food Hydrocoll. 2015, 43, 636–648. [Google Scholar] [CrossRef]
- Senevirathna, S.S.J.; Ramli, N.S.; Azman, E.M.; Juhari, N.H.; Karim, R. Optimization of the Drum Drying Parameters and Citric Acid Level to Produce Purple Sweet Potato (Ipomoea batatas L.) Powder Using Response Surface Methodology. Foods 2021, 10, 1378. [Google Scholar] [CrossRef]
- Suthiluk, P.; Naradisorn, M.; Setha, S. Assessing the Impact of Drum Drying on the Nutritional Properties of Pineapple Pomace-Fortified Crispy Mushroom Sheets. Front. Sustain. Food Syst. 2023, 7, 1253597. [Google Scholar] [CrossRef]
- Chong, S.Y.; Wong, C.W. Effect of Spray Dryer Inlet Temperature and Maltodextrin Concentration on Colour Profile and Total Phenolic Content of Sapodilla (Manilkara zapota) Powder. Int. Food Res. J. 2017, 24, 2543–2548. [Google Scholar]
- Drozłowska, E.; Starowicz, M.; Śmietana, N.; Krupa-Kozak, U.; Łopusiewicz, Ł. Spray-Drying Impact the Physicochemical Properties and Formation of Maillard Reaction Products Contributing to Antioxidant Activity of Camelina Press Cake Extract. Antioxidants 2023, 12, 919. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Bao, F.; Zhang, T.; Cheng, Y.; Zhang, W.; Lu, Y. Chemometric Analysis Illuminates the Relationship Among Browning, Polyphenol Degradation, Maillard Reaction and Flavor Variation of 5 Jujube Fruits during Air-Impingement Jet Drying. Food Chem. X 2024, 22, 101425. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Jiang, W.; Su, J.; Yu, F. Recent Advances in Drying Technologies for Orange Products. Foods 2025, 14, 3051. [Google Scholar] [CrossRef]
- Samborska, K.; Jedlińska, A.; Wiktor, A.; Derewiaka, D.; Wołosiak, R.; Matwijczuk, A.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Błażowski, Ł.; et al. The Effect of Low-Temperature Spray Drying with Dehumidified Air on Phenolic Compounds, Antioxidant Activity, and Aroma Compounds of Rapeseed Honey Powders. Food Bioprocess Technol. 2019, 12, 919–932. [Google Scholar] [CrossRef]
- Wijayanti, N.; Widyaningsih, T.D.; Wulan, S.N.; Rifai, M. Influence of Spray Drying Inlet Temperature on the Physical Properties and Antioxidant Activity of Black Garlic Extract Powder. Trends Sci. 2023, 21, 7247. [Google Scholar] [CrossRef]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Saud Alhegaili, A.; Sarwat, M.; Kesari, K.; Kumar, D.; Kumar Jha, N.; Ruokolainen, J. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef]
- Ngamsuk, S.; Huang, T.C.; Hsu, J.L. Determination of Phenolic Compounds, Procyanidins, and Antioxidant Activity in Processed Coffea arabica L. Leaves. Foods 2019, 8, 389. [Google Scholar] [CrossRef]
- Pinthong, S.; Judprasong, K.; Tangsuphoom, N.; Jittinandana, S.; Nakngamanong, Y. Effect of Different Drying Processes on Physical Properties and Carotenoid Content of Gac Fruit (Momordica cochinchinensis Spreng.). J. Food Sci. Agric. Technol. 2019, 5, 61–70. [Google Scholar]
- Millinia, B.L.; Mashithah, D.; Nawatila, R.; Kartini, K. Microencapsulation of Roselle (Hibiscus sabdariffa L.) Anthocyanins: Effects of Maltodextrin and Trehalose Matrix on Selected Physicochemical Properties and Antioxidant Activities of Spray-Dried Powder. Future Foods 2024, 9, 100300. [Google Scholar] [CrossRef]
- Anis, S.; Urfi, S.M.; Kusumastuti, A.; Baskoro, W.W. Analysis of Inlet Temperature and Airflow Rate on Drying Process in a Spray Dryer Using Computational Fluid Dynamics Method. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 94, 163–171. [Google Scholar] [CrossRef]
- Mohammadi, X.; Deng, Y.; Matinfar, G.; Singh, A.; Mandal, R.; Pratap--Singh, A. Impact of Three Different Dehydration Methods on Nutritional Values and Sensory Quality of Dried Broccoli, Oranges, and Carrots. Foods 2020, 9, 1464. [Google Scholar] [CrossRef]
- Ferreira, S.; Piovanni, G.M.O.; Malacrida, C.R.; Nicoletti, V.R. Influence of Emulsification Methods and Spray Drying Parameters on the Microencapsulation of Turmeric Oleoresin. Emir. J. Food Agric. 2019, 31, 491–500. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Łopusiewicz, Ł. Formulation and Evaluation of Spray-Dried Reconstituted Flaxseed Oil-in-Water Emulsions Based on Flaxseed Oil Cake Extract as Emulsifying and Stabilizing Agent. Foods 2021, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Oh, H.J.; Han, S.H.; Lim, S. Bin Effects of Hot Air and Freeze Drying Methods on Physicochemical Properties of Citrus “Hallabong” Powders. Food Sci. Biotechnol. 2012, 21, 1633–1639. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Jannah, H.N.; Indrianingsih, A.W.; Anggoro, D.D. Phycocyanin Encapsulation in Carboxymethyl Chitosan and Whey Protein Isolate as a Strategy to Enhance Physicochemical Stability as a Food Coloring: A Review. Int. J. Chem. Biochem. Sci. (IJCBS) 2024, 25, 656–668. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.d.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Čulina, P.; Zorić, Z.; Garofulić, I.E.; Repajić, M.; Dragović-Uzelac, V.; Pedisić, S. Optimization of the Spray-Drying Encapsulation of Sea Buckthorn Berry Oil. Foods 2023, 12, 2448. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, Z.; Xiang, F.; Liu, Y.; Yan, J.; Wang, J. Fabrication and Encapsulation of Soy Peptide Nanoparticles Using Ultrasound Followed by Spray Drying. Foods 2024, 13, 3967. [Google Scholar] [CrossRef]
- Singh, P.; Krishnaswamy, K. The Influence of Flavoring Components on the Physicochemical Properties of Spray-Dried High Oleic (HO) and Tofu Line (TL) Soymilk Powder. Front. Food Sci. Technol. 2023, 3, 1070453. [Google Scholar] [CrossRef]
- Taşoyan, İ.C.; Turabi Yolacaner, E. Physical Properties of Some Soy Powders and Functional and Sensory Properties of Milk Chocolates Prepared with These Powders. Turk. J. Agric.—Food Sci. Technol. 2023, 11, 246–257. [Google Scholar] [CrossRef]
- Rahman Mazumder, M.A.; Ranganathan, T.V. Encapsulation of Isoflavone with Milk, Maltodextrin and Gum Acacia Improves Its Stability. Curr. Res. Food Sci. 2020, 2, 77. [Google Scholar] [CrossRef] [PubMed]

| Temperature (°C) | Weeks | Procyanidins (mg PC/g) | % Retention |
|---|---|---|---|
| 0 (control) | 0.92 ± 0.01 a | 100 | |
| 2 | 0.90 ± 0.01 a | 97.8 ± 0.87 a | |
| 25 | 4 | 0.90 ± 0.01 a | 97.6 ± 0.08 a |
| 6 | 0.81 ± 0.01 b | 88.3 ± 0.43 b | |
| 8 | 0.77 ± 0.01 c | 83.9 ± 0.16 b | |
| 0 (control) | 0.92 ± 0.01 a | 100 | |
| 2 | 0.92 ± 0.01 a | 99.8 ± 0.10 a | |
| 35 | 4 | 0.81 ± 0.01 b | 87.9 ± 1.46 b |
| 6 | 0.77 ± 0.01 c | 83.9 ± 0.53 b | |
| 8 | 0.78 ± 0.01 bc | 84.3 ± 0.14 b | |
| 0 (control) | 0.92 ± 0.01 a | 100 | |
| 2 | 0.73 ± 0.01 c | 78.2 ± 1.31 c | |
| 45 | 4 | 0.64 ± 0.01 d | 69.1 ± 0.70 d |
| 6 | 0.61 ± 0.01 d | 66.9 ± 0.65 d | |
| 8 | 0.65 ± 0.01 d | 70.5 ± 0.44 cd |
| Bioactive Compounds | Drying Process | |||
|---|---|---|---|---|
| Drum-Drying | Spray-Drying | |||
| 2 rpm | 3 rpm | 180 °C | 200 °C | |
| TPC (mg GAE/g) | 0.81 ± 0.01 b | 0.95 ± 0.02 a | 0.32 ± 0.01 b | 0.66 ± 0.01 a |
| TFC (mg CAE/g) | 0.31 ± 0.02 a | 0.32 ± 0.01 a | 0.15 ± 0.01 b | 0.17 ± 0.01 a |
| Procyanidins (mg PC/g) | 0.21 ± 0.01 b | 0.43 ± 0.01 a | 0.14 ± 0.01 b | 0.28 ± 0.01 a |
| DPPH (µM Trolox eq/g) | 1412 ± 8.31 b | 1477 ± 7.80 a | 341.1 ± 4.87 b | 565.5 ± 8.71 a |
| FRAP (µM Trolox eq/g) | 1617 ± 24.9 b | 1902 ± 2.26 a | 1064 ± 4.67 b | 1248 ± 11.5 a |
| Drying Process | Compounds | Concentration Level of Procyanidins Fortification in GSP (%) | |||
|---|---|---|---|---|---|
| 0 (Control) | 1 | 3 | 5 | ||
| Drum-drying | TPC (mg GAE/g) | 0.95 ± 0.02 c | 2.62 ± 0.01 b | 2.69 ± 0.02 a | 2.71 ± 0.05 a |
| TFC (mg CAE/g) | 0.27 ± 0.01 c | 0.33 ± 0.01 b | 0.34 ± 0.012 a | 0.35 ± 0.01 a | |
| Procyanidins (mg PC/g) | 0.42 ± 0.01 c | 0.54 ± 0.01 b | 0.62 ± 0.01 a | 0.63 ± 0.01 a | |
| Quercetin (mg/g) | 0.04 ± 0.01 c | 0.05 ± 0.01 b | 0.07 ± 0.01 a | 0.07 ± 0.01 a | |
| Rutin (mg/g) | 0.03 ± 0.01 c | 0.21 ± 0.01 b | 0.22 ± 0.01 a | 0.22 ± 0.01 a | |
| Kaempferol (mg/g) | 0.04 ± 0.01 c | 0.08 ± 0.01 b | 0.08 ± 0.01 ab | 0.09 ± 0.01 a | |
| DPPH (µM Trolox eq/g) | 1067 ± 2.80 c | 1305 ± 3.51 b | 1325 ± 3.31 a | 1383 ± 6.10 a | |
| FRAP (µM Trolox eq/g) | 1502 ± 1.26 c | 2114 ± 1.20 b | 2129 ± 6.30 ab | 2151 ± 2.56 a | |
| Spray-drying | TPC (mg GAE/g) | 0.46 ± 0.01 c | 0.65 ± 0.01 b | 0.65 ± 0.01 ab | 0.66 ± 0.02 a |
| TFC (mg CAE/g) | 0.13 ± 0.01 c | 0.21 ± 0.01 b | 0.21 ± 0.01 ab | 0.23 ± 0.01 a | |
| Procyanidins (mg PC/g) | 0.13 ± 0.01 c | 0.14 ± 0.01 b | 0.14 ± 0.01 a | 0.15 ± 0.01 a | |
| Quercetin (mg/g) | 0.02 ± 0.01 c | 0.03 ± 0.01 b | 0.03 ± 0.01 b | 0.03 ± 0.01 a | |
| Rutin (mg/g) | 0.02 ± 0.01 c | 0.11 ± 0.01 b | 0.12 ± 0.01 ab | 0.13 ± 0.01 a | |
| Kaempferol (mg/g) | 0.07 ± 0.01 c | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.08 ± 0.01 a | |
| DPPH (µM Trolox eq/g) | 165.48 ± 3.21 c | 299.2 ± 4.83 b | 387.6 ± 1.55 ab | 417.5 ± 3.23 a | |
| FRAP (µM Trolox eq/g) | 1216 ± 19.5 c | 1345 ± 10.8 b | 1387 ± 5.02 b | 1438 ± 5.93 a | |
| Drying Process | Compounds | Concentration Level of Procyanidins Fortification in GSP (%) | |||
|---|---|---|---|---|---|
| 0 (Control) | 1 | 3 | 5 | ||
| Drum-drying | L | 74.0 ± 0.21 b | 74.1 ± 0.24 b | 74.8 ± 0.09 ab | 75.2 ± 0.48 a |
| a* | −9.81 ± 0.11 a | −9.76 ± 0.07 a | −9.65 ± 0.06 a | −9.80 ± 0.15 a | |
| b* | 32.5 ± 0.22 a | 32.3 ± 0.26 a | 31.6 ± 0.16 a | 31.8 ± 0.66 a | |
| aw | 0.22 ± 0.01 a | 0.22 ± 0.01 a | 0.22 ± 0.01 a | 0.22 ± 0.01 a | |
| Moisture content (%) | 3.25 ± 0.12 a | 3.28 ± 0.14 a | 3.43 ± 0.21 a | 3.44 ± 1.20 a | |
| Creaming index (%) | 66.2 ± 0.23 a | 66.0 ± 0.23 a | 64.3 ± 0.37 b | 62.6 ± 2.75 c | |
| Water solubility index (%) | 2.02 ± 0.31 a | 2.03 ± 0.31 a | 2.02 ± 0.36 a | 2.05 ± 0.08 a | |
| Hygroscopicity (%) | 6.41 ± 1.65 d | 7.21 ± 1.45 c | 8.46 ± 1.70 b | 9.09 ± 1.84 a | |
| Spray-drying | L | 91.1 ± 0.22 a | 91.4 ± 0.15 a | 91.2 ± 0.14 a | 91.6 ± 0.11 a |
| a* | −5.81 ± 0.03 b | −5.92 ± 0.05 ab | −6.02 ± 0.21 a | −6.00 ± 0.09 a | |
| b* | 14.8 ± 0.10 a | 14.9 ± 0.14 a | 15.1 ± 0.63 a | 15.1 ± 0.24 a | |
| aw | 0.17 ± 0.04 a | 0.16 ± 0.07 a | 0.14 ± 0.03 b | 0.15 ± 0.02 ab | |
| Moisture content (%) | 2.19 ± 0.06 a | 2.49 ± 0.02 a | 2.54 ± 0.28 a | 2.98 ± 0.29 a | |
| Creaming index (%) | 59.4 ± 0.28 a | 57.4 ± 0.38 b | 54.4 ± 3.26 b | 53.7 ± 4.88 b | |
| Water solubility index (%) | 6.67 ± 0.88 b | 6.67 ± 0.17 b | 7.95 ± 1.11 ab | 8.71 ± 0.03 a | |
| Hygroscopicity (%) | 10.3 ± 0.47 b | 11.3 ± 0.67 b | 13.2 ± 0.26 a | 13.5 ± 1.67 a | |
| Nutritional Value (%) | Drying Method | |
|---|---|---|
| Drum-Drying | Spray-Drying | |
| Carbohydrate | 20.0 ± 0.03 a | 12.5 ± 0.01 b |
| Protein | 36.9 ± 0.02 a | 35.8 ± 0.02 a |
| Fat | 18.4 ± 0.01 a | 15.5 ± 0.02 b |
| Fiber | 14.5 ± 0.01 a | 11.7 ± 0.01 b |
| Ash | 4.26 ± 0.01 a | 2.23 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosonphong, S.; Leksawasdi, N.; Sommano, S.R.; Techapun, C.; Rachtanapun, P.; Sumonsiri, N.; Khemacheewakul, J. Production and Evaluation of Green Soybean (Glycine max L.) Powder Fortified with Encapsulated Crude Procyanidin Extract Powder. Processes 2025, 13, 2955. https://doi.org/10.3390/pr13092955
Kosonphong S, Leksawasdi N, Sommano SR, Techapun C, Rachtanapun P, Sumonsiri N, Khemacheewakul J. Production and Evaluation of Green Soybean (Glycine max L.) Powder Fortified with Encapsulated Crude Procyanidin Extract Powder. Processes. 2025; 13(9):2955. https://doi.org/10.3390/pr13092955
Chicago/Turabian StyleKosonphong, Saritanot, Noppol Leksawasdi, Sarana Rose Sommano, Charin Techapun, Pornchai Rachtanapun, Nutsuda Sumonsiri, and Julaluk Khemacheewakul. 2025. "Production and Evaluation of Green Soybean (Glycine max L.) Powder Fortified with Encapsulated Crude Procyanidin Extract Powder" Processes 13, no. 9: 2955. https://doi.org/10.3390/pr13092955
APA StyleKosonphong, S., Leksawasdi, N., Sommano, S. R., Techapun, C., Rachtanapun, P., Sumonsiri, N., & Khemacheewakul, J. (2025). Production and Evaluation of Green Soybean (Glycine max L.) Powder Fortified with Encapsulated Crude Procyanidin Extract Powder. Processes, 13(9), 2955. https://doi.org/10.3390/pr13092955











