1. Introduction
Burn injuries represent a significant occupational hazard across multiple industries, with textiles often acting as a primary vector for flame propagation due to their inherent flammability. Natural fibers such as cotton, widely used for clothing and protective apparel, are particularly susceptible to ignition and rapid combustion, posing substantial risks to workers and environments exposed to heat or flame sources [
1]. To mitigate these hazards, flame-retardant (FR) treatments have been extensively developed to improve the fire-resistance of textiles, increasing both safety and material longevity in high-risk applications [
2,
3,
4].
Conventional flame retardants applied to textiles include halogenated compounds, phosphorus-based additives, and ammonium salts, which act by promoting char formation, releasing inert gases, or interrupting the combustion chain reaction [
5]. While effective, these compounds have raised environmental and health concerns due to their potential toxicity, persistence in ecosystems, and release of hazardous byproducts during degradation or incineration [
6]. This has led to growing interest in the development of more sustainable, non-toxic, and environmentally benign flame-retardant strategies that align with circular economy principles and regulatory demands for safer materials [
7].
One promising approach involves the functionalization of textile surfaces through the incorporation of biopolymers and naturally derived materials that provide fire resistance while minimizing ecological impact. Layer-by-layer (LbL) assembly has emerged as a particularly versatile and efficient method for textile functionalization, allowing for the controlled deposition of alternating layers of cationic and anionic species at the nanoscale [
8,
9]. This technique facilitates the integration of a wide range of functional agents and enables the precise tailoring of the surface chemistry and properties of fibers without substantially compromising their mechanical performance or handle [
10].
Among the natural and renewable materials explored for flame retardancy, eggshell waste has attracted attention due to its high content of calcium carbonate, a mineral known for its thermal stability and char-promoting behavior during combustion. Eggshell is an abundant agro-industrial byproduct, predominantly composed of calcium carbonate (~94%) and minor fractions of organic matter and magnesium carbonate [
11]. Its use as an additive in flame retardant formulations offers a sustainable alternative to mined carbonate sources and contributes to waste valorization. Studies have demonstrated the ability of calcium carbonate to act as a heat sink and form a protective barrier on the surface of polymeric substrates, thereby reducing thermal degradation and slowing flame propagation.
In parallel, biopolymers such as chitosan have been widely investigated for textile applications due to their inherent film-forming ability, biodegradability, and capability to interact chemically with cellulosic fibers. Chitosan can serve as a cationic matrix in LbL processes, enabling the immobilization of inorganic particles and additional functional agents such as silanes and phosphorus compounds [
8,
12]. Citric acid is often employed to enhance chitosan solubility and facilitate its crosslinking on the fiber surface, further improving durability and performance. The addition of silane coupling agents, such as (3-aminopropyl) triethoxysilane (APTES), can increase interfacial adhesion and anchoring of inorganic particles to the cellulose matrix, whereas phytic acid, a phosphorus-rich natural compound, contributes synergistically by promoting char formation through intumescence [
13,
14].
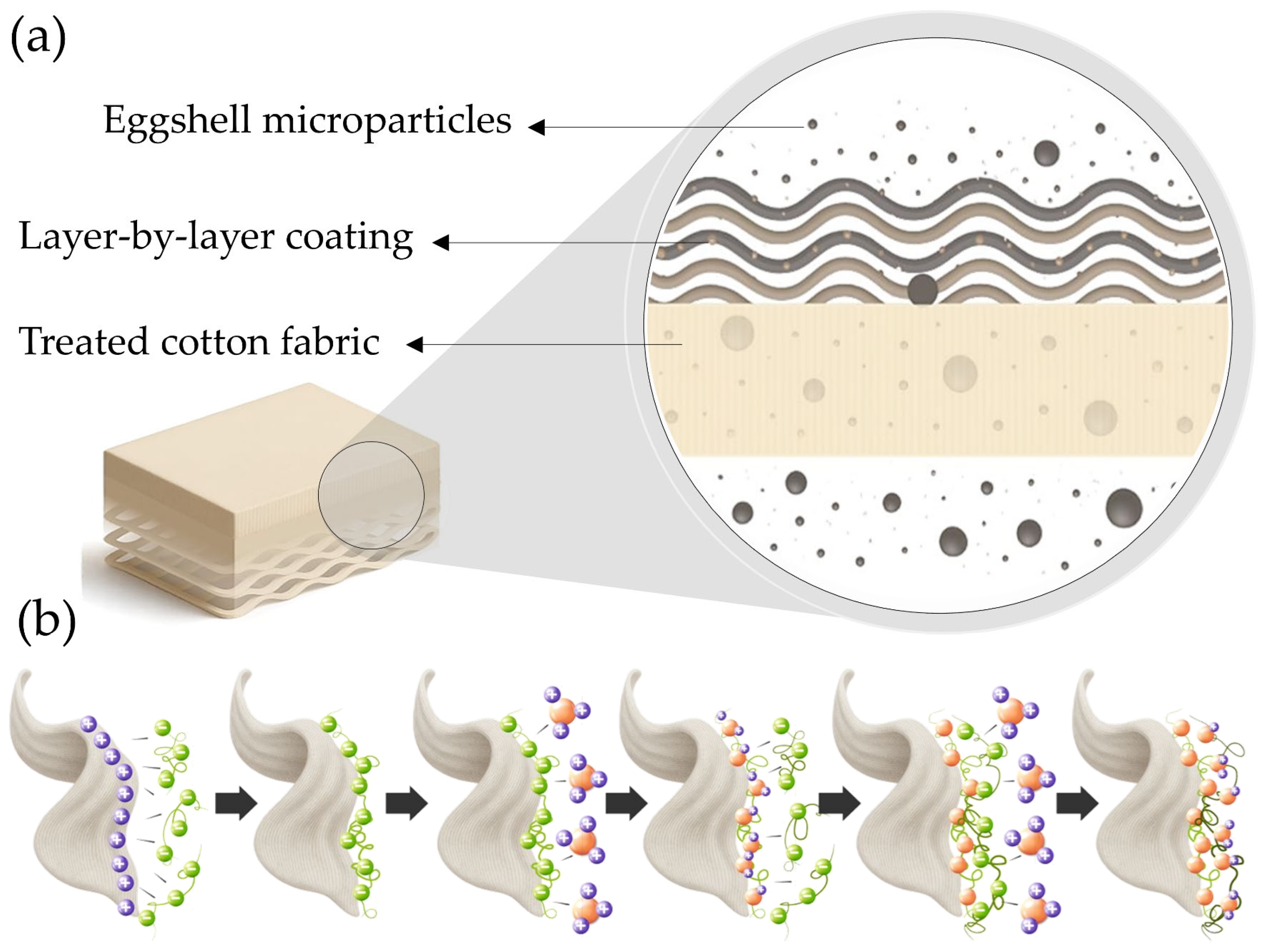
This study explores the combined use of these sustainable agents—eggshell microparticles, chitosan, APTES, urea, and phytic acid—applied via LbL deposition to impart flame-retardant functionality to cotton fabrics (see
Figure 1a). The cotton substrate, owing to its high cellulose content and hydrophilic character, provides a reactive surface conducive to chemical modification. The choice of eggshell microparticles as a filler material aligns with the principles of resource efficiency and waste valorization, offering a renewable and low-cost alternative to synthetic fillers [
15]. Furthermore, the multilayer architecture of the LbL (see
Figure 1b) approach enables fine-tuning of the deposition process to achieve optimal performance with minimal impact on fabric aesthetics and mechanical properties.
In this work, cotton fabrics were pre-treated with urea to enhance the accessibility of hydroxyl groups and then functionalized with layer-by-layer assemblies incorporating chitosan solutions containing 2% or 4% eggshell microparticles, alternated with APTES and phytic acid layers. Up to two LbL cycles were applied to assess the effect of increasing deposition on flame retardancy and overall fabric performance. The treated fabrics were evaluated by flame resistance testing according to NFPA 701 [
16], tensile strength measurements, thermogravimetric analysis (TGA), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and colorimetric analysis using a Datacolor spectrophotometer.
The development of sustainable flame-retardant finishes based on renewable and non-toxic materials represents a critical step toward safer and more environmentally responsible textile products. By leveraging abundant agro-industrial waste streams and green chemistry principles, the present study demonstrates a feasible pathway for producing functional cotton fabrics that meet stringent safety standards while supporting the transition to a circular textile economy. The findings contribute to the growing body of knowledge on eco-friendly textile functionalization, providing insights into material selection, process optimization, and performance characterization of sustainable flame-retardant systems.
2. Materials and Methods
2.1. Materials
Commercially available pre-bleached 100% cotton fabrics (Blumenau, SC, Brazil) were used, cut into samples measuring 30 × 7.6 cm for the vertical flame resistance tests, and into appropriate sizes for the other analyses. Eggshell microparticles were obtained from local commercial waste. The chemical reagents employed included chitosan (Sigma-Aldrich, Duque de Caxias, RJ, Brazil), citric acid (Neon, Suzano, SP, Brazil), APTES (Sigma-Aldrich, Duque de Caxias, RJ, Brazil), and phytic acid (Sigma-Aldrich, Duque de Caxias, RJ, Brazil). All reagents were used as received without further purification. Distilled water was used to prepare all solutions.
2.2. Preparation of Eggshell Microparticles
Eggshells were initially cleaned using distilled water to remove organic residues and then calcinated in a muffle furnace (SPLabor, Presidente Prudente, SP, Brazil) at 500 °C (±50 °C) for 1 h to ensure complete decomposition of residual organic matter. Subsequently, the shells were washed with distilled water, dried in an oven (Thermo Fisher Scientific, Waltham, MA, USA) at 120 °C for 1 h, and ground using a bench-top planetary ball mill (Matoli, Carapicuiba, SP, Brazil) with porcelain jars and balls to obtain a fine powder. The resulting particles were sieved through a 45 µm mesh, and only particles smaller than 45 µm were used in the experiments.
2.3. Fabric Pre-Treatment
The cotton samples were initially treated with urea to promote the formation of carbamyl groups (-CONH2) on the substrate (pre-carbamylation). Each fabric sample (approximately 15 g) was placed in stainless-steel jars of a lab beaker dyeing machine (HT IR Dyer Texcontrol 2200, São Paulo, SP, Brazil), operating by exhaustion, containing an aqueous urea solution (1 g/L) with a liquor ratio of 1:10. The treatment was performed at 135 °C, with a heating rate of 3 °C·min−1, for 60 min. After treatment, the samples were squeezed in a laboratory padder foulard (fvh-B-350, Mathis, São Paulo, SP, Brazil) (1.5 bar, 2 m/min) to remove excess solution and subsequently dried in an oven at 90 °C for 10 min.
2.4. Layer-by-Layer Deposition
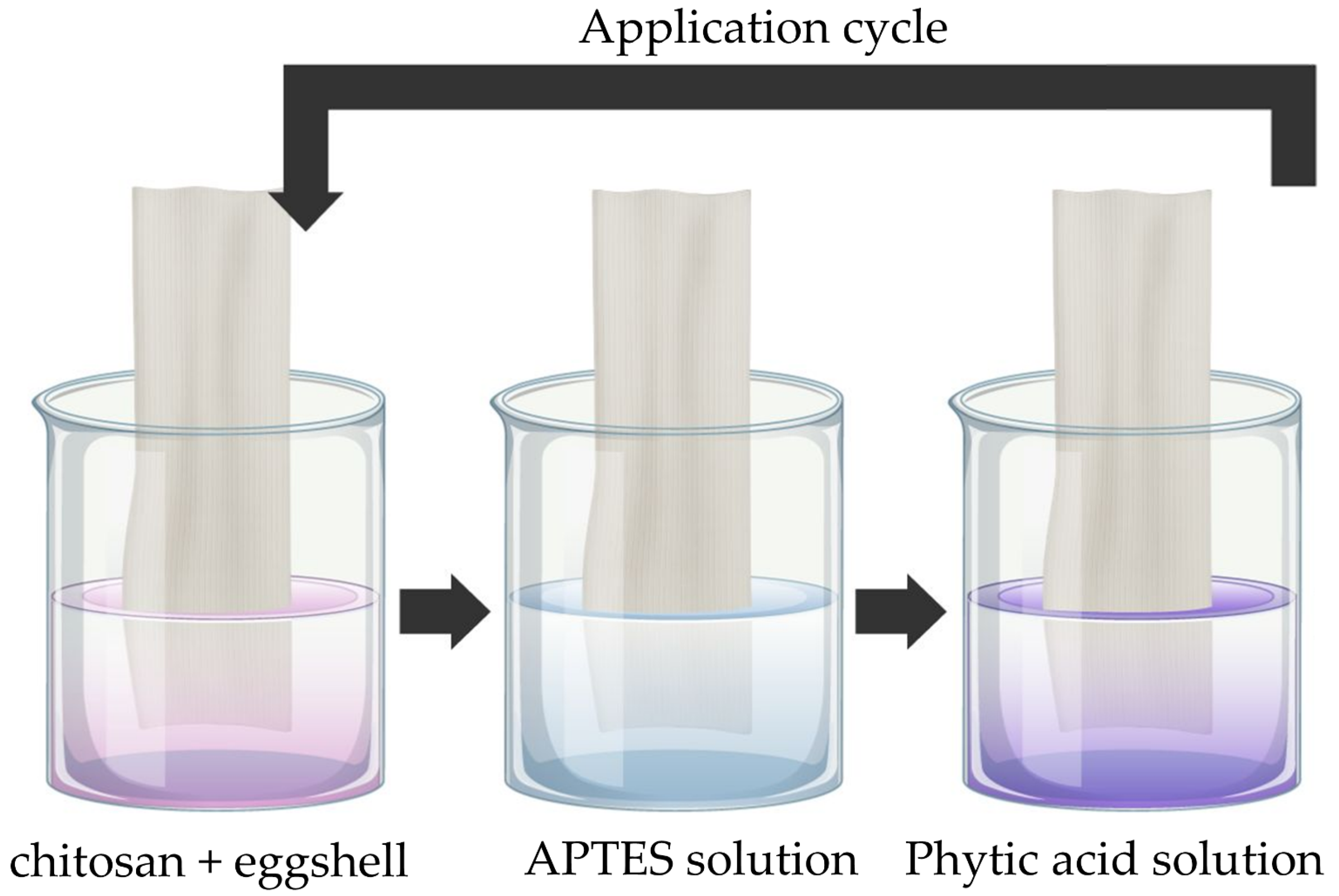
The functionalization process was carried out using the LbL deposition technique (see
Figure 2). Initially, a solution was prepared by dissolving chitosan at 2% (
w/
v) in 10% (
w/
v) citric acid, kept under magnetic stirring for 12 h to ensure complete solubilization and pH adjustment to approximately 5. Eggshell microparticles were then added at final concentrations of 2% and 4% (
w/
v), followed by homogenization.
The pre-treated and dried cotton samples were immersed in the chitosan/eggshell solution for 5 min under gentle stirring, squeezed in the foulard (1.5 bar, 2 m/min), and dried in an oven at 100 °C for 5 min. Next, the samples were immersed for 5 min in an aqueous solution of APTES at 0.1% (w/v), squeezed in the pad-foulard, and dried under the same conditions (100 °C for 5 min). Finally, the samples were immersed in a 10% (v/v) phytic acid solution for 5 min, squeezed, and dried using a convection oven. This sequence constituted one deposition cycle, which was repeated to obtain samples with one or two cycles.
To facilitate the discussion of the results, the reference samples, i.e., untreated (original pre-bleached fabric), were designated as S-REF; the fabric samples treated with 2% eggshell microparticles with 1 and 2 application cycles were designated as S2%-I and S2%-II, respectively; and the fabric samples treated with 4% eggshell microparticles with 1 and 2 application cycles were designated as S4%-I and S4%-II, respectively.
3. Characterization
The flame resistance was evaluated according to ASTM D6413-2008 [
17] (vertical flame test). The treated samples (30 cm × 7.6 cm) were fixed on a metallic template positioned vertically inside a fume hood. Each sample was exposed to the flame of a Bunsen burner for 12 s at a distance of 1 cm, with the flame height adjusted to approximately 4 cm. The after-flame time and char length were measured after flame removal.
The thermal behavior of the samples was analyzed by TGA using a Jupiter STA-449 F3 instrument (Netzsch, Pomerode, SC, Brazil). The samples were heated from room temperature to 800 °C at a rate of 10 °C/min under a synthetic air atmosphere, with a flow rate of 20 mL/min. This analysis allowed for the quantification of thermal stability and mass loss of treated and untreated samples.
The mechanical properties of the samples were evaluated according to ISO 13934-1:2016 using a 3400 Series Universal Testing Machine (Instron, São José dos Pinhais, PR, Brazil). Each sample measured 50 × 100 mm, with a thickness of 0.77 mm, and was tested at a speed of 100 mm/min, with one clamp positioned 30 mm from the lower edge and the other clamp 30 mm from the upper edge.
The surface chemical characterization was performed by FTIR using a Frontier FTIR spectrometer (PerkinElmer, Waltham, MA, USA), acquiring spectra in the range of 4000–400 cm−1 with a resolution of 2 cm−1 to identify the functional groups incorporated.
The morphology of the treated and untreated samples was characterized by SEM using a JSM-6701F microscope (Jeol, Tokyo, Japan) equipped with a NanoTrace™ EDS X-ray detector (Thermo Scientific, Waltham, MA, USA) for elemental analysis.
The color changes of the samples after treatment were quantified by visible spectrophotometry using a Datacolor®500 (Datacolor, Lucerne, Switzerland), recording the CIE Lab* coordinates and the color strength index (K/S).
4. Results
4.1. Mass Gain of the Treated Samples
After each treatment condition, the samples were properly weighed, and the average mass gain percentage relative to their initial mass was calculated. The results presented in
Table 1 show that all treated samples exhibited an increase in mass compared with the reference sample (S-REF), as expected, due to the layer-by-layer functionalization process. This mass gain results from the successive deposition of reagents—chitosan, eggshell microparticles, APTES, and phytic acid—onto the surface of the cotton fibers.
It can be observed that the samples treated with 2% eggshell microparticles (S2%-I and S2%-II) showed higher mass gains (18.21% and 21.74%, respectively) than those treated with 4% microparticles (S4%-I and S4%-II), which exhibited gains of 14.52% and 15.81%, respectively. Furthermore, increasing the number of deposition cycles (from I to II) resulted in additional mass gain for both concentrations. This trend can be interpreted in light of two concurrent phenomena. First, repeating the LbL cycle naturally leads to incremental material deposition on the substrate, justifying the observed increase from cycle I to cycle II in both concentrations. Second, the lower incorporation in samples treated with 4% microparticles may be explained by surface saturation or agglomeration of microparticles in the higher-concentration solution. Such agglomeration can reduce the homogeneity of deposition and hinder effective penetration of the particles into the internal structure of the fibers, resulting in a relatively lower mass gain compared with the 2% samples. Therefore, the results suggest that the condition with 2% microparticles combined with two deposition cycles constitutes the most efficient configuration for uniform incorporation of the functionalization agents, possibly due to better dispersion of the microparticles and greater accessibility to fiber surfaces, without significant saturation or agglomeration.
4.2. Vertical Burning Tests of Untreated and Treated Cotton Fabric
The results of the flammability tests revealed marked differences between the untreated samples (S-REF) and those treated with 2% and 4% eggshell powder (2%ES and 4%ES) in one and two application cycles, as shown in
Table 2 and the post-test images of the specimens. The S-REF samples exhibited total carbonization, being entirely consumed by the flame. The prolonged afterflame time (65–70 s) following flame removal demonstrates that the material continued burning, failing to meet the NFPA 701 standard criteria (see
Video S1a,b). This behavior confirms the high flammability of raw cotton and the absence of any flame-retardant effect.
The 2%ES-treated samples showed substantially improved performance. In both application cycles (S2%-I and S2%-II), the flame exposure time was limited to 12 s, and no afterflame propagation was observed, meeting the NFPA 701 self-extinguishing requirement. The mass loss ranged between 0.10 g and 0.38 g, and the char length varied from 12.00 to 14.00 cm, all within the 16.5 cm limit established by the standard (
Video S1a,b).
The 2%ES treatment not only prevented flame propagation, but also maintained controlled carbonization, indicating the formation of an effective protective barrier (see
Figure 3), consistent with the TGA/dTGA results that demonstrated higher char yield for these samples.
The 4%ES-treated samples also exhibited self-extinguishing behavior after 12 s of exposure, confirming the effectiveness of the treatment in suppressing combustion. However, the char lengths were higher in some cases. The S4%-I sample reached 17 cm, exceeding the NFPA 701 maximum limit of 16.5 cm, whereas S4%-II showed values between 13 and 16 cm, close to the threshold (see
Figure 4). The mass loss was slightly higher (0.32–0.45 g), suggesting that the higher concentration of inorganic particles increased the extent of carbonization, possibly due to the formation of a thicker layer that retains heat and prolongs pyrolysis, even while reducing flame spread [
18].
Overall, all treatments significantly reduced the fabric’s flammability compared with that of untreated cotton, meeting the NFPA 701 self-extinguishing requirement. However, the best overall performance in the vertical flammability test was achieved by the S2%-I and S2%-II samples, which exhibited controlled carbonization and lower mass loss, balancing flame-retardant efficiency with structural preservation. These findings are consistent with the thermal analysis results, where the 2% eggshell powder system with two application cycles demonstrated greater thermal stability, reinforcing its suitability as a flame-retardant formulation for cotton fabrics.
4.3. TGA-DTG Analysis
The TGA of the treated and untreated cotton fabric samples allowed for the evaluation of the thermal behavior and the potential flame-retardant effect of the applied coatings (see
Figure 5a). The curve corresponding to the S-REF sample, composed of pure cotton, exhibited the typical cellulose profile, with an initial weight loss between 30 and 120 °C, attributed to the evaporation of absorbed moisture [
1]. The primary decomposition stage occurred between 280 and 380 °C (~365 °C, see
Figure 5b), with a sharp mass loss resulting from the thermal degradation of the cellulosic backbone and the formation of combustible volatile compounds. After this stage, the residual mass was practically negligible, indicating complete combustion of the material and reflecting the high flammability of untreated cotton.
In contrast, the curves of the samples treated with chitosan, phytic acid, APTES, and different concentrations of eggshell powder (2% and 4%, with one or two application cycles) revealed significant changes. In the 30–120 °C range, all treated samples exhibited slightly higher weight loss, indicative of greater moisture retention due to the presence of hydrophilic groups (–OH, –NH
2, and –POH) introduced by the coating components. Subsequently, between 280 and 290 °C, an additional degradation stage appeared, absent in the S-REF sample, attributed to the thermal decomposition of the coating constituents such as chitosan and phytic acid. Phytic acid on the surface contributed to phosphorus, which promotes the formation of a protective char layer through catalytic dehydration of chitosan and cellulose [
1,
15,
18]. This stage may also involve the onset of the thermal reaction of calcium carbonate (CaCO
3) from the eggshell powder, which acts as a heat sink and releases CO
2, aiding in the dilution of combustible gases such as O
2 and CO in the gas phase and reinforcing the flame-retardant effect. The shift of the initial degradation peak to a slightly lower temperature in the treated samples allows for a slower subsequent decomposition of cellulose [
1,
7,
11,
18]. This occurs because the prior degradation of surface components forms a carbonaceous char layer that acts as a barrier, protecting the fibers and hindering their combustion. This behavior suggests that the coatings provided improved thermal stability to the substrate by forming a protective layer that limits oxygen feedback to the condensed phase and restricts the release of flammable volatiles into the gas phase, thus retarding combustion.
The main cellulose degradation stage, centered between 280 and 380 °C, showed relevant alterations in the treated samples, with a slight shift in the maximum peak to higher temperatures and a reduction in the mass loss rate. The most remarkable difference among the samples was associated with the final residue observed near 600 °C. While the S-REF sample exhibited negligible residue, the treated samples presented progressively higher values consistent with the eggshell content and the number of application cycles. The S2%-I and S2%-II samples retained approximately 15% final residue, whereas the S4%-I and S4%-II samples exhibited higher values, between 18% and 25%. This increase is attributed to the presence of non-combustible inorganic materials, such as calcium carbonate, and the enhanced char formation promoted by the synergistic action between phytic acid and the other coating components.
Overall, the TGA data indicate that the applied treatments effectively enhanced the thermal performance of the cotton fabric, particularly by reducing the decomposition rate and significantly increasing the char residue. Among the tested conditions, the S2%-II and S4%-II samples, which underwent two applications of the 2% and 4% eggshell powder formulations (resulting in slower degradation), exhibited the best performance, as evidenced by their higher thermal stability and identical final residue at 800 °C. These findings demonstrate that the combination of chitosan, phytic acid, APTES, and mineral particles acts synergistically and efficiently as a flame-retardant system, showing strong potential for applications in technical textiles requiring enhanced thermal behavior.
4.4. Tensile Strength Test
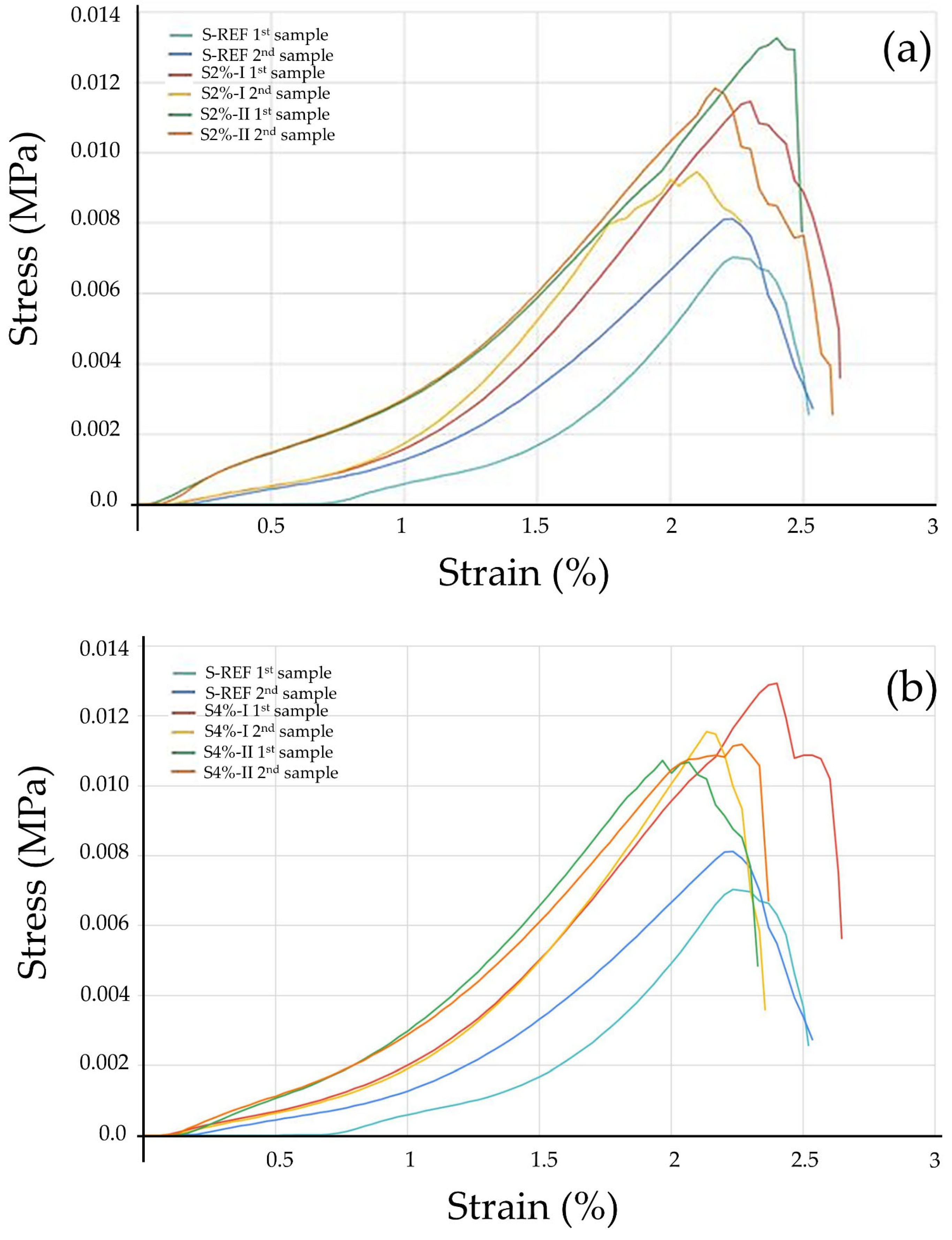
The analysis of the tensile strength tests allowed for the evaluation of the impact of treatments with different concentrations of eggshell powder on the structural properties of the cotton fabric. The untreated samples (S-REF), shown in
Figure 6, exhibited a maximum strain of approximately 2.5% and a breaking stress of around 0.008 MPa. Failure occurred predominantly near the grip fixation points, suggesting a lack of uniform stress distribution and highlighting the vulnerability of raw cotton under mechanical load.
The samples treated with 2% eggshell powder (
Figure 7a) displayed a distinct behavior. For the specimens subjected to one application cycle (S2%-I), failure occurred closer to the central region of the fabric, indicating a more uniform stress distribution and a possible improvement in structural cohesion provided by the coating. In contrast, the samples with two application cycles (S2%-II) showed failure points closer to the grips, a behavior similar to that of the untreated fabric, which may have been related to the increased stiffness resulting from the higher amount of deposited material. The stress–strain curves (
Figure 8a) confirmed these patterns: the treated samples exhibited higher breaking stress values compared with raw cotton, ranging from 0.010 to 0.012 MPa, with S2%-I standing out as the sample reaching the highest stress before failure. The exception was the duplicate of S2%-I, which showed lower performance, possibly due to variations in coating deposition or heterogeneity in the applied layers.
The samples containing 4% eggshell powder (
Figure 7b) presented different results. The stress–strain curves (
Figure 8b) showed that, although the maximum stress of the S4%-I and S4%-II samples remained at levels similar to those of the 2% samples, a reduction in strain at the breaking point was observed, especially in the samples with two cycles. This behavior suggests that the higher content of inorganic particles produced a thicker and more rigid layer on the fibers, reducing the fabric’s ductility and causing a more abrupt fracture. The images of the test specimens corroborate this interpretation: failures in the 4% eggshell powder samples occurred closer to the grip regions and showed more defined tears, indicating lower elongation prior to rupture.
Overall, the treatment with 2% eggshell powder, particularly with a single application cycle, provided the best balance between mechanical strength and strain capacity, suggesting that a thinner coating layer maintains the structural integrity of cotton while improving fiber cohesion. Increasing the concentration to 4% and applying multiple cycles resulted in a stiffer fabric with lower elongation and more localized failure, which may be advantageous for specific technical applications, but indicates a reduction in ductility compared with the lower-concentration treatments.
4.5. FTIR Analysis
The FTIR analysis (see
Figure 9) for the untreated cotton fabric (sample S-REF) and the fabrics treated with different formulations reveals significant changes in the surface composition of the samples. The S-REF sample exhibits characteristic cellulose bands, particularly the broad band centered around 3330 cm
−1, attributed to hydroxyl groups (O–H) stretching in the cellulose structure. This band becomes more intense and broader in the treated samples, indicating the additional presence of hydroxyl and amino groups derived from chitosan, phytic acid, and hydrolyzed silane groups from APTES. These functional groups may form extensive hydrogen bonding networks and contribute to chemical interactions with the cellulose substrate. In the 2920–2850 cm
−1 region, corresponding to the stretching of aliphatic C–H groups, a slight increase in intensity is observed in the treated samples. This can be attributed to the methylene chains of the propyl group from APTES (–CH
2–CH
2–CH
2–NH
2) and the organic chains from chitosan. This alteration confirms the incorporation of organic components do not present in the reference sample [
4].
In the 1740–1715 cm
−1 region, low-intensity bands in some treated samples suggest the presence of C=O bonds, possibly resulting from small amounts of carboxylic groups in phytic acid or surface oxidation of cellulose during the treatment process. The 1650–1550 cm
−1 region shows marked differences. A band around 1630 cm
−1 is observed in the S-REF sample, mainly attributed to absorbed water or cellulose vibration. In the treated samples, this band becomes more intense and slightly shifted, indicating the contribution of new vibrational modes—especially Amide I (C=O) and Amide II (N–H) from chitosan. The free amino groups from APTES (–NH
2) also contribute to this region, reinforcing the hypothesis of hydrogen bonding and acid–base interactions between the coating components and the substrate [
3].
The 1250–950 cm
−1 region is particularly significant, as it reflects an overlap of bands from cellulose, phytic acid, and siloxane bonds derived from APTES. The increased intensity of bands in this region indicates the formation of P=O and P–O–C bonds from phytic acid, as well as Si–O–Si and Si–O–C linkages, resulting from the condensation of silane groups from APTES with the fabric surface or other components in the formulation. The band centered between 1070 and 1040 cm
−1 can be attributed to Si–O–C bonding with cellulose, evidencing the role of APTES as a coupling agent between organic and inorganic components. Finally, the bands at 875 and 712 cm
−1—absent in the S-REF sample and well-defined in samples treated with eggshell powder (particularly those with higher concentrations or two applications)—are assigned to carbonate stretching of calcium carbonate (CaCO
3), the main mineral component of eggshells. These bands confirm the effective incorporation of eggshell powder into the fabric and, together with the siloxane bands from APTES, suggest efficient fixation of inorganic materials onto the organic matrix, possibly through interactions with chitosan and the phosphorylated groups of phytic acid [
3].
Overall, the FTIR results confirm that the treatments significantly modified the cotton surface, with spectroscopic evidence of all the applied components. The variations observed among the samples with different eggshell powder concentrations (2% and 4%) and different numbers of applications (I and II) suggest that the coating thickness and the intensity of chemical interactions increase with the amount of deposited material. This may directly influence the final properties of the fabric, such as adhesion, functionality, and performance in specific tests, including thermal resistance, antimicrobial activity, or flame retardancy.
4.6. SEM-EDS Analysis
The analysis of the SEM micrographs reveals significant differences between the untreated cotton fabric sample (see
Figure 10a) and the sample treated with the formulation containing 2% eggshell powder in two application cycles (S2%-II) (see
Figure 10b,c).
In the image of the untreated sample, the fabric surface exhibits the typical morphology of cotton fibers, with bundles of smooth and twisted microfibrils without any apparent deposits. The well-defined individualized fiber structure evidences the absence of layers or surface coatings. This morphology is characteristic of unmodified cotton fabrics, whose exposed surface favors both moisture absorption and rapid ignition due to the lack of physical barriers to protect the substrate.
In contrast, the micrograph of the S2%-II sample shows a heterogeneous coating covering the fibers, formed by the deposition of the formulation containing chitosan, phytic acid, APTES, and eggshell powder particles. A continuous layer can be observed partially covering the fibers, along with adhered particles integrated into the surface, suggesting good interaction between the coating and the cellulosic substrate. These particles, likely composed of calcium carbonate, create surface irregularities that may contribute to the formation of a thermal barrier upon heat exposure. Furthermore, overlapping the two application layers partially fills the interfibrillar spaces, producing a thicker and semi-cohesive film. The direct comparison between the images confirms that the treatment with 2% eggshell powder and two application cycles substantially modifies the cotton surface, creating a hybrid organic–inorganic structure. This modification is consistent with the TGA and dTGA results (presented in
Section 4.4), which indicated greater char formation and enhanced flame-retardant behavior for this sample. The deposition visible in the SEM suggests that the combination of coating components acts not only as a physical barrier but also as a support matrix for the chemical reactions that promote the formation of protective char during thermal decomposition.
4.7. Colorimetric Analysis
Table 3 presents the colorimetric analysis results of the samples compared with the untreated cotton fabric (S-REF), used as the reference. In the CIELAB color space, the
L coordinate represents lightness, indicating how light or dark the tone is; the
a coordinate expresses the variation between red (positive values) and green (negative values); and the
b coordinate reflects the variation between yellow (positive) and blue (negative) tones [
19,
20,
21].
All treated samples exhibited lower L values than the reference sample (92.62 and 92.44), ranging from 87.00 to 89.98. This reduction in lightness indicates a darkening of the fabric, associated with the deposition of chitosan, phytic acid, and eggshell powder layers on the fibers, regarding the b coordinate, which measures the yellow hue, a consistent increase was observed in all treated samples (15.27–16.22) compared with the control (11.72–12.26). This behavior suggests that the presence of yellowish components, such as phytic acid, chitosan, and eggshell powder, contributed to the observed chromatic shift. This effect intensified with higher concentrations and multiple application cycles. The a coordinate, associated with the red–green axis, showed positive values in all treated samples (0.39–1.37), in contrast to the slightly negative values of the untreated fabric (−0.37 and −0.29). This indicates a slight shift toward reddish tones induced by the coating, more pronounced in the S2%-I samples, with a value above 1.0. In samples with higher cycle numbers and 4% eggshell powder concentration, the values tended to decrease, suggesting that thicker layers may attenuate the initial reddish hue and enhance the predominance of yellow tones.
Regarding color strength (K/S), all treated samples exhibited substantially higher values compared with the untreated fabric duplicate (104.60), ranging from 204.40 to 241.07. The highest K/S value was observed for the S2%-II duplicate (241.07), followed by the 4%ES two-cycle samples (223.75–224.29). This increase is directly associated with the greater deposition of material on the fabric surface, which enhances absorption and reduces diffuse reflection, intensifying color perception. The correlation between concentration, number of application cycles, and higher K/S values confirms that the applied formulation forms a continuous and visually perceptible layer, with its hue influenced by chitosan and phytic acid. Overall, the results indicate that both the eggshell powder concentration and the number of application cycles significantly affect the fabric’s colorimetric response by reducing lightness, enhancing yellow tones, and increasing color strength, demonstrating the effective deposition of the formulation on the fibers.
5. Discussion
The results demonstrated the effectiveness of combining chitosan, phytic acid, APTES, and eggshell powder as a multifunctional coating for cotton fabrics. FTIR analyses confirmed the deposition of the hybrid layer through the presence of phosphate, amine, and carbonate bands absent in raw cotton, in agreement with previous studies on bio–mineral coatings in textiles [
8,
12,
13].
TGA and dTGA analyses showed a significant improvement in the thermal stability of the treated samples. The initial degradation step (~280–300 °C) and the increase in char residue, especially in S4%-II, indicate intumescent behavior promoted by phytic acid and CaCO
3, consistent with phosphorus–nitrogen systems reported in the literature [
8,
11,
15]. The vertical flammability test confirmed these effects: all treated samples exhibited self-extinguishing behavior within 12 s, with S2%-I and S2%-II showing the lowest char lengths and mass loss. The 4%ES samples, particularly S4%-I, exhibited larger carbonized areas, suggesting that higher mineral content retains heat and prolongs pyrolysis, as observed in other CaCO
3-based systems [
22,
23]
In the mechanical tests, 2%ES with one cycle (S2%-I) exhibited the highest tensile strength (~0.0115 MPa) and better stress distribution, whereas 4%ES and two applications reduced strain at break, increasing stiffness. This behavior aligns with reports on mineral-loaded flame-retardant finishes that enhance thermal stability but decrease ductility. SEM images corroborated these findings, showing a cohesive organic–inorganic layer with embedded CaCO3 particles, consistent with the higher char formation observed in TGA. Colorimetric analysis indicated a decrease in lightness, an increase in yellow hue, and higher K/S values in the treated samples, confirming uniform coating deposition and the influence of chitosan and phytic acid. These results are consistent with those for bio-based flame-retardant finishes, where optical changes indicate coating homogeneity.
The observed agglomeration at the 4% eggshell microparticle concentration suggests limitations in the homogeneity and effectiveness of the coating at higher loads. To overcome this issue, future studies should consider strategies such as the use of eco-friendly dispersants, application of ultrasonic dispersion techniques (or high rpm using ultraturrax), or more controlled particle size reduction processes to improve particle stability in suspension and ensure uniform distribution on the fabric surface. Such improvements may enhance coating uniformity, reduce char length variation, and potentially allow for higher inorganic content without compromising fabric performance.
These findings highlight an inherent trade-off between eggshell microparticle concentration and the physical properties of the fabric. While higher concentrations (4%) can improve flame retardancy and increase final char yield due to the greater presence of inorganic content, they also tend to form thicker and stiffer layers on the fiber surface. This leads to reduced strain at break and increased brittleness, as observed in the tensile tests. In contrast, the 2% concentration, particularly with two deposition cycles, maintained better mechanical flexibility and more uniform stress distribution, while still achieving effective flame-retardant behavior. Future formulation optimization must consider this balance to tailor treatments according to the specific performance requirements of each textile application.
Preliminary washability tests were conducted to assess the durability of the flame-retardant treatment under washing conditions. As expected, the treated fabrics did not maintain their flame-retardant properties after laundering due to the natural origin and weak chemical bonding of the biopolymeric components—such as chitosan and phytic acid—and the absence of conventional crosslinkers. After a single washing cycle, the previously treated samples were subjected to the vertical flammability test and exhibited complete combustion. Although the burning process occurred more slowly than with the untreated cotton, the fabrics no longer met the self-extinguishing criteria defined by the NFPA 701 standard. These results reinforce the intended application indicated in the manuscript title, which emphasizes using this bio-based flame-retardant system for dry-use textiles such as upholstery, decorative coverings, or other non-washable applications where durability against laundering is not required.
Overall, the findings confirm the synergistic effect of the proposed formulation, combining thermal protection, flame retardancy, and structural reinforcement. The use of eggshell powder also highlights the potential of agro-industrial waste in sustainable textile finishing. Future research should focus on optimizing the mineral content and assessing the durability of the coating after washing to enable its industrial application.
6. Conclusions
The treatment with chitosan, phytic acid, APTES, and eggshell powder proved effective in improving the thermal stability and reducing the flammability of cotton. The samples with 2% eggshell powder, especially those with two application cycles, showed the best balance between flame retardancy and preservation of mechanical properties. Despite these promising results, this study presents limitations regarding washing resistance, as the natural components of the formulation do not promote the same type of chemical bonding as halogenated or aldehyde-based compounds, which are typically more durable but toxic and polluting. However, the samples remained stable over time, with no signs of staining or microbial growth, making this approach suitable for further development and for application in upholstery and covering textiles that are not intended for domestic washing.
Author Contributions
Conceptualization, C.R.S.d.O. and R.F.d.S.B.; methodology, C.R.S.d.O.; validation, C.R.S.d.O., C.L.d.A. and T.M.C.; formal analysis, E.C.N., T.M.C., and M.J.G.; investigation, C.R.S.d.O., R.F.d.S.B., and A.H.d.S.J.; resources, C.R.S.d.O., E.C.N., and T.M.C.; data curation, C.R.S.d.O., C.L.d.A., and R.S.; writing—original draft preparation, C.R.S.d.O. and R.F.d.S.B.; writing—review and editing, C.R.S.d.O.; visualization, T.C.N., R.S., and M.E.P.M.; supervision, C.R.S.d.O. and C.L.d.A.; project administration, C.R.S.d.O.; funding acquisition, C.R.S.d.O. and A.H.d.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/
Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Federal University of Santa Catarina (UFSC), the Regional University of Blumenau (FURB), and the UniSENAI University Center for providing access to their laboratories and facilities, which were essential for carrying out the experiments and developing this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Price, D.; Horrocks, A.R. Combustion Processes of Textile Fibres. In Handbook of Fire Resistant Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–25. [Google Scholar]
- Zhang, X.; Huang, G.; Wang, G. Preparation of Phosphorus/Hollow Silica Microsphere Modified Polyacrylonitrile-Based Carbon Fiber Composites and Their Thermal Insulation and Flame Retardant Properties. Processes 2024, 12, 2489. [Google Scholar] [CrossRef]
- de Oliveira, C.R.S.; Batistella, M.A.; Guelli Ulson de Souza, S.M.D.A.; Ulson de Souza, A.A. Functionalization of Cellulosic Fibers with a Kaolinite-TiO2 Nano-Hybrid Composite via a Solvothermal Process for Flame Retardant Applications. Carbohydr. Polym. 2021, 266, 118108. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.R.S.; Batistella, M.A.; Lourenço, L.A.; de Souza, S.M.D.A.G.U.; de Souza, A.A.U. Cotton Fabric Finishing Based on Phosphate/Clay Mineral by Direct-Coating Technique and Its Influence on the Thermal Stability of the Fibers. Prog. Org. Coat. 2021, 150, 105949. [Google Scholar] [CrossRef]
- Haase, J. Flame Resistant Clothing Standards and Regulations. In Handbook of Fire Resistant Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 364–414. [Google Scholar]
- Hirschler, M.M. Safety, Health and Environmental Aspects of Flame Retardants. In Handbook of Fire Resistant Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 108–173. [Google Scholar]
- Joseph, P.; Tretsiakova-McNally, S. Chemical Modification of Natural and Synthetic Textile Fibres to Improve Flame Retardancy. In Handbook of Fire Resistant Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 37–67. [Google Scholar]
- Liu, Y.; Wang, Q.-Q.; Jiang, Z.-M.; Zhang, C.-J.; Li, Z.-F.; Chen, H.-Q.; Zhu, P. Effect of Chitosan on the Fire Retardancy and Thermal Degradation Properties of Coated Cotton Fabrics with Sodium Phytate and APTES by LBL Assembly. J. Anal. Appl. Pyrolysis 2018, 135, 289–298. [Google Scholar] [CrossRef]
- Li, Z.-F.; Zhang, C.-J.; Cui, L.; Zhu, P.; Yan, C.; Liu, Y. Fire Retardant and Thermal Degradation Properties of Cotton Fabrics Based on APTES and Sodium Phytate through Layer-by-Layer Assembly. J. Anal. Appl. Pyrolysis 2017, 123, 216–223. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, W.; Li, J.; Liu, H.; Liu, X. Eco-Friendly Flame Retardant and Dripping-Resistant of Polyester/Cotton Blend Fabrics through Layer-by-Layer Assembly Fully Bio-Based Chitosan/Phytic Acid Coating. Int. J. Biol. Macromol. 2021, 175, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.S.; Kamil, F.H.; Hasso, A.A.; Abduljawaad, A.N.; Saleh, T.F.; Mahmood, S.K. Calcium Carbonate Nanoparticles of Quail’s Egg Shells: Synthesis and Characterizations. J. Mech. Behav. Mater. 2022, 31, 1–7. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Liu, Y.-Y.; Xu, Y.-J.; Jiang, Z.-M.; Dong, C.-H.; Zhang, L.; Liu, Y.; Zhu, P. Fully Bio-Based Coating from Chitosan and Phytate for Fire-Safety and Antibacterial Cotton Fabrics. Carbohydr. Polym. 2020, 237, 116173. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Q. Flame Resistant Cotton. In Handbook of Fire Resistant Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 177–220. [Google Scholar]
- Taib, M.N.A.M.; Yee, T.S.; Trache, D.; Hazwan Hussin, M. Modification on Nanocellulose Extracted from Kenaf (Hibiscus Cannabinus) with 3-Aminopropyltriethoxysilane for Thermal Stability in Poly (Vinyl Alcohol) Thin Film Composites. Cellulose 2024, 31, 997–1015. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Guan, J.-P.; Yang, X.-H.; Tang, R.-C.; Yao, F. A Bio-Resourced Phytic Acid/Chitosan Polyelectrolyte Complex for the Flame Retardant Treatment of Wool Fabric. J. Clean. Prod. 2019, 223, 342–349. [Google Scholar] [CrossRef]
- NFPA 701; Standard Methods of Fire Tests for Flame Propagation of Textiles and Films. National Fire Protection Association (NFPA): Quincy, MA, USA, 2019.
- ASTM D6413-2008; Standard Test Method for Flame Resistance of Textiles (Vertical Test). ASTM International: West Conshohocken, PA, USA, 2008.
- Broughton, R.; Cerkez, I. Burning Mechanisms of Fibers. In Handbook of Fire Resistant Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 26–36. [Google Scholar]
- Chakraborty, J.N. An Overview of Dye Fastness Testing. In Handbook of Textile and Industrial Dyeing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 207–224. [Google Scholar]
- Chattopadhyay, D.P. Chemistry of Dyeing. In Handbook of Textile and Industrial Dyeing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 150–183. [Google Scholar]
- Clark, M. Fundamental Principles of Dyeing. In Handbook of Textile and Industrial Dyeing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–27. [Google Scholar]
- Xu, Z.; Chu, Z.; Yan, L.; Chen, H.; Jia, H.; Tang, W. Effect of Chicken Eggshell on the Flame-retardant and Smoke Suppression Properties of an Epoxy-based Traditional APP-PER-MEL System. Polym. Compos. 2019, 40, 2712–2723. [Google Scholar] [CrossRef]
- Urtekin, G.; Hazer, S.; Aytac, A. Effect of Eggshell and Intumescent Flame Retardant on the Thermal and Mechanical Properties of Plasticised PLA. Plast. Rubber Compos. 2021, 50, 127–136. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).