Promotion Effect and Mechanism Analysis of Different Strain Pre-Treatment on Methane Conversion from Lignite
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Experimental Procedures
2.2.2. Experimental Testing Methods
- (1)
- Gas Composition and Concentration Analysis: The gas composition and concentration were determined using gas chromatography (Agilent7890GC, Agilent Technologies Inc., Santa Clara, CA, USA).
- (2)
- Ultimate Analysis: Ultimate analysis was conducted using a Thermo Scientific FLASH 2000 CHNS/O analyzer (Waltham, MA, USA). Both pre- and post-treatment lignite samples were washed with distilled water, placed in a vacuum-drying oven, and dried at 105 °C for 6 h. Ultimate analysis accorded to the standard (GB/T 31391-2015) [14], and changes in carbon, hydrogen, oxygen, nitrogen, and sulfur content in the treated lignite samples were compared.
- (3)
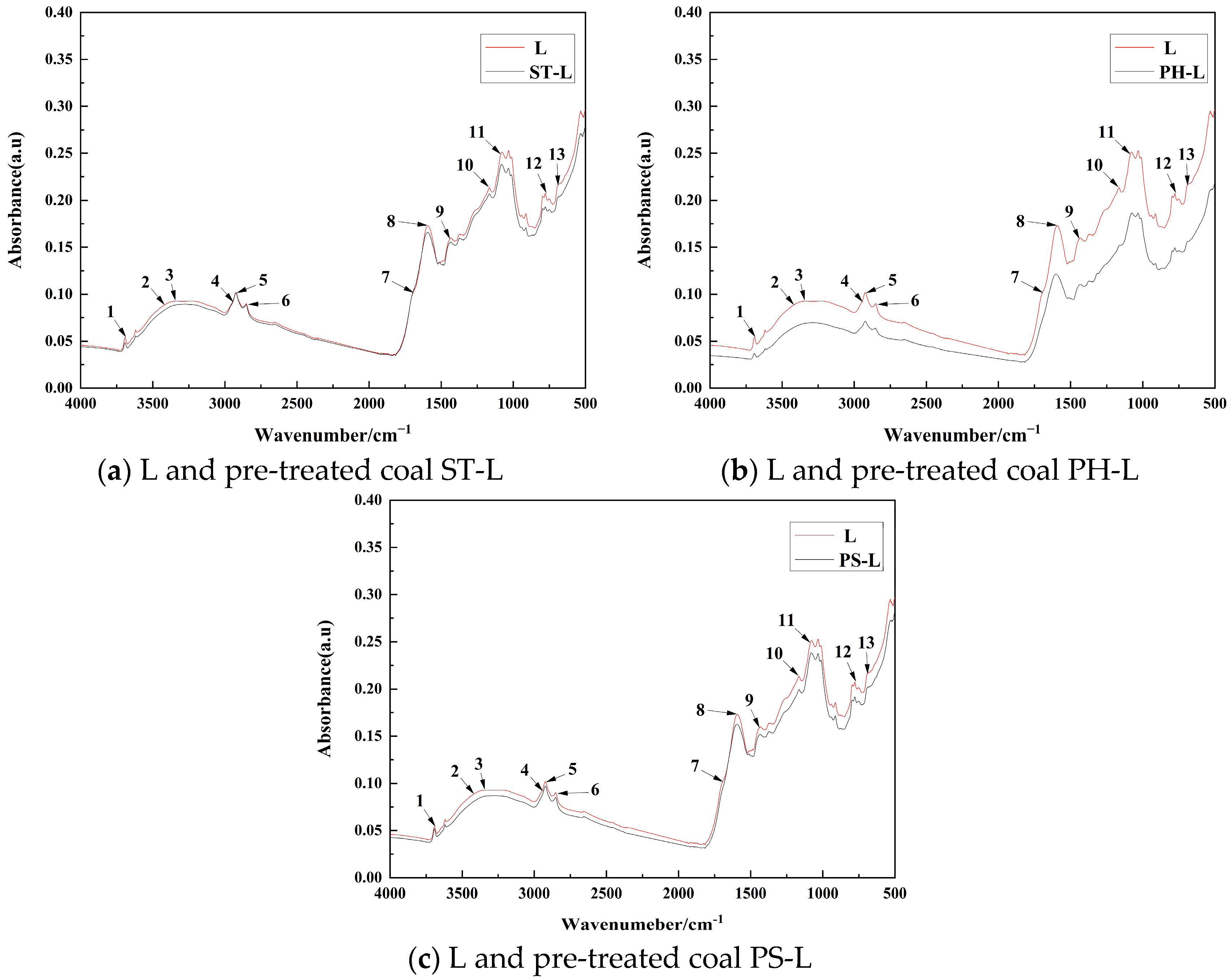
- Analysis of Lignite Functional Groups: The analysis of lignite functional groups was performed using an AVATAR 360 Fourier-transform infrared (FTIR) spectrometer (Nicolet Instrument Corporation, Madison, WI, USA). The scanning parameters included 32 scans, a resolution of 4 cm−1, and a scanning range of 400–4000 cm−1.
- (4)
- Pore Structure Analysis of Lignite: The pore structure of the lignite was evaluated using an AutoPore IV 9505 mercury intrusion porosimeter (Micromeritics Instrument Corporation, Norcross, GA, USA). The accuracy of the mercury volume measurement during both intrusion and extrusion was less than 0.1 μL. The contact angle between the mercury and the sample was 130°, with a surface tension of 0.485 N/m.
- (5)
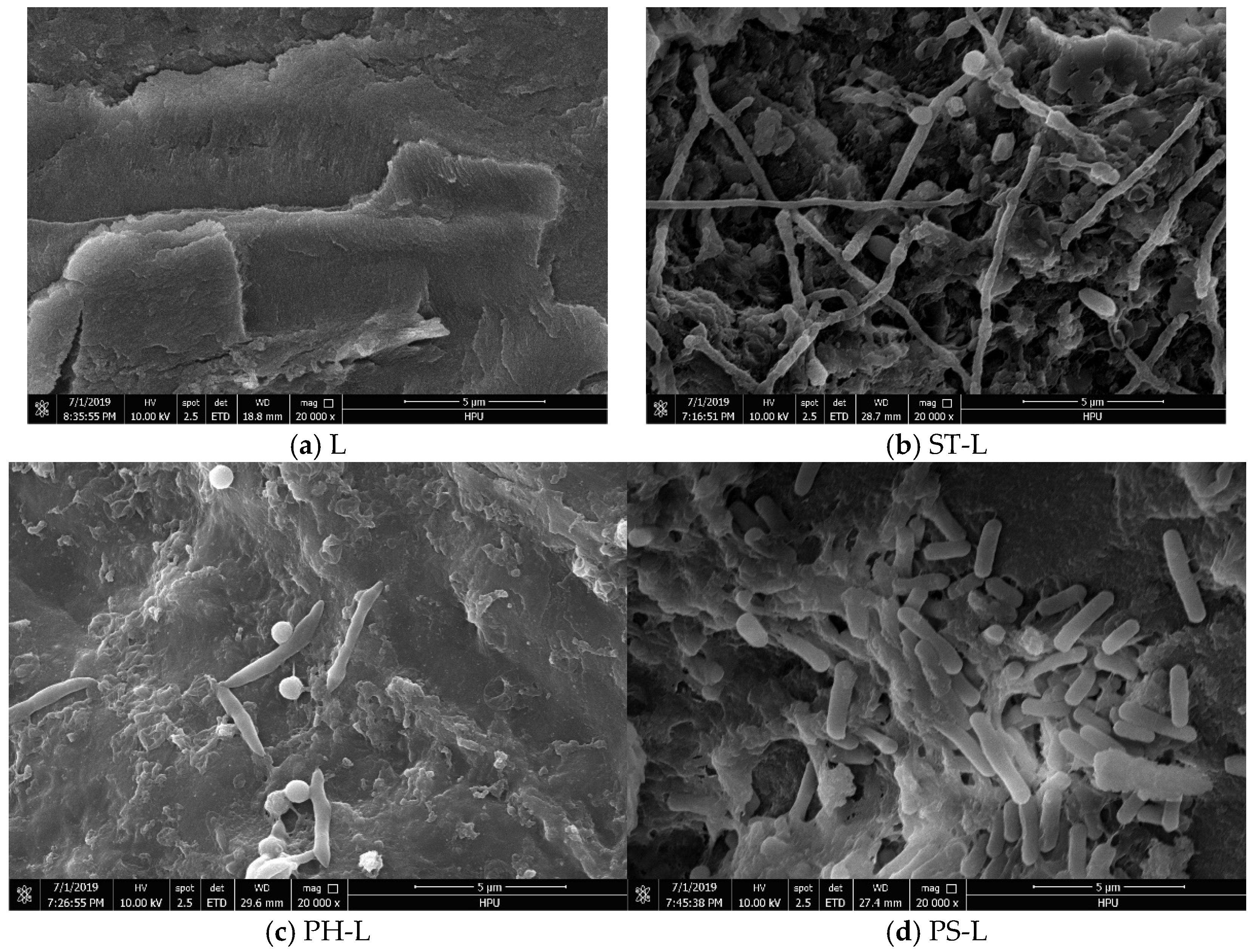
- SEM Analysis: The surface morphology of coal samples was examined using a Scanning Electron Microscope (SEM, QUANTA FEG 250, Thermo Fisher Scientific, Waltham, MA, USA). Coal samples were fixed with 2.5% glutaraldehyde, dehydrated through a graded ethanol series, dried, and gold-coated for SEM observation.
3. Experimental Results and Analysis
3.1. Effect of Different Microbial Pre-Treatment on Lignite Degradation Rate
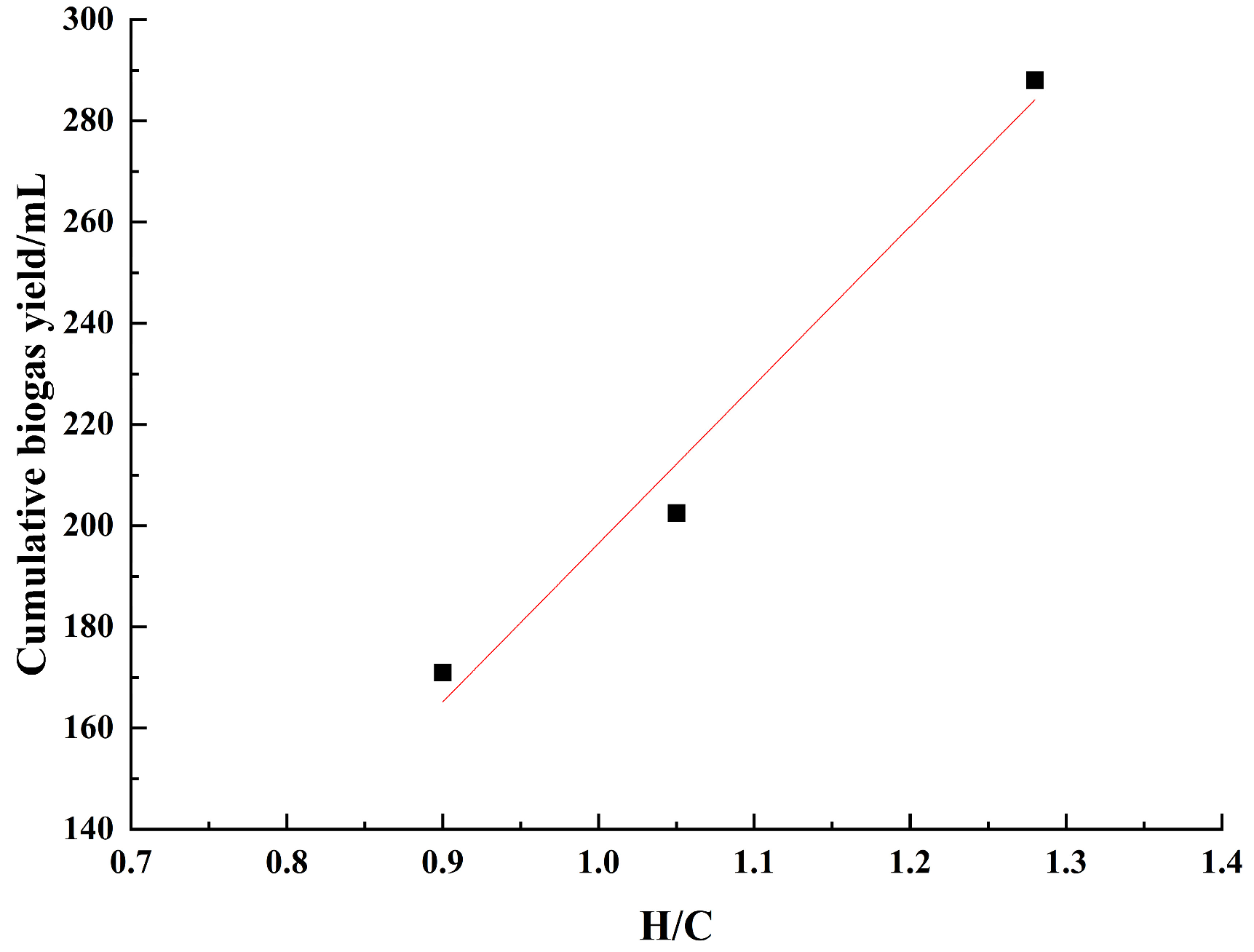
3.2. Ultimate Analysis
3.3. Influence of Lignite Chemical Functional Groups
3.4. Analysis of Lignite Porosity
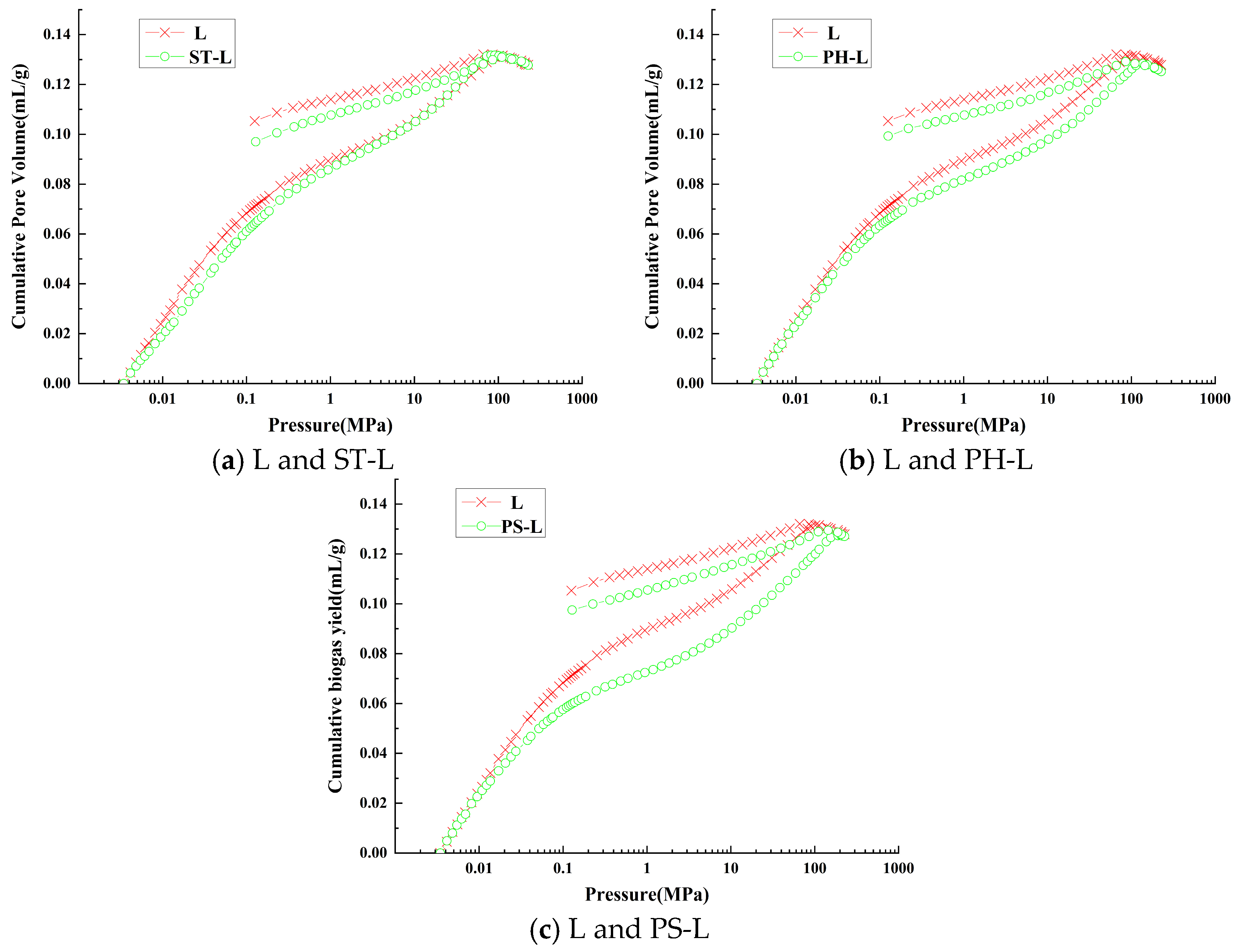
3.4.1. Pore Volume Analysis
3.4.2. Specific Surface Area Analysis
3.4.3. Mercury Intrusion–Extrusion Curve Analysis
3.4.4. SEM Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, Z.; Yu, Y.; Yan, J. Best available techniques assessment for coal gasification to promote cleaner production based on the ELECTRE-II method. J. Clean. Prod. 2016, 129, 12–22. [Google Scholar] [CrossRef]
- Keboletse, K.P.; Ntuli, F.; Oladijo, O.P. Influence of coal properties on coal conversion processes-coal carbonization, carbon fiber production, gasification and liquefaction technologies: A review. Int. J. Coal Sci. Technol. 2021, 8, 817–843. [Google Scholar] [CrossRef]
- Xu, T.; Wu, Y.; Bhattacharya, S. Release of nitrogen and sulphur species from low-rank coal during entrained flow pyrolysis and gasification. J. Anal. Appl. Pyrolysis 2022, 168, 105741. [Google Scholar] [CrossRef]
- Scott, A.R. Improving coal gas recovery with microbially enhanced coalbed methane. In Coalbed Methane: Scientific, Environmental and Economic Evaluation; Springer: Dordrecht, The Netherlands, 1999; pp. 89–110. [Google Scholar]
- Flores, R.M.; Rice, C.A.; Stricker, G.D.; Warden, A.; Ellis, M.S. Methanogenic pathways of coal-bed gas in the Powder River Basin, United States: The geologic factor. Int. J. Coal Geol. 2008, 76, 52–75. [Google Scholar] [CrossRef]
- Vinson, D.S.; Blair, N.E.; Martini, A.M.; Larter, S.; Orem, W.H.; McIntosh, J.C. Microbial methane from in situ biodegradation of coal and shale: A review and reevaluation of hydrogen and carbon isotope signatures. Chem. Geol. 2017, 453, 128–145. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Xie, Y. Degradation of Shanxi lignite by Trichoderma citrinoviride. Fuel 2021, 291, 120204. [Google Scholar] [CrossRef]
- Li, X.-H.; Li, H.-J.; Wang, R.-Q.; Feng, J.; Li, W.-Y. Acid pretreatment effect on oxygen migration during lignite pyrolysis. Fuel 2020, 262, 116650. [Google Scholar] [CrossRef]
- Zhang, D.; He, H.; Ren, Y.; Haider, R.; Urynowicz, M.; Fallgren, P.H.; Jin, S.; Ishtiaq Ali, M.; Jamal, A.; Huang, Z.; et al. A mini review on biotransformation of coal to methane by enhancement of chemical pretreatment. Fuel 2022, 308, 121961. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, G.; Zhu, G.; Zhao, P.; Ma, Z.; Zhang, B. Using microwave pretreatment to improve the high-gradient magnetic-separation desulfurization of pulverized coal before combustion. Fuel 2020, 274, 117826. [Google Scholar] [CrossRef]
- Li, Y.; Tang, S.; Chen, J.; Zhang, S. A review on microbial metabolism to increase coalbed methane generation and coal pretreatment to improve its bioavailability. Front. Earth Sci. 2023, 17, 218–229. [Google Scholar] [CrossRef]
- Xia, D.; Niu, Y.; Tian, J.; Su, X.; Wei, G.; Jian, K.; Wang, Z.; Zhang, Y.; Zhao, W. Degradation metabolic pathway of low-rank coal using single hydrolytic bacteria. Fuel 2024, 364, 130917. [Google Scholar] [CrossRef]
- Dong, Z.; Guo, H.; Zhang, M.; Xia, D.; Yin, X.; Lv, J. Enhancing biomethane yield of coal in anaerobic digestion using iron/copper nanoparticles synthesized from corn straw extract. Fuel 2022, 319, 123664. [Google Scholar] [CrossRef]
- GB/T 31391-2015; Ultimate Analysis of Coal. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China; China Coal Industry Press: Beijing, China, 2015.
- Wang, B.; Yu, Z.; Zhang, Y.; Zhang, H. Microbial communities from the Huaibei Coalfield alter the physicochemical properties of coal in methanogenic bioconversion. Int. J. Coal Geol. 2019, 202, 85–94. [Google Scholar] [CrossRef]
- Zhang, J.; Anderson, K.; Britt, D.; Liang, Y. Sustaining biogenic methane release from Illinois coal in a fermentor for one year. Fuel 2018, 227, 27–34. [Google Scholar] [CrossRef]
- Dong, X.; Wang, F.; Guo, L.; Han, T.; Dong, X.; Hu, X. The inhibitory effect of microbes on coal spontaneous combustion. Fuel 2024, 375, 132658. [Google Scholar] [CrossRef]
- Li, D.; Bao, Y.; Wang, Y.; An, C.; Chang, J. Multiple-experimental investigation on the physicochemical structures alternation during coal biogasification. Fuel 2023, 339, 127433. [Google Scholar] [CrossRef]
- Bao, Y.; Li, Z.; Meng, J.; Chen, X.; Liu, X. Reformation of coal reservoirs by microorganisms and its significance in CBM exploitation. Fuel 2024, 360, 130642. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Zhu, W.; Li, T.; Liu, Z.; Yang, H.; Liu, J. Effects of indigenous microorganisms on the CO2 adsorption capacity of coal. Chem. Eng. J. 2024, 500, 156894. [Google Scholar] [CrossRef]
- Zheng, C.; Li, J.; Xue, S.; Jiang, B.; Liu, B. Experimental study on changes in components and pore characteristics of acidified coal treated by organic solvents. Fuel 2023, 353, 129215. [Google Scholar] [CrossRef]
- Haider, R.; Ghauri, M.A.; Rahim, M.U. On Comparison Between Fungal and Bacterial Pretreatment of Coal for Enhanced Biogenic Methane Generation. Geomicrobiol. J. 2018, 35, 432–437. [Google Scholar] [CrossRef]
- Xia, D.; Lv, H.; Chen, Z.; Chen, H.; Su, P. Bioconversion of coal to Biogas: Insights into the microbial degradation mechanisms and molecular structure transformations. Chem. Eng. Sci. 2025, 312, 121706. [Google Scholar] [CrossRef]
- Li, S.; Xia, D.; Chen, Z.; Zhao, W.; Chen, L.; Lin, J. Experimental study on the change of coal structure and microbial community structure during supercritical-CO2-H2O-microorganisms-coal interaction process. Environ. Technol. Innov. 2023, 30, 103036. [Google Scholar] [CrossRef]
- Guo, H.; Li, Y.; Wang, Q.; Zhao, W.; Jia, J.; Lv, J.; Liu, S.; Xia, D. Feasibility analysis of the in situ conversion of biomethane in surface weathered coal. Fuel 2020, 268, 117273. [Google Scholar] [CrossRef]
- Zhao, W.; Su, X.; Xia, D.; Hou, S.; Wang, Q.; Zhou, Y. Enhanced coalbed methane recovery by the modification of coal reservoir under the supercritical CO2 extraction and anaerobic digestion. Energy 2022, 259, 124914. [Google Scholar] [CrossRef]
| Sample | Ro,ran% | Mad/% | Aad/% | Vad/% | FCad/% | Cdaf% | Hdaf% | Odaf% | Ndaf% | Sdaf% |
|---|---|---|---|---|---|---|---|---|---|---|
| Lignite | 0.43 | 7.46 | 10.71 | 44.58 | 37.25 | 40.43 | 4.40 | 21.47 | 0.40 | 1.34 |
| Strains | Phylum | Class | Order | Family | Genus | Strain Source | Culture Medium |
|---|---|---|---|---|---|---|---|
| Streptomyces viridosporus | Actinobacteriota | Actinomycetes | Actinomycetales | Streptomycetaceae | Streptomyces | CGMCC 4.1770 | 0038 ISP-2 medium |
| Phanerochaete chrysosporium | Basidiomycota | Agaricomycetes | Polyporales | Phanerochaetaceae | White rot fungi | BNCC336257 | Comprehensive potato medium |
| Pseudomonas | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | GSICC 31603 | CM0841 culture medium |
| Sample Number | Sample Information |
|---|---|
| L | Lignite |
| ST-L | Streptomyces viridosporus + Lignite |
| PH-L | Phanerochaete chrysosporium + Lignite |
| PS-L | Pseudomonas + Lignite |
| Sample Number | Total Gas Production/mL | CH4 Concentration/% | Concentration of Other Gas/% | CH4 Generation/mL |
|---|---|---|---|---|
| L | 151.50 | 34.54 | 65.46 | 52.33 |
| ST-L | 202.50 | 35.37 | 64.63 | 71.62 |
| PH-L | 288.00 | 48.20 | 51.80 | 138.82 |
| PS-L | 171.00 | 51.77 | 48.23 | 88.53 |
| Sample Number | C% | H% | O% | N% | S% | H/C Atomic Ratio | O/C Atomic Ratio |
|---|---|---|---|---|---|---|---|
| L | 40.43 | 4.40 | 21.47 | 0.40 | 1.34 | 1.31 | 0.40 |
| ST-L | 58.25 | 5.08 | 20.46 | 0.83 | 0 | 1.05 | 0.26 |
| PH-L | 58.83 | 6.27 | 19.59 | 1.37 | 0 | 1.28 | 0.25 |
| PS-L | 71.80 | 5.41 | 20.02 | 1.40 | 0 | 0.90 | 0.21 |
| Sample Number | Porosity/% | Total Intrusion Volume/mL/g | Total Hole Area/m2/g | Average Pore Size/nm | Starting Pressure/kPa | Discontinuation Pressure/kPa |
|---|---|---|---|---|---|---|
| L | 15.0153 | 0.1316 | 3.378 | 346.0 | 0.689 | 414,300 |
| ST-L | 14.9303 | 0.1314 | 2.986 | 555.1 | 0.689 | 414,300 |
| PH-L | 14.6621 | 0.1284 | 4.879 | 167.7 | 0.689 | 413,400 |
| PS-L | 15.1088 | 0.1271 | 7.088 | 71.7 | 0.689 | 414,300 |
| Sample Number | Macropore cm3·g−1 | Ratio % | Mesopore cm3·g−1 | Ratio % | Small Pores cm3·g−1 | Ratio % | Micropore cm3·g−1 | Ratio % | Total cm3·g−1 |
|---|---|---|---|---|---|---|---|---|---|
| L | 0.0710 | 53.95 | 0.0349 | 26.52 | 0.0257 | 19.53 | 0.0000 | 0.00 | 0.1316 |
| ST-L | 0.0640 | 48.71 | 0.0411 | 31.28 | 0.0263 | 20.02 | 0.0000 | 0.00 | 0.1314 |
| PH-L | 0.0656 | 51.09 | 0.0324 | 25.23 | 0.0296 | 23.05 | 0.0008 | 0.62 | 0.1284 |
| PS-L | 0.0596 | 46.89 | 0.0307 | 24.15 | 0.0315 | 24.78 | 0.0053 | 4.17 | 0.1271 |
| Sample Number | Macropore m2/g | Ratio/% | Mesopore m2/g | Ratio/% | Small Pore m2/g | Ratio/% | Micropore m2/g | Ratio/% | Total |
|---|---|---|---|---|---|---|---|---|---|
| L | 0.038 | 1.12 | 0.310 | 9.18 | 3.030 | 89.70 | 0.000 | 0.00 | 3.378 |
| ST-L | 0.045 | 1.51 | 0.332 | 11.12 | 2.609 | 87.37 | 0.000 | 0.00 | 2.986 |
| PH-L | 0.034 | 0.70 | 0.288 | 5.90 | 4.233 | 86.76 | 0.324 | 6.64 | 4.879 |
| PS-L | 0.030 | 0.42 | 0.338 | 4.77 | 4.471 | 63.08 | 2.249 | 31.73 | 7.088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Z.; Guo, H.; Xu, Q.; Wang, S.; Bai, X.; Zhang, Z.; Yang, H.; Wang, Z.; Ren, S.; et al. Promotion Effect and Mechanism Analysis of Different Strain Pre-Treatment on Methane Conversion from Lignite. Processes 2025, 13, 2581. https://doi.org/10.3390/pr13082581
Li Y, Wang Z, Guo H, Xu Q, Wang S, Bai X, Zhang Z, Yang H, Wang Z, Ren S, et al. Promotion Effect and Mechanism Analysis of Different Strain Pre-Treatment on Methane Conversion from Lignite. Processes. 2025; 13(8):2581. https://doi.org/10.3390/pr13082581
Chicago/Turabian StyleLi, Yongchen, Zebin Wang, Hongyu Guo, Qiang Xu, Shuai Wang, Xiujia Bai, Zhengguang Zhang, Haorui Yang, Zheng Wang, Shan Ren, and et al. 2025. "Promotion Effect and Mechanism Analysis of Different Strain Pre-Treatment on Methane Conversion from Lignite" Processes 13, no. 8: 2581. https://doi.org/10.3390/pr13082581
APA StyleLi, Y., Wang, Z., Guo, H., Xu, Q., Wang, S., Bai, X., Zhang, Z., Yang, H., Wang, Z., Ren, S., Zhao, G., & Zhang, B. (2025). Promotion Effect and Mechanism Analysis of Different Strain Pre-Treatment on Methane Conversion from Lignite. Processes, 13(8), 2581. https://doi.org/10.3390/pr13082581









