Carbon-Based Heterogeneous Catalysis for Biomass Conversion to Levulinic Acid: A Special Focus on the Catalyst
Abstract
1. Introduction
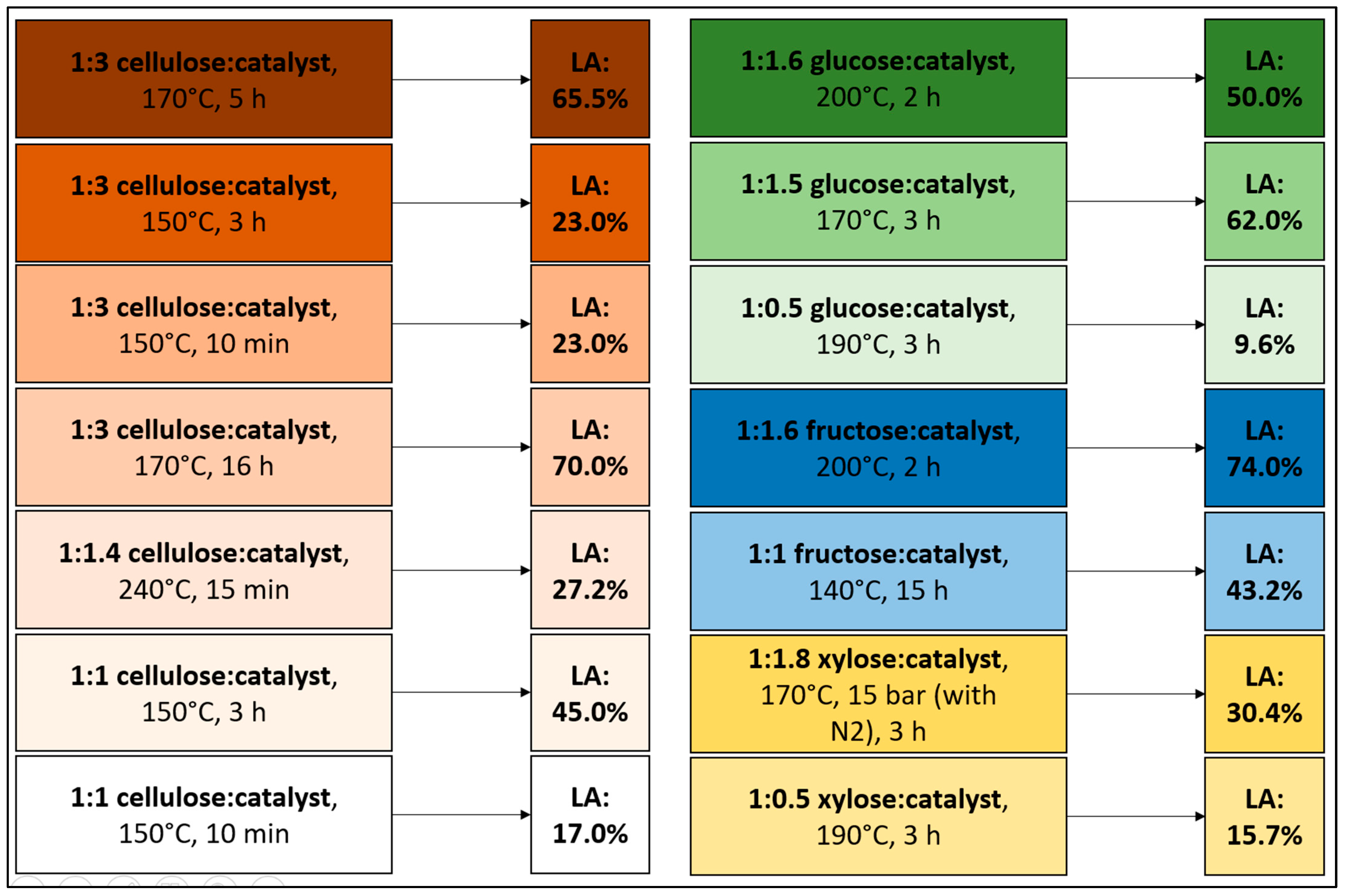
2. Reaction Conditions and General Considerations
| Raw Material | Reaction Conditions | Yield | Ref. |
|---|---|---|---|
| Cellulose | 2 g of cellulose, 2 g of zirconium dioxide, 100 mL of water, 180 °C, 3 h | 53.90 mol% | [48] |
| Cellulose | 2 wt.% of cellulose, 0.2 g of Amberlyst 70, 3 mL of solvent (90 wt.% GVL/10 wt.% water), 180 °C, 16 h | 69.00% | [76] |
| Cellulose | 2 wt.% of cellulose, 0.2 g of C–SO3H, 3 mL of solvent (90 wt.% GVL/10 wt.% water), 180 °C, 16 h | 56.00% | [76] |
| Cellulose | 0.05 g cellulose, 0.1 g of C–SO3H, 5 mL of water, 180 °C, 12 h | 51.50% | [77] |
| Cellulose | 1 g of cellulose, 1.5 g of Al-doped mesoporous niobium phosphate, 15 mL of water, at 150 °C, 3 h, total acid density: 1.09 mmol H+/g | 52.90% | [78] |
| Cellulose | 0.5 g of cellulose, 0.4 g of H-ZSM-5 (Al/Si = 1/25), 10 mL of water, 180 °C, 24 h | 27.00% | [79] |
| Cellobiose | 100 mg of cellobiose, 200 mg of CP-SO3H, 2 mL of water, 180 °C, 5 h, total acid density: 1.69 mmol H+/g | 40.00% | [68] |
| Glucose | 30 g of glucose, 0.5 g of graphene oxide, 200 mL of water, 200 °C, 2 h, total acid density: 2.70 mmol H+/g | 78% | [28] |
| Glucose | 30 g of glucose, 0.5 g of sulfonated graphene (GO–SO3H), 200 mL of water, 200 °C, 2 h, total acid density: 2.70 mmol H+/g | 78.00% | [28] |
| Glucose | 1 g of glucose, 1 g of 10%Fe/HY zeolite, in 50 mL of water, 180 °C, 180 min, total acid density: 2.68 mmol H+/g | 62% | [40] |
| Glucose | 1 g of glucose, 1 g of 10% Fe/HY, 50 mL of water, 180 min, total acid density: 2.30 mmol H+/g | 62.00% | [40] |
| Glucose | Catalyst/glucose ratio: 1:1, CrCl3/HY zeolite (2:1), water 180 °C, 160 min, total acid density: 2.94 mmol H+/g | 44.00% | [53] |
| Glucose | 1.5 mL of glucose, 0.3 g of CH3-SBA-15-SO3H, 13.5 mL of GVL/water, 180 °C, 150 min | 60.00% | [71] |
| Glucose | 0.05 g glucose, 0.1 g of C–SO3H, 5 mL of water, 180 °C, 12 h | 61.05% | [77] |
| Glucose | 100 mg of glucose, 0.05 g of Fe-NbP, 10 mL of water, 180 °C, 3 h, total acid density: 3.59 mmol H+/g, Brønsted/Lewis ratio: 1:2 | 64.20% | [80] |
| Glucose | 11.9 g of glucose, 18.1 g of Amberlyst 70, 85 mL of water, 180 °C, 120 min | 55.00% | [81] |
| Fructose | 1 g of fructose, 1 g of LZY zeolite, 140 °C, 15 h | 43.20% | [82] |
| Fructose | 9 wt.%, 0.28 mmol of fructose, Fructose: Amberlyst 15 (1:1), 0.5 mL of water, 120 °C, 24 h | 55.00 mol% | [83] |
| Mixture of pentoses | Pentoses: 1.56 g/100 g biomass, Y zeolite treated with 0.25 M NaOH, 190 °C, 180 min | 4.71 g/100 g of biomass | [47] |
| Xylose | 0.9 g of xylose and 0.5 g of catalyst, Y zeolite treated with 0.25 M NaOH, 170 °C, 3 h, N2 at 15 bar, total acid density: 1.409 mmol H+/g | 30.40% | [84] |
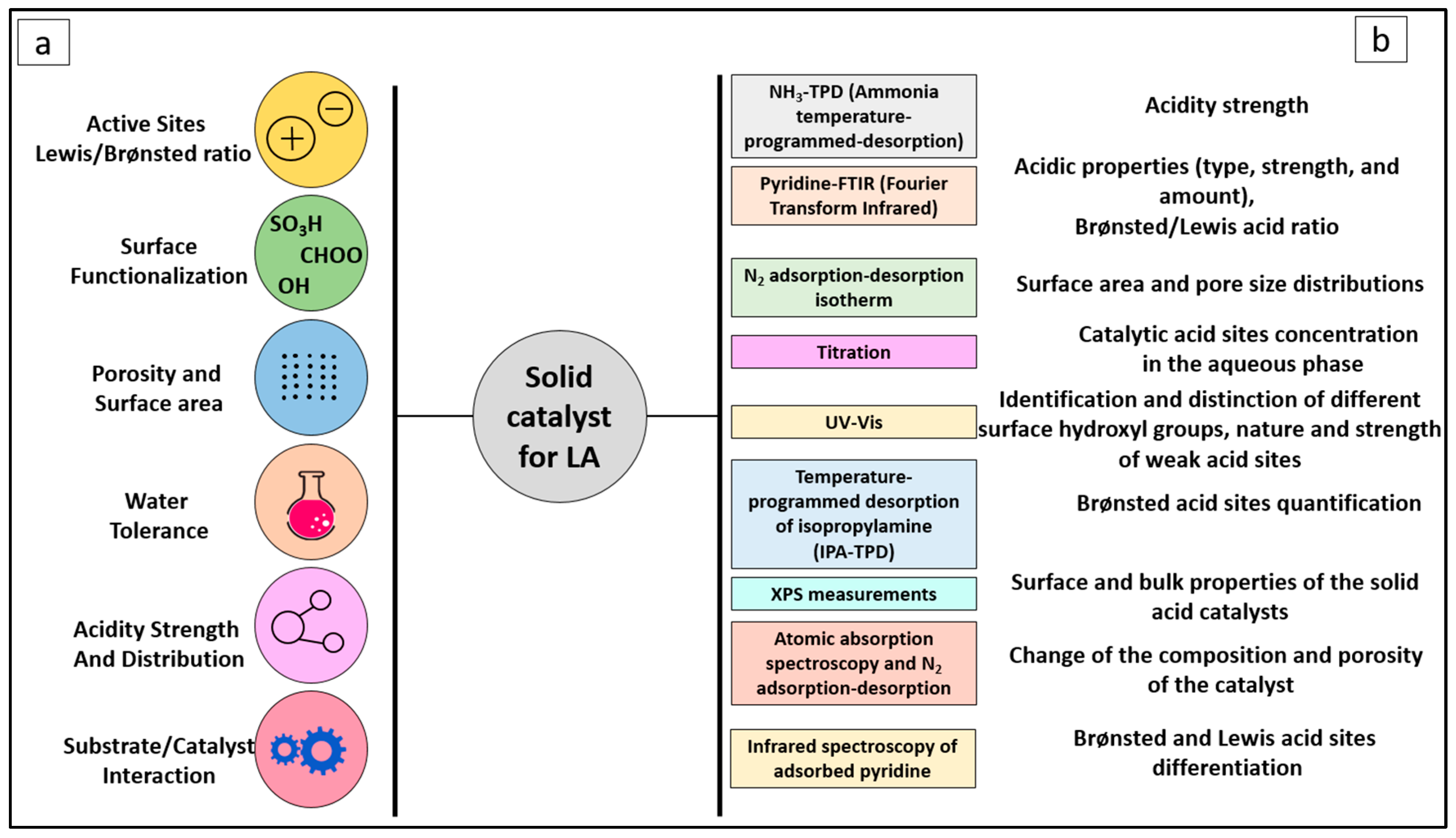
3. Catalyst Design and Characterization
3.1. Active Sites on the Catalyst
3.2. Sulfonation of Carbon-Based Catalyst
| Sulfonating Agent | Sulfonation Conditions | Total Acid Density | Ref. |
|---|---|---|---|
| H2SO4 | 1 g of carbonized lignin, 10 mL of H2SO4, 200 °C, 5 h | 1.34 mmol H+/g | [26] |
| H2SO4 | 1 g of char, 10 mL of H2SO4, 150 °C, 14 h, N2 | 0.02 S/C ratio | [33] |
| H2SO4 | 150 °C, 24 h, 60 mmol H2SO4/g carbon | 0.90 mmol H+/g | [37] |
| H2SO4 | fast pyrolysis biochar (7 g) and 150 mL of H2SO4, 80 °C, 10 h, N2 | - | [96] |
| H2SO4 | 2.6 g of sucrose, 0.25 g of H2SO4 and 4.2 g of distilled water, 160 °C, 15 h | 0.90 mmol H+/g | [128] |
| H2SO4 | 1 g of alkaline lignin, 10 mL of H2SO4, 150 °C, 10 h, N2 | 6.21 mmol H+/g | [134] |
| H2SO4 | 20 g sample of D-glucose powder, 200 cm3 of concentrated H2SO4 (> 96%), 150 °C, 15 h, N2 | 0.75 mmol/g a | [140] |
| H2SO4 | 50 mg of reduced graphene oxide, 20 mL of H2SO4, 150 °C, 15 h | - | [141] |
| H2SO4 | 12.5 g of biochar, 12 mL of H2SO4, 100 °C, 15 h | 0.65 mmol H+/g | [142] |
| H2SO4 | 1 g of ordered mesoporous carbon, 15 mL of H2SO4, 200 °C, N2 (30 mL/min), 16 h | 1.82 mmol H+/g | [143] |
| H2SO4 | 1 g of polyethylene pellets, 35 g of H2SO4, 120 °C, 3 h | 85% b | [144] |
| H2SO4 | 1:10 carbon: H2SO4, 100 °C, 5 h | 1.15 mmol H+/g | [145] |
| H2SO4 | 1 g of carbon, 20 mL of H2SO4, 150 °C, 13 h, N2 | 0.64 mmol H+/g | [146] |
| H2SO4 | 300 mg of lignin-based carbon, 10 mL H2SO4, 150 °C, 8 h | 1.58 mmol H+/g | [147] |
| H2SO4 | 20 mL of H2SO4/g of glucose-based carbon, 150 °C, 15 h Ar | 5.43 mmol H+/g | [148] |
| H2SO4 | 0.2 g of carbon, 10 mL of H2SO4, 80 °C, 8 h under reflux | 1.12 mmol H+/g | [149] |
| H2SO4 | 1 g of lignin-based carbon, 20 mL of H2SO4, 180 °C, 12 h, N2 | 1.46 mmol H+/g | [150] |
| H2SO4 | carbonized cellulose, 15 wt.% of H2SO4, 80 °C, 10 h | 0.92 mmol/g a | [151] |
| H2SO4 | cup-stack carbon nanotubes, plasma- 100 mL of 1 M H2SO4, 30 min | 1.60 wt.% c | [152] |
| 4-benzene diazonium sulfonate | Fe3O4-reduced graphene oxide, 50 °C, 1.5 h | 1.06 mmol/g a | [153] |
| 4-benzene-diazonium sulfonate | 100 mL of water, 12 g of 4-benzene-diazonium sulfonate, 2 g of activated carbon, 30 min | 1.01 mmol H+/g | [154] |
| Fe(SO4)3/H2SO4 | 1 g of carbon material, 10 mL of the sulfonating mixture, 190 °C, 10 h | 1.15 wt.% of sulfur | [155] |
| Sulfanilic acid | 373 mg of graphene, 789 g of sulfanilic acid, 25 °C, 24 h | 1.75 mmol/g a | [156] |
| 5-sulfosalicylic acid dihydrate | 32 g of cellulose, 50 mL of aqueous solution of 20 g of 5-sulfosalicylic acid dihydrate; stainless-steel autoclave for 4 h at 180 °C, with stirring | 3.54 mmol H+/g | [157] |
| Na2SO3 | 0.4 ratio of Na2SO3 to lignin in weight, 90 °C, 5 h | 1.55 mmol/g a | [133] |
| SO3/H2SO4 | 0.3 g of mesoporous carbon, 5 mL 50 wt% SO3/H2SO4, Teflon-lined autoclave at 60 °C, 48 h | 1.30 mmol H+/g | [158] |
| Ferrous sulfide | 1 g of carbonized lignin, 25 g/L of ferrous sulfide solution, 105 °C, 10 h, N2 | 1.79 wt.% c | [159] |
| Chlorosulfonic acid | 1 g of graphene oxide, 0.5 g of chlorosulfonic acid, 50 mL of Chloroform, 1 h, 70 °C, 4 h ultrasound | 2.70 mmol H+/g | [28] |
3.3. Catalyst Porosity
3.4. Substrate/Catalyst Ratio
4. Challenges and Perspectives
4.1. Catalyst Deactivation
4.2. Conventional Catalysts vs. Biobased Catalysts
4.3. Technological Challenges of Carbon-Based Catalysts and Integration Opportunities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guleria, A.; Kumari, G.; Saravanamurugan, S. Chapter 17. Cellulose valorization to potential platform chemicals. Biomass Biofuels Biochem. Recent Adv. Dev. Platf. Chem. 2019, 433–457. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Cellulose Depolymerization over Heterogeneous Catalysts. Accunts Chem. Res. 2018, 51, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Atanda, L.; Silahua, A.; Mukundan, S.; Shrotri, A.; Torres-Torres, G.; Beltramini, J. Catalytic behaviour of TiO2-ZrO2 binary oxide synthesized by sol-gel process for glucose conversion to 5-hydroxymethylfurfural. RSC Adv. 2015, 5, 80346–80352. [Google Scholar] [CrossRef]
- Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P. Brönsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural. Chem. Eng. J. 2016, 303, 22–30. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Fukuoka, A.; Dhepe, P.L. Catalytic conversion of cellulose into sugar alcohols. Angew. Chem. Int. Ed. 2006, 45, 5161–5163. [Google Scholar] [CrossRef]
- Ya’aini, N.; Amin, N.A.S. Catalytic performance of hybrid nanocatalyst for levulinic acid production from glucose. AIP Conf. Proc. 2012, 1502, 442–453. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Q.; Wang, Y. Catalytic transformations of cellulose and cellulose-derived carbohydrates into organic acids. Catal. Today 2014, 234, 31–41. [Google Scholar] [CrossRef]
- Chambon, F.; Rataboul, F.; Pinel, C.; Cabiac, A.; Guillon, E.; Essayem, N. Cellulose hydrothermal conversion promoted by heterogeneous Brønsted and Lewis acids: Remarkable efficiency of solid Lewis acids to produce lactic acid. Appl. Catal B Environ. 2011, 105, 171–181. [Google Scholar] [CrossRef]
- Choudhary, V.; Pinar, A.B.; Lobo, R.F.; Vlachos, D.G.; Sandler, S.I. Comparison of homogeneous and heterogeneous catalysts for glucose-to-fructose isomerization in aqueous media. ChemSusChem 2013, 6, 2369–2376. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transformation of levulinic acid: A platform for speciality chemicals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Isomerization of Biomass-Derived Aldoses: A Review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef]
- Caratzoulas, S.; Davis, M.E.; Gorte, R.J.; Gounder, R.; Lobo, R.F.; Nikolakis, V.; Caratzoulas, S.; Davis, M.E.; Gorte, R.J.; Gounder, R.; et al. Challenges of and Insights into Acid-Catalyzed Transformations of Sugars. J. Phys. Chem. C 2014, 118, 22815–22833. [Google Scholar] [CrossRef]
- Khan, M.A.; Dharmalingam, B.; Chuetor, S.; Cheng, Y.S.; Sriariyanun, M. Comprehensive review on effective conversion of lignocellulosic biomass to levulinic acid. Biomass Convers. Biorefinery 2023, 1–16. [Google Scholar] [CrossRef]
- Azlan, N.S.M.; Yap, C.L.; Gan, S.; Rahman, M.B.A. Recent advances in the conversion of lignocellulosic biomass and its degraded products to levulinic acid: A synergy of Brønsted-Lowry acid and Lewis acid. Ind. Crops Prod. 2022, 181, 114778. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. Kinetic Study on the Acid-Catalyzed Hydrolysis of Cellulose to Levulinic Acid. Ind. Eng. Chem. Res. 2007, 46, 1696–1708. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts. Appl. Catal. B Environ. 2015, 174–175, 225–243. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kaiki, H.; Shrotri, A.; Techikawara, K.; Fukuoka, A. Hydrolysis of woody biomass by a biomass-derived reusable heterogeneous catalyst. Chem. Sci. 2015, 7, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, Y.; Sakata, S.; Yoshinaga, Y.; Ohnishi, R.; Okuhara, T. Zirconium Phosphate with a High Surface Area as a Water-Tolerant Solid Acid. Catal. Lett. 2004, 94, 45–47. [Google Scholar] [CrossRef]
- Ilgen, F.; Ott, D.; Kralisch, D.; Reil, C.; Palmberger, A.; König, B. Conversion of carbohydrates into 5-hydroxymethylfurfural in highly concentrated low melting mixtures. Green Chem. 2009, 11, 1948–1954. [Google Scholar] [CrossRef]
- Lai, D.M.; Deng, L.; Li, J.; Liao, B.; Guo, Q.X.; Fu, Y. Hydrolysis of cellulose into glucose by magnetic solid acid. ChemSusChem 2011, 4, 55–58. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef]
- Guo, F.; Fang, Z.; Zhou, T.J. Conversion of fructose and glucose into 5-hydroxymethylfurfural with lignin-derived carbonaceous catalyst under microwave irradiation in dimethyl sulfoxide-ionic liquid mixtures. Bioresour. Technol. 2012, 112, 313–318. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Upare, P.P.; Yoon, J.W.; Kim, M.Y.; Kang, H.Y.; Hwang, D.W.; Hwang, Y.K.; Upare, P.P.; Kung, H.H.; Chang, J.S. Chemical conversion of biomass-derived hexose sugars to levulinic acid over sulfonic acid-functionalized graphene oxide catalysts. Green Chem. 2013, 15, 2935–2943. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, R.; Ma, Z.; Wu, T.; Wu, Y. Conversion of cellulose to HMF in ionic liquid catalyzed by bifunctional ionic liquids. Bioresour. Technol. 2013, 129, 450–455. [Google Scholar] [CrossRef]
- Mosier, N.S.; Ladisch, C.M.; Ladisch, M.R. Characterization of acid catalytic domains for cellulose hydrolysis and glucose degradation. Biotechnol. Bioeng. 2002, 79, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.S.; Sarikaya, A.; Ladisch, C.M.; Ladisch, M.R. Characterization of dicarboxylic acids for cellulose hydrolysis. Biotechnol. Prog. 2001, 17, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Lindman, B. Competing forces during cellulose dissolution: From solvents to mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Sangsiri, P.; Laosiripojana, N.; Daorattanachai, P. Synthesis of sulfonated carbon-based catalysts from organosolv lignin and methanesulfonic acid: Its activity toward esterification of stearic acid. Renew. Energy 2022, 193, 113–127. [Google Scholar] [CrossRef]
- Pandey, S.; Karakoti, M.; Bhardwaj, D.; Tatrari, G.; Sharma, R.; Pandey, L.; Lee, M.J.; Sahoo, N.G. Recent advances in carbon-based materials for high-performance perovskite solar cells: Gaps, challenges and fulfillment. Nanoscale Adv. 2023, 5, 1492–1526. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Carbon Dot-Based Fluorescent Detection of Biothiols. Biosensors 2023, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, D.; Hayashi, S.; Suganuma, S.; Kato, H.; Kitano, M.; Nakajima, K.; Hara, M. Adsorption-Enhanced Hydrolysis of β-1,4-Glucan on Graphene-Based Amorphous Carbon Bearing SO3H, COOH, and OH Groups. Langmuir 2009, 25, 5068–5075. [Google Scholar] [CrossRef]
- Li, S.; Gu, Z.; Bjornson, B.E.; Muthukumarappan, A. Biochar based solid acid catalyst hydrolyze biomass. J. Environ. Chem. Eng. 2013, 1, 1174–1181. [Google Scholar] [CrossRef]
- Park, M.R.; Kim, S.K.; Jeong, G.T. Production of levulinic acid from glucosamine using zirconium oxychloride. J. Ind. Eng. Chem. 2018, 61, 119–123. [Google Scholar] [CrossRef]
- Gong, R.; Ma, Z.; Wang, X.; Han, Y.; Guo, Y.; Sun, G.; Li, Y.; Zhou, J. Sulfonic-acid-functionalized carbon fiber from waste newspaper as a recyclable carbon based solid acid catalyst for the hydrolysis of cellulose. RSC Adv. 2019, 9, 28902–28907. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Fe/HY zeolite as an effective catalyst for levulinic acid production from glucose: Characterization and catalytic performance. Appl. Catal. B Environ. 2015, 163, 487–498. [Google Scholar] [CrossRef]
- Garces, D.; Faba, L.; Díaz, E.; Ordóñez, S. Aqueous-Phase Transformation of Glucose into Hydroxymethylfurfural and Levulinic Acid by Homogeneous and Heterogeneous Catalysis. ChemSusChem 2019, 12, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Lourvanij, K.; Rorrer, G.L. Dehydration of glucose to organic acids in microporous pillared clay catalysts. Appl. Catal. A Gen. 1994, 109, 147–165. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, S. Experimental and kinetic study of glucose conversion to levulinic acid catalyzed by synergy of Lewis and Brønsted acids. Chem. Eng. J. 2017, 307, 389–398. [Google Scholar] [CrossRef]
- Potvin, J.; Sorlien, E.; Hegner, J.; DeBoef, B.; Lucht, B.L. Effect of NaCl on the Conversion of cellulose to glucose and levulinic acid via solid supported acid catalysis. Tetrahedron Lett. 2011, 52, 5891–5893. [Google Scholar] [CrossRef]
- Wei, W.; Wu, S. Experimental and kinetic study of glucose conversion to levulinic acid in aqueous medium over Cr/HZSM-5 catalyst. Fuel 2018, 225, 311–321. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Jeong, H.; Park, S.-Y.; Ryu, G.-H.; Choi, J.-H.; Kim, J.-H.; Choi, W.-S.; Lee, S.M.; Choi, J.W.; Choi, I.-G. Catalytic conversion of hemicellulosic sugars derived from biomass to levulinic acid. Catal. Commun. 2018, 117, 19–25. [Google Scholar] [CrossRef]
- Joshi, S.S.; Zodge, A.D.; Pandare, K.V.; Kulkarni, B.D. Efficient Conversion of Cellulose to Levulinic Acid by Hydrothermal Treatment Using Zirconium Dioxide as a Recyclable Solid Acid Catalyst. Ind. Eng. Chem. Res. 2014, 53, 18796–18805. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Van Der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. Fructose dehydration to 5-hydroxymethylfurfural over solid acid catalysts in a biphasic system. ChemSusChem 2012, 5, 1812–1819. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. Green chemicals: A kinetic study on the conversion of glucose to levulinic acid. Chem. Eng. Res. Des. 2006, 84, 339–349. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Lund, C.R.F. Formation and growth of humins via aldol addition and condensation during acid-catalyzed conversion of 5-hydroxymethylfurfural. Energy Fuels 2011, 25, 4745–4755. [Google Scholar] [CrossRef]
- Peng, L.; Lin, L.; Zhang, J.; Zhuang, J.; Zhang, B.; Gong, Y. Catalytic conversion of cellulose to levulinic acid by metal chlorides. Molecules 2010, 15, 5258–5272. [Google Scholar] [CrossRef]
- Ya’Aini, N.; Amin, N.A.S.; Endud, S. Characterization and performance of hybrid catalysts for levulinic acid production from glucose. Microporous Mesoporous Mater. 2013, 171, 14–23. [Google Scholar] [CrossRef]
- Saunders, G.J.; Kendall, K. Reactions of hydrocarbons in small tubular SOFCs. J. Power Sources 2002, 106, 258–263. [Google Scholar] [CrossRef]
- Ahlkvist, J.; Wärnå, J.; Salmi, T.; Mikkola, J.P. Heterogeneously catalyzed conversion of nordic pulp to levulinic and formic acids. React. Kinet. Mech. Catal. 2016, 119, 415–427. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Xing, R.; Conner, W.C.; Huber, G.W. Kinetics and reaction engineering of levulinic acid production from aqueous glucose solutions. ChemSusChem 2012, 5, 1280–1290. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Doherty, W.O.S. A review on the production of levulinic acid and furanics from sugars. In Proceedings of the 34th Annual Conference of the Australian Society of Sugar Cane Technologists 2012, ASSCT 2012, Palm Cove, Australia, 1–4 May 2012; Volume 34, pp. 605–613. [Google Scholar]
- Weingarten, R.; Kim, Y.T.; Tompsett, G.A.; Fernández, A.; Han, K.S.; Hagaman, E.W.; Conner, W.C.; Dumesic, J.A.; Huber, G.W. Conversion of glucose into levulinic acid with solid metal(IV) phosphate catalysts. J. Catal. 2013, 304, 123–134. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Bai, X.; Du, Y. Conversion of biomass into 5-hydroxymethylfurfural using solid acid catalyst. Bioresour. Technol. 2011, 102, 3424–3429. [Google Scholar] [CrossRef]
- Yuan, Z.; Xu, C.; Cheng, S.; Leitch, M. Catalytic conversion of glucose to 5-hydroxymethyl furfural using inexpensive co-catalysts and solvents. Carbohydr. Res. 2011, 346, 2019–2023. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Chong, Y.; Dumesic, J.A. Production of levulinic acid and gamma-valerolactone (GVL) from cellulose using GVL as a solvent in biphasic systems. Energy Environ. Sci. 2012, 5, 8199–8203. [Google Scholar] [CrossRef]
- Gürbüz, E.I.; Wettstein, S.G.; Dumesic, J.A. Conversion of hemicellulose to furfural and levulinic acid using biphasic reactors with alkylphenol solvents. ChemSusChem 2012, 5, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Z.; Hou, W.; Shen, H. Direct conversion of lignocellulose to levulinic acid catalyzed by ionic liquid. Carbohydr. Polym. 2018, 181, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Jakob, A.; Grilc, M.; Teržan, J.; Likozar, B. Solubility Temperature Dependence of Bio-Based Levulinic. Processes 2021, 9, 924. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, Y.; Fu, Y. Catalytic conversion of cellulose into levulinic acid by a sulfonated chloromethyl polystyrene solid acid catalyst. ChemCatChem 2014, 6, 753–757. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Alonso, D.M.; Luterbacher, J.S.; Gallo, J.M.R.; Dumesic, J.A. Effects of γ-valerolactone in hydrolysis of lignocellulosic biomass to monosaccharides. Green Chem. 2014, 16, 4659–4662. [Google Scholar] [CrossRef]
- Victor, A.; Sharma, P.; Pulidindi, I.N.; Gedanken, A. Levulinic Acid Is a Key Strategic Chemical from Biomass. Catalysts 2022, 12, 909. [Google Scholar] [CrossRef]
- Cheng, X.; Feng, Q.; Ma, D.; Xing, F.; Zeng, X.; Huang, X.; Teng, J.; Feng, L. Kinetics for glucose conversion to levulinic acid over solid acid catalyst in γ-valerolactone solution. Biochem. Eng. J. 2022, 180, 108360. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, J.; Lin, L.; Liu, S.; Zhang, Z. Conversion of D-xylose into furfural with mesoporous molecular sieve MCM-41 as catalyst and butanol as the extraction phase. Biomass Bioenergy 2012, 39, 73–77. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.; Abu-Omar, M.M. Conversion of glucose into furans in the presence of AlCl3 in an ethanol-water solvent system. Bioresour. Technol. 2012, 116, 190–194. [Google Scholar] [CrossRef]
- Mahmoud, E. Glucose Conversion to Furans in Alcohols Catalyzed by Lewis Acidic Beta Zeolites and Brønsted Acidic Resins. ChemistrySelect 2017, 2, 10336–10339. [Google Scholar] [CrossRef]
- Jia, S.; Xu, Z.; Zhang, Z.C. Catalytic conversion of glucose in dimethylsulfoxide/water binary mix with chromium trichloride: Role of water on the product distribution. Chem. Eng. J. 2014, 254, 333–339. [Google Scholar] [CrossRef]
- Alonso, D.M.; Gallo, J.M.R.; Mellmer, M.A.; Wettstein, S.G.; Dumesic, J.A. Direct conversion of cellulose to levulinic acid and gamma-valerolactone using solid acid catalysts. Catal. Sci. Technol. 2013, 3, 927–931. [Google Scholar] [CrossRef]
- Shen, F.; Smith, R.L.; Li, L.; Yan, L.; Qi, X. Eco-friendly Method for Efficient Conversion of Cellulose into Levulinic Acid in Pure Water with Cellulase-Mimetic Solid Acid Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2421–2427. [Google Scholar] [CrossRef]
- Lai, D.M.; Deng, L.; Guo, Q.X.; Fu, Y. Hydrolysis of biomass by magnetic solid acid. Energy Environ. Sci. 2011, 4, 3552–3557. [Google Scholar] [CrossRef]
- Ding, D.; Wang, J.; Xi, J.; Liu, X.; Lu, G.; Wang, Y. High-yield production of levulinic acid from cellulose and its upgrading to γ-valerolactone. Green Chem. 2014, 16, 3846–3853. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; He, J.; Zhao, W.; Yang, T.; Yang, S. Catalytic conversion of carbohydrates to levulinic acid with mesoporous niobium-containing oxides. Catal. Commun. 2017, 93, 20–24. [Google Scholar] [CrossRef]
- Hu, X.; Wang, S.; Westerhof, R.J.M.; Wu, L.; Song, Y.; Dong, D.; Li, C.Z. Acid-catalyzed conversion of C6 sugar monomer/oligomers to levulinic acid in water, tetrahydrofuran and toluene: Importance of the solvent polarity. Fuel 2015, 141, 56–63. [Google Scholar] [CrossRef]
- Jow, J.; Rorrer, G.L.; Hawley, M.C.; Lamport, D.T.A. Dehydration of d-fructose to levulinic acid over LZY zeolite catalyst. Biomass 1987, 14, 185–194. [Google Scholar] [CrossRef]
- Thapa, I.; Mullen, B.; Saleem, A.; Leibig, C.; Baker, R.T.; Giorgi, J.B. Efficient green catalysis for the conversion of fructose to levulinic acid. Appl. Catal. A Gen. 2017, 539, 70–79. [Google Scholar] [CrossRef]
- Chamnankid, B.; Ratanatawanate, C.; Faungnawakij, K. Conversion of xylose to levulinic acid over modified acid functions of alkaline-treated zeolite Y in hot-compressed water. Chem. Eng. J. 2014, 258, 341–347. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glucose dehydration to 5-hydroxymethylfurfural on zirconium containing mesoporous MCM-41 silica catalysts. Fuel 2014, 118, 265–271. [Google Scholar] [CrossRef]
- Vilcocq, L.; Castilho, P.C.; Carvalheiro, F.; Duarte, L.C. Hydrolysis of oligosaccharides over solid acid catalysts: A review. ChemSusChem 2014, 7, 1010–1019. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Geboers, J.; Jacobs, P.A.; Sels, B.F. Recent Advances in the Catalytic Conversion of Cellulose. ChemCatChem 2011, 3, 82–94. [Google Scholar] [CrossRef]
- Chen, D.; Liang, F.; Feng, D.; Xian, M.; Zhang, H.; Liu, H.; Du, F. An efficient route from reproducible glucose to 5-hydroxymethylfurfural catalyzed by porous coordination polymer heterogeneous catalysts. Chem. Eng. J. 2016, 300, 177–184. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, X.; Liu, X.; Xia, Y.; Hu, B.; Lu, G.; Wang, Y. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurural over water-tolerant niobium-based catalysts. Fuel 2015, 139, 301–307. [Google Scholar] [CrossRef]
- Peng, K.; Li, X.; Liu, X.; Wang, Y. Hydrothermally stable Nb-SBA-15 catalysts applied in carbohydrate conversion to 5-hydroxymethyl furfural. Mol. Catal. 2017, 441, 72–80. [Google Scholar] [CrossRef]
- Weingarten, R.; Tompsett, G.A.; Conner, W.C.; Huber, G.W. Design of solid acid catalysts for aqueous-phase dehydration of carbohydrates: The role of Lewis and Brønsted acid sites. J. Catal. 2011, 279, 174–182. [Google Scholar] [CrossRef]
- Velthoen, M.E.Z.; Nab, S.; Weckhuysen, B.M. Probing acid sites in solid catalysts with pyridine UV-Vis spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 21647–21659. [Google Scholar] [CrossRef] [PubMed]
- Macht, J.; Carr, R.T.; Iglesia, E. Functional assessment of the strength of solid acid catalysts. J. Catal. 2009, 264, 54–66. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; He, H.; Wang, L. Glucose production from hydrolysis of cellulose over a novel silica catalyst under hydrothermal conditions. J. Environ. Sci. 2011, 23, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Dora, S.; Bhaskar, T.; Singh, R.; Naik, D.V.; Adhikari, D.K. Effective catalytic conversion of cellulose into high yields of methyl glucosides over sulfonated carbon based catalyst. Bioresour. Technol. 2012, 120, 318–321. [Google Scholar] [CrossRef]
- Guo, H.; Qi, X.; Li, L.; Smith, R.L. Hydrolysis of cellulose over functionalized glucose-derived carbon catalyst in ionic liquid. Bioresour. Technol. 2012, 116, 355–359. [Google Scholar] [CrossRef]
- Rezayan, A.; Zhang, Y.; Li, B.; Xu, C.C.X. Catalytic Conversion of Cellulose to 5-Hydroxymethylfurfural: Advancements in Heterogeneous Catalysts and Cutting-Edge Hydrolysis Strategies. ChemCatChem 2023, 15, e202300973. [Google Scholar] [CrossRef]
- Gupta, S.; Gambhire, A.B.; Jain, R. Conversion of carbohydrates (glucose and fructose) into 5-HMF over solid acid loaded natural zeolite (PMA/NZ) catalyst. Mater. Lett. X 2022, 13, 100119. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.-W.; Abu-Omar, M.M. Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3·6H2O catalyst in a biphasic solvent system. Green Chem. 2012, 14, 509–513. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Xie, Q.; Wang, J.; Hou, B.; Jia, L.; Cui, J.; Li, D. Conversion of fructose into furfural or 5-hydroxymethylfurfural over HY zeolites selectively in Γ-butyrolactone. Appl. Catal. A Gen. 2019, 572, 51–60. [Google Scholar] [CrossRef]
- Tao, X.; Li, H.; Chang, K.; Huang, L.; Wang, L. Regulation of Brønsted-Lewis acid sites by combination of Amberlyst-15 and Cr-MOFs based catalysts for glucose conversion. J. Phys. Chem. Solids 2024, 193, 112159. [Google Scholar] [CrossRef]
- Akiyama, T.; Mori, K. Stronger Brønsted Acids: Recent Progress. Chem. Rev. 2015, 115, 9277–9306. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Hara, M. Amorphous Carbon with SO3H Groups as a Solid Brønsted Acid Catalyst. ACS Catal. 2018, 2, 1296–1304. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, Z.; Xue, L.; Wang, X.; Jiang, Z. Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction. Appl. Catal. B Environ. 2016, 196, 50–56. [Google Scholar] [CrossRef]
- Katada, N.; Tsubaki, T.; Niwa, M. Measurements of number and strength distribution of Brønsted and Lewis acid sites on sulfated zirconia by ammonia IRMS-TPD method. Appl. Catal. A Gen. 2008, 340, 76–86. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610–626. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wang, J.; Wang, Y.; Wang, M.; Ciu, H.; Song, F.; Sun, X.; Xie, Y.; Yi, W. Al2O3-TiO2 Modified Sulfonated Carbon with Hierarchically Ordered Pores for Glucose Conversion to 5-HMF. ChemistrySelect 2019, 4, 5724–5731. [Google Scholar] [CrossRef]
- Taghavi, S.; Pizzolitto, C.; Ghedini, E.; Menegazzo, F.; Cruciani, G.; Peurla, M.; Eränen, K.; Heinmaa, I.; Aho, A.; Kumar, N.; et al. Levulinic Acid Production: Comparative Assessment of Al-Rich Ordered Mesoporous Silica and Microporous Zeolite. Catal. Lett. 2023, 153, 41–53. [Google Scholar] [CrossRef]
- Otomo, R.; Yokoi, T.; Kondo, J.N.; Tatsumi, T. Dealuminated Beta zeolite as effective bifunctional catalyst for direct transformation of glucose to 5-hydroxymethylfurfural. Appl. Catal. A Gen. 2014, 470, 318–326. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Effect of Lewis and BrØnsted acidity on glucose conversion to 5-HMF and lactic acid in aqueous and organic media. Appl. Catal. A Gen. 2018, 555, 75–87. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Glucose to Fructose Isomerization in Aqueous Media over Homogeneous and Heterogeneous Catalysts. ChemCatChem 2016, 8, 1100–1110. [Google Scholar] [CrossRef]
- Enslow, K.R.; Bell, A.T. SnCl4-catalyzed isomerization/dehydration of xylose and glucose to furanics in water. Catal. Sci. Technol. 2015, 5, 2839–2847. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Davis, M.E. Activation of carbonyl-containing molecules with solid lewis acids in aqueous media. ACS Catal. 2011, 1, 1566–1580. [Google Scholar] [CrossRef]
- Huang, Y.B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Acid hydrolysis of cellulose as the entry point into biorefinery schemes. ChemSusChem 2009, 2, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Shi, T.; Guan, H.; Wang, S.; Wang, X.; Jiang, Z. Clean production of glucose from polysaccharides using a micellar heteropolyacid as a heterogeneous catalyst. Appl. Catal. B Environ. 2011, 107, 104–109. [Google Scholar] [CrossRef]
- Kang, S.; Ye, J.; Chang, J. Recent Advances in Carbon-Based Sulfonated Catalyst: Preparation and Application. Int. Rev. Chem. Eng. 2013, 5, 133–144. [Google Scholar]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Albers, P.W.; Pietsch, J.; Krauter, J.; Parker, S.F. Investigations of activated carbon catalyst supports from different natural sources. Phys. Chem. Chem. Phys. 2003, 5, 1941–1949. [Google Scholar] [CrossRef]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated carbon as catalyst support: Precursors, preparation, modification and characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef]
- Shao, S.; Yang, Y.; Guo, S.; Hao, S.; Yang, F.; Zhang, S.; Ren, Y.; Ke, Y. Highly active and stable Co nanoparticles embedded in nitrogen-doped mesoporous carbon nanofibers for aqueous-phase levulinic acid hydrogenation. Green Energy Environ. 2021, 6, 567–577. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, Z.; Wang, Y.; Wang, T.; Du, Q.; Wang, Y.; Qu, J. Graphene-based metal/acid bifunctional catalyst for the conversion of levulinic acid to γ-valerolactone. ACS Sustain. Chem. Eng. 2017, 5, 1538–1548. [Google Scholar] [CrossRef]
- Pang, J.; Wang, A.; Zheng, M.; Zhang, T. Hydrolysis of cellulose into glucose over carbons sulfonated at elevated temperatures. Chem. Commun. 2010, 46, 6935–6937. [Google Scholar] [CrossRef]
- Hassan, A.H.; Zainol, M.M.; Zainuddin, K.R.; Rosmadi, H.A.; Asmadi, M.; Rahman, N.A.; Amin, N.A.S. A Review on Alkyl Levulinates Synthesis from Renewable Levulinic Acid using Various Modified Carbon-Based Catalysts. Malays. J. Chem. 2022, 24, 264–282. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, A.; Meng, Y.; Wang, L.; Jiang, H.; Li, G. Catalytic Performance of Biomass Carbon-Based Solid Acid Catalyst for Esterification of Free Fatty Acids in Waste Cooking Oil. Catal. Surv. Asia 2015, 19, 61–67. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Mikkola, J.P. SO3H-Containing Functional Carbon Materials: Synthesis, Structure, and Acid Catalysis. Chem. Rev. 2019, 119, 11576–11630. [Google Scholar] [CrossRef] [PubMed]
- Qian, E.W.; Sukma, L.P.P.; Li, S.; Higashi, A. Saccharification of cellulosic biomass using sulfonated mesoporous carbon-based catalysts. Environ. Prog. Sustain. Energy 2015, 35, 574–581. [Google Scholar] [CrossRef]
- Chen, S.S.; Maneerung, T.; Tsang, D.C.W.; Ok, Y.S.; Wang, C.H. Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 2017, 328, 246–273. [Google Scholar] [CrossRef]
- Kitano, M.; Arai, K.; Kodama, A.; Kousaka, T.; Nakajima, K.; Hayashi, S.; Hara, M. Preparation of a sulfonated porous carbon catalyst with high specific surface area. Catal. Lett. 2009, 131, 242–249. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Cao, Q.; Mu, X. Acid functionalized, highly dispersed carbonaceous spheres: An effective solid acid for hydrolysis of polysaccharides. J. Nanopart. Res. 2011, 13, 463–469. [Google Scholar] [CrossRef]
- Ogino, I.; Suzuki, Y.; Mukai, S.R. Esterification of levulinic acid with ethanol catalyzed by sulfonated carbon catalysts: Promotional effects of additional functional groups. Catal. Today 2018, 314, 62–69. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Yuan, Z.; Fang, J.; Chang, L.; Zhang, H.; Li, C. Role of sulfonation in lignin-based material for adsorption removal of cationic dyes. Int. J. Biol. Macromol. 2019, 135, 1171–1181. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Yuan, B.; Yu, F.; Xie, C.; Yu, S. Lignin-based sulfonated carbon as an efficient biomass catalyst for clean benzylation of benzene ring compounds. J. Ind. Eng. Chem. 2022, 111, 369–379. [Google Scholar] [CrossRef]
- Yu, H.; Jin, Y.; Li, Z.; Peng, F.; Wang, H. Synthesis and characterization of sulfonated single-walled carbon nanotubes and their performance as solid acid catalyst. J. Solid State Chem. 2008, 181, 432–438. [Google Scholar] [CrossRef]
- Na, S.; Minhua, Z.; Xiuqin, D.; Lingtao, W. Preparation of sulfonated ordered mesoporous carbon catalyst and its catalytic performance for esterification of free fatty acids in waste cooking oils. RSC Adv. 2019, 9, 15941–15948. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Zhang, L.; Li, C. Functionalized periodic mesoporous organosilicas for catalysis. J. Mater. Chem. 2009, 19, 1945–1955. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Shi, M.; Du, S.; Su, Y.; Yang, X.; Xu, J. Sulfonated hierarchical H-USY zeolite for efficient hydrolysis of hemicellulose/cellulose. Carbohydr. Polym. 2013, 98, 146–151. [Google Scholar] [CrossRef]
- Du, C.Y.; Zhao, T.S.; Liang, Z.X. Sulfonation of carbon-nanotube supported platinum catalysts for polymer electrolyte fuel cells. J. Power Sources 2008, 176, 9–15. [Google Scholar] [CrossRef]
- Okamura, M.; Takagaki, A.; Toda, M.; Kondo, J.N.; Domen, K.; Tatsumi, T.; Hara, M.; Hayashi, S. Acid-catalyzed reactions on flexible polycyclic aromatic carbon in amorphous carbon. Chem. Mater. 2006, 18, 3039–3045. [Google Scholar] [CrossRef]
- Morales-Acosta, D.; Flores-Oyervides, J.D.; Rodríguez-González, J.A.; Sánchez-Padilla, N.M.; Benavides, R.; Fernán-dez-Tavizón, S.; Mercado-Silva, J.A. Comparative methods for reduction and sulfonation of graphene oxide for fuel cell electrode applications. Int. J. Hydrogen Energy 2019, 44, 12356–12364. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- Hou, K.; Zhang, A.; Gu, L.; Liu, M.; Guo, X. Efficient synthesis and sulfonation of ordered mesoporous carbon materials. J. Colloid Interface Sci. 2012, 377, 18–26. [Google Scholar] [CrossRef]
- Li, C.; Zhu, H.; Salim, N.V.; Fox, B.L.; Hameed, N. Preparation of microporous carbon materials via in-depth sulfonation and stabilization of polyethylene. Polym. Degrad. Stab. 2016, 134, 272–283. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Montes, V.; Caballero, A.; Bautista, F.M. Sulfonated carbons from olive stones as catalysts in the microwave-assisted etherification of glycerol with tert-butyl alcohol. Mol. Catal. 2020, 488, 110921. [Google Scholar] [CrossRef]
- Mo, X.; López, D.E.; Suwannakarn, K.; Liu, Y.; Lotero, E.; Goodwin, J.G.; Lu, C. Activation and deactivation characteristics of sulfonated carbon catalysts. J. Catal. 2008, 254, 332–338. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Luo, B.; Chen, C.; Wang, S.; Min, D. Lignin-based carbon solid acid catalyst prepared for selectively converting fructose to 5-hydroxymethylfurfural. Ind. Crop. Prod. 2020, 145, 111920. [Google Scholar] [CrossRef]
- Roldán, L.; Pires, E.; Fraile, J.M.; García-Bordejé, E. Impact of sulfonated hydrothermal carbon texture and surface chemistry on its catalytic performance in esterification reaction. Catal. Today 2015, 249, 153–160. [Google Scholar] [CrossRef]
- Tamborini, L.H.; Militello, M.P.; Balach, J.; Moyano, J.M.; Barbero, C.A.; Acevedo, D.F. Application of sulfonated nanoporous carbons as acid catalysts for Fischer esterification reactions. Arab. J. Chem. 2015, 12, 3172–3182. [Google Scholar] [CrossRef]
- Zhu, J.; Gan, L.; Li, B.; Yang, X. Synthesis and characteristics of lignin-derived solid acid catalysts for microcrystalline cellulose hydrolysis. Korean J. Chem. Eng. 2017, 34, 110–117. [Google Scholar] [CrossRef]
- Fukuhara, K.; Nakajima, K.; Kitano, M.; Kato, H.; Hayashi, S.; Hara, M. Structure and catalysis of cellulose-derived amorphous carbon bearing SO3H groups. ChemSusChem 2011, 4, 778–784. [Google Scholar] [CrossRef]
- Li, O.L.; Qin, L.; Takeuchi, N.; Kim, K.; Ishizaki, T. Effect of hydrophilic/hydrophobic properties of carbon materials on plasma-sulfonation process and their catalytic activities in cellulose conversion. Catal. Today 2019, 337, 155–161. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Qi, W.; Tong, L.; Su, R.; He, Z. Hydrolysis of cellulose by sulfonated magnetic reduced graphene oxide. Chem. Eng. J. 2015, 280, 90–98. [Google Scholar] [CrossRef]
- Liu, X.Y.; Huang, M.; Ma, H.L.; Zhang, Z.Q.; Gao, J.M.; Zhu, Y.L.; Han, X.J.; Guo, X.Y. Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Koskin, A.P.; Larichev, Y.V.; Mishakov, I.V.; Mel’gunov, M.S.; Vedyagin, A.A. Synthesis and characterization of carbon nanomaterials functionalized by direct treatment with sulfonating agents. Microporous Mesoporous Mater. 2020, 299, 110130. [Google Scholar] [CrossRef]
- Nongbe, M.C.; Ekou, T.; Ekou, L.; Yao, K.B.; Le Grognec, E.; Felpin, F.X. Biodiesel production from palm oil using sulfonated graphene catalyst. Renew. Energy 2017, 106, 135–141. [Google Scholar] [CrossRef]
- Guo, H.; Hirosaki, Y.; Qi, X.; Smith, R.L. Synthesis of ethyl levulinate over amino-sulfonated functional carbon materials. Renew. Energy 2020, 157, 951–958. [Google Scholar] [CrossRef]
- Xing, R.; Liu, Y.; Wang, Y.; Chen, L.; Wu, H.; Jiang, Y.; He, M.; Wu, P. Active solid acid catalysts prepared by sulfonation of carbonization-controlled mesoporous carbon materials. Microporous Mesoporous Mater. 2007, 105, 41–48. [Google Scholar] [CrossRef]
- Han, Y.; Ye, L.; Gu, X.; Zhu, P.; Lu, X. Lignin-based solid acid catalyst for the conversion of cellulose to levulinic acid using γ-valerolactone as solvent. Ind. Crop. Prod. 2019, 127, 88–93. [Google Scholar] [CrossRef]
- Csicsery, S.M. Shape-selective catalysis in zeolites. Zeolites 1984, 4, 202–203. [Google Scholar] [CrossRef]
- Lima, S.; Fernandes, A.; Antunes, M.M.; Pillinger, M.; Ribeiro, F.; Valente, A.A. Dehydration of xylose into furfural in the presence of crystalline microporous silicoaluminophosphates. Catal. Lett. 2010, 135, 41–47. [Google Scholar] [CrossRef]
- Xu, H.; Miao, Z.; Zhao, H.; Yang, J.; Zhao, J.; Song, H.; Liang, N.; Chou, L. Dehydration of fructose into 5-hydroxymethylfurfural by high stable ordered mesoporous zirconium phosphate. Fuel 2015, 145, 234–240. [Google Scholar] [CrossRef]
- Kruger, J.S.; Choudhary, V.; Nikolakis, V.; Vlachos, D.G. Elucidating the roles of zeolite H-BEA in aqueous-phase fructose dehydration and HMF rehydration. ACS Catal. 2013, 3, 1279–1291. [Google Scholar] [CrossRef]
- Nalawade, K.S.; Gogate, P.R. Understanding the effect of reaction parameters on the production of levulinic acid from glucose. Can. J. Chem. Eng. 2024, 102, 3713–3722. [Google Scholar] [CrossRef]
- Wang, P.; Zhan, S.H.; Yu, H.B. Production of Levulinic Acid from Cellulose Catalyzed by Environmental-Friendly Catalyst. Adv. Mater. Res. 2010, 96, 183–187. [Google Scholar] [CrossRef]
- Chen, S.S.; Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Khan, E.; Wang, L.; Ok, Y.S.; Poon, C.S. Valorization of cellulosic food waste into levulinic acid catalyzed by heterogeneous BrØnsted acids: Temperature and solvent effects. Chem. Eng. J. 2017, 327, 328–335. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Optimization of renewable levulinic acid production from glucose conversion catalyzed by Fe/HY zeolite catalyst in aqueous medium. Energy Convers. Manag. 2015, 95, 10–19. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A: Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Boskovic, G.; Baerns, M. Catalyst deactivation. Springer Ser. Chem. Phys. 2004, 75, 477–503. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Tang, P.; Yu, J. Kinetic analysis on deactivation of a solid Brønsted acid catalyst in conversion of sucrose to levulinic acid. Ind. Eng. Chem. Res. 2014, 53, 11629–11637. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, K.; Zhang, L.; Xu, Q.; Zhang, Z.; Li, Q.; Hu, X. Balanced distribution of Brønsted acidic sites and Lewis acidic sites for highly selective conversion of xylose into levulinic acid/ester over Zr-beta catalysts. Green Chem. 2019, 21, 6634–6645. [Google Scholar] [CrossRef]
- Bakhtiari, B.; Najafi Chermahini, A.; Babaei, Z. Design of an acidic sulfonated mesoporous carbon catalyst for the synthesis of butyl levulinate from levulinic acid. Environ. Prog. Sustain. Energy 2021, 40, e13721. [Google Scholar] [CrossRef]
- Burmana, A.D.; Tambun, R.; Haryanto, B.; Sarah, M.; Alexander, V. Recycling heterogeneous catalyst waste in biodiesel production using methanol and hydrochloric acid: A case study on the washing effect with lauric acid as raw material. Case Stud. Chem. Environ. Eng. 2023, 8, 100510. [Google Scholar] [CrossRef]
- Cheng, Z.; Everhart, J.L.; Tsilomelekis, G.; Nikolakis, V.; Saha, B.; Vlachos, D.G. Structural analysis of humins formed in the Brønsted acid catalyzed dehydration of fructose. Green Chem. 2018, 20, 997–1006. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Peng, J.; Ding, M. Advances in catalytic valorization of cellulose into value-added chemicals and fuels over heterogeneous catalysts. Catal. Today 2023, 408, 92–110. [Google Scholar] [CrossRef]
- Oliveira, B.L.; Da Silva, V.T. Sulfonated carbon nanotubes as catalysts for the conversion of levulinic acid into ethyl levulinate. Catal. Today 2014, 234, 257–263. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Jiang, Y.; Mu, X.; Liu, H. Insights into deactivation mechanism of sulfonated carbonaceous solid acids probed by cellulose hydrolysis. Catal. Today 2019, 319, 25–30. [Google Scholar] [CrossRef]
- Pratama, A.P.; Rahayu, D.U.C.; Krisnandi, Y.K. Levulinic acid production from delignified rice husk waste over manganese catalysts: Heterogeneous versus homogeneous. Catalysts 2020, 10, 327. [Google Scholar] [CrossRef]
- Perveen, F.; Farooq, M.; Ramli, A.; Naeem, A.; Khan, I.W.; Saeed, T.; Khan, J. Levulinic Acid Production from Waste Corncob Biomass Using an Environmentally Benign WO3Grafted ZnCo2O4@CeO2 Bifunctional Heterogeneous Catalyst. ACS Omega 2023, 8, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liang, B.; Zhu, Y.; Chen, J.; Gan, T.; Hu, H.; Zhang, Y.; Huang, Z.; Qin, Y. Direct and efficient conversion of cellulose to levulinic acid catalyzed by carbon foam-supported heteropolyacid with Brønsted–Lewis dual-acidic sites. Bioresour. Technol. 2023, 387, 129600. [Google Scholar] [CrossRef]
- Covinich, L.G.; Clauser, N.M.; Felissia, F.E.; Vallejos, M.E.; Area, M.C. The challenge of converting biomass polysaccharides into levulinic acid through heterogeneous catalytic processes. Biofuels Bioprod. Biorefining 2019, 14, 417–445. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Singh, A.K.; Ganachari, S.V.; Pengadeth, D.; Mohanakrishna, G.; Aminabhavi, T.M. Biobased heterogeneous renewable catalysts: Production technologies, innovations, biodiesel applications and circular bioeconomy. Environ. Res. 2024, 261, 119745. [Google Scholar] [CrossRef]
- Changmai, B.; Vanlalveni, C.; Ingle, A.P.; Bhagat, R.; Rokhum, L. Widely used catalysts in biodiesel production: A review. RSC Adv. 2020, 10, 41625–41679. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Andas, J.; Ma, Y.K.; Phoon, B.L.; Batagarawa, S.M.; Khoerunnisa, F.; Hazwan Hussin, M.; Ng, E.P. Recent advances in heterogeneous catalysts for the synthesis of alkyl levulinate biofuel additives from renewable levulinic acid: A comprehensive review. Fuel 2022, 323, 124362. [Google Scholar] [CrossRef]
- Chaffey, D.R.; Bere, T.; Davies, T.E.; Apperley, D.C.; Taylor, S.H.; Graham, A.E. Conversion of levulinic acid to levulinate ester biofuels by heterogeneous catalysts in the presence of acetals and ketals. Appl. Catal. B Environ. 2021, 293, 120219. [Google Scholar] [CrossRef]
- Maleki, B.; Talesh, S.S.A.; Mansouri, M. Comparison of catalysts types performance in the generation of sustainable biodiesel via transesterification of various oil sources: A review study. Mater. Today Sustain. 2022, 18, 100157. [Google Scholar] [CrossRef]
- Global Heterogeneous Catalyst Market Size & Outlook. Available online: https://www.grandviewresearch.com/horizon/outlook/heterogeneous-catalyst-market-size/global (accessed on 5 August 2025).
- Heterogeneous Catalyst Market Report 2025. Available online: https://www.researchandmarkets.com/report/heterogeneous-catalyst (accessed on 5 August 2025).
- Global Carbon-Based Catalyst Supports Market Insights, Forecast to 2030. Available online: https://reports.valuates.com/market-reports/QYRE-Auto-36A9611/global-carbon-based-catalyst-supports2025:9611 (accessed on 5 August 2025).
- Offerings, O.; Us, A.; Platform, I.; Trends, M.; Store, R. Bio-Based-Materials-Global-Market-Report 2025. pp. 1–18. Available online: https://www.thebusinessresearchcompany.com/report/bio-based-materials-global-market-report (accessed on 5 August 2025).
- Wurster, S.; Ladu, L.; Arisaktiwardhana, D. Bio-Based Products. Int. J. Stand. Res. 2019, 17, 23–39. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrogen Energy 2015, 40, 948–979. [Google Scholar] [CrossRef]
- Saperi, N.Q.; Hassan, A.H.; Zainol, M.M.; Asmadi, M.; Mohamad Daud, A.R.; Yusof, M.Y.; Anggoro, D.D. Catalytic Reaction Study of Levulinic Acid Esterification To Ethyl Levulinate Using Modified Carbon Catalyst and Acidic Des. Malays. J. Anal. Sci. 2024, 28, 724–736. [Google Scholar]
- Jędrzejczyk, M.; Soszka, E.; Goscianska, J.; Kozanecki, M.; Grams, J.; Ruppert, A.M. The Influence of Carbon Nature on the Catalytic Performance of Ru/C in Levulinic Acid Hydrogenation with Internal Hydrogen Source. Molecules 2020, 25, 5362. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Zhang, W.; Yi, S.; Chen, H.; Su, Z. Defect engineering in carbon materials for electrochemical energy storage and catalytic conversion. Mater. Adv. 2023, 4, 835–867. [Google Scholar] [CrossRef]
- Signoretto, M.; Taghavi, S.; Ghedini, E.; Menegazzo, F. Catalytic Production of Levulinic Acid (LA) from Actual Biomass. Molecules 2019, 24, 2760. [Google Scholar] [CrossRef]
- Sessa, A.; Prete, P.; Cespi, D.; Scotti, N.; Tabanelli, T.; Antonetti, C.; Russo, V.; Cucciniello, R. Levulinic acid biorefinery in a life cycle perspective. Curr. Opin. Green Sustain. Chem. 2024, 50, 100963. [Google Scholar] [CrossRef]
- Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E.; Gutiérrez, S. An energy-saving re-use strategy for the treatment of lignocellulosic biomass applied to the production of levulinc acid. J. Clean. Prod. 2020, 257, 120549. [Google Scholar] [CrossRef]
- Hurst, G.; Peeters, M.; Tedesco, S. Integration of Catalytic Biofuel Production and Anaerobic Digestion for Biogas Production. In Energy and Sustainable Futures, Proceedings of the 3rd International Conference on Energy and Sustainable Futures(ICESF), Coventry University, UK, 7–8 September 2022; Springer: Berlin/Heidelberg, Germany, 2021; pp. 125–131. [Google Scholar] [CrossRef]
- Clauser, N.M.; Gutiérrez, S.; Area, M.C.; Felissia, F.E.; Vallejos, M.E. Techno-economic assessment of carboxylic acids, furfural, and pellet production in a pine sawdust biorefinery. Biofuels Bioprod. Biorefining 2018, 12, 997–1012. [Google Scholar] [CrossRef]
- Thakkar, A.; Shell, K.M.; Bertosin, M.; Rodene, D.D.; Amar, V.; Bertucco, A.; Gupta, R.B.; Shende, R.; Kumar, S. Production of levulinic acid and biocarbon electrode material from corn stover through an integrated biorefinery process. Fuel Process. Technol. 2021, 213, 106644. [Google Scholar] [CrossRef]


| Aspect | Conventional Catalysts | Biobased Catalysts |
|---|---|---|
| Source | Synthetic and inorganic materials. | Renewable materials (lignin). |
| Sustainability | Energy-intensive. Use of rare materials. | Biomass-derived, reduced waste. |
| Reusability | Moderate (in some cases higher than biobased); 14 cycles for biodiesel. | Reported values between 5 (for LA) and 9 cycles (for biodiesel). |
| Performance | Up to 80% LA yield from cellulose. | 61% of LA yield from cellulose. |
| Status | Commercial availability. | Under development. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covinich, L.G.; Clauser, N.M.; Area, M.C. Carbon-Based Heterogeneous Catalysis for Biomass Conversion to Levulinic Acid: A Special Focus on the Catalyst. Processes 2025, 13, 2582. https://doi.org/10.3390/pr13082582
Covinich LG, Clauser NM, Area MC. Carbon-Based Heterogeneous Catalysis for Biomass Conversion to Levulinic Acid: A Special Focus on the Catalyst. Processes. 2025; 13(8):2582. https://doi.org/10.3390/pr13082582
Chicago/Turabian StyleCovinich, Laura G., Nicolás M. Clauser, and María C. Area. 2025. "Carbon-Based Heterogeneous Catalysis for Biomass Conversion to Levulinic Acid: A Special Focus on the Catalyst" Processes 13, no. 8: 2582. https://doi.org/10.3390/pr13082582
APA StyleCovinich, L. G., Clauser, N. M., & Area, M. C. (2025). Carbon-Based Heterogeneous Catalysis for Biomass Conversion to Levulinic Acid: A Special Focus on the Catalyst. Processes, 13(8), 2582. https://doi.org/10.3390/pr13082582