Growth and Characterization of Myristic Acid Crystals Doped with Co and Cu and Microbiological Assays for Potential Antimicrobial Applications
Abstract
1. Introduction
2. Experimental Procedures
2.1. Crystal Synthesis
2.2. X-Ray Powder Diffraction (XRPD) Analysis
2.3. Raman Spectroscopy
2.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.5. Morphological Characterization
2.6. Thermogravimetric-Differential Thermal Analysis (TG-DTA)
2.7. Microbiological Assay (Minimal Inhibitory Concentration, MIC Test)
3. Results and Discussion
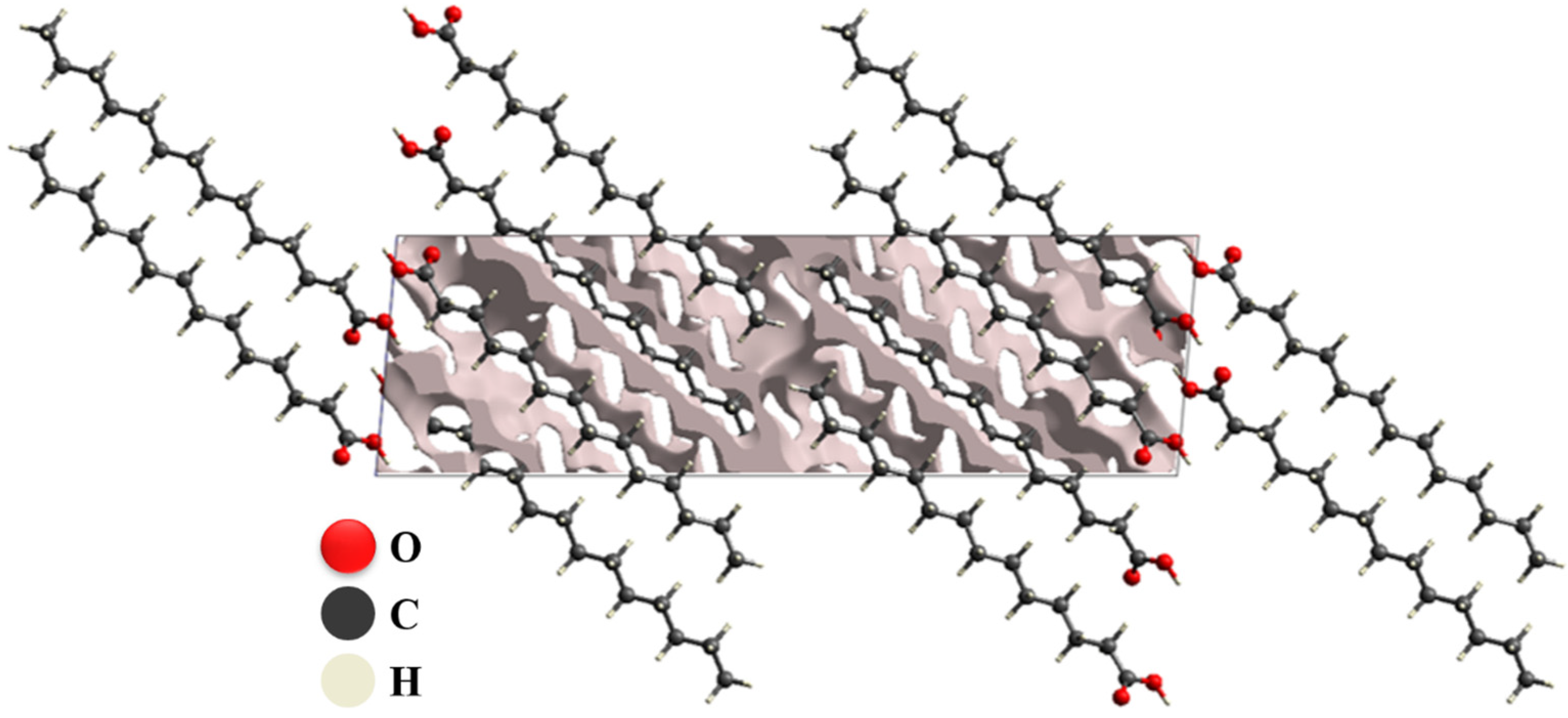
3.1. Crystal Voids
3.2. SEM and EDS Analyses
3.3. XRPD Analysis
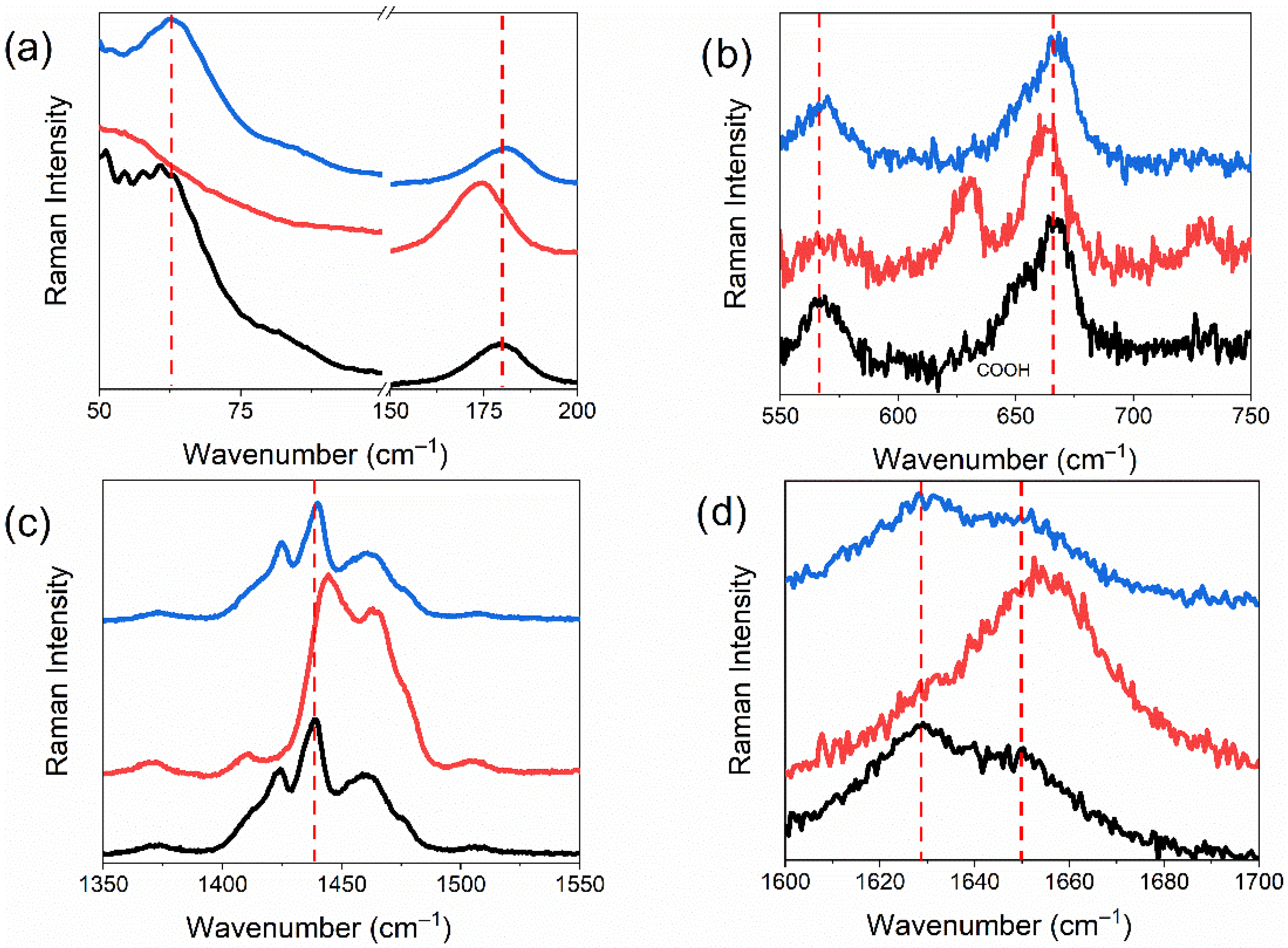
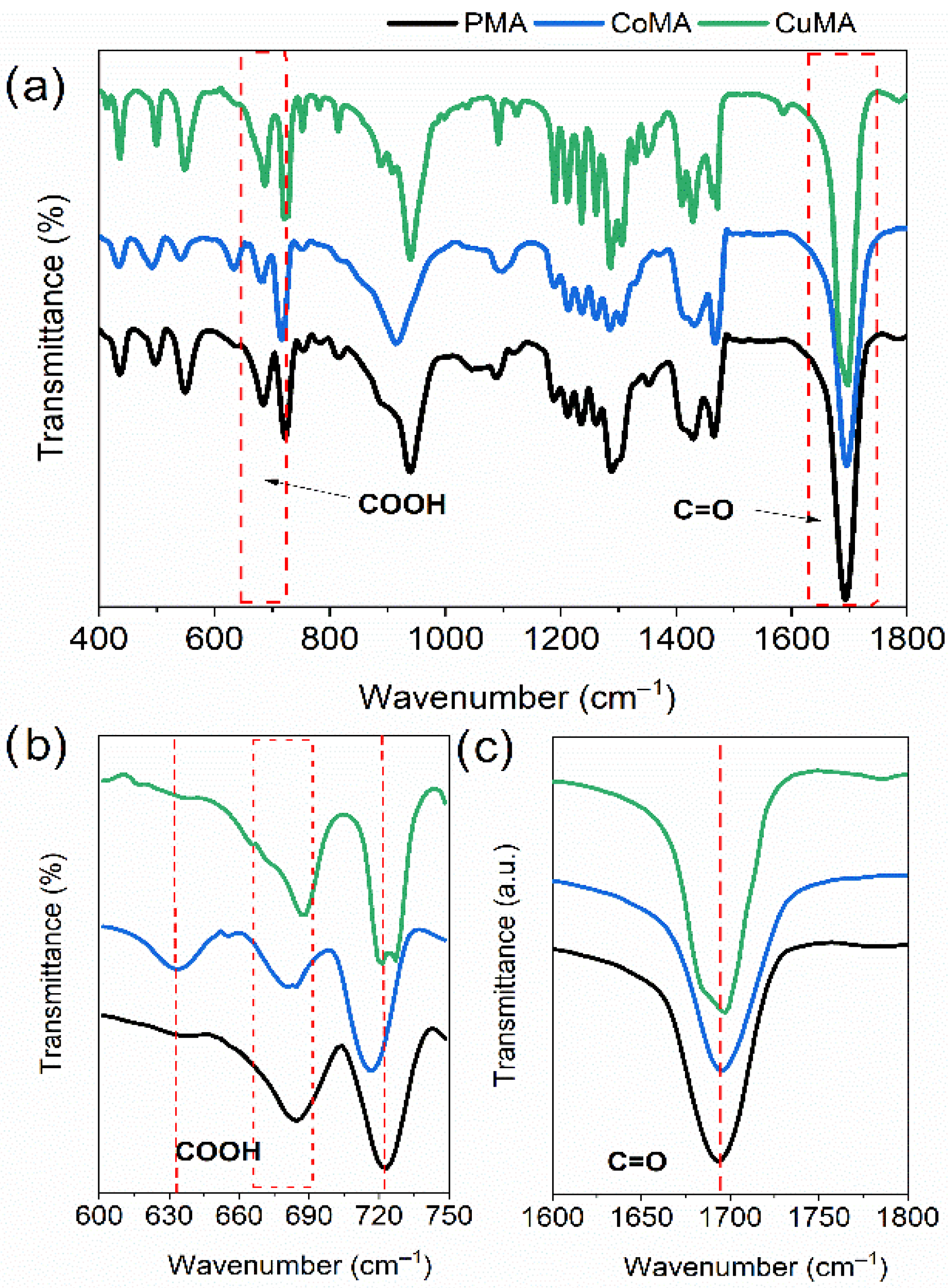
3.4. Raman and FT-IR Spectroscopy
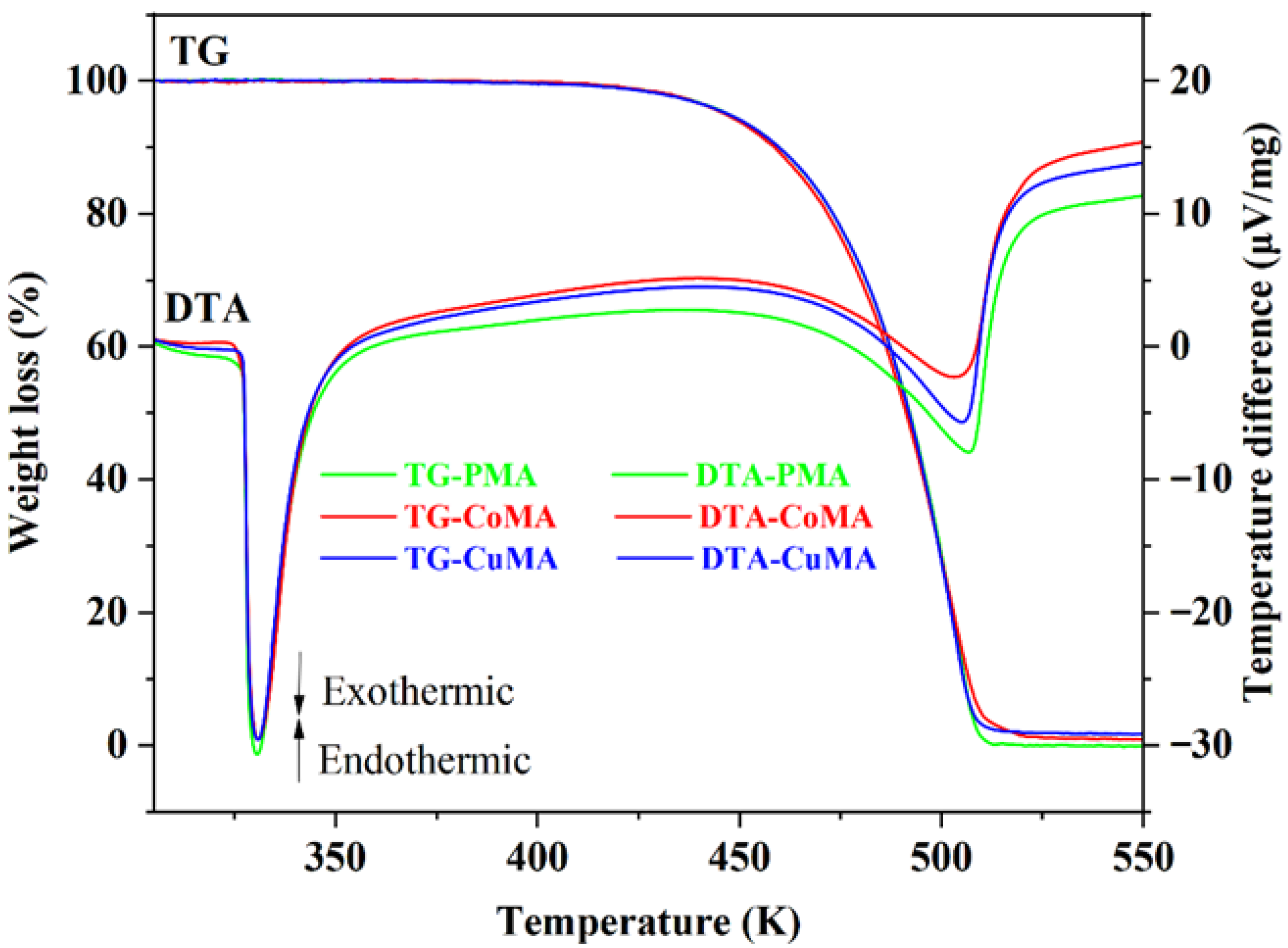
3.5. TG and DTA Analyses
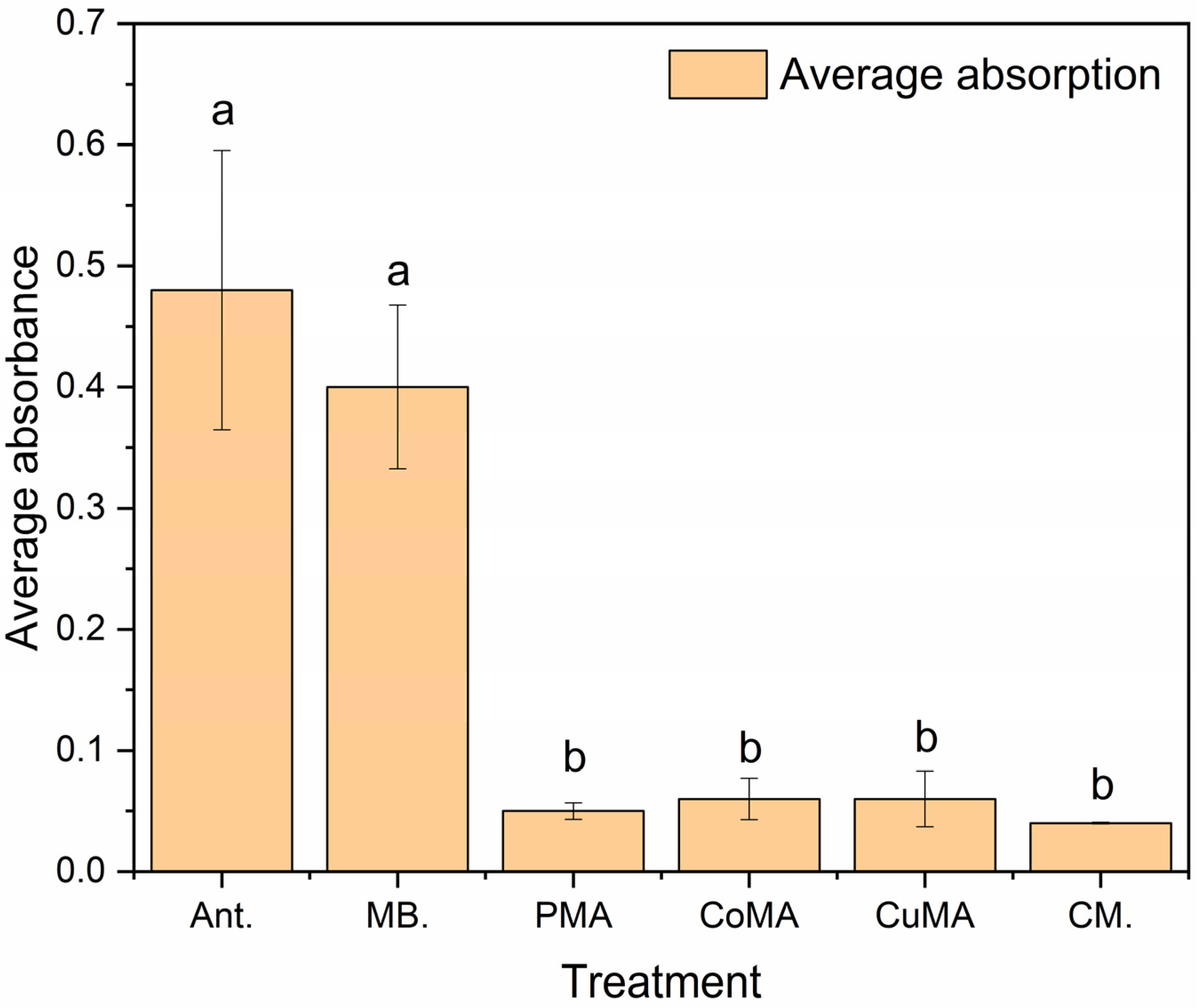
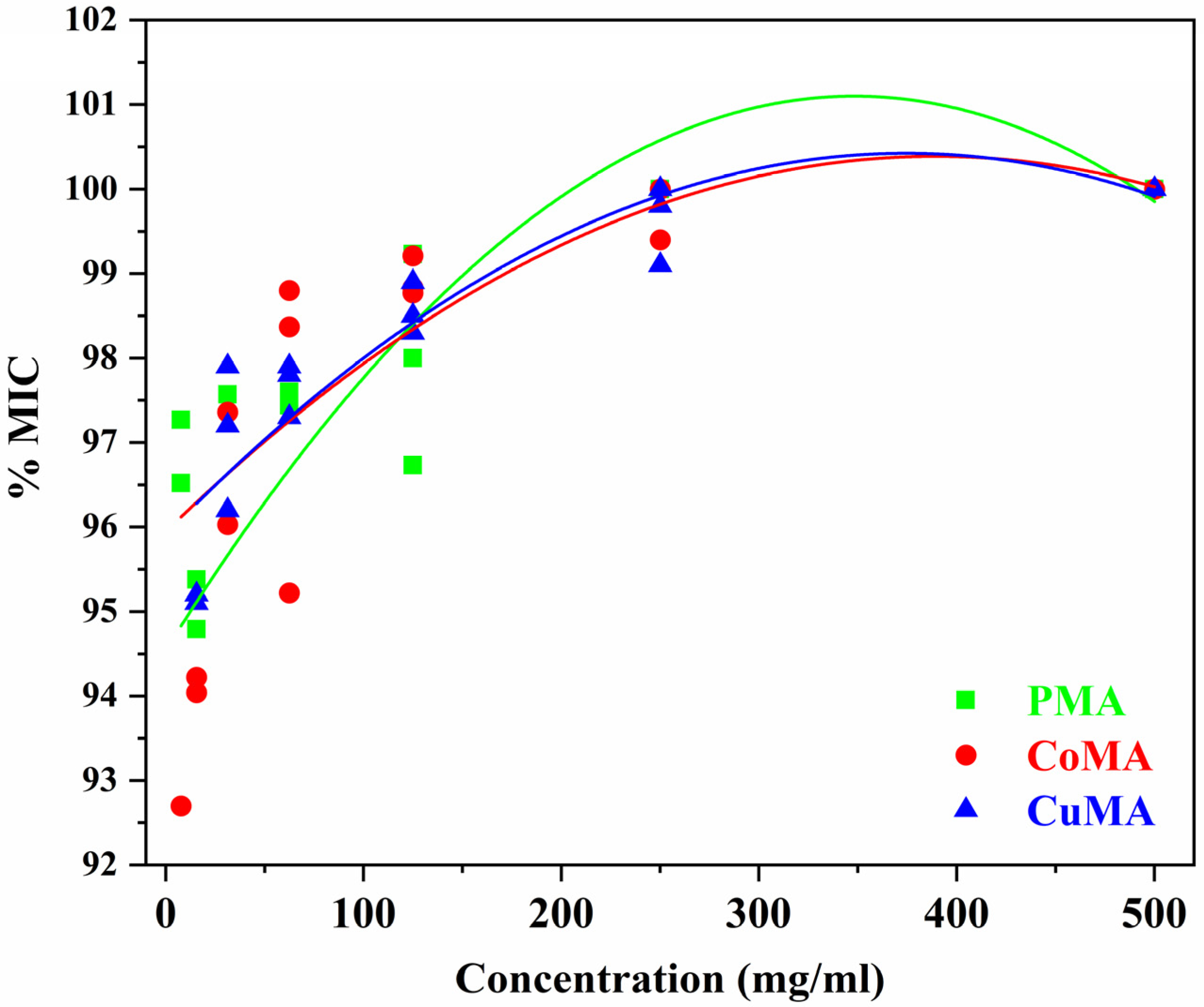
3.6. Biological Assay
Statistical Analysis of the Microbiological Assay
- Figure 10 (green line)—PMA sample:
- Figure 10 (blue line)—CuMA sample:
- Figure 10 (red line)—CoMA sample:
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Wang, Y.; Wang, Y.; Cui, H.; Zhao, G.; Guo, Y.; Wen, J. Effect of myristic acid supplementation on triglyceride synthesis and related genes in the pectoral muscles of broiler chickens. Poult. Sci. 2024, 103, 104038. [Google Scholar] [CrossRef]
- Saraswathi, V.; Kumar, N.; Ai, W.; Gopal, T.; Bhatt, S.; Harris, E.N.; Talmon, G.A.; Desouza, C.V. Myristic acid supplementation aggravates high fat diet-induced adipose inflammation and systemic insulin resistance in mice. Biomolecules 2022, 12, 739. [Google Scholar] [CrossRef]
- Miranda, J.R.S.; de Castro, A.J.R.; da Silva Filho, J.G.; Freire, P.T.C.; Pinheiro, G.S.; Moreira, S.G.C.; Saraiva, G.D.; de Sousa, F.F. Phase transformation in the C form of myristic-acid crystals and DFT calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 208, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Calvo, E.; Gbabode, G.; Cordobilla, R.; Calvet, T.; Cuevas-Diarte, M.À.; Negrier, P.; Mondieig, D. Competing intermolecular interactions in the high-temperature solid phases of even saturated carboxylic acids (C10H19O2H to C20H39O2H). Chem. A Eur. J. 2009, 15, 13141–13149. [Google Scholar] [CrossRef] [PubMed]
- Vandana, L.P.; Ramadoss, R. Herbal myristic acid blended with expanded graphite for thermal regulation in construction materials. J. Energy Storage 2023, 65, 107258. [Google Scholar] [CrossRef]
- Agarwal, P.; Svirskis, D.; Nieuwoudt, M.K. Thermodynamic and spectroscopic evaluation of the eutectic mixture of myristic acid and the local anaesthetics, bupivacaine and ropivacaine. RSC Pharm. 2024, 1, 296–304. [Google Scholar] [CrossRef]
- Javid, S.; Shanmugarajan, D.; Kumar, H.Y.; Arivuselvam, R.; Anjum, N.F.; Purohit, M.N.; Susil, A.; Harindranath, H.; Nilugal, K.C.; Nagojappa, N.B.S.; et al. Rational design, synthesis, analysis and antifungal activity of novel myristic acid derivatives as n-myristoyltransferase inhibitors. J. Mol. Struct. 2024, 1303, 137568. [Google Scholar] [CrossRef]
- Vijayarohini, P.; Kavitha, G.; Bangaru Sudarsan Alwar, S.; Mercy, A.S.C. Antimicrobial activity of selective transition metal co-ordination complexes of myristic acid. Mater. Today Proc. 2020, 33, 4198–4205. [Google Scholar] [CrossRef]
- Bashiri Rezaie, A.; Montazer, M.; Mahmoudi Rad, M. Facile fabrication of cytocompatible polyester fiber composite incorporated via photocatalytic nano copper ferrite/myristic-lauric fatty acids coating with antibacterial and hydrophobic performances. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109888. [Google Scholar] [CrossRef]
- Ling, L.; Cai, S.; Zuo, Y.; Meng, T.; Tian, H.; Bao, X.; Xu, G. Wettability transition of superhydrophobic myristic acid/dodecyl mercaptan/Cu@ZIF-8/HA coating modified magnesium alloy driven by visible light for antibacterial and osteogenic applications. Prog. Org. Coat. 2023, 184, 107828. [Google Scholar] [CrossRef]
- Habib, M.L.; Disha, S.A.; Sahadat Hossain, M.; Uddin, M.N.; Ahmed, S. Enhancement of antimicrobial properties by metals doping in nano-crystalline hydroxyapatite for efficient biomedical applications. Heliyon 2024, 10, e23845. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Moroda, M.D.; Leta Deressa, T.; Tiwikrama, A.H.; Chala, T.F. Green synthesis of copper oxide nanoparticles using rosmarinus officinalis leaf extract and evaluation of its antimicrobial activity. Next Mater. 2025, 7, 100337. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Effect of catalysis on the stability of metallic nanoparticles: Suzuki reaction catalyzed by PVP-palladium nanoparticles. J. Am. Chem. Soc. 2003, 125, 8340–8347. [Google Scholar] [CrossRef]
- Ameen, F.; Majrashi, N. Recent trends in the use of cobalt ferrite nanoparticles as an antimicrobial agent for disability infections: A review. Inorg. Chem. Commun. 2023, 156, 111187. [Google Scholar] [CrossRef]
- Şahin, N.; Üstün, E.; Özdemir, İ.; Günal, S.; Özdemir, N.; Bülbül, H.; Gürbüz, N.; Özdemir, İ.; Sémeril, D. Antimicrobial activities of bis-(n-alkylbenzimidazole)-cobalt(II) and zinc(II) complexes. Inorg. Chem. Commun. 2023, 157, 111396. [Google Scholar] [CrossRef]
- Khan, R.T.; Rasool, S. Nanotechnology: A new strategy to combat bacterial infections and antibiotic resistant bacteria. In Nanotechnology and Human Health: Current Research and Future Trends; Elsevier: Amsterdam, The Netherlands, 2023; pp. 167–190. [Google Scholar] [CrossRef]
- Kado, C.I.; Heskett, M.G. Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathology 1970, 60, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-candida activity of brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef]
- Abreu, D.C.; Filho, P.F.F.; Pinheiro, G.S.; Freire, P.T.C.; Moreira, S.G.C.; dos Santos, A.O.; de Sousa, F.F. Polymorphism at hexadecanoic-acid crystals investigated through structural and vibrational studies. Vib. Spectrosc. 2022, 121, 103402. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Kumari, M.; Sheoran, S.; Prakash, D.; Yadav, D.B.; Yadav, P.K.; Jat, M.K.; Ankit; Apurva. Long-term application of organic manures and chemical fertilizers improve the organic carbon and microbiological properties of soil under pearl millet-wheat cropping system in north-western india. Heliyon 2024, 10, e25333. [Google Scholar] [CrossRef]
- Bilen, M.V.; Uzun, P.; Yıldız, H.; Fındık, B.T. Evaluation of the effect of active essential oil components added to pickled-based marinade on beef stored under vacuum packaging: Insight into physicochemical and microbiological quality. Int. J. Food Microbiol. 2024, 418, 110733. [Google Scholar] [CrossRef]
- Agnoli, S.; Favaro, M. Doping graphene with boron: A review of synthesis methods, physicochemical characterization, and emerging applications. J. Mater. Chem. A Mater. 2016, 4, 5002–5025. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Visualisation and characterisation of voids in crystalline materials. CrystEngComm 2011, 13, 1804–1813. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. Crystalexplorer: A program for hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Soldatov, D.V.; Moudrakovski, I.L.; Grachev, E.V.; Ripmeester, J.A. Micropores in crystalline dipeptides as seen from the crystal structure, He pycnometry, and 129Xe NMR spectroscopy. J. Am. Chem. Soc. 2006, 128, 6737–6744. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.M.; Patil, S.S.; Sonawane, K.D.; More, V.B.; Patil, P.S. Hydrothermally synthesized copper oxide nanoparticles: Rietveld analysis and antimicrobial studies. Surf. Interfaces 2024, 51, 104598. [Google Scholar] [CrossRef]
- Bin Mobarak, M.; Hossain, M.S.; Chowdhury, F.; Ahmed, S. Synthesis and characterization of CuO nanoparticles utilizing waste fish scale and exploitation of XRD peak profile analysis for approximating the structural parameters. Arab. J. Chem. 2022, 15, 104117. [Google Scholar] [CrossRef]
- Santos Souza, G.D.; Amado, A.M.; Teixeira, A.M.R.; Freire, P.T.C.; Saraiva, G.D.; Pinheiro, G.S.; Moreira, S.G.C.; De Sousa, F.F.; Nogueira, C.E.S. Low-temperature phase transition of dodecanoic acid crystals: A study using raman, powder x-ray diffraction, and density functional theory calculations. Cryst. Growth Des. 2019, 20, 281–290. [Google Scholar] [CrossRef]
- de Matos, F.C.; da Costa, M.C.; de Almeida Meirelles, A.J.; Batista, E.A.C. Binary solid–liquid equilibrium systems containing fatty acids, fatty alcohols and triolein by differential scanning calorimetry. Fluid. Phase Equilib. 2015, 404, 1–8. [Google Scholar] [CrossRef]
- Wigzell, F.A.; Jackson, S.D. The genesis of supported cobalt catalysts. Appl. Petrochem. Res. 2016, 7, 9–21. [Google Scholar] [CrossRef]
- Leong, K.Y.; Abdul Rahman, M.R.; Gurunathan, B.A. Nano-enhanced phase change materials: A review of thermo-physical properties, applications and challenges. J. Energy Storage 2019, 21, 18–31. [Google Scholar] [CrossRef]
- Freeman, T.B.; Foster, K.E.O.; Troxler, C.J.; Irvin, C.W.; Aday, A.; Boetcher, S.K.S.; Mahvi, A.; Smith, M.K.; Odukomaiya, A.; Freeman, T.B.; et al. Advanced materials and additive manufacturing for phase change thermal energy storage and management: A review. Adv. Energy Mater. 2023, 13, 2204208. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rocha, V.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and antiadhesive properties of a biosurfactant isolated from lactobacillus paracasei ssp. paracasei A20. Lett. Appl. Microbiol. 2010, 50, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Lingaraj, B.; Naik, M.K.; Ajithkumar, K.; Yenjerappa, S.T.; Pampanna, Y.; Kisan, B.; Muthusamy, A.; Murali, T.S. Molecular characterization and exploration of streptomycin resistance genes in Xanthomonas campestris pv. campestris isolates from diverse agro climatic regions of karnataka state, southern india. Eur. J. Plant Pathol. 1–11. [CrossRef]
- Silva, L.A.L.; Silva, A.A.L.; Rios, M.A.S.; Brito, M.P.; Araújo, A.R.; Silva, D.A.; Peña-Garcia, R.R.; Silva-Filho, E.C.; Magalhães, J.L.; Matos, J.M.E.; et al. Insights into the antimicrobial activity of hydrated cobaltmolybdate doped with copper. Molecules 2021, 26, 1267. [Google Scholar] [CrossRef] [PubMed]
- Panicker, S.; Mathew, A.; Dalvi, S.; Chattaraj, M. Medicinal properties of cobalt and copper nanoparticles synthesized using limonia acidissima leaf extract. Biotechnol. J. Int. 2024, 28, 36–45. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial poperties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Pu, Y.; Sun, L.; Wang, Y.; Qi, D.; Chen, D.; Liu, H.; Xu, D.; Deng, C.; Li, J. Modeling inhibitory activity of a novel antimicrobial peptide AMPNT-6 from bacillus subtilis against vibrio parahaemolyticus in shrimp under various environmental conditions. Food Control 2013, 33, 249–253. [Google Scholar] [CrossRef]
- Adamson, R.H. The acute lethal dose 50 (LD50) of caffeine in albino rats. Regul. Toxicol. Pharmacol. 2016, 80, 274–276. [Google Scholar] [CrossRef] [PubMed]




| CIF [4] | PMA | CoMA | CuMA | |
|---|---|---|---|---|
| a (Å) | 31.628(2) | 31.701(5) | 31.647(8) | 31.570(7) |
| b (Å) | 4.966(9) | 4.957(1) | 4.932(7) | 4.939(2) |
| c (Å) | 9.492(2) | 9.470(5) | 9.385(5) | 9.456(1) |
| α° | 90.000(0) | 90.000(0) | 90.000(0) | 90.000(0) |
| β° | 95.105(7) | 95.347(0) | 94.284(0) | 95.422(0) |
| γ° | 90.000(0) | 90.000(0) | 90.000(0) | 90.000(0) |
| Volume (Å)3 | 1485.25 | 1481.84 | 1461.10 | 1467.96 |
| Crystallite (nm) | 675.14 | 312.14 | 165.55 | 307.26 |
| C. Structure | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/c | P21/c |
| Raman-Active Modes | IR-Active Modes | |||||
|---|---|---|---|---|---|---|
| (cm−1) | Assignments | (cm−1) | Assignments | |||
| 50 | XX | 600 | XX | |||
| 161 | τ(CCCC)(60) | 672 | sc(C14O2)(61) | |||
| 167 | τ(CCCC)(65) | 687 | sc(C14O2)(61) | |||
| 181 | δ(CCC)(78) | 720 | τ(C13C14O15H)(80) | |||
| 187 | δ(CCC)(78) | 728 | τ(C13C14O15H)(80) | |||
| 200 | XX | 750 | XX | |||
| 550 | XX | 1600 | XX | |||
| 571 | δ(CCC)(64) | 1678 | ν(C=O)(83) | |||
| 584 | δ(CCC)(64) | 1717 | ν(C=O)(83) | |||
| 654 | sc(C14O2)(61) | 1800 | XX | |||
| 671 | τ(HO15C14C13)(80) | |||||
| 742 | ρ(CH2)(60) | |||||
| 750 | XX | |||||
| 1350 | XX | |||||
| 1372 | wag(C1H3)(84) | |||||
| 1381 | XX | |||||
| 1411 | sc(C13H2)(88) | |||||
| 1437 | sc(CH2)(69) | |||||
| 1450 | sc(CH2)(73) | |||||
| 1462 | sc(CH2)(55) | |||||
| 1475 | sc(CH2)(64) | |||||
| 1507 | sc(CH2)(71) | |||||
| 1550 | XX | |||||
| 1600 | XX | |||||
| 1626 | ν(C=O)(83) | |||||
| 1649 | ν(C=O)(83) | |||||
| 1700 | XX | |||||
| TG | DTA | ||||
|---|---|---|---|---|---|
| Tonset (K) | Tmidset (K) | Tendset (K) | Weight Loss (mg) | T (K) | |
| PMA | 480.3 | 492.2 | 509.8 | 4.00 | 331 |
| CoMA | 477.4 | 491.3 | 509.8 | 3.99 | |
| CuMA | 484.9 | 492.2 | 508.5 | 3.92 | |
| Samples | Inhibitory Activity | ||||||
|---|---|---|---|---|---|---|---|
| Concentrations | |||||||
| A | B | C | D | E | F | G | |
| 500 µg/mL | 250 µg/mL | 125 µg/mL | 62.5 µg/mL | 31.3 µg/mL | 15.6 µg/mL | 7.8 µg/mL | |
| PMA | * | * | * | * | * | ** | ** |
| CoMA | * | * | * | * | * | * | * |
| CuMA | * | * | * | * | * | * | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, L.A.C.; de Oliveira Neto, J.G.; de Souza Junior, M.V.; dos Santos, A.O.; Batista, T.F.V.; Moreira, S.G.C.; de Sousa, F.F.; Paschoal, W., Jr. Growth and Characterization of Myristic Acid Crystals Doped with Co and Cu and Microbiological Assays for Potential Antimicrobial Applications. Processes 2025, 13, 3481. https://doi.org/10.3390/pr13113481
Vieira LAC, de Oliveira Neto JG, de Souza Junior MV, dos Santos AO, Batista TFV, Moreira SGC, de Sousa FF, Paschoal W Jr. Growth and Characterization of Myristic Acid Crystals Doped with Co and Cu and Microbiological Assays for Potential Antimicrobial Applications. Processes. 2025; 13(11):3481. https://doi.org/10.3390/pr13113481
Chicago/Turabian StyleVieira, Luiz A. Cohen, João G. de Oliveira Neto, Marinaldo V. de Souza Junior, Adenilson O. dos Santos, Telma F. Vieira Batista, Sanclayton G. Carneiro Moreira, Francisco F. de Sousa, and Waldomiro Paschoal, Jr. 2025. "Growth and Characterization of Myristic Acid Crystals Doped with Co and Cu and Microbiological Assays for Potential Antimicrobial Applications" Processes 13, no. 11: 3481. https://doi.org/10.3390/pr13113481
APA StyleVieira, L. A. C., de Oliveira Neto, J. G., de Souza Junior, M. V., dos Santos, A. O., Batista, T. F. V., Moreira, S. G. C., de Sousa, F. F., & Paschoal, W., Jr. (2025). Growth and Characterization of Myristic Acid Crystals Doped with Co and Cu and Microbiological Assays for Potential Antimicrobial Applications. Processes, 13(11), 3481. https://doi.org/10.3390/pr13113481









