Optimizing Aspergillus oryzae Inoculation Dosage and Fermentation Duration for Enhanced Protein Content in Soybean Meal and Its Influence on Dog Food Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Spore Suspension Preparation
2.2. Fermentation of Soybean Meal
2.3. Chemical Analysis
2.4. In Vitro Protein Digestibility
2.5. Diet Formulation
2.6. Rapid Viscosity Analysis
2.7. Particle Size Distribution
2.8. Extrusion Processing
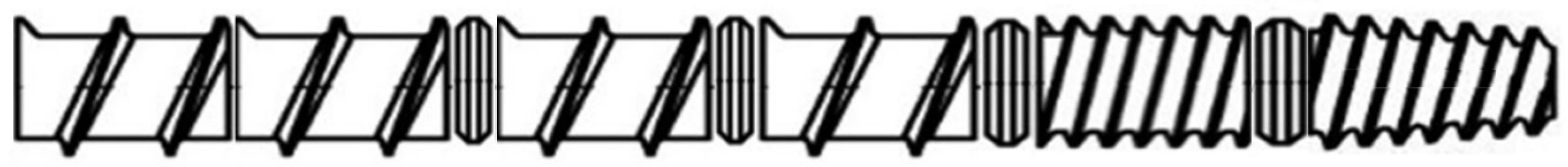
- Screw element 1: inlet screw, single-flight full-pitch;
- Screw element 2: single-flight, full-pitch screw;
- Screw element 3: small steam lock;
- Screw element 4: single-flight full-pitch screw;
- Screw element 5: small steam lock;
- Screw element 6: single-flight, full-pitch screw;
- Screw element 7: medium steam lock;
- Screw element 8: double-flight, ½-pitch screw;
- Screw element 9: large steam lock;
- Screw element 10: double--flight, ½-pitch, cone screw.
2.9. Kibble Physical Characteristics
2.10. Statistical Analysis
3. Results
3.1. Optimal Inoculation Dosage and Fermentation Duration (Experiment 1)
3.2. Impact of Fermentation on Nutrient Profiles and Antinutritional Factors (Experiment 2)
3.3. Raw Mix Characteristics
3.3.1. Rapid Viscosity Analysis
3.3.2. Particle Size Distribution
3.4. Extrusion Performance of A. oryzae Fermented Soybean Meal (Experiment 3)
3.4.1. Extrusion Processing
3.4.2. Kibble Characteristics
3.4.3. Experimental Diets
4. Discussion
4.1. Optimal Inoculation Dosage and Fermentation Duration (Experiment 1)
4.1.1. Crude Protein
4.1.2. In Vitro Protein Digestibility
4.1.3. Fiber Composition
4.2. Impact of Fermentation on Nutrient Profiles and Antinutritional Factors (Experimental 2)
4.2.1. Changes of Macronutrient Contents
4.2.2. Amino Acid Composition
4.2.3. Oligosaccharides
4.2.4. Trypsin Inhibitor
4.3. Extrusion Performance of A. oryzae Fermented Soybean Meal (Experiment 3)
4.3.1. Raw Mix Characteristics and Extrusion Process
4.3.2. Kibble Characteristics
4.3.3. Experimental Diets
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TI | Trypsin inhibitor |
| OS | Oligosaccharides |
| KTI | Kunitz trypsin inhibitor |
| BBI | Bowman–Birk trypsin inhibitor |
| DM | Dry matter |
| CP | Crude protein |
| CF | Crude fat |
| OM | Organic matter |
| TDF | Total dietary fiber |
| IDF | Insoluble dietary fiber |
| SDF | Soluble dietary fiber |
| GE | Gross energy |
| SEI | Sectional expansion index |
| SME | Specific mechanical energy |
| RVA | Rapid viscosity analysis |
| PSD | Particle size distribution |
References
- USDA FAS Soybeans|USDA Foreign Agricultural Service. Available online: https://fas.usda.gov/data/production/commodity/2222000 (accessed on 6 June 2024).
- Grieshop, C.M.; Kadzere, C.T.; Clapper, G.M.; Flickinger, E.A.; Bauer, L.L.; Frazier, R.L.; Fahey, G.C. Chemical and Nutritional Characteristics of United States Soybeans and Soybean Meals. J. Agric. Food Chem. 2003, 51, 7684–7691. [Google Scholar] [CrossRef]
- Ruiz, N.; Parsons, C.M.; Stein, H.H.; Coon, C.N.; van Eys, J.E.; Miles, R.D. A Historical Look at the Soybean and Its Use for Animal Feed. Available online: https://nutrition.ansci.illinois.edu/node/1566 (accessed on 14 July 2024).
- Stein, H.H.; Berger, L.L.; Drackley, J.K.; Fahey, G.C.; Hernot, D.C.; Parsons, C.M. Nutritional Properties and Feeding Values of Soybeans and Their Coproducts. In Soybeans; Johnson, L.A., White, P.J., Galloway, R., Eds.; AOCS Press: Champaign, IL, USA, 2008; pp. 613–660. ISBN 978-1-893997-64-6. [Google Scholar]
- Gomi, K. Aspergillus: Aspergillus oryzae. In Encyclopedia of Food Microbiology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 92–96. [Google Scholar]
- Frias, J.; Song, Y.S.; Martínez-Villaluenga, C.; De Mejia, E.G.; Vidal-Valverde, C. Immunoreactivity and Amino Acid Content of Fermented Soybean Products. J. Agric. Food Chem. 2008, 56, 99–105. [Google Scholar] [CrossRef]
- Hong, K.-J.; Lee, C.-H.; Kim, S.W. Aspergillus oryzae GB-107 Fermentation Improves Nutritional Quality of Food Soybeans and Feed Soybean Meals. J. Med. Food 2004, 7, 430–435. [Google Scholar] [CrossRef]
- Teng, D.; Gao, M.; Yang, Y.; Liu, B.; Tian, Z.; Wang, J. Bio-Modification of Soybean Meal with Bacillus Subtilis or Aspergillus oryzae. Biocatal. Agric. Biotechnol. 2012, 1, 32–38. [Google Scholar] [CrossRef]
- Yang, H.; Qu, Y.; Li, J.; Liu, X.; Wu, R.; Wu, J. Improvement of the Protein Quality and Degradation of Allergens in Soybean Meal by Combination Fermentation and Enzymatic Hydrolysis. LWT 2020, 128, 109442. [Google Scholar] [CrossRef]
- Sukhikh, S.; Kalashnikova, O.; Ivanova, S.; Prosekov, A.; Krol, O.; Kriger, O.; Fedovskikh, N.; Babich, O. Evaluating the Influence of Microbial Fermentation on the Nutritional Value of Soybean Meal. Fermentation 2022, 8, 458. [Google Scholar] [CrossRef]
- Beloshapka, A.N.; De Godoy, M.R.C.; Detweiler, K.B.; Newcomb, M.; Ellegård, K.H.; Fahey, G.C.; Swanson, K.S. Apparent Total Tract Macronutrient Digestibility, Fecal Characteristics, and Fecal Fermentative End-Product Concentrations of Healthy Adult Dogs Fed Bioprocessed Soy Protein1. J. Anim. Sci. 2016, 94, 3826–3834. [Google Scholar] [CrossRef]
- Cervantes-Pahm, S.K.; Stein, H.H. Ileal Digestibility of Amino Acids in Conventional, Fermented, and Enzyme-Treated Soybean Meal and in Soy Protein Isolate, Fish Meal, and Casein Fed to Weanling Pigs1. J. Anim. Sci. 2010, 88, 2674–2683. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.R.; Lu, Y.P.; Liu, Y.Y. The Effect of Aspergillus oryzae Fermented Soybean Meal on Growth Performance, Digestibility of Dietary Components and Activities of Intestinal Enzymes in Weaned Piglets. Anim. Feed. Sci. Technol. 2007, 134, 295–303. [Google Scholar] [CrossRef]
- Sumi, H.; Hamada, H.; Nakanishi, K.; Hiratani, H. Enhancement of the Fibrinolytic Activity in Plasma by Oral Administration of Nattokinase. Acta Haematol. 1990, 84, 139–143. [Google Scholar] [CrossRef]
- Kim, M.H.; Yun, C.H.; Lee, C.H.; Ha, J.K. The Effects of Fermented Soybean Meal on Immunophysiological and Stress-Related Parameters in Holstein Calves after Weaning. J. Dairy Sci. 2012, 95, 5203–5212. [Google Scholar] [CrossRef]
- Yuan, L.; Chang, J.; Yin, Q.; Lu, M.; Di, Y.; Wang, P.; Wang, Z.; Wang, E.; Lu, F. Fermented Soybean Meal Improves the Growth Performance, Nutrient Digestibility, and Microbial Flora in Piglets. Anim. Nutr. 2017, 3, 19–24. [Google Scholar] [CrossRef]
- Tsumura, K.; Saito, T.; Tsuge, K.; Ashida, H.; Kugimiya, W.; Inouye, K. Functional Properties of Soy Protein Hydrolysates Obtained by Selective Proteolysis. LWT-Food Sci. Technol. 2005, 38, 255–261. [Google Scholar] [CrossRef]
- Ojokoh, A.O.; Yimin, W.; Eromosele, O.S. Effect of Some Extrusion Variables on Fermented Maize-Soybean Blend. J. Food. Sci. Technol. 2015, 52, 5763–5771. [Google Scholar] [CrossRef]
- Jin, Z.; Hsieh, F.; Huff, H.E. Effects of Soy Fiber, Salt, Sugar and Screw Speed on Physical Properties and Microstructure of Corn Meal Extrudate. J. Cereal Sci. 1995, 22, 185–194. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Zhu, Q.; Qian, G. Optimization of Solid-State Fermentation with Lactobacillus Brevis and Aspergillus oryzae for Trypsin Inhibitor Degradation in Soybean Meal. J. Integr. Agric. 2013, 12, 869–876. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2019. [Google Scholar]
- Bhatti, T.; Chambers, R.E.; Clamp, J.R. The Gas Chromatographic Properties of Biologically Important N-Acetylglucosamine Derivatives, Monosaccharides, Disaccharides, Trisaccharides, Tetrasaccharides and Pentasaccharides. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1970, 222, 339–347. [Google Scholar] [CrossRef]
- Kim, S.; Krishnan, H.B. A Fast and Cost-Effective Procedure for Reliable Measurement of Trypsin Inhibitor Activity in Soy and Soy Products. In Methods in Enzymology; Biochemical Pathways and Environmental Responses in Plants: Part, B; Jez, J., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 680, pp. 195–213. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- de Godoy, M.R.C.; Bauer, L.L.; Parsons, C.M.; Fahey, G.C., Jr. Select Corn Coproducts from the Ethanol Industry and Their Potential as Ingredients in Pet Foods. J. Anim. Sci. 2009, 87, 189–199. [Google Scholar] [CrossRef]
- AAFCO Dog Food Nutrient Profiles Based on Dry Matter. 2016 Official Publication; Association of American Feed Control Officials Inc: Champaign, IL, USA, 2016; pp. 158–159. [Google Scholar]
- Patwa, A.; Malcolm, B.; Wilson, J.; Ambrose, R.P.K. Particle Size Analysis of Two Distinct Classes of Wheat Flour by Sieving. ASABE 2014, 57, 151–159. [Google Scholar] [CrossRef]
- Chutmanop, J.; Chuichulcherm, S.; Chisti, Y.; Srinophakun, P. Protease Production by Aspergillus oryzae in Solid-state Fermentation Using Agroindustrial Substrates. J. Chem. Technol. Biotechnol. 2008, 83, 1012–1018. [Google Scholar] [CrossRef]
- Das, P.P.; Xu, C.; Lu, Y.; Khorsandi, A.; Tanaka, T.; Korber, D.; Nickerson, M.; Rajagopalan, N. Snapshot of Proteomic Changes in Aspergillus oryzae during Various Stages of Fermentative Processing of Pea Protein Isolate. Food Chem. Mol. Sci. 2023, 6, 100169. [Google Scholar] [CrossRef]
- Eugster, P.J.; Salamin, K.; Grouzmann, E.; Monod, M. Production and Characterization of Two Major Aspergillus oryzae Secreted Prolyl Endopeptidases Able to Efficiently Digest Proline-Rich Peptides of Gliadin. Microbiology 2015, 161, 2277–2288. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Reis, A.P.; Guimarães, V.M.; Falkoski, D.L.; da Silva Fialho, L.; de Rezende, S.T. Purification and Characterization of Aspergillus Terreus α-Galactosidases and Their Use for Hydrolysis of Soymilk Oligosaccharides. Appl. Biochem. Biotechnol. 2011, 164, 1111–1125. [Google Scholar] [CrossRef]
- Hoa, B.T.; Hung, P.V. Optimization of Nutritional Composition and Fermentation Conditions for Cellulase and Pectinase Production by Aspergillus oryzae Using Response Surface Methodology. Int. Food Res. J. 2013, 20, 3269–3274. [Google Scholar]
- Samarntarn, W.; Cheevadhanarak, S.; Tanticharoen, M. Production of Alkaline Protease by a Genetically Engineered Aspergillus oryzae U1521. J. Gen. Appl. Microbiol. 1999, 45, 99–103. [Google Scholar] [CrossRef]
- Shankar, S.K.; Mulimani, V.H. α-Galactosidase Production by Aspergillus oryzae in Solid-State Fermentation. Bioresour. Technol. 2007, 98, 958–961. [Google Scholar] [CrossRef]
- Vishwanatha, K.S.; Rao, A.G.A.; Singh, S.A. Acid Protease Production by Solid-State Fermentation Using Aspergillus oryzae MTCC 5341: Optimization of Process Parameters. J. Ind. Microbiol. Biotechnol. 2010, 37, 129–138. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, L.-L.; Pan, Z.-H.; Kong, D.-H.; Hadiatullah, H.; Fan, Z.-C. Proteinase and Glycoside Hydrolase Production Is Enhanced in Solid-State Fermentation by Manipulating the Carbon and Nitrogen Fluxes in Aspergillus oryzae. Food Chem. 2019, 271, 606–613. [Google Scholar] [CrossRef]
- Suprayogi, W.P.S.; Ratriyanto, A.; Akhirini, N.; Hadi, R.F.; Setyono, W.; Irawan, A. Changes in Nutritional and Antinutritional Aspects of Soybean Meals by Mechanical and Solid-State Fermentation Treatments with Bacillus subtilis and Aspergillus oryzae. Bioresour. Technol. Rep. 2022, 17, 100925. [Google Scholar] [CrossRef]
- Sandhya, C.; Sumantha, A.; Szakacs, G.; Pandey, A. Comparative Evaluation of Neutral Protease Production by Aspergillus oryzae in Submerged and Solid-State Fermentation. Process Biochem. 2005, 40, 2689–2694. [Google Scholar] [CrossRef]
- Battaglino, R.A.; Huergo, M.; Pilosof, A.M.R.; Bartholomai, G.B. Culture Requirements for the Production of Protease by Aspergillus oryzae in Solid State Fermentation. Appl. Microbiol. Biotechnol. 1991, 35, 292–296. [Google Scholar] [CrossRef]
- Park, M.K.; Seo, J.-A.; Kim, Y.-S. Comparative Study on Metabolic Changes of Aspergillus oryzae Isolated from Fermented Foods According to Culture Conditions. Int. J. Food Microbiol. 2019, 307, 108270. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.W.; Drouliscos, N.J.; Worgan, J.T. Composition and Nutritional Evaluation of Aspergillus oryzae Biomass Grown on Palm Oil Processing Effluents. J. Sci. Food Agric. 1981, 32, 1014–1020. [Google Scholar] [CrossRef]
- Sardjono; Zhu, Y.; Knol, W. Comparison of Fermentation Profiles between Lupine and Soybean by Aspergillus oryzae and Aspergillus Sojae in Solid-State Culture Systems. J. Agric. Food Chem. 1998, 46, 3376–3380. [Google Scholar] [CrossRef]
- Amadou, I.; Amza, T.; Foh, M.; Le, M. Influence of Lactobacillus Plantarum Lp6 Fermentation on the Functional Properties of Soybean Protein Meal. Emir. J. Food Agric. 2010, 22, 456. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Zou, X.; He, J.; Xu, X.; Zhou, G.; Li, C. In Vitro Protein Digestibility of Pork Products Is Affected by the Method of Processing. Food Res. Int. 2017, 92, 88–94. [Google Scholar] [CrossRef]
- Wei, Z.; Kang, J.; Liao, M.; Ju, H.; Fan, R.; Shang, J.; Ning, X.; Li, M. Investigating Changes of Proteome in the Bovine Milk Serum after Retort Processing Using Proteomics Techniques. Food Sci. Nutr. 2021, 10, 307–316. [Google Scholar] [CrossRef]
- Choct, M. Feed Non-Starch Polysaccharides: Chemical Structures and Nutritional Significance. Feed Milling Int. 1997, 191, 13–26. [Google Scholar]
- Choct, M.; Dersjant-Li, Y.; Mcleish, J.; Peisker, M. Soy Oligosaccharides and Soluble Non-Starch Polysaccharides: A Review of Digestion, Nutritive and Anti-Nutritive Effects in Pigs and Poultry. Asian-Australas. J. Anim. Sci. 2010, 23, 1386–1398. [Google Scholar] [CrossRef]
- Freire, J.P.B.; Guerreiro, A.J.G.; Cunha, L.F.; Aumaitre, A. Effect of Dietary Fibre Source on Total Tract Digestibility, Caecum Volatile Fatty Acids and Digestive Transit Time in the Weaned Piglet. Anim. Feed. Sci. Technol. 2000, 87, 71–83. [Google Scholar] [CrossRef]
- Smiricky, M.R.; Grieshop, C.M.; Albin, D.M.; Wubben, J.E.; Gabert, V.M.; Fahey, G.C., Jr. The Influence of Soy Oligosaccharides on Apparent and True Ileal Amino Acid Digestibilities and Fecal Consistency in Growing Pigs12. J. Anim. Sci. 2002, 80, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Smiricky-Tjardes, M.R.; Flickinger, E.A.; Grieshop, C.M.; Bauer, L.L.; Murphy, M.R.; Fahey, G.C., Jr. In Vitro Fermentation Characteristics of Selected Oligosaccharides by Swine Fecal Microflora1. J. Anim. Sci. 2003, 81, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Varel, V.H.; Kreikemeier, K.K.; Jung, H.-J.G.; Hatfield, R.D. In Vitro Stimulation of Forage Fiber Degradation by Ruminal Microorganisms with Aspergillus oryzae Fermentation Extract. Appl. Environ. Microbiol. 1993, 59, 3171–3176. [Google Scholar] [CrossRef]
- Mohammadi, M.; Norlander, S.; Hedström, M.; Adlercreutz, P.; Grey, C. Xylanases and High-Degree Wet Milling Improve Soluble Dietary Fibre Content in Liquid Oat Base. Food Chem. 2024, 442, 138619. [Google Scholar] [CrossRef]
- Souza, J.M.; Souza, J.C.S.M.; Sousa, D.O.; Del Valle, T.A.; Ghizzi, L.G.; Alcântara, A.H.D.; Mesquita, L.G.; Sousa, R.L.M.; Bueno, I.C.S.; Balieiro, J.C.C. The Effects of Compound Treatment of Aspergillus oryzae and Fibrolytic Enzyme on in Vitro Degradation, Gas Production and Fermentative Profile of Maize Silage and Sugarcane Silage. J. Agric. Sci. 2021, 159, 147–158. [Google Scholar] [CrossRef]
- de Fátima Viana, S.; Guimarães, V.M.; José, I.C.; de Almeida e Oliveira, M.G.; Brunoro Costa, N.M.; de Barros, E.G.; Moreira, M.A.; de Rezende, S.T. Hydrolysis of Oligosaccharides in Soybean Flour by Soybean α-Galactosidase. Food Chem. 2005, 93, 665–670. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Pedersen, H.; Carlsen, M.; Nielsen, J. Identification of Enzymes and Quantification of Metabolic Fluxes in the Wild Type and in a Recombinant Aspergillus oryzae Strain. Appl. Environ. Microbiol. 1999, 65, 11–19. [Google Scholar] [CrossRef]
- Chen, L.; Madl, R.L.; Vadlani, P.V. Nutritional Enhancement of Soy Meal via Aspergillus oryzae Solid-State Fermentation. Cereal Chem. 2013, 90, 529–534. [Google Scholar] [CrossRef]
- Choi, D.; Alshannaq, A.F.; Yu, J.-H. Safe and Effective Degradation of Aflatoxins by Food-Grade Culture Broth of Aspergillus oryzae. PNAS Nexus 2024, 3, pgae271. [Google Scholar] [CrossRef]
- Akinola, S.A.; Ateba, C.N.; Mwanza, M. Polyphasic Assessment of Aflatoxin Production Potential in Selected Aspergilli. Toxins 2019, 11, 692. [Google Scholar] [CrossRef]
- Massarolo, K.C.; Mendoza, J.R.; Verma, T.; Kupski, L.; Badiale-Furlong, E.; Bianchini, A. Fate of Aflatoxins in Cornmeal during Single-Screw Extrusion: A Bioaccessibility Approach. LWT 2021, 138, 110734. [Google Scholar] [CrossRef]
- Berry, T.H.; Becker, D.E.; Rasmussen, O.G.; Jensen, A.H.; Norton, H.W. The Limiting Amino Acids in Soybean Protein. J. Anim. Sci. 1962, 21, 558–561. [Google Scholar] [CrossRef]
- Zarkadas, C.G.; Gagnon, C.; Gleddie, S.; Khanizadeh, S.; Cober, E.R.; Guillemette, R.J.D. Assessment of the Protein Quality of Fourteen Soybean [Glycine max (L.) Merr.] Cultivars Using Amino Acid Analysis and Two-Dimensional Electrophoresis. Food Res. Int. 2007, 40, 129–146. [Google Scholar] [CrossRef]
- Yu, J.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Keller, N.P.; Chu, F.S. Cloning and Characterization of a cDNA from Aspergillus Parasiticus Encoding an O-Methyltransferase Involved in Aflatoxin Biosynthesis. Appl. Environ. Microbiol. 1993, 59, 3564–3571. [Google Scholar] [CrossRef]
- Kacprzak, M.M.; Lewandowska, I.; Matthews, R.G.; Paszewski, A. Transcriptional Regulation of Methionine Synthase by Homocysteine and Choline in Aspergillus Nidulans. Biochem. J. 2003, 376, 517–524. [Google Scholar] [CrossRef]
- Kim, H.S.; Titgemeyer, E.C.; Aldrich, C.G. Evaluation of Fermentability of Whole Soybeans and Soybean Oligosaccharides by a Canine In Vitro Fermentation Model. Fermentation 2023, 9, 414. [Google Scholar] [CrossRef]
- Opazo, R.; Ortúzar, F.; Navarrete, P.; Espejo, R.; Romero, J. Reduction of Soybean Meal Non-Starch Polysaccharides and α-Galactosides by Solid-State Fermentation Using Cellulolytic Bacteria Obtained from Different Environments. PLoS ONE 2012, 7, e44783. [Google Scholar] [CrossRef]
- Birk, Y. Purification and Some Properties of a Highly Active Inhibitor of Trypsin and α-Chymotrypsin from Soybeans. Biochim. Biophys. Acta 1961, 54, 378–381. [Google Scholar] [CrossRef]
- Kunitz, M. Crystallization of a Trypsin Inhibitor from Soybean. Science 1945, 101, 668–669. [Google Scholar] [CrossRef]
- Huang, H.; Kwok, K.-C.; Liang, H.-H. Inhibitory Activity and Conformation Changes of Soybean Trypsin Inhibitors Induced by Ultrasound. Ultrason. Sonochem. 2008, 15, 724–730. [Google Scholar] [CrossRef]
- Vajda, T.; Szabó, T. Specificity of Trypsin and Alpha-Chymotrypsin towards Neutral Substrates. Acta Biochim. Biophys. Acad. Sci. Hung. 1976, 11, 287–294. [Google Scholar]
- Murphy, P.A. Soybean Proteins. In Soybeans; Johnson, L.A., White, P.J., Galloway, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 229–267. ISBN 978-1-893997-64-6. [Google Scholar]
- Zhang, W.; Zhao, D.; Dong, Z.; Li, J.; Zhang, B.; Yu, W. The Consistency Factor and the Viscosity Exponent of Soybean-Protein-Isolate/Wheat-Gluten/Corn-Starch Blends by Using a Capillary Rheometry. Molecules 2022, 27, 6693. [Google Scholar] [CrossRef]
- Al-Rabadi, G.J.; Torley, P.J.; Williams, B.A.; Bryden, W.L.; Gidley, M.J. Particle Size of Milled Barley and Sorghum and Physico-Chemical Properties of Grain Following Extrusion. J. Food Eng. 2011, 103, 464–472. [Google Scholar] [CrossRef]
- Carvalho, C.W.P.; Takeiti, C.Y.; Onwulata, C.I.; Pordesimo, L.O. Relative Effect of Particle Size on the Physical Properties of Corn Meal Extrudates: Effect of Particle Size on the Extrusion of Corn Meal. J. Food Eng. 2010, 98, 103–109. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Hanna, M.A. Influence of Process and Product Variables on Extrusion Energy and Pressure Requirements. J. Food Eng. 1987, 6, 153–163. [Google Scholar] [CrossRef]
- Torrezan, R.; Tham, W.P.; Bell, A.E.; Frazier, R.A.; Cristianini, M. Effects of High Pressure on Functional Properties of Soy Protein. Food Chem. 2007, 104, 140–147. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Tatsumi, E.; Isobe, S. High-Pressure Treatment Effects on Proteins in Soy Milk. LWT-Food Sci. Technol. 2005, 38, 7–14. [Google Scholar] [CrossRef]
- Guy, R. Extrusion Cooking: Technologies and Applications; Woodhead Publishing: Boca Raton, FL, USA, 2001; ISBN 978-1-85573-559-0. [Google Scholar]
- Robin, F.; Schuchmann, H.P.; Palzer, S. Dietary Fiber in Extruded Cereals: Limitations and Opportunities. Trends Food Sci. Technol. 2012, 28, 23–32. [Google Scholar] [CrossRef]
- Kumar, A.; Ganjyal, G.M.; Jones, D.D.; Hanna, M.A. Experimental Determination of Longitudinal Expansion During Extrusion of Starches. Cereal Chem. 2007, 84, 480–484. [Google Scholar] [CrossRef]
- Calabrò, S.; Carciofi, A.C.; Musco, N.; Tudisco, R.; Gomes, M.O.S.; Cutrignelli, M.I. Fermentation Characteristics of Several Carbohydrate Sources for Dog Diets Using the In Vitro Gas Production Technique. Ital. J. Anim. Sci. 2013, 12, e4. [Google Scholar] [CrossRef]



| Ingredient, % DM | SBM | AMF | FSBM | SPI |
|---|---|---|---|---|
| Soybean meal | 30.00 | 30.00 | 0.00 | 0.00 |
| Amaferm® | 0.00 | 1.00 | 0.00 | 0.00 |
| Fermented soybean meal | 0.00 | 0.00 | 30.00 | 0.00 |
| Soybean protein isolate | 0.00 | 0.00 | 0.00 | 18.00 |
| Brewer’s rice | 0.00 | 0.00 | 0.00 | 10.68 |
| Corn | 37.23 | 35.87 | 39.11 | 40.00 |
| Beet pulp | 3.79 | 3.79 | 3.79 | 3.79 |
| Poultry by-produce meal | 19.31 | 19.25 | 16.00 | 16.06 |
| Chicken fat | 5.82 | 6.06 | 6.07 | 6.00 |
| Dog dry digest | 1.00 | 1.00 | 1.00 | 1.00 |
| Fish oil | 1.00 | 1.00 | 1.00 | 1.00 |
| Calcium carbonate | 0.47 | 0.51 | 0.60 | 0.68 |
| Dicalcium phosphate | 0.37 | 0.31 | 0.82 | 0.84 |
| Potassium chloride | 0.00 | 0.20 | 0.60 | 0.95 |
| Salt | 0.25 | 0.25 | 0.25 | 0.25 |
| Choline chloride | 0.10 | 0.10 | 0.10 | 0.10 |
| Titanium dioxide | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin Premix 1 | 0.15 | 0.15 | 0.15 | 0.15 |
| Trace mineral premix 2 | 0.10 | 0.10 | 0.10 | 0.10 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient | U-SBM | F-SBM | Difference 1 | SEM of Difference 2 | p-Value |
|---|---|---|---|---|---|
| Organic matter, %DM | 92.52 | 91.51 | −1.01 | 0.001 | <0.001 |
| Crude protein, %DM | 50.05 | 54.69 | 4.63 | 0.004 | <0.001 |
| Crude fat, %DM | 2.21 | 2.40 | 0.19 | 0.002 | 0.2874 |
| TDF, %DM | 25.06 | 23.88 | −1.18 | 0.009 | 0.2104 |
| IDF, %DM | 19.32 | 15.29 | −4.03 | 0.009 | 0.0026 |
| SDF, %DM | 5.74 | 8.59 | 2.85 | 0.005 | <0.001 |
| GE, kcal/kg DM | 4720.2 | 4555.6 | −164.6 | 13.78 | <0.001 |
| Chemicals | U-SBM | F-SBM | |
|---|---|---|---|
| Amino acids, % DM | |||
| Arginine | 3.64 | 3.46 | |
| Histidine | 1.34 | 1.36 | |
| Isoleucine | 2.46 | 2.72 | |
| Leucine | 3.99 | 4.43 | |
| Lysine | 3.21 | 2.96 | |
| Methionine | 0.72 | 0.81 | |
| Phenylalanine | 2.64 | 2.92 | |
| Threonine | 1.96 | 2.17 | |
| Tryptophan | 0.63 | 0.63 | |
| Valine | 2.55 | 2.84 | |
| Alanine | 2.23 | 2.52 | |
| Aspartic acid | 5.69 | 6.21 | |
| Cysteine | 0.76 | 0.82 | |
| Glutamic acid | 9.34 | 10.11 | |
| Glycine | 2.14 | 2.35 | |
| Serine | 2.15 | 2.33 | |
| Proline | 2.56 | 2.85 | |
| Tyrosine | 1.90 | 2.11 | |
| Hydroxylysine | 0.02 | 0.01 | |
| Hydroxyproline | 0.07 | 0.08 | |
| Taurine | 0.09 | 0.06 | |
| Lanthionine | 0.00 | 0.07 | |
| Ornithine | 0.03 | 0.08 | |
| Sugars, % DM | |||
| Sucrose | 6.58 | 0.00 | |
| Raffinose | 0.95 | 0.12 | |
| Stachyose | 2.93 | 0.14 | |
| Verbascose | 0.55 | 0.32 | |
| Trypsin inhibitor, TIU/g DM | U-SBM | R-SBM | F-SBM |
| Kunitz TI | 3.17 | Not detected | Not detected |
| Bowman–Birk TI | 2.29 | Not detected | Not detected |
| SBM | AMF | FSBM | SPI | |
|---|---|---|---|---|
| Dgw | 382.8 | 347.1 | 286.8 | 223.1 |
| Sgw | 300.6 | 284.3 | 240.8 | 206.9 |
| Parameter | SBM | AMF | FSBM | SPI | SEM 1 | p-Value |
|---|---|---|---|---|---|---|
| Feed rate (as-is), kg/h | 113.05 | 117.00 | 124.20 | 98.85 | 4.583 | 0.0681 |
| PC water injection, kg/h | 18.50 a | 19.30 a | 11.27 b | 13.07 b | 0.604 | <0.001 |
| PC discharge temperature, °C | 93.9 | 92.7 | 95.0 | 94.3 | 0.71 | 0.1723 |
| EX Screw speed, rpm | 636 ab | 637 a | 636 b | 342 c | 0.2 | <0.001 |
| EX Water injection, kg/h | 12.60 | 13.23 | 11.60 | 11.90 | 0.533 | 0.2109 |
| Motor power, kW | 5.90 b | 7.57 b | 10.57 a | 10.03 a | 0.512 | <0.001 |
| Die temperature, °C | 100.33 b | 104.67 b | 117.00 a | 117.33 a | 1.581 | <0.001 |
| Die pressure, psig | 200 b | 250 a | 210 b | 250 a | 5.0 | <0.001 |
| In-barrel moisture, % | 34.46 a | 34.69 a | 28.21 c | 33.45 b | 0.002 | <0.001 |
| SME, kJ/kg | 83.7 c | 115.9 bc | 198.7 b | 305.3 a | 14.57 | 0.0015 |
| Wet kibble characteristics | ||||||
| SEI, mm2/mm2 | 1.44 c | 1.50 c | 1.87 b | 2.45 a | 0.030 | <0.001 |
| Piece density, g/cm3 | 0.788 a | 0.759 a | 0.575 b | 0.449 c | 0.0227 | <0.001 |
| Specific length, mm/g | 22.95 b | 22.69 b | 25.75 a | 23.68 ab | 0.651 | 0.0049 |
| Bulk density, g/L | 520.7 a | 484.0 ab | 419.0 bc | 348.3 c | 17.31 | <0.001 |
| Dry kibble characteristics | ||||||
| SEI, mm2/mm2 | 1.30 c | 1.31 c | 1.80 b | 2.55 a | 0.024 | <0.001 |
| Piece density, g/cm3 | 0.74 a | 0.68 b | 0.49 c | 0.41 d | 0.010 | <0.001 |
| Specific length, mm/g | 26.63 b | 28.60 a | 29.19 a | 24.26 c | 0.314 | <0.001 |
| Bulk density, g/L | 520.3 a | 481.0 b | 385.0 c | 324.7 d | 5.73 | <0.001 |
| Nutrient | SBM | AMF | FSBM | SPI |
|---|---|---|---|---|
| DM, % | 93.63 | 93.89 | 95.99 | 93.89 |
| Crude protein, % DM | 33.21 | 33.82 | 31.46 | 35.04 |
| Crude fat, % DM | 12.41 | 12.32 | 11.51 | 10.95 |
| Ash, % DM | 7.78 | 7.28 | 8.61 | 7.18 |
| Total dietary fiber, % DM | 17.90 | 17.60 | 19.80 | 14.00 |
| Insoluble dietary fiber, % DM | 13.30 | 13.70 | 14.70 | 9.40 |
| Soluble dietary fiber, % DM | 4.60 | 3.90 | 5.10 | 4.60 |
| Gross energy, kcal/kg DM | 4955.7 | 4975.6 | 4829.5 | 4938.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Weiss, T.; Wang, D.; Alavi, S.; Aldrich, C.G. Optimizing Aspergillus oryzae Inoculation Dosage and Fermentation Duration for Enhanced Protein Content in Soybean Meal and Its Influence on Dog Food Extrusion. Processes 2025, 13, 2441. https://doi.org/10.3390/pr13082441
Chen Y, Weiss T, Wang D, Alavi S, Aldrich CG. Optimizing Aspergillus oryzae Inoculation Dosage and Fermentation Duration for Enhanced Protein Content in Soybean Meal and Its Influence on Dog Food Extrusion. Processes. 2025; 13(8):2441. https://doi.org/10.3390/pr13082441
Chicago/Turabian StyleChen, Youhan, Thomas Weiss, Donghai Wang, Sajid Alavi, and Charles Gregory Aldrich. 2025. "Optimizing Aspergillus oryzae Inoculation Dosage and Fermentation Duration for Enhanced Protein Content in Soybean Meal and Its Influence on Dog Food Extrusion" Processes 13, no. 8: 2441. https://doi.org/10.3390/pr13082441
APA StyleChen, Y., Weiss, T., Wang, D., Alavi, S., & Aldrich, C. G. (2025). Optimizing Aspergillus oryzae Inoculation Dosage and Fermentation Duration for Enhanced Protein Content in Soybean Meal and Its Influence on Dog Food Extrusion. Processes, 13(8), 2441. https://doi.org/10.3390/pr13082441







