Photolysis, Photocatalysis, and Sorption of Caffeine in Aqueous Media in the Presence of Chitosan Membrane and Chitosan/TiO2 Composite Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Chitosan Membranes
2.3. Characterization of Membranes
2.4. Degree of Swelling
2.5. Point of Zero Charge
2.6. Caffeine Removal Experiments
2.7. Reuse of the Catalytic Membrane
2.8. Kinetics of Caffeine Removal from Aqueous Medium
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Chitosan Membranes
3.1.1. FTIR Spectra
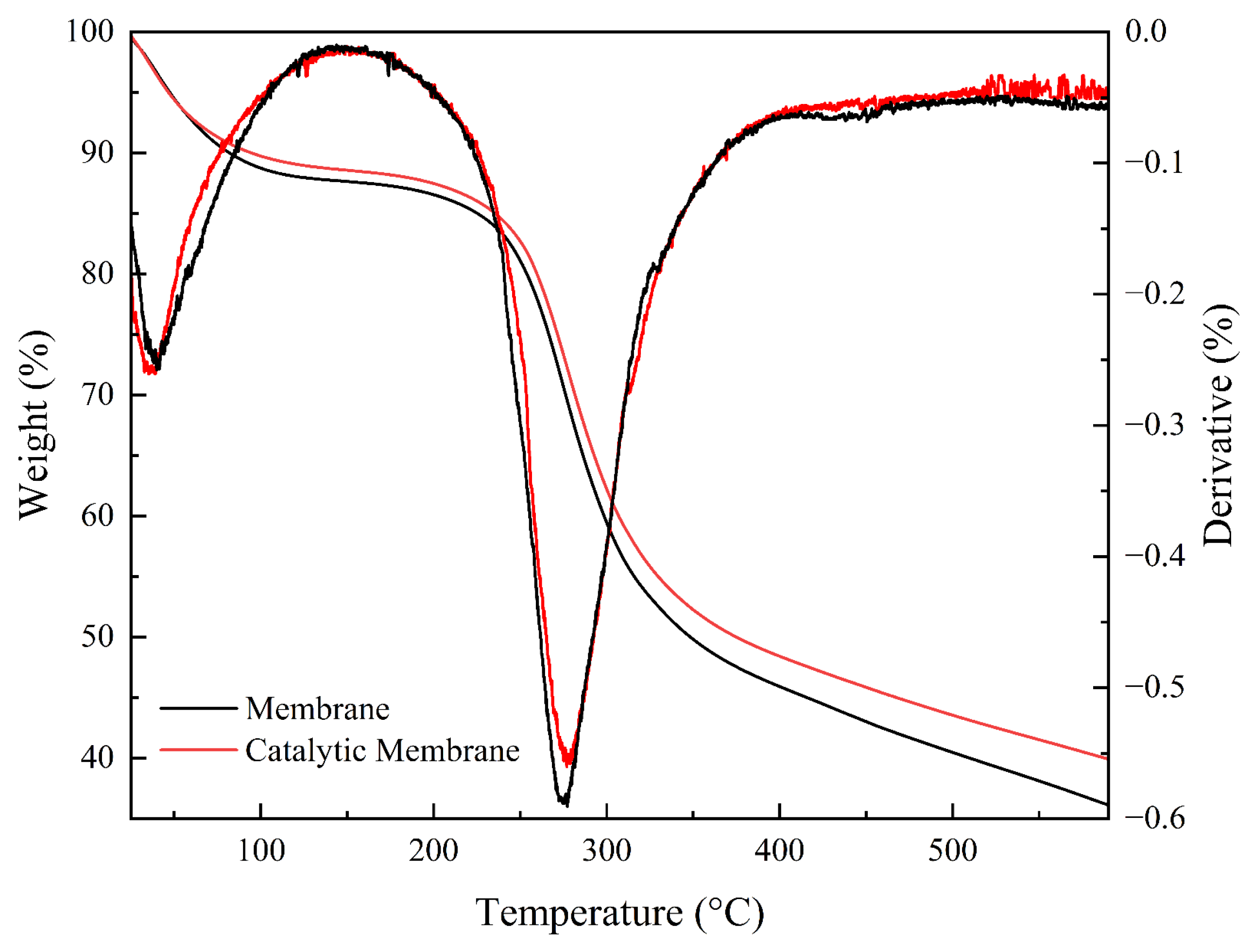
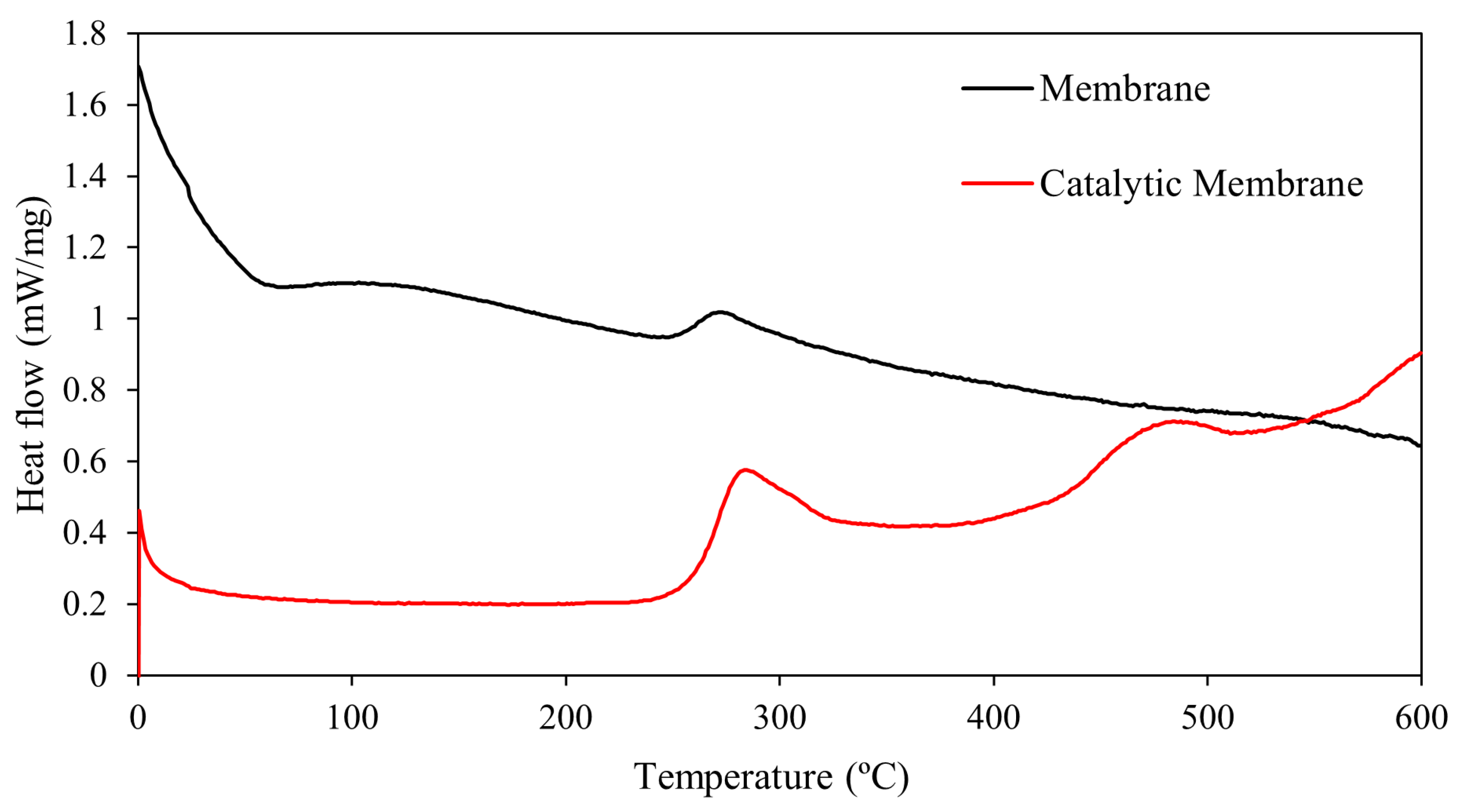
3.1.2. TGA and DSC
3.1.3. X-Ray Diffractograms
3.1.4. Degree of Swelling
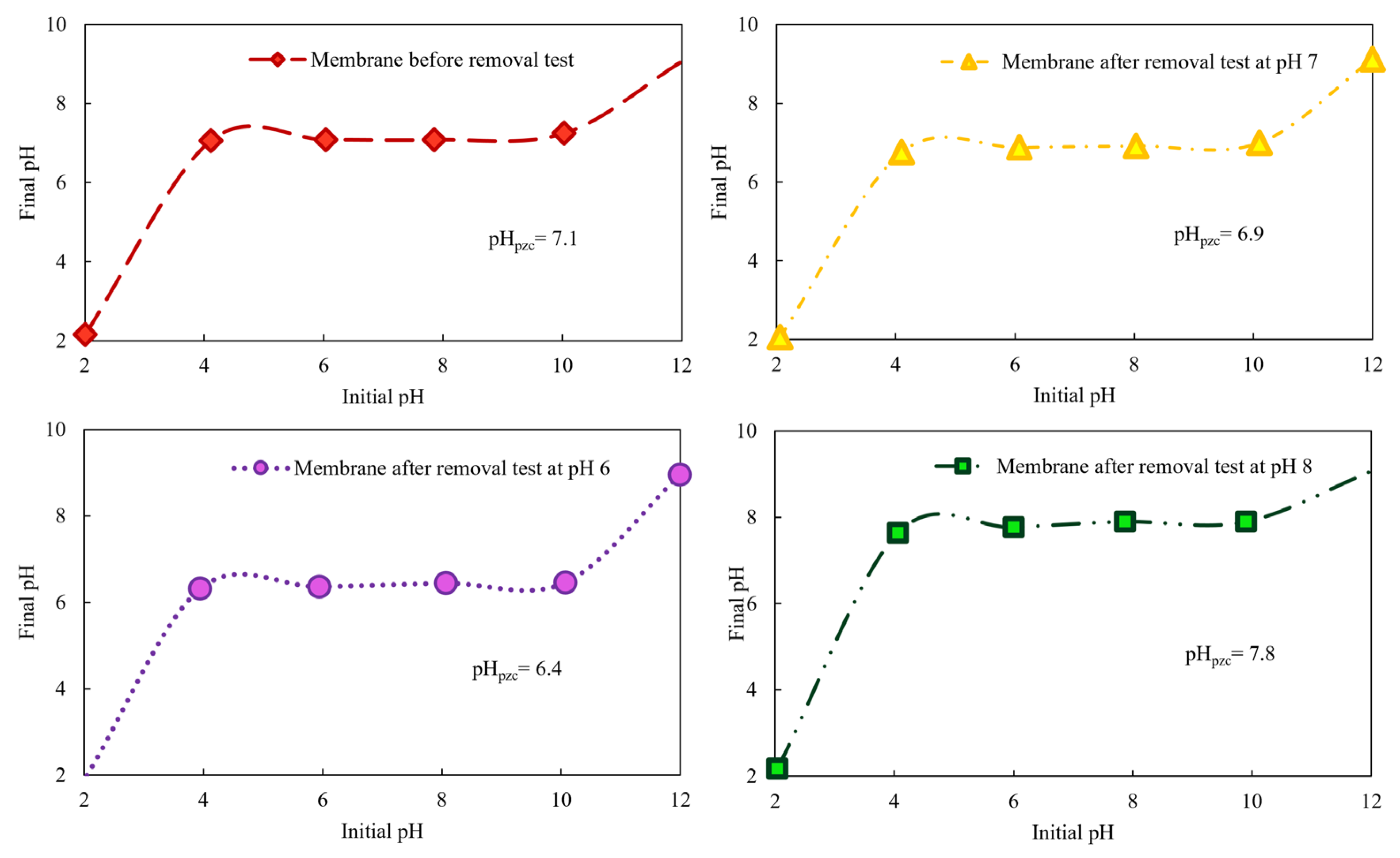
3.1.5. Point of Zero Charge (pHPZC)
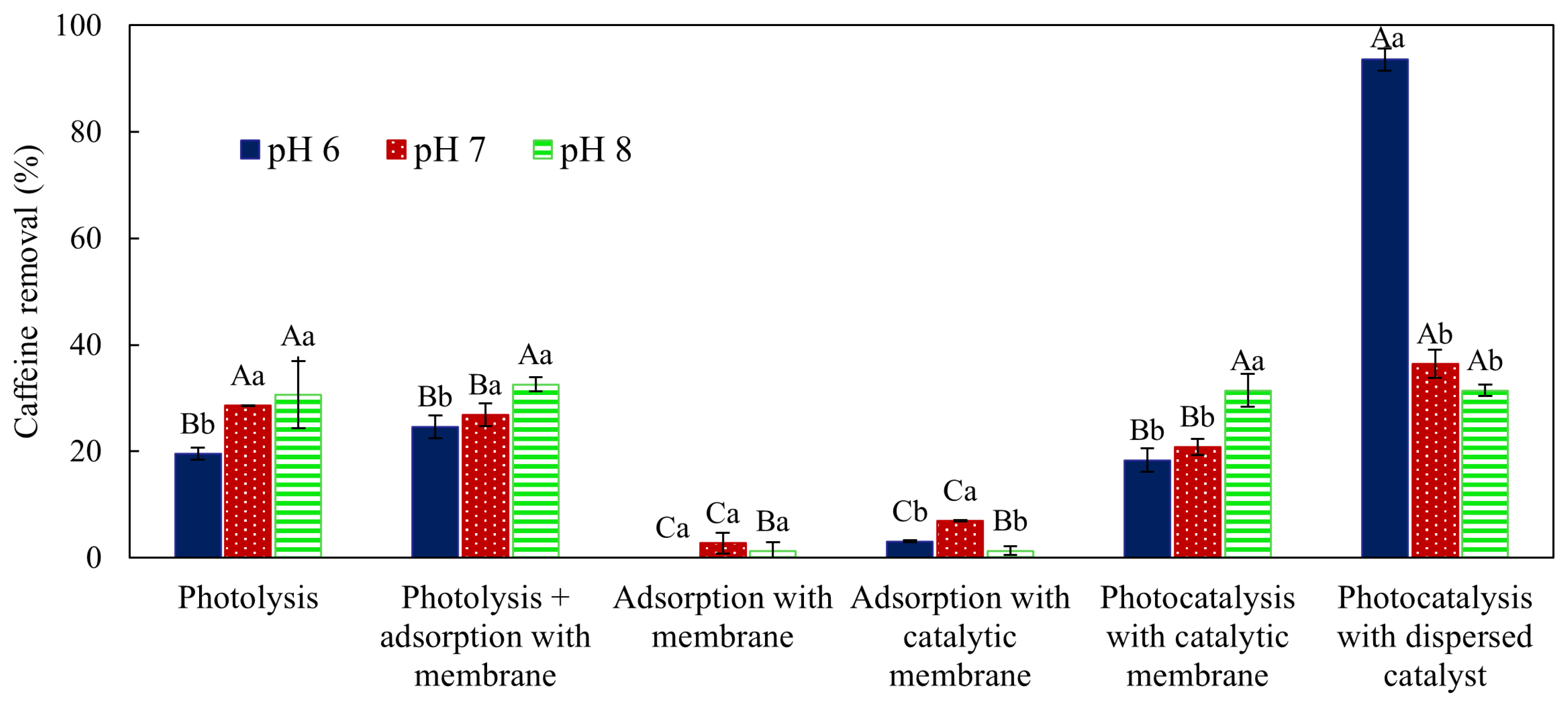
3.2. Caffeine Removal from Water
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, J.O.; Strieder, M.M.; Silva, L.F.O.; dos Reis, G.S.; Dotto, G.L. Advanced Technologies in Water Treatment: Chitosan and Its Modifications as Effective Agents in the Adsorption of Contaminants. Int. J. Biol. Macromol. 2024, 270, 132307. [Google Scholar] [CrossRef] [PubMed]
- Arfanis, M.K.; Adamou, P.; Moustakas, N.G.; Triantis, T.M.; Kontos, A.G.; Falaras, P. Photocatalytic Degradation of Salicylic Acid and Caffeine Emerging Contaminants Using Titania Nanotubes. Chem. Eng. J. 2017, 310, 525–536. [Google Scholar] [CrossRef]
- de Oliveira, C.P.M.; Sperle, P.; Arcanjo, G.S.; Koch, K.; Viana, M.M.; Drewes, J.E.; Amaral, M.C.S. Successful Application of Photocatalytic Recycled TiO2-GO Membranes for the Removal of Trace Organic Compounds from Tertiary Effluent. Chemosphere 2024, 362, 142730. [Google Scholar] [CrossRef]
- da Silva Vasconcelos de Almeida, A.; Vieira, W.T.; Bispo, M.D.; de Melo, S.F.; da Silva, T.L.; Balliano, T.L.; Vieira, M.G.A.; Soletti, J.I. Caffeine Removal Using Activated Biochar from Açaí Seed (Euterpe oleracea Mart): Experimental Study and Description of Adsorbate Properties Using Density Functional Theory (DFT). J. Environ. Chem. Eng. 2021, 9, 104891. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Zakharova, A.; Tynysbek, A.; Koole, L.H.; Atabaev, T.S.; Poulopoulos, S.G. Polymer Immobilized TiO2 Microparticles for Photocatalytic Degradation of Caffeine. Mater. Today Proc. 2022, 71, 119–123. [Google Scholar] [CrossRef]
- Zanella, H.G.; Spessato, L.; Lopes, G.K.P.; Yokoyama, J.T.C.; Silva, M.C.; Souza, P.S.C.; Ronix, A.; Cazetta, A.L.; Almeida, V.C. Caffeine Adsorption on Activated Biochar Derived from Macrophytes (Eichornia crassipes). J. Mol. Liq. 2021, 340, 117206. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Ince, N.H.; Ozon, E.; Arslan, E.; Aviyente, V.; Savun-Hekimoğlu, B.; Erdincler, A. Oxidative Decomposition and Mineralization of Caffeine by Advanced Oxidation Processes: The Effect of Hybridization. Ultrason. Sonochem. 2021, 76, 105635. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Chen, B.; Bu, Y.; Chen, Y.; Ma, J.; Rosario-Ortiz, F.L. Photolysis and Photocatalysis of Haloacetic Acids in Water: A Review of Kinetics, Influencing Factors, Products, Pathways, and Mechanisms. J. Hazard. Mater. 2020, 391, 122143. [Google Scholar] [CrossRef]
- Nawaz, T.; Sengupta, S. Contaminants of Emerging Concern: Occurrence, Fate, and Remediation. In Advances in Water Purification Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–114. [Google Scholar]
- Venecia-Nuñez, J.D.; Ramírez-Parra, L.T.; Lara-Ramos, J.A.; Machuca-Martinez, F.; Colina-Márquez, J.Á.; Mueses, M.A. Evaluation of Photocatalysis Based on Ozone/Goethite/Ultraviolet C for Caffeine Degradation. Chem. Eng. Technol. 2023, 46, 1845–1853. [Google Scholar] [CrossRef]
- Tayebee, R.; Esmaeili, E.; Maleki, B.; Khoshniat, A.; Chahkandi, M.; Mollania, N. Photodegradation of Methylene Blue and Some Emerging Pharmaceutical Micropollutants with an Aqueous Suspension of WZnO-NH2@H3PW12O40 Nanocomposite. J. Mol. Liq. 2020, 317, 113928. [Google Scholar] [CrossRef]
- Zubieta, C.E.; Messina, P.V.; Luengo, C.; Dennehy, M.; Pieroni, O.; Schulz, P.C. Reactive Dyes Remotion by Porous TiO2-Chitosan Materials. J. Hazard. Mater. 2008, 152, 765–777. [Google Scholar] [CrossRef]
- Simionato, J.I.; Paulino, A.T.; Garcia, J.C.; Nozaki, J. Adsorption of Aluminium from Wastewater by Chitin and Chitosan Produced from Silkworm Chrysalides. Polym. Int. 2006, 55, 1243–1248. [Google Scholar] [CrossRef]
- Saiyad, M.; Shah, N.; Joshipura, M.; Dwivedi, A.; Pillai, S. Chitosan and Its Derivatives in Wastewater Treatment Application. Mater. Today Proc. 2024, 99, 190–194. [Google Scholar] [CrossRef]
- Alshahrani, H.; Abdulhameed, A.S.; Khan, M.K.A.; Al Omari, R.H.; Algburi, S. Functionalization of Chitosan/Alumina Nanoparticles with Carboxylic Groups Using Phthalic Anhydride for Adsorption of Methylene Blue Dye via Response Surface Methodology. Polym. Bull. 2025, 82, 87–109. [Google Scholar] [CrossRef]
- Pavoni, J.M.F.; dos Santos, N.Z.; May, I.C.; Pollo, L.D.; Tessaro, I.C. Impact of Acid Type and Glutaraldehyde Crosslinking in the Physicochemical and Mechanical Properties and Biodegradability of Chitosan Films. Polym. Bull. 2021, 78, 981–1000. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, F.; Dong, H.; Zhang, X.; Gao, S.; Zhang, S.; Jin, J. Ultrasmall Cu3(PO4)2 Nanoparticles Reinforced Hydrogel Membrane for Super-Antifouling Oil/Water Emulsion Separation. ACS Nano 2022, 16, 20786–20795. [Google Scholar] [CrossRef]
- Amiri, S.; Vatanpour, V.; He, T. Antifouling Thin-Film Nanocomposite NF Membrane with Polyvinyl Alcohol-Sodium Alginate-Graphene Oxide Nanocomposite Hydrogel Coated Layer for As(III) Removal. Chemosphere 2023, 322, 138159. [Google Scholar] [CrossRef]
- Rajabi, N.; Masrournia, M.; Abedi, M. Measuring and Pre-Concentration of Lanthanum Using Fe3O4@Chitosan Nanocomposite with Solid-Phase Microextraction for ICP-OES Determination. Arab. J. Sci. Eng. 2020, 45, 121–129. [Google Scholar] [CrossRef]
- Yang, J.M.; Su, W.Y. Preparation and Characterization of Chitosan Hydrogel Membrane for the Permeation of 5-Fluorouracil. Mater. Sci. Eng. C 2011, 31, 1002–1009. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yusuf, N.M.; Ooi, B.S. Preparation and Modification of Poly (Vinyl) Alcohol Membrane: Effect of Crosslinking Time towards Its Morphology. Desalination 2012, 287, 35–40. [Google Scholar] [CrossRef]
- Haripriyan, U.; Gopinath, K.P.; Arun, J. Chitosan Based Nano Adsorbents and Its Types for Heavy Metal Removal: A Mini Review. Mater. Lett. 2022, 312, 131670. [Google Scholar] [CrossRef]
- Matsuyama, H.; Shiraishi, H.; Kitamura, Y. Effect of Membrane Preparation Conditions on Solute Permeability in Chitosan Membrane. J. Appl. Polym. Sci. 1999, 73, 2715–2725. [Google Scholar] [CrossRef]
- Prieto-Rodriguez, L.; Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Puma, G.L.; Malato, S. Treatment of Emerging Contaminants in Wastewater Treatment Plants (WWTP) Effluents by Solar Photocatalysis Using Low TiO2 Concentrations. J. Hazard. Mater. 2012, 211–212, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An Overview of Photocatalytic Degradation: Photocatalysts, Mechanisms, and Development of Photocatalytic Membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, A. Photocatalytic Degradation Properties. Charact. Appl. Nanomater. 2024, 7, 5523. [Google Scholar] [CrossRef]
- Aoudjit, L.; Zioui, D.; Touahra, F.; Mahidine, S.; Bachari, K. Photocatalytic Degradation of Tartrazine Dyes Using TiO2–Chitosan Beads under Sun Light Irradiation. Russ. J. Phys. Chem. A 2021, 95, 1069–1076. [Google Scholar] [CrossRef]
- Savun-Hekimoğlu, B.; Eren, Z.; Ince, N.H. Photocatalytic Destruction of Caffeine on Sepiolite-Supported TiO2 Nanocomposite. Sustainability 2020, 12, 10314. [Google Scholar] [CrossRef]
- Giri, R.R.; Ozaki, H.; Ota, S.; Taniguchi, S.; Takanami, R. Influence of Inorganic Solids on Photocatalytic Oxidation of 2,4-Dichlorophenoxyacetic Acid with UV and TiO2 Fiber in Aqueous Solution. Desalination 2010, 255, 9–14. [Google Scholar] [CrossRef]
- Marques, R.R.N.; Sampaio, M.J.; Carrapiço, P.M.; Silva, C.G.; Morales-Torres, S.; Dražić, G.; Faria, J.L.; Silva, A.M.T. Photocatalytic Degradation of Caffeine: Developing Solutions for Emerging Pollutants. Catal. Today 2013, 209, 108–115. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Ravichandar, S.; Lim, Y.C. Combined Effects of Adsorption and Photocatalysis by Hybrid TiO2/ZnO-Calcium Alginate Beads for the Removal of Copper. J. Environ. Sci. 2017, 55, 214–223. [Google Scholar] [CrossRef]
- Paz, M.J.; Vieira, T.; Enzweiler, H.; Paulino, A.T. Chitosan/Wood Sawdust/Magnetite Composite Membranes for the Photodegradation of Agrochemicals in Water. J. Environ. Chem. Eng. 2022, 10, 106967. [Google Scholar] [CrossRef]
- Aparecida Matias, C.; Vilela, P.B.; Becegato, V.A.; Paulino, A.T. Adsorption Kinetic, Isotherm and Thermodynamic of 2,4-Dichlorophenoxyacetic Acid Herbicide in Novel Alternative Natural Adsorbents. Water Air Soil Pollut. 2019, 230, 276. [Google Scholar] [CrossRef]
- Das, R.S.; Kumar, A.; Wankhade, A.V.; Peshwe, D.R. ZrO2@chitosan Composite for Simultaneous Photodegradation of Three Emerging Contaminants and Antibacterial Application. Carbohydr. Polym. 2022, 278, 118940. [Google Scholar] [CrossRef]
- Zioui, D.; Aoudjit, L.; Touahra, F.; Bachari, K. Preparation and characterization of TiO2-chitosan composite films and application for tartrazine dye degradation. Cellul. Chem. Technol. 2022, 56, 1101–1107. [Google Scholar] [CrossRef]
- Costa, E.d.S., Jr.; Mansur, H.S. Preparação e Caracterização de Blendas de Quitosana/Poli(Álcool Vinílico) Reticuladas Quimicamente Com Glutaraldeído Para Aplicação Em Engenharia de Tecido. Química Nova 2008, 31, 1460–1466. [Google Scholar] [CrossRef]
- da Cruz, L.F.; Polizeli, A.G.; Enzweiler, H.; Paulino, A.T. Chitosan-Co-GLU/Eucalyptus Residue Composite Membrane for the Stabilization of β-d-Galactosidase in Aqueous Solutions. Polym. Bull. 2024, 81, 6437–6455. [Google Scholar] [CrossRef]
- Vieira, T.; Becegato, V.A.; Paulino, A.T. Equilibrium Isotherms, Kinetics, and Thermodynamics of the Adsorption of 2,4-Dichlorophenoxyacetic Acid to Chitosan-Based Hydrogels. Water Air Soil Pollut. 2021, 232, 60. [Google Scholar] [CrossRef]
- Banafati Zadeh, F.; Zamanian, A. Glutaraldehyde: Introducing Optimum Condition for Cross-Linking the Chitosan/Gelatin Scaffolds for Bone Tissue Engineering. Int. J. Eng. 2022, 35, 1967–1980. [Google Scholar] [CrossRef]
- Wegrzynowska-Drzymalska, K.; Grebicka, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications. Materials 2020, 13, 3413. [Google Scholar] [CrossRef]
- Geeta; Shivani; Devi, N.; Shayoraj; Bansal, N.; Sharma, S.; Dubey, S.K.; Kumar, S. Novel Chitosan-Based Smart Bio-Nanocomposite Films Incorporating TiO2 Nanoparticles for White Bread Preservation. Int. J. Biol. Macromol. 2024, 267, 131367. [Google Scholar] [CrossRef]
- Alzahrani, E.; Ahmed, R.A.; Sattam Alotibi, R. TiO2NPs Embedded in Chitosan Membrane for Efficient Photodegradation of Various Dyes. Orient. J. Chem. 2020, 36, 144–160. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ruvalcaba-Gómez, J.M.; Maytorena-Verdugo, C.I.; González-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Pérez-Larios, A.; Montalvo-González, E. Chitosan-TiO2: A Versatile Hybrid Composite. Materials 2020, 13, 811. [Google Scholar] [CrossRef]
- Vilela, P.B.; Matias, C.A.; Dalalibera, A.; Becegato, V.A.; Paulino, A.T. Polyacrylic Acid-Based and Chitosan-Based Hydrogels for Adsorption of Cadmium: Equilibrium Isotherm, Kinetic and Thermodynamic Studies. J. Environ. Chem. Eng. 2019, 7, 103327. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M. Chitosan-Titanium Oxide Fibers Supported Zero-Valent Nanoparticles: Highly Efficient and Easily Retrievable Catalyst for the Removal of Organic Pollutants. Sci. Rep. 2018, 8, 6260. [Google Scholar] [CrossRef] [PubMed]
- Wongniramaikul, W.; Kleangklao, B.; Sadegh, F.; Sadegh, N.; Choodum, A. Highly Efficient Phosphate Adsorption Using Calcium Silicate Hydrate-Embedded Calcium Crosslinked Polyvinyl Alcohol Thin Film. Environ. Res. 2024, 263, 120229. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, P.; Wang, J.; Zhang, L.; Huang, R. Phosphate Adsorption from Aqueous Solutions by Zirconium (IV) Loaded Cross-Linked Chitosan Particles. J. Taiwan. Inst. Chem. Eng. 2016, 59, 311–319. [Google Scholar] [CrossRef]
- Isidoro Ribeiro, N.; Barreto Pessanha, O.; Luiza Gomes Soares Pessanha, M.; Guimarães, D. Efficient Phosphate Adsorption by a Composite Composed of Mg6Al2(CO3)(OH)16·4H2O LDH and Chitosan: Kinetic, Thermodynamic, Desorption, and Characterization Studies. Sep. Purif. Technol. 2023, 307, 122717. [Google Scholar] [CrossRef]
- Banu, H.T.; Meeenakshi, S. Effective Removal of Phosphate from Aqueous Solution by Lanthanum Loaded Bio-Polymeric Composite. Mater. Today Proc. 2018, 5, 16165–16171. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Removal of Phosphate Anions Using the Modified Chitosan Beads: Adsorption Kinetic, Isotherm and Mechanism Studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Abd El-Monaem, E.M.; Elshishini, H.M.; El-Aqapa, H.G.; Hosny, M.; Abdelfatah, A.M.; Ahmed, M.S.; Hammad, E.N.; El-Subruiti, G.M.; Fawzy, M.; et al. Recent Developments in Alginate-Based Adsorbents for Removing Phosphate Ions from Wastewater: A Review. RSC Adv. 2022, 12, 8228–8248. [Google Scholar] [CrossRef]
- Stefanowska, K.; Woźniak, M.; Sip, A.; Mrówczyńska, L.; Majka, J.; Kozak, W.; Dobrucka, R.; Ratajczak, I. Characteristics of Chitosan Films with the Bioactive Substances—Caffeine and Propolis. J. Funct. Biomater. 2023, 14, 358. [Google Scholar] [CrossRef]
- Lewandowska, K. Miscibility and Thermal Stability of Poly(Vinyl Alcohol)/Chitosan Mixtures. Thermochim. Acta 2009, 493, 42–48. [Google Scholar] [CrossRef]
- Paulino, A.T.; Pereira, A.G.B.; Fajardo, A.R.; Erickson, K.; Kipper, M.J.; Muniz, E.C.; Belfiore, L.A.; Tambourgi, E.B. Natural Polymer-Based Magnetic Hydrogels: Potential Vectors for Remote-Controlled Drug Release. Carbohydr. Polym. 2012, 90, 1216–1225. [Google Scholar] [CrossRef]
- Guinesi, L.S.; Cavalheiro, É.T.G. The Use of DSC Curves to Determine the Acetylation Degree of Chitin/Chitosan Samples. Thermochim. Acta 2006, 444, 128–133. [Google Scholar] [CrossRef]
- Zong, Z.; Kimura, Y.; Takahashi, M.; Yamane, H. Characterization of Chemical and Solid State Structures of Acylated Chitosans. Polymer 2000, 41, 899–906. [Google Scholar] [CrossRef]
- Farzana, M.H.; Meenakshi, S. Synergistic Effect of Chitosan and Titanium Dioxide on the Removal of Toxic Dyes by the Photodegradation Technique. Ind. Eng. Chem. Res. 2014, 53, 55–63. [Google Scholar] [CrossRef]
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of Silver/Titanium Dioxide/Chitosan Adipate Nanocomposite as an Antibacterial Coating for Fruit Storage. LWT—Food Sci. Technol. 2015, 63, 1206–1213. [Google Scholar] [CrossRef]
- Fahmy, T.; Sarhan, A. Characterization and Molecular Dynamic Studies of Chitosan–Iron Complexes. Bull. Mater. Sci. 2021, 44, 142. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, S.S.; Nair, A.S. Fabrication of a Bioadhesive Transdermal Device from Chitosan and Hyaluronic Acid for the Controlled Release of Lidocaine. Carbohydr. Polym. 2016, 152, 687–698. [Google Scholar] [CrossRef]
- Paulino, A.T.; Guilherme, M.R.; Mattoso, L.H.C.; Tambourgi, E.B. Smart Hydrogels Based on Modified Gum Arabic as a Potential Device for Magnetic Biomaterial. Macromol. Chem. Phys. 2010, 211, 1196–1205. [Google Scholar] [CrossRef]
- Barleany, D.R.; Jayanudin, J.; Nasihin, N.; Widiawati, M.; Yulvianti, M.; Sari, D.K.; Gunawan, A. Hydrogel Preparation from Shrimp Shell-Based Chitosan: The Degree of Crosslinking and Swelling Study. ASEAN J. Chem. Eng. 2023, 23, 28. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Gierszewska, M.; Pieróg, M. PH-Responsive Hydrogel Membranes Based on Modified Chitosan: Water Transport and Kinetics of Swelling. J. Polym. Res. 2015, 22, 153. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Han, Q. Modulation of Swelling of PVA Hydrogel by Polymer and Crosslinking Agent Concentration. Polym. Bull. 2023, 80, 1303–1320. [Google Scholar] [CrossRef]
- Rani, C.N. Feasibility Study on the Utilization of Low-Cost Sawdust for Adsorption of Caffeine: Equilibrium, Optimization, and Response Surface Modeling. Desalination Water Treat. 2022, 276, 271–281. [Google Scholar] [CrossRef]
- Hu, P.; Liu, Q.; Wang, J.; Huang, R. Phosphate Removal by Ce(III)-impregnated Crosslinked Chitosan Complex from Aqueous Solutions. Polym. Eng. Sci. 2017, 57, 44–51. [Google Scholar] [CrossRef]
- Miotto, A.C.; Orchulhak, A.P.; Enzweiler, H.; Paulino, A.T.; Visioli, L.J. Caffeine Removal from Water Using Rice Husk Residue Biochar: Characterization, Kinetics and Sorption Isotherms. Int. J. Environ. Sci. Technol. 2025, 22, 7197–7210. [Google Scholar] [CrossRef]
- Castañeda, C.; Martínez, J.J.; Santos, L.; Rojas, H.; Osman, S.M.; Gómez, R.; Luque, R. Caffeine Photocatalytic Degradation Using Composites of NiO/TiO2–F and CuO/TiO2–F under UV Irradiation. Chemosphere 2022, 288, 132506. [Google Scholar] [CrossRef]
- Shu, Z.; Bolton, J.R.; Belosevic, M.; Gamal El Din, M. Photodegradation of Emerging Micropollutants Using the Medium-Pressure UV/H2O2 Advanced Oxidation Process. Water Res. 2013, 47, 2881–2889. [Google Scholar] [CrossRef]
- Lakshmipathy, K.; Sindhu, S.; Singh, A.; Chikkaballapur Krishnappa, S.; Duggonahally Veeresh, C. A Review on Pesticides Degradation by Using Ultraviolet Light Treatment in Agricultural Commodities. eFood 2024, 5, e129. [Google Scholar] [CrossRef]
- Ferens, T.F.; Visioli, L.J.; Paulino, A.T.; Enzweiler, H. Photodegradation of Ibuprofen by Pd-TiO2/ZSM-5 Catalyst. Int. J. Environ. Sci. Technol. 2025, 22, 6759–6768. [Google Scholar] [CrossRef]
- Minyeong Hong, A.Z. Insights into the Eco-Friendly Adsorption of Caffeine from Contaminated Solutions by Using Hydrogel Beads. J. Environ. Anal. Chem. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Copello, G.J.; Mebert, A.M.; Raineri, M.; Pesenti, M.P.; Diaz, L.E. Removal of Dyes from Water Using Chitosan Hydrogel/SiO2 and Chitin Hydrogel/SiO2 Hybrid Materials Obtained by the Sol–Gel Method. J. Hazard. Mater. 2011, 186, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Young, T.A.; Heidler, J.; Matos-Pérez, C.R.; Sapkota, A.; Toler, T.; Gibson, K.E.; Schwab, K.J.; Halden, R.U. Ab Initio and in Situ Comparison of Caffeine, Triclosan, and Triclocarban as Indicators of Sewage-Derived Microbes in Surface Waters. Environ. Sci. Technol. 2008, 42, 3335–3340. [Google Scholar] [CrossRef]
- Sanford, S.; Singh, K.S.; Chaini, S.; LeClair, G. Study of Natural Adsorbent Chitosan and Derivatives for the Removal of Caffeine from Water. Water Qual. Res. J. 2012, 47, 80–90. [Google Scholar] [CrossRef]
- Sowmya, A.; Meenakshi, S. An Efficient and Regenerable Quaternary Amine Modified Chitosan Beads for the Removal of Nitrate and Phosphate Anions. J. Environ. Chem. Eng. 2013, 1, 906–915. [Google Scholar] [CrossRef]
- Do, T.M.P.; Nguyen, X.L. Adsorption Capacity of Chitosan Hydrogel Beads Fabricated from Penaeus Monodon Shrimp Shell Waste for Safranin o Dye. Hue Univ. J. Sci. Nat. Sci. 2023, 132, 23–32. [Google Scholar] [CrossRef]
- Elhalil, A.; Elmoubarki, R.; Farnane, M.; Machrouhi, A.; Sadiq, M.; Mahjoubi, F.Z.; Qourzal, S.; Barka, N. Photocatalytic Degradation of Caffeine as a Model Pharmaceutical Pollutant on Mg Doped ZnO-Al2O3 Heterostructure. Environ. Nanotechnol. Monit. Manag. 2018, 10, 63–72. [Google Scholar] [CrossRef]
- Kumeda, T.; Sakaushi, K. Joint Kinetic/In Situ Spectrometric Investigation of the Multielectron/Multiproton-Transfer-Based Adsorption Electrode Process of Phosphate Anions on the Ir(111) Surface across a Comprehensive PH Range. J. Phys. Chem. C 2023, 127, 10341–10354. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, S.; Xie, H.; Zhang, Y. Utilization and Value-Adding of Waste: Fabrication of Porous Material from Chitosan for Phosphate Capture and Energy Storage. Int. J. Biol. Macromol. 2024, 268, 131944. [Google Scholar] [CrossRef]
- G., G.; D’Souza, J.Q.; Sundaram, N.G. UV Induced Photocatalytic Degradation of Caffeine Using TiO2–H-Beta Zeolite Composite. Minerals 2023, 13, 465. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, M.; Rong, H.; Jiang, P.; Liao, Q.; Zhang, C.; Chen, Y. Photocatalyst Immobilized by Hydrogel, Efficient Degradation and Self Regeneration: A Review. Mater. Sci. Semicond. Process. 2022, 150, 106929. [Google Scholar] [CrossRef]
- Nirmala Rani, C. Photocatalytic Degradation of Caffeine in a Slurry Reactor with Intermittent UV Irradiation: Optimization and Response Surface Modelling. Water Pract. Technol. 2022, 17, 517–528. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Wang, Y.; Ji, H.; Ma, W.; Zang, L.; Zhao, J. Surface Modification of TiO2 by Phosphate: Effect on Photocatalytic Activity and Mechanism Implication. J. Phys. Chem. C 2008, 112, 5993–6001. [Google Scholar] [CrossRef]
- Muangmora, R.; Kemacheevakul, P.; Punyapalakul, P.; Chuangchote, S. Enhanced Photocatalytic Degradation of Caffeine Using Titanium Dioxide Photocatalyst Immobilized on Circular Glass Sheets under Ultraviolet C Irradiation. Catalysts 2020, 10, 964. [Google Scholar] [CrossRef]
- Rahman, T.U.; Roy, H.; Shoronika, A.Z.; Fariha, A.; Hasan, M.; Islam, M.S.; Marwani, H.M.; Islam, A.; Hasan, M.M.; Alsukaibi, A.K.D.; et al. Sustainable Toxic Dye Removal and Degradation from Wastewater Using Novel Chitosan-Modified TiO2 and ZnO Nanocomposites. J. Mol. Liq. 2023, 388, 122764. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of Water Matrix on the Removal of Micropollutants by Advanced Oxidation Technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Heydari Orojlou, S.; Rastegarzadeh, S.; Zargar, B. Experimental and Modeling Analyses of COD Removal from Industrial Wastewater Using the TiO2–Chitosan Nanocomposites. Sci. Rep. 2022, 12, 11088. [Google Scholar] [CrossRef]
- Almeida, L.N.B.; Pietrobelli, J.M.T.A.; Lenzi, G.G.; Santos, O.A.A. Degradation of Caffeine by Heterogeneous Photocatalysis Using ZnO with Fe and Ag. Braz. Arch. Biol. Technol. 2020, 63, 1–9. [Google Scholar] [CrossRef]
- Gonçalves, M.; Guerreiro, M.C.; Oliveira, L.C.A.; Rocha, C.L. da Materiais à Base de Óxido de Ferro Para Oxidação de Compostos Presentes No Efluente Da Despolpa Do Café. Química Nova 2008, 31, 1636–1640. [Google Scholar] [CrossRef][Green Version]
- Reinehr, I.L.; Capra, D.F.; Kempka, A.P.; Visioli, L.J.; Paulino, A.T.; Enzweiler, H. Innovative Chitosan/TiO2 Composite Membrane for the Sustainable Photocatalytic Purification of Water Contaminated with Emerging Micropollutants. Polym. Bull. 2025; in press. [Google Scholar] [CrossRef]
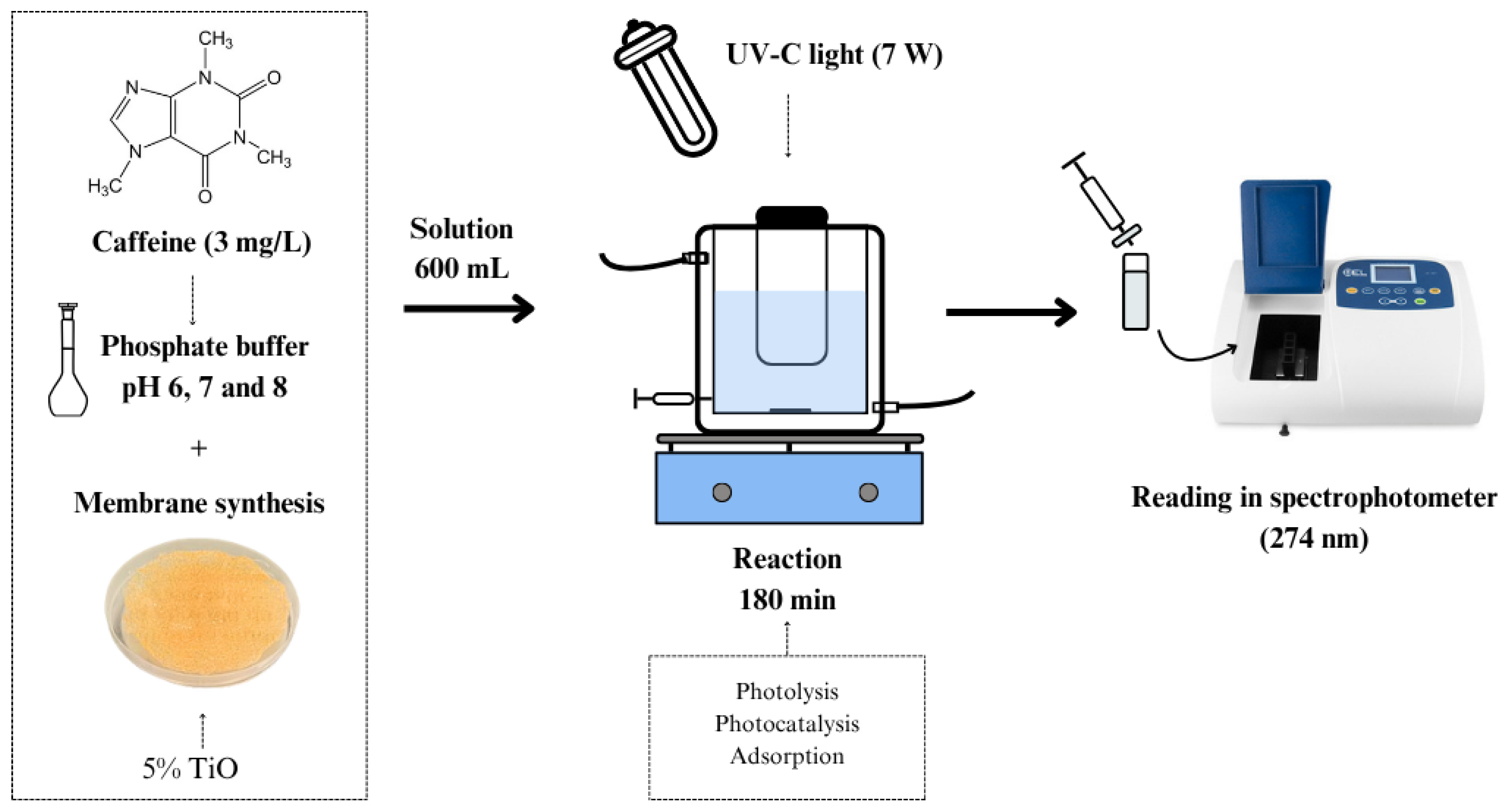
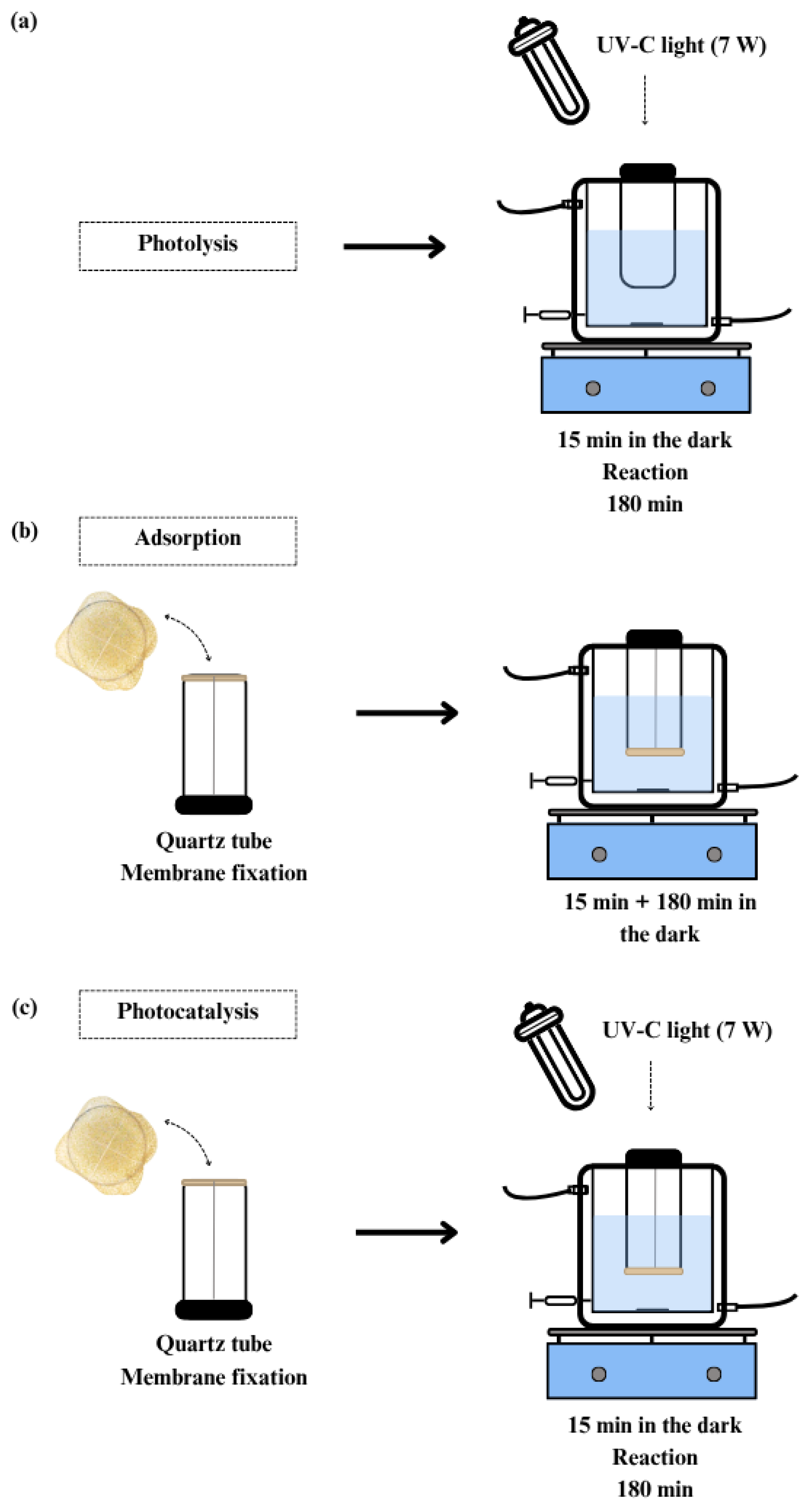


| Process | Description |
|---|---|
| Photolysis | Use of irradiation source alone |
| Photolysis + sorption in membrane | Combined use of irradiation source and non-catalytic membrane |
| Sorption in membrane | Use of non-catalytic membrane in the dark |
| Sorption in catalytic membrane | Use of catalytic membrane in the dark |
| Photocatalysis with catalytic membrane | Combined use of irradiation source and catalytic membrane |
| Photocatalysis with dispersed catalyst | Use of catalyst dispersed in reaction medium |
| Sample | Degree of Swelling (%) | |||
|---|---|---|---|---|
| H2O | pH 6 | pH 7 | pH 8 | |
| Chitosan | 481.59 ± 74.26 a | 138.20 ± 6.51 b | 171.17 ± 40.92 b | 180.50 ± 38.53 b |
| Composite | 376.25 ± 8.31 a | 115.42 ± 10.11 c | 158.81 ± 7.77 b | 131.20 ± 5.95 b |
| Process | k Values (mg L−1h−1) | ||
|---|---|---|---|
| pH = 6 | pH = 7 | pH = 8 | |
| Photolysis | 0.063 ± 0.004 Ba | 0.093 ± 0.013 Aa | 0.111 ± 0.030 Aa |
| Photolysis + sorption | 0.081 ± 0.004 Ba | 0.084 ± 0.017 Aa | 0.114 ± 0.008 Aa |
| Sorption in chitosan membrane | 0.008 ± 0.005 Ca | 0.015 ± 0.004 Ba | 0.010 ± 0.011 Ba |
| Sorption in composite membrane | 0.014 ± 0.014 Ca | 0.027 ± 0.013 Ba | 0.001 ± 0.000 Ba |
| Photocatalysis with composite membrane | 0.060 ± 0.008 Bb | 0.075 ± 0.004 Aa | 0.108 ± 0.017 Aa |
| Photocatalysis with dispersed catalyst | 0.366 ± 0.034 Aa | 0.111 ± 0.004 Ab | 0.105 ± 0.004 Ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prando, J.; Reinehr, I.L.; Visioli, L.J.; Paulino, A.T.; Enzweiler, H. Photolysis, Photocatalysis, and Sorption of Caffeine in Aqueous Media in the Presence of Chitosan Membrane and Chitosan/TiO2 Composite Membrane. Processes 2025, 13, 2439. https://doi.org/10.3390/pr13082439
Prando J, Reinehr IL, Visioli LJ, Paulino AT, Enzweiler H. Photolysis, Photocatalysis, and Sorption of Caffeine in Aqueous Media in the Presence of Chitosan Membrane and Chitosan/TiO2 Composite Membrane. Processes. 2025; 13(8):2439. https://doi.org/10.3390/pr13082439
Chicago/Turabian StylePrando, Juliana, Ingrid Luíza Reinehr, Luiz Jardel Visioli, Alexandre Tadeu Paulino, and Heveline Enzweiler. 2025. "Photolysis, Photocatalysis, and Sorption of Caffeine in Aqueous Media in the Presence of Chitosan Membrane and Chitosan/TiO2 Composite Membrane" Processes 13, no. 8: 2439. https://doi.org/10.3390/pr13082439
APA StylePrando, J., Reinehr, I. L., Visioli, L. J., Paulino, A. T., & Enzweiler, H. (2025). Photolysis, Photocatalysis, and Sorption of Caffeine in Aqueous Media in the Presence of Chitosan Membrane and Chitosan/TiO2 Composite Membrane. Processes, 13(8), 2439. https://doi.org/10.3390/pr13082439








