Simple Boronic Acid-Appended Sensor Array for Saccharides by Linear Discriminant Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, X.; Han, Y.; Li, J.; Tang, M.; Qing, G. Sensitive and Specific Detection of Saccharide Species Based on Fluorescence: Update from 2016. Anal. Bioanal. Chem. 2023, 415, 4061–4077. [Google Scholar] [CrossRef]
- Zhai, W.; Sun, X.; James, T.D.; Fossey, J.S. Boronic Acid-Based Carbohydrate Sensing. Chem. Asian J. 2015, 10, 1836–1848. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.T.; Kedge, J.L.; Fossey, J.S. Molecular Boronic Acid-Based Saccharide Sensors. ACS Sens. 2021, 6, 1508–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, G.; Ning, Z.; Xing, G. Boronic Acid-Based Chemical Sensors for Saccharides. Carbohydr. Res. 2017, 452, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Xing, G. Aggregates-Based Boronlectins with Pyrene as Fluorophore: Multichannel Discriminative Sensing of Monosaccharides and Their Applications. ACS Appl. Mater. Interfaces 2016, 8, 12007–12017. [Google Scholar] [CrossRef]
- Sasaki, Y.; Lyu, X.; Zhou, Q.; Minami, T. Indicator Displacement Assay-Based Chemosensor Arrays for Saccharides Using Off-the-Shelf Materials toward Simultaneous On-Site Detection on Paper. Chem. Lett. 2021, 50, 987–995. [Google Scholar] [CrossRef]
- Lyu, X.; Hamedpour, V.; Sasaki, Y.; Zhang, Z.; Minami, T. 96-Well Microtiter Plate Made of Paper: A Printed Chemosensor Array for Quantitative Detection of Saccharides. Anal. Chem. 2020, 93, 1179–1184. [Google Scholar] [CrossRef]
- Long, Z.; Fang, D.-C.; Ren, H.; Ouyang, J.; He, L.; Na, N. Excited Oxidized-Carbon Nanodots Induced by Ozone from Low-Temperature Plasma to Initiate Strong Chemiluminescence for Fast Discrimination of Metal Ions. Anal. Chem. 2016, 88, 7660–7666. [Google Scholar] [CrossRef]
- Wang, F.; Xiong, S.; Wang, T.; Hou, Y.; Li, Q. Discrimination of Cis-Diol-Containing Molecules Using Fluorescent Boronate Affinity Probes by Principal Component Analysis. Anal. Methods 2023, 15, 5803–5812. [Google Scholar] [CrossRef]
- Edwards, N.Y.; Sager, T.W.; McDevitt, J.T.; Anslyn, E.V. Boronic Acid Based Peptidic Receptors for Pattern-Based Saccharide Sensing in Neutral Aqueous Media, an Application in Real-Life Samples. J. Am. Chem. Soc. 2007, 129, 13575–13583. [Google Scholar] [CrossRef]
- Sasaki, Y.; Leclerc, É.; Hamedpour, V.; Kubota, R.; Takizawa, S.; Sakai, Y.; Minami, T. Simplest Chemosensor Array for Phosphorylated Saccharides. Anal. Chem. 2019, 91, 15570–15576. [Google Scholar] [CrossRef]
- Yang, J.; He, X.; Chen, L.; Zhang, Y. The Selective Detection of Galactose Based on Boronic Acid Functionalized Fluorescent Carbon Dots. Anal. Methods 2016, 8, 8345–8351. [Google Scholar] [CrossRef]
- Ashokkumar, P.; Bell, J.; Buurman, M.; Rurack, K. Analytical Platform for Sugar Sensing in Commercial Beverages Using a Fluorescent BODIPY “Light-up” Probe. Sens. Actuators B Chem. 2018, 256, 609–615. [Google Scholar] [CrossRef]
- Inci, F.; Resendez, A.; Karaaslan, M.G.; Pandrala, M.; Kojouri, A.M.; Ahmed, R.; Ogut, M.G.; Singaram, B.; Malhotra, S.V.; Demirci, U. A Smart Probe for Detection of Sugar Markers for Applications in Gastrointestinal Barrier Dysfunction. Biosens. Bioelectron. 2025, 272, 117040. [Google Scholar] [CrossRef] [PubMed]
- Resendez, A.; Malhotra, S.V. Boronic Acid Appended Naphthyl-Pyridinium Receptors as Chemosensors for Sugars. Sci. Rep. 2019, 9, 6651. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y. Self-Assembled Nanorods of Phenylboronic Acid Functionalized Pyrene for In Situ Two-Photon Imaging of Cell Surface Sialic Acids and Photodynamic Therapy. Anal. Chem. 2021, 93, 7029–7036. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mizuta, Y.; Mikagi, A.; Misawa-Suzuki, T.; Tsuchido, Y.; Sugaya, T.; Hashimoto, T.; Ema, K.; Hayashita, T. Recognition of D-Glucose in Water with Excellent Sensitivity, Selectivity, and Chiral Selectivity Using γ-Cyclodextrin and Fluorescent Boronic Acid Inclusion Complexes Having a Pseudo-Diboronic Acid Moiety. ACS Sens. 2023, 8, 218–227. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, B.; Ji, D.-K.; Chen, Q.; He, X.-P.; Chen, G.-R.; James, T.D. Selective Fluorescence Detection of Monosaccharides Using a Material Composite Formed between Graphene Oxide and Boronate-Based Receptors. ACS Appl. Mater. Interfaces 2014, 6, 10078–10082. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, G.; Li, C.; Liu, Y.; Hou, M.; Xing, G. Water-Soluble Glucosamine-Coated AIE-Active Fluorescent Organic Nanoparticles: Design, Synthesis and Assembly for Specific Detection of Heparin Based on Carbohydrate–Carbohydrate Interactions. Chem. Asian. J. 2019, 14, 3295–3300. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Xing, G. Novel Boronlectins Based on Bispyridium Salt with a Flexible Linker: Discriminative Sensing of Lactose and Other Monosaccharides and Disaccharides in Aqueous Solution. Chem. Asian. J. 2015, 10, 2594–2598. [Google Scholar] [CrossRef]
- Liu, G.-J.; Long, Z.; Lv, H.; Li, C.; Xing, G. A Dialdehyde–Diboronate-Functionalized AIE Luminogen: Design, Synthesis and Application in the Detection of Hydrogen Peroxide. Chem. Commun. 2016, 52, 10233–10236. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Xing, G. Boronlectin/Polyelectrolyte Ensembles as Artificial Tongue: Design, Construction, and Application for Discriminative Sensing of Complex Glycoconjugates from Panax Ginseng. ACS Appl. Mater. Interfaces 2017, 9, 3368–3375. [Google Scholar] [CrossRef]
- Jia, L.; Liu, G.; Ji, Y.; Zhang, Y.; Xing, G. Assembly of Water-Soluble Sugar-Coated AIE-Active Fluorescent Organic Nanoparticles for the Detection of Ginsenosides Based on Carbohydrate-Carbohydrate Interactions. ChemNanoMat 2020, 6, 368–372. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Mohadjerani, M.; Pooryousef, M. A New Selective Fluorene-Based Fluorescent Internal Charge Transfer (ICT) Sensor for Sugar Alcohols in Aqueous Solution. Anal. Bioanal. Chem. 2016, 408, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, K.O.; Lee, J.J. D-Glucose Recognition Based on Phenylboronic Acid-Functionalized Polyoligomeric Silsesquioxane Fluorescent Probe. Mat. Sci. Eng. C 2019, 95, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Michida, W.; Nagai, A.; Sakuragi, M.; Okobira, T.; Kusakabe, K. Fluorescence Emission Behaviors of the L-Cysteine/Au(I) Complex in a Cyclodextrin-Based Metal-Organic Framework. Processes 2020, 8, 1555. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; He, M.; Huang, J. Synthesis of a Benzothiadiazole-Based D−A Molecule with Aggregation-Induced Emission and Controlled Assembly Properties. Processes 2021, 9, 1094. [Google Scholar] [CrossRef]
- Chua, M.H.; Hui, B.Y.K.; Chin, K.L.O.; Zhu, Q.; Liu, X.; Xu, J. Recent Advances in Aggregation-Induced Emission (AIE)-Based Chemosensors for the Detection of Organic Small Molecules. Mater. Chem. Front. 2023, 7, 5561–5660. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Zhou, W.; Feng, G.; Xing, G. Recent Advances in Sugar-Based AIE Luminogens and Their Applications in Sensing and Imaging. Chem. Commun. 2024, 60, 11899–11915. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, C.; Tang, L.; Qin, A.; Hu, R.; Sun, J.Z.; Tang, B.Z. Specific Detection of D-Glucose by a Tetraphenylethene-Based Fluorescent Sensor. J. Am. Chem. Soc. 2011, 133, 660–663. [Google Scholar] [CrossRef]
- Suenaga, H.; Mikami, M.; Sandanayake, K.R.A.S.; Shinkai, S. Screening of Fluorescent Boronic Acids for Sugar Sensing Which Show a Large Fluorescence Change. Tetrahedron Lett. 1995, 36, 4825–4828. [Google Scholar] [CrossRef]
- Otsuka, H.; Uchimura, E.; Koshino, H.; Okano, T.; Kataoka, K. Anomalous Binding Profile of Phenylboronic Acid with N-Acetylneuraminic Acid (Neu5Ac) in Aqueous Solution with Varying pH. J. Am. Chem. Soc. 2003, 125, 3493–3502. [Google Scholar] [CrossRef]
- Crista, D.M.A.; Mello, G.P.C.; Shevchuk, O.; Sendão, R.M.S.; Simões, E.F.C.; Leitão, J.M.M.; da Silva, L.P.; Esteves da Silva, J.C.G. 3-Hydroxyphenylboronic Acid-Based Carbon Dot Sensors for Fructose Sensing. J. Fluoresc. 2019, 29, 265–270. [Google Scholar] [CrossRef]
- Penavic, A.; Radujevic, A.; Pushina, M.; Anzenbacher, P., Jr. Binaphthalene Boronic Acid Sensor for Saccharides and D-Fructose Determination in Beverages. Anal. Sens. 2022, 2, e202100068. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Diamond, D.; Florea, L. Fluorescent Probes for Sugar Detection. ACS Appl. Mater. Interfaces 2018, 10, 38431–38437. [Google Scholar] [CrossRef]
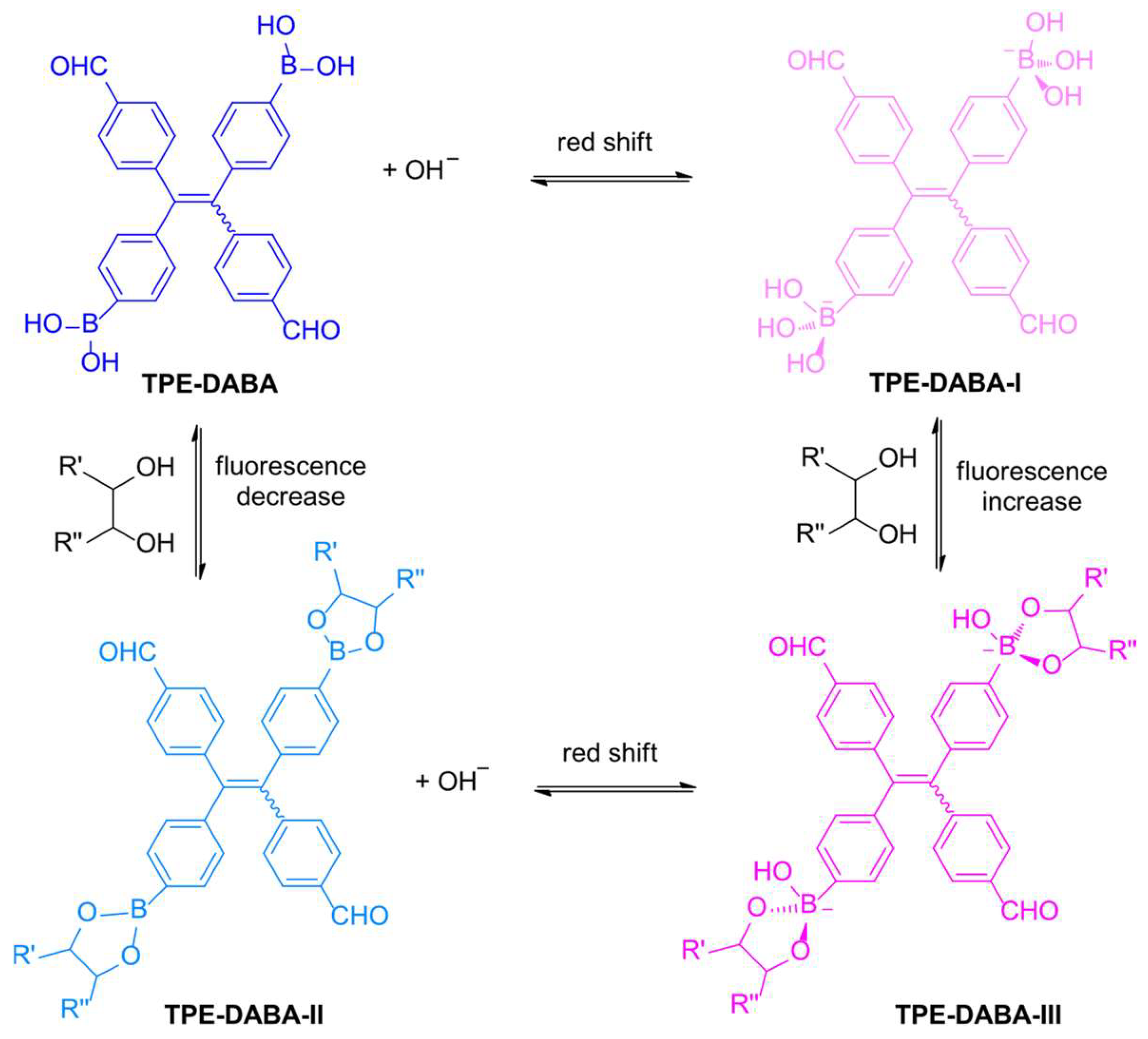
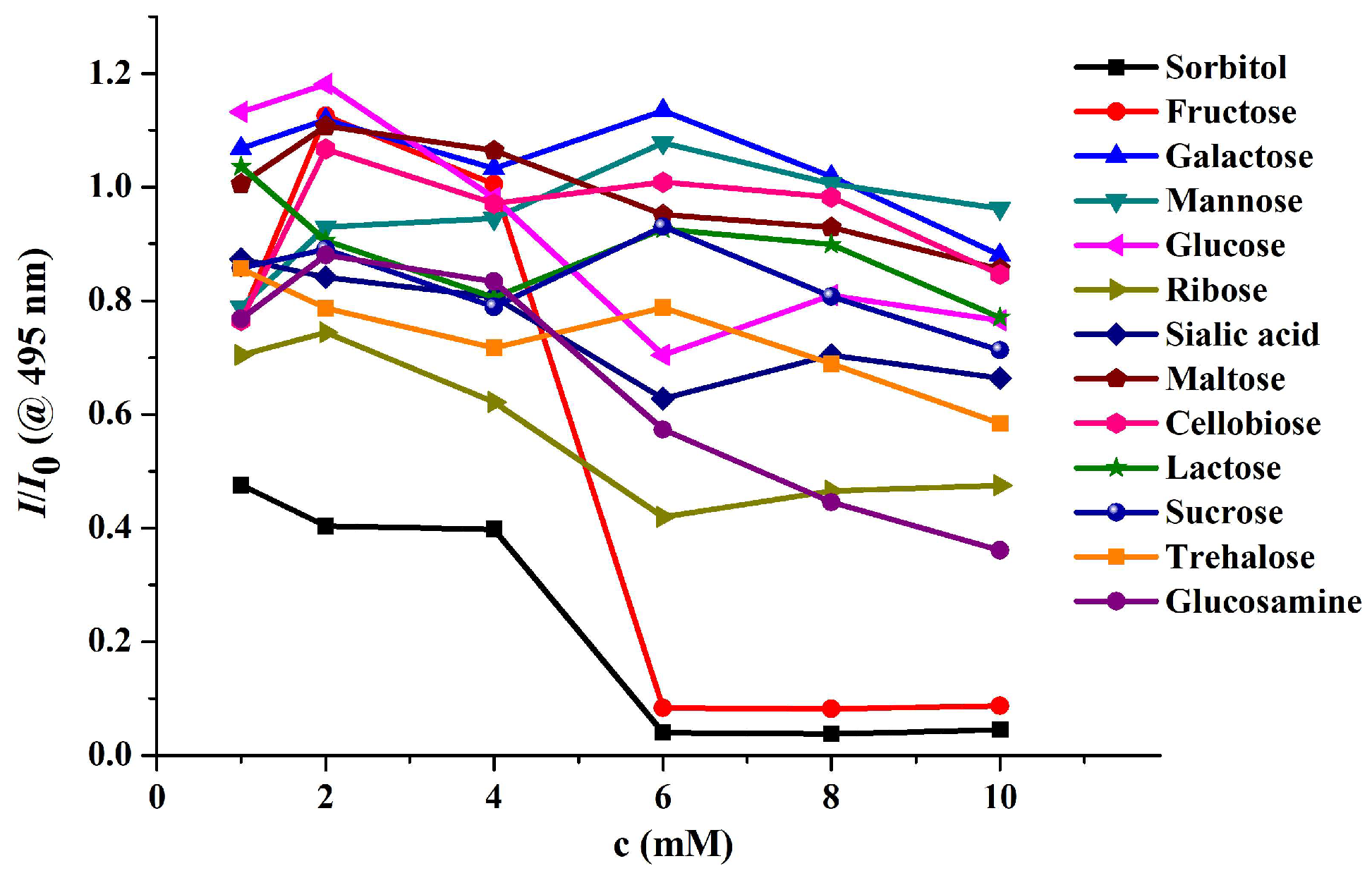
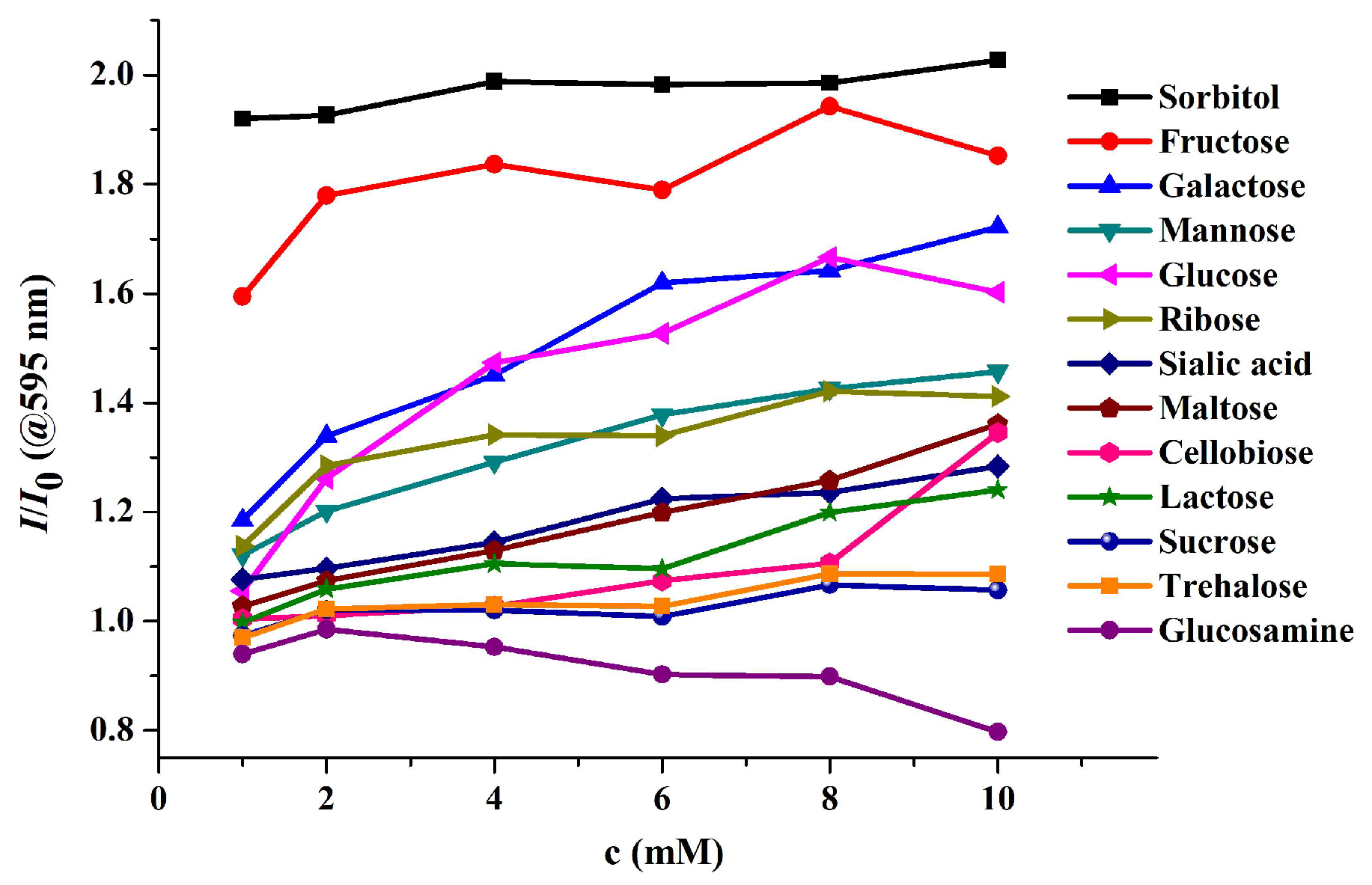
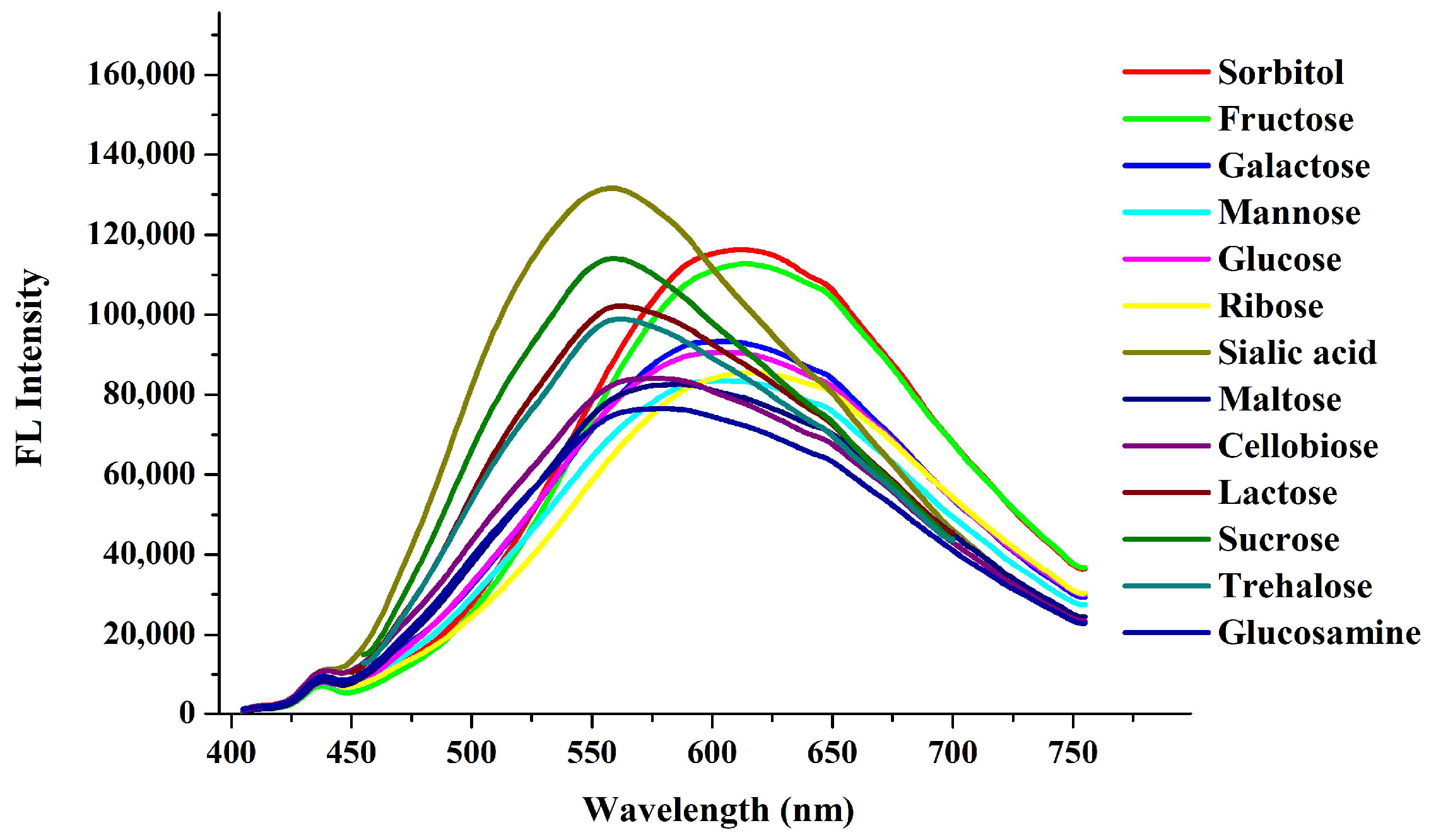

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Long, Z.; Xing, G. Simple Boronic Acid-Appended Sensor Array for Saccharides by Linear Discriminant Analysis. Processes 2025, 13, 2323. https://doi.org/10.3390/pr13082323
Liu G, Long Z, Xing G. Simple Boronic Acid-Appended Sensor Array for Saccharides by Linear Discriminant Analysis. Processes. 2025; 13(8):2323. https://doi.org/10.3390/pr13082323
Chicago/Turabian StyleLiu, Guangjian, Zi Long, and Guowen Xing. 2025. "Simple Boronic Acid-Appended Sensor Array for Saccharides by Linear Discriminant Analysis" Processes 13, no. 8: 2323. https://doi.org/10.3390/pr13082323
APA StyleLiu, G., Long, Z., & Xing, G. (2025). Simple Boronic Acid-Appended Sensor Array for Saccharides by Linear Discriminant Analysis. Processes, 13(8), 2323. https://doi.org/10.3390/pr13082323





