Development and Characterization of Dairy Compound with Goat Milk Powder and Rice Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Dairy Compounds: Goat’s Milk and Rice Flour
2.2. Reagents
2.3. Physicochemical Analysis
2.4. Physical Analysis
2.4.1. Density
2.4.2. Fluidity and Cohesiveness
2.4.3. Flowability
2.4.4. Wettability
2.5. Total Phenolic Content
2.6. Individual Phenolic Compounds
2.7. Multi-Element Profile
2.8. Scanning Electron Microscopy (SEM)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis
3.2. Physical Analysis
3.2.1. Density
3.2.2. Fluidity and Cohesiveness
3.2.3. Flowability
3.2.4. Wettability
3.3. Total Phenolic Content
3.4. Individual Phenolic Compounds
3.5. Multi-Element Profile
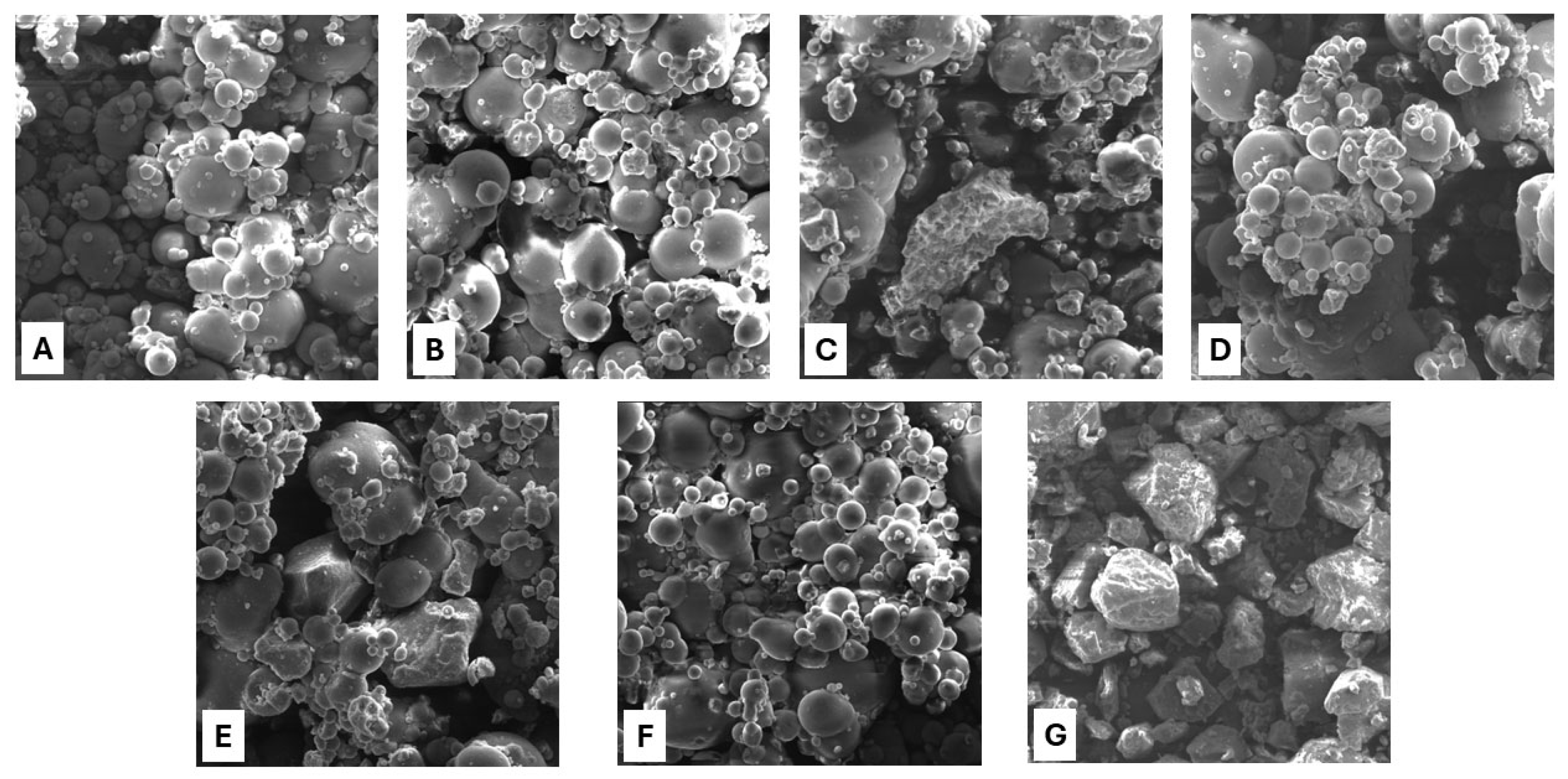
3.6. Scanning Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballabio, C.; Chessa, S.; Rignanese, D.; Gigliotti, C.; Pagnacco, G.; Terracciano, L.; Fiocchi, A.; Restani, P.; Caroli, A.M. Goat milk allergenicity as a function of αS1-casein genetic polymorphism. J. Dairy Sci. 2011, 94, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Fialho, T.L.; Nogueira, M.H.; Moreau, A.; Delaplace, G.; Schuck, P.; Perrone, Í.T.; de Carvalho, A.F.; de Sá Peixoto Júnior, P.P. Sugar type matters in spray drying: Homogeneous distribution in milk powder favors repulsive interactions between proteins. Food Struct. 2019, 22, 100132. [Google Scholar] [CrossRef]
- Wang, H.J.; Lee, D.S. Packaging and the Shelf Life of Milk Powder Products. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- INMETRO. Leite em pó Integral. 2020. Available online: https://bibliosus.saude.gov.br/lis/resource/?id=17605 (accessed on 20 July 2025).
- Verruck, S.; de Liz, G.R.; Dias, C.O.; de Mello Castanho Amboni, R.D.; Prudencio, E.S. Effect of full-fat goat’s milk and prebiotics use on Bifidobacterium BB-12 survival and on the physical properties of spray-dried powders under storage conditions. Food Res. Int. 2019, 119, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Kostić, A.Ž.; Gašić, U.M.; Lević, S.; Stanojević, S.P.; Barać, M.B.; Tešić, Ž.L.; Nedović, V.; Pešić, M.B. Skimmed Goat’s Milk Powder Enriched with Grape Pomace Seed Extract: Phenolics and Protein Characterization and Antioxidant Properties. Biomolecules 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Pavan Kumar, M.V.; Rohini Kumar, B. Investigating the impact of spray drying parameters on milk powder bio fortification with curcumin and veld grape extract. Food Hum. 2025, 4, 100572. [Google Scholar] [CrossRef]
- Yu, T.; Jing, S.; Jiaxin, L.; Aixia, W.; Mengzi, N.; Xue, G.; Lili, W.; Liya, L.; Fengzhong, W.; Litao, T. Effects of Milling Methods on Rice Flour Properties and Rice Product Quality: A Review. Rice. Sci. 2024, 31, 33–46. [Google Scholar] [CrossRef]
- Brazil. Instrução Normativa MAPA no 28, de 12 de junho de 2007: Regulamento Técnico Para Fixação de Identidade e Qualidade de Composto Lácteo. 2007. Available online: https://www.normasbrasil.com.br/norma/?id=76488 (accessed on 20 July 2025).
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Reddy, R.S.; Ramachandra, C.T.; Hiregoudar, S.; Nidoni, U.; Ram, J.; Kammar, M. Influence of processing conditions on functional and reconstitution properties of milk powder made from Osmanabadi goat milk by spray drying. Small Rumin. Res. 2014, 119, 130–137. [Google Scholar] [CrossRef]
- Hailu, Y.; Maidannyk, V.A.; Murphy, E.G.; McCarthy, N.A. Improving the physical and wettability properties of skim milk powders through agglomeration and lecithination. J. Food Eng. 2023, 357, 111597. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Burin, V.M.; Ferreira-Lima, N.E.; Panceri, C.P.; Bordignon-Luiz, M.T. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: Evaluation of different extraction methods. Microchem. J. 2014, 114, 155–163. [Google Scholar] [CrossRef]
- Prestes, A.A.; de Kacia Souza Coelho, B.; de Oliveira Mindelo, L.J.; Carvalho, A.C.F.; de Gois, J.S.; Andrade, D.R.M.; de Souza, C.K.; Helm, C.V.; da Cruz, A.G.; Prudencio, E.S. Development and chemical, physical, functional, and multi-element profile characterization of Requeijão with guabiroba pulp. Processes 2025, 13, 1322. [Google Scholar] [CrossRef]
- Carvalho, A.C.F.; Hoffmann, T.G.; Helm, C.V.; Prudêncio, E.S.; Roy, S.; de Souza, C.K. Smart biopolymer based on rice husk extracts and Pinhão failure applied as an interlayer for sliced mozzarella cheese. J. Vinyl Addit. Technol. 2025, 1–14. [Google Scholar] [CrossRef]
- da Cunha, T.M.P.; da Silva Haas, I.C.; da Costa, M.A.J.L.; Luna, A.S.; de Gois, J.S.; Amboni, R.D.D.M.C.; Prudencio, E.S. Dairy powder enriched with a soy extract (Glycine max): Physicochemical and polyphenolic characteristics, physical and rehydration properties and multielement composition. Food Res. Int. 2022, 162, 112144. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.; Cabassi, G.; Chiavaro, E.; Paciulli, M.; Carini, E.; Mucchetti, G. Physical characterization of whole and skim dried milk powders. J. Food Sci. Technol. 2017, 54, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Saipriya, K.; Deshwal, G.K.; Singh, A.K.; Kapila, S.; Sharma, H. Effect of dairy unit operations on immunoglobulins, colour, rheology and microbiological characteristics of goat milk. Int. Dairy, J. 2021, 121, 105118. [Google Scholar] [CrossRef]
- Lee, H.S.; Coates, G.A. Effect of thermal pasteurization on Valencia orange juice color and pigments. LWT-Food Sci. Technol. 2003, 36, 153–156. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, W.J.; Guo, L.; Shao, Z.P.; Xu, X.J. Comparative study on the characteristics and oxidation stability of commercial milk powder during storage. J. Dairy Sci. 2019, 102, 8785–8797. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Boeneke, C.; King, J.M. Comparison of Storage Stability and In Vitro Digestion of Rice Flour-Based Yogurt Alternatives Made with Lactobacillus rhamnosus Lgg to Milk-Based Yogurt. Foods 2025, 14, 1129. [Google Scholar] [CrossRef] [PubMed]
- Alsaleem, K.A.; Hamouda, M.E.A. Optimizing probiotic low-fat yogurt: The benefits of incorporating defatted rice bran for enhanced quality and functionality. Food Sci. Nutr. 2024, 12, 10242–10254. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, G.K.; Singh, A.K.; Kumar, D.; Sharma, H. Effect of spray and freeze drying on physico-chemical, functional, moisture sorption and morphological characteristics of camel milk powder. LWT 2020, 134, 110117. [Google Scholar] [CrossRef]
- Lamolha, M.A.; Serra, C.H.D.R. Avaliação das propriedades de fluxo dos granulados e dissolução de comprimidos de hidroclorotiazida 50 mg obtidos por granulação úmida. Rev. Bras. Cienc. Farm. 2007, 43, 435–446. [Google Scholar] [CrossRef]
- Lin, Q.; Xiao, H.; Fu, X.; Tian, W.; Li, L.; Yu, F. Physico-chemical properties of flour, starch, and modified starch of two rice varieties. Agric. Sci. China 2011, 10, 960–968. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Aalim, H.; Islam Shishir, M.R.; Yosri, N.; Arslan, M.; Tahir, H.E.; Hashim, S.B.H.; Karim, N.; Zhai, X.; Li, Z.; Zhou, C.; et al. Systematic review of the digestive fate of rice phenolic compounds: Insights into bioavailability, influencing factors, encapsulation strategies, and health implications. Trends Food Sci. Technol. 2025, 156, 104833. [Google Scholar] [CrossRef]
- Ansori, F.A.Z.; Nisa, F.C.; Mubarok, A.Z. Functional products based on goat milk with the addition of natural antioxidants: Scientific review. J. Ilmu Teknol. Hasil Ternak 2024, 19, 217–229. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Noriega, L.G.; Morales-Romero, A.M.; Nieto-Camacho, A.; Granados-Portillo, O.; Rodríguez-López, L.A.; Alemán, G.; Furuzawa-Carballeda, J.; Tovar, A.R.; Cisneros-Zevallos, L.; et al. Goat’s milk intake prevents obesity, hepatic steatosis and insulin resistance in mice fed a high-fat diet by reducing inflammatory markers and increasing energy expenditure and mitochondrial content in skeletal muscle. Int. J. Mol. Sci. 2020, 21, 5530. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Puga, C.; Cuchillo-Hilario, M.; León-Ortiz, L.; Ramírez-Rodríguez, A.; Cabiddu, A.; Navarro-Ocaña, A.; Morales-Romero, A.M.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Goats’ feeding supplementation with Acacia farnesiana pods and their relationship with milk composition: Fatty acids, polyphenols, and antioxidant activity. Animals 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Al-Wraikat, M.; Shu, Q.; Yang, X.; Liu, Y. An overview of interactions between goat milk casein and other food components: Polysaccharides, polyphenols, and metal ions. Foods 2024, 13, 2903. [Google Scholar] [CrossRef] [PubMed]
- Avinash, G.; Sharma, N.; Prasad, K.R.; Kaur, R.; Singh, G.; Pagidipala, N.; Thulasinathan, T. Unveiling the distribution of free and bound phenolic acids, flavonoids, anthocyanins, and proanthocyanidins in pigmented and non-pigmented rice genotypes. Front. Plant Sci. 2024, 15, 1324825. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 2014, 143, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Li, Z.; Deng, L. Analysis of 17 elements in cow, goat, buffalo, yak, and camel milk by inductively coupled plasma mass spectrometry (ICP-MS). RSC Adv. 2020, 10, 6736–6742. [Google Scholar] [CrossRef] [PubMed]
- Currò, S.; De Marchi, M.; Claps, S.; Salzano, A.; De Palo, P.; Manuelian, C.; Neglia, G. Differences in the detailed milk mineral composition of Italian local and Saanen goat breeds. Animals 2019, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yu, Z.; Dai, J.; Jiang, H.; Shi, C.; Du, Q.; Zhu, W.; Bari, L.; Fan, R.; Wang, J.; et al. Effect of processing methods on the distribution of mineral elements in goat milk fractions. J. Dairy Sci. 2024, 107, 5449–5459. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, M.; Dirchwolf, P.M.; Rodríguez-Negrín, Z.; Pellerano, R.G. Assessing mineral profiles for rice flour fraud detection by principal component analysis based data fusion. Food Chem. 2021, 339, 128125. [Google Scholar] [CrossRef] [PubMed]

| Sample | Goat’s Milk Powder (%) (m/m) | Rice Flour (%) (m/m) |
|---|---|---|
| Control 1 | 100 | 0 |
| Control 2 | 0 | 100 |
| DC 10 | 90 | 10 |
| DC 20 | 80 | 20 |
| DC 30 | 70 | 30 |
| DC 40 | 60 | 40 |
| DC 49 | 51 | 49 |
| Results | Control 1 | Control 2 | DC 10 | DC 20 | DC 30 | DC 40 | DC 49 | |
|---|---|---|---|---|---|---|---|---|
| Water activity (aw) | 0.33 b ± 0.01 | 0.42 a ± 0.01 | 0.28 c ± 0.01 | 0.31 bc ± 0.01 | 0.30 c ± 0.01 | 0.31 bc ± 0.01 | 0.33 b ± 0.01 | |
| Total solids content (g/100 g) | 10.0 a ± 0.5 | 10.1 a ± 0.6 | 10.1 a ± 0.5 | 10.0 a ± 0.5 | 10.0 a ± 0.5 | 10.0 a ± 0.5 | 10.0 a ± 0.6 | |
| Total protein content (g/100 g) | 27.0 a ± 0.2 | 9.3 g ± 0.3 | 25.2 b ± 0.2 | 23.5 c ± 0.2 | 21.7 d ± 0.2 | 19.9 e ± 0.2 | 18.3 f ± 0.2 | |
| Total lipid content (g/100 g) | 32.0 a ± 0.2 | 1.0 g ± 0.1 | 28.9 b ± 0.2 | 25.6 c ± 0.2 | 22.7 d ± 0.2 | 19.6 e ± 0.2 | 16.8 f ± 0.2 | |
| Carbohydrates (g/100 g) | 31.0 g ± 0.4 | 79.6 a ± 0.8 | 35.8 f ± 0.4 | 40.7 e ± 0.5 | 45.6 d ± 0.5 | 50.4 c ± 0.6 | 54.8 b ± 0.6 | |
| Energy value | (kcal) | 520.0 a ± 2.3 | 362.2 g ± 1.1 | 504.1 b ± 1.4 | 487.2 c ± 1.5 | 473.5 d ± 1.5 | 457.6 e ± 1.6 | 443.6 f ± 1.6 |
| (kJ) | 2170.0 a ± 9.6 | 1538.1 g ± 4.7 | 2150.8 b ± 4.2 | 2038.6 c ± 6.4 | 1950.9 d ± 6.4 | 1920.3 e ± 7.0 | 1864.3 f ± 12.7 | |
| Sample | Color Parameters | ||||
|---|---|---|---|---|---|
| L* | a* | b* | ΔE*1 | ΔE*2 | |
| Control 1 | 95.57 a ± 0.12 | −3.26 a ± 0.05 | 11.09 a ± 0.12 | - | 6.62 |
| Control 2 | 93.81 b ± 0.26 | −0.17 f ± 0.04 | 5.51 d ± 0.16 | 6.62 | - |
| DC 10 | 95.37 a ± 0.17 | −3.40 a ± 0.39 | 10.12 b ± 0.66 | 1.00 | 5.84 |
| DC 20 | 95.52 a ± 0.33 | −2.72 b ± 0.05 | 10.01 b ± 0.26 | 1.20 | 5.45 |
| DC 30 | 95.54 a ± 0.33 | −2.51 c ± 0.07 | 9.71 b ± 0.36 | 1.57 | 5.11 |
| DC 40 | 95.54 a ± 0.52 | −2.34 d ± 0.09 | 9.46 b ± 0.29 | 1.87 | 4.83 |
| DC 49 | 94.93 a ± 0.51 | −2.07 e ± 0.01 | 8.81 c ± 0.04 | 2.65 | 3.97 |
| Physical Properties | Samples | ||||||
|---|---|---|---|---|---|---|---|
| Control 1 | Control 2 | DC 10 | DC 20 | DC 30 | DC 40 | DC 49 | |
| Aerated bulk density (g/cm3) | 0.47 d ± 0.01 | 0.63 a ± 0.02 | 0.43 d ± 0.05 | 0.45 d ± 0.01 | 0.50 c ± 0.01 | 0.51 c ± 0.01 | 0.50 b ± 0.01 |
| Compacted density (g/cm3) | 0.52 c ± 0.03 | 0.77 a ± 0.02 | 0.48 c ± 0.04 | 0.53 c ± 0.01 | 0.59 b ± 0.03 | 0.60 b ± 0.01 | 0.60 b ± 0.02 |
| Carr index (%) | 10.64 d ± 0.23 | 22.22 a ± 0.42 | 11.63 d ± 0.90 | 17.78 c ± 0.13 | 18.00 c ± 0.21 | 17.64 c ± 0.15 | 20.00 b ± 0.50 |
| Hausner ratio | 1.11 a ± 0.11 | 1.22 b ± 0.03 | 1.12 a ± 0.01 | 1.18 b ± 0.01 | 1.18 b ± 0.01 | 1.18 b ± 0.01 | 1.20 b ± 0.02 |
| Flowability (°) | 84.00 a ± 1.00 | 87.00 a ± 2.00 | 83.00 a ± 2.00 | 83.00 a ± 2.00 | 83.00 a ± 2.00 | 83.00 a ± 2.00 | 84.00 a ± 1.00 |
| Wettability (s) | 120.0 a ± 1.10 | 25.5 g ± 0.50 | 91.0 b ± 0.80 | 70.0 c ± 0.40 | 51.0 d ± 0.50 | 47.4 e ± 0.30 | 34.9 f ± 0.30 |
| Sample | TPC (mg GAE·100 g−1) |
|---|---|
| Control 1 | 37.98 ± 1.45 d |
| Control 2 | 33.77 ± 1.94 e |
| DC 10 | 48.53 ± 4.64 a |
| DC 20 | 44.49 ± 3.02 b |
| DC 30 | 44.56 ± 6.42 b |
| DC 40 | 40.33 ± 1.57 c |
| DC 49 | 39.16 ± 2.67 cd |
| Phenolic Compound Detected | LOQ (µg/g) | LOD (µg/g) | Quantification (µg/g) |
|---|---|---|---|
| Gallic acid | 0.07 ± 0.01 | 0.02 ± 0.001 | 0.08 ± 0.01 |
| Protocatechuic acid | 0.06 ± 0.01 | 0.02 ± 0.001 | 0.07 ± 0.01 |
| Vanillic acid | 0.60 ± 0.01 | 0.2 ± 0.001 | 0.72 ± 0.05 |
| Siringic acid | 0.50 ± 0.01 | 0.2 ± 0.001 | 0.61 ± 0.04 |
| trans-Cinnamic acid | 0.40 ± 0.01 | 0.1 ± 0.001 | 0.47 ± 0.03 |
| Caffeic acid | 0.006 ± 0.001 | 0.002 ± 0.0001 | 0.008 ± 0.001 |
| p-Coumaric acid | 0.07 ± 0.01 | 0.02 ± 0.001 | 0.08 ± 0.01 |
| Rutin | 0.03 ± 0.01 | 0.01 ± 0.001 | 0.05 ± 0.01 |
| Quercetin | 0.60 ± 0.01 | 0.2 ± 0.001 | 0.67 ± 0.05 |
| Element (µg/g) | Samples | ||||||
|---|---|---|---|---|---|---|---|
| Control 1 | Control 2 | DC 10 | DC 20 | DC 30 | DC 40 | DC 49 | |
| Ca | 9485 ± 89 a | 47 ± 5.4 g | 8566 ± 184 b | 7656 ± 123 c | 6680 ± 211 d | 5756 ± 146 e | 4693 ± 44 f |
| Cu | 0.84 ± 0.04 g | 3.1 ± 0.1 a | 0.95 ± 0.04 f | 1.2 ± 0.05 e | 1.4 ± 0.1 d | 1.6 ± 0.1 c | 1.8 ± 0.1 b |
| Fe | Present | 11.5 ± 0.8 a | 2.7 ± 0.3 b | 1.4 ± 0.1 d | Present | 2.3 ± 0.2 c | 2.4 ± 0.4 c |
| K | 16,883 ± 189 a | 465 ± 9 g | 15,302 ± 299 b | 13,655 ± 206 c | 12,003 ± 367 d | 10,358 ± 268 e | 8525 ± 67 f |
| Mg | 1074 ± 4.0 a | 174 ± 0.1 f | 938 ± 12 b | 858 ± 13 c | 843 ± 31 c | 725 ± 8.0 d | 631 ± 34 e |
| Mn | 0.35 ± 0.03 g | 6.1 ± 0.1 a | 1.0 ± 0.1 f | 1.4 ± 0.1 e | 2.1 ± 0.1 d | 2.5 ± 0.1 c | 3.0 ± 0.2 b |
| Na | 3274 ± 32 a | Present | 2954 ± 54 b | 2628 ± 46 c | 2281 ± 68 d | 1943 ± 60 e | 1580 ± 18 f |
| P | 18,116 ± 121 a | 1346 ± 25 g | 16,286 ± 331 b | 14,745 ± 216 c | 13,160 ± 427 d | 11,456 ± 247 e | 9667 ± 96 f |
| Zn | 27.8 ± 0.2 a | 18.9 ± 0.3 f | 26.4 ± 0.3 b | 25.1 ± 0.3 c | 26.3 ± 0.9 b | 24.7 ± 0.4 d | 23.2 ± 1.1 e |
| Al | Present | Present | Present | Present | Present | Present | Present |
| Cd | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Cr | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.C.F.; Vieira, G.M.N.; Prestes, A.A.; Marafon, K.; de Souza, C.K.; Andrade, D.R.M.; Helm, C.V.; de Gois, J.S.; Prudêncio, E.S. Development and Characterization of Dairy Compound with Goat Milk Powder and Rice Flour. Processes 2025, 13, 2324. https://doi.org/10.3390/pr13082324
Carvalho ACF, Vieira GMN, Prestes AA, Marafon K, de Souza CK, Andrade DRM, Helm CV, de Gois JS, Prudêncio ES. Development and Characterization of Dairy Compound with Goat Milk Powder and Rice Flour. Processes. 2025; 13(8):2324. https://doi.org/10.3390/pr13082324
Chicago/Turabian StyleCarvalho, Ana Caroline Ferreira, Gilberto Marques Nunes Vieira, Amanda Alves Prestes, Karine Marafon, Carolina Krebs de Souza, Dayanne Regina Mendes Andrade, Cristiane Vieira Helm, Jefferson Santos de Gois, and Elane Schwinden Prudêncio. 2025. "Development and Characterization of Dairy Compound with Goat Milk Powder and Rice Flour" Processes 13, no. 8: 2324. https://doi.org/10.3390/pr13082324
APA StyleCarvalho, A. C. F., Vieira, G. M. N., Prestes, A. A., Marafon, K., de Souza, C. K., Andrade, D. R. M., Helm, C. V., de Gois, J. S., & Prudêncio, E. S. (2025). Development and Characterization of Dairy Compound with Goat Milk Powder and Rice Flour. Processes, 13(8), 2324. https://doi.org/10.3390/pr13082324






