Sustainable Bamboo-Based Packaging and Passive Modified Atmosphere: A Strategy to Preserve Strawberry Quality During Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Sample and Experimental Design
2.2. Physico-Chemical Traits
2.3. Bioactive Compounds
2.4. Enzymatic Activity
2.5. Polyphenoloxidase and Lipoxygenase Activity and MDA Content
2.6. Statistical Analyses
3. Results
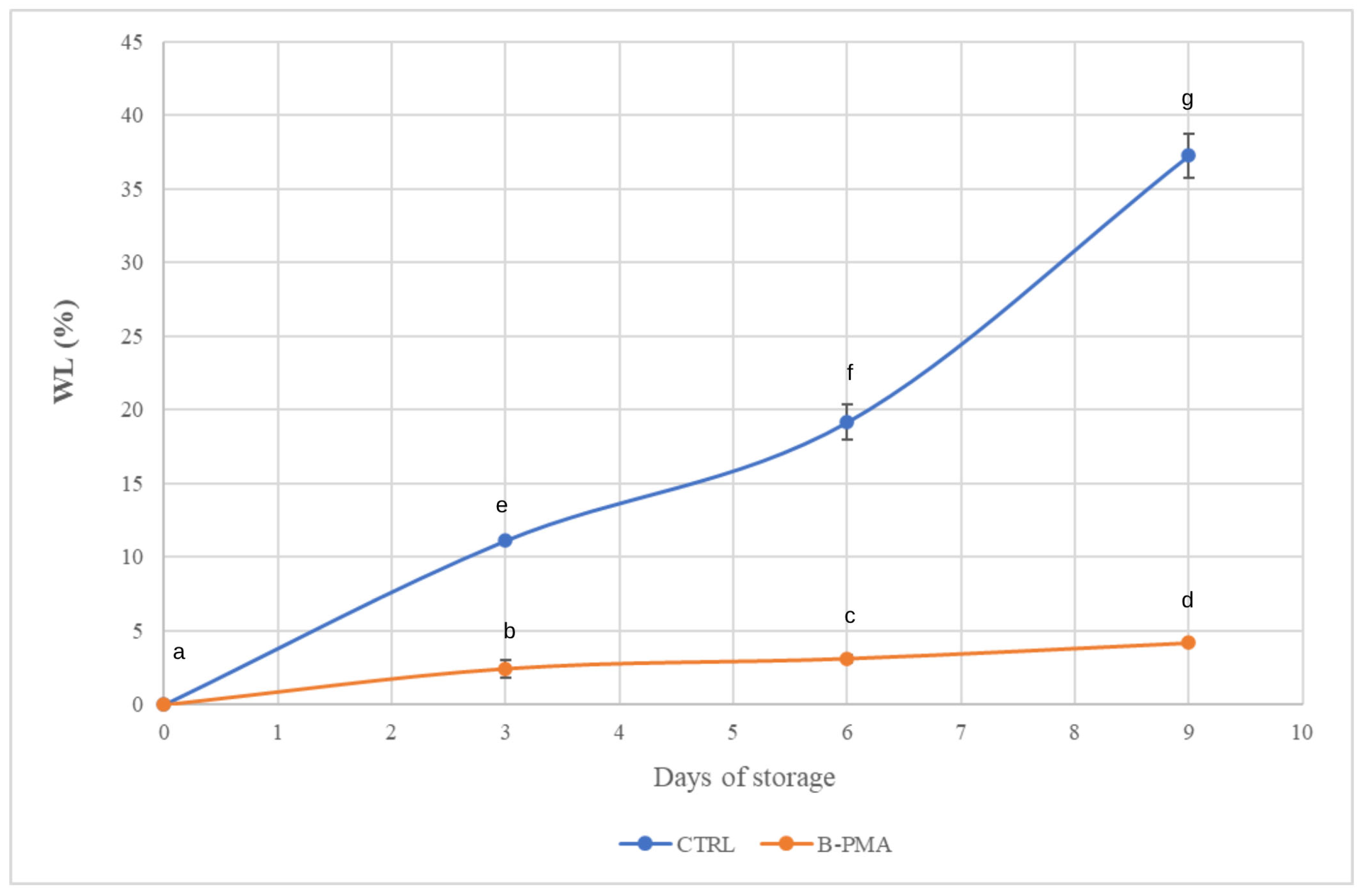
3.1. Effect of Packaging and MAP on Strawberry Physicochemical Features
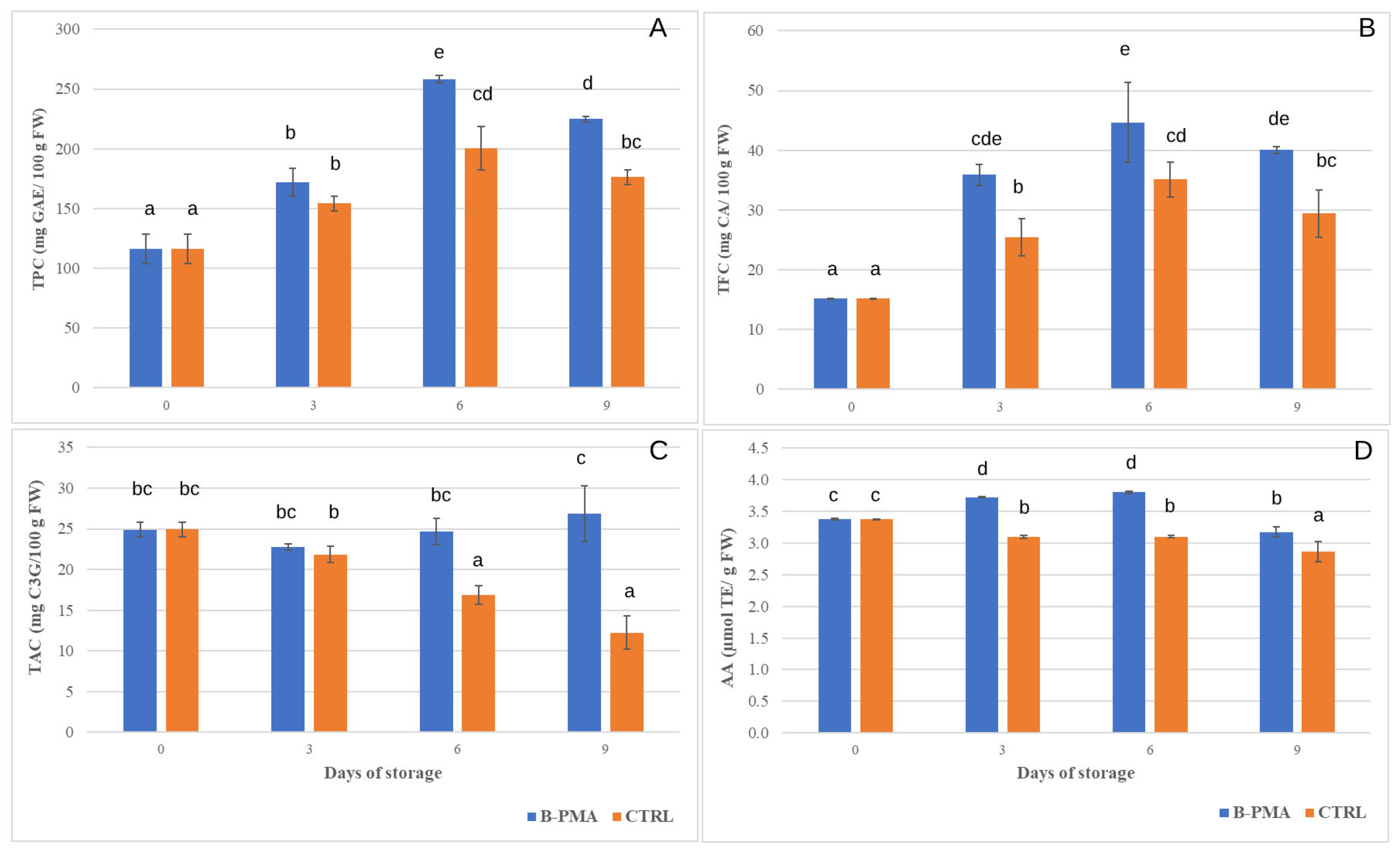
3.2. Influence of Packaging and MAP on Strawberry Bioactive Compounds
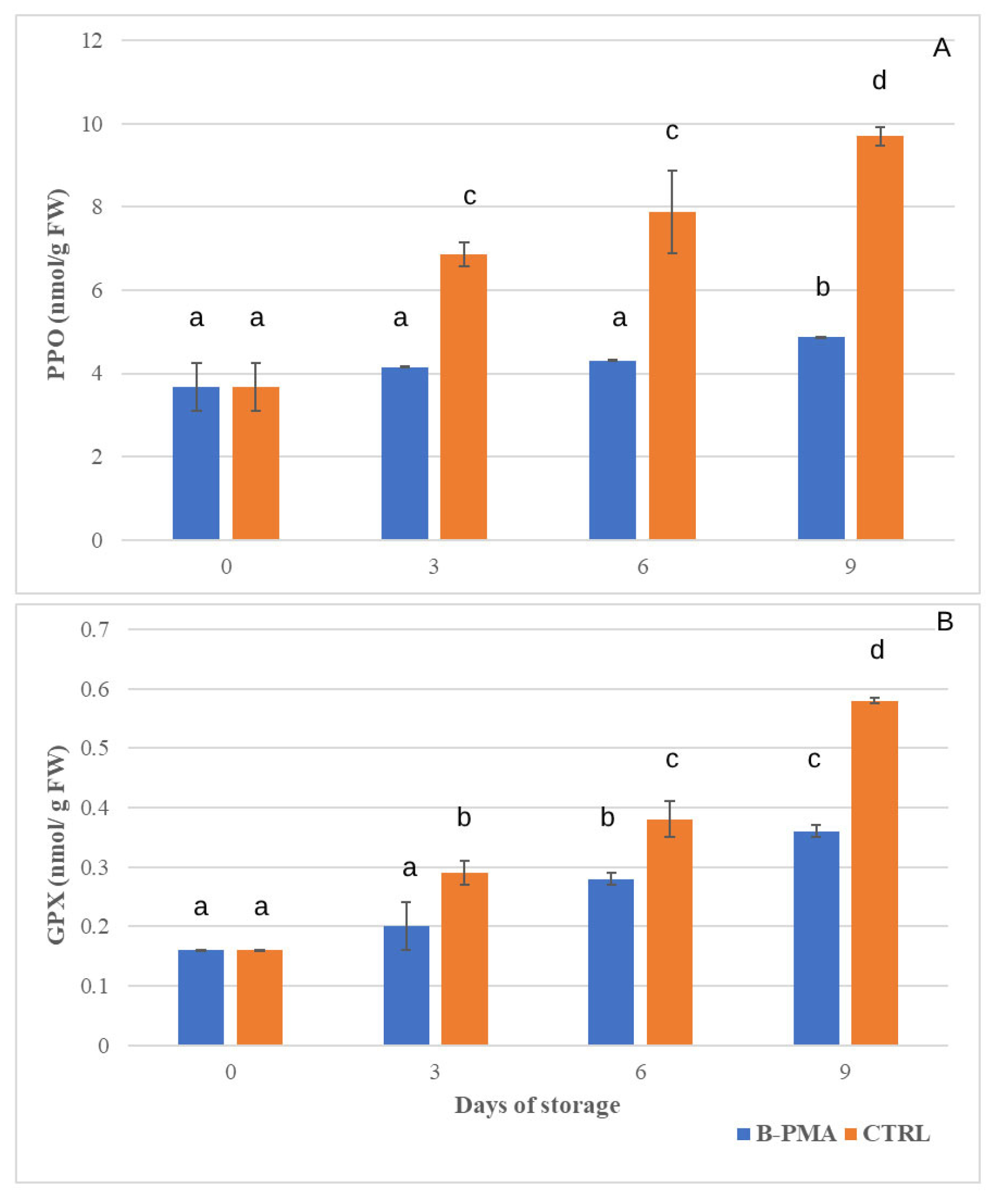
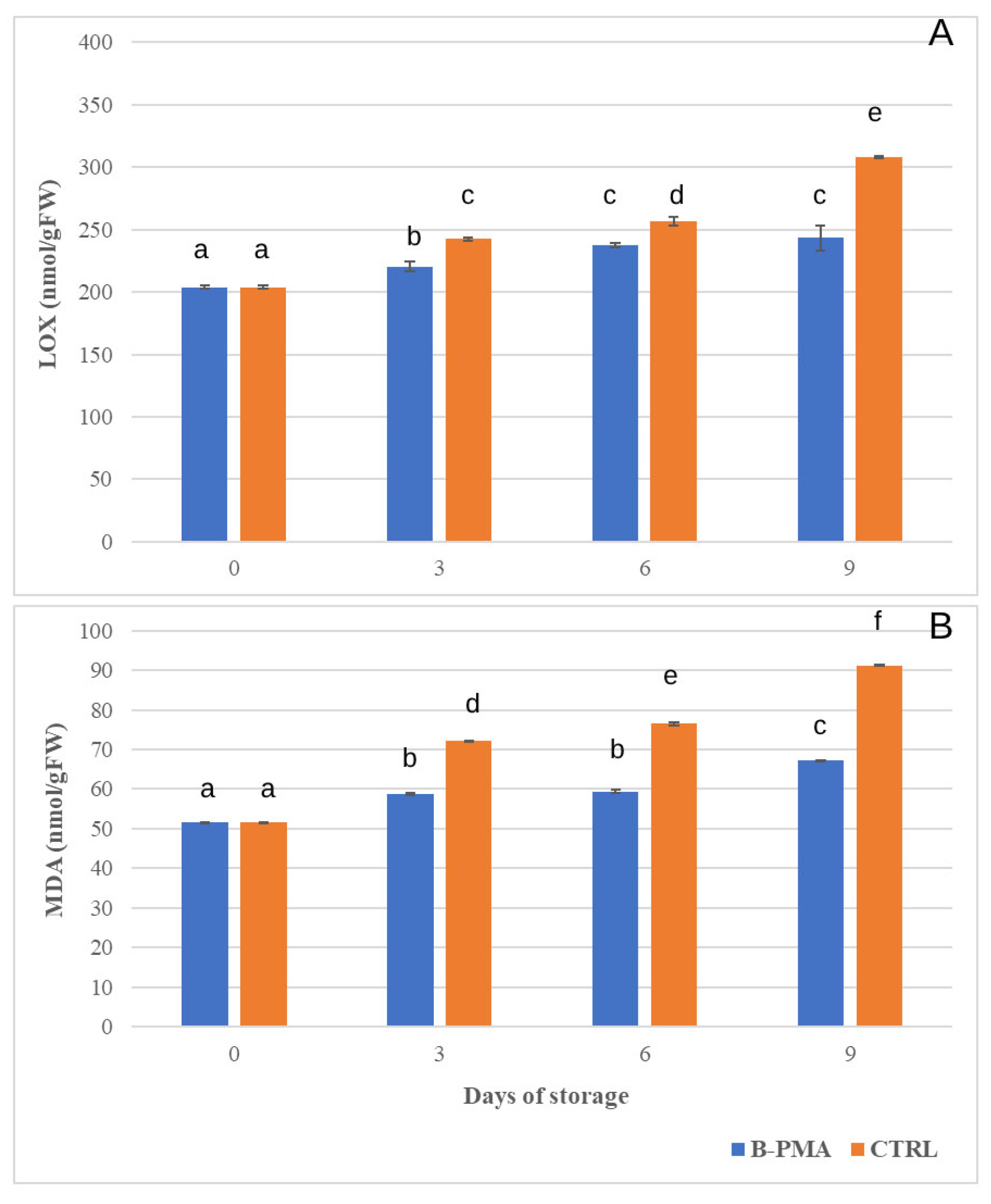
3.3. Influence of Packaging and MAP on Antioxidant Enzymes and Membrane Damage
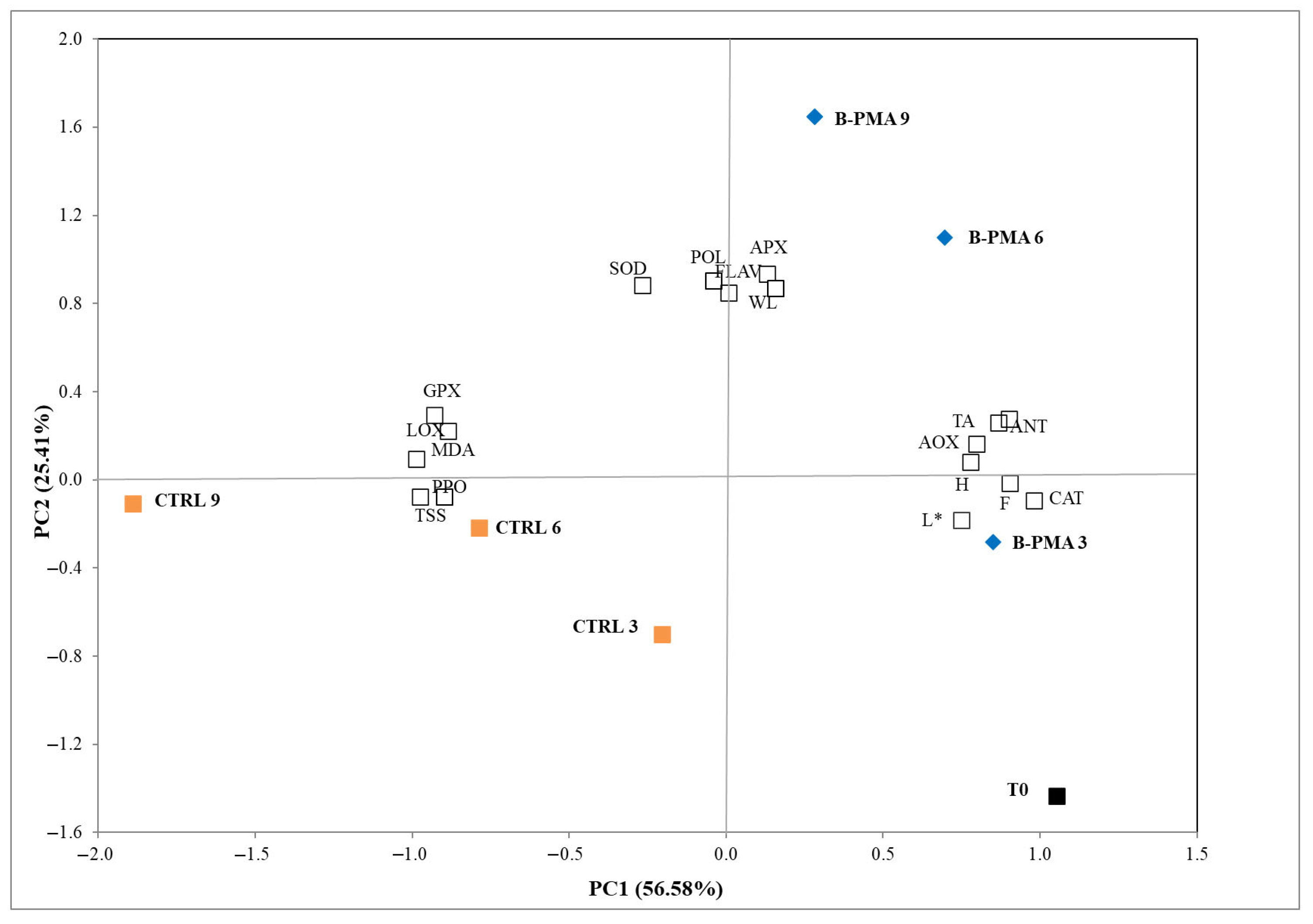
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT, 2020 FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Division. 2020. Available online: http://faostat.fao.org (accessed on 30 April 2025).
- De Souza, V.R.; Pereira, P.A.; Da Silva, T.L.; Lima, L.C.O.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, Y.; Cao, Z.; Jiang, S.; Chen, Y.; Shao, X. The jasmonic acid signaling pathway is associated with terpinen-4-ol-induced disease resistance against Botrytis cinerea in strawberry fruit. J. Agric. Food. Chem. 2021, 69, 10678–10687. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red Fruits Composition and Their Health Benefits-A Review. Foods 2022, 11, 644. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Deng, M.; Gui, R.; Liu, Y.; Chen, X.; Lin, Y.; Li, M.; Wang, Y.; Tang, H.; et al. Blue light combined with salicylic acid treatment maintained the postharvest quality of strawberry fruit during refrigerated storage. Food Chem X 2022, 15, 10038. [Google Scholar] [CrossRef]
- Chen, T.; Ji, D.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Advances and strategies for controlling the quality and safety of postharvest fruit. Eng 2021, 7, 1177–1184. [Google Scholar] [CrossRef]
- Dixon, H.; Mullins, R.; Wakefield, M.; Hill, D. Encouraging the consumption of fruit and vegetables by older Australians: An experimental study. J. Nutr. Educ. Behav. 2004, 36, 245–249. [Google Scholar] [CrossRef]
- Mai, R.; Symmank, C.; Seeberg-Elverfeldt, B. Light and pale colors in food packaging: When does this package cue signal superior healthiness or inferior tastiness? J. Retail. 2016, 92, 426–444. [Google Scholar] [CrossRef]
- Vila-López, N.; Küster-Boluda, I. Commercial versus technical cues to position a new product: Do hedonic and functional/healthy packages differ? Soc. Sci. Med. 2018, 198, 85–94. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, M.; Wei, X.; Xu, C.; Luo, Z.; Mao, L. Consolidated cold and modified atmosphere package system for fresh strawberry supply chains. LWT 2019, 109, 207–215. [Google Scholar] [CrossRef]
- Verghese, K.; Lewis, H.; Lockrey, S.; Williams, H. Packaging’s role in minimizing food loss and waste across the supply chain. Packag. Technol. Sci. 2015, 28, 603–620. [Google Scholar] [CrossRef]
- Wikström, F.; Verghese, K.; Auras, R.; Olsson, A.; Williams, H.; Wever, R.; Grönman, K.; Pettersen, M.K.; Møller, H.; Soukka, R. Packaging strategies that save food: A research agenda for 2030. J. Ind. Ecol. 2019, 23, 532–540. [Google Scholar] [CrossRef]
- Yin, Y.; Woo, M.W. Transitioning of petroleum-based plastic food packaging to sustainable bio-based alternatives. Sustain. Food Technol. 2024, 2, 548–566. [Google Scholar] [CrossRef]
- Ho, M.P.; Lau, K.T.; Wang, H.; Hui, D. Improvement on the properties of polylactic acid (PLA) using bamboo charcoal particles. Compos. Part B-Eng. 2015, 81, 14–25. [Google Scholar] [CrossRef]
- Nurul Fazita, M.R.; Jayaraman, K.; Bhattacharyya, D.; Mohamad Haafiz, M.K.; Saurabh, C.K.; Hussin, M.H.; HPS, A.K. Green composites made of bamboo fabric and poly (lactic) acid for packaging applications—A review. Materials 2016, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Yang, C.; Lu, J.H.; Xu, M.T.; Shi, X.C.; Song, Z.W.; Chen, T.M.; Herrera-Balandrano, D.D.; Zhang, Y.-J.; Laborda, P.; Wang, S.Y.; et al. Evaluation of chitosan coatings enriched with turmeric and green tea extracts on postharvest preservation of strawberries. LWT 2022, 163, 113551. [Google Scholar] [CrossRef]
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, I.; Ejaz, S. Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef]
- Lee, D.; Shayan, M.; Gwon, J.; Picha, D.H.; Wu, Q. Effectiveness of cellulose and chitosan nanomaterial coatings with essential oil on postharvest strawberry quality. Carbohydr. Polym. 2022, 298, 120101. [Google Scholar] [CrossRef]
- Huang, J.; Wu, W.; Niu, B.; Fang, X.; Chen, H.; Wang, Y.; Gao, H. Characterization of Zizania latifolia polysaccharide-corn starch composite films and their application in the postharvest preservation of strawberries. LWT 2023, 173, 114332. [Google Scholar] [CrossRef]
- Rashvand, M.; Matera, A.; Altieri, G.; Genovese, F.; Fadiji, T.; Opara, U.L.; Mohamadifar, M.A.; Feyissa, A.H.; Di Renzo, G.C. Recent advances in the potential of modeling and simulation to assess the performance of modified atmosphere packaging (MAP) systems for the fresh agricultural product: Challenges and development. Trends Food Sci. Technol. 2023, 136, 48–63. [Google Scholar] [CrossRef]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 59, 411–422. [Google Scholar] [CrossRef]
- Afifi, E.H. Effect of active and passive modified atmosphere packaging on quality attributes of strawberry fruits during cold storage. Arab Univ. J. Agric. Sci. 2016, 24, 157–168. [Google Scholar] [CrossRef]
- Matar, C.; Salou, T.; Hélias, A.; Pénicaud, C.; Gaucel, S.; Gontard, N.; Guilbert, S.; Guillard, V. Benefit of modified atmosphere packaging on the overall environmental impact of packed strawberries. Postharvest Biol. Technol. 2021, 177, 111521. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ. Effects of lemongrass oil application and modified atmosphere packaging on the postharvest life and quality of strawberry fruits. Sci. Hortic. 2019, 256, 108527. [Google Scholar] [CrossRef]
- Adiletta, G.; Gliottone, G.; Di Matteo, M.; Petriccione, M. Response of Qualitative Traits and Antioxidant Systems to Chitosan Postharvest Treatment in ‘Black Golden’ Japanese Plum. Foods 2022, 11, 853. [Google Scholar] [CrossRef]
- Adiletta, G.; Petriccione, M.; Di Matteo, M. Effects of Passive Modified Atmosphere Packaging on Physico-Chemical Traits and Antioxidant Systems of ‘Dottato’ Fresh Fig. Horticulturae 2022, 8, 709. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colourimetry of Total Phenolics with Phosphomolybdic- phosphotungstic Acid Reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1-2. [Google Scholar] [CrossRef]
- Petriccione, M.; De Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life. Food Bioprocess Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Bao, A.K.; Wang, S.M.; Wu, G.Q.; Xi, J.J.; Zhang, J.L.; Wang, C.M. Over expression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2009, 176, 232–240. [Google Scholar] [CrossRef]
- Joshi, K.; Tiwari, B.; Cullen, P.J.; Frias, J.M. Predicting quality attributes of strawberry packed under modified atmosphere throughout the cold chain. Food Packag. Shelf Life 2019, 21, 100354. [Google Scholar] [CrossRef]
- Parvez, S.; Wani, I.A. Postharvest Biology and Technology of Strawberry. In Postharvest Biology and Technology of Temperate Fruits, 1st ed.; Mir, S., Shah, M., Mir, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 331–348. [Google Scholar]
- Priyadarshi, R.; El-Araby, A.; Rhim, J.-W. Chitosan-based sustainable packaging and coating technologies for strawberry preservation: A review. Int. J. Biol. Macromol. 2024, 278, 134859. [Google Scholar] [CrossRef]
- Robinson, J.E.; Browne, K.M.; Burton, W.G. Storage characteristics of some vegetables and soft fruits. Ann. Appl. Biol. 2010, 81, 399–408. [Google Scholar] [CrossRef]
- Paulsen, E.; Barrios, S.; Bogdanoff, N.; Leandro, G.C.; Valencia, G.A. Recent progress in modified atmosphere packaging and biopolymeric films and coatings for fresh strawberry shelf-life extension. Packag. Technol. Sci. 2024, 37, 619–640. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes in bioactive composition of fresh-cut strawberries stored under superatmospheric oxygen, low-oxygen or passive atmospheres. J. Food Compos. Anal. 2010, 23, 37–43. [Google Scholar] [CrossRef]
- Nielsen, T.; Leufvén, A. The effect of modified atmosphere packaging on the quality of Honeoye and Korona strawberries. Food Chem. 2008, 107, 1053–1063. [Google Scholar] [CrossRef]
- Korte, I.; Albrecht, A.; Mittler, M.; Waldhans, C.; Kreyenschmidt, J. Influence of different bio-based and conventional packaging trays on the quality loss of fresh cherry tomatoes during distribution and storage. Packag. Technol. Sci. 2023, 36, 569–583. [Google Scholar] [CrossRef]
- Garavito, J.; Mendoza, S.M.; Castellanos, D.A. Configuration of biodegradable equilibrium modified atmosphere packages, including a moisture absorber for fresh cape gooseberry (Physalis peruviana L.) fruits. J. Food Eng. 2022, 314, 110761. [Google Scholar] [CrossRef]
- Lei, T.T.; Qian, J.; Yin, C. Equilibrium modified atmosphere packaging on postharvest quality and antioxidant activity of strawberry. Int. J. Food Sci. Technol. 2022, 57, 7125–7134. [Google Scholar] [CrossRef]
- Pasha, H.Y.; Mohtasebi, S.S.; Tajeddin, B.; Taherimehr, M.; Tabatabaeekoloor, R.; Firouz, M.S.; Javadi, A. The effect of a new bionanocomposite packaging film on postharvest quality of strawberry at modified atmosphere condition. Food Bioprocess Technol. 2023, 16, 1246–1257. [Google Scholar] [CrossRef]
- Baratter, M.; Weschenlfelder, E.F.; Stoffel, F.; Zeni, M.; Piemolini-Barreto, L.T. Analysis and evaluation of cassava starch-based biodegradable trays as an alternative packaging to fresh strawberry (Fragaria ananassa cv San Andreas). Am. J. Polym. Sci. Technol. 2017, 3, 76–81. [Google Scholar] [CrossRef]
- Dladla, S.S.; Workneh, T.S. Evaluation of the effects of different packaging materials on the quality attributes of the tomato fruit. Appl. Sci. 2023, 13, 2100. [Google Scholar] [CrossRef]
- Pinto, L.; Palma, A.; Cefola, M.; Pace, B.; D’Aquino, S.; Carboni, C.; Baruzzi, F. Effect of modified atmosphere packaging (MAP) and gaseous ozone pre-packaging treatment on the physico-chemical, microbiological and sensory quality of small berry fruit. Food Packag. Shelf Life 2020, 26, 100573. [Google Scholar] [CrossRef]
- Koyama, R.; Ishibashi, M.; Fukuda, I.; Okino, A.; Osawa, R.; Uno, Y. Pre-and post-harvest conditions affect polyphenol content in strawberry (Fragaria × ananassa). Plants 2022, 11, 2220. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Zamindar, N.; Paidari, S.; Ibrahim, S.A.; Mohammadi Nafchi, A. The synergistic effects of aloe vera gel and modified atmosphere packaging on the quality of strawberry fruit. J. Food Process Preserv. 2021, 45, e16003. [Google Scholar] [CrossRef]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Zampella, L.; Mastrobuoni, F.; Di Matteo, M. Overall quality and antioxidant enzymes of ready-to-eat ‘Purple Queen’pomegranate arils during cold storage. Postharvest Biol. Technol. 2019, 155, 20–28. [Google Scholar] [CrossRef]
- Mahecha-Rubiano, Y.R.; Garavito, J.; Castellanos, D.A. Configuration of a combined active packaging system with modified atmospheres and antimicrobial control to preserve fresh strawberry fruits. Food Packag. Shelf Life 2024, 46, 101390. [Google Scholar] [CrossRef]
- Manda-Hakki, K.; Hassanpour, H. Effect of L-glutathione treatment on biochemical properties, antioxidant capacity and antioxidant enzymes activity in strawberry fruits during storage. Heliyon 2024, 10, e38046. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anjum, M.A.; Nawaz, A.; Shah, H.M.S. Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage. Sci. Hortic. 2019, 254, 14–20. [Google Scholar] [CrossRef]
- De Reuck, K.; Sivakumar, D.; Korsten, L. Effect of integrated application of chitosan coating and modified atmosphere packaging on overall quality retention in litchi cultivars. J. Sci. Food Agric. 2009, 89, 915–920. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Aghdam, M.S.; Farmani, B.; Maggi, F.; Morshedloo, M.R. β-Aminobutyric acid treatment confers decay tolerance in strawberry fruit by warranting sufficient cellular energy providing. Sci. Hortic. 2018, 240, 249–257. [Google Scholar] [CrossRef]
- Ozcan, G.; Barringer, S. Effect of enzymes on strawberry volatiles during storage, at different ripeness level, in different cultivars, and during eating. J. Food Sci. 2011, 76, C324–C333. [Google Scholar] [CrossRef]
- Cice, D.; Ferrara, E.; Pecoraro, M.T.; Capriolo, G.; Petriccione, M. An Innovative Layer-by-Layer Edible Coating to Regulate Oxidative Stress and Ascorbate–Glutathione Cycle in Fresh-Cut Melon. Horticulturae 2024, 10, 465. [Google Scholar] [CrossRef]
| Samples | Days | Firmness (N) | TSS (° Brix) | TA (G Citric Acid per Juice Liter) | L* | H |
|---|---|---|---|---|---|---|
| CTRL | 0 | 5.14 ± 0.61 c | 7.03 ± 0.21 a | 5.56 ± 0.02 c | 30.94 ± 2.33 c | 33.78 ± 0.29 d |
| 3 | 4.65 ± 0.09 bc | 7.47 ± 0.15 a | 4.63 ± 0.05 b | 25.14 ± 1.66 ab | 30.19 ± 2.62 bc | |
| 6 | 3.65 ± 0.14 ab | 8.10 ± 0.20 b | 4.45 ± 0.18 ab | 26.12 ± 1.64 ab | 29.60 ± 2.07 b | |
| 9 | 3.13 ± 0.10 a | 11.77 ± 0.20c | 4.36 ± 0.05 a | 24.00 ± 1.24 a | 26.09 ± 2.30 a | |
| B-PMA | 0 | 5.14 ± 0.61 c | 7.03 ± 0.21 a | 5.56 ± 0.02 c | 30.94 ± 2.33 | 33.78 ± 0.29 d |
| 3 | 5.24 ± 0.39 c | 7.07 ± 0.11 a | 5.54 ± 0.02 c | 31.53 ± 1.50 c | 33.96 ± 1.97 d | |
| 6 | 5.56 ± 0.53 c | 7.10 ± 0.10 a | 5.60 ± 0.04 c | 30.73 ± 2.27 c | 32.18 ± 1.99 bcd | |
| 9 | 5.33 ± 1.11 c | 7.13 ± 0.15 a | 5.51 ± 0.13 c | 28.56 ± 2.36 bc | 33.19 ± 1.92 cd |
| Samples | Days | SOD | CAT | APX |
|---|---|---|---|---|
| CTRL | 0 | 0.45 ± 0.01 a | 2.61 ± 0.06 e | 0.18 ± 0.01 a |
| 3 | 0.46 ± 0.02 a | 1.80 ± 0.07 c | 0.22 ± 0.01 a | |
| 6 | 0.57 ± 0.01 b | 1.53 ± 0.04 b | 0.23 ± 0.01 a | |
| 9 | 0.66 ± 0.02 c | 1.14 ± 0.10 a | 0.25 ± 0.02 a | |
| B-PMA | 0 | 0.45 ± 0.01 a | 2.61 ± 0.06 e | 0.18 ± 0.01 a |
| 3 | 0.56 ± 0.02 b | 2.47 ± 0.07 e | 0.24 ± 0.02 a | |
| 6 | 0.66 ± 0.02 c | 2.23 ± 0.04 d | 0.34 ± 0.02 b | |
| 9 | 0.72 ± 0.01 d | 2.06 ± 0.07 d | 0.48 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adiletta, G.; Di Matteo, M.; De Filippis, G.; Di Grazia, A.; Ciambelli, P.; Petriccione, M. Sustainable Bamboo-Based Packaging and Passive Modified Atmosphere: A Strategy to Preserve Strawberry Quality During Cold Storage. Processes 2025, 13, 2262. https://doi.org/10.3390/pr13072262
Adiletta G, Di Matteo M, De Filippis G, Di Grazia A, Ciambelli P, Petriccione M. Sustainable Bamboo-Based Packaging and Passive Modified Atmosphere: A Strategy to Preserve Strawberry Quality During Cold Storage. Processes. 2025; 13(7):2262. https://doi.org/10.3390/pr13072262
Chicago/Turabian StyleAdiletta, Giuseppina, Marisa Di Matteo, Giuseppe De Filippis, Antonio Di Grazia, Paolo Ciambelli, and Milena Petriccione. 2025. "Sustainable Bamboo-Based Packaging and Passive Modified Atmosphere: A Strategy to Preserve Strawberry Quality During Cold Storage" Processes 13, no. 7: 2262. https://doi.org/10.3390/pr13072262
APA StyleAdiletta, G., Di Matteo, M., De Filippis, G., Di Grazia, A., Ciambelli, P., & Petriccione, M. (2025). Sustainable Bamboo-Based Packaging and Passive Modified Atmosphere: A Strategy to Preserve Strawberry Quality During Cold Storage. Processes, 13(7), 2262. https://doi.org/10.3390/pr13072262









