Experimental Study on the Influence of Magnesium on the Separation of Carbon Dioxide from Gas Mixtures with Nitrogen by Combustion Processes
Abstract
1. Introduction
2. Materials and Methods
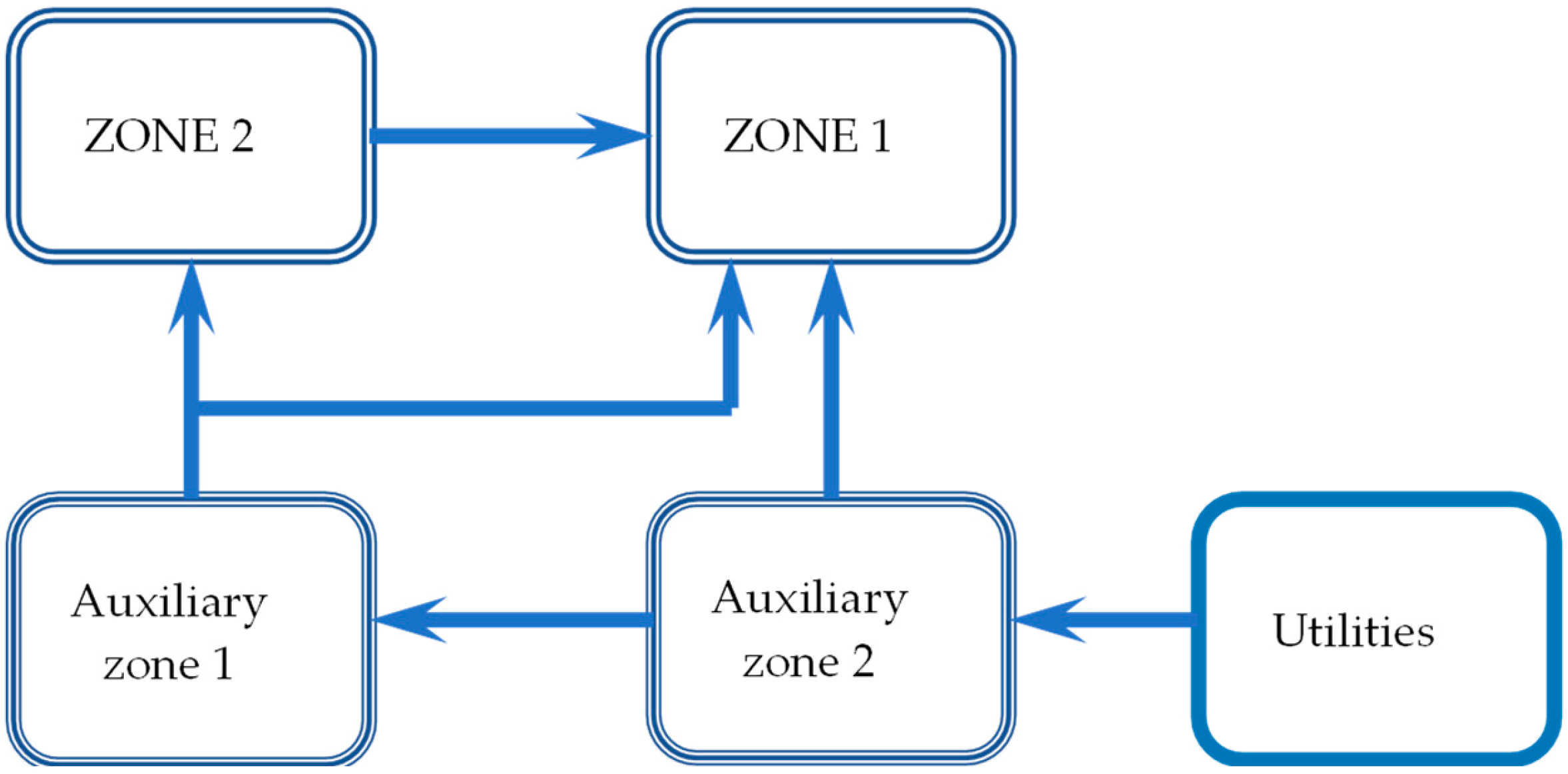
2.1. Laboratory Experimental Set-Up
2.2. Combustion Tests
3. Results
3.1. Influence of Gas Composition of Mg Combustion Parameters
3.2. Mechanism of Mg Combustion Process in CO2-N2
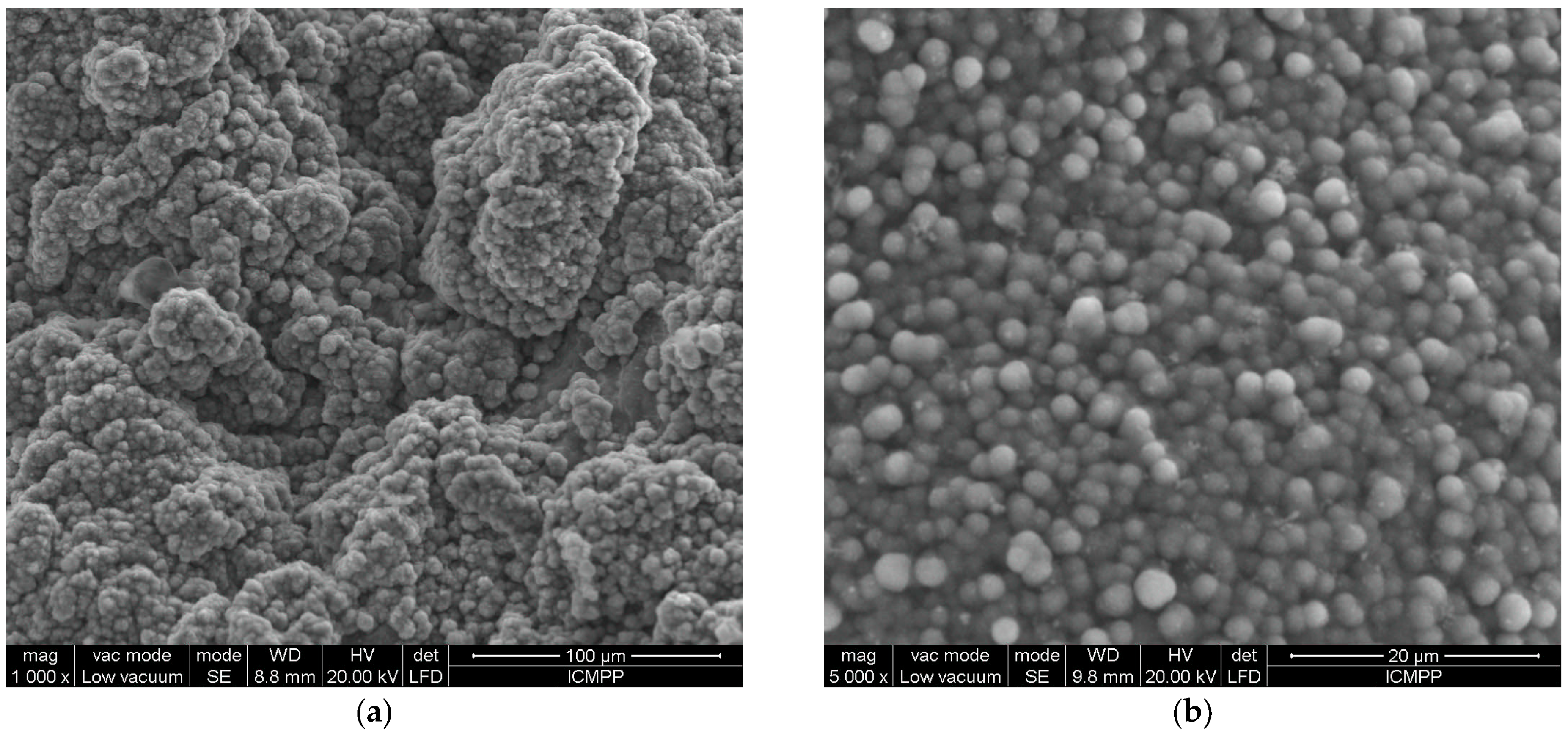
3.3. Characterization of the Solid Residue
Mg: 24 + 24a = 24(1 + a)
presence of only MgCO3, where R = 3 · 16/24 = 2,
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, J.; Yang, Y.; Wen, C.; Chen, J.; Zhou, G.; Jiang, B.; Peng, X.; Pan, F. Applications of magnesium alloys for aerospace: A review. J. Magnes. Alloy 2023, 11, 3609–3619. [Google Scholar] [CrossRef]
- Nam, K.-H.; Lee, J.-S.; Park, H.-J. Understanding Combustion Mechanism of Magnesium for Better Safety Measures: An Experimental Study. Safety 2022, 8, 11. [Google Scholar] [CrossRef]
- Farooq, M.Z.; Wu, Y.; Lu, L.; Zheng, M. Combustion phases of magnesium alloys based on predicted heating rate using machine learning. Measurement 2025, 242 Pt E, 116192. [Google Scholar] [CrossRef]
- Nam, K.-H.; Park, J.K.; Lee, J.-S. Thermal phenomena and size effects of Mg powder in combustion process. PLoS ONE 2024, 19, e0310185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, H.; Min, L. Ignition Temperature of Magnesium Powder and Pyrotechnic Composition. J. Energetic Mater. 2013, 32, 219–226. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, D.; Li, C.; Li, G. Ignition behavior of magnesium powder layers on a plate heated at constant temperature. J. Hazar. Mater. 2013, 246–247, 283–290. [Google Scholar]
- Zuo, D.; Ding, H.; Zhi, M.; Xu, Y.; Zhang, Z.; Zhang, M. Research Progress on the Oxidation Behavior of Ignition-Proof Magnesium Alloy and Its Effect on Flame Retardancy with Multi-Element Rare Earth Additions: A Review. Materials 2024, 17, 3183. [Google Scholar] [CrossRef]
- Horste, F.E.; Mordike, B.L. Magnesium Technology Metallurgy, Design Data, Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 469–632. [Google Scholar]
- Wu, L.; Han, F.; Liu, G. Comprehensive Utilization of Magnesium Slag by Pidgeon Process; Springer Briefs in Materials, Open Access Springer: Singapore, 2021; pp. 5–18. [Google Scholar]
- Paul, B. A Study of the Flammability of Magnesium; Federal Aviation Agency: Washington, DC, USA, 1964.
- Dupre, B.R.; Streiff, R. The Kinetics of Magnesium Nitriding by Pure Nitrogen. Oxid. Met. 1970, 2, 155–160. [Google Scholar] [CrossRef]
- Gurevich, M.A.; Lydkin, V.M.; Stepanov, A.M. Ignition and Combustion of a Gas Suspention of Magnesium Particles. Fiz. Goreniya Vzryva 1970, 6, 335–342. [Google Scholar]
- Tsuyoshi, M.; Itatani, K.F.; Howell, S.; Kishioka, A.; Kinoshita, M. Preparation of Magnesium Nitride Powder by Low-Pressure Chemical Vapor Deposition. JACerS 1993, 76, 2909–2911. [Google Scholar]
- Cui, S.; Liao, S.; Zhang, Y.; Xu, Y.; Miao, Y.; Lu, M.; Fan, Y.; Guo, W. Synthesis of nanometric-size magnesium nitride by nitriding of pre-activates magnesium powder. J. Mater. Sci. 1999, 34, 5601–5604. [Google Scholar] [CrossRef]
- Han, D.; Zhang, J.; Huang, J.; Lian, Y.; He, G. A review on ignition mechanisms and characteristics of magnesium alloys. J. Magnes. Alloy 2020, 8, 329–344. [Google Scholar] [CrossRef]
- Feng, Y.C.; Xia, Z.X.; Huang, L.Y.; Ma, L.K.; Yang, D.L. Experimental Investigation on the Ignition and Combustion Characteristics of a Single Magnesium Particle in Air. Combust. Explos. Shock Waves 2019, 55, 210–219. [Google Scholar] [CrossRef]
- Aleksandrova, Y.P.; Roshchina, I.N. Interaction of Magnesium with Gases. Metalloved. Termicheskaya Obrab. Met. 1977, 3, 52–55. [Google Scholar] [CrossRef]
- Perez Ramirez, L.; Bournel, F.; Gallet, J.J.; Dudy, L.; Rochet, F. Testing the Cabrera–Mott Oxidation Model for Aluminum under Realistic Conditions with Near-Ambient Pressure Photoemission. J. Phys. Chem. C. 2022, 126, 2517–2530. [Google Scholar] [CrossRef]
- Shafirovich, E.Y.; Gol’dshleger, U.I. Ignition and Burning of Magnesium Particles in Oxides of Carbon. Fiz. Goreniya Vzryva 1990, 26, 3–10. [Google Scholar] [CrossRef]
- Barabulica, I.; Secula, M.S.; Asoltanei, M.D.; Iacob-Tudose, E.T.; Lisa, G.; Mamaliga, I. Experimental Study on the Reaction of Magnesium in Carbon Dioxide and Nitrogen Atmosphere. ChemEngineering 2024, 8, 41. [Google Scholar] [CrossRef]
- Barabulica, I. Potential Applications of Magnesium in Industrial Gas Separation. Ph.D. Thesis, Chemical Engineering, Gheorghe Asachi Technical University of Iasi, Iasi, Romania, 2025. [Google Scholar]
- Negoescu, C.C.; Mamaliga, I. Sorption Kinetics and Thermodynamic Equilibrium of Some Solvents in Polymer Films Using the Pressure Decay Method at Low Pressures. Mater. Plast. 2013, 50, 303–306. [Google Scholar]
- Cozma, P.; Wukovits, W.; Mamaliga, I.; Friedl, A.; Gavrilescu, M. Modeling and simulation of high- pressure water scrubbing technology applied for biogas upgrading. Clean Technol. Environ. Policy 2014, 17, 373–391. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Qian, J.; Zhang, X.; Liu, Z. Review of research progress and stability studies of amine-based biphasic absorbents for CO2 capture. J. Ind. Eng. Chem. 2024, 134, 28–50. [Google Scholar] [CrossRef]
- Peng, X.D.; Edwards, D.S.; Barteau, M.A. Formation of magnesium nitride layers on the Mg(0001) surface by ion implantation. Surf. Sci. 1987, 185, 227–248. [Google Scholar]
- Gol’dshleger, U.I.; Shafirovich, E.Y. Combustion Regimes of Magnesium in Carbon Oxides. 1. Combustion in CO2. Combust. Explos. Shock Waves 1999, 35, 37–644. [Google Scholar] [CrossRef]
- Merrill, K. A Simplified Two-Reaction Zone Model of Magnesium Combustion in Carbon Dioxide. Proc. Combust. Inst. 2002, 29, 2931–2938. [Google Scholar]



 and
and  —tests conducted at 0.3 MPa with gas mixture prepared in the mixing chamber increasing and, decreasing N2 fraction, respectively;
—tests conducted at 0.3 MPa with gas mixture prepared in the mixing chamber increasing and, decreasing N2 fraction, respectively;  —tests conducted at 0.2 MPa;
—tests conducted at 0.2 MPa;  —combustion tests using high weight Mg samples (40 mg vs. 10 mg).
—combustion tests using high weight Mg samples (40 mg vs. 10 mg).
 and
and  —tests conducted at 0.3 MPa with gas mixture prepared in the mixing chamber increasing and, decreasing N2 fraction, respectively;
—tests conducted at 0.3 MPa with gas mixture prepared in the mixing chamber increasing and, decreasing N2 fraction, respectively;  —tests conducted at 0.2 MPa;
—tests conducted at 0.2 MPa;  —combustion tests using high weight Mg samples (40 mg vs. 10 mg).
—combustion tests using high weight Mg samples (40 mg vs. 10 mg).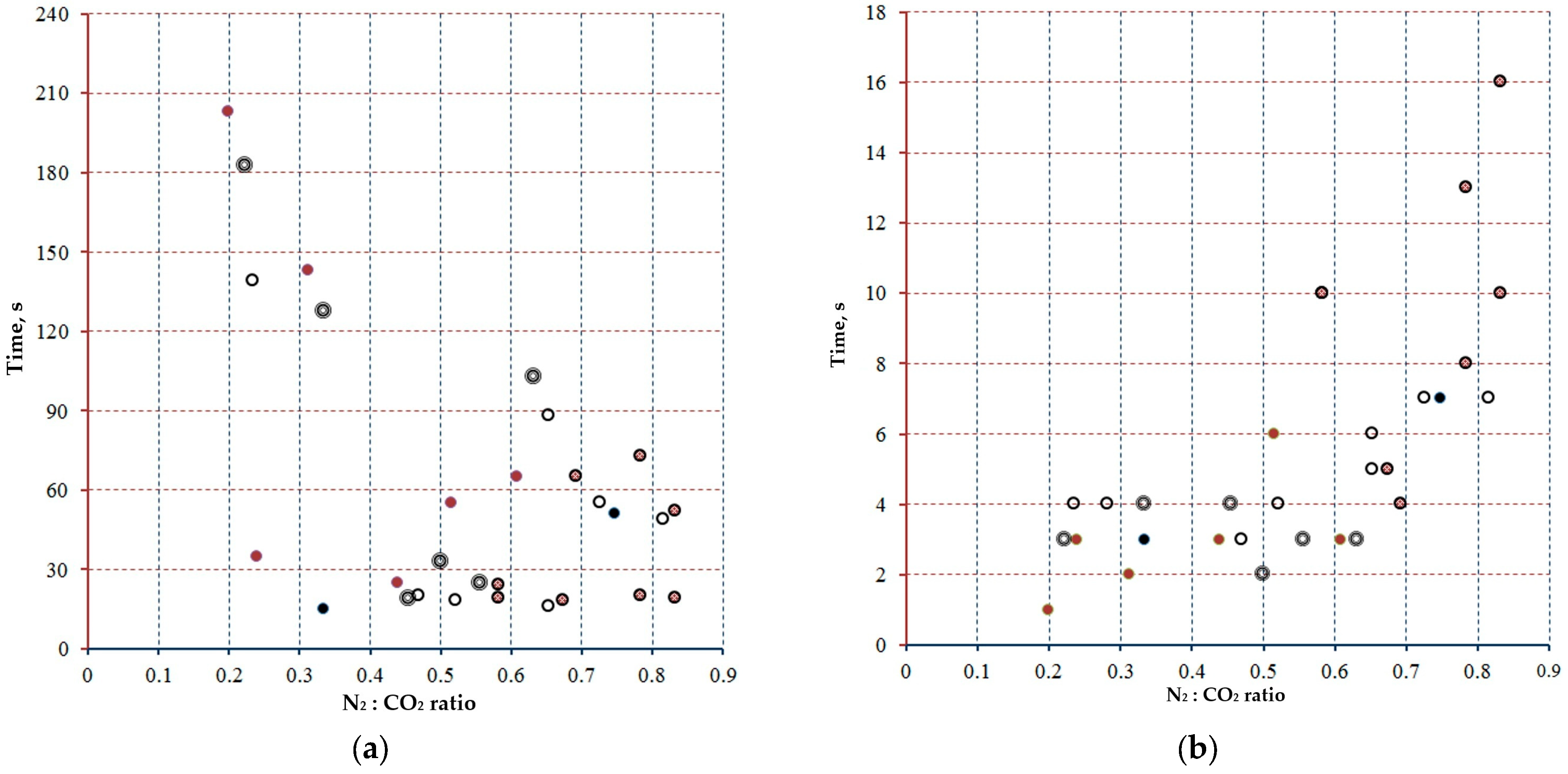
| Run | Mg Weight, mg | Initial Absorbed CO2, vol.% | Final Absorbed CO2, vol.% | Concentration Change, vol.% |
|---|---|---|---|---|
| 1 | 43.2 | 72.52 | 59.86 | 12.66 |
| 2 | 45.8 | 54.81 | 35.95 | 18.86 |
| 3 | 44.2 | 52.02 | 41.43 | 10.57 |
| 4 | 44.6 | 14.12 | 1.58 | 12.54 |
| 5 | 41.2 | 37.9 | 35.96 | 1.94 |
| 6 | 43.3 | 19.68 | 3.99 | 15.69 |
| 7 | 44.5 | 39.3 | 7.01 | 32.29 |
| 8 | 44.4 | 41.43 | 16.47 | 24.98 |
| Sample | Analyses | C, wt.% | N, wt.% | O, wt.% | Mg, wt.% | Cr, wt.% | Fe, wt.% |
|---|---|---|---|---|---|---|---|
| 1 | a | 7.86 | 1.29 | 35.97 | 54.75 | 0.12 | 0 |
| b | 16.58 | 1.2 | 32.58 | 49.47 | 0.17 | 0 | |
| c | 30.93 | 1.57 | 28.69 | 38.52 | 0.29 | 0 | |
| 2 | a | 12.44 | 1.33 | 33.54 | 52.69 | 0 | 0 |
| b | 28.15 | 1.82 | 29.18 | 40.48 | 0.37 | 0 | |
| c | 22.65 | 1.45 | 33.32 | 42.52 | 0.06 | 0 | |
| 3 | a | 28.66 | 2.31 | 27.32 | 41.44 | 0.2 | 0.06 |
| b | 26.9 | 1.08 | 25.42 | 45.74 | 0.68 | 0.19 | |
| c | 15.22 | 1.72 | 34.75 | 48.2 | 0.11 | 0 | |
| 4 | a | 19.78 | 1.2 | 25.4 | 53.36 | 0.26 | 0 |
| b | 37.68 | 1.93 | 21.43 | 38.47 | 0.43 | 0.06 | |
| c | 16.56 | 1.91 | 30.21 | 51.06 | 0.26 | 0 | |
| 5 | a | 26.56 | 1.89 | 33.82 | 37.55 | 0.18 | 0 |
| b | 31.78 | 1.86 | 34.24 | 31.95 | 0.17 | 0 | |
| c | 23.11 | 1.32 | 31.46 | 43.88 | 0.23 | 0 | |
| 6 | a | 25.51 | 2.43 | 30.94 | 40.82 | 0.23 | 0.07 |
| b | 25.77 | 1.62 | 31.04 | 41.41 | 0.15 | 0 | |
| c | 16.02 | 1.68 | 33.52 | 48.58 | 0.2 | 0 | |
| 7 | a | 31 | 3.13 | 30.05 | 35.23 | 0.45 | 0.14 |
| b | 19.2 | 1.56 | 34.32 | 44.79 | 0.13 | 0 | |
| c | 24.24 | 1.5 | 29.39 | 44.48 | 0.4 | 0 | |
| 8 | a | 9.8 | 1.81 | 31.15 | 57.05 | 0.19 | 0 |
| b | 10.57 | 1.85 | 16.06 | 71.08 | 0.44 | 0 | |
| c | 12.41 | 1.02 | 16.71 | 69.65 | 0.21 | 0 |
| Sample | %N2 | %Mg | Mg3N2 | Mg in Mg3N2 | Remaining Mg | |
|---|---|---|---|---|---|---|
| 1 | a | 1.29 | 54.75 | 4.61 | 3.32 | 51.43 |
| b | 1.2 | 49.47 | 4.29 | 3.09 | 46.38 | |
| c | 1.57 | 38.52 | 5.61 | 4.04 | 34.48 | |
| 2 | a | 1.33 | 52.69 | 4.75 | 3.42 | 49.27 |
| b | 1.82 | 40.48 | 6.50 | 4.68 | 35.80 | |
| c | 1.45 | 42.52 | 5.18 | 3.73 | 38.79 | |
| 3 | a | 2.31 | 41.44 | 8.25 | 5.94 | 35.50 |
| b | 1.08 | 45.74 | 3.86 | 2.78 | 42.96 | |
| c | 1.72 | 48.2 | 6.14 | 4.42 | 43.78 | |
| 4 | a | 1.2 | 53.36 | 4.29 | 3.09 | 50.27 |
| b | 1.93 | 38.47 | 6.89 | 4.96 | 33.51 | |
| c | 1.91 | 51.06 | 6.82 | 4.91 | 46.15 | |
| 5 | a | 1.89 | 37.55 | 6.75 | 4.86 | 32.69 |
| b | 1.86 | 31.95 | 6.64 | 4.78 | 27.17 | |
| c | 1.32 | 43.88 | 4.71 | 3.39 | 40.49 | |
| 6 | a | 2.43 | 40.82 | 8.68 | 6.25 | 34.57 |
| b | 1.62 | 41.41 | 5.79 | 4.17 | 37.24 | |
| c | 1.68 | 48.58 | 6.00 | 4.32 | 44.26 | |
| 7 | a | 3.13 | 35.23 | 11.18 | 8.05 | 27.18 |
| b | 1.56 | 44.79 | 5.57 | 4.01 | 40.78 | |
| c | 1.5 | 44.48 | 5.36 | 3.86 | 40.62 | |
| 8 | a | 1.81 | 57.05 | 6.46 | 4.65 | 52.40 |
| b | 1.85 | 71.08 | 6.61 | 4.76 | 66.32 | |
| c | 1.02 | 69.65 | 3.64 | 2.62 | 67.03 | |
| Sample | %O | O/Mg Ratio | a | % MgCO3 | MgO | Mg in MgO | Unreacted Mg | Unreacted %Mg | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | a | 35.97 | 0.69936 | 39.79 | 2.51 | - | - | - | - |
| b | 32.58 | 0.70024 | 36.32 | 2.75 | - | - | - | - | |
| c | 28.69 | 0.83 | 7.06 | 14.16 | - | - | - | - | |
| 2 | a | 33.54 | 0.68 | 93.75 | 1.07 | - | - | - | - |
| b | 29.18 | 0.82 | 7.98 | 12.53 | - | - | - | - | |
| c | 33.32 | 0.86 | 5.93 | 16.85 | - | - | - | - | |
| 3 | a | 27.32 | 0.77 | 11.96 | 8.36 | - | - | - | - |
| b | 25.42 | 0.59 | - | - | 63.55 | 38.13 | 4.83 | 7.07 | |
| c | 34.75 | 0.79 | 9.49 | 10.54 | - | - | - | - | |
| 4 | a | 25.4 | 0.51 | - | - | 63.5 | 38.1 | 12.17 | 16.09 |
| b | 21.43 | 0.64 | - | - | 53.575 | 32.145 | 1.36 | 2.48 | |
| c | 30.21 | 0.65 | - | - | 75.525 | 45.315 | 0.83 | 1.09 | |
| 5 | a | 33.82 | 1.03 | 2.62 | 38.11 | - | - | - | - |
| b | 34.24 | 1.26 | 1.25 | 80.26 | - | - | - | - | |
| c | 31.46 | 0.78 | 11.08 | 9.03 | - | - | - | - | |
| 6 | a | 30.94 | 0.89 | 4.84 | 20.66 | - | - | - | - |
| b | 31.04 | 0.83 | 7.00 | 14.29 | - | - | - | - | |
| c | 33.52 | 0.76 | 13.70 | 7.30 | - | - | - | - | |
| 7 | a | 30.05 | 1.11 | 2.04 | 49.06 | - | - | - | - |
| b | 34.32 | 0.84 | 6.62 | 15.10 | - | - | - | - | |
| c | 29.39 | 0.72 | 22.47 | 4.45 | - | - | - | - | |
| 8 | a | 31.15 | 0.59 | - | - | 77.875 | 46.725 | 5.67 | 6.79 |
| b | 16.06 | 0.24 | - | - | 40.15 | 24.09 | 42.23 | 51.26 | |
| c | 16.71 | 0.25 | - | - | 41.775 | 25.065 | 41.96 | 50.11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barabulica, I.; Secula, M.S.; Iacob-Tudose, E.T.; Mamaliga, I. Experimental Study on the Influence of Magnesium on the Separation of Carbon Dioxide from Gas Mixtures with Nitrogen by Combustion Processes. Processes 2025, 13, 2054. https://doi.org/10.3390/pr13072054
Barabulica I, Secula MS, Iacob-Tudose ET, Mamaliga I. Experimental Study on the Influence of Magnesium on the Separation of Carbon Dioxide from Gas Mixtures with Nitrogen by Combustion Processes. Processes. 2025; 13(7):2054. https://doi.org/10.3390/pr13072054
Chicago/Turabian StyleBarabulica, Ioan, Marius Sebastian Secula, Eugenia Teodora Iacob-Tudose, and Ioan Mamaliga. 2025. "Experimental Study on the Influence of Magnesium on the Separation of Carbon Dioxide from Gas Mixtures with Nitrogen by Combustion Processes" Processes 13, no. 7: 2054. https://doi.org/10.3390/pr13072054
APA StyleBarabulica, I., Secula, M. S., Iacob-Tudose, E. T., & Mamaliga, I. (2025). Experimental Study on the Influence of Magnesium on the Separation of Carbon Dioxide from Gas Mixtures with Nitrogen by Combustion Processes. Processes, 13(7), 2054. https://doi.org/10.3390/pr13072054








