Recent Progress and Challenges in Microbial Defluorination and Degradation for Sustainable Remediation of Fluorinated Xenobiotics
Abstract
1. Introduction
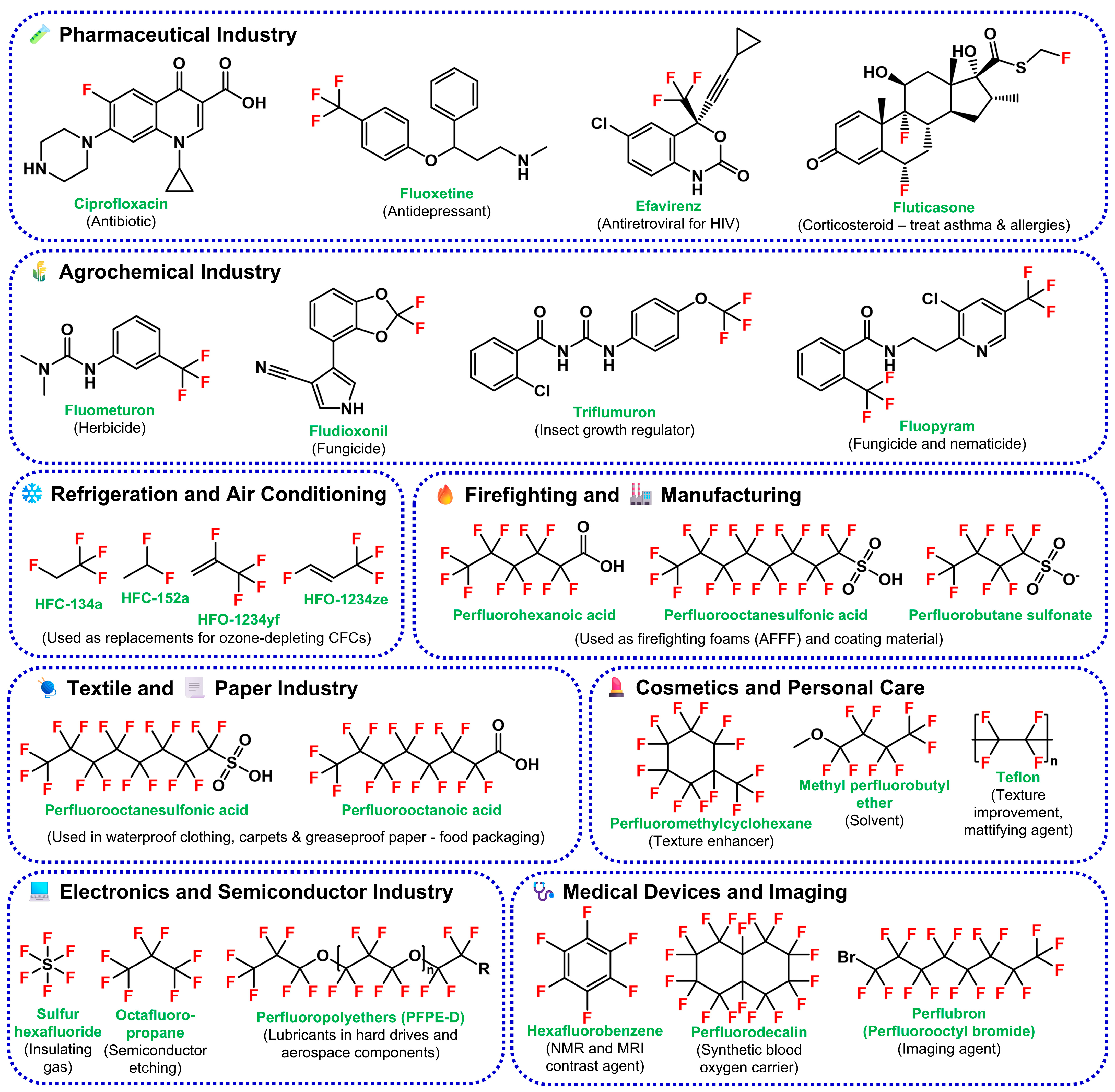
2. Fluorinated Xenobiotics: Chemistry and Environmental Impact
2.1. Properties of the Carbon–Fluorine (C–F) Bond
2.2. Environmental Occurrence and Pathways of Contamination
2.2.1. Sources of Fluorinated Xenobiotics
2.2.2. Environmental Pathways and Fate of Fluorinated Xenobiotics
2.3. Health and Ecological Risks Associated with Fluorinated Xenobiotics
2.3.1. Human Health Risks
2.3.2. Ecological Risks
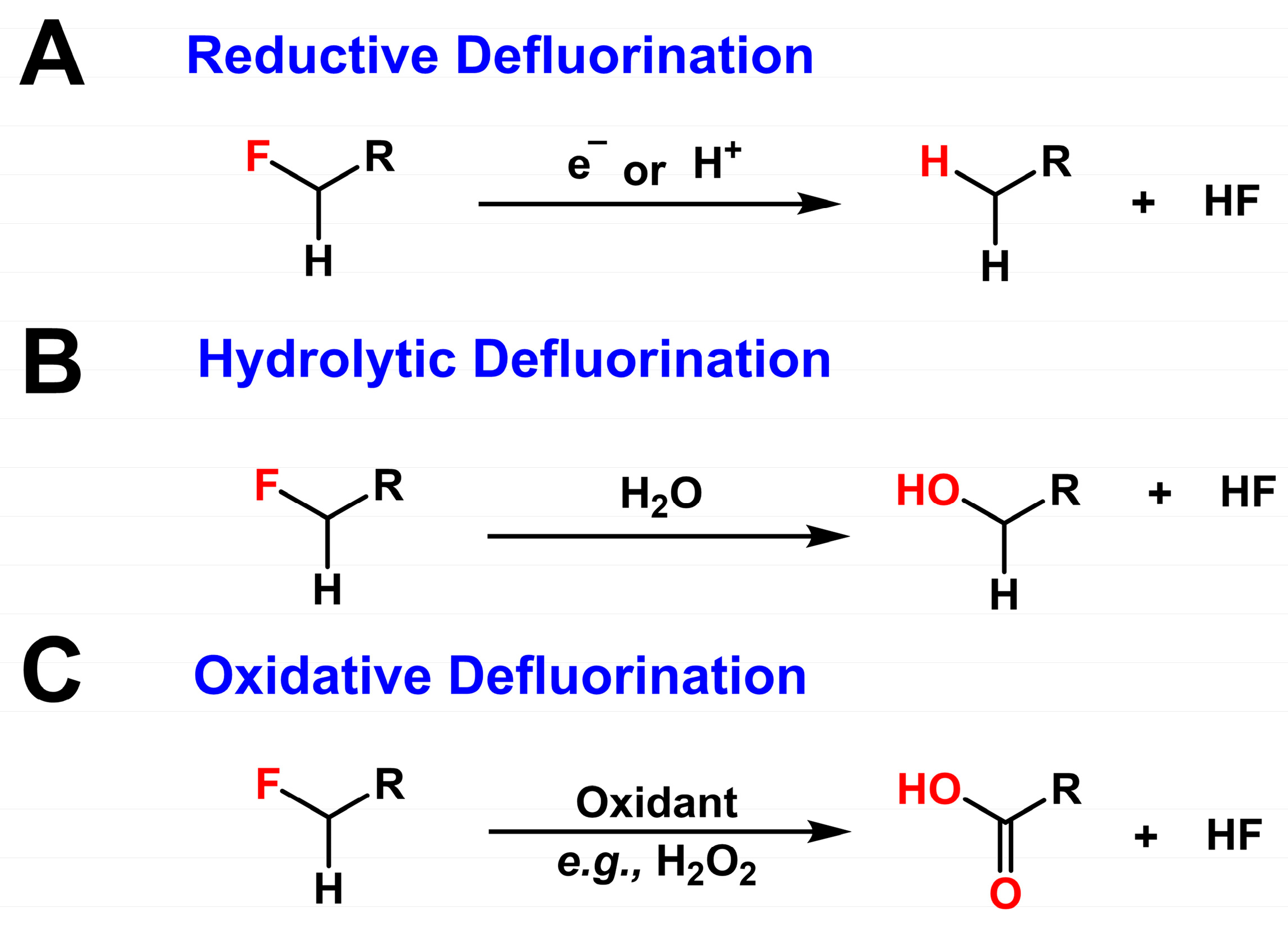
3. Microbial Defluorination: Mechanisms of C–F Bond Cleavage
3.1. Reductive Defluorination
3.2. Hydrolytic Defluorination
3.3. Oxidative Defluorination
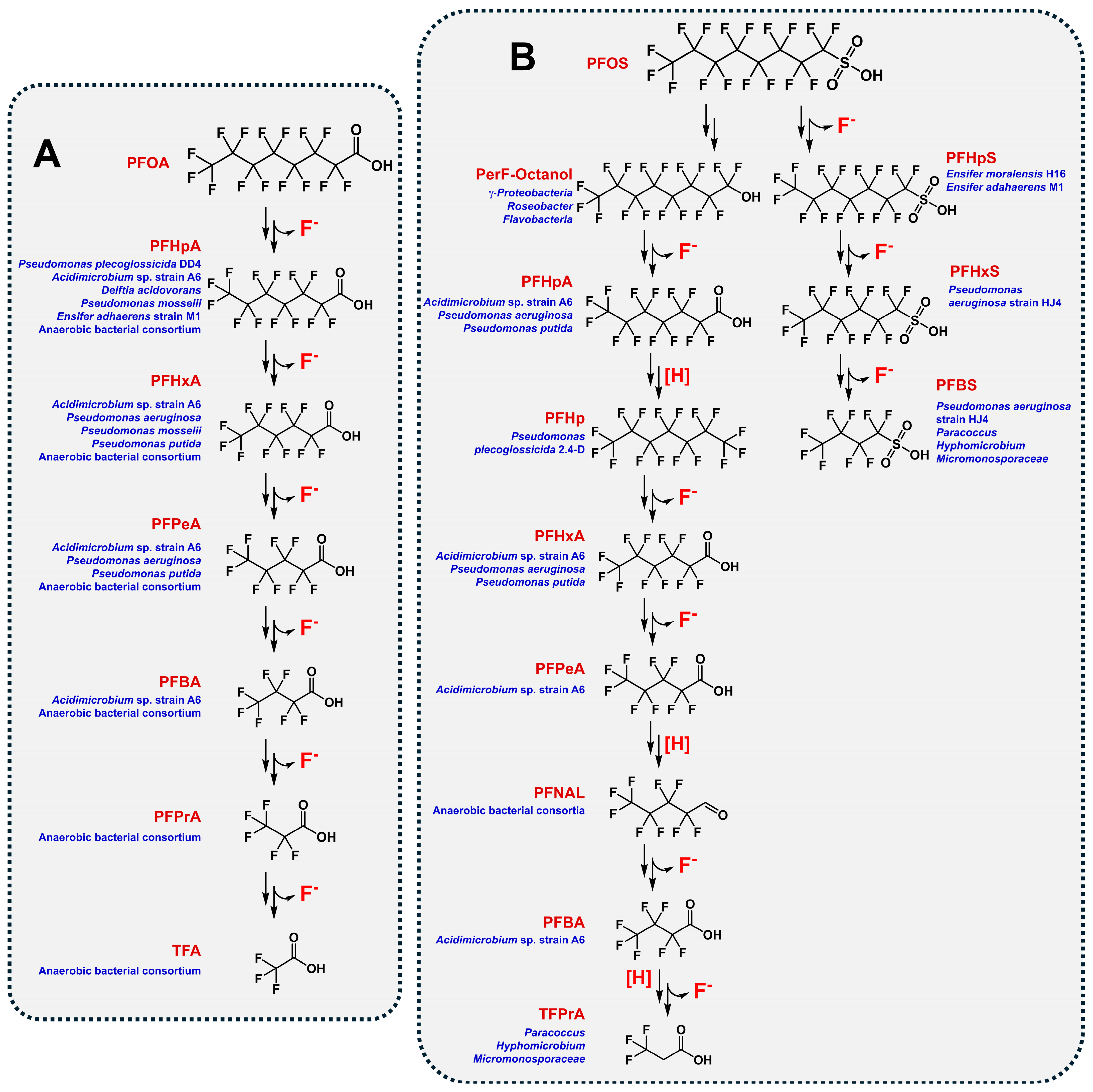
4. Microbial Degradation of Fluorinated Xenobiotics
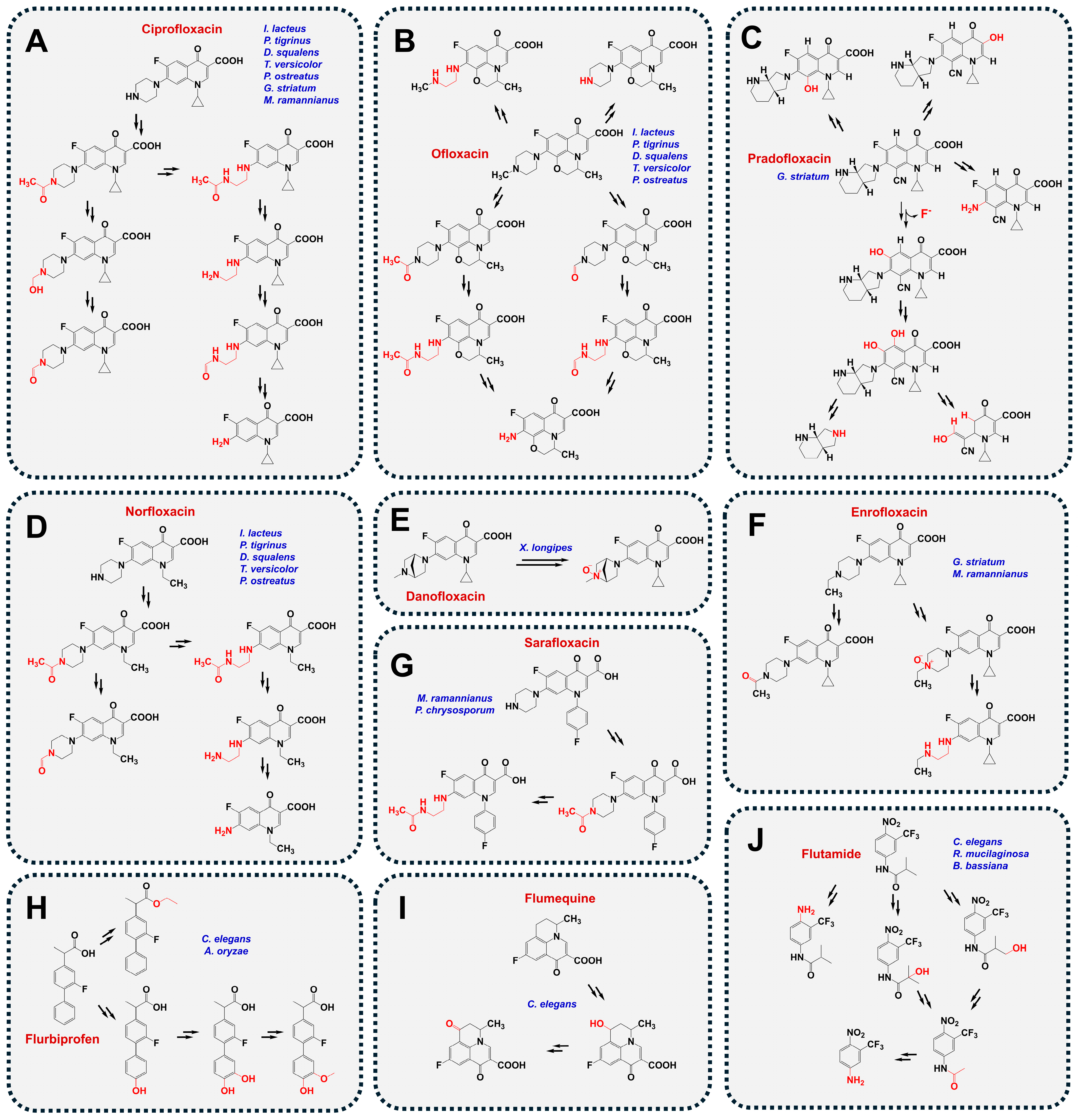
4.1. Bacterial Defluorination and Degradation
4.2. Fungal Defluorination and Degradation
4.3. Algal Defluorination and Degradation
4.4. Microbial Consortia and Syntrophic Interactions
5. Enzymatic Defluorination of Fluorinated Xenobiotics
5.1. Dehalogenases
5.1.1. Haloacid Dehalogenases
5.1.2. Reductive Dehalogenases
5.2. Cytochrome P450s and Monooxygenases
5.3. Peroxidases
5.4. Laccases
5.5. Enzymatic and Synthetic Biology Approaches to Improve Microbial Defluorination
5.5.1. Thermodynamic and Environmental Factors Influencing Microbial Defluorination
5.5.2. Engineering Microbial Hosts and Discovery of Novel Defluorinases
5.5.3. Enzymatic Mechanisms and Structure-Guided Enzyme Engineering
5.5.4. Synthetic Biology and Microbial Consortia for Scalable Bioremediation
6. Challenges and Limitations
6.1. Challenges in Microbial Defluorination
6.2. Challenges in Enzymatic Defluorination
7. Conclusions and Future Directions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dias, D.; Bons, J.; Kumar, A.; Kabir, M.H.; Liang, H. Forever Chemicals, Per- and Polyfluoroalkyl Substances (PFAS), in Lubrication. Lubricants 2024, 12, 114. [Google Scholar] [CrossRef]
- Du, Y.; Bian, Y.; Baecker, D.; Dhawan, G.; Semghouli, A.; Kiss, L.; Zhang, W.; Sorochinsky, A.E.; Soloshonok, V.A.; Han, J. Fluorine in the Pharmaceutical Industry: FDA-Approved Fluorine-Containing Drugs in 2024. Chem. Eur. J. 2025, 31, e202500662. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F. Recent Advances in Microbial Enzyme Applications for Sustainable Textile Processing and Waste Management. Science 2025, 7, 46. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Khan, M.F.; Hof, C.; Niemcova, P.; Murphy, C.D. Biotransformation of Fluorinated Drugs and Xenobiotics by the Model Fungus Cunninghamella elegans. Methods Enzymol. 2024, 696, 251–285. [Google Scholar]
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.A.; Khanna, S.; Rawat, G.; Panda, A.K.; Bisht, S.S.; Upadhyay, J.; Ansari, M.N. Degradation of Xenobiotic Pollutants: An Environmentally Sustainable Approach. Metabolites 2022, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef]
- Sun, J.M.; Kelly, B.C.; Gobas, F.A.P.C.; Sunderland, E.M. A Food Web Bioaccumulation Model for the Accumulation of Per- and Polyfluoroalkyl Substances (PFAS) in Fish: How Important Is Renal Elimination? Environ. Sci. Process. Impacts 2022, 24, 1152–1164. [Google Scholar] [CrossRef]
- Blake, B.E.; Fenton, S.E. Early Life Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Latent Health Outcomes: A Review Including the Placenta as a Target Tissue and Possible Driver of Peri- and Postnatal Effects. Toxicology 2020, 443, 152565. [Google Scholar] [CrossRef]
- Ahn, C.; Jeung, E.-B. Endocrine-Disrupting Chemicals and Disease Endpoints. Int. J. Mol. Sci. 2023, 24, 5342. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Ye, C.; Wang, T.; Li, X.; Luo, Y. Toxicity of Per- and Polyfluoroalkyl Substances to Aquatic Invertebrates, Planktons, and Microorganisms. Int. J. Environ. Res. Public Health 2022, 19, 16729. [Google Scholar] [CrossRef]
- Glassmeyer, S.T.; Burns, E.E.; Focazio, M.J.; Furlong, E.T.; Gribble, M.O.; Jahne, M.A.; Keely, S.P.; Kennicutt, A.R.; Kolpin, D.W.; Medlock Kakaley, E.K.; et al. Water, Water Everywhere, but Every Drop Unique: Challenges in the Science to Understand the Role of Contaminants of Emerging Concern in the Management of Drinking Water Supplies. Geohealth 2023, 7, e2022GH000716. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Wei, T.; Shen, C. Leading Techniques for Per- and Polyfluoroalkyl Substances (PFASs) Remediating in Water and Wastewater. Water 2025, 17, 1319. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Fathima, J.; Varghese, S.; Chatterjee, P.; Gadhamshetty, V. Advances in Bioremediation Strategies for PFAS-Contaminated Water and Soil. Soil Environ. Health 2024, 3, 100126. [Google Scholar] [CrossRef]
- Natarajan, R.; Azerad, R.; Badet, B.; Copin, E. Microbial Cleavage of CF Bond. J. Fluor. Chem. 2005, 126, 424–435. [Google Scholar] [CrossRef]
- Seong, H.J.; Kwon, S.W.; Seo, D.C.; Kim, J.H.; Jang, Y.S. Enzymatic Defluorination of Fluorinated Compounds. Appl. Biol. Chem. 2019, 62. [Google Scholar] [CrossRef]
- Khan, M.F.; Chowdhary, S.; Koksch, B.; Murphy, C.D. Biodegradation of Amphipathic Fluorinated Peptides Reveals a New Bacterial Defluorinating Activity and a New Source of Natural Organofluorine Compounds. Environ. Sci. Technol. 2023, 57, 9762–9772. [Google Scholar] [CrossRef]
- Alexandrino, D.A.; Carvalho, M.F. Defluorination as the Key Trait to Gauge the Biodegradability of Fluorinated Pollutants in Environmental Microbial Communities. In Methods in Enzymology, 1st ed.; Academic Press: London, UK, 2024; Volume 696, pp. 321–338. [Google Scholar]
- Khan, M.F. Fungi for Sustainable Pharmaceutical Remediation: Enzymatic Innovations, Challenges, and Applications—A Review. Processes 2025, 13, 1034. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Bacterial Degradation of the Anti-depressant Drug Fluoxetine Produces Trifluoroacetic Acid and Fluoride Ion. Appl. Microbiol. Biotechnol. 2021, 105, 9359–9369. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Cunninghamella spp. Produce Mammalian-Equivalent Metabolites from Fluorinated Pyrethroid Pesticides. AMB Express 2021, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, S.; Kumar, A.; Reetu, R.; Singh, R. Environment-Friendly Refrigerants for Sustainable Refrigeration and Air Conditioning: A Review. Curr. World Environ. 2023, 18, 933–947. [Google Scholar] [CrossRef]
- Céline, C.; Catherine, B.; Romane, C.; Laurence, C. Per- and Polyfluoroalkyls Used as Cosmetic Ingredients—Qualitative Study of 765 Cosmetic Products. Food Chem. Toxicol. 2024, 187, 114625. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, Y. Sustainable Transition of the Global Semiconductor Industry: Challenges, Strategies, and Future Directions. Sustainability 2025, 17, 3160. [Google Scholar] [CrossRef]
- Cosco, D.; Fattal, E.; Fresta, M.; Tsapis, N. Perfluorocarbon-Loaded Micro and Nanosystems for Medical Imaging: A State of the Art. J. Fluor. Chem. 2015, 171, 18–26. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Haswell, A.; Hopkinson, M.N. C−F Bond Insertion: An Emerging Strategy for Constructing Fluorinated Molecules. Chem. Eur. J. 2024, 30, e202304229. [Google Scholar] [CrossRef]
- Tong, W.; Huang, Q.; Li, M.; Wang, J.B. Enzyme-catalyzed C–F bond formation and cleavage. Bioresour. Bioprocess. 2019, 6, 46. [Google Scholar] [CrossRef]
- Hunter, L. The C–F bond as a conformational tool in organic and biological chemistry. Beilstein J. Org. Chem. 2010, 6, 38. [Google Scholar] [CrossRef]
- Dasu, K.; Xia, X.; Siriwardena, D.; Klupinski, T.P.; Seay, B. Concentration profiles of per- and polyfluoroalkyl substances in major sources to the environment. J. Environ. Manag. 2022, 301, 113879. [Google Scholar] [CrossRef]
- Sharifan, H.; Bagheri, M.; Wang, D.; Burken, J.G.; Higgins, C.P.; Liang, Y.; Liu, J.; Schaefer, C.E.; Blotevogel, J. Fate and transport of per- and polyfluoroalkyl substances (PFASs) in the vadose zone. Sci. Total Environ. 2021, 771, 145427. [Google Scholar] [CrossRef] [PubMed]
- Evich, M.G.; Davis, M.J.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of poly- and perfluoroalkyl substances (PFASs) in US drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Environmental impacts, exposure pathways, and health effects of PFOA and PFOS. Ecotoxicol. Environ. Saf. 2023, 267, 115663. [Google Scholar] [CrossRef]
- Young, C.J.; Mabury, S.A. Atmospheric Perfluorinated Acid Precursors: Chemistry, Occurrence, and Impacts. In Reviews of Environmental Contamination and Toxicology, 1st ed.; De Voogt, P., Ed.; Springer: New York, NY, USA, 2010; Volume 208, pp. 1–109. [Google Scholar]
- Vierke, L.; Ahrens, L.; Shoeib, M.; Reiner, E.J.; Guo, R.; Palm, W.U.; Ebinghaus, R.; Harner, T. Air concentrations and particle–gas partitioning of polyfluoroalkyl compounds at a wastewater treatment plant. Environ. Chem. 2011, 8, 363–371. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Leung, K.M.; Lam, P.K.; Ruan, Y. Per- and polyfluoroalkyl substances (PFAS) in the marine environment: An overview and prospects. Mar. Pollut. Bull. 2025, 216, 117993. [Google Scholar] [CrossRef]
- Fischer, F.C.; Ludtke, S.; Thackray, C.; Pickard, H.M.; Haque, F.; Dassuncao, C.; Endo, S.; Schaider, L.; Sunderland, E.M. Binding of per- and polyfluoroalkyl substances (PFAS) to serum proteins: Implications for toxicokinetics in humans. Environ. Sci. Technol. 2024, 58, 1055–1063. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Hong, M.S.; Lee, J.S.; Lee, M.C.; Lee, J.S. Ecotoxicological effects of per- and polyfluoroalkyl substances in aquatic organisms: A review. Mar. Pollut. Bull. 2025, 214, 117678. [Google Scholar] [CrossRef]
- Wasel, O.; King, H.; Choi, Y.J.; Lee, L.S.; Freeman, J.L. Differential developmental neurotoxicity and tissue uptake of the per- and polyfluoroalkyl substance alternatives, GenX and PFBS. Environ. Sci. Technol. 2023, 57, 19274–19284. [Google Scholar] [CrossRef]
- Hu, M.; Scott, C. Toward the development of a molecular toolkit for the microbial remediation of per- and polyfluoroalkyl substances. Appl. Environ. Microbiol. 2024, 90, e00157-24. [Google Scholar] [CrossRef]
- Fincker, M.; Spormann, A.M. Biochemistry of catabolic reductive dehalogenation. Annu. Rev. Biochem. 2017, 86, 357–386. [Google Scholar] [CrossRef]
- Jin, B.; Liu, H.; Che, S.; Gao, J.; Yu, Y.; Liu, J.; Men, Y. Substantial defluorination of polychlorofluorocarboxylic acids triggered by anaerobic microbial hydrolytic dechlorination. Nat. Water 2023, 1, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Guan, X.; Rodrigues, R.; Yu, Y.; Xie, Y.; Liu, C.; Men, Y. Synergistic material–microbe interface toward deeper anaerobic defluorination. Proc. Natl. Acad. Sci. USA 2024, 121, e2400525121. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, K.; Li, Z.; Ren, C.; Chen, J.; Lin, Y.H.; Liu, J.; Men, Y. Microbial cleavage of C–F bonds in two C6 per- and polyfluorinated compounds via reductive defluorination. Environ. Sci. Technol. 2020, 54, 14393–14402. [Google Scholar] [CrossRef] [PubMed]
- Haschimi, B.; Willecke, F.; Mundinger, S.; Hüttel, W.; Jessen, H.; Müller, M.; Auwärter, V. Enzymatic defluorination of a terminally monofluorinated pentyl moiety: Oxidative or hydrolytic mechanism? Drug Metab. Dispos. 2024, 52, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Hof, C.; Niemcová, P.; Murphy, C.D. Recent advances in fungal xenobiotic metabolism: Enzymes and applications. World J. Microbiol. Biotechnol. 2023, 39, 296. [Google Scholar] [CrossRef]
- Idris, O.A.; Erasmus, M. Degradation pathways of perfluoroalkyl and polyfluoroalkyl compounds: Removal in water and soil using fungi and plant-based remediation. Environ. Adv. 2024, 18, 100598. [Google Scholar] [CrossRef]
- Doi, R.; Yasuda, M.; Kajita, N.; Koh, K.; Ogoshi, S. Nickel-catalyzed exhaustive hydrodefluorination of perfluoroalkyl arenes. J. Am. Chem. Soc. 2023, 145, 11449–11456. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Wijayahena, M.K.; Moreira, I.S.; Castro, P.M.; Dowd, S.; Marciesky, M.I.; Ng, C.; Aga, D.S. PFAS biodegradation by Labrys portucalensis F11: Evidence of chain shortening and identification of metabolites of PFOS, 6:2 FTS, and 5:3 FTCA. Sci. Total Environ. 2025, 959, 178348. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, F.; Zhao, W.; Thoma, C.; Che, S.; Richman, J.E.; Jin, B.; Zhu, Y.; Xing, Y.; Wackett, L.; et al. Electron bifurcation and fluoride efflux systems implicated in defluorination of perfluorinated unsaturated carboxylic acids by Acetobacterium spp. Sci. Adv. 2024, 10, eado2957. [Google Scholar]
- Huang, S.; Pilloni, G.; Key, T.A.; Jaffé, P.R. Defluorination of various perfluoro alkyl acids and selected PFOA and PFOS monomers by Acidimicrobium sp. Strain A6 enrichment cultures. J. Hazard. Mater. 2024, 480, 136426. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Bottos, E.M.; Bilbey, N.J.; Brewer, S.E. Genomic and proteomic characterization of Gordonia sp. NB4-1Y in relation to 6:2 fluorotelomer sulfonate biodegradation. Microbiology 2013, 159, 1618–1628. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Stoica, C.; Iftode, C.; Pirvu, F.; Petre, V.A.; Paun, I.; Pascu, L.F.; Vasile, G.G.; Nita-Lazar, M. Bacterial biodegradation of perfluorooctanoic acid (PFOA) and perfluorosulfonic acid (PFOS) using pure Pseudomonas strains. Sustainability 2023, 15, 14000. [Google Scholar] [CrossRef]
- Chetverikov, S.P.; Sharipov, D.A. Biodegradation of perfluorooctanoic acid by Pseudomonas plecoglossicida strain DD4. Baghdad Sci. J. 2022, 19, 1502. [Google Scholar] [CrossRef]
- Harris, J.D.; Coon, C.M.; Doherty, M.E.; McHugh, E.A.; Warner, M.C.; Walters, C.L.; Orahood, O.M.; Loesch, A.E.; Hatfield, D.C.; Sitko, J.C.; et al. Engineering and characterization of dehalogenase enzymes from Delftia acidovorans in bioremediation of perfluorinated compounds. Synth. Syst. Biotechnol. 2022, 7, 671–676. [Google Scholar] [CrossRef]
- Chetverikov, S.; Hkudaigulov, G.; Sharipov, D.; Starikov, S. Probable New Species of Bacteria of the Genus Pseudomonas Accelerates and Enhances the Destruction of Perfluorocarboxylic Acids. Toxics 2024, 12, 930. [Google Scholar] [CrossRef]
- Chetverikov, S.P.; Loginov, O.N. A new Ensifer adhaerens strain M1 is capable of transformation of perfluorocarboxylic acids. Microbiology 2019, 88, 115–117. [Google Scholar] [CrossRef]
- Jaffé, P.R.; Huang, S.; Park, J.; Ruiz-Urigüen, M.; Shuai, W.; Sima, M. Defluorination of PFAS by Acidimicrobium sp. strain A6 and potential applications for remediation. Methods Enzymol. 2024, 696, 287–320. [Google Scholar]
- Kognou, A.L.M.; Ngane, R.A.N.; Jiang, Z.H.; Xu, C.C.; Qin, W.; Inui, H. Harnessing the power of microbial consortia for the biodegradation of per- and polyfluoroalkyl substances: Challenges and opportunities. Chemosphere 2025, 374, 144221. [Google Scholar] [CrossRef]
- Chetverikov, S.; Hkudaigulov, G. New Ensifer moralensis H16 strain for perfluorooctane sulfonate destruction. Fluoride 2021, 54, 309–320. [Google Scholar]
- Chetverikov, S.P.; Sharipov, D.A.; Korshunova, T.Y.; Loginov, O.N. Degradation of perfluorooctanyl sulfonate by strain Pseudomonas plecoglossicida 2.4-D. Appl. Biochem. Microbiol. 2017, 53, 533–538. [Google Scholar] [CrossRef]
- Sorn, S.; Hara-Yamamura, H.; Vet, S.; Xiao, M.; Hoek, E.M.; Honda, R. Biological treatment of perfluorooctanesulfonic acid (PFOS) using microbial capsules of a polysulfone membrane. Chemosphere 2023, 329, 138585. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.F.; Maiangwa, J.; Salleh, A.B.; Normi, Y.M.; Leow, T.C. Dehalogenases: From improved performance to potential microbial dehalogenation applications. Molecules 2018, 23, 1100. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Gao, Z.; Xiang, X.; Wang, Q.; Song, X.; Wu, Y.; Löffler, F.E.; Zeng, J.; Lin, X. Defluorination of monofluorinated alkane by Rhodococcus sp. NJF-7 isolated from soil. AMB Express 2024, 14, 65. [Google Scholar] [CrossRef]
- Tiedt, O.; Mergelsberg, M.; Boll, K.; Müller, M.; Adrian, L.; Jehmlich, N.; von Bergen, M.; Boll, M. ATP-dependent C–F bond cleavage allows the complete degradation of 4-fluoroaromatics without oxygen. mBio 2016, 7, e01128. [Google Scholar] [CrossRef]
- Murphy, C.D.; Quirke, S.; Balogun, O. Degradation of fluorobiphenyl by Pseudomonas pseudoalcaligenes KF707. FEMS Microbiol. Lett. 2008, 286, 45–49. [Google Scholar] [CrossRef]
- Schmidt, S.; Fortnagel, P.; Wittich, R. Biodegradation and transformation of 4,4′- and 2,4-dihalodiphenyl ethers by Sphingomonas sp. strain SS33. Appl. Environ. Microbiol. 1993, 59, 3931–3933. [Google Scholar] [CrossRef]
- Yi, L.B.; Chai, L.Y.; Xie, Y.; Peng, Q.J.; Peng, Q.Z. Isolation, identification, and degradation performance of a PFOA-degrading strain. Genet. Mol. Res. 2016, 15, 235–246. [Google Scholar] [CrossRef]
- Kwon, B.G.; Lim, H.-J.; Na, S.-H.; Choi, B.-I.; Shin, D.-S.; Chung, S.-Y. Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant. Chemosphere 2014, 109, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Munoz, G.; Bottos, E.M.; Duy, S.V.; Sauvé, S.; Liu, J.; Van Hamme, J.D. Degradation and defluorination of 6:2 fluorotelomer sulfonamidoalkyl betaine and 6:2 fluorotelomer sulfonate by Gordonia sp. strain NB4-1Y under sulfur-limiting conditions. Sci. Total Environ. 2019, 647, 690–698. [Google Scholar] [CrossRef]
- Kim, M.H.; Wang, N.; Chu, K.H. 6:2 Fluorotelomer alcohol (6:2 FTOH) biodegradation by multiple microbial species under different physiological conditions. Appl. Microbiol. Biotechnol. 2014, 98, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.J.; Li, J.; Li, C.X.; Tang, X.D.; Yu, G.W.; Wang, Y. Study of ciprofloxacin biodegradation by a Thermus sp. isolated from pharmaceutical sludge. J. Hazard. Mater. 2018, 343, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lutterbeck, C.A.; Wilde, M.L.; Baginska, E.; Leder, C.; Machado, Ê.L.; Kümmerer, K. Degradation of cyclophosphamide and 5-fluorouracil by UV and simulated sunlight treatments: Assessment of the enhancement of the biodegradability and toxicity. Environ. Pollut. 2016, 208, 467–476. [Google Scholar] [CrossRef]
- Bright, T.V.; Clark, B.R.; O’Brien, E.; Murphy, C.D. Bacterial production of hydroxylated and amidated metabolites of flurbiprofen. J. Mol. Catal. B Enzym. 2011, 72, 116–121. [Google Scholar] [CrossRef]
- Herath, W.; Khan, I.A. Microbial metabolism. Part 11. Metabolites of flutamide. Chem. Pharm. Bull. 2010, 58, 562–564. [Google Scholar] [CrossRef][Green Version]
- Lara-Moreno, A.; Morillo, E.; Merchán, F.; Madrid, F.; Villaverde, J. Bioremediation of a trifluralin contaminated soil using bioaugmentation with novel isolated bacterial strains and cyclodextrin. Sci. Total Environ. 2022, 840, 156695. [Google Scholar] [CrossRef]
- Khan, M.F.; Liao, J.; Liu, Z.; Chugh, G. Bacterial Cytochrome P450 Involvement in the Biodegradation of Fluorinated Pyrethroids. J. Xenobiot. 2025, 15, 58. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, L.; Chen, F.; Chen, L.; Yin, J.; Huang, X. Detoxification of diphenyl ether herbicide lactofen by Bacillus sp. Za and enantioselective characteristics of an esterase gene lacE. J. Hazard. Mater. 2018, 341, 336–345. [Google Scholar] [CrossRef]
- Liang, B.; Lu, P.; Li, H.; Li, R.; Li, S.; Huang, X. Biodegradation of fomesafen by strain Lysinibacillus sp. ZB-1 isolated from soil. Chemosphere 2009, 77, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.S.; Lee, Y.J.; Kim, J.H. Metabolism of nitrodiphenyl ether herbicides by dioxin-degrading bacterium Sphingomonas wittichii RW1. J. Agric. Food Chem. 2008, 56, 9146–9151. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Gul, P. Fungicide isopyrazam degradative response toward extrinsically added fungal and bacterial strains. J. Basic Microbiol. 2020, 60, 484–493. [Google Scholar] [CrossRef]
- Strunk, N.; Engesser, K.H. Degradation of fluorobenzene and its central metabolites 3-fluorocatechol and 2-fluoromuconate by Burkholderia fungorum FLU100. Appl. Microbiol. Biotechnol. 2013, 97, 5605–5614. [Google Scholar] [CrossRef] [PubMed]
- Misiak, K.; Casey, E.; Murphy, C.D. Factors influencing 4-fluorobenzoate degradation in biofilm cultures of Pseudomonas knackmussii B13. Water Res. 2011, 45, 3512–3520. [Google Scholar] [CrossRef]
- Hasan, S.A.; Ferreira, M.I.M.; Koetsier, M.J.; Arif, M.I.; Janssen, D.B. Complete biodegradation of 4-fluorocinnamic acid by a consortium comprising Arthrobacter sp. strain G1 and Ralstonia sp. strain H1. Appl. Environ. Microbiol. 2011, 77, 572–579. [Google Scholar] [CrossRef]
- Wackett, L.P. Why is the biodegradation of polyfluorinated compounds so rare? mSphere 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M. Genetically engineered microorganisms for environmental remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farradá, G.; Thijs, S.; Rineau, F.; Guerra, G.; Vangronsveld, J. White rot fungi as tools for the bioremediation of xenobiotics: A review. J. Fungi 2024, 10, 167. [Google Scholar] [CrossRef]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar] [CrossRef]
- Prieto, A.; Möder, M.; Rodil, R.; Adrian, L.; Marco-Urrea, E. Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products. Bioresour. Technol. 2011, 102, 10987–10995. [Google Scholar] [CrossRef]
- Gao, N.; Liu, C.X.; Xu, Q.M.; Cheng, J.S.; Yuan, Y.J. Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co-producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus. Chemosphere 2018, 195, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Čvančarová, M.; Moeder, M.; Filipová, A.; Cajthaml, T. Biotransformation of fluoroquinolone antibiotics by ligninolytic fungi—Metabolites, enzymes and residual antibacterial activity. Chemosphere 2015, 136, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Rusch, M.; Kauschat, A.; Spielmeyer, A.; Römpp, A.; Hausmann, H.; Zorn, H.; Hamscher, G. Biotransformation of the antibiotic danofloxacin by Xylaria longipes leads to an efficient reduction of its antibacterial activity. J. Agric. Food Chem. 2015, 63, 6897–6904. [Google Scholar] [CrossRef]
- Parshikov, I.A.; Freeman, J.P.; Lay, J.O., Jr.; Beger, R.D.; Williams, A.J.; Sutherland, J.B. Microbiological transformation of enrofloxacin by the fungus Mucor ramannianus. Appl. Environ. Microbiol. 2000, 66, 2664–2667. [Google Scholar] [CrossRef] [PubMed]
- Wetzstein, H.G.; Schmeer, N.; Karl, W. Degradation of the fluoroquinolone enrofloxacin by the brown rot fungus Gloeophyllum striatum: Identification of metabolites. Appl. Environ. Microbiol. 1997, 63, 4272–4281. [Google Scholar] [CrossRef]
- Marengo, J.R.; Kok, R.A.; Burrows, L.A.; Velagaleti, R.R.; Stamm, J.M. Biodegradation of 14C-sarafloxacin hydrochloride, a fluoroquinolone antimicrobial by Phanerochaete chrysosporium. J. Sci. Ind. Res. 2001, 60, 121–130. [Google Scholar]
- Parshikov, I.A.; Freeman, J.P.; Lay, J.O., Jr.; Moody, J.D.; Williams, A.J.; Beger, R.D.; Sutherland, J.B. Metabolism of the veterinary fluoroquinolone sarafloxacin by the fungus Mucor ramannianus. J. Ind. Microbiol. Biotechnol. 2001, 26, 140–144. [Google Scholar] [CrossRef]
- Amadio, J.; Gordon, K.; Murphy, C.D. Biotransformation of flurbiprofen by Cunninghamella species. Appl. Environ. Microbiol. 2010, 76, 6299–6303. [Google Scholar] [CrossRef]
- Tamborini, L.; Romano, D.; Pinto, A.; Contente, M.; Iannuzzi, M.C.; Conti, P.; Molinari, F. Biotransformation with whole microbial systems in a continuous flow reactor: Resolution of (RS)-flurbiprofen using Aspergillus oryzae by direct esterification with ethanol in organic solvent. Tetrahedron Lett. 2013, 54, 6090–6093. [Google Scholar] [CrossRef]
- Amadio, J.; Murphy, C.D. Production of human metabolites of the anti-cancer drug flutamide via biotransformation in Cunninghamella species. Biotechnol. Lett. 2011, 33, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Deck, J.; Freeman, J.P.; Chiarelli, M.P.; Adjei, M.D.; Heinze, T.M.; Sutherland, J.B. Biotransformation of flumequine by the fungus Cunninghamella elegans. Chemosphere 2007, 67, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Tseng, N.; Wang, N.; Szostek, B.; Mahendra, S. Biotransformation of 6:2 fluorotelomer alcohol (6:2 FTOH) by a wood-rotting fungus. Environ. Sci. Technol. 2014, 48, 4012–4020. [Google Scholar] [CrossRef]
- Tseng, N.S.L. Feasibility of Biodegradation of Polyfluoroalkyl and Perfluoroalkyl Substances. Ph.D. Thesis, UCLA, Los Angeles, CA, USA, 2012. [Google Scholar]
- Ayala-Cabrera, J.F.; Santos, F.J.; Moyano, E. Fragmentation studies of neutral per- and polyfluoroalkyl substances by atmospheric pressure ionization-multiple-stage mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 7357–7373. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Murphy, C.D. Fluorotelomer alcohols are efficiently biotransformed by Cunninghamella elegans. Environ. Sci. Pollut. Res. 2023, 30, 23613–23623. [Google Scholar] [CrossRef]
- Khan, M.F.; Paul Guin, J.; Thampi, R.K.; Sullivan, J.A.; Murphy, C.D. Enhanced Removal of Perfluorooctanoic Acid with Sequential Photocatalysis and Fungal Treatment. Environ. Sci. Pollut. Res. 2023, 30, 91478–91486. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Cytochrome P450 5208A3 is a promiscuous xenobiotic biotransforming enzyme in Cunninghamella elegans. Enzym. Microb. Technol. 2022, 161, 110102. [Google Scholar] [CrossRef]
- Birolli, W.G.; Vacondio, B.; Alvarenga, N.; Seleghim, M.H.R.; Porto, A.L.M. Enantioselective biodegradation of the pyrethroid (+/)-lambda-cyhalothrin by marine-derived fungi. Chemosphere 2018, 197, 651–660. [Google Scholar] [CrossRef]
- Alvarenga, N.; Birolli, W.G.; Seleghim, M.H.; Porto, A.L. Biodegradation of methyl parathion by whole cells of marine-derived fungi Aspergillus sydowii and Penicillium decaturense. Chemosphere 2014, 117, 47–52. [Google Scholar] [CrossRef]
- Bhatt, P.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Chen, S. Biodegradation of Allethrin by a Novel Fungus Fusarium proliferatum Strain CF2, Isolated from Contaminated Soils. Microorganisms 2020, 8, 593. [Google Scholar] [CrossRef]
- Thoa, L.T.K.; Thao, T.T.P.; Nguyen-Thi, M.-L.; Chung, N.D.; Ooi, C.W.; Park, S.-M.; Lan, T.T.; Quang, H.T.; Khoo, K.S.; Show, P.L.; et al. Microbial biodegradation of recalcitrant synthetic dyes from textile-enriched wastewater by Fusarium oxysporum. Chemosphere 2023, 325, 138392. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mao, C.; Zong, S.; Khan, A.; Wang, W.; Yun, H.; Zhang, P.; Shigaki, T.; Fang, Y.; Han, H.; et al. Transcriptome analysis reveals self-redox mineralization mechanism of azo dyes and novel decolorizing hydrolases in Aspergillus tabacinus LZ-M. Environ. Pollut. 2023, 325, 121459. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.J.; Doerge, D.R.; Cerniglia, C.E. Biotransformation of malachite green by the fungus Cunninghamella elegans. Appl. Environ. Microbiol. 2001, 67, 4358–4360. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Quinn, L.; Li, J.J.; Casey, E.; Murphy, C.D. Simultaneous removal of malachite green and hexavalent chromium by Cunninghamella elegans biofilm in a semi-continuous system. Int. Biodeterior. Biodegrad. 2017, 125, 142–149. [Google Scholar] [CrossRef]
- Munck, C.; Thierry, E.; Grassle, S.; Chen, S.H.; Ting, A.S.Y. Biofilm formation of filamentous fungi Coriolopsis sp. on simple muslin cloth to enhance removal of triphenylmethane dyes. J. Environ. Manag. 2018, 214, 261–266. [Google Scholar] [CrossRef]
- Park, H.; Min, B.; Jang, Y.; Kim, J.; Lipzen, A.; Sharma, A.; Andreopoulos, B.; Johnson, J.; Riley, R.; Spatafora, J.W.; et al. Comprehensive genomic and transcriptomic analysis of polycyclic aromatic hydrocarbon degradation by a mycoremediation fungus, Dentipellis sp. Appl. Microbiol. Biotechnol. 2019, 109, 8145–8155. [Google Scholar] [CrossRef]
- Baldantoni, D.; Morelli, R.; Bellino, A.; Prati, M.V.; Alfani, A.; De Nicola, F. Anthracene and benzo(a)pyrene degradation in soil is favoured by compost amendment: Perspectives for a bioremediation approach. J. Hazard. Mater. 2017, 339, 395–400. [Google Scholar] [CrossRef]
- Mitra, S.; Pramanik, A.; Banerjee, S.; Haldar, S.; Gachhui, R.; Mukherjee, J. Enhanced biotransformation of fluoranthene by intertidally derived Cunninghamella elegans under biofilm-based and niche-mimicking conditions. Appl. Environ. Microbiol. 2013, 79, 7922–7930. [Google Scholar] [CrossRef]
- Chun, S.C.; Muthu, M.; Hasan, N.; Tasneem, S.; Gopal, J. Mycoremediation of PCBs by Pleurotus ostreatus: Possibilities and prospects. Appl. Sci. 2019, 9, 4185. [Google Scholar] [CrossRef]
- Kordon, K.; Mikolasch, A.; Schauer, F. Oxidative dehalogenation of chlorinated hydroxybiphenyls by laccases of white-rot fungi. Int. Biodeterior. Biodegrad. 2010, 64, 203–209. [Google Scholar] [CrossRef]
- Germain, J.; Raveton, M.; Binet, M.N.; Mouhamadou, B. Screening and metabolic potential of fungal strains isolated from contaminated soil and sediment in the polychlorinated biphenyl degradation. Ecotoxicol. Environ. Saf. 2021, 208, 111703. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef] [PubMed]
- Velkova, Z.; Lazarova, K.; Kirova, G.; Gochev, V. Recent advances in pharmaceuticals biosorption on microbial and algal-derived biosorbents. Processes 2025, 13, 561. [Google Scholar] [CrossRef]
- Marchetto, F.; Roverso, M.; Righetti, D.; Bogialli, S.; Filippini, F.; Bergantino, E.; Sforza, E. Bioremediation of per- and polyfluoroalkyl substances (PFAS) by Synechocystis sp. PCC 6803: A chassis for a synthetic biology approach. Life 2021, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shang, Z.; Ma, Y.; Zhao, H.; Ni, Z.; Wei, Z.; Zhang, X. Tolerance mechanisms and removal efficiency of Chlorella pyrenoidosa in treating 3-fluorophenol pollution. Metabolites 2024, 14, 449. [Google Scholar] [CrossRef] [PubMed]
- Abate, R.; Oon, Y.S.; Oon, Y.L.; Bi, Y. Microalgae-bacteria nexus for environmental remediation and renewable energy resources: Advances, mechanisms and biotechnological applications. Heliyon 2024, 10, e31170. [Google Scholar] [CrossRef]
- Blair, E.M.; Dickson, K.L.; O’Malley, M.A. Microbial communities and their enzymes facilitate degradation of recalcitrant polymers in anaerobic digestion. Curr. Opin. Microbiol. 2021, 64, 100–108. [Google Scholar] [CrossRef]
- Niu, Q.; Lin, X.; Zheng, X.; Wu, Y.; Long, M.; Chen, Y. Aerobic or anaerobic? Microbial degradation of per- and polyfluoroalkyl substances: A review. J. Hazard. Mater. 2024, 480, 136173. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Field, J.A.; Luna-Velasco, A.; Sierra-Alvarez, R. Microbial toxicity and biodegradability of perfluorooctane sulfonate (PFOS) and shorter chain perfluoroalkyl and polyfluoroalkyl substances (PFASs). Environ. Sci. Process. Impacts 2016, 18, 1236–1246. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Application of microbial biofilms in biocatalysis and biodegradation. In Enzymes for Pollutant Degradation; Springer Nature: Singapore, 2022; pp. 93–118. [Google Scholar]
- Kouzuma, A.; Kato, S.; Watanabe, K. Microbial interspecies interactions: Recent findings in syntrophic consortia. Front. Microbiol. 2015, 6, 477. [Google Scholar] [CrossRef]
- Wackett, L.P. Nothing lasts forever: Understanding microbial biodegradation of polyfluorinated compounds and perfluorinated alkyl substances. Microb. Biotechnol. 2022, 15, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ma, A. Investigating the degradation potential of microbial consortia for perfluorooctane sulfonate through a functional "top-down" screening approach. PLoS ONE 2024, 19, e0303904. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Duarte, P.; Almeida, C.M.R.; Carvalho, M.F.; Mucha, A.P. Potential of bacterial consortia obtained from different environments for bioremediation of paroxetine and bezafibrate. J. Environ. Chem. Eng. 2020, 8, 103881. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, H.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B. Metagenomic analysis of soil microbial community under PFOA and PFOS stress. Environ. Res. 2020, 188, 109838. [Google Scholar] [CrossRef] [PubMed]
- Sorn, S.; Matsuura, N.; Honda, R. Metagenome-Assembled Genomes and Metatranscriptome Analysis of Perfluorooctane Sulfonate-Reducing Bacteria Enriched From Activated Sludge. Environ. Microbiol. 2025, 27, e70087. [Google Scholar] [CrossRef]
- Kurihara, T.; Esaki, N.; Soda, K. Bacterial 2-haloacid dehalogenases: Structures and reaction mechanisms. J. Mol. Catal. B Enzym. 2000, 10, 57–65. [Google Scholar] [CrossRef]
- Kurihara, T.; Esaki, N. Bacterial hydrolytic dehalogenases and related enzymes: Occurrences, reaction mechanisms, and applications. Chem. Rec. 2008, 8, 67–74. [Google Scholar] [CrossRef]
- O’Hagan, D.; Harper, D.B. Fluorine-containing natural products. J. Fluor. Chem. 1999, 100, 127–133. [Google Scholar] [CrossRef]
- Tonomura, K.; Futai, F.; Tanabe, O.; Yamaoka, T. Defluorination of monofluoroacetate by bacteria: Part I. Isolation of bacteria and their activity of defluorination. J. Agric. Food Chem. 1965, 29, 124–128. [Google Scholar] [CrossRef]
- Sota, M.; Endo, M.; Nitta, K.; Kawasaki, H.; Tsuda, M. Characterization of a class II defective transposon carrying two haloacetate dehalogenase genes from Delftia acidovorans plasmid pUO1. Appl. Environ. Microbiol. 2002, 68, 2307–2315. [Google Scholar] [CrossRef]
- Kurihara, T.; Yamauchi, T.; Ichiyama, S.; Takahata, H.; Esaki, N. Purification, characterization, and gene cloning of a novel fluoroacetate dehalogenase from Burkholderia sp. FA1. J. Mol. Catal. B Enzym. 2003, 23, 347–355. [Google Scholar] [CrossRef]
- Chan, W.Y.; Wong, M.; Guthrie, J.; Savchenko, A.V.; Yakunin, A.F.; Pai, E.F.; Edwards, E.A. Sequence- and activity-based screening of microbial genomes for novel dehalogenases. Microb. Biotechnol. 2010, 3, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.S.; Wu, J.Y.; Sun, M.; Zheng, Q.C.; Sun, C.C. Homology modeling and SN2 displacement reaction of fluoroacetate dehalogenase from Burkholderia sp. FA1. Biochem. Biophys. Res. Commun. 2004, 325, 414–420. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Kurihara, T.; Ichiyama, S.; Miyagi, M.; Tsunasawa, S.; Kawasaki, H.; Soda, K.; Esaki, N. Reaction mechanism of fluoroacetate dehalogenase from Moraxella sp. B. J. Biol. Chem. 1998, 273, 30897–30902. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, T.; Nakayama, T.; Shitamichi, O.; Jitsumori, K.; Kurihara, T.; Esaki, N.; Yoshizawa, K. The catalytic mechanism of fluoroacetate dehalogenase: A computational exploration of biological dehalogenation. Chemistry 2009, 15, 7394–7403. [Google Scholar] [CrossRef]
- Nakayama, T.; Kamachi, T.; Jitsumori, K.; Omi, R.; Hirotsu, K.; Esaki, N.; Kurihara, T.; Yoshizawa, K. Substrate specificity of fluoroacetate dehalogenase: An insight from crystallographic analysis, fluorescence spectroscopy, and theoretical computations. Chemistry 2012, 18, 8392–8402. [Google Scholar] [CrossRef]
- Schanstra, J.P.; Janssen, D.B. Kinetics of halide release of haloalkane dehalogenase: Evidence for a slow conformational change. Biochemistry 1996, 35, 5624–5632. [Google Scholar] [CrossRef]
- Krooshof, G.H.; Kwant, E.M.; Damborský, J.; Koca, J.; Janssen, D.B. Repositioning the catalytic triad aspartic acid of haloalkane dehalogenase: Effects on stability, kinetics, and structure. Biochemistry 1997, 36, 9571–9580. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yue, Y.; Zhang, H.; Yang, Z.; Wang, H.; Tian, S.; Wang, W. Harnessing fluoroacetate dehalogenase for defluorination of fluorocarboxylic acids: In silico and in vitro approach. Environ. Int. 2019, 131, 104999. [Google Scholar] [CrossRef]
- Sima, M.W.; Huang, S.; Jaffé, P.R. Modeling the kinetics of perfluorooctanoic and perfluorooctane sulfonic acid biodegradation by Acidimicrobium sp. Strain A6 during the feammox process. J. Hazard. Mater. 2023, 448, 130903. [Google Scholar] [CrossRef]
- Yue, Y.; Fan, J.; Xin, G.; Huang, Q.; Wang, J.B.; Li, Y.; Zhang, Q.; Wang, W. Comprehensive understanding of fluoroacetate dehalogenase-catalyzed degradation of fluorocarboxylic acids: A QM/MM approach. Environ Sci Technol. 2021, 55, 9817–9825. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.W.Y.; Chakrabarti, N.; Ing, C.; Halgas, O.; To, T.K.W.; Wälti, M.; Petit, A.-P.; Tran, C.; Savchenko, A.; Yakunin, A.F.; et al. Defluorination capability of L-2-haloacid dehalogenases in the HAD-like hydrolase superfamily correlates with active site compactness. Chembiochem 2022, 23, e202100414. [Google Scholar] [CrossRef]
- Lewis, M.; Kim, M.-H.; Wang, N.; Chu, K.-H. Engineering artificial communities for enhanced FTOH degradation. Sci. Total Environ. 2016, 572, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Kim, M.H.; Liu, E.J.; Wang, N.; Chu, K.-H. Biotransformation of 6:2 polyfluoroalkyl phosphates (6:2 PAPs): Effects of degradative bacteria and co-substrates. J. Hazard. Mater. 2016, 320, 479–486. [Google Scholar] [CrossRef]
- Farajollahi, S.; Lombardo, N.V.; Crenshaw, M.D.; Guo, H.B.; Doherty, M.E.; Davison, T.R.; Steel, J.J.; Almand, E.A.; Varaljay, V.A.; Suei-Hung, C.; et al. Defluorination of organofluorine compounds using dehalogenase enzymes from Delftia acidovorans (D4B). ACS Omega 2024, 9, 28546–28555. [Google Scholar] [CrossRef]
- Oyewusi, H.A.; Wahab, R.A.; Huyop, F. Dehalogenase-producing halophiles and their potential role in bioremediation. Mar. Pollut. Bull. 2020, 160, 111603. [Google Scholar] [CrossRef]
- Müller, J.A.; Rosner, B.M.; Von Abendroth, G.; Meshulam-Simon, G.; McCarty, P.L.; Spormann, A.M. Molecular identification of the catabolic vinyl chloride reductase from Dehalococcoides sp. strain VS and its environmental distribution. Appl. Environ. Microbiol. 2004, 70, 4880–4888. [Google Scholar] [CrossRef]
- Hug, L.A.; Maphosa, F.; Leys, D.; Löffler, F.E.; Smidt, H.; Edwards, E.A.; Adrian, L. Overview of organohalide-respiring bacteria and a proposal for a classification system for reductive dehalogenases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120322. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, T.; Fisher, K.; Payne, K.A.P.; Rigby, S.E.J.; Leys, D. Catabolic reductive dehalogenase substrate complex structures underpin rational repurposing of substrate scope. Microorganisms 2020, 8, 1344. [Google Scholar] [CrossRef]
- Kunze, C.; Bommer, M.; Hagen, W.R.; Uksa, M.; Dobbek, H.; Schubert, T.; Diekert, G. Cobamide-mediated enzymatic reductive dehalogenation via long-range electron transfer. Nat. Commun. 2017, 8, 15858. [Google Scholar] [CrossRef]
- Parsons, J.R.; Sáez, M.; Dolfing, J.; de Voogt, P. Biodegradation of perfluorinated compounds. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2008; Volume 196, pp. 53–71. [Google Scholar]
- Liu, J.; Van Hoomissen, D.J.; Liu, T.; Maizel, A.; Huo, X.; Fernández, S.R.; Ren, C.; Xiao, X.; Fang, Y.; Schaefer, C.E.; et al. Reductive defluorination of branched per- and polyfluoroalkyl substances with cobalt complex catalysts. Environ. Sci. Technol. Lett. 2018, 5, 289–294. [Google Scholar] [CrossRef]
- Park, S.; de Perre, C.; Lee, L.S. Alternate reductants with VB12 to transform C8 and C6 perfluoroalkyl sulfonates: Limitations and insights into isomer-specific transformation rates, products and pathways. Environ. Sci. Technol. 2017, 51, 13869–13877. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.K.; Webb, R.I.; Sly, L.I.; Denman, S.E.; McSweeney, C.S. Isolation and survey of novel fluoroacetate-degrading bacteria belonging to the phylum Synergistetes. FEMS Microbiol. Ecol. 2012, 80, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jaffé, P.R. Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef]
- Guo, H.-B.; Varaljay, V.A.; Kedziora, G.; Taylor, K.; Farajollahi, S.; Lombardo, N.; Harper, E.; Hung, C.; Gross, M.; Perminov, A.; et al. Accurate prediction by AlphaFold2 for ligand binding in a reductive dehalogenase and implications for PFAS biodegradation. Sci. Rep. 2023, 13, 4082. [Google Scholar]
- Bommer, M.; Kunze, C.; Fesseler, J.; Schubert, T.; Diekert, G.; Dobbek, H. Structural basis for organohalide respiration. Science 2014, 346, 455–458. [Google Scholar] [CrossRef]
- Yu, Y.; Che, S.; Ren, C.; Jin, B.; Tian, Z.; Liu, J.; Men, Y. Microbial defluorination of unsaturated per- and polyfluorinated carboxylic acids under anaerobic and aerobic conditions: A structure specificity study. Environ. Sci. Technol. 2022, 56, 4894–4904. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases in biotechnology and synthetic biology. Trends Biotechnol. 2019, 37, 882–897. [Google Scholar] [CrossRef]
- Guengerich, F.P. Intersection of the roles of cytochrome P450 enzymes with xenobiotic and endogenous substrates: Relevance to toxicity and drug interactions. Chem. Res. Toxicol. 2017, 30, 2–12. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Zhang, M.; Zhu, Y.; Zhuo, R. Removal of heavy-metal pollutants by white rot fungi: Mechanisms, achievements, and perspectives. J. Clean. Prod. 2022, 354, 131681. [Google Scholar] [CrossRef]
- Gangola, S.; Joshi, S.; Kumar, S.; Pandey, S.C. Comparative analysis of fungal and bacterial enzymes in biodegradation of xenobiotic compounds. In Smart Bioremediation Technologies; Bhatt, P., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 169–189. [Google Scholar]
- Durairaj, P.; Hur, J.-S.; Yun, H. Versatile biocatalysis of fungal cytochrome P450 monooxygenases. Microb. Cell Fact. 2016, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Murphy, C.D. Nitroreduction of flutamide by Cunninghamella elegans NADPH: Cytochrome P450 reductase. Biochem. Biophys. Rep. 2022, 29, 101209. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, L.; Cai, J.; Xu, Z.; Lian, J. Functional expression of eukaryotic cytochrome P450s in yeast. Biotechnol. Bioeng. 2021, 118, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Theron, C.W.; Labuschagné, M.; Gudiminchi, R.; Albertyn, J.; Smit, M.S. A broad-range yeast expression system reveals Arxula adeninivorans expressing a fungal self-sufficient cytochrome P450 monooxygenase as an excellent whole-cell biocatalyst. FEMS Yeast Res. 2014, 14, 556–566. [Google Scholar] [CrossRef]
- Permana, D.; Niesel, K.; Ford, M.J.; Ichinose, H. Latent functions and applications of cytochrome P450 monooxygenases from Thamnidium elegans: A novel biocatalyst for 14α-hydroxylation of testosterone. ACS Omega 2022, 7, 13932–13941. [Google Scholar] [CrossRef]
- Kharasch, E.D.; Thummel, K.E. Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology 1993, 79, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Harkey, A.; Kim, H.-J.; Kandagatla, S.; Raner, G.M. Defluorination of 4-fluorophenol by cytochrome P450(BM3)-F87G: Activation by long chain fatty aldehydes. Biotechnol. Lett. 2012, 34, 1725–1731. [Google Scholar] [CrossRef]
- Li, S.; Wackett, L.P. Reductive dehalogenation by cytochrome P450CAM: Substrate binding and catalysis. Biochemistry 1993, 32, 9355–9361. [Google Scholar] [CrossRef]
- Yang, S.H.; Shi, Y.; Strynar, M.; Chu, K.H. Desulfonation and defluorination of 6:2 fluorotelomer sulfonic acid (6:2 FTSA) by Rhodococcus jostii RHA1: Carbon and sulfur sources, enzymes, and pathways. J. Hazard. Mater. 2022, 423, 127052. [Google Scholar] [CrossRef]
- Merino, N.; Wang, N.; Gao, Y.; Wang, M.; Mahendra, S. Roles of various enzymes in the biotransformation of 6:2 fluorotelomer alcohol (6:2 FTOH) by a white-rot fungus. J. Hazard. Mater. 2023, 450, 131007. [Google Scholar] [CrossRef]
- Wang, V.C.C.; Maji, S.; Chen, P.P.Y.; Lee, H.K.; Yu, S.S.F.; Chan, S.I. Alkane oxidation: Methane monooxygenases, related enzymes, and their biomimetics. Chem. Rev. 2017, 117, 8574–8621. [Google Scholar] [CrossRef] [PubMed]
- Sluis, M.K.; Sayavedra-Soto, L.A.; Arp, D.J. Molecular analysis of the soluble butane monooxygenase from ‘Pseudomonas butanovora’. Microbiology 2002, 148, 3617–3629. [Google Scholar] [CrossRef]
- Halsey, K.H.; Doughty, D.M.; Sayavedra-Soto, L.A.; Bottomley, P.J.; Arp, D.J. Evidence for modified mechanisms of chloroe-thene oxidation in Pseudomonas butanovora mutants containing single amino acid substitutions in the hydroxylase α-subunit of butane monooxygenase. J. Bacteriol. 2007, 189, 5068–5074. [Google Scholar] [CrossRef]
- Takahashi, J. Production of intracellular and extracellular protein from n-butane by Pseudomonas butanovora sp. nov. In Advances in Applied Microbiology, 1st ed.; Perlman, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1980; Volume 26, pp. 117–127. [Google Scholar]
- Tsai, Y.F.; Luo, W.I.; Chang, J.L.; Chang, C.W.; Chuang, H.C.; Ramu, R.; Wei, G.T.; Zen, J.M.; Yu, S.S.F. Electrochemical hydroxylation of C3–C12 n-alkanes by recombinant alkane hydroxylase (AlkB) and rubredoxin-2 (AlkG) from Pseudomonas putida GPo1. Sci. Rep. 2017, 7, 8369. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Wang, N.; McDonald, T.; Chu, K.H. Biodefluorination and biotransformation of fluorotelomer alcohols by two alkane-degrading Pseudomonas strains. Biotechnol. Bioeng. 2012, 109, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Folsom, P.W.; Wolstenholme, B.W.; Sun, H.; Wang, N.; Buck, R.C. 6:2 fluorotelomer alcohol biotransformation in an aerobic river sediment system. Chemosphere 2013, 90, 203–209. [Google Scholar] [CrossRef]
- Porter, J.L.; Boon, P.L.; Murray, T.P.; Huber, T.; Collyer, C.A.; Ollis, D.L. Directed evolution of new and improved enzyme functions using an evolutionary intermediate and multidirectional search. ACS Chem. Biol. 2015, 10, 611–621. [Google Scholar] [CrossRef]
- Bansal, N.; Kanwar, S.S. Peroxidase(s) in environment protection. Sci. World J. 2013, 2013, 714639. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Falade, A.O.; Nwodo, U.U.; Iweriebor, B.C.; Green, E.; Mabinya, L.V.; Okoh, A.I. Lignin peroxidase functionalities and prospective applications. MicrobiologyOpen 2017, 6, e00394. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Environmental remediation by novel nanomaterials and fungi with high-degradation capacity of hazardous contaminants. In Bio and Nanoremediation of Hazardous Environmental Pollutants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023; pp. 283–310. [Google Scholar]
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging contaminants of high concern and their enzyme-assisted biodegradation—A review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Y.; Sastri, C.V.; de Visser, S.P. Defluorination of fluorophenols by a heme dehaloperoxidase: Insights into the defluorination mechanism. ACS Catal. 2025, 15, 3898–3912. [Google Scholar] [CrossRef]
- Son, H.; Seo, H.; Han, S.; Kim, S.M.; Khan, M.F.; Sung, H.J.; Kang, S.H.; Kim, K.J.; Kim, Y.H. Extra disulfide and ionic salt bridge improves the thermostability of lignin peroxidase H8 under acidic condition. Enzym. Microb. Technol. 2021, 148, 109803. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shah, K.; Kwok, I.; Wang, M.; Rome, L.H.; Mahendra, S. Immobilized fungal enzymes: Innovations and potential applications in biodegradation and biosynthesis. Biotechnol. Adv. 2022, 57, 107936. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Kaur, N.; Selvaraj, M.; Ghramh, H.A.; Al-Shehri, B.M.; Singh, G.; Arya, S.K.; Bhatt, K.; Ghotekar, S.; Mani, R.; et al. Laccase-assisted degradation of emerging recalcitrant compounds—A review. Bioresour. Technol. 2022, 364, 128031. [Google Scholar] [CrossRef]
- Tromp, S.A.; Matijošytė, I.; Sheldon, R.A.; Arends, I.W.C.E.; Mul, G.; Kreutzer, M.T.; Moulijn, J.A.; de Vries, S. Mechanism of laccase–TEMPO-catalyzed oxidation of benzyl alcohol. ChemCatChem 2010, 2, 827–833. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. TrAC Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Ashraf, S.S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Zhang, H.; Wang, Z.; Feng, M.; Chiang, S.-Y.D.; Woodward, D.; Huang, Q. Laccase-catalyzed degradation of perfluorooctanoic acid. Environ. Sci. Technol. Lett. 2015, 2, 198–203. [Google Scholar] [CrossRef]
- Luo, Q.; Yan, X.; Lu, J.; Huang, Q. Perfluorooctanesulfonate degrades in a laccase-mediator system. Environ. Sci. Technol. 2018, 52, 10617–10626. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, Z.; Feng, M.; Chiang, D.; Woodward, D.; Liang, S.; Lu, J.; Huang, Q. Factors controlling the rate of perfluorooctanoic acid degradation in laccase-mediator systems: The impact of metal ions. Environ. Pollut. 2017, 224, 649–657. [Google Scholar] [CrossRef]
- Luo, Q.; Liang, S.; Huang, Q. Laccase induced degradation of perfluorooctanoic acid in a soil slurry. J. Hazard. Mater. 2018, 359, 241–247. [Google Scholar] [CrossRef]
- Kurniati, A.; Puspaningsih, N.N.T.; Putri, K.D.A.; Damayanti, M.; Purwani, N.N.; Rahmah, S.A.; Fujiyama, K.; Sakka, M.; Sakka, K.; Kimura, T.; et al. Heterologous fusion gene expression and characterization of a novel carbohydrate binding module (Cbm36) to laccase (Lcc2). Biocatal. Agric. Biotechnol. 2022, 42, 102377. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Bai, Y.; Li, Y.; Tang, T.; Liang, H.; Gao, D. Immobilized enzyme with sustainable chestnut biochar to remediate polycyclic aromatic hydrocarbons contaminated soils. Environ. Technol. 2024, 45, 2034–2044. [Google Scholar] [CrossRef]
- Grgas, D.; Petrina, A.; Štefanac, T.; Bešlo, D.; Landeka Dragičević, T. A review: Per- and polyfluoroalkyl substances—Biological degradation. Toxics 2023, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Griffith, W.P.; Davis, I.; Shin, I.; Wang, J.; Li, F.; Wang, Y.; Wherritt, D.J.; Liu, A. Cleavage of a carbon-fluorine bond by an engineered cysteine dioxygenase. Nat. Chem. Biol. 2018, 14, 853–860. [Google Scholar] [CrossRef]
- McCoy, J.G.; Bailey, L.J.; Bitto, E.; Bingman, C.A.; Aceti, D.J.; Fox, B.G.; Phillips, G.N. Structure and mechanism of mouse cysteine dioxygenase. Proc. Natl. Acad. Sci. USA 2006, 103, 3084–3089. [Google Scholar] [CrossRef]
- Liu, L.; Mi, H.; Zhang, M.; Sun, F.; Zhan, R.; Zhao, H.; He, S.; Zhou, L. Efficient moxifloxacin degradation by CoFe2O4 magnetic nanoparticles activated peroxymonosulfate: Kinetics, pathways and mechanisms. Chem. Eng. J. 2021, 407, 127201. [Google Scholar] [CrossRef]
- Kang, Y.; Li, M.; Guo, Z.; Zhang, Y.; Li, W.; Wu, H.; Zhang, J. Effect of electron shuttles on typical perfluoroalkyl substance removal via iron oxide reduction in wetland sediment. J. Clean. Prod. 2022, 365, 132821. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Biswas, J.K.; Datta, R. Biodegradation of per-and polyfluoroalkyl substances (PFAS): A review. Bioresour. Technol. 2022, 344, 126223. [Google Scholar] [CrossRef] [PubMed]
- Stockbridge, R.B.; Wackett, L.P. The link between ancient microbial fluoride resistance mechanisms and bioengineering organofluorine degradation or synthesis. Nat. Commun. 2024, 15, 4593. [Google Scholar] [CrossRef]
- McIlwain, B.C.; Ruprecht, M.T.; Stockbridge, R.B. Membrane exporters of fluoride ion. Annu. Rev. Biochem. 2021, 90, 559–579. [Google Scholar] [CrossRef]
- Stockbridge, R.B.; Lim, H.H.; Otten, R.; Williams, C.; Shane, T.; Weinberg, Z.; Miller, C. Fluoride resistance and transport by riboswitch-controlled CLC antiporters. Proc. Natl. Acad. Sci. USA 2012, 109, 15289–15294. [Google Scholar] [CrossRef] [PubMed]
- Stockbridge, R.B.; Robertson, J.L.; Kolmakova-Partensky, L.; Miller, C. A family of fluoride-specific ion channels with dual-topology architecture. eLife 2013, 2, e01084. [Google Scholar] [CrossRef]
- Mukherjee, S.; Seshadri, R.; Varghese, N.J.; Eloe-Fadrosh, E.A.; Meier-Kolthoff, J.P.; Göker, M.; Coates, R.C.; Hadjithomas, M.; Pavlopoulos, G.A.; Paez-Espino, D.; et al. 1003 reference genomes of bacterial and archaeal isolates expand coverage of the tree of life. Nat. Biotechnol. 2017, 35, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Calero, P.; Volke, D.C.; Lowe, P.T.; Gotfredsen, C.H.; O’Hagan, D.; Nikel, P.I. A fluoride-responsive genetic circuit enables in vivo biofluorination in engineered Pseudomonas putida. Nat. Commun. 2020, 11, 5045. [Google Scholar] [CrossRef]
- Flournoy, D.S.; Frey, P.A. Pyruvate dehydrogenase and 3-fluoropyruvate: Chemical competence of 2-acetylthiamin pyrophosphate as an acetyl group donor to dihydrolipoamide. Biochemistry 1986, 25, 6036–6043. [Google Scholar] [CrossRef]
- Kertesz, M.A. Riding the sulfur cycle—Metabolism of sulfonates and sulfate esters in Gram-negative bacteria. FEMS Microbiol. Rev. 2000, 24, 135–175. [Google Scholar]
- Ellis, H.R. Mechanism for sulfur acquisition by the alkanesulfonate monooxygenase system. Bioorg. Chem. 2011, 39, 178–184. [Google Scholar] [CrossRef]
- Marciesky, M.; Aga, D.S.; Bradley, I.M.; Aich, N.; Ng, C. Mechanisms and opportunities for rational in silico design of enzymes to degrade per-and polyfluoroalkyl substances (PFAS). J. Chem. Inf. Model. 2023, 63, 7299–7319. [Google Scholar] [CrossRef] [PubMed]
- Mekureyaw, M.F.; Junker, A.L.; Bai, L.; Zhang, Y.; Wei, Z.; Guo, Z. Laccase based per-and polyfluoroalkyl substances degradation: Status and future perspectives. Water Res. 2025, 271, 122888. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Tiwari, A.; Anisha, G.S.; Chen, C.W.; Singh, A.; Haldar, D.; Patel, A.K.; Singhania, R.R. Laccase: A potential biocatalyst for pollutant degradation. Environ. Pollut. 2023, 319, 120999. [Google Scholar] [CrossRef]
- Khersonsky, O.; Lipsh, R.; Avizemer, Z.; Ashani, Y.; Goldsmith, M.; Leader, H.; Dym, O.; Rogotner, S.; Trudeau, D.L.; Prilusky, J.; et al. Automated design of efficient and functionally diverse enzyme repertoires. Mol. Cell 2018, 72, 178–186.e5. [Google Scholar] [CrossRef]
- Goldenzweig, A.; Fleishman, S.J. Principles of protein stability and their application in computational design. Annu. Rev. Biochem. 2018, 87, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Wackett, L.P.; Robinson, S.L. A prescription for engineering PFAS biodegradation. Biochem. J. 2024, 481, 1757–1770. [Google Scholar] [CrossRef]
- Bygd, M.D.; Aukema, K.G.; Richman, J.E.; Wackett, L.P. Microwell fluoride screen for chemical, enzymatic, and cellular reactions reveals latent microbial defluorination capacity for –CF groups. Appl. Environ. Microbiol. 2022, 88, e0028822. [Google Scholar] [CrossRef]
- Husser, C.; Vuilleumier, S.; Ryckelynck, M. Fluormango, an RNA-based fluorogenic biosensor for the direct and specific detection of fluoride. Small 2023, 19, e2205232. [Google Scholar] [CrossRef]
- Mazurenko, S.; Prokop, Z.; Damborsky, J. Machine learning in enzyme engineering. ACS Catal. 2019, 10, 1210–1223. [Google Scholar] [CrossRef]
- Nayak, S.K.; Yamijala, S.S. Computing accurate bond dissociation energies of emerging per-and polyfluoroalkyl substances: Achieving chemical accuracy using connectivity-based hierarchy schemes. J. Hazard. Mater. 2024, 468, 133804. [Google Scholar] [CrossRef]
- Harris, B.A.; Zhou, J.; Clarke, B.O.; Leung, I.K. Enzymatic degradation of PFAS: Current status and ongoing challenges. ChemSusChem 2025, 18, e202401122. [Google Scholar] [CrossRef]
- Rust, S.; Randau, L. Golden Gate Cloning of Synthetic CRISPR RNA Spacer Sequences. In Golden Gate Cloning: Methods and Protocols; Springer: New York, NY, USA, 2024; pp. 297–306. [Google Scholar]
- Allred, B.M.; Lang, J.R.; Barlaz, M.A.; Field, J.A. Physical and biological release of poly- and perfluoroalkyl substances (PFASs) from municipal solid waste in anaerobic model landfill reactors. Environ. Sci. Technol. 2015, 49, 7648–7656. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.N.; Jilani, M.I.; Imtiaz, F.; Ahmed, T.; Arshad, M.B.; Mudassar, M.; Sharif, M.N. Advances in the removal of polyfluoroalkyl substances (PFAS) from water using destructive and non-destructive methods. Green. Anal. Chem. 2025, 12, 100225. [Google Scholar] [CrossRef]
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response: A review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef]
- Carvalho, M.F.; Alves, C.C.T.; Ferreira, M.I.M.; De Marco, P.; Castro, P. Isolation and initial characterization of a bacterial consortium able to mineralize fluorobenzene. Appl. Environ. Microbiol. 2002, 68, 102–105. [Google Scholar] [CrossRef]
- Brase, R.A.; Mullin, E.J.; Spink, D.C. Legacy and emerging per- and polyfluoroalkyl substances: Analytical techniques, environmental fate, and health effects. Int. J. Mol. Sci. 2021, 22, 995. [Google Scholar] [CrossRef] [PubMed]
- Garavagno, M.d.l.A.; Holland, R.; Khan, M.A.H.; Orr-Ewing, A.J.; Shallcross, D.E. Trifluoroacetic Acid: Toxicity, Sources, Sinks and Future Prospects. Sustainability 2024, 16, 2382. [Google Scholar] [CrossRef]
- Howard, S.A.; McCarthy, R.R. Modulating biofilm can potentiate activity of novel plastic-degrading enzymes. NPJ Biofilms Microbiomes 2023, 9, 72. [Google Scholar] [CrossRef]
- Wackett, L.P. Confronting PFAS persistence: Enzymes catalyzing C–F bond cleavage. Trends Biochem. Sci. 2024, 49, 71–83. [Google Scholar] [CrossRef]
- Hvizdak, M.; Kandel, S.E.; Work, H.M.; Gracey, E.G.; McCullough, R.L.; Lampe, J.N. Per- and polyfluoroalkyl substances (PFAS) inhibit cytochrome P450 CYP3A7 through direct coordination to the heme iron and water displacement. J. Inorg. Biochem. 2023, 240, 112120. [Google Scholar] [CrossRef]
- Waugh, D.T. Fluoride exposure induces inhibition of sodium- and potassium-activated adenosine triphosphatase (Na+, K+-ATPase) enzyme activity: Molecular mechanisms and implications for public health. Int. J. Environ. Res. Public Health 2019, 16, 1427. [Google Scholar] [CrossRef] [PubMed]
- Al-Maqdi, K.A.; Elmerhi, N.; Athamneh, K.; Bilal, M.; Alzamly, A.; Ashraf, S.S.; Shah, I. Challenges and recent advances in enzyme-mediated wastewater remediation—A review. Nanomaterials 2021, 11, 3124. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Microbial Defluorination | Physicochemical Treatments |
|---|---|---|
| Mechanism | Enzymatic C–F cleavage occurs via hydrolytic (haloacid dehalogenases from Burkholderia sp. and Streptomyces; fluoroacetate), reductive (reductive dehalogenases from Cloacibacillus and Dehalococcoides; PFOA), oxidative (cytochrome P450s from Thamnidium and Cunninghamella; fluorobenzene), and radical-mediated (peroxidases and laccases from Phanerochaete and Pleurotus; fluoxetine) pathways | Advanced oxidation (UV, ozone, and persulfate), pyrolysis, UV photolysis, and plasma treatment |
| Target Specificity | High; enzyme-substrate specificity allows for targeted degradation (e.g., laccases in Pleurotus ostreatus active on fluorinated phenols) | Moderate; broad-spectrum but non-specific |
| Scalability | Moderate; requires controlled bioreactor conditions, strain enrichment, and biofilm optimisation | High; widely used in industrial-scale wastewater treatment and soil remediation |
| Cost-Effectiveness | Generally low-cost; uses renewable inputs, but may incur time and optimisation costs for strain development and process integration | High cost; requires significant energy and chemical inputs |
| Environmental Impact | Low; biologically mediated, minimal secondary pollution, and manageable fluoride release | Risk of forming toxic by-products and greenhouse gases |
| Degradation Rate | Slower; it often ranges from days to weeks, depending on the organism and conditions | Rapid (minutes to hours), though sometimes incomplete |
| By-Product Toxicity | Low; fluoride ion is the main by-product, which can be managed biologically | Variable; potentially toxic intermediates may form |
| Adaptability to Mixed Pollutants | High; microbial consortia (e.g., enriched sludge with alkane sulfonate monooxygenase and cytochrome P450—PFOS degradation) allow for flexibility and syntrophic interactions | Limited; often requires sequential or combined treatments |
| Limitations | Requires microbial adaptation, pathway elucidation, and system engineering; uncultivated species and enzymes remain poorly characterised | High energy demand, incomplete mineralisation, and limited selectivity |
| Example Applications | Bioreactors, constructed wetlands, and engineered consortia for PFAS/pharmaceutical removal | Industrial oxidation units, incineration plants, and chemical reactors |
| Reference | [16,17,18,19] | [13,14,15] |
| Feature | Reductive Defluorination | Hydrolytic Defluorination | Oxidative Defluorination |
|---|---|---|---|
| Basic mechanism | Electron- and proton-mediated replacement of F with H | Nucleophilic substitution of F by OH− or H2O | Attack by reactive oxygen species (ROS) or oxidants, leading to cleavage of C–F |
| Type of reaction | Redox (reduction) | Hydrolysis (nucleophilic substitution) | Redox (oxidation) |
| Electron flow | Electron gain at carbon (reduction) | No net redox change | Electron loss from carbon (oxidation) |
| Typical conditions | Anaerobic/reducing (e.g., presence of H2 or electron donors) | Mild aqueous or basic conditions, sometimes enzymatic | Aerobic/oxidative (e.g., presence of H2O2, ROS, or photolysis) |
| Catalysts/enzymes | Reductive dehalogenases, metal catalysts (e.g., Pd/C and Ni), and microbes like Dehalococcoides | Defluorinases, hydrolytic enzymes, and strong bases | Peroxidases (e.g., lignin peroxidase and Mn peroxidase), Fenton reagent, laccases, and photocatalysts |
| By-products | HF (gas or aqueous), alkane, or partially defluorinated hydrocarbons | F− (aqueous), alcohols, or carboxylic acids | F− and oxidised intermediates (e.g., aldehydes, ketones, and acids) |
| Environmental relevance | Anaerobic biodegradation (e.g., groundwater and sediments) | Biodegradation by specific microbes in aerobic/neutral pH conditions | Abiotic or enzymatic degradation under aerobic/oxidative conditions |
| Rate and relative efficiency | Typically slow; depends on electron donor availability and anaerobic conditions; moderate to low efficiency | Moderate; enzyme-dependent with limited substrate scope and availability | Broad substrate range with fast degradation rates, but potentially toxic by-products |
| Substrate preference | Perfluoroalkyls with terminal halogens or activated groups | Fluorinated aromatics and short-chain fluorinated carboxylates | Perfluorinated carboxylic/sulfonic acids and fluorinated aromatics |
| Limitations | Slow reaction rates; requires strict anaerobic conditions; limited knowledge of many reductive enzymes | Narrow substrate specificity; enzyme stability issues; less effective for highly fluorinated compounds | Possible formation of toxic by-products; high energy input; not always fully mineralising |
| Typical applications | Anaerobic bioreactors for halogenated organics and PFAS remediation | Enzymatic treatment of mild fluorinated pollutants; biocatalysis in wastewater treatment | Advanced oxidation processes (AOPs) and photolytic degradation in water treatment and soil remediation |
| Examples | - Dehalococcoides reducing C–F in fluoroacetate | - Enzymatic hydrolysis of fluoroacetate | - Laccase-mediator oxidation of fluorophenols |
| - Ni-based catalysts in PFAS | - Base-catalysed cleavage of aryl-F | - H2O2/UV treatment of PFOA | |
| References | [43,44,45,46,47,51] | [17,43,48] | [49,50,51] |
| Compound | Bacterial Strain(s) | Degradation Outcome | Reference |
|---|---|---|---|
| PFAS (per- and polyfluoroalkyl substances) | |||
| Perfluorooctanoic acid (PFOA) | Pseudomonas parafulva strain YAB1 | Partial defluorination (48%) | [72] |
| Perfluorooctane sulfonate (PFOS) | Pseudomonas aeruginosa strain HJ4 | Minimal biodegradation | [73] |
| 6:2 Fluorotelomersulfonic acid (6:2 FTSA) | Gordonia sp. strain NB4-1Y | Degradation up to ∼88% | [74] |
| 6:2 Fluorotelomer alcohol (6:2 FTOH) | Pseudomonas fluorescens DSM 83413, Pseudomonas butanovora, Pseudomonas oleovorans, Mycobacterium vaccae JOB5 | Degradation up to ∼80–100% | [75] |
| Fluorinated pharmaceuticals | |||
| Fluoxetine (Prozac) | Bacillus subtilis DSM 3477, Comamonas testosteroni DSM 12678, Pseudomonas knackmussii B-13 DSM 6978 | Produce trifluoroacetic acid and fluoride ion | [21] |
| Ciprofloxacin, ofloxacin, norfloxacin, and enrofloxacin | Thermus thermophilus strain C419 | Attenuated antibacterial activity | [76] |
| Fluorouracil (5-FU) | Vibrio fischeri | Via uracil metabolism | [77] |
| Flurbiprofen | Streptomyces griseus DSM40236 and ATCC13273, and Streptomyces subrutilis DSM40445 Bacillus subtilis IM7, Bacillus megaterium NCIMB8291 and B. megaterium ATTC14581 | Produce hydroxylated and amidated metabolites | [78] |
| Flutamide | Rhodotorula mucilaginosa ATCC 20129 | Produce three metabolites via hydrolysis, nitroreduction, and N-acetylation | [79] |
| Fluorinated pesticides and agrochemicals | |||
| Trifluralin | Bacillus sp. TF-1 and Arthrobacter aurescens CTFL7 | Biodegradation up to 88% | [80] |
| β-cyfluthrin and λ-cyhalothrin | Bacillus sp. MFK14 | Fluoride from β-cyfluthrin and TFA from λ-cyhalothrin are end-products. | [81] |
| Acifluorfen | Bacillus sp. Za | Aminoacifluorfen is produced as a non-toxic end-product | [82] |
| Fomesafen | Lysinibacillus sp. ZB-1 | Degradation up to 81.32% | [83] |
| Oxyfluorfen | Sphingomonas wittichii RW1 | Degradation up to 75% | [84] |
| Isopyrazam | Xanthomonas axonopodis and Pseudomonas syringae | Degradation up to 80% and 86%, respectively | [85] |
| Fluorinated industrial compounds and intermediates | |||
| Fluorobenzene and 3-fluorocatechol | Burkholderia fungorum FLU100 | Complete metabolism via 2-fluoromuconate pathway | [86] |
| 4-Fluorobenzoate | Pseudomonas knackmussii B-13 DSM 6978 | Conversion to 4-fluorocatechol | [87] |
| 4-Fluorobenzaldehyde | Arthrobacter sp. strain G1 and Ralstonia sp. strain H1 | Degradation via ortho-cleavage pathway | [88] |
| Feature | Haloacid Dehalogenases | Reductive Dehalogenases | Cytochrome P450s and Monooxygenases | Oxidative Enzymes (Peroxidases and Laccases) |
|---|---|---|---|---|
| Examples | L-haloacid dehalogenases D-haloacid dehalogenases DL-2-haloacid dehalogenases Fluoroacetate dehalogenases (FA1, H-1, RPA1163, POL0530) | T7RdhA RDase A6RdhA RDase Other RDases | CYP5208A3 P450BM3-F87G P450CAM Alkane monooxygenase Butane monooxygenase | Peroxidases (lignin peroxidase, manganese peroxidase, versatile peroxidase) Laccases (multi-copper oxidases) |
| Mechanism | SN2 hydrolysis via enzyme-ester intermediate | Reductive cleavage via cobalamin + [4Fe–4S] clusters | Monooxygenation via Compound I (Fe4+=O), hydroxylation via electron transfer | Electron transfer oxidations, radical generation, substrate oxidation |
| Microbial sources | Streptomyces cattleya Pseudomonas spp. Serratia liquefaciens Delftia acidovorans Burkholderia sp. FA1 Rhodopseudomonas palustris Polaromonas sp. Rhodococcus jostii RHA1 | Cloacibacillus porcorum Acidimicrobium sp. A6 Dehalococcoides-containing consortium KB1 | Thamnidium elegans Cunninghamella elegans Phanerochaete chrysosporium Pseudomonas putida Bacillus megaterium Pseudomonas butanovora | Phanerochaete chrysosporium Pleurotus ostreatus Pycnoporus sp. SYBC-L3 Trametes versicolor Bacteria Plants |
| Substrate scope and examples | Fluoroacetate 2-FPA Difluoroacetate 6:2 FTOH 6:2 PAPs | MFA TFA PFOA PFOS C6–C8 unsaturated PFAS | 6:2 FTOH 6:2 FTSA 4-Fluorophenol Alkanes (C2–C12) fluorotelomer alcohols | Aromatic compounds Phenolics PFOA PFOS Fluorinated phenols |
| PFAS relevance/key findings | Demonstrated defluorination of fluoroacetate and analogues Enzyme engineering (e.g., in E. coli) shows PFOA defluorination potential | Emerging evidence—that vitamin B12 stimulation enhances PFAS defluorination—indirect links to RDases Some stoichiometric MFA defluorination | Confirmed defluorination of 6:2 FTOH and FTSA Potential co-metabolic PFAS transformation due to substrate similarity | Some evidence of PFAS precursor oxidation Partial defluorination via radical attack Enhances biodegradation with mediators |
| References | [140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160] | [161,162,163,164,165,166,167,168,169,170,171,172] | [173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194] | [195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213] |
| Challenge | Description | Possible Solutions/Alternatives | Reference |
|---|---|---|---|
| Microbial defluorination | |||
| High stability of the C–F bond | Exceptional bond strength makes microbial cleavage thermodynamically unfavourable | Use engineered or naturally evolved enzymes (e.g., dehalogenases and cytochrome P450s); apply redox mediators or co-substrates to drive reaction thermodynamics | [27,28] |
| Fluoride toxicity | Released fluoride ions inhibit microbial growth and metabolism | Engineer fluoride-exporting proteins or fluoride-resistant strains; integrate fluoride-trapping materials (e.g., calcium salts and biochar) in bioreactors | [89,232] |
| Isolation of efficient strains | Few strains degrade fluorinated compounds; often narrow substrate range | Metagenomic mining, directed evolution, or synthetic biology to discover or engineer strains with broader defluorination capacity | [18,233] |
| Environmental variability | Inconsistent pH, redox, nutrients, and microbial competition limit effectiveness | Use adaptive consortia, in situ conditioning (bioaugmentation and biostimulation), and microencapsulation for environmental stability | [235] |
| Incomplete mineralisation | Many pathways leave partially degraded products | Combine aerobic and anaerobic treatments; co-culture with complementary degraders; apply sequential bioreactor systems | [60,61] |
| Formation of persistent intermediates | Transformation may yield short-chain PFAS, still persistent and toxic | Apply oxidative post-treatment (e.g., UV, ozone, and AOPs); design enzymes targeting terminal groups of intermediates | [60,61,235] |
| Anaerobic vs. Aerobic conditions | Differing strategies complicate unified bioremediation approaches | Design modular treatment systems that switch between aerobic and anaerobic conditions; engineer facultative microbes | [179] |
| Co-metabolism dependency | Requires primary substrates to co-metabolise fluorinated compounds | Provide low-cost, renewable co-substrates (e.g., glycerol and acetate); develop strains capable of autonomous degradation | [55] |
| Strain & compound specificity | Activity is highly specific to the organism and compound | Develop multi-strain consortia; use machine learning to predict enzyme–substrate compatibility; engineer broad-spectrum enzymes | [138,140,194] |
| Reproducibility in complex environments | Lab-scale degradation is often not replicable in the field | Use pilot-scale microcosms to test real conditions; employ microbial encapsulation and bioaugmentation with native-strain compatibility screening | [242] |
| Algal biosorption, not degradation | Algae adsorb rather than degrade fluorinated compounds | Combine algae with bacteria for biosorption and biodegradation; functionalise algae to express defluorinating enzymes | [126] |
| Dependency on microbial consortia | Algal/fungal degradation depends on bacterial interactions | Engineer synthetic microbial consortia with defined roles; identify keystone strains for co-culture optimisation. | [133,134,135,136,137,138,139] |
| Limited mechanistic insight (fungi/algae) | Degradation pathways are poorly characterised | Apply omics (transcriptomics, proteomics, and metabolomics); use isotope tracing and pathway reconstruction | [89] |
| Challenges in biofilm engineering | Biofilm development for defluorination is underdeveloped | Use microfluidic systems to select effective biofilm formers; apply material science for tailored scaffolds; use quorum sensing modulators | [244] |
| Enzymatic defluorination | |||
| Thermodynamic barrier of C–F bond | High bond dissociation energy limits cleavage under physiological conditions | Use oxidoreductases coupled to redox-active cofactors; apply artificial electron donors/acceptors; couple with energy-yielding pathways | [28,29,30] |
| Limited discovery of specific enzymes | Few enzymes have been identified for C–F cleavage | Metagenomics, functional screening of extreme environments, and AI-based enzyme prediction models can accelerate discovery | [245] |
| Substrate specificity vs. promiscuity | Overly narrow or broad specificities limit use | Engineer enzyme active sites via directed evolution or rational design for enhanced selectivity; develop modular domains for substrate targeting. | [150,250] |
| Fluoride-mediated inhibition | Accumulated fluoride ions inhibit or denature enzymes | Use fluoride scavengers or precipitation strategies (e.g., with Ca2+); engineer fluoride-resistant enzymes or fluoride-export mechanisms | [251] |
| Cofactor dependency | Expensive/unstable cofactors like flavins and cobalamin are required | Engineer cofactor regeneration systems (e.g., NAD(P)H cycles); use cell-free lysates or co-expression of cofactor biosynthesis pathways | [49,166,176] |
| Limited stability of isolated enzymes | Enzymes often lose activity outside their native contexts | Immobilise enzymes on stable carriers; engineer thermostable and solvent-tolerant variants | [251] |
| Oxygen sensitivity (e.g., RDases) | Many enzymes require strict anaerobic conditions | Use anaerobic reactors; engineer oxygen-tolerant variants or apply encapsulation to shield enzymes from oxygen | [162] |
| Difficulties in heterologous expression | Functional expression is often challenging | Use optimised expression hosts (e.g., S. cerevisiae and P. pastoris); co-express chaperones and their redox partners, as well as cofactor assembly proteins; codon optimisation | [110] |
| Lack of direct biochemical evidence | Proposed pathways lack in vitro validation | Reconstitute systems using purified components; conduct enzyme kinetics and structural studies with fluorinated model substrates | [18,233] |
| High cost and low scalability | Industrial enzyme production is often too costly | Develop low-cost fermentation systems; use cell lysates or whole-cell biocatalysts; implement scalable immobilisation and reuse systems | [251] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.F. Recent Progress and Challenges in Microbial Defluorination and Degradation for Sustainable Remediation of Fluorinated Xenobiotics. Processes 2025, 13, 2017. https://doi.org/10.3390/pr13072017
Khan MF. Recent Progress and Challenges in Microbial Defluorination and Degradation for Sustainable Remediation of Fluorinated Xenobiotics. Processes. 2025; 13(7):2017. https://doi.org/10.3390/pr13072017
Chicago/Turabian StyleKhan, Mohd Faheem. 2025. "Recent Progress and Challenges in Microbial Defluorination and Degradation for Sustainable Remediation of Fluorinated Xenobiotics" Processes 13, no. 7: 2017. https://doi.org/10.3390/pr13072017
APA StyleKhan, M. F. (2025). Recent Progress and Challenges in Microbial Defluorination and Degradation for Sustainable Remediation of Fluorinated Xenobiotics. Processes, 13(7), 2017. https://doi.org/10.3390/pr13072017








