Characteristics of Food Industry Wastewaters and Their Potential Application in Biotechnological Production
Abstract
1. Introduction
2. Food Industry Wastewaters
2.1. Starch Industry Wastewater
2.2. Winery Industry Wastewater
2.3. Confectionery Industry Wastewater
2.4. Other Food Industry Wastewaters
2.5. Comparative Analysis of Physicochemical Characteristics of Food Industry Wastewaters
2.6. Potential Applications of Wastewaters from the Food Industry
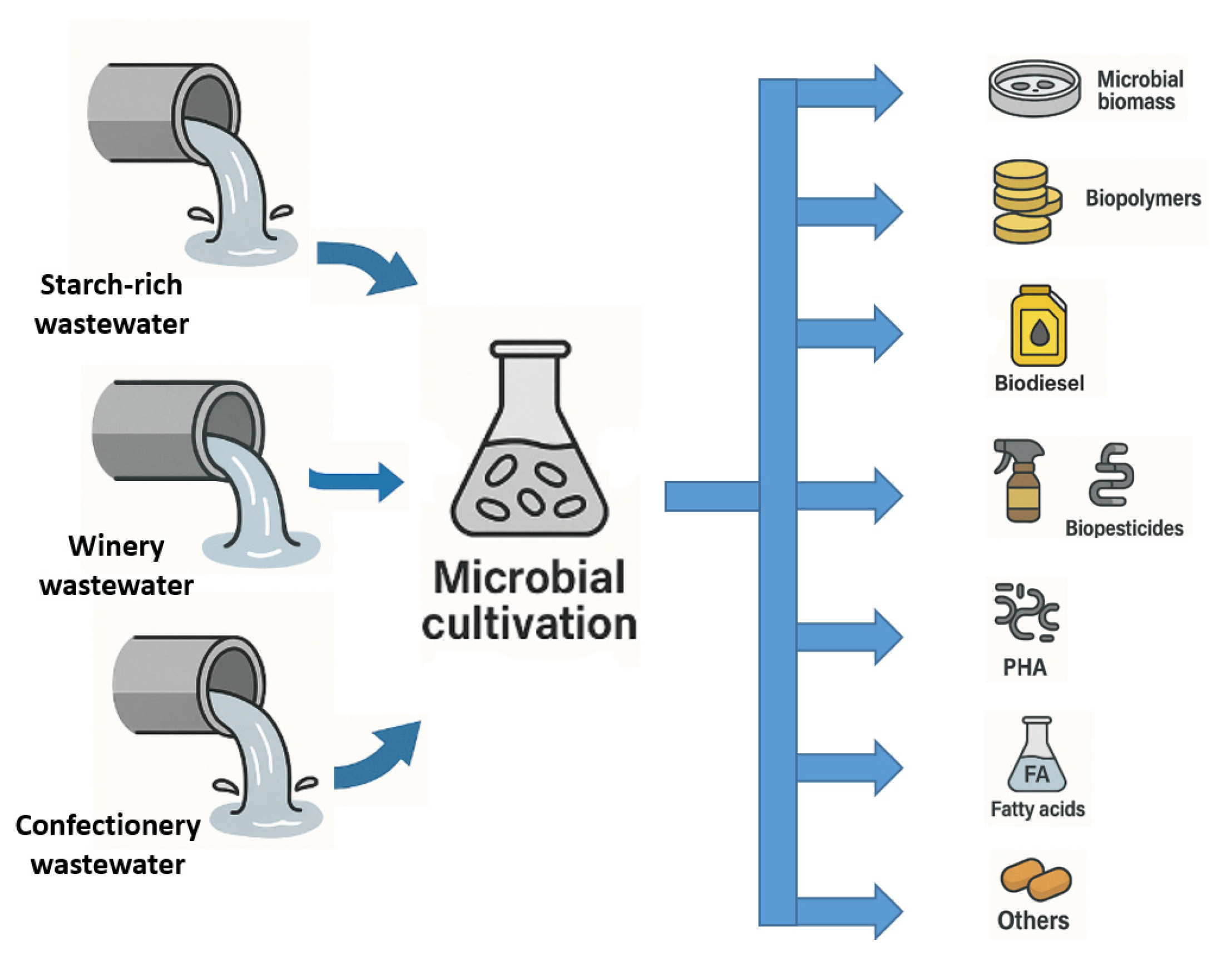
3. Valorization of Wastewater in Biotechnological Production
3.1. Valorization of Starch-Rich Wastewater
3.2. Valorization of Winery Wastewater
| High-Value Product | Applied Wastewater | Main Carbon Source | Wastewater Availability | Usage | Microorganism Producer | References |
|---|---|---|---|---|---|---|
| Biodiesel | Potato processing wastewater | starch | All year | Fuel | Aspergillus oryzae | [20] |
| Biomass/protein | Potato chips industry wastewater | starch | All year | Feed supplement | A. niger ITCC 2012 A. foetidus MTCC 508 | [120] |
| Biomass/protein | Wheat-starch plant wastewater | starch | Seasonal | Feed supplement | A.oryzae Rhizopus oryzae | [112] |
| Biodiesel/fatty acid | Deproteinated potato wastewater (DPW) | starch | All year | Fuel | Rhodotorula glutinis var. rubescens LOCKR13 | [113] |
| Biodiese/lipids | Corn starch wastewater | starch | Seasonal | Fuel | R.glutinis | [114] |
| Lipid/carotenoid | Potato wastewater | starch | All year | Food, pharmaceuticals, and cosmetics industy | R. gracilis ATCC 10788 | [115] |
| Palmitic, oleic, linoleic and α-linolenic acids | DPW medium enriched with glucose | starch, glucose | All year | Acids | Trichosporon domesticum PCM 2960 | [116] |
| Biofungicides | Potato processing wastewater | starch | All year | Plant protection | Trichoderma harzianum K179 | [109] |
| Lactic acid | Potato processing wastewater | starch | All year | Acids | Rhizopus oligosporus, R. arrhizus, R. oryzae | [19] |
| Biomass/protein | Potato processing wastewater/with glycerol | starch, glycerol | All year | Feed supplement | Candida utilis ATCC 9950 | [117] |
| Carotenoid | Potato processing wastewater/with glycerol | starch, glycerol | All year | Food, pharmaceuticals, and cosmetics industy | R. glutinis | [118] |
| Biomass/protein | Winery wastewater | glucose, fructose | Seasonal | Feed supplement | A.s oryzae WEBL0401, A. niger WEBL0901 T. viride WEBL0702, | [25] |
| Biodiesel | Winery wastewater | glucose, fructose | Seasonal | Fuel | Co-cultures Arthrospira platensis and Chlorella vulgaris | [122] |
| Biomass/protein | Winery wastewater | glucose, fructose | Seasonal | Feed supplement | A. platensis, C. vulgaris | [123,124,125] |
| Hydrogen | Winery wastewater | glucose, fructose | Seasonal | Energy source | Klebsiella pneumoniae MF101, Rhodopseudomonas sp. BR0Y6 | [126] |
| Biodiesel | Wine and raisin industries wastewater | glucose, fructose | Seasonal | Fuel | Leptolyngbya sp. | [127] |
| Xanthan | Winery wastewater | glucose, fructose | Seasonal | Biopolymer | Xanthomonas campestris | [128,129,130] |
| Kombucha beverage | Winery wastewater | glucose, fructose | Seasonal | Fermented beverage | Mixed microbial consortium | [131,132] |
| Xanthan | Confectionary industry wastewater | sucrose, glucose, fructose | All year/seasonal | Biopolymer | Xanthomonas campestris | [133,134] |
| Biofungicides | Confectionary industry wastewater | sucrose, glucose, fructose | All year/seasonal | Plant protection | Bacillus sp. | [135] |
| Bioethanol | Confectionary industry wastewater | sucrose, glucose, fructose | All year/seasonal | Fuel | Zymomonas mobilis ATCC 31821 | [136] |
| Polyglutamic acid (PGA) | Chocolate, candy and marshmallow wastewater industry | sucrose, glucose, fructose | All year/seasonal | Cosmetics industy | B. licheniformis JCM 2505 | [136] |
| Polyhydroxyalkanoates (PHA, PHB) | Chocolate and candy-based industry wastewater | sucrose, glucose, fructose | All year/seasonal | Bioplastic | Alcaligenes latus DSM 1123, Cupriavidus necator DSM 428 | [136] |
3.3. Valorization of Confectionery Industry Wastewater
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, J.; Thakur, A.; Goyal, A. Industrial wastewater and its toxic effects. In Biological Treatment of Industrial Wastewater; Shah, M.P., Ed.; The Royal Society of Chemistry: London, UK, 2021; pp. 1–14. [Google Scholar] [CrossRef]
- Aderibigbe, D.O.; Giwa, A.R.A.; Bello, I.A. Characterization and treatment of wastewater from food processing industry: A review. Imam J. Appl. Sci. 2017, 2, 27–36. [Google Scholar] [CrossRef]
- Kato, S.; Kansha, Y. Comprehensive review of industrial wastewater treatment techniques. Environ. Sci. Pollut. Res. 2024, 31, 51064–51097. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fatah, M.A. Integrated management of industrial wastewater in the food sector. Sustainability 2023, 15, 16193. [Google Scholar] [CrossRef]
- Hanchang, S.H.I. Industrial wastewater-types, amounts and effects. In Point Sources of Pollution: Local Effects and Their Control; Yi, Q., Ed.; EOLSS Publisher Co., Ltd.: Oxford, UK, 2009; Volume 2, p. 191. [Google Scholar]
- Mukherjee, J.; Lodh, B.K.; Sharma, R.; Mahata, N.; Shah, M.P.; Mandal, S.; Ghanta, S.; Bhunia, B. Advanced oxidation process for the treatment of industrial wastewater: A review on strategies, mechanisms, bottlenecks and prospects. Chemosphere 2023, 345, 140473. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, V.; Ali, I.; Marjub, M.M.; Rene, E.R.; Soto, A.M.F. Wastewater in the food industry: Treatment technologies and reuse potential. Chemosphere 2022, 293, 133553. [Google Scholar] [CrossRef]
- Sehar, S.; Nasser, H.A.A. Wastewater treatment of food industries through constructed wetland: A review. Int. J. Environ. Sci. Technol. 2019, 16, 6453–6472. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sirohi, R.; Udayan, A.; Yadav, P.; Raj, A.; Sim, S.J.; Pandey, A. Sustainable microalgal biomass production in food industry wastewater for low-cost biorefinery products: A review. Phytochem. Rev. 2023, 22, 969–991. [Google Scholar] [CrossRef]
- Cristian, O. Characteristics of the untreated wastewater produced by food industry. Analele Univ. Din Oradea Fasc. Protecţia Mediu. 2010, 15, 709–714. [Google Scholar]
- Bader, A.C.; Hussein, H.J.; Jabar, M.T. BOD: COD ratio as indicator for wastewater and industrial water pollution. Int. J. Spec. Educ. 2022, 37, 2164–2171. [Google Scholar]
- Sirohi, R.; Joun, J.; Lee, J.Y.; Yu, B.S.; Sim, S.J. Waste mitigation and resource recovery from food industry wastewater employing microalgae-bacterial consortium. Bioresour. Technol. 2022, 352, 127129. [Google Scholar] [CrossRef]
- Rekrak, A.Z.; Fellah, A.C. Dependability and purification performance of a semi-arid zone: A case study of Algeria’s wastewater treatment plant. Egypt. J. Aquat. Res. 2020, 46, 41–47. [Google Scholar] [CrossRef]
- Devi, M.K.; Manikandan, S.; Oviyapriya, M.; Selvaraj, M.; Assiri, M.A.; Vickram, S.; Subbaiya, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; et al. Recent advances in biogas production using Agro-Industrial Waste: A comprehensive review outlook of Techno-Economic analysis. Bioresour. Technol. 2022, 363, 127871. [Google Scholar] [CrossRef]
- Aguilar-Torrejón, J.A.; Balderas-Hernández, P.; Roa-Morales, G. Relationship, importance, and development of analytical techniques: COD, BOD, and, TOC in water—An overview through time. SN Appl. Sci. 2023, 5, 118. [Google Scholar] [CrossRef]
- Abdullah, R.M.; Ali, H.; Mohammed, M. Characteristics of Erbil Wastewater. Bachelor’s Thesis, Salahaddin University-Erbil College of Engineering Civil Engineering Department, Erbil, Iraq, 2020. Available online: https://www.researchgate.net/publication/342004577_CHARACTERISTICS_OF_ERBIL_WASTEWATER (accessed on 15 May 2025).
- Kukwa, R.E.; Ukpoko, U.W.; Leke, L. Assessment of physicochemical and minerals of wastewaters from food industries in Makurdi, for irrigation purposes. Water Sci. 2024, 38, 475–484. [Google Scholar] [CrossRef]
- Kot, A.M.; Pobiega, K.; Piwowarek, K.; Kieliszek, M.; Błażejak, S.; Gniewosz, M.; Lipińska, E. Biotechnological methods of management and utilization of potato industry waste—A review. Potato Res. 2020, 63, 431–447. [Google Scholar] [CrossRef]
- Hung, Y.-T.; Lo, H.H.; Awad, A.; Salman, H. Potato Wastewater Treatment. In Handbook of Industrial and Hazardous Wastes Treatment, 2nd ed.; Wang, L.K., Hung, Y.-T., Lo, H.H., Yapijakis, C., Eds.; Imprint CRC Press: Boca Raton, FL, USA, 2004; pp. 193–254. [Google Scholar]
- Muniraj, I.K.; Xiao, L.; Hu, Z.; Zhan, X.; Shi, J. Microbial lipid production from potato processing wastewater using oleaginous filamentous fungi Aspergillus oryzae. Water Res. 2013, 47, 3477–3483. [Google Scholar] [CrossRef]
- Huang, L.P.; Jin, B.; Lant, P.; Zhou, J. Biotechnological production of lactic acid integrated with potato wastewater treatment by Rhizopus arrhizus. J. Chem. Technol. Biotechnol. 2003, 78, 899–906. [Google Scholar] [CrossRef]
- Fooladgar, E.; Taheriyoun, M.; Bakhodaei, D. Evaluation of Electro-chemical Methods in Wastewater Treatment of Wheat Starch Industry. Water Air Soil Pollut. 2025, 236, 131. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Yang, H.; Xie, X.; Zhu, Y.; Xu, G.; Hu, X.; Jin, Z.; Hu, Y.; Hai, Z.; et al. Treatment of potato starch wastewater by dual natural flocculants of chitosan and poly-glutamic acid. J. Clean. Prod. 2020, 264, 121641. [Google Scholar] [CrossRef]
- Skornia, K.; Safferman, S.I.; Rodriguez-Gonzalez, L.; Ergas, S.J. Treatment of winery wastewater using bench-scale columns simulating vertical flow constructed wetlands with adsorption media. Appl. Sci. 2020, 10, 1063. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jin, B.; Bai, Z.H.; Wang, X.Y. Production of fungal biomass protein using microfungi from winery wastewater treatment. Bioresour. Technol. 2008, 99, 3871–3876. [Google Scholar] [CrossRef] [PubMed]
- Colin, T.; Bories, A.; Sire, Y.; Perrin, R. Treatment and valorisation of winery wastewater by a new biophysical process (ECCF). Water Sci. Technol 2005, 51, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Malandra, L.; Wolfaardt, G.; Zietsman, A.; Viljoen-Bloom, M. Microbiology of a biological contactor for winery wastewater treatment. Water Res. 2003, 37, 4125–4134. [Google Scholar] [CrossRef] [PubMed]
- Miklas, V.; Touš, M.; Miklasová, M.; Máša, V.; Hornák, D. Winery wastewater treatment technologies: Current trends and future perspective. CET J-Chem. Eng. Trans. 2022, 94, 847–852. [Google Scholar] [CrossRef]
- Johnson, M.B.; Mehrvar, M. Characterizing winery wastewater composition to optimize treatment and reuse. Aust. J. Grape Wine Res. 2020, 26, 410–416. [Google Scholar] [CrossRef]
- Vlotman, D.E.; Key, D.; Bladergroen, B.J. Technological advances in winery wastewater treatment: A comprehensive review. S. Afr. J. Enol. Vitic. 2022, 43, 58–80. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Zieliński, M. Methane production from confectionery wastewater treated in the anaerobic labyrinth-flow bioreactor. Energies 2023, 16, 571. [Google Scholar] [CrossRef]
- Mikheeva, E.R.; Katraeva, I.V.; Vorozhtsov, D.L.; Kovalev, D.A.; Kovalev, A.A.; Grigoriev, V.S.; Litti, Y.V. Dark fermentative biohydrogen production from confectionery wastewater in continuous-flow reactors. Int. J. Hydrogen Energy 2022, 47, 22348–22358. [Google Scholar] [CrossRef]
- Zajda, M.; Aleksander-Kwaterczak, U. Wastewater treatment methods for effluents from the confectionery industry–an overview. J. Ecol. Eng. 2019, 20, 293–304. [Google Scholar] [CrossRef]
- Nasr, F.A.; Abdelfattah, I.; El-Shafai, S.A. Cost effective management of confectionery industrial wastewater. Egypt J. Chem. 2022, 65, 391–399. [Google Scholar] [CrossRef]
- Puchlik, M.; Struk-Sokołowska, J. Comparison of the composition of wastewater from fruit and vegetables as well as dairy industry. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2017; Volume 17, p. 00077. [Google Scholar] [CrossRef]
- Asgharnejad, H.; Khorshidi Nazloo, E.; Madani Larijani, M.; Hajinajaf, N.; Rashidi, H. Comprehensive review of water management and wastewater treatment in food processing industries in the framework of water-food-environment nexus. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4779–4815. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Tian, J.; Shi, J.; Linhardt, R.J.; Ye, T.D.X.; Chen, S. Recovery of high value-added nutrients from fruit and vegetable industrial wastewater. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1388–1402. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Ma, S.; Li, K.; Zhang, X.; Hou, D.; Zheng, X.; Wang, C.; Lyu, P.; Xu, S.; et al. Recovery and utilization of phosphorus from fruit and vegetable wastewater. Sci. Rep. 2022, 12, 617. [Google Scholar] [CrossRef]
- Zelenina, V.A.; Vizir, A.D.; Basamykina, A.N. Adoption of optimal wastewater treatment system for fruit and vegetable production. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2024; Volume 103, p. 00042. [Google Scholar] [CrossRef]
- Singh, S.P.; Rathinam, K.; Gupta, T.; Agarwal, A.K. Pollution Control Technologies: Current Status and Future Prospects. In Pollution Control Technologies: Energy, Environment, and Sustainability; Singh, S.P., Rathinam, K., Gupta, T., Agarwal, A.K., Eds.; Springer: Singapore, 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Mishra, S.; Bindhani, S.K.; Jena, H. Statistical analysis of COD and BOD removal from dairy waste water. Int. J. Membr. Sci. Technol. 2023, 10, 3457–3462. [Google Scholar] [CrossRef]
- Handayani, T.; Mulyanto, A.; Priyanto, F.E.; Nugroho, R. Utilization of dairy industry wastewater for nutrition of microalgae Chlorella vulgaris. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; Volume 1655, p. 012123. [Google Scholar] [CrossRef]
- Sathya, K.; Nagarajan, K.; Carlin Geor Malar, G.; Rajalakshmi, S.; Raja Lakshmi, P. A comprehensive review on comparison among effluent treatment methods and modern methods of treatment of industrial wastewater effluent from different sources. Appl. Water Sci. 2022, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- You, K.; Ge, F.; Wu, X.; Song, K.; Yang, Z.; Zhang, Q.; Liu, Y.; Ruan, R.; Zheng, H. Nutrients recovery from piggery wastewater and starch wastewater via microalgae-bacteria consortia. Algal Res. 2021, 60, 102551. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Liu, J.; Han, L. Research progress on corn starch wastewater treatment process. In Proceedings of the 2011 International Conference on New Technology of Agricultural, Zibo, China, 27–29 May 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 620–625. [Google Scholar] [CrossRef]
- Zhang, C.; Singh, R.P.; Yadav, P.; Kumar, I.; Kaushik, A.; Roychowdhury, R.; Mubeen, M.; Singh, S.K.; Kumar, A.; Wang, J. Recent advances in biotechnology and bioengineering for efficient microalgal biofuel production. Fuel Process. Technol. 2025, 270, 108199. [Google Scholar] [CrossRef]
- Cai, T.; Lin, H.; Liu, Z.; Chen, K.; Lin, Y.; Xi, Y.; Chhuond, K. Starch wastewater treatment technology. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 358, p. 022054. [Google Scholar] [CrossRef]
- Drosg, B.; Neubauer, M.; Marzynski, M.; Meixner, K. Valorisation of starch wastewater by anaerobic fermentation. Appl. Sci. 2021, 11, 10482. [Google Scholar] [CrossRef]
- Hedayati Moghaddam, A.; Sargolzaei, J. A mini-review over diverse methods used in starchy wastewater treatment. Recent Pat. Chem. Eng. 2012, 5, 95–102. [Google Scholar] [CrossRef]
- Suo, N.; Li, X.; Wu, J. Effects of different flocculants and environment on the flocculation effect of potato starch-processing wastewater. Water Qual. Res. J. 2024, 59, 43–61. [Google Scholar] [CrossRef]
- Dorantes-Fuertes, M.G.; López-Méndez, M.C.; Martínez-Castellanos, G.; Meléndez-Armenta, R.Á.; Jiménez-Martínez, H.E. Starch extraction methods in tubers and roots: A systematic review. Agronomy 2024, 14, 865. [Google Scholar] [CrossRef]
- Devereux, S.; Shuttleworth, P.S.; Macquarrie, D.J.; Paradisi, F. Isolation and characterization of recovered starch from industrial wastewater. J. Polym. Environ. 2011, 19, 971–979. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.; Li, L.; Zhang, M.; Tian, S.; Wang, D.; Liu, K.; Liu, H.; Zhu, W.; Wang, X. Comprehensive utilization of corn starch processing by-products: A review. Grain Oil Sci. Technol. 2021, 4, 89–107. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, L.; Qin, J.Y. Application of Membrane Biological Reactor on Purifying Wastewater from Corn Starch Processing. In Proceedings of the 2011 Third International Conference on Measuring Technology and Mechatronics Automation, Shanghai, China, 6–7 January 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 543–546. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Zhang, G.; Liu, W.; Xu, J.; Tian, Y.; Wang, Y.; Xie, X.; Peng, Z.; Li, A.; et al. Efficient treatment of the starch wastewater by enhanced flocculation–coagulation of environmentally benign materials. Sep. Purif. Technol. 2023, 307, 122788. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Puma, G.L.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Latessa, S.H.; Hanley, L.; Tao, W. Characteristics and practical treatment technologies of winery wastewater: A review for wastewater management at small wineries. J. Environ. Manag. 2023, 342, 118343. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Symeonidou, M.P.; Matis, K.A. Technologies of winery wastewater treatment: A critical approach. Desalin. Water Treat. 2016, 57, 3372–3386. [Google Scholar] [CrossRef]
- Pascual, A.; Pena, R.; Gómez-Cuervo, S.; de la Varga, D.; Alvarez, J.A.; Soto, M.; Arias, C.A. Nature based solutions for winery wastewater valorisation. Ecol. Eng. 2021, 169, 106311. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Gomes, A.; Peres, J.A.; Lucas, M.S. Winery wastewater: Challenges and perspectives. Eng. Proc. 2023, 56, 267. [Google Scholar] [CrossRef]
- Davididou, K.; Frontistis, Z. Advanced oxidation processes for the treatment of winery wastewater: A review and future perspectives. J. Chem. Technol. Biotechnol. 2021, 96, 2436–2450. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Guimarães, V.; Lucas, M.S.; Peres, J.A. Treatment of winery wastewater with a combination of adsorption and thermocatalytic processes. Processes 2022, 10, 75. [Google Scholar] [CrossRef]
- Dostiyev, H.; Gönder, Z.B. A comparative investigation of chemical coagulation and electrocoagulation for the post-treatment of confectionery wastewater. Int. J. Environ. Sci. Technol. 2024, 21, 3661–3674. [Google Scholar] [CrossRef]
- Patsialou, S.; Politou, E.; Nousis, S.; Liakopoulou, P.; Vayenas, D.V.; Tekerlekopoulou, A.G. Hybrid treatment of confectionery wastewater using a biofilter and a cyanobacteria-based system with simultaneous valuable metabolic compounds production. Algal Res. 2024, 79, 103483. [Google Scholar] [CrossRef]
- Marszałek, A.; Puszczało, E. Effect of photooxidation on nanofiltration membrane fouling during wastewater treatment from the confectionery industry. Water 2020, 12, 793. [Google Scholar] [CrossRef]
- Park, E.; Enander, R.; Barnett, S.M.; Lee, C. Pollution prevention and biochemical oxygen demand reduction in a squid processing facility. J. Clean. Prod. 2001, 9, 341–349. [Google Scholar] [CrossRef]
- Zieliński, M.; Kazimierowicz, J.; Dębowski, M. Advantages and limitations of anaerobic wastewater treatment—Technological basics, development directions, and technological innovations. Energies 2022, 16, 83. [Google Scholar] [CrossRef]
- Tikariha, A.; Sahu, O. Study of characteristics and treatments of dairy industry waste water. J. App. Environ. Microbiol. 2014, 2, 16–22. [Google Scholar] [CrossRef]
- Kaur, N. Different treatment techniques of dairy wastewater. Groundw. Sust. Dev. 2021, 14, 100640. [Google Scholar] [CrossRef]
- Passeggi, M.; López, I.; Borzacconi, L. Integrated anaerobic treatment of dairy industrial wastewater and sludge. Water Sci. Technol. 2009, 59, 501–506. [Google Scholar] [CrossRef]
- Alalam, S.; Ben-Souilah, F.; Lessard, M.H.; Chamberland, J.; Perreault, V.; Pouliot, Y.; Labrie, S.; Doyen, A. Characterization of chemical and bacterial compositions of dairy wastewaters. Dairy 2021, 2, 179–190. [Google Scholar] [CrossRef]
- Singh, P.; Mohanty, S.S.; Mohanty, K. Comprehensive assessment of microalgal-based treatment processes for dairy wastewater. Front. Bioeng. Biotechnol. 2024, 12, 1425933. [Google Scholar] [CrossRef]
- Kushwaha, J.P.; Srivastava, V.C.; Mall, I.D. An Overview of Various Technologies for the Treatment of Dairy Wastewaters. Crit. Rev. Food Sci. 2011, 51, 442–452. [Google Scholar] [CrossRef]
- Bella, K.; Rao, P.V. Anaerobic digestion of dairy wastewater: Effect of different parameters and co-digestion options—A review. Biomass Convers. Biorefinery 2023, 13, 2527–2552. [Google Scholar] [CrossRef]
- Shete, B.S.; Shinkar, N.P. Dairy industry wastewater sources, characteristics its effects on environment. Int. J. Curr. Eng. Technol. 2013, 3, 1611–1615. [Google Scholar]
- Tabelini, D.B.; Lima, J.P.P.; Borges, A.C.; Aguiar, A. A review on the characteristics and methods of dairy industry wastewater treatment in the state of Minas Gerais, Brazil. J. Water Process. Eng. 2023, 53, 103779. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic treatment of dairy wastewaters: A review. Process. Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Deka, A.; Rasul, A.; Baruah, A.; Malakar, H.; Basumatary, A.K. Treatment of dairy wastewater with tubular ceramic membrane. Mater. Today Proc. 2023, 72, 2773–2779. [Google Scholar] [CrossRef]
- Akansha, J.; Nidheesh, P.V.; Gopinath, A.; Anupama, K.V.; Kumar, M.S. Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 2020, 253, 126652. [Google Scholar] [CrossRef]
- Kolev Slavov, A. General characteristics and treatment possibilities of dairy wastewater–a review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef]
- Ramsuroop, J.; Gutu, L.; Ayinde, W.B.; Basitere, M.; Manono, M.S. A review of biological processes for dairy wastewater treatment and the effect of physical parameters which affect their efficiency. Water 2024, 16, 537. [Google Scholar] [CrossRef]
- Al-Tayawi, A.N.; Sisay, E.J.; Beszédes, S.; Kertész, S. Wastewater treatment in the dairy industry from classical treatment to promising technologies: An overview. Processes 2023, 11, 2133. [Google Scholar] [CrossRef]
- Irshad, A.; Sureshkumar, S.; Raghunath, B.V.; Rajarajan, G.; Mahesh Kumar, G. Treatment of Waste Water from Meat Industry. In Integrated Waste Management in India: Environmental Science and Engineering; Prashanthi, M., Sundaram, R., Eds.; Springer: Cham, Switzerland, 2016; pp. 251–263. [Google Scholar] [CrossRef]
- Harris, P.W.; McCabe, B.K. Process optimisation of anaerobic digestion treating high-strength wastewater in the Australian red meat processing industry. Appl. Sci. 2020, 10, 7947. [Google Scholar] [CrossRef]
- Philipp, M.; Masmoudi Jabri, K.; Wellmann, J.; Akrout, H.; Bousselmi, L.; Geißen, S.U. Slaughterhouse wastewater treatment: A review on recycling and reuse possibilities. Water 2021, 13, 3175. [Google Scholar] [CrossRef]
- Yeoh, J.X.; Md Jamil, S.N.A.; Syukri, F.; Koyama, M.; Nourouzi Mobarekeh, M. Comparison between conventional treatment processes and advanced oxidation processes in treating slaughterhouse wastewater: A review. Water 2022, 14, 3778. [Google Scholar] [CrossRef]
- Fatima, F.; Du, H.; Kommalapati, R.R. Treatment of poultry slaughterhouse wastewater with membrane technologies: A review. Water 2021, 13, 1905. [Google Scholar] [CrossRef]
- Ng, M.; Dalhatou, S.; Wilson, J.; Kamdem, B.P.; Temitope, M.B.; Paumo, H.K.; Djelal, H.; Assadi, A.A.; Nguyen-Tri, P.; Kane, A. Characterization of slaughterhouse wastewater and development of treatment techniques: A review. Processes 2022, 10, 1300. [Google Scholar] [CrossRef]
- Šereš, Z.; Maravić, N.; Takači, A.; Nikolić, I.; Šoronja-Simović, D.; Jokić, A.; Hodur, C. Treatment of vegetable oil refinery wastewater using alumina ceramic membrane: Optimization using response surface methodology. J. Clean. Prod. 2016, 112, 3132–3137. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Oulego, P.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Towards the implementation of circular economy in the wastewater sector: Challenges and opportunities. Water 2020, 12, 1431. [Google Scholar] [CrossRef]
- Dridi, N.; Romdhane, L.; Ferreira, R.; Sleimi, N. Fertilizer effect of composted sewage sludge and cattle manure on Pelargonium growth. J. Water Sanit. Hyg. Dev. 2020, 10, 1019–1025. [Google Scholar] [CrossRef]
- Sugurbekova, G.; Nagyzbekkyzy, E.; Sarsenova, A.; Danlybayeva, G.; Anuarbekova, S.; Kudaibergenova, R.; Frochot, C.; Acherar, S.; Zhatkanbayev, Y.; Moldagulova, N. Sewage sludge management and application in the form of sustainable fertilizer. Sustainability 2023, 15, 6112. [Google Scholar] [CrossRef]
- Shi, W.; Healy, M.G.; Ashekuzzaman, S.M.; Daly, K.; Leahy, J.J.; Fenton, O. Dairy processing sludge and co-products: A review of present and future re-use pathways in agriculture. J. Clean. Prod. 2021, 314, 128035. [Google Scholar] [CrossRef]
- Nafez, A.H.; Nikaeen, M.; Kadkhodaie, S.; Hatamzadeh, M.; Moghim, S. Sewage sludge composting: Quality assessment for agricultural application. Environ. Monit. Assess. 2015, 187, 709. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; García, A.; Fernández-Bolaños, J. Novel pectin present in new olive mill wastewater with similar emulsifying and better biological properties than citrus pectin. Food Hydrocolloid 2015, 50, 237–246. [Google Scholar] [CrossRef]
- Bethi, C.M.; Narayan, B.; Martin, A.; Kudre, T.G. Recovery, physicochemical and functional characteristics of proteins from different meat processing wastewater streams. Environ. Sci. Pollut. Res. 2020, 27, 25119–25131. [Google Scholar] [CrossRef] [PubMed]
- Bethi, C.M.; Jayprakash, G.; Muthukumar, S.P.; Kudre, T.G. Application of proteins from different meat processing wastewater streams as a dietary protein source in animal feed. J. Environ. Manag. 2021, 299, 113662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Serventi, L. Sustainability of dairy and soy processing: A review on wastewater recycling. J. Clean. Prod. 2019, 237, 117821. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. 2018, 58, 2829–2841. [Google Scholar] [CrossRef]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Barbera, M. Reuse of food waste and wastewater as a source of polyphenolic compounds to use as food additives. J. AOAC Int. 2020, 103, 906–914. [Google Scholar] [CrossRef]
- Santos, J.R.; Rodrigues, R.P.; Quina, M.J.; Gando-Ferreira, L.M. Recovery of value-added compounds from winery wastewater: A review and bibliometric analysis. Water 2023, 15, 1110. [Google Scholar] [CrossRef]
- Chang, Z.; Long, G.; Zhou, J.L.; Ma, C. Valorization of sewage sludge in the fabrication of construction and building materials: A review. Res. Conserv. Recycl. 2020, 154, 104606. [Google Scholar] [CrossRef]
- De Souza, J.M.; Ramos Filho, R.E.B.; Duarte, J.B.; da Silva, V.M.; do Rêgo, S.R.; Lucena, L.D.F.L.; Acchar, W. Mechanical and durability properties of compressed stabilized earth brick produced with cassava wastewater. J. Build. Eng. 2021, 44, 103290. [Google Scholar] [CrossRef]
- Meesad, S.; Rodsrida, C.; Srivichai, P. Utilization of Waste-Activated Sludge as A Substitute in Fired Clay Brick Production. Burapha Sci. J. 2023, 28, 1491–1503. [Google Scholar]
- Kundu, D.; Dutta, D.; Samanta, P.; Dey, S.; Sherpa, K.C.; Kumar, S.; Dubey, B.K. Valorization of wastewater: A paradigm shift towards circular bioeconomy and sustainability. Sci. Total Environ. 2022, 848, 157709. [Google Scholar] [CrossRef] [PubMed]
- Lad, B.C.; Coleman, S.M.; Alper, H.S. Microbial valorization of underutilized and nonconventional waste streams. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab056. [Google Scholar] [CrossRef] [PubMed]
- Donohue, T.J. Producing transportation fuels, electrical power, and chemicals in a circular Bioeconomy. Bridge 2023, 53, 41–46. [Google Scholar]
- Mitrović, I.; Tančić Živanov, S. Biotechnological production of Trichoderma biofungicide on starch-rich wastewater. In Proceedings of the 3rd International Conference “Conference On Advances in Science and Technology“ COAST 2024, Herceg Novi, Montenegro, 29 May–1 June 2024. [Google Scholar]
- Nigam, P.; Singh, D. Processes of fermentative production of xylitol—A sugar substitute. Process. Biochem. 1995, 30, 117–124. [Google Scholar] [CrossRef]
- Mishra, B.K.; Arora, A.L. Optimization of a biological process for treating potato chips industry wastewater using a mixed culture of Aspergillus foetidus and Aspergillus niger. Biores. Technol. 2004, 94, 9–12. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Zamani, A.; Taherzadeh, M.J. Edible protein production by filamentous fungi using starch plant wastewater. Waste Biomass Valoriz. 2019, 10, 2487–2496. [Google Scholar] [CrossRef]
- Gientka, I.; Duda, M.; Bzducha-Wróbel, A.; Błażejak, S. Deproteinated potato wastewater as a low-cost nitrogen substrate for very high yeast biomass quantities: Starting point for scaled-up applications. Eur. Food Res. Technol. 2019, 245, 919–928. [Google Scholar] [CrossRef]
- Xue, F.; Gao, B.; Zhu, Y.; Zhang, X.; Feng, W.; Tan, T. Pilotscale production of microbial lipid using starch wastewater as raw material. Biores. Technol. 2010, 101, 6092–6095. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Piwowarek, K.; Brzezińska, R. Production of lipids and carotenoids by Rhodotorula gracilis ATCC 10788 yeast in a bioreactor using low—cost wastes. Biocatal. Agric. Biotechnol. 2020, 26, 101634. [Google Scholar] [CrossRef]
- Gientka, I.; Aleksandrzak-Piekarczyk, T.; Bzducha-Wróbel, A.; Synowiec, A.; Błażejak, S. Deproteinated Potato Wastewater as a Sustainable Nitrogen Source in Trichosporon domesticum Yeast Lipids Biosynthesis-a Concept of Valorization of Wastewater from Starch Industry. Potato Res. 2019, 62, 221–237. [Google Scholar] [CrossRef]
- Kurcz, A.; Błażejak, S.; Kot, A.M.; Bzducha-Wróbel, A.; Kieliszek, M. Application of industrial wastes for the production of microbial single-cell protein by fodder yeast Candida utilis. Waste Biomass Valoriz. 2018, 9, 57–64. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Bryś, J.; Gientka, I.; Bzducha-Wróbel, A.; Maliszewska, M.; Reczek, L. Effect of initial pH of medium with potato wastewater and glycerol on protein, lipid and carotenoid biosynthesis by Rhodotorula glutinis. Electron. J. Biotechnol. 2017, 27, 25–31. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Mohanta, Y.K.; Reddy, C.N.; Reddy, S.D.M.; Mandal, S.K.; Yadavalli, R.; Sarma, H. Valorization of agro-industrial biowaste to biomaterials: An innovative circular bioeconomy approach. Circ. Econ. 2023, 2, 100050. [Google Scholar] [CrossRef]
- Hultberg, M.; Bodin, H. Fungi-based treatment of real brewery waste streams and its effects on water quality. Bioproc. Biosyst. Eng. 2019, 42, 1317–1324. [Google Scholar] [CrossRef]
- Spennati, E.; Alberto Casazza, A.; Converti, A. Winery Wastewater Treatment by Microalgae to Produce Low-Cost Biomass for Energy Production Purposes. Energies 2020, 13, 2490. [Google Scholar] [CrossRef]
- Casazzaa, A.A.; Ferraria, P.F.; Aliakbariana, B.; Comottoa, M.; Peregoa, P. Microalgae Growth using Winery Wastewater for Energetic and Environmental Purposes. Chem. Eng. Trans. 2016, 49, 565–570. [Google Scholar] [CrossRef]
- Spennatia, R.; Casazzaa, A.A.; Peregoa, P.; Solisioa, C.; Busca, G.; Converti, A. Microalgae Growth in Winery Wastewater under Dark Conditions. Chem. Eng. Trans. 2019, 74, 1471–1476. [Google Scholar] [CrossRef]
- Sousa, A.C.; Dias, C.; Martins, A.R.; Gomes, A.G.; Santos, C.A. Using winery effluents for cultivating microalgae as bio-additives for vineyards. J. App. Phycol. 2025, 37, 1–14. [Google Scholar] [CrossRef]
- Policastro, G.; Carraturo, F.; Compagnone, M.; Guida, M.; Fabbricino, M. Enhancing hydrogen production from winery wastewater through fermentative microbial culture selection. Biores. Technol. Rep. 2022, 19, 101196. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Aggelis, G.; Genitsaris, S.; Moustaka-Gouni, M.; Vayenas, D.V. Biotreatment of raisin and winery wastewaters and simultaneous biodiesel production using a Leptolyngbya-based microbial consortium. J. Clean. Prod. 2017, 148, 185–193. [Google Scholar] [CrossRef]
- Bajić, B.; Rončević, Z.; Puškaš, V.; Miljić, U.; Dodić, S.; Grahovac, J.; Dodić, J. White wine production effluents used for biotechnological production of xanthan. J. Process. Energy Agric. 2015, 19, 52–55. [Google Scholar]
- Rončević, Z.; Grahovac, J.; Dodić, S.; Vučurović, D.; Dodić, J. Utilisation of winery wastewater for xanthan production in stirred tank bioreactor: Bioprocess modelling and optimization. Food Bioprod. Process. 2019, 117, 113–125. [Google Scholar] [CrossRef]
- Trivunović, Z.; Mitrović, I.; Puškaš, V.; Bajić, B.; Miljić, U.; Dodić, J. Utilization of wastewaters from red wine technology for xanthan production in laboratory bioreactor. J. Food Process. Preserv. 2021, 46, e15849. [Google Scholar] [CrossRef]
- Vukmanović, S.; Vitas, V.; Kravić, S.; Stojanović, Z.; Đurović, A.; Cvetković, B.; Malbaša, M. Influence of main production variables on nutritional characteristics of winery effluent kombucha. Chem. Ind. Chem. Eng. Q. 2024, 30, 285–294. [Google Scholar] [CrossRef]
- Vitas, J.; Vukmanović, S.; Malbaša, R. Antioxidant Potential and Composition of Winery Effluent Based Kombucha Products. Waste Biomass Valoriz. 2023, 14, 4187–4200. [Google Scholar] [CrossRef]
- Bajić, B.; Dodić, J.; Rončević, Z.; Grahovac, J.; Dodić, S.; Vučurović, D.; Tadijan, I. Biosynthesis of xanthan gum on wastewater from confectionary industry. Anal. Tech. Szeged. 2014, 8, 13–17. [Google Scholar] [CrossRef]
- Bajić, B.; Vučurović, D.; Dodić, S.; Grahovac, J.; Dodić, J. Process model economics of xanthan production from confectionery industry wastewaters. J. Environ. Manag. 2017, 203, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Dujković, T.; Danilov, I.; Vlajkov, V.; Gladikostić, N.; Dmitrović, S.; Lukić, N.; Jokić, A.; Grahovac, J. Investigation of Microbial Biocontrol Agents and Essential Oils Synergism in Suppression of Aflatoxigenic Aspergillus flavus. Acta Period. Technol. 2024, 55, 235–245. [Google Scholar] [CrossRef]
- Harrison, S.T.L.; Johnstone-Robertson, M.; Rademeyer, S.; Murhonyi, L.; Ngwenya, C.; Horn, C.; Rumjeet, S.; Smart, M. Value recovery from Solid Confectionery Waste; Technical Report; Centre for Bioprocess Engineering, University of Cape Town: Cape Town, South Africa, 2019. [Google Scholar]
- Rangel, C.; Carvalho, G.; Oehmen, A.; Frison, N.; Lourenço, N.D.; Reis, M.A.M. Polyhydroxyalkanoates production from ethanol- and lactate-rich fermentate of confectionary industry effluents. Int. J. Biol. Macromol. 2023, 229, 713–723. [Google Scholar] [CrossRef]
- Simó-Cabrera, L.; García-Chumillas, S.; Benitez-Benitez, S.J.; Cánovas, V.; Monzó, F.; Pire, C.; Martínez-Espinosa, R.M. Production of Poly(3-hydroxybutyrate-co-3- hydroxyvalerate) (PHBV) by Haloferax mediterranei Using Candy Industry Waste as Raw Materials. Bioengineering 2024, 11, 870. [Google Scholar] [CrossRef]
- Kora, E.; Antonopoulou, G.; Zhang, Y.; Yan, Q.; Lyberatos, G.; Ntaikou, I. Investigating the efficiency of a two-stage anaerobic-aerobic process for the treatment of confectionery industry wastewaters with simultaneous production of biohydrogen and polyhydroxyalkanoates. Environ. Res. 2024, 248, 118526. [Google Scholar] [CrossRef]
- Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC—Commission Declaration. Off. J. L 2001, 106, 0001–0039.
- Council Directive 91/271/EEC of 21 May 1991 concerning urban waste-water treatment. Off. J. L 1991, 135, 0040–0052.
- U.S. EPA Standards, 5 November 2024. Available online: https://www.epa.gov/data/data-standards (accessed on 5 November 2024).
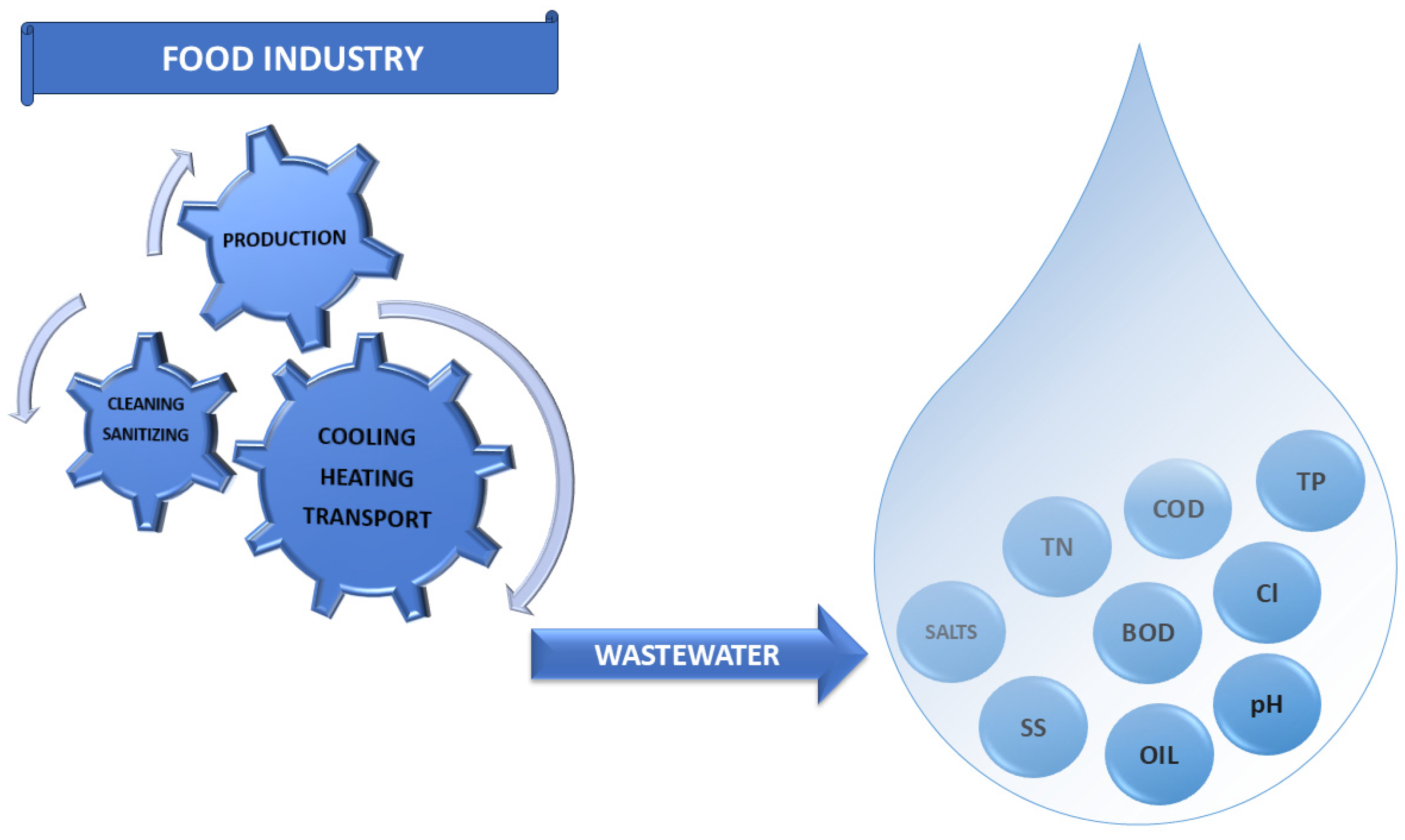
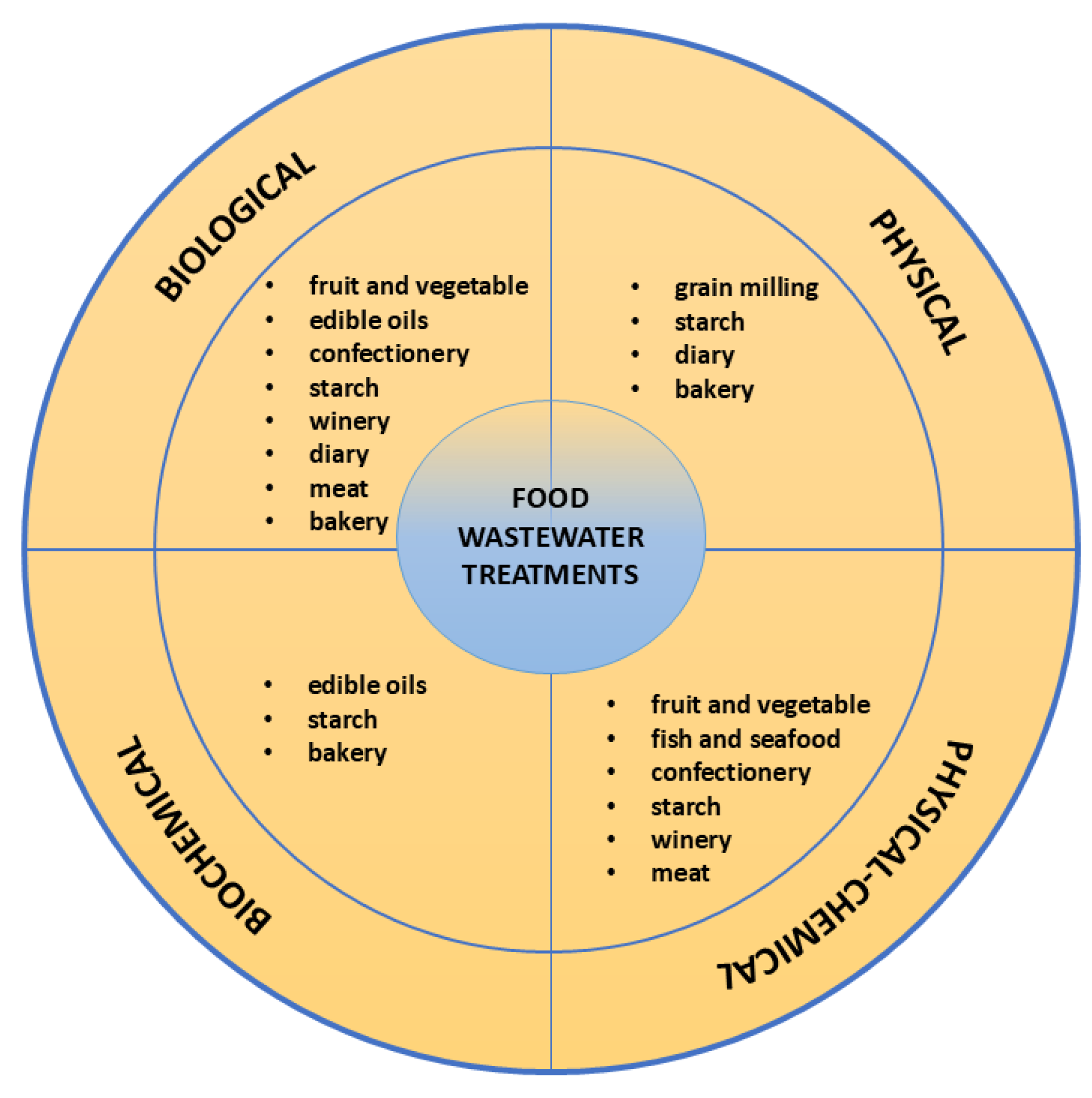
| Food Industry Wastewaters | Starch | Winery | Confectionery | Fruit and Vegetable | Diary | Meat |
|---|---|---|---|---|---|---|
| Parameters | ||||||
| Main organic load | Starch, glucose, dextrins | Sugars (glucose, fructose), ethanol, polyphenols | Sugars (sucrose, glucose), fats, proteins | Fibers, phenolic compounds, acids, minerals | Proteins, lactose, lipids, detergents | Proteins, fats, minerals |
| COD (g/L) | 8.1–37 | 0.32–12.7 | 2.5–20.02 | 0.8–7.7 | 0.43–95 | 1.6–15 |
| BOD5 (g/L) | 0.005–5.4 | 0.125–130 | 3.132–8 | 0.5–6.1 | 0.35–48 | 0.6–8 |
| Total solids (g/L) | 0.26–42 | 1.602–79.635 | 11.1–44.6 | 0.2–0.4 | 0.2–5.8 | 0.25–6.4 |
| Suspended solids (g/L) | 0.007–6.4 | 0.06–30.300 | 0.47–1.31 | - | 0.8–4.4 | 0.22–9.3 |
| Total nitrogen (g/L) | 0.02–0.87 | 0–0.415 | 0.171–0.225 | 0.8–1.2 | 0.01–1.12 | 0.6–2.7 |
| Total phosphorus (g/L) | 0.014–0.160 | 0.003–0.188 | 0.01–0.028 | 0.045–0.5 | 0.05–0.55 | 0.15–0.32 |
| Volatile solids (g/L) | 0.45–6.685 | 0.13–54.952 | 0.0273–0.0301 | - | - | - |
| Starch (g/L) | 19.47–31.2 | - | - | - | - | - |
| Total sugars (g/L) | - | 8.1–13.2 | 7.4 | - | - | - |
| pH (1) | 4–10 | 3.93–12.9 | 3.9–9.5 | 4.6–7.9 | 6.8–9.4 | 6.5–9 |
| References | [9,18,19,20,21,22,23] | [24,25,26,27,28,29,30] | [31,32,33,34] | [35,36,37,38,39] | [2,9,10,40,41] | [9,10,40,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, I.; Mijić, K.; Mitrović, I. Characteristics of Food Industry Wastewaters and Their Potential Application in Biotechnological Production. Processes 2025, 13, 2401. https://doi.org/10.3390/pr13082401
Nikolić I, Mijić K, Mitrović I. Characteristics of Food Industry Wastewaters and Their Potential Application in Biotechnological Production. Processes. 2025; 13(8):2401. https://doi.org/10.3390/pr13082401
Chicago/Turabian StyleNikolić, Ivana, Kosta Mijić, and Ivana Mitrović. 2025. "Characteristics of Food Industry Wastewaters and Their Potential Application in Biotechnological Production" Processes 13, no. 8: 2401. https://doi.org/10.3390/pr13082401
APA StyleNikolić, I., Mijić, K., & Mitrović, I. (2025). Characteristics of Food Industry Wastewaters and Their Potential Application in Biotechnological Production. Processes, 13(8), 2401. https://doi.org/10.3390/pr13082401










