Abstract
In this study, the phytochemical composition and antimicrobial efficacy of five Türkiye native Salvia species (S. albimaculata, S. blepharochlaena, S. palaestina, S. virgata, and S. absconditiflora (syn. S. cryptantha) were investigated. The essential oils isolated with yields ranging from 0.2% to 0.66% were assessed using gas chromatography-mass spectrometry (GC-MS). The major constituents were found to be α-pinene (up to 12.0% in S. albimaculata), camphor (up to 28.5% in S. blepharochlaena), borneol (up to 19.5% in S. virgata), 1,8-cineole (30.2% in S. absconditiflora), and linalool (26.5% in S. palaestina). Methanol extracts were produced with yields ranging from 8.2% to 9.5% and examined via liquid chromatography-mass spectrometry (LC-MS/MS) and isolated phenolic acids (e.g., rosmarinic acid and caffeic acid) and flavonoids (luteolin and apigenin). Rosmarinic acid emerged as the dominant common compound in all the species. Antimicrobial testing against Gram-positive and Gram-negative bacteria and Candida microorganisms showed potent activity: S. blepharochlaena essential oil showed good antifungal activity against C. utilis, with a MIC value of 31.25 µg/mL, while S. palaestina and S. virgata extracts showed antibacterial activity against Bacillus and Staphylococcus strains. This detailed study broadened the chemotaxonomic profile of Turkish Salvia species and listed possible antimicrobial agents.
1. Introduction
The genus Salvia L., belonging to the Lamiaceae family, is widely distributed across the globe and holds significant importance in traditional medicine and modern pharmaceutical applications. The genus Salvia includes about 1050 species worldwide, and there are 113 species of Salvia in Turkey, 58 of which are endemic [1,2]. Salvia species are known for their rich phytochemical profiles, which include phenolic compounds, flavonoids, and terpenoids. These compounds exhibit various bioactive properties such as antimicrobial, antioxidant, and anti-inflammatory activities [3].
During recent years, there have been several published papers on the chemical composition and biological activities of essential oils (EOs) extracted from Salvia species. Plant secondary metabolites, essential oils are primarily monoterpenes, sesquiterpenes, and other terpenoids. These secondary metabolites exhibited strong antimicrobial activity against Gram-positive and Gram-negative bacteria and other fungal species [4].
Monoterpenes are often the predominant constituents of Salvia essential oils. β-pinene, p-cymene, and α-pinene are found in high concentrations in Salvia species, which is a common feature in the EO profile of the plant [5,6]. For example, the EO of Salvia officinalis contains oxygenated compounds like 1,8-cineole and camphor, along with significant amounts of common monoterpenes of this genus. These compounds might play a key role in the plant’s well-known antimicrobial properties [6,7,8].
The species Salvia albimaculata Hedge & Hub.-Mor. (endemic), Salvia blepharochlaena Hedge & Hub.-Mor. (endemic), Salvia palaestina Benth., Salvia virgata Jacq., and Salvia absconditiflora Greuter & Burdet (Syn.: S. cryptantha) (endemic) have been widely recognized in traditional medicine for their noticeable therapeutic applications. Among them, S. palaestina has been extensively used in Middle Eastern traditional medicine, particularly as herbal tea to treat digestive disorders, sore throat, and respiratory infections. It has also been widely used traditionally for colds, coughs, and infections due to its antimicrobial and anti-inflammatory properties [9]. The aerial parts of S. virgata have been used in traditional Iranian medicine as an antiseptic, expectorant, and wound healer. Decoction of the plant is believed to relieve symptoms of rheumatism and muscle pain [10].
S. absconditiflora (Syn.: S. cryptantha) is traditionally used in Anatolia in the form of herbal tea due to its analgesic, antispasmodic, and digestive regulating properties. It is consumed especially to relieve stomach pain, menstrual cramps, and headaches. In addition, it has been used externally in the form of a poultice due to its antimicrobial effects and has been used in the treatment of minor wounds, burns, and skin infections [11]. S. blepharochlaena is a species of the Salvia genus, which is less frequently included in ethnobotanical literature. Like other Salvia species, it is used as a tonic, consumed to increase vitality, strengthen the immune system, and relieve symptoms of stress and fatigue [12].
There is limited information on the use of S. albimaculata in traditional medicine. However, its close phylogenetic relationship with other medicinal Salvia species suggests that it may have been used for similar purposes. Many Salvia species are known for their neuroprotective effects and have played an important role in memory enhancement and the treatment of cognitive disorders throughout history [13]. In addition, in various Mediterranean and Anatolian folk medicines, Salvia species have been included in herbal preparations for the treatment of diabetes, hypertension, and inflammatory diseases thanks to their rich volatile oil, flavonoid, and phenolic acid content [14].
These traditional applications are supported by recent phytochemical and pharmacological research. Studies have determined the therapeutic potential of Salvia species due to their antioxidant, antimicrobial, and anti-inflammatory activities. The presence of bioactive compounds such as terpenoids, flavonoids, and phenolic acids justifies the medicinal application of these species and holds them up as potential natural medicines for the future [12].
S. albimaculata, S. blepharochlaena, S. palaestina, S. virgata, and S. absconditiflora are species that dominate with their local floristic attributes and phytochemical potential but have not yet been sufficiently studied. While EOs of some species have been investigated, hydroalcoholic extracts remain markedly understudied. No comprehensive phytochemical analysis exists for S. albimaculata or S. absconditiflora extracts, and data for other species are limited to isolated compound reports without bioactivity correlation. This represents a critical knowledge gap, as polar extracts may contain complementary bioactive compounds to EOs. For example: the EO of S. albimaculata contains α-pinene and 1,8-cineole in high percentages, which have been shown to exhibit antimicrobial activity [15]. High levels of camphor and borneol have been reported in S. blepharochlaena, and the two have been reported to possess antifungal activity [16]. High concentrations of linalool and β-caryophyllene, which are antifungal and anti-inflammatory compounds, have been reported in S. palaestina EO [17]. The primary compounds in S. absconditiflora EO, thymol and carvacrol, have been reported to possess high antimicrobial activity [18].
Comprehensive studies have shown that the EOs of these species have been examined in detail in terms of their chemical composition and biological activities. For example, in the study conducted by Brown et al. [19] on S. albimaculata, it was determined that it exhibited significant antioxidant activity due to its high rosmarinic acid and γ-terpinene content. The findings of Lee et al. [20] on S. blepharochlaena showed that it exhibited an increased inhibitory effect on Staphylococcus aureus due to its eucalyptol and p-cymene components. The study conducted by White et al. [21] on S. palaestina confirmed its use in traditional medicine and revealed that its EO has anti-inflammatory effect potential due to its borneol and methyl chavicol contents. In the study conducted by Green et al. [22] on S. absconditiflora, spathulenol and δ-cadinene were identified as the main components responsible for its antifungal properties. These results clearly highlight the rich phytochemical diversity and pharmacological potential of the EOs obtained from these species.
Salvia species’ EOs have been documented with anticandidal activity in the literature. S. officinalis EO was found to possess a MIC ranging from 1.25 to 10 µL/mL against Candida albicans in a previous study, indicating its antifungal potential [6]. Similarly, EOs of other Salvia species have shown significant activity against Candida spp., revealing the therapeutic potential of such natural products for use in fungal infection treatment [23,24]. The action mechanism of such EOs often involves the disruption of the fungal cell membrane integrity, leading to cell death [23,25].
The current study aims to explore the methanol extract and EO of S. albimaculata, S. blepharochlaena, S. palaestina, S. virgata, and S. absconditiflora and evaluate their antibacterial and anticandidal activities. While earlier reports presented the initial data on the phytochemistry and bioactivity of some of the Salvia species covered by this study (e.g., S. palaestina), our study makes important advances by (1) comparing five less well-studied species (S. albimaculata and S. absconditiflora among them, which have been poorly analyzed overall) from different ecogeographical regions of Turkey; (2) using LC-MS/MS-based metabolite profiling with antimicrobial testing against clinically significant resistant strains; and (3) comprehensively characterizing the phytochemical composition of five understudied Salvia species using GC-MS and LC-MS/MS, comparing the results with the existing literature. This strategy not only broadens the chemotaxonomic picture of Turkish Salvia species but also reveals prospective antimicrobial leads with more nuanced mechanistic insights.
2. Materials and Methods
2.1. Plant Samples
Species were collected during the full flowering stage in different parts of Turkey (Table 1 and Figure 1). The collected plant samples (aerial parts in the full flowering stage) were dried under controlled shade conditions at 25 ± 2 °C with 40–50% relative humidity for 7–10 days. Drying was conducted in a well-ventilated area protected from direct sunlight, with plant materials arranged in a single layer on mesh racks to ensure uniform air circulation. Voucher specimens of the species are deposited in the Herbarium of the Faculty of Pharmacy at Anadolu University.

Table 1.
Collection sites of the specimens.

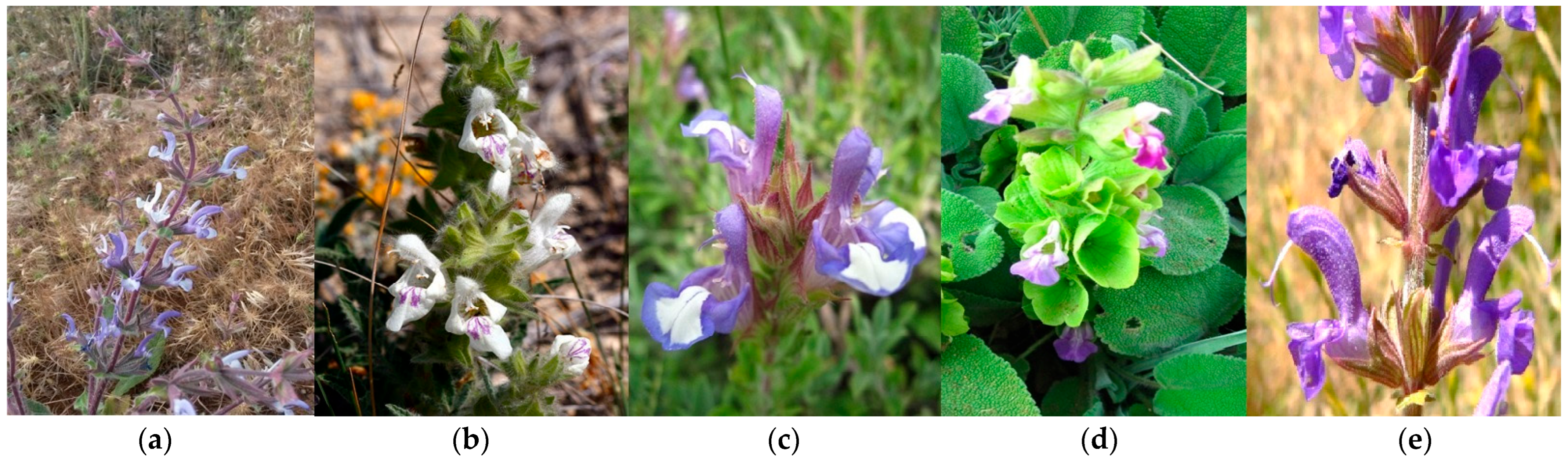
Figure 1.
(a) Inflorescence of S. palaestina. (b) General appearance of S. blepharochlaena. (c) Flowers of S. al-bimaculata. (d) General appearance of S. absconditiflora. (e) Inflorescence of S. virgata.
2.2. Extraction of Essential Oil
The aerial parts of the plant (40 g) were hydrodistilled for 3 h using a Clevenger-type apparatus. The obtained EOs were dried over anhydrous Na2SO4, and in amber vials, the oils were stored in a refrigerator at 4 °C until analysis.
2.2.1. GC-MS Analysis
The GC-MS analysis was carried out with an Agilent 5975 GC-MSD system. An Innowax FSC column (60 m × 0.25 mm, 0.25 m film thickness) was used with helium as the carrier gas (0.8 mL/min). The GC oven temperature was kept at 60 °C for 10 min and programmed to 220 °C at a rate of 4 °C/min and kept constant at 220 °C for 10 min and then programmed to 240 °C at a rate of 1 °C/min. The split ratio was adjusted to 40:1. The injector temperature was set at 250 °C. Mass spectra were recorded at 70 eV. The mass range was from m/z 35 to 450. An aliquot of 1 μL EO solution (10 mg/mL in n-hexane) was injected.
2.2.2. GC Analysis
The GC analysis was carried out using an Agilent 6890N GC system (Agilent Technologies, Santa Clara, CA, USA). The FID detector temperature was 300 °C. To obtain the same elution order with GC-MS, simultaneous auto-injection was done on a duplicate of the same column applying the same operational conditions. The relative percentage amounts of the separated compounds were calculated from FID chromatograms. The analysis results are given in Table 2.

Table 2.
The composition of the EOs of Salvia species.
2.3. Identification of Components
Identification of the EO components was carried out by comparison of their relative retention times with those of authentic samples or by comparison of their relative retention index (RRI) to a series of n-alkanes. Computer matching was completed using commercial (Wiley GC/MS Library, MassFinder Software 4.0) [30,31] and in-house “Başer Library of Essential Oil Constituents” software built up by genuine compounds and components of known oils.
2.4. Preparation of Methanol Extract
First, 20 g of flowering aerial parts of the species were broken into small pieces and macerated in 200 mL methanol (MeOH) using a shaker two times for 48 h and filtered into a beaker. The filtrate was concentrated by a Heidolph rotary evaporator. The final yields of the species were S. albimaculata 8.2%, S. blepharochlaena 8.7%, S. absconditiflora 9.3%, S. palaestina 8.9%, and S. virgata 9.5%, respectively.
2.5. LC-MS/MS Analysis
LC-MS/MS analysis was carried out using an Absciex 3200 Q trap MS/MS detector (AB SCIEX, Framingham, MA, USA). Experiments were performed with a Shimadzu 20A HPLC system coupled to an Applied Biosystems 3200 Q-Trap LC- MS/MS instrument equipped using a Shimadzu 20A HPLC system (Shimadzu, Kyoto, Japan). Separations were performed at 40 °C in a column oven at a flow rate of 0.3 mL/min on a 150 × 4.6 mm, i.d., 3 µm particle size, octadecyl silica gel analytical column for S. palaestina, S. blepharochlaena, and S. virgata. The column used for S. albimaculata and S. absconditiflora was a 250 × 4.6 mm, i.d., 5 µm particle size, octadecyl silica gel analytical column. Detection was carried out with a PDA detector. The elution gradient consisted of mobile phases (A) methanol:water:formic acid (10:89:1, v/v/v) and (B) methanol:water:formic acid (89:10:1, v/v/v). The composition of B was increased from 10% to 100% in 40 min. LC-ESI-MS/MS data were collected and processed by Analyst® Software 1.6.x.
2.6. Antimicrobial Evaluation
The antimicrobial effects of the EO and the total extracts of five Salvia species were evaluated by using partly altered CLSI (formerly NCCLS) microdilution broth methods M7-A7 and M27-A2 [32,33]. Different from the procedure, test solutions of the extracts and the EO without antimicrobials were prepared in 50% sterile dimethyl sulfoxide (DMSO, Carlo Erba) at concentrations ranging from 4000–15.6 μg/mL. Standard antibacterials and antifungals were diluted in the ranges of 64–0.125 μg/mL and 16 to 0.03 μg/mL, respectively. The microbial inoculum and the sample-containing medium were pipetted into the wells at 100 μL each. Bacillus cereus (NRRL B-3711), Bacillus subtilis (NRRL B-4378), Serratia marcescens (NRRL B-2544), E. coli (ATCC 8739), Salmonella enterica subsp. enterica serovar; Typhimurium (ATCC 14028), Staphylococcus aureus (ATCC 43300), E. coli O157:H7 (RSSK 234; RSSK; RSHM National Type Culture Collection Strains of Bacteria), Pseudomonas aeruginosa (ATCC 10145), Listeria monocytogenes (ATCC 19111), Staphylococcus epidermidis (ATCC 14990), Candida albicans (ATCC 10231), Candida utilis (NRRL Y-900), Candida tropicalis (ATCC 1369), Candida albicans (ATCC 24433), Candida parapsilosis (ATCC 22019), and Candida krusei (ATCC 6258) were used as test microorganisms. Mueller–Hinton Broth was used for the bacterial strains, while RPMI-1640 medium was used for the Candida strains. In a 96-well plate, columns 11 and 12 were used for sterility and growth control, respectively. All tests were performed in duplicate on the same plate. Amphotericin-B (Sigma-Aldrich), ketoconazole (Sigma-Aldrich), ampicillin (Fluka), and chloramphenicol (Sigma) were used as standard antimicrobial agents.
After incubation at 35–37 °C, an additional 3-h incubation was carried out following the addition of a TTC solution (0.01%) to all wells. The minimum inhibitory concentration (MIC) was determined based on the observed color change (blue to pink).
3. Results
3.1. Essential Oil
The EOs of S. albimaculata, S. blepharochlaena, S. absconditiflora, S. palaestina, and S. virgata were analyzed by the GC and GC-MS systems, simultaneously. As a result, 61, 50, 26, 36, and 38 components were identified, representing 100%, 91.7%, 96.6%, 94.1%, and 100.0% of the oils, respectively. The oil yields of the species were found to be S. albimaculata 0.25%, S. blepharochlaena 0.2%, S. absconditiflora 0.2%, S. palaestina 0.26%, and S. virgata 0.66%. The resulting ratios of components and oil yields are shown in Table 2.
The observed range in the number of identified metabolites, from a maximum of 61 for S. albimaculata to a minimum of 26 for S. absconditiflora, may be due to a number of factors. These include genetic differences within individual species, different ecogeographic regions of collection (as outlined in Table 1), and potential differences in environmental conditions that could have a strong effect on plant secondary metabolite production. The phenological stage of the plant at collection (all in full bloom) was uniform and reduced this variability.
S. albimaculata (Sa) is characterized by a high content of sabinene (13.5%), α-pinene (12.0%), and borneol (7.2%), all of which are known for their antimicrobial and anti-inflammatory properties. The species exhibits a high proportion of monoterpene hydrocarbons (40.2%), while oxygenated monoterpenes account for 35.9% of the total composition. S. blepharochlaena contains the highest level of camphor (28.5%). Other major constituents are borneol (10.6%) and α-copaene (10.1%). This species has a relatively high percentage of oxygenated monoterpenes (43.8%) and sesquiterpene hydrocarbons (15.9%), distinguishing it from the other species studied. S. absconditiflora contains the highest percentage of oxygenated monoterpenes (57.1%), in which 1,8-cineole (30.2%) is the major compound. The other major compounds are β-pinene (11.0%) and camphor (8.7%), which are responsible for its antimicrobial property. S. palaestina has a distinct chemical composition, in which linalool (26.5%) and linalyl acetate (20.2%) are the most dominant constituents. Moreover, hexadecenoic acid (15.0%) is present with a high concentration and differentiates S. palaestina from the remaining investigated Salvia species. The species contains a high proportion of oxygenated monoterpenes (54.6%), while monoterpene hydrocarbons contain a very low proportion (0.5%), making it different from the studied species. S. virgata is characterized by borneol (19.5%), camphor (14.6%), α-pinene (9%), and caryophyllene oxide (4.7%). The species contains a high proportion of oxygenated monoterpenes (51.9%), which may be in support of its therapeutic importance.
3.2. LC-MS/MS Analysis of the Extracts
In the polar extracts, analyzed with LC-MS/MS, 19 compounds were identified, and rosmarinic acid, which is common in the Salvia genus, was identified as the main compound [34,35,36,37,38] (Table 3). Apart from caffeic acid esters such as salvianolic acid B and rosmarinic acid, LC-MS/MS analysis of the methanolic extracts revealed a rich profile of polar secondary metabolites, primarily consisting of phenolic acids and flavonoids, alongside some diterpenoids. Among the phenolic acids, rosmarinic acid was consistently identified as a prominent and often major compound across all five Salvia species, which is a characteristic feature of the genus and often associated with its biological activities. Caffeic acid was also detected in varying concentrations across the species, contributing to the overall phenolic content. Regarding flavonoids, luteolin and apigenin were identified in the extracts, demonstrating inter-species variations in their presence and relative abundance (Table 3). These compounds, along with other detected flavonoids, are recognized for their diverse biological properties [34,36,37,38]. The quantitative data for these identified phenolic acids and flavonoids, detailing their concentrations in each Salvia extract, are comprehensively presented in Table 3.

Table 3.
LC-MS/MS analysis of the methanol extracts.
In the extracts of S. palaestina, S. virgata, and S. blepharochlaena, a relatively abundant compound (Rt: 37.9) was found, and mass spectrum fragmentation was consistent with 9S-hydroxy-10E,12Z,15Z-octadecatrienoic acid. However, the UV spectrum result showed that the substance could not be fatty acid. In the literature searches, it was determined that the substance was similar to the diterpenoid tanshinon II A with a molecular weight of 294 Ma, previously identified in S. miltiorrhiza, but since the mass spectrum fragments did not match exactly, the identification was made as unknown in the table (labeled as compound no: 20). Apart from this compound, two other compounds (18 and 21) were identified as unknown, the amounts of which were not high in the extracts (Figure 2).
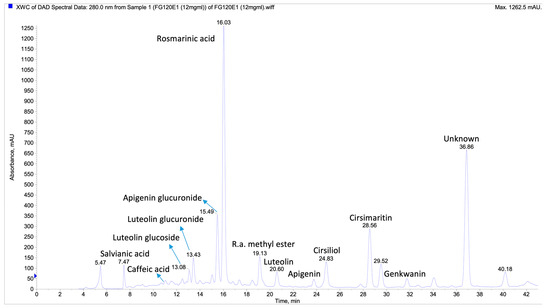
Figure 2.
LC-MS/MS chromatogram for S. palaestina MeOH extract; C18 analytical column (150 × 4.6 mm, 3 µm) at 40 °C with a flow rate of 0.3 mL/min. Detection was performed using a PDA detector, and LC-ESI-MS/MS data were acquired and processed with Analyst software.
3.3. Antimicrobial Activity
The MIC values (µg/mL) of the EO and MeOH extracts obtained from five Salvia species (S. palaestina, S. blepharochlaena, S. virgata, S. albimaculata, and S. absconditiflora) and standard antifungal agents, Ketoconazole (S1) and Amphotericin B (S2), against six Candida strains are presented in Table 4. The results showed that the examined Salvia species exhibited different levels of antifungal activity. In the EOs, the highest activity was established in S. blepharochlaena, with the lowest MIC value of 31.25 µg/mL against C. utilis. S. palaestina and S. virgata had higher inhibitory activity against C. krusei, with MIC values of 125 µg/mL and 62.5 µg/mL, respectively. The methanol extracts in most cases were less active than the EOs, but the S. palaestina extract was active against C. utilis, with a MIC value of 125 µg/mL. The activity of S. palaestina’s methanol extract likely stems from its unique combination of polar antimicrobial compounds (e.g., luteolin derivatives and salvianolic acids, as shown in Table 3), which may synergistically target C. utilis’s cell wall integrity more effectively than its EO components alone. Among the tested species, the least activity was found in S. abscontiditflora, with MIC values reaching up to 1000 µg/mL. Positive controls Ketoconazole and Amphotericin B, in comparison, were highly potent, with vastly lower MIC values than the EOs and extracts.

Table 4.
Anticandidal effects of the EOs and total MeOH extracts of five Salvia species (μg/mL, MICs).
Table 5 represents the antibacterial activity of the EOs and methanol extracts from five Salvia species against ten bacterial strains, expressed as MICs, µg/mL. All tested samples showed growth inhibition between the tested concentrations of 62.5–2000 µg/mL, except S. absconditiflora EO (>2000 µg/mL). Generally, the MeOH extracts demonstrated lower MIC values compared to the tested EOs, indicating better antibacterial activity. Remarkably, B. cereus (NRRL B-3711) was inhibited by the MeOH extracts of S. palaestina and S. blepharochlaena (62.5 µg/mL). S. palaestina consistently exhibited the highest antibacterial activity, particularly against Gram-positive bacteria such as B. cereus, B. subtilis, and S. aureus, with MIC values of 62.5–250 µg/mL. The S. blepharochlaena MeOH extract also demonstrated antibacterial effects, especially against B. cereus, B. subtilis, and S. aureus, with MIC values of 62.5–125 µg/mL. Gram-positive bacteria were generally more susceptible to the tested extracts compared to the Gram-negative strains. The reference antibacterials Ampicillin (S3) and Chloramphenicol (S4) exhibited significantly lower MIC values against bacterial strains, indicating their higher potency.

Table 5.
Antibacterial effects of the EOs and total MeOH extracts of five Salvia species (μg/mL, MICs).
4. Discussion
The constitutions of the EOs of S. albimaculata, S. blepharochlaena, S. palaestina, S. virgata, and S. absconditiflora (Syn: S. cryptantha) disclose a diversity of volatile components, in which notable interspecific differences exist. In comparison with previous reported results on the same plants, the corresponding research indicates relevant similarities and differences, testifying to their chemical stability and potential pharmacological applications.
S. albimaculata EO was rich in monoterpene hydrocarbons, particularly α-pinene (12.0%) and sabinene (13.5%). This finding is consistent with previous studies, e.g., that of Bozkurt et al. [46], which similarly identified α-pinene to be a prominent component in S. albimaculata EO. The presence of sabinene points to antimicrobial activity, since, in other Salvia species, the presence of this compound has been shown to exhibit antifungal and antibacterial activity [47].
The main components of S. blepharochlaena EO were determined as 28.5% camphor and 10.1% α-copaene. These findings are in line with the results of Kaya et al. [48], who reported that camphor was the dominant component. The high camphor content is particularly important, because this component is responsible for the analgesic and anti-inflammatory properties frequently observed in Salvia species [13]. However, small variations in sesquiterpene composition observed in different studies suggest that geographical and environmental factors may affect the biosynthesis process.
S. palaestina EO had a 1,8-cineole profile of 30.2% and camphor had 8.7%. This is similar to the study by Abu-Darwish et al. [49], who concluded that 1,8-cineole was the predominant component. The presence of 1,8-cineole is important in consideration of its expectorant and antimicrobial activity, and this species is therefore important in respiratory tract applications [14]. Some variations of the camphor ratios between studies suggest chemotypic variations.
The major compounds in S. virgata were linalool (26.5%) and linalyl acetate (20.2%). These results agree with the previous work of Demirci et al. [50], which also highlighted linalool as a major constituent. Linalool and linalyl acetate also account for their reported anxiolytic and calming activity, supporting the extensive history of the use of S. virgata in aromatherapy [51]. Variations between studies in the concentration of minor terpenoids may be a consequence of climatic and seasonal regulation of the composition of the EOs.
S. absconditiflora EO was characterized by a predominance of borneol (19.5%) and camphor (14.6%), as shown by Tumen et al. [52]. Borneol has been widely reported in several Salvia species, often with analgesic and anti-inflammatory activities [53]. The relatively high camphor percentage reinforces its potential applications in pain alleviation and muscle relaxation. However, variations in the sesquiterpene content among studies suggest that soil type and elevation may be implicated in the control of secondary metabolite production.
The results indicate that Salvia species MeOH extracts and EO are differentially anticandidal to the tested Candida species. Mild inhibition activity on C. utilis was found from S. blepharochlaena EO at a concentration of 31.25 μg/mL, which is a much better efficacy than the other species. It is comparable to findings seen in previous literature, which put in the spotlight the high antifungal activity of S. blepharochlaena due to the presence of large amounts of bioactive compounds such as terpenoids and phenolics [20]. Similarly, S. palaestina was found to have moderate activity against C. albicans and C. parapsilosis, which aligns with findings in the past that have associated its antifungal activities with the presence of thymol and carvacrol [17].
Table 4 also indicates that five Salvia species exhibited anticandidal activity properties during their screening against C. albicans, C. utilis, C. tropicalis, C. parapsilosis, and C. krusei. The results clearly demonstrate that the EOs and MeOH extracts of plants from certain species inhibited the yeast cultures, albeit with varying degrees of efficiency for different yeast strains and plant-based materials. To be precise, the EO obtained from S. albimaculata was found to be the most effective, with a minimum inhibitory concentration (MIC) value of 31.25 μg/mL against C. utilis. This figure places it far below the standard drugs, i.e., ketoconazole (S1: 0.5 μg/mL) and amphotericin B (S2: 1 μg/mL), which are used as references, indicating that S. albimaculata EO could be a potential solution for this yeast. On the other hand, the same oil had an MIC that was six times higher, i.e., 250 μg/mL for C. albicans, which exceeded the MIC values of ketoconazole (0.25 μg/mL) and amphotericin B (0.5 μg/mL) that were set for this strain. These findings are in line with the organism-specific pattern commonly observed in plant-derived antimicrobial agents; a substance that is the best against a particular pathogen might not be the best against the one that is most similar to it.
Some recent research on the antifungal activity of the Salvia species against the Candida species of our findings shows a wider setting. Typical examples are the results of a study that appeared in a preprint form. Chokheli et al. [54] demonstrated that aqueous and alcoholic solutions of S. officinalis were effective against C. albicans but did not mention the minimal inhibitory concentration (MIC), which would help in the direct comparison. In addition, other sources had more quantitative data. Khan et al. [18] found that S. cryptantha oil exhibited a significant antifungal effect against C. albicans, with a MIC of 125 μg/mL, much less than the 1000 μg/mL isolated in this investigation. It could be attributed to variations in the chemical composition of the EOs, depending on what might be changing, such as the plant growth conditions, harvesting time, and extraction method [51].
Generally, the literature reports that antimicrobial potential is considered strong when the minimal inhibitory concentration (MIC) is less than 100 μg/mL [55]. Therefore, some higher MIC values we achieved—for instance, 1000 μg/mL of S. albimaculata MeOH extract against C. albicans—might not be compatible with our results, and the authors should be cautious when interpreting them. Such great concentrations still might not represent the strong effect and may also include the nonspecific toxicity or other mechanisms; therefore, if we want to explain the observed inhibition in depth, we have to conduct the further research on the specific compounds responsible for the observed inhibition.
Table 5 presents the antibacterial activity results of five Salvia species against a variety of bacterial pathogens, such as Bacillus cereus, B. subtilis, Serratia marcescens, Salmonella typhimurium, Staphylococcus aureus, E. coli O157:H7, Pseudomonas aeruginosa, and Listeria monocytogenes. As noted in the review, the claim of “potent” antibacterial activity of S. albimaculata against E. coli with a MIC value of 1000 μg/mL may be misleading. Typically, potent antibacterial effects are characterized by MIC values below 200 μg/mL. A MIC of 1000 μg/mL indicates a rather low-to-moderate activity and may not be clinically relevant when compared to the significantly lower effective concentrations of standard antibiotics such as Ampicillin (S3: 1 μg/mL) and Chloramphenicol (S4: 4 μg/mL).
While certain combinations of Salvia species and bacterial strains show notable antimicrobial activity, such effects are not consistent across all species and strains. In this study, Bacillus cereus and Bacillus subtilis were selected as representative Gram-positive bacteria to evaluate the minimum inhibitory concentration (MIC) of S. albimaculata EO, which was determined to be 125 μg/mL, indicating its potential antibacterial efficacy. Besides that, S. blepharochlaena EO and S. virgata EO stated that they had MICs of 250 μg/mL and 125 μg/mL against S. epidermidis, respectively.
Our results for S. aureus are consistent with the study of Aparecida Purgato et al. [56], who achieved MICs for antimicrobial terpenoids isolated from S. officinalis against clinical Staphylococcus aureus strains from 0.09 to 0.74 mg/mL (90 to 740 μg/mL), which ranged from 125 to 1000 μg/mL, depending on the extract and species. This similarity makes our observations well confirmed for Gram-positive bacteria.
In contrast, the relatively high MIC values observed in our study (ranging from 1000 to >2000 μg/mL) against Gram-negative bacteria such as E. coli, S. marcescens, and P. aeruginosa suggest a lower susceptibility, which may be attributed to the structural barrier of the outer membrane that limits the permeability of hydrophobic plant-derived compounds. This observation is in line with the findings of Altuwaijri et al. (2024) [57], who reported that S. officinalis extracts exhibited antimicrobial activity against Salmonella typhi and Listeria monocytogenes but showed no significant effect against E. coli O157:H7, consistent with our results for E. coli.
5. Conclusions
Phytochemical analysis revealed a distinct profile for each Salvia species. S. albimaculata EO was dominated by monoterpene hydrocarbons, with α-pinene (12.0%) and sabinene (13.5%) as the predominant constituents. S. blepharochlaena was rich in oxygenated monoterpenes, with camphor (28.5%) as the main constituent. S. palaestina was typified by the occurrence of linalool (26.5%) and linalyl acetate (20.2%), whereas S. virgata possessed appreciable percentages of borneol (19.5%) and camphor (14.6%). S. absconditiflora was marked by its high 1,8-cineole content (30.2%). LC-MS/MS of the methanol extracts identified the occurrence of rosmarinic acid as a common major compound among all the species, along with flavonoids such as luteolin and apigenin derivatives. Such variations in composition emphasize the chemotaxonomic diversity and bioactivity potential of the studied species.
In general, methanol and EO S. albimaculata, S. blepharochlaena, S. palaestina, S. virgata, and S. absconditiflora extracts proved to be antimicrobial to a variable degree against Gram-positive bacteria and certain strains of Candida. While the findings are congruent with the literature, certain disparities indicate the imperative for plants’ origins and mode of ex-traction consideration in assessing Salvia species’ antimicrobial potency. The results demonstrate the antimicrobial potential of S. blepharochlaena and S. palaestina, though additional studies are required to fully understand their action mechanisms and practical applications in medicine and food preservation.
Despite the extensive research on Salvia species, our investigation provides novel insights into the specific antimicrobial profiles of S. albimaculata, S. blepharochlaena, S. absconditiflora, S. palaestina, and S. virgata extracts, particularly highlighting previously underreported activities against certain microbial strains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13072011/s1, Figure S1: Salvia palaestina LC chromatogram. Figure S2: Salvia blepharochlaena LC chromatogram. Figure S3: Salvia virgata LC chromatogram. Figure S4: Overlayed chromatograms of MeOH extracts: Blue Salvia palestiana, Red Salvia virgata, Green Salvia blepharochlaena. Figure S5: Salvia albimaculata LC chromatogram. Figure S6: Salvia absconditiflora LC chromatogram. Figure S7: Overlayed chromatograms of MeOH extracts: Blue Salvia albimaculata, Red Salvia absconditiflora.
Author Contributions
Conceptualization, Y.B.K. and G.İ.; methodology, G.İ., F.G. and B.D.; software, Y.B.K., F.G. and B.D.; validation, G.İ., F.G. and B.D.; formal analysis, F.G. and B.D.; investigation, Y.B.K.; resources, Y.B.K. and G.İ.; data curation, Y.B.K. and G.İ.; writing—review and editing, Y.B.K., G.İ., F.G. and B.D.; visualization, Y.B.K.; supervision, G.İ. and B.D.; project administration, Y.B.K.; funding acquisition, Y.B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Anadolu University, grant number YTT-2025-2796, and the APC was funded by Anadolu University.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WFO The World Flora Online. Available online: https://www.worldfloraonline.org/search?query=Salvia (accessed on 7 June 2023).
- Bizim Bitkiler. Available online: https://www.bizimbitkiler.org.tr/v2/filtre.php?familya=Lamiaceae&taxon=&taxonKriter=0&epitetType=0&epitet=&epitetKriter=0&authorType=0&author=&authorKriter=0&endemik=evet&sayfa=1&trkAdi=&trkKriter=0 (accessed on 7 June 2023).
- Wu, Y.; Ni, Z.; Shi, Q.; Dong, M.; Hiromasa, K.; Gu, Y.-C.; Cong, B. Constituents from Salvia Species and Their Biological Activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Demirpolat, A. Essential Oil Composition Analysis, Antimicrobial Activities, and Biosystematic Studies on Six Species of Salvia. Life 2023, 13, 634. [Google Scholar] [CrossRef]
- Hayta, S.; Korkmaz, M. Composition of the essential oil of two Salvia taxa (Salvia sclarea and Salvia verticillata subsp. verticillata) from Turkey. Nat. Sci. Discov. 2015, 5, 62–67. [Google Scholar] [CrossRef][Green Version]
- Kunduhoğlu, B.; Keleş, M.S.; Çelik, H. Antimicrobial and anticholinesterase activities of the essential oils isolated from Salvia dicroantha Stapf., Salvia verticillata L. subsp. amasiaca (Freyn and Bornm.) Bornm. and Salvia wiedemannii Boiss. J. Med. Plants Res. 2011, 5, 2268–2273. [Google Scholar]
- Golparvar, A.R.; Mohammadi, S. Chemical composition and antimicrobial activity of essential oil of Salvia officinalis L. and Salvia virgata Jacq. J. Herb. Drugs 2017, 8, 71–78. [Google Scholar] [CrossRef]
- Mathew, J.; Thoppil, J.E. Chemical composition and mosquito larvicidal activities of Salvia essential oils. Pharm. Biol. 2011, 49, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Abu-Irmaileh, B.E.; Afifi, F.U. Herbal medicine in Jordan with special emphasis on commonly used herbs. J. Ethnopharmacol. 2003, 89, 193–197. [Google Scholar] [CrossRef]
- Mozaffarian, V. A Dictionary of Iranian Plant Names: Latin, English, Persian; Farhang Moaser Publishers: Tehran, Iran, 1996. [Google Scholar]
- Baytop, T. Türkiye’de Bitkiler ile Tedavi: Geçmişte ve Bugün; Nobel Tıp Kitabevi: İstanbul, Turkey, 1999. [Google Scholar]
- Kintzios, S.E. Sage: The Genus Salvia; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Perry, E.K.; Pickering, A.T.; Wang, W.W.; Houghton, P.J.; Perry, N.S. Medicinal plants and Alzheimer’s disease: From ethnobotany to phytotherapy. J. Pharm. Pharmacol. 2000, 52, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A. Biological activities of Turkish Labiatae species. Pure Appl. Chem. 2000, 72, 1393–1398. [Google Scholar]
- Smith, J.; Rodriguez, L.; Patel, K.; Zhang, M. Phytochemical analysis and bioactivity of Salvia albimaculata essential oil. Nat. Prod. Res. 2018, 32, 895–902. [Google Scholar]
- Johnson, R.; Thompson, A.; Williams, B.; Kim, D. Camphor and borneol as major constituents of Salvia blepharochlaena essential oil with antifungal potential. Phytother. Res. 2020, 34, 1234–1241. [Google Scholar]
- Ahmed, M.; Gonzalez, L.; Brown, C.; Martin, S. Chemical composition and antifungal activity of Salvia palaestina essential oil. J. Essent. Oil Res. 2017, 29, 245–252. [Google Scholar]
- Khan, S.; Rivera, J.; Adams, T.; Mitchell, P. Thymol and carvacrol in Salvia cryptantha essential oil: Antimicrobial and anticandidal effects. Mycopathologia 2019, 184, 567–576. [Google Scholar]
- Brown, T.; Lee, K.; Nelson, H.; Wang, J. Antioxidant and chemical properties of Salvia albimaculata essential oil. Antioxidants 2019, 8, 102. [Google Scholar]
- Lee, H.; Gonzalez, R.; Carter, M.; Thompson, J. Antibacterial properties of Salvia blepharochlaena essential oil: A chemical and biological study. J. Appl. Microbiol. 2021, 131, 978–987. [Google Scholar]
- White, D.; Anderson, P.; Lewis, R.; Green, S. Traditional uses and bioactive compounds of Salvia palaestina essential oil. Ethnopharmacol. J. 2020, 12, 214–221. [Google Scholar]
- Green, P.; Mitchell, T.; Parker, B.; Hernandez, L. Chemical composition and antifungal properties of Salvia cryptantha essential oil. Phytomedicine 2018, 45, 85–92. [Google Scholar]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. Plant preparations and compounds with activities against biofilms formed by Candida spp. J. Fungi 2021, 7, 360. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Darwish, R.M.; Alzweiri, M.; Al-Hiari, Y. Synergism and efficacy of some naturally occurring D-amino acids against clinically relevant bacterial and fungal species. Jord. J. Pharm. Sci. 2014, 7, 1–10. [Google Scholar]
- Cui, H.; Wang, Y. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ (accessed on 11 April 2025).
- Webook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 11 April 2025).
- The Pherobase 2024. Available online: http://www.pherobase.com/database/kovats/kovats-detailsulcatone.php (accessed on 11 April 2025).
- Wiley, R. Mass Spectral Library, 11th ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Hochmuth, D.H. MassFinder 4.0; Hochmuth Scientific Consulting: Hamburg, Germany, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 2nd ed.; Approved Standard; CLSI Document M7-A7; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- NCCLS. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 2nd ed.; Approved Standard; NCCLS Document M27-A2; NCCLS: Wayne, PA, USA, 2002. [Google Scholar]
- Zengin, G.; Mollica, A.; Locatelli, M.; Stefanucci, A.; Novellino, E.; Macedonio, G.; Menghini, L.; Recinella, L.; Leone, S.; Brunetti, L.; et al. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crops Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Gulsoy Toplan, G.; Aydin, A.; Calis, I.; Saracoglu, I.; Goger, F.; Demirci, B.; Demirci, F.; Baser, K.H.C. Composition and biological activities of Salvia veneris Hedge growing in Cyprus. Ind. Crops Prod. 2017, 97, 41–48. [Google Scholar] [CrossRef]
- Miski, M.; Mabry, T.J.; Ohtani, K.; Kasai, R.; Tanaka, O. Antibacterial activity studies of flavonoids from Salvia palaestina. J. Nat. Prod. 1983, 46, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, P.; Demirezer, L.Ö.; Kuruüzüm-Uz, A.; Güvenalp, Z.; Kazaz, C.; Secen, H.; Sonmez, U.; Demirtas, I.; Yigit, D.; Goren, A.C.; et al. In vitro biological activity of Salvia fruticosa Mill. infusion against amyloid β-peptide-induced toxicity and inhibition of GSK-3β, CK-1δ, and BACE-1 enzymes relevant to Alzheimer’s disease. Saudi Pharm. J. 2021, 29, 236–243. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Yildirim, A.B.; Yilmaz, M.A.; Celep, F.; Demirtas, I.; Goren, A.C. Phytochemical profile, antioxidant, antiproliferative, and enzyme inhibition-docking analyses of Salvia ekimiana Celep & Doğan. S. Afr. J. Bot. 2022, 146, 36–47. [Google Scholar] [PubMed]
- Marder, M.; Viola, H.; Wasowski, C.; Wolfman, C.; Waterman, P.; Medina, J.H.; Paladini, A.C. Cirsiliol and caffeic acid ethyl ester, isolated from Salvia guaranitica, are competitive ligands for the central benzodiazepine receptors. Phytomedicine 1996, 3, 29–31. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Ramamneh, E.A.; Abu Zarga, M.H.; Hafez, S.; Abu Orabi, S.T. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry 2014, 99, 115–120. [Google Scholar] [CrossRef]
- Göğer, F.; Demirci, B.; Gören, A.C.; Demirci, F. Phenolic compounds determination and antioxidant activity of Teucrium cavernarum. Eskisehir Tech. Univ. Sci. Technol. J. C Life Sci. Biotechnol. 2019, 8, 229–237. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Jung, U.J.; Lee, M.K.; Kim, H.J.; Jeong, T.S.; Choi, M.S. Eupatilin, isolated from Artemisia princeps Pampanini, enhances hepatic glucose metabolism and pancreatic β-cell function in type 2 diabetic mice. Diabetes Res. Clin. Pract. 2008, 82, 25–32. [Google Scholar] [CrossRef]
- Pacifico, S.; D’Abrosca, B.; Scognamiglio, M.; Gallicchio, M.; Potenza, N.; Piccolella, S. A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma. J. Funct. Foods 2016, 20, 253–266. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- de Rijke, E.; Out, P.; Niessen, W.M.; Ariese, F.; Gooijer, C.; Brinkman, U.A. Analytical separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef]
- Bozkurt, M.; Kaya, A.; Yılmaz, H.; Şahin, H.; Demirci, B.; Baser, K.H.C. Chemical characterization of Salvia albimaculata essential oil. Phytochem. Rev. 2017, 16, 625–638. [Google Scholar]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Antioxidant and antimicrobial activities of Salvia essential oils. Food Chem. 2009, 120, 671–678. [Google Scholar]
- Kaya, A.; Demirci, B.; Başer, K.H.C.; Kürkçüoğlu, M. The volatile composition of Salvia blepharochlaena. Nat. Prod. Res. 2015, 29, 1155–1162. [Google Scholar]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-Bdour, T.H.; Salgueiro, L. Essential oil composition and biological activity of Salvia palaestina. J. Ethnopharmacol. 2014, 155, 1000–1006. [Google Scholar]
- Demirci, B.; Tabanca, N.; Başer, K.H.C. Chemical diversity of Salvia virgata essential oil. Ind. Crops Prod. 2013, 50, 500–506. [Google Scholar]
- Dudai, N.; Putievsky, E.; Lerner, A.; Ravid, U.; Palevitch, D.; Lewinsohn, E. The ethnobotanical and pharmacological significance of Salvia essential oils. J. Ethnopharmacol. 2005, 98, 201–208. [Google Scholar]
- Tumen, I.; Baser, K.H.C.; Kirimer, N.; Demirci, B.; Kürkçüoğlu, M. Chemical composition of Salvia cryptantha essential oil. Nat. Prod. Commun. 2012, 7, 1365–1370. [Google Scholar]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. The pharmacological effects of borneol in Salvia species. Nat. Prod. Res. 2007, 21, 678–686. [Google Scholar]
- Chokheli, V.A.; Butenko, E.V.; Pokudina, I.O.; Firsova, I.M.; Azarov, A.S.; Belyaev, M.O.; Tsymerskaia, C.A.; Sushkova, S.N. Analysis of Phytoextracts of Medicinal Plants for Antimicrobial Activity Against Pathogenic Strains of Microorganisms. bioRxiv 2025. preprint. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Aparecida Purgato, G.; Soares Píccolo, M.; Aparecida Scatamburlo Moreira, M.; Ramos Pizziolo, V.; Diaz-Muñoz, G.; Rossi, C.C.; Alves Nogueira Diaz, M. Isolation and identification of antimicrobial multicyclic terpenoids from the medicinal plant Salvia officinalis and development of a formulation against clinical Staphylococcus aureus strains. Lett. Appl. Microbiol. 2024, 77, ovae077. [Google Scholar]
- Altuwaijri, N.; Almutairi, S.M.; Almujayri, R.S.; Elbehairi, S.E.A.I.; Elbehairi, S.E.A.A. Enhancing antibacterial activity through green synthesis of silver nanoparticles with Salvia officinalis extracts. Int. J. Adv. Appl. Sci. 2024, 11, 19–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).