Integrating Machine Learning and In Vitro Screening to Evaluate Drought and Temperature Stress Responses for Vicia Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sterilization Process
2.3. Germination Medium Preparation and Culture Conditions
2.4. Drought Stress Application Using PEG-6000
2.5. Data Collection and Experimental Design
2.6. Statistical Analysis
2.7. Machine Learning Approaches
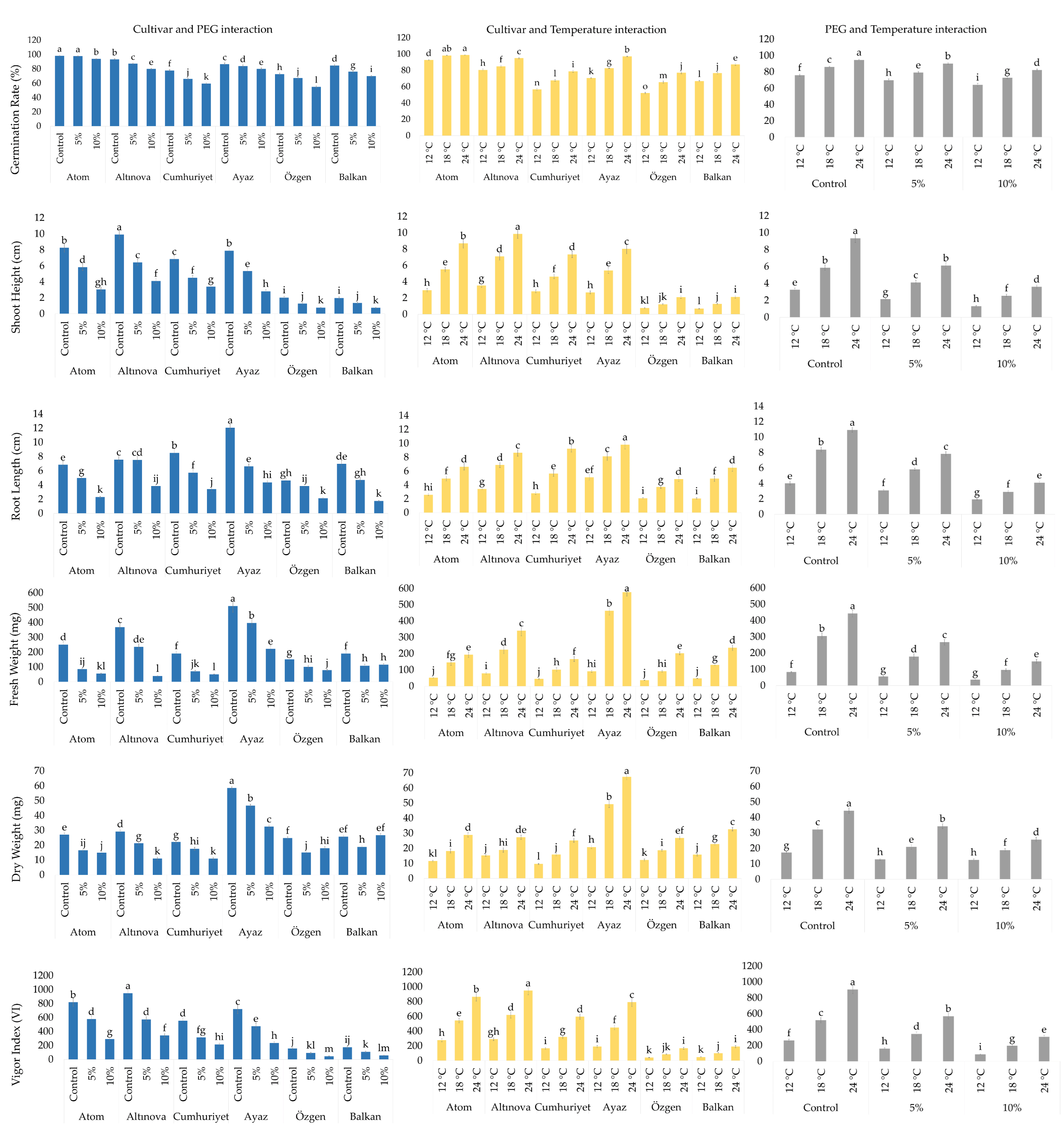
3. Results
3.1. Variance Analysis
3.2. Correlation and PCA Analysis
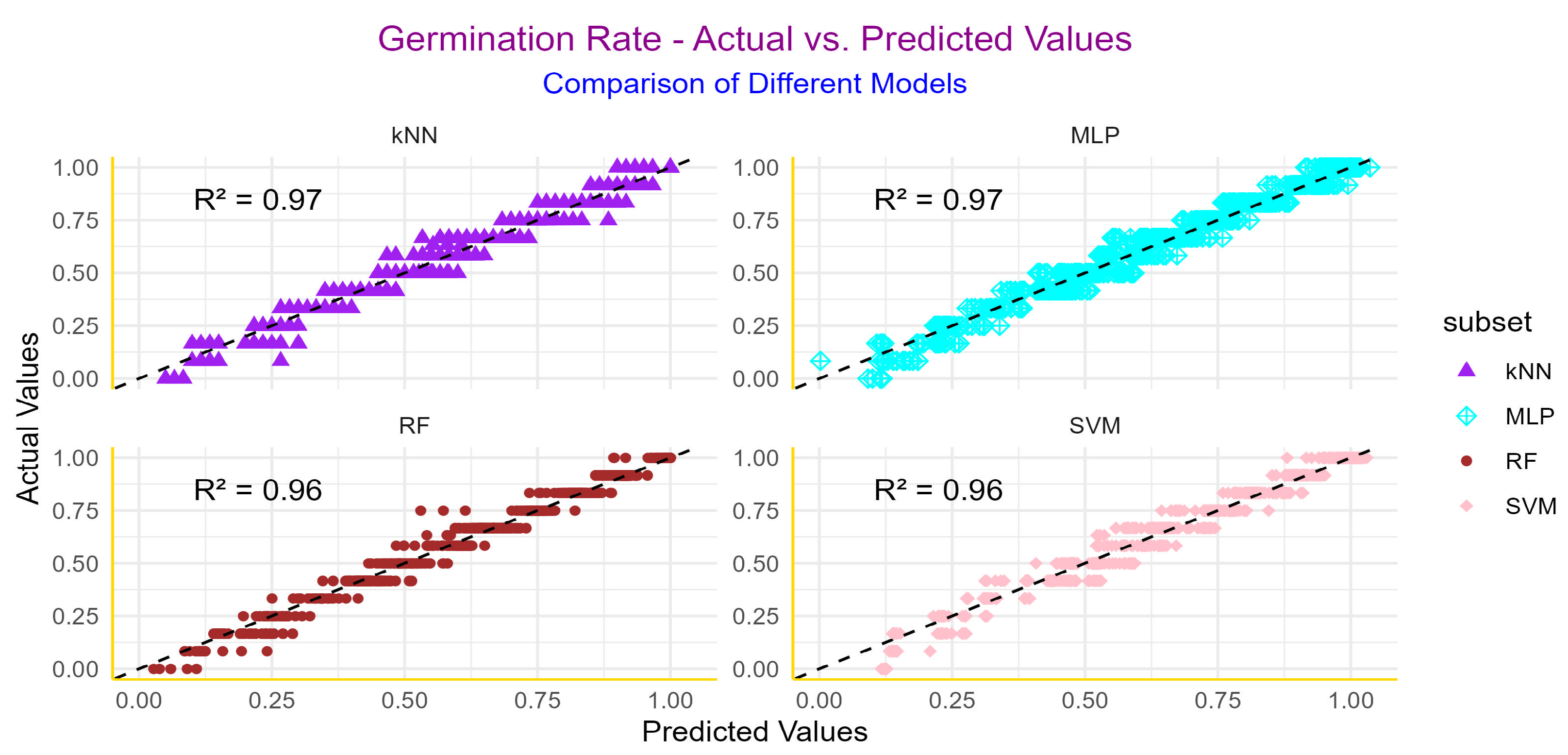
3.3. Machine Learning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leht, M. Phylogenetics of Vicia (Fabaceae) based on morphological data. Feddes Repert. 2009, 120, 379–393. [Google Scholar] [CrossRef]
- Yadollahi, P.; Eshghizadeh, H.R.; Razmjoo, J.; Zahedi, M.; Majidi, M.M.; Gheysari, M. Drought stress tolerance in vetch plants (Vicia sp.): Agronomic evidence and physiological signatures. J. Agric. Sci. 2024, 163, 1–14. [Google Scholar] [CrossRef]
- Nguyen, V.; Riley, S.; Nagel, S.; Fisk, I.; Searle, I.R. Common vetch: A drought tolerant, high protein neglected leguminous crop with potential as a sustainable food source. Fron. Plant Sci. 2020, 11, 818. [Google Scholar] [CrossRef]
- Huang, Y.F.; Gao, X.L.; Nan, Z.B.; Zhang, Z.X. Potential value of the common vetch (Vicia sativa L.) as an animal feedstuff: A review. J. Anim. Phys. Anim. Nutr. 2017, 101, 807–823. [Google Scholar] [CrossRef]
- Albayrak, S.; Sevimay, C.S.; Töngel, Ö. Effects of inoculation with rhizobium on seed yield and yield components of common vetch (Vicia sativa L.). Turk. J. Agri. Forest. 2006, 30, 31–37. [Google Scholar]
- Ramírez-Parra, E.; De la Rosa, L. Designing novel strategies for improving old legumes: An overview from common vetch. Plants 2023, 12, 1275. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Vetch Production Statistics (2023). FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 March 2025).
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Fron. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Paranychianakis, N.V.; Angelakis, A.N. Water supply and water scarcity. Water 2020, 12, 2347. [Google Scholar] [CrossRef]
- Ashraf, M. Inducing drought tolerance in plants: Recent advances. Biotechnol. Advanc. 2010, 28, 169–183. [Google Scholar] [CrossRef]
- Sabagh, A.E.; Hossain, A.; Islam, M.S.; Iqbal, M.A.; Amanet, K.; Mubeen, M.; Nasim, W.; Wasaya, A.; Llanes, A.; Ratnasekera, D.; et al. Prospective role of plant growth regulators for tolerance to abiotic stresses. In Plant Growth Regulators: Signalling Under Stress Conditions; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–38. [Google Scholar]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Internation. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resourc. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Micheletto, S.; Rodriguez-Uribe, L.; Hernandez, R.; Richins, R.D.; Curry, J.; O’Connell, M.A. Comparative transcript profiling in roots of Phaseolus acutifolius and P. vulgaris under water deficit stress. Plant Sci. 2007, 173, 510–520. [Google Scholar] [CrossRef]
- Fang, X.; Turner, N.C.; Yan, G.; Li, F.; Siddique, K.H. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J. Exper Bot. 2010, 61, 335–345. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Chai, X.; Dong, R.; Liu, W.; Wang, Y.; Liu, Z. Optimizing sample size to assess the genetic diversity in common vetch (Vicia sativa L.) populations using start codon targeted (SCoT) markers. Molecules 2017, 22, 567. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Z.; Nan, Z.; Unkovich, M.; Coulter, J.A. Effects of cultivar and growing degree day accumulations on forage partitioning and nutritive value of common vetch (Vicia sativa L.) on the Tibetan plateau. J. Sci. Food Agric. 2021, 101, 3749–3757. [Google Scholar] [CrossRef]
- Qu, A.L.; Ding, Y.F.; Jiang, Q.; Zhu, C. Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 2013, 432, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Sharavdorj, K.; Jang, Y.; Byambadorj, S.O.; Cho, J.W. Understanding seed germination of forage crops under various salinity and temperature stress. J. Crop Sci. Biotechnol. 2021, 24, 545–554. [Google Scholar] [CrossRef]
- Gurvich, D.E.; Pérez-Sánchez, R.; Bauk, K.; Jurado, E.; Ferrero, M.C.; Funes, G.; Flores, J. Combined effect of water potential and temperature on seed germination and seedling development of cacti from a mesic Argentine ecosystem. Flora 2017, 227, 18–24. [Google Scholar] [CrossRef]
- Guo, M.; Zong, J.; Zhang, J.; Wei, L.; Wei, W.; Fan, R.; Zhang, T.; Tang, Z.; Zhang, G. Effects of temperature and drought stress on the seed germination of a peatland lily (Lilium concolor var. megalanthum). Fron. Plant Sci. 2024, 15, 1462655. [Google Scholar] [CrossRef]
- Guedes, R.S.; Alves, E.U.; Viana, J.S.; Goncalves, E.P.; de Lima, C.R.; Nascimento dos Santos, S. Germination and vigor of Apeiba tibourbou seeds submitted to water stress and to different temperatures. Cienc. Florest. 2013, 23, 45–53. [Google Scholar] [CrossRef]
- Vicente, M.J.; Martínez-Díaz, E.; Martínez-Sánchez, J.J.; Franco, J.A.; Bañón, S.; Conesa, E. Effect of light, temperature, and salinity and drought stresses on seed germination of Hypericum ericoides, a wild plant with ornamental potential. Sci. Hortic. 2020, 270, 109433. [Google Scholar] [CrossRef]
- Basal, O.; Szabó, A.; Veres, S. PEG-induced drought stress effects on soybean germination parameters. J. Plant Nutr. 2020, 43, 1768–1779. [Google Scholar] [CrossRef]
- Demirel, S.; Eroğlu, A.; Eren, B.; Demirel, F. DNA methylation change and antioxidant enzyme activity of drought stress and putrescine treatment in ancient wheat (Triticum monococcum L.). Cereal Res. Commun. 2025, 53, 771–781. [Google Scholar] [CrossRef]
- Ceylan, H.A.; Türkan, I.; Sekmen, A.H. Effect of coronatine on antioxidant enzyme response of chickpea roots to combination of PEG-induced osmotic stress and heat stress. J. Plant Growth Reg. 2013, 32, 72–82. [Google Scholar] [CrossRef]
- Rouhi, V.; Samson, R.; Lemeur, R.; Van Damme, P. Stomatal resistance under drought stress conditions induced by PEG 6000 on wild almond. Commun. Agric. Appl. Biol. Sci. 2006, 71, 269–273. [Google Scholar]
- Caruso, A.; Chefdor, F.; Carpin, S.; Depierreux, C.; Delmotte, F.M.; Kahlem, G.; Morabito, D. Physiological characterization and identification of genes differentially expressed in response to drought induced by PEG 6000 in Populus canadensis leaves. J. Plant Phy. 2008, 165, 932–941. [Google Scholar] [CrossRef]
- Chen, J.; Wu, W.; Zheng, Y.; Hou, K.; Xu, Y.; Zai, J. Drought resistance of Angelica dahurica during seedling stage under polyethylene glycol (PEG-6000)-simulated drought stress. China J. Chin. Mater. Medica 2010, 35, 149–153. (In Chinese) [Google Scholar]
- Piwowarczyk, B.; Kamińska, I.; Rybiński, W. Influence of PEG generated osmotic stress on shoot regeneration and some biochemical parameters in Lathyrus culture. Czech J. Genet. Plant Breed 2014, 50, 77–83. [Google Scholar] [CrossRef]
- Sajid Aqeel Ahmad, M.; Javed, F.; Ashraf, M. Iso-osmotic effect of NaCl and PEG on growth, cations and free proline accumulation in callus tissue of two indica rice (Oryza sativa L.) genotypes. Plant Growth Reg. 2007, 53, 53–63. [Google Scholar] [CrossRef]
- Lawlor, D.W. Absorption of polyethylene glycols by plants and their effects on plant growth. New Phytol. 1970, 69, 501–513. [Google Scholar] [CrossRef]
- Palaz, E.B.; Demirel, S.; Popescu, G.C.; Demirel, F.; Uğur, R.; Yaman, M.; Say, A.; Şimşek, Ö.; Tunç, Y. Refinement of surface sterilization protocol for in vitro olive (Olea europaea L.) shoot proliferation and optimizing by machine learning techniques. Hortic. Environ. BioTechnol. 2025, 1–16. [Google Scholar] [CrossRef]
- Ramezanpour, M.R.; Farajpour, M. Application of artificial neural networks and genetic algorithm to predict and optimize greenhouse banana fruit yield through nitrogen, potassium and magnesium. PLoS ONE 2022, 17, e0264040. [Google Scholar] [CrossRef]
- Jafari, M.; Paul, N.; Hesami, M.; Jones, A.M.P. Machine learning-aided optimization of in vitro tetraploid induction in Cannabis. Int. J. Mol. Sci. 2025, 26, 1746. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Alizadeh, M.; Jones, A.M.P.; Torkamaneh, D. Machine learning: Its challenges and opportunities in plant system biology. Appl. Microbiol. BioTechnol. 2022, 106, 3507–3530. [Google Scholar] [CrossRef]
- Rezaei, H.; Mirzaie-Asl, A.; Abdollahi, M.R.; Tohidfar, M. Comparative analysis of different artificial neural networks for predicting and optimizing in vitro seed germination and sterilization of petunia. PLoS ONE 2023, 18, e0285657. [Google Scholar] [CrossRef]
- Tütüncü, M.; Isak, M.A.; İzgü, T.; Dönmez, D.; Kaçar, Y.A.; Şimşek, Ö. Assessing cadmium stress resilience in myrtle genotypes using machine learning predictive models: A comparative in vitro analysis. Horticulturae 2024, 10, 542. [Google Scholar] [CrossRef]
- Şimşek, Ö. Machine learning offers insights into the impact of in vitro drought stress on strawberry cultivars. Agriculture 2024, 14, 294. [Google Scholar] [CrossRef]
- Schmider, E.; Ziegler, M.; Danay, E.; Beyer, L.; Bühner, M. Is it really robust? Reinvestigating the Robustness of ANOVA against Violations of the Normal Distribution Assumption. Methodology 2010, 6, 147–151. [Google Scholar] [CrossRef]
- Blanca Mena, M.J.; Alrcòn Postigo, M.; Arnau Gras, J.; Bono Cabré, R.; Bendayan, R. Non-normal data: Is ANOVA still a valid option? Psicothema 2017, 29, 552–557. [Google Scholar] [CrossRef]
- Isak, M.A.; Bozkurt, T.; Tütüncü, M.; Dönmez, D.; İzgü, T.; Şimşek, Ö. Leveraging machine learning to unravel the impact of cadmium stress on goji berry micropropagation. PLoS ONE 2024, 19, e0305111. [Google Scholar] [CrossRef]
- Sadat-Hosseini, M.; Arab, M.M.; Soltani, M.; Eftekhari, M.; Soleimani, A.; Vahdati, K. Predictive modeling of Persian walnut (Juglans regia L.) in vitro proliferation media using machine learning approaches: A comparative study of ANN, KNN and GEP models. Plant Methods 2022, 181, 48. [Google Scholar] [CrossRef] [PubMed]
- Aydinoğlu, B.; Shabani, A.; Safavi, S.M. Impact of priming on seed germination, seedling growth and gene expression in common vetch under salinity stress. Cell. Mol. Biol. 2019, 65, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Çifçi, H.; Açıkbaş, S. Effect of drought stress on germination and seedling growth of common vetch (Vicia sativa L.) cultivars. Turk. J. Agric. Res. 2023, 10, 288–299. (In Turkish) [Google Scholar]
- Zeng, Y.; Liu, Z.; Chen, W.; Qv, K.; Huang, Y.; Ade, L.; Hou, F. Methane pulse spray and irrigation promote seed germination and seedling growth of common vetch. BMC Plant Bio. 2024, 24, 971. [Google Scholar] [CrossRef]
- Tilhou, N.; Kucek, L.K.; Moore, V.; Hanson, S.; Reberg-Horton, S.C.; Ryan, M.R.; Ehlke, N.; Bartow, A.; Carr, B.; Douglas, J.; et al. Seed size has a major impact on fall seedling vigor in the cover crop hairy vetch (Vicia villosa Roth). Crop Sci. 2025, 65, e21439. [Google Scholar] [CrossRef]
- Odat, N.; Tawaha, A.M.; Hasan, M.; Al-Tawaha, A.R.; Thangadurai, D.; Sangeetha, J.; Rauf, A.; Khalid, S.; Saranraj, P.; Al-Taey, D.K.A.; et al. Seed priming with chitosan alleviates salinity stress by improving germination and early growth parameters in common vetch (Vicia sativa). In IOP Conference Series: Earth and Environmental Science, Proceedings of the 3rd International Conference of Animal Science and Technology, Makassar, Indonesia, 3–4 November 2020; IOP Publishing: Bristol, UK, 2021; Volume 788, p. 012059. [Google Scholar]
- Shi, J.; Jin, F.; Ma, S.; Liu, X.; Leng, X. Effect of high voltage discharge on germination characteristics of vetch seeds at high altitude. J. Phys. D Appl. Phys. 2024, 57, 175401. [Google Scholar] [CrossRef]
- Skamarokhova, A.S.; Siyukhov, H.R.; Yurina, N.A.; Petenko, A.I.; Anisimova, E.Y.; Mosolova, N.I.; Surkova, S.A. Effect of new complex bio-fertilizer on seed germination of different varieties of winter vetch. IOP Conf. Ser. Earth Environ. Sci. 2021, 848, 012108. [Google Scholar] [CrossRef]
- Zarbakhsh, S.; Shahsavar, A.R.; Afaghi, A.; Hasanuzzaman, M. Predicting and optimizing reactive oxygen species metabolism in Punica granatum L. through machine learning: Role of exogenous GABA on antioxidant enzyme activity under drought and salinity stress. BMC Plant Biol. 2024, 24, 65. [Google Scholar] [CrossRef]
- Okyere, F.G.; Cudjoe, D.K.; Virlet, N.; Castle, M.; Riche, A.B.; Greche, L.; Okyere, F.G.; Riche, A.B. Hyperspectral imaging for phenotyping plant drought stress and nitrogen interactions using multivariate modeling and machine learning techniques in wheat. Remote Sens. 2024, 16, 3446. [Google Scholar] [CrossRef]
- Kirtis, A.; Aasim, M.; Katırcı, R. Application of artificial neural network and machine learning algorithms for modeling the in vitro regeneration of chickpea (Cicer arietinum L.). Plant Cell Tissue Organ. Cul. 2022, 150, 141–152. [Google Scholar] [CrossRef]
- Shwetabh, K.; Ambhaikar, A. Modeling and studying in vitro regeneration in common bean breeding using artificial neural networks and machine learning algorithms. Agric. BioTechnol. J. 2024, 16, 283–299. [Google Scholar]











| Species | Cultivar | Institution | |
|---|---|---|---|
| Common Vetch | Vicia sativa | Ayaz08 | Ankara Field Crops Central Research Institute ‘2008’ |
| Cumhuriyet99 | Ege Field Crops Central Research Institute ‘1999’ | ||
| Hungarian Vetch | Vicia pannonica Crantz | Atom | Yonca Agriculture Products ‘2020’ |
| Altınova2002 | General Directorate of Agricultural Enterprises ‘2007’ | ||
| Narbon Vetch | Vicia narbonensis | Balkan | Transitional Zone Agricultural Research Institute ‘2011’ |
| Özgen | Dicle University Faculty of Agriculture ‘2009’ | ||
| Training Set | Testing Set | |||||
|---|---|---|---|---|---|---|
| R2 | RMSE | MAE | R2 | RMSE | MAE | |
| Germination Rate | ||||||
| RF | 0.96 | 0.05 | 0.04 | 0.90 | 0.08 | 0.06 |
| k-NN | 0.97 | 0.04 | 0.02 | 0.93 | 0.08 | 0.06 |
| MLP | 0.96 | 0.05 | 0.04 | 0.95 | 0.06 | 0.05 |
| SVM | 0.97 | 0.04 | 0.03 | 0.94 | 0.07 | 0.06 |
| Shoot Length | ||||||
| RF | 0.99 | 0.01 | 0.007 | 0.98 | 0.02 | 0.02 |
| k-NN | 0.94 | 0.05 | 0.03 | 0.93 | 0.05 | 0.04 |
| MLP | 0.98 | 0.03 | 0.02 | 0.97 | 0.03 | 0.03 |
| SVM | 0.98 | 0.03 | 0.02 | 0.95 | 0.05 | 0.04 |
| Root Length | ||||||
| RF | 0.84 | 0.08 | 0.07 | 0.81 | 0.10 | 0.08 |
| k-NN | 0.86 | 0.08 | 0.06 | 0.76 | 0.11 | 0.09 |
| MLP | 0.85 | 0.09 | 0.07 | 0.82 | 0.10 | 0.07 |
| SVM | 0.87 | 0.08 | 0.06 | 0.83 | 0.09 | 0.07 |
| Fresh Weight | ||||||
| RF | 0.95 | 0.05 | 0.03 | 0.91 | 0.06 | 0.05 |
| k-NN | 0.97 | 0.04 | 0.02 | 0.90 | 0.07 | 0.05 |
| MLP | 0.94 | 0.06 | 0.04 | 0.92 | 0.06 | 0.05 |
| SVM | 0.96 | 0.04 | 0.03 | 0.97 | 0.05 | 0.03 |
| Dry Weight | ||||||
| RF | 0.95 | 0.04 | 0.03 | 0.93 | 0.06 | 0.04 |
| k-NN | 0.94 | 0.03 | 0.02 | 0.86 | 0.07 | 0.05 |
| MLP | 0.93 | 0.05 | 0.04 | 0.88 | 0.06 | 0.05 |
| SVM | 0.95 | 0.04 | 0.03 | 0.91 | 0.05 | 0.04 |
| Vigor Index | ||||||
| RF | 0.99 | 0.01 | 0.006 | 0.98 | 0.03 | 0.02 |
| k-NN | 0.96 | 0.04 | 0.03 | 0.91 | 0.06 | 0.04 |
| MLP | 0.99 | 0.009 | 0.006 | 0.99 | 0.02 | 0.01 |
| SVM | 0.98 | 0.03 | 0.02 | 0.97 | 0.04 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okumuş, O.; Şimşek, Ö.; Isak, M.A.; Şahin, N.K.; Aydin, A.; Eren, B.; Demirel, F.; Kahramanoğulları, C.T.; Uzun, S.; Yaman, M. Integrating Machine Learning and In Vitro Screening to Evaluate Drought and Temperature Stress Responses for Vicia Species. Processes 2025, 13, 1845. https://doi.org/10.3390/pr13061845
Okumuş O, Şimşek Ö, Isak MA, Şahin NK, Aydin A, Eren B, Demirel F, Kahramanoğulları CT, Uzun S, Yaman M. Integrating Machine Learning and In Vitro Screening to Evaluate Drought and Temperature Stress Responses for Vicia Species. Processes. 2025; 13(6):1845. https://doi.org/10.3390/pr13061845
Chicago/Turabian StyleOkumuş, Onur, Özhan Şimşek, Musab A. Isak, Nilüfer Koçak Şahin, Adnan Aydin, Barış Eren, Fatih Demirel, Cansu Telci Kahramanoğulları, Satı Uzun, and Mehmet Yaman. 2025. "Integrating Machine Learning and In Vitro Screening to Evaluate Drought and Temperature Stress Responses for Vicia Species" Processes 13, no. 6: 1845. https://doi.org/10.3390/pr13061845
APA StyleOkumuş, O., Şimşek, Ö., Isak, M. A., Şahin, N. K., Aydin, A., Eren, B., Demirel, F., Kahramanoğulları, C. T., Uzun, S., & Yaman, M. (2025). Integrating Machine Learning and In Vitro Screening to Evaluate Drought and Temperature Stress Responses for Vicia Species. Processes, 13(6), 1845. https://doi.org/10.3390/pr13061845










