Efficient Preparation and Bioactivity Evaluation of Aglycone Soy Isoflavones via a Multi-Enzyme Synergistic Catalysis Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Enzymatic Hydrolysis Process of Soy Isoflavones
2.3. Condition Optimization for Preparing Aglycone Soy Isoflavones Using Composite Enzymes
2.4. Aglycone Soy Isoflavone Purification Process
2.5. Detection Method for Soy Isoflavones
2.6. In Vitro Antioxidant Activity Determination
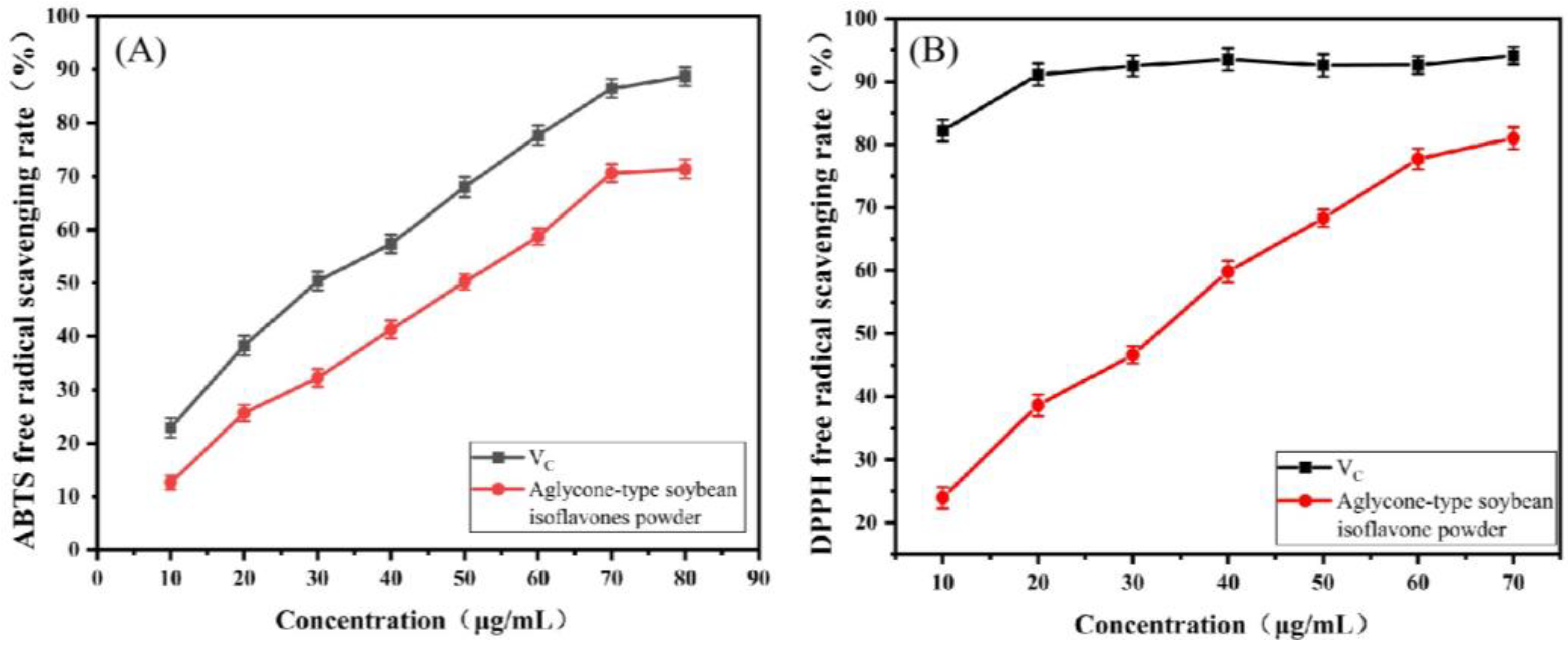
2.6.1. DPPH Free Radical Scavenging Rate
2.6.2. ABTS Radical Scavenging Rate
2.7. Evaluating Central Nervous System-Protective Effects in Zebrafish
2.7.1. Sample Solution Preparation
2.7.2. Animals and Treatments
2.7.3. Maximum Test Concentration (MTC) Determination
2.7.4. Protective Effect on CNS Injury
2.8. Statistical Analysis
3. Results and Discussion
3.1. Composite Enzyme Ratio Optimization
3.2. Composite Enzyme Reaction Condition Optimization
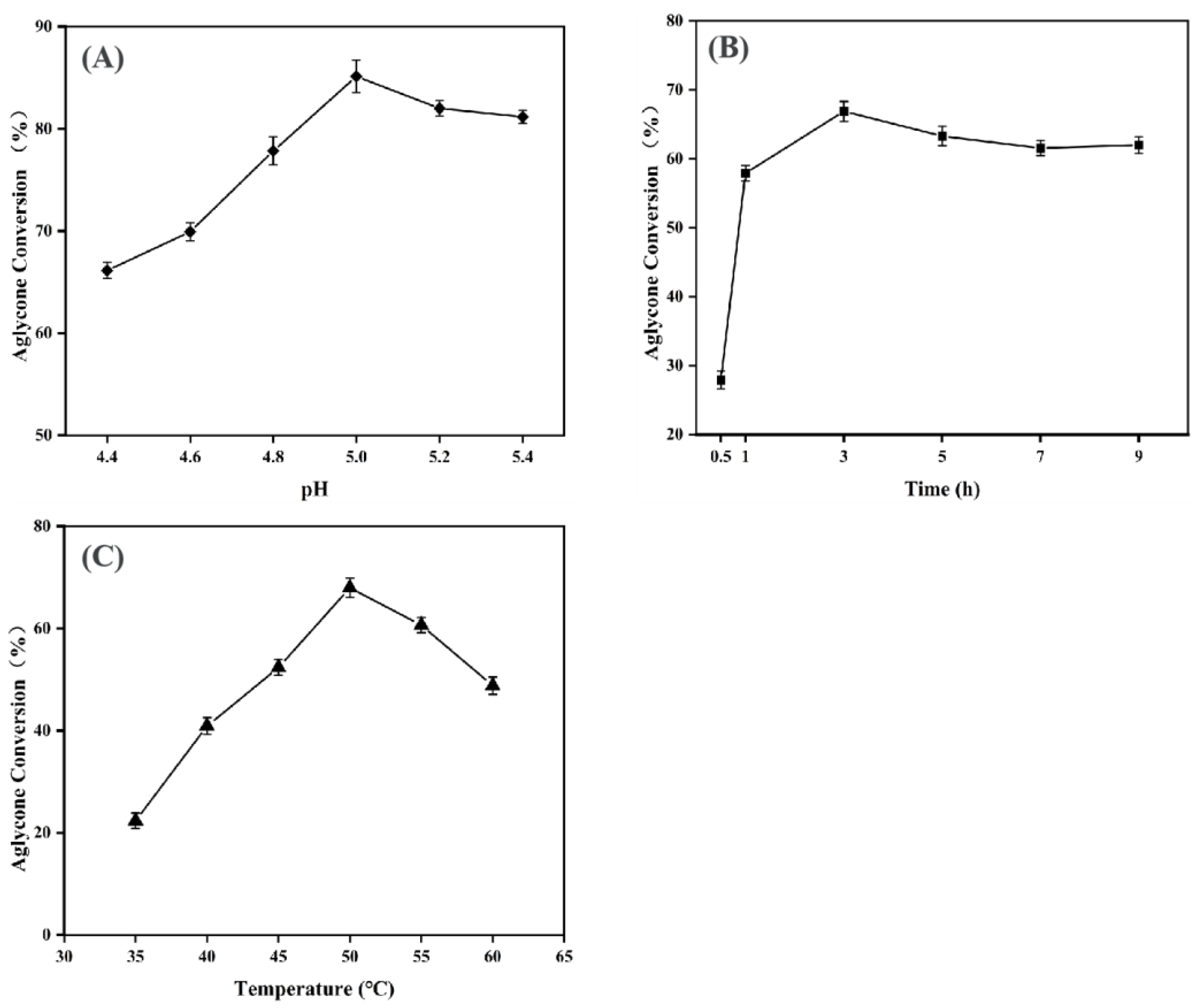
3.2.1. Analysis of Single-Factor Experiment Results
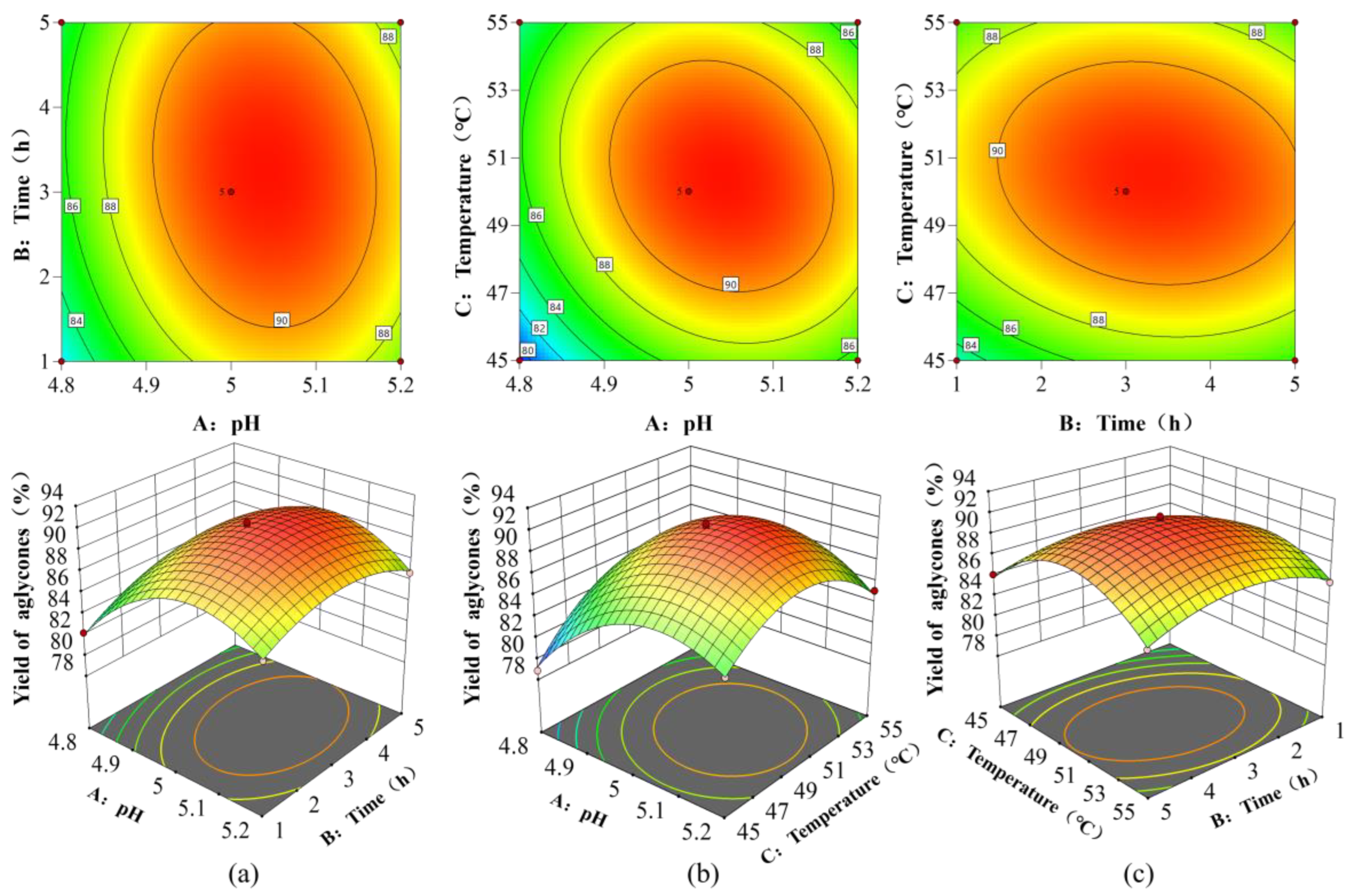
3.2.2. Response Surface Model Validation and Optimal Parameters
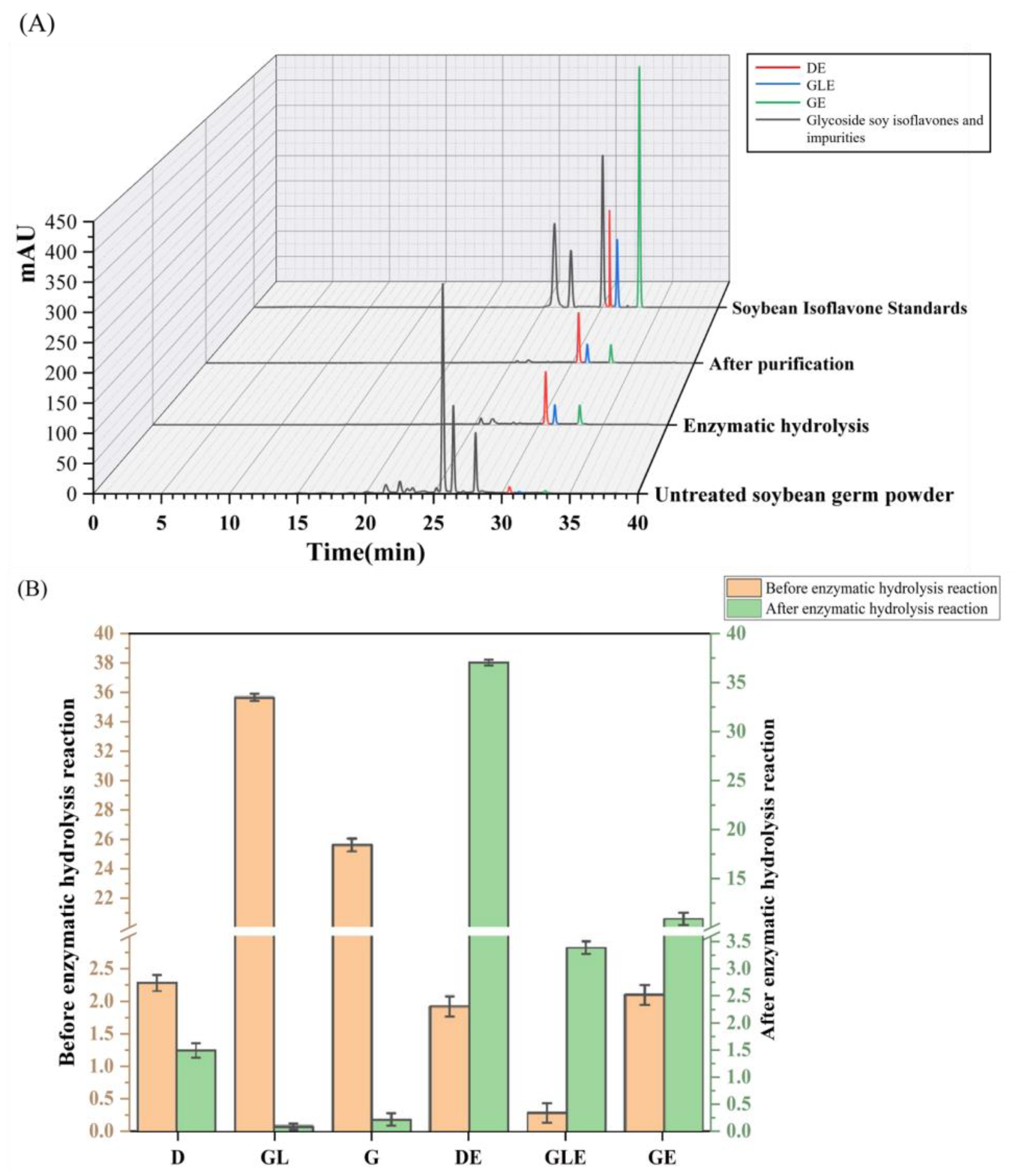
3.3. Purification Results of Aglycone Soy Isoflavones
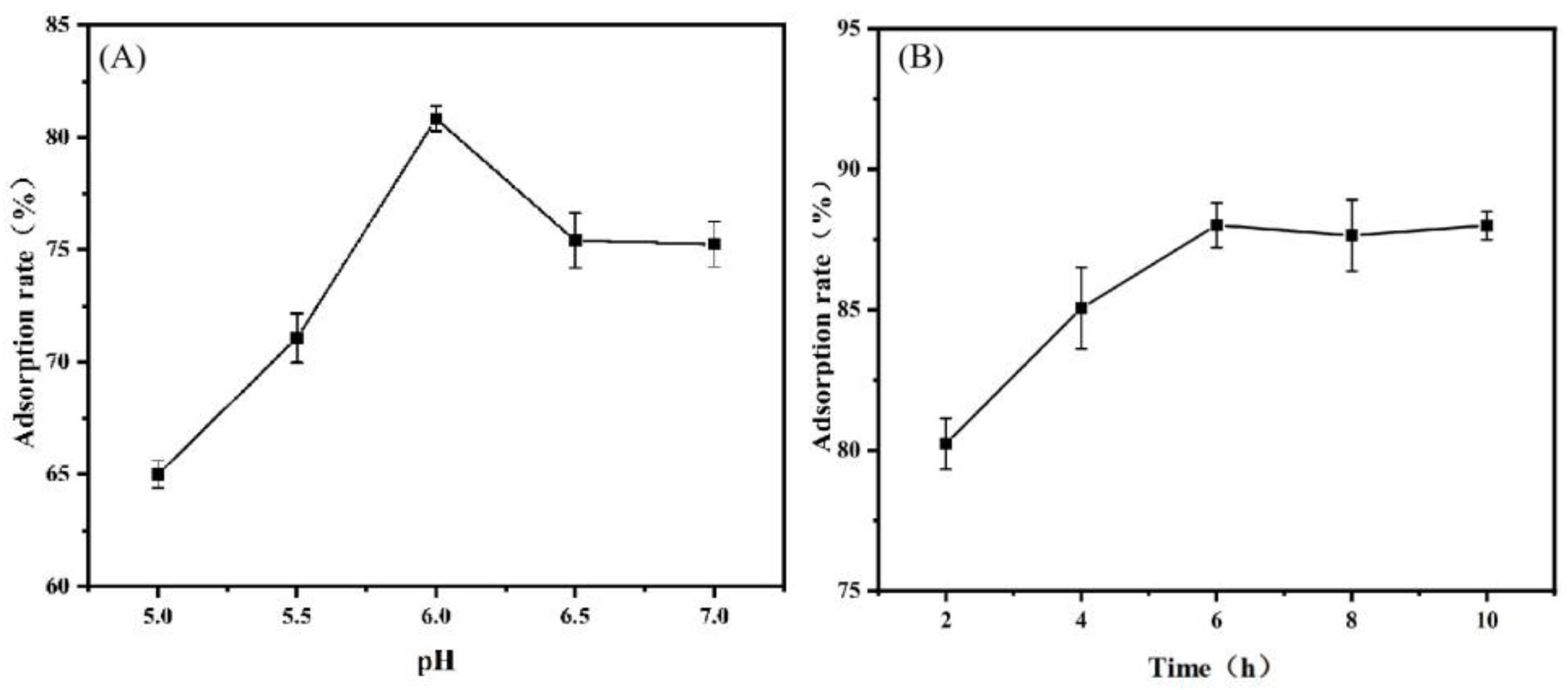
3.3.1. Effects of Different Adsorption Conditions on Adsorption Rate
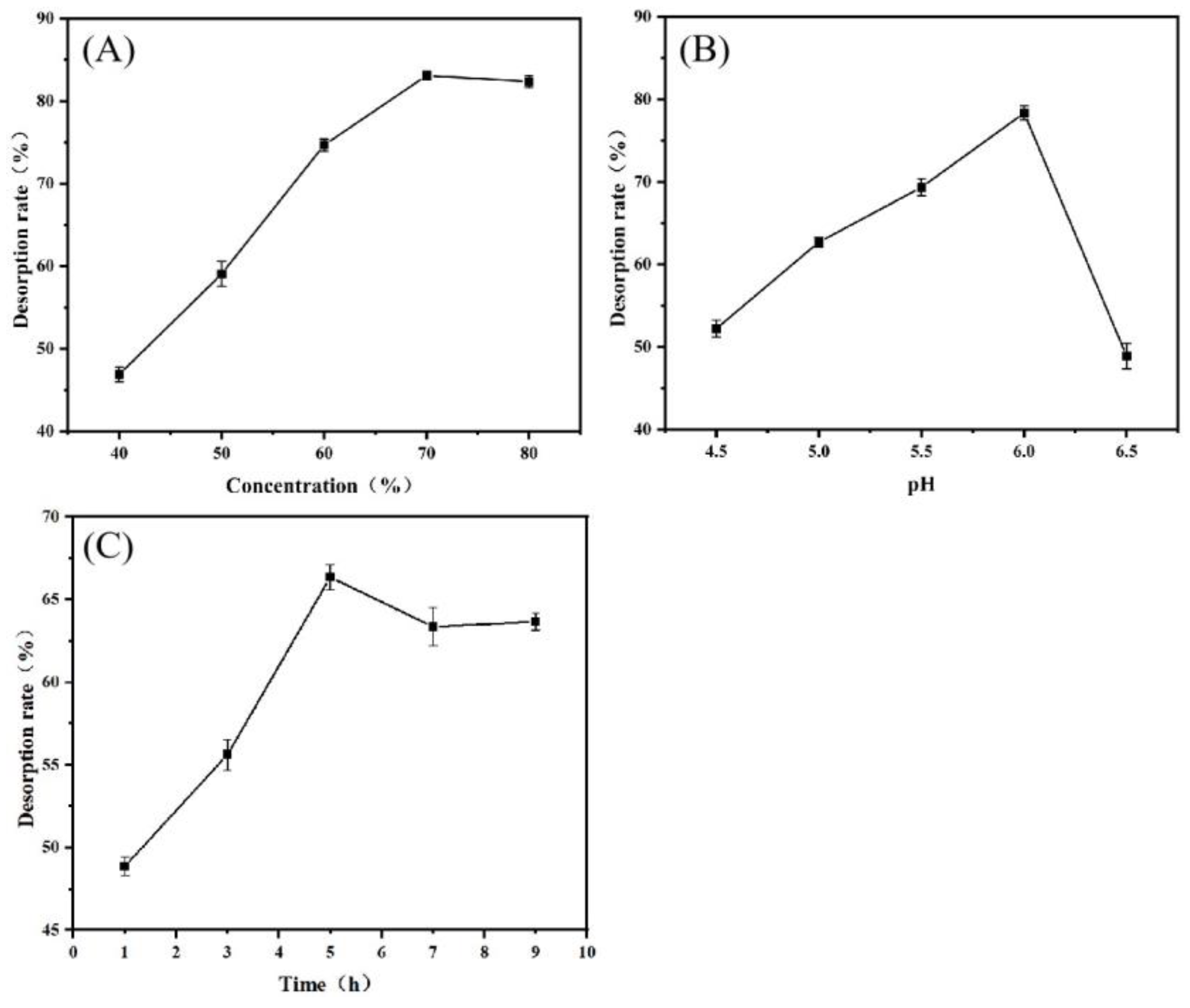
3.3.2. Influence of Different Desorption Conditions on Desorption Rate
3.3.3. Effects of Multi-Enzyme Synergistic Catalysis and Purification on Aglycone Soy Isoflavones
3.4. Evaluation of Aglycone Soy Isoflavone Bioactivity
3.4.1. In Vitro Antioxidant Activity
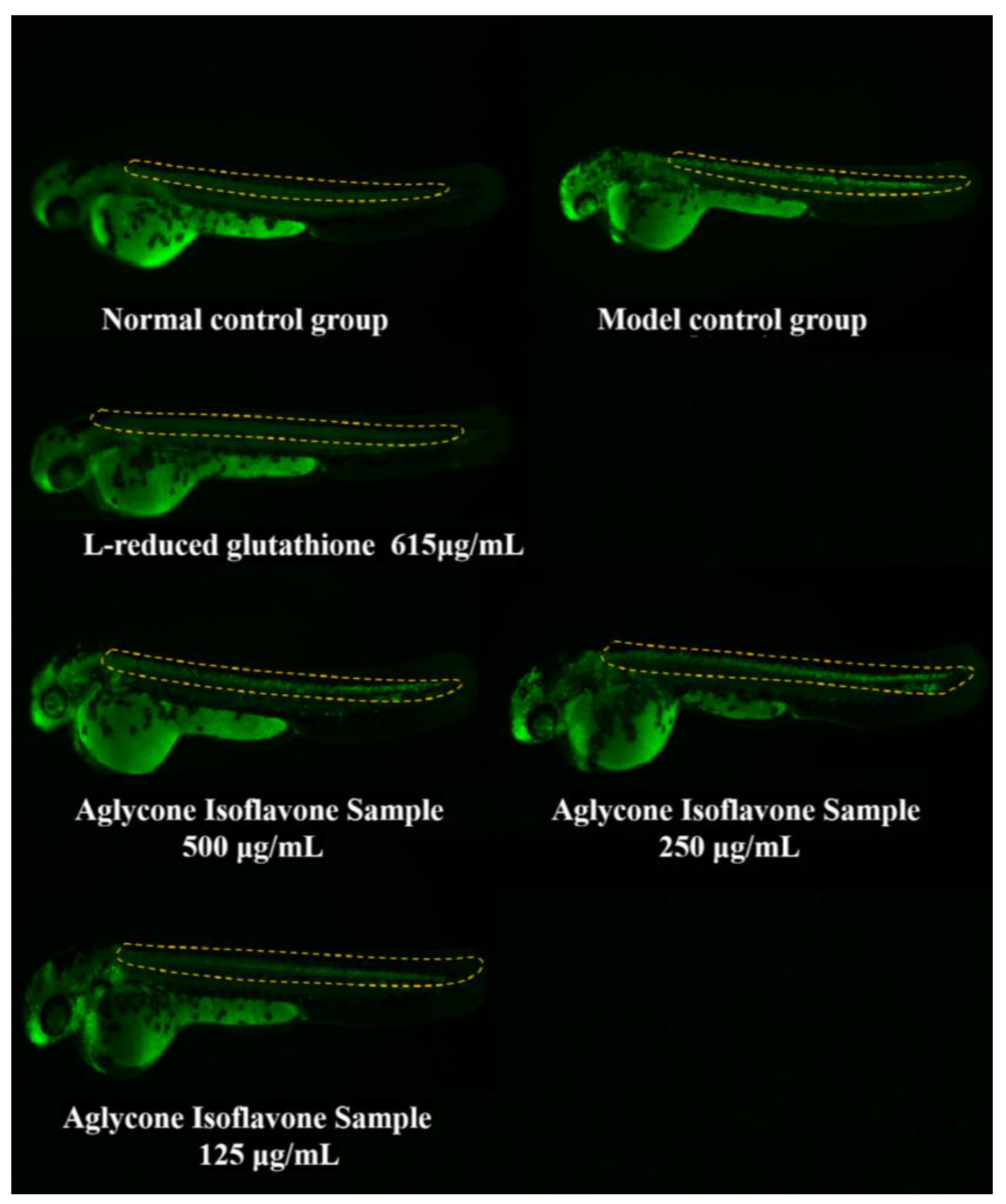
3.4.2. CNS Protection in Zebrafish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azinobis |
| AO | Acridine orange |
| CNS | Central nervous system |
| D | Daidzin |
| DE | Daidzein |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| G | Glycitin |
| GE | Glycitein |
| GL | Genistin |
| GLE | Genistein |
| IMPDH | Inosine monophosphate dehydrogenase |
| MMF | Mycophenolate mofetil |
| MTC | Maximum test concentration |
| VC | Vitamin C |
References
- Leuner, O.; Havlik, J.; Hummelova, J.; Prokudina, E.; Novy, P.; Kokoska, L. Distribution of isoflavones and coumestrol in neglected tropical and subtropical legumes. J. Sci. Food Agric. 2013, 93, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Chen, K.H. Utilization of isoflavones in soybeans for women with menopausal syndrome: An overview. Int. J. Mol. Sci. 2021, 22, 3212. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zhang, Y.J.; Zhu, G.Y.; Shi, X.C.; Chen, X.; Herrera-Balandrano, D.D.; Liu, F.Q.; Laborda, P. Occurrence of isoflavones in soybean sprouts and strategies to enhance their content: A review. J. Food Sci. 2022, 87, 1961–1982. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T. Emerging signal regulating potential of genistein against Alzheimer’s disease: A promising molecule of interest. Front. Cell Dev. Biol. 2019, 7, 197. [Google Scholar] [CrossRef]
- Hu, C.; Wong, W.T.; Wu, R.; Lai, W.F. Biochemistry and use of soybean isoflavones in functional food development. Crit. Rev. Food Sci. Nutr. 2020, 60, 2098–2112. [Google Scholar] [CrossRef]
- Pei, R.; Zhang, J.; Tian, L.; Zhang, S.; Han, F.; Yan, S.; Wang, L.; Li, B.; Sun, J. Identification of novel QTL associated with soybean isoflavone content. Crop J. 2018, 6, 244–252. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, X.; Tian, X.; Zhang, Y.; Zhang, J.; Zhang, P. Soy isoflavone: The multipurpose phytochemical (Review). Biomed. Rep. 2013, 1, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Yang, L.; Xu, J.Z. Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem. 2005, 90, 735–741. [Google Scholar] [CrossRef]
- Chen, P.; Sun, J.W.; Liang, Z.Q.; Xu, H.; Du, P.; Li, A.; Meng, Y.; Reshetnik, E.I.; Liu, L.; Li, C. The bioavailability of soy isoflavones in vitro and their effects on gut microbiota in the simulator of the human intestinal microbial ecosystem. Food Res. Int. 2022, 152, 110868. [Google Scholar] [CrossRef]
- Wójciak, M.; Drozdowski, P.; Skalska-Kamińska, A.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Latalska, M. Protective, anti-inflammatory, and anti-aging effects of soy isoflavones on skin cells: An overview of in vitro and in vivo studies. Molecules 2024, 29, 5790. [Google Scholar] [CrossRef]
- Lee, J.H.; Choung, M.G. Determination of optimal acid hydrolysis time of soybean isoflavones using drying oven and microwave assisted methods. Food Chem. 2011, 129, 577–582. [Google Scholar] [CrossRef]
- Sun, X.D. Enzymatic hydrolysis of soy proteins and the hydrolysates utilisation. Int. J. Food Sci. Technol. 2011, 46, 2447–2459. [Google Scholar] [CrossRef]
- Chien, H.J.; Wang, C.S.; Chen, Y.H.; Toh, J.T.; Zheng, Y.F.; Hong, X.G.; Lin, H.Y.; Lai, C.C. Rapid determination of isoflavones and other bioactive compounds in soybean using SWATH-MS. Anal. Chim. Acta 2020, 1103, 122–133. [Google Scholar] [CrossRef]
- Silva, M.B.R.; Falcão, H.G.; Kurozawa, L.E.; Prudencio, S.H.; de Camargo, A.C.; Shahidi, F.; Ida, E.I. Ultrasound and hemicellulase-assisted extraction increase β-glucosidase activity, the content of isoflavone aglycones and antioxidant potential of soymilk. J. Food Bioact. 2019, 6, 140–147. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ashton, J.F.; Shah, N.P. Isoflavone phytoestrogen degradation in fermented soymilk with selected β-glucosidase producing L. acidophilus strains during storage at different temperatures. Int. J. Food Microbiol. 2007, 115, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.J.; Ng, K.M.; Luo, K.Q. Extraction and purification of isoflavones from soybeans and characterization of their estrogenic activities. J. Agric. Food Chem. 2007, 55, 6940–6950. [Google Scholar] [CrossRef]
- Nemitz, M.C.; Moraes, R.C.; Koester, L.S.; Bassani, V.L.; von Poser, G.L.; Teixeira, H.F. Bioactive soy isoflavones: Extraction and purification procedures, potential dermal use and nanotechnology-based delivery systems. Phytochem. Rev. 2015, 14, 849–869. [Google Scholar] [CrossRef]
- Chea, J.D.; Lehr, A.L.; Stanzione, J.F., III; Yenkie, K.M. Evaluation of isoflavone extraction options at commercial scale. Biofuel Bioprod. Biorefin. 2022, 16, 1708–1725. [Google Scholar] [CrossRef]
- Xi, L.; Mu, T.; Sun, H. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, M.; Lin, L. Adsorption and desorption characteristics of adlay bran free phenolics on macroporous resins. Food Chem. 2016, 194, 900–907. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, M.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef]
- Chang, X.L.; Wang, D.; Chen, B.Y.; Feng, Y.M.; Wen, S.H.; Zhan, P.Y. Adsorption and desorption properties of macroporous resins for anthocyanins from the calyx extract of roselle (Hibiscus sabdariffa L.). J. Agric. Food Chem. 2012, 60, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Meenu, M.; Xu, B.; Yu, H. Impact of processing technologies on isoflavones, phenolic acids, and antioxidant capacities of soymilk prepared from 15 soybean varieties. Food Chem. 2021, 345, 128612. [Google Scholar] [CrossRef] [PubMed]
- Szeja, W.; Grynkiewicz, G.; Rusin, A. Isoflavones, their glycosides and glycoconjugates. Synthesis and biological activity. Curr. Org. Chem. 2017, 21, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Zhang, X.; Veliky, C.V.; Birru, R.L.; Barinas-Mitchell, E.; Magnani, J.W.; Sekikawa, A. Potential protective effects of equol (soy isoflavone metabolite) on coronary heart diseases—From molecular mechanisms to studies in humans. Nutrients 2021, 13, 3739. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Geraldi, M.V.; Maróstica Júnior, M.R.; Shahidi, F.; Schwember, A.R. Is chickpea a potential substitute for soybean? Phenolic bioactives and potential health benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef]
- Wang, W.-L.; Chen, S.-M.; Lee, Y.-C.; Chang, W.-W. Stigmasterol inhibits cancer stem cell activity in endometrial cancer by repressing IGF1R/mTOR/AKT pathway. J. Funct. Foods. 2022, 99, 105338. [Google Scholar] [CrossRef]
- Popović, D.B.; Sinkjær, T. Central nervous system lesions leading to disability. J. Autom. Control 2008, 18, 11–23. [Google Scholar] [CrossRef]
- Jurisch-Yaksi, N.; Yaksi, E.; Kizil, C. Radial glia in the zebrafish brain: Functional, structural, and physiological comparison with the mammalian glia. Glia 2020, 68, 2451–2470. [Google Scholar] [CrossRef]
- Jiang, L.-L.; Liu, M.-H.; Li, J.-Y.; He, Z.-H.; Li, H.; Shen, N.; Wei, P.; He, M.-F. Mycophenolic acid-induced developmental defects in zebrafish embryos. Int. J. Toxicol. 2016, 35, 712–718. [Google Scholar] [CrossRef]
- Magiera, S.; Sobik, A. Ionic liquid-based ultrasound-assisted extraction coupled with liquid chromatography to determine isoflavones in soy foods. J. Food Compos. Anal. 2017, 57, 94–101. [Google Scholar] [CrossRef]
- Macedo, G.A.; Caria, C.R.E.P.; Barbosa, P.P.M.; Mazine, M.R.; Gambero, A. Bioaccessibility evaluation of soymilk isoflavones with biotransformation processing. Foods 2023, 12, 3401. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jung, Y.S.; Jang, D.; Cho, C.H.; Lee, S.H.; Han, N.S.; Kim, D.O. Antioxidant capacity of 12 major soybean isoflavones and their bioavailability under simulated digestion and in human intestinal Caco-2 cells. Food Chem. 2022, 374, 131493. [Google Scholar] [CrossRef]
- Lee, D.P.S.; Gan, A.X.; Kim, J.E. Incorporation of biovalorised okara in biscuits: Improvements of nutritional, antioxidant, physical, and sensory properties. LWT 2020, 134, 109902. [Google Scholar] [CrossRef]
- Ma, W.; Ding, B.; Yu, H.; Yuan, L.; Xi, Y.; Xiao, R. Genistein alleviates β-amyloid-induced inflammatory damage through regulating toll-like receptor 4/nuclear factor κ B. J. Med. Food 2015, 18, 273–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Dong, J.; Xia, X. Soy isoflavones protect neuronal PC12 cells against hypoxic damage through Nrf2 activation and suppression of p38 MAPK and AKT–mTOR pathways. Antioxidants 2022, 11, 2037. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
| No. | A: β-Glucosidase (U/mL) | B: Cellulase (U/mL) | C: Hemicellulase (U/mL) | D: β-Galactosidase (U/mL) | Aglycone Soy Isoflavone Yield (%) |
|---|---|---|---|---|---|
| 1 | 1 (20) | 1 (150) | 1 (200) | 1 (500) | 82.57 |
| 2 | 1 | 2 (200) | 2 (400) | 2 (700) | 85.29 |
| 3 | 1 | 3 (250) | 3 (600) | 3 (900) | 83.57 |
| 4 | 2 (25) | 1 | 2 | 3 | 88.62 |
| 5 | 2 | 2 | 3 | 1 | 89.68 |
| 6 | 2 | 3 | 1 | 2 | 87.42 |
| 7 | 3 (30) | 1 | 3 | 2 | 85.85 |
| 8 | 3 | 2 | 1 | 3 | 87.09 |
| 9 | 3 | 3 | 2 | 1 | 86.63 |
| K1 | 251.43 | 257.04 | 257.08 | 258.88 | |
| K2 | 265.72 | 262.06 | 260.54 | 258.56 | |
| K3 | 259.57 | 257.62 | 259.1 | 259.28 | |
| k1 | 83.81 | 85.68 | 85.693 | 86.293 | |
| k2 | 88.573 | 87.353 | 86.847 | 86.187 | |
| k3 | 86.523 | 85.873 | 86.367 | 86.427 |
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Intercept | 197,939.741 | 1 | 197,939.741 | 506,148.652 | <0.01 ** |
| β-glucosidase | 99.411 | 2 | 49.706 | 127.102 | <0.01 ** |
| Cellulase | 15.544 | 2 | 7.772 | 19.873 | <0.01 ** |
| Hemicellulase | 5.974 | 2 | 2.987 | 7.638 | <0.01 ** |
| β-galactosidase | 0.367 | 2 | 0.184 | 0.470 | 0.633 |
| Residual | 7.039 | 18 | 0.391 |
| Sequence | Three Factors with Three Levels | Response Values | ||
|---|---|---|---|---|
| A: pH | B: Time (h) | C: Temperature (°C) | Aglycone Conversion (%) | |
| 1 | 5.2 | 5 | 50 | 86.77 |
| 2 | 5 | 5 | 45 | 86.12 |
| 3 | 4.8 | 3 | 45 | 78.85 |
| 4 | 4.8 | 1 | 50 | 82.23 |
| 5 | 5.2 | 1 | 50 | 86.78 |
| 6 | 5 | 5 | 55 | 86.24 |
| 7 | 5 | 3 | 50 | 92.05 |
| 8 | 5 | 1 | 55 | 86.19 |
| 9 | 5.2 | 3 | 45 | 85.34 |
| 10 | 4.8 | 3 | 55 | 83.82 |
| 11 | 5 | 3 | 50 | 91.58 |
| 12 | 5 | 3 | 50 | 91.47 |
| 13 | 5 | 3 | 50 | 91.19 |
| 14 | 5 | 3 | 50 | 91.82 |
| 15 | 5.2 | 3 | 55 | 85.17 |
| 16 | 4.8 | 5 | 50 | 84.87 |
| 17 | 5 | 1 | 45 | 83.23 |
| Constituencies | Concentration (µg/mL) | Deaths (tail) | Mortality (%) | Phenotype |
|---|---|---|---|---|
| Normal control group | - | 0 | 0 | No significant abnormalities were observed |
| Model control group | - | 0 | 0 | No significant abnormalities were observed |
| Aglycone soy isoflavone samples | 125 | 0 | 0 | The status was similar to that of the model control group |
| 250 | 0 | 0 | The status was similar to that of the model control group | |
| 500 | 0 | 0 | The status was similar to that of the model control group | |
| 1000 | 6 | 20 | - | |
| 2000 | 30 | 100 | - |
| Constituencies | Concentration (µg/mL) | Neuraxial Apoptosis Fluorescence Intensity (Pixel, Mean ± SE) | CNS Protection Rate (%) |
|---|---|---|---|
| Normal control group | - | 81,656 ± 2131 *** | |
| Model control group | - | 162,661 ± 6501 | |
| Glutathione | 615 | 107,491 ± 5303 *** | 68.11 ± 0.27 |
| Aglycone-type soybean isoflavone sample | 125 | 140,627 ± 6099 | 27.20 ± 0.09 |
| 250 | 135,166 ± 6174 | 33.94 ± 0.08 | |
| 500 | 100,628 ± 1809 *** | 76.58 ± 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Fu, Y.; Du, P.; Li, N.; Lv, Y.; Hao, L.; Liu, W.; Xiao, J. Efficient Preparation and Bioactivity Evaluation of Aglycone Soy Isoflavones via a Multi-Enzyme Synergistic Catalysis Strategy. Processes 2025, 13, 1831. https://doi.org/10.3390/pr13061831
Zhao Y, Fu Y, Du P, Li N, Lv Y, Hao L, Liu W, Xiao J. Efficient Preparation and Bioactivity Evaluation of Aglycone Soy Isoflavones via a Multi-Enzyme Synergistic Catalysis Strategy. Processes. 2025; 13(6):1831. https://doi.org/10.3390/pr13061831
Chicago/Turabian StyleZhao, Yating, Yanhong Fu, Peng Du, Nan Li, Yaru Lv, Lizhen Hao, Wenlong Liu, and Jing Xiao. 2025. "Efficient Preparation and Bioactivity Evaluation of Aglycone Soy Isoflavones via a Multi-Enzyme Synergistic Catalysis Strategy" Processes 13, no. 6: 1831. https://doi.org/10.3390/pr13061831
APA StyleZhao, Y., Fu, Y., Du, P., Li, N., Lv, Y., Hao, L., Liu, W., & Xiao, J. (2025). Efficient Preparation and Bioactivity Evaluation of Aglycone Soy Isoflavones via a Multi-Enzyme Synergistic Catalysis Strategy. Processes, 13(6), 1831. https://doi.org/10.3390/pr13061831






