1. Introduction
The modernization and sustainable growth of the global food industry increasingly rely on maximizing the use of agricultural raw materials, minimizing waste, and promoting food security. One of the most promising areas in this context is the valorization of by-products from meat processing [
1,
2,
3]. Animal blood is produced in large volumes by modern slaughterhouses and remains underutilized despite containing high-quality proteins and bioactive compounds. Researchers have demonstrated that bovine blood plasma, especially, can be harnessed to improve the nutritional value and functional properties of finished products, including meat emulsions, comminuted products, and plant-based alternatives [
4,
5,
6,
7].
Large volumes of by-products are generated throughout the meat industry production cycle, including blood, bones, skulls, trimmings, organs, viscera, and other offal, all of which must be processed or disposed of in an environmentally safe manner [
8,
9]. The blood of slaughtered animals and their fractions, obtained during cattle slaughter, contain relatively high levels of protein, the nutritional value of which is comparable to that of meat [
10,
11]. However, these blood proteins differ in amino acid composition: in particular, plasma, by its fibrinogen content, a complete protein, differs from hemoglobin by having higher concentrations of essential amino acids such as tryptophan and methionine, as well as isoleucine, which is absent from hemoglobin [
12,
13]. Through their favorable functional attributes, blood proteins can enhance meat’s water-binding capacity and improve the quality of meat products.
Blood from slaughtered animals is a truly unique protein resource—often referred to as “liquid meat”. Its potential applications increase considerably when separated into fractions. Blood plasma, in particular, is rich in high-value, easily digestible protein, and its physicochemical properties make it well-suited for developing a range of products. However, both blood and its fractions also exhibit known limitations: they are highly susceptible to microbial spoilage, and incorporating more than 1% whole blood or 10% plasma into sausage formulations can adversely affect key sensory attributes, including texture, juiciness, and color. Consequently, these challenges account for the currently low level of blood fraction utilization in meat production and highlight the need for methods to modify their properties [
14].
Despite these advantages, at present, only about 20% of the available blood protein resource is used for food purposes [
15]. The principal cause of this inefficiency lies in the limited scientific data characterizing its functional-technological properties and biotechnological potential, knowledge that would be instrumental in developing fundamentally new technologies for meat products [
16].
Various methods exist for structuring blood plasma, but many rely on chemical-based preparations. Among the currently available modification strategies, a notable approach involves structuring plasma with solutions of calcium chloride, calcium phosphate, or calcium lactate [
17,
18]. While these methods are relatively simple, they often overlook how various plasma preparation techniques may influence the structuring process. Moreover, relying on chemical additives is generally considered undesirable in food production. Structured plasma thus has potential applications as a stabilizer, a source of bioactive compounds, and a functional protein component, ultimately contributing to improved meat product quality [
19]. A significant advantage of using plasma proteins in food formulations arises from their ability to enhance water-binding, emulsification, and gelling properties, thereby improving yield, texture, and moisture retention [
20,
21].
Recent studies have demonstrated the potential of blood plasma as an ingredient that can effectively substitute fat and phosphates in meat products, leading to a 5% increase in protein content and a 10–15% reduction in calorie density, without compromising sensory characteristics [
22,
23,
24]. Awan et al. further observed that incorporating blood plasma into fermented sausages enhances texture by approximately 12%, improves flavor, and promotes the development of a desirable microbiome through pH adjustments of 0.2–0.4, thereby increasing product safety [
25]. In works [
26,
27], blood plasma addition was shown to improve emulsion stability and textural properties, reduce moisture loss by 15–20% during thermal processing, and increase emulsion viscosity by about 10%.
Collectively, these findings underscore the substantial promise of utilizing blood plasma in diverse aspects of meat product production, including enhanced textural attributes, stability, nutritional value, and product safety. Nonetheless, the successful implementation of this technology necessitates careful consideration of factors such as dosage, processing methods, and their influence on the final product’s organoleptic qualities.
Among potential plant-based thickeners and stabilizers (e.g., guar, xanthan, or pectin), hydrated flaxseed cake flour offers a unique combination of high residual protein content, mucilage-rich polysaccharides, and dietary fiber [
28]. These constituents reinforce the fibrin network through protein–polysaccharide interactions. In addition, flaxseed meal delivers antioxidant lignans and dietary fibers, and studies report that defatted flaxseed cake achieves superior oil absorption (14.33%) and water-binding capacity compared with mustard and soybean meals [
29,
30]. As an agro-industrial by-product, it supports sustainable valorization while providing both techno-functional and nutritional advantages for stabilizing bovine plasma gels.
Accordingly, this research focuses on optimizing the structuring and stabilization of bovine blood plasma using lactic acid bacterial fermentation in conjunction with flaxseed oil cake flour at varying concentrations. By evaluating clot presence, gel formation time, yield stress, and syneresis, we aim to elucidate how plant-protein and microbial interactions can improve plasma’s functional attributes.
2. Materials and Methods
2.1. Blood Sampling
Blood was collected from four Kazakh White-Headed bulls at the “MPK Bizhan” meat-processing complex in Kokshetau, Kazakhstan. Each animal was under three years of age, a stage at which blood protein content is relatively high compared to older cattle (as cattle age, their blood water content decreases and overall solids increase). A total of 9 L of blood was drawn, with 2–3 L taken from each bull (equivalent to approximately 10–15% of total blood volume), which is considered safe for animals of this age group.
Immediately following collection, blood samples (at 25–35 °C or 8–12 °C) were transferred to vacuum-sealed bags and placed in thermally insulated containers. Transport was conducted at 2–6 °C to ensure a stable temperature. Partial hemolysis was carried out by mixing the blood with filtered water at 25–35 °C (5% of the total blood volume).
The study protocols and experimental procedures were approved by the Local Ethical Commission of the Institutional Review Board of S. Seifullin Kazakh Agro Technical University (Regulations on the Ethics Committee of S. Seifullin Kazakh Agro Technical University No. PLEC VND 03.3017—2021 dated 21 September 2023).
2.2. Separation of Plasma from Blood
Blood plasma was obtained under laboratory conditions by centrifugation of cooled or thawed blood samples. Centrifuge rotor speeds varied from 1100 to 3500 rpm, with a marked increase in the removal of erythrocyte fragments observed at speeds above 2800 rpm. To ensure the separation of intact erythrocytes, the final speed was set at 3500 rpm.
Cooled blood (centrifuged at 1100–3500 rpm): centrifugation time: 10–15 min; gelation time following centrifugation: ~3 h.
Thawed blood (centrifuged at 1100–3000 rpm): centrifugation time: 10–15 min; gelation time following centrifugation: ~7 h.
The choice of specific rotor speed and processing time ensured effective separation of plasma from cellular components, allowing for reproducible plasma preparation in both cooled and thawed states. Centrifugation at 1100–3500 rpm is fully compatible with industrial blood processing. Commercial slaughterhouses and blood—fractionation plants employ continuous—flow disc—stack and decanter centrifuges operating in the 1000–5500 rpm, capable of handling several cubic meters per hour [
31]. By matching the relative centrifugal force and residence time parameters used in the laboratory, these large—scale systems achieve equivalent erythrocyte removal and high plasma yield.
2.3. Technological Procedure for Preparing a Blood Plasma-Based Composition
A structured composition based on blood plasma was produced using lactic acid bacteria (Bifidobacterium lactis, Bifidobacterium longum, Pediococcus pentosaseus, Staphylococcus xylosus), hydrated flaxseed oil cake flour, and blood plasma.
Microorganism Characteristics
Lactobacillus casei (MicroMilk PR C, Italy) is produced by MicroMilk (Cremona, Italy) as a monospecies concentrate containing Lactobacillus casei. It is commonly used for fortification and cold ripening in fermented dairy products such as cultured milk and various cheeses, where it expedites cheese ripening and enhances sensory attributes.
Lactobacillus curvatus (B-8949, Russia) originates from the National Bioresource Center (Moscow, Russia), All-Russian Collection of Industrial Microorganisms (BRC VKPM), with the strain lineage “Bactoferm F-SC-111”. It is employed in fermented products to develop flavor and maintain stable fermentation processes.
Pediococcus acidilactici (Choozit Flav 43 LYO 5D, France) is manufactured by Danisco (Paris, France). It is a starter culture frequently used in Cheddar-type, semi-hard, and hard cheeses, providing a sweet, intensified flavor and mitigating bitter off-flavors. This culture also helps reduce cheese aging times.
Pediococcus pentosaseus (Qingzhou, Shandong, China) has a live count of 1.0 × 1010 to 1011 CFU/g. As a gram-positive, non-motile, spore-free coccoid bacterium, it belongs to the lactic acid bacteria group. It is commonly involved in both natural and controlled fermentations of vegetables, sausages, and silage inoculants, contributing to desirable flavors and improved microbial stability.
Bifidobacterium lactis (BB12, Germany), produced by Chr. Hansen (Nienburg, Germany), is a well-known probiotic strain widely applied in dairy fermentations for both technological and probiotic benefits.
Bifidobacterium longum (BB46, Germany), also from Chr. Hansen, is another prominent bifidobacterial strain frequently used in probiotic food formulations to enhance gut health and aid fermentation processes.
Staphylococcus xylosus (Hansen MIC SALSA-1, Denmark) is manufactured by Chr. Hansen (Hoersholm, Denmark) as a specialized ripening culture typically used in cheese production to generate sulfurous or raw milk–like aromas. In addition, it modifies cheese texture and surface coloration, particularly in surface-ripened varieties.
Combined Culture (BB12 + BB46 with Bactoferm T-SPX, Chr. Hansen, Hørsholm, Denmark) is a synergistic blend of Bifidobacterium lactis (BB12), Bifidobacterium longum (BB46), Staphylococcus xylosus, and Pediococcus pentosaseus. It is formulated to optimize fermentation parameters in various products by enhancing flavor development, texture, and microbial stability.
All strains in the mixed starter—Lactobacillus casei (MicroMilk PR C), L. curvatus B-8949, Pediococcus acidilactici (Choozit Flav 43), P. pentosaceus, Bifidobacterium lactis BB-12, B. longum BB-46, and Staphylococcus xylosus SALSA-1—are approved for food use and carry established safety designations. The lactic acid bacteria and pediococci appear on the European Food Safety Authority’s Qualified Presumption of Safety (QPS) list for use as starter cultures in fermented meat and dairy products. Both B. lactis BB-12 and B. longum BB-46 are classified as Generally Recognized As Safe (GRAS) by the U.S. Food and Drug Administration. Staphylococcus xylosus (e.g., SALSA-1) holds a favorable regulatory status, being covered by EFSA’s Qualified Presumption of Safety (QPS) in the EU and typically by FDA’s Generally Recognized As Safe (GRAS) affirmation in the US, allowing its widespread use in food fermentation. These regulatory statuses ensure that the mixed starter meets current probiotic and starter-culture requirements for industrial meat and dairy applications.
The process involves the following steps:
First, skim milk was sterilized at 120 °C and 1.2 atm to eliminate pathogenic microorganisms. After sterilization, the milk was cooled to 30 °C and inoculated with starter cultures (Bifidobacterium lactis, Bifidobacterium longum, Pediococcus pentosaceus, and Staphylococcus xylosus). The inoculated milk was then incubated at 37 °C for 12–24 h.
The nine experimental variants were used to study how different microorganisms (both single-strain and combined cultures) influence blood plasma gelation. Sample 1 served as a control (recalcified plasma without microbial addition), while Samples 2–9 involved introducing lyophilized microbial strains into plasma at four incremental concentrations (5%, 10%, 15%, and 20%). Inoculation percentages refer to the amount of lyophilized microbial starter added as a percentage of the plasma’s weight (
w/
w). Each microorganism or microorganism mix was allowed to interact with the plasma under standardized conditions in order to assess effects on clot formation, gel stability, and other relevant rheological properties (
Table 1).
2.4. Structurization of Blood Plasma Using Lactic Acid Bacteria and Flaxseed Cake Flour
Separately, flaxseed oil cake flour was hydrated by mixing it with water heated to 70 ± 2 °C in a 1:1.5 ratio. During hydration, components of the flour (proteins, fiber, and mucilaginous substances) bind water to form a gel-like structure. The resulting hydrated flaxseed flour and the lactic starter culture were combined with blood plasma to create a homogenous mixture (
Figure 1). This mixture was maintained at 20–22 °C until an elastic gel was formed.
The five experimental samples were designed to evaluate how varying levels of hydrated flaxseed oil cake flour (ranging from 0% to 10%) influence structure formation and coagulation stability in blood plasma (
Table 2). Each sample included a consistent 20% addition of a multi-strain starter culture (
Bifidobacterium lactis,
Bifidobacterium longum,
Pediococcus pentosaseus, and
Staphylococcus xylosus). The first sample, containing no flaxseed flour, served as the control; subsequent samples progressively increased flaxseed flour content to observe changes in gelation behavior, texture, and syneresis.
2.5. Determination of Structure Formation
Structure formation proceeds in several stages. Initially, the appearance of discrete particles indicates the nucleation phase, signified by slight turbidity and subtle changes in viscosity, as nascent structural centers begin to form [
32]. During the subsequent growth phase, the structure expands, leading to a noticeable increase in viscosity. A three-dimensional network develops, and turbidity intensifies. In this study, structure formation was evaluated visually. After the addition of the structuring agents, cloudiness, sediment deposition, and the emergence of a dense clot were observed. In the final phase, the system reaches a stable state of organization.
2.6. Determination of pH
The pH value was measured potentiometrically using a pH-Tester 340 (Infraspak Analyt, Novosibirsk, Russia). This method relies on measuring the electromotive force of a cell composed of a reference electrode (with a known potential) and a glass electrode. The glass electrode’s potential is determined by the concentration of ions in the test sample.
Prior to measurement, the instrument was calibrated with a standard solution and distilled water to ensure the accuracy of pH readings [
33].
2.7. Determination of Yield Stress
Yield stress was measured with an ST-2 structurometer (LLC Laboratory of Quality, Moscow, Russia) fitted with a 60° cone indenter. Minced—meat batter was packed into a cylindrical cup with a spatula, taking care to eliminate air pockets and obtain a level surface. The filled cup was centred on the instrument table so that its axis coincided with that of the cone. After the structurometer was started, the cone was driven downward at the preset speed; the accompanying software “Algorithm-1” continuously recorded force as a function of penetration depth. When full immersion was reached the run was stopped, and the software automatically calculated yield stress and exported the results as tables and graphs for subsequent analysis [
34].
The yield stress
θ0 (in Pa) was determined by the depth of cone immersion and calculated by Equation (1):
where
F—loading value (N);
h—total immersion depth of the cone (m);
K—cone constant, which is dependent on the cone angle α at the apex.
Since the device provides the load in grams and the cone depth in millimeters, for convenience in calculations, grams must be converted to newtons. The result is expressed in pascals (Pa).
The constant
K, which corresponds to the cone angle
αα at the top, is calculated using the formula shown in Equation (2):
where
α is the cone’s vertex angle. In this case, the vertex angle of the cone was 60°.
2.8. Determination of Time Required for Fixed Structure Formation
The time needed for blood plasma to form a stable gel after the introduction of structuring agents was measured visually by documenting when a fully formed plasma coagulate (gel) appeared. The objective was to evaluate how long it takes for plasma to develop a non-flowing, gel-like structure once specific agents are added. After preparing samples by combining blood plasma with the chosen structuring agents, the mixture was maintained at the designated temperature (for example, 37 °C to simulate physiological coagulation) or placed in a water bath if heating was required. At regular intervals (for instance, every five minutes), the samples were checked visually for changes in consistency. The endpoint was reached when the plasma no longer flowed and maintained its shape (i.e., it did not spread when the container was tilted) [
35]. For each sample, the time was recorded from the initial addition of the structuring agents until the formation of a rigid, non-flowing gel.
2.9. Statistical Analysis
All measurements were carried out in triplicate (n = 3 for each sample and concentration), and results are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed to assess differences among treatment groups, with statistical significance defined at p < 0.05. Where significant differences were found, post hoc comparisons were conducted using appropriate tests (Tukey’s HSD), ensuring robust evaluation of variability and treatment effects.
3. Results and Discussion
3.1. Study of Procoagulation Activity of Lactic Acid Microorganisms
A key observation was that several strains demonstrated minimal clotting activity at lower concentrations (5%), whereas higher concentrations (≥10%) significantly accelerated gel formation and clot presence (
Table 3). For example,
Lactobacillus curvatus (Sample 2) showed no clot and no gelation when added at 5% or 10%. However, at 15%, a clot eventually appeared (33 h), indicating that this strain’s activity is concentration-dependent. A similar pattern was observed for
Pediococcus acidilactici (Sample 3), which showed no gelation at lower concentrations but progressively shortened clotting times with increasing inoculation rates.
Strains such as Bifidobacterium lactis, Bifidobacterium longum, Staphylococcus xylosus, and Pediococcus pentosaseus (Samples 5–8) were more effective than Lactobacillus curvatus and Pediococcus acidilactici in promoting clot formation, even at lower concentrations (5%). Notably, Bifidobacterium longum (Sample 7) and Staphylococcus xylosus (Sample 8) achieved clotting at 5% inoculation, although the time to full gelation was relatively prolonged (24 and 35 h, respectively). As the concentration increased to 10%, these strains induced clot formation in significantly shorter times (16 and 12 h, respectively).
Among the single-strain treatments,
Bifidobacterium longum at 20% resulted in a clot in 3 h, emphasizing the high reactivity of this strain at elevated dosages.
Lactobacillus casei (Sample 4) also notably decreased gelation times with an increase in concentration—from no clot at 5% to 4 h at 20%. The mechanisms behind these reductions in gelation time likely include enhanced acidification, proteolytic activity, and possible production of exopolysaccharides, all of which contribute to protein network formation in blood plasma [
36].
A crucial finding was the markedly reduced gelation times when strains were used in combination (Sample 9:
Bifidobacterium lactis + Bifidobacterium longum + Pediococcus pentosaseus + Staphylococcus xylosus). At 5% addition of this combined culture, a clot formed within 24 h, whereas at 20% concentration, gelation was complete in 1 h. These results strongly suggest that synergistic interactions among different bacterial strains enhance the rate of protein matrix formation in the plasma. Cooperative enzymatic pathways, co-production of acidity, and complementary metabolic by-products may collectively accelerate clot development and stabilize the gel structure [
37].
This synergy is important from both a biochemical and a technological standpoint. When different strains work together, they can more effectively break down plasma proteins and produce structural components, such as exopolysaccharides, thereby hastening network formation and creating a more robust gel. In contrast, single strains acting in isolation often require higher inoculum levels or extended incubation times to achieve similar results [
38].
Proteolysis and acidification are major factors, as most lactic acid bacteria (e.g.,
Lactobacillus,
Pediococcus, and
Bifidobacterium spp.) break down proteins in blood plasma to generate peptides and free amino acids. They also typically lower the pH by producing lactic acid, and this combined effect of protein breakdown and pH reduction helps destabilize plasma proteins, promoting clot formation [
39]. Enzymatic cross-linking may also play a role. Some bacterial strains secrete enzymes (for example, transglutaminase-like activities in certain microflora) or produce co-factors that facilitate protein cross-linking, ultimately enhancing the formation of a stable gel network. In addition, exopolysaccharide production by lactic and bifidobacteria can reinforce protein networks. This process, often noted in fermented dairy products, may function similarly in blood plasma, contributing to the final gel’s consistency and firmness [
40].
3.2. Sensory and Physical Characteristics
The color of the resulting gels ranged from cream to light brown, with higher inoculum concentrations generally producing a paler, brighter appearance. This trend may be related to protein–protein interactions, pigment variations in the plasma, and the by-products of microbial metabolism that can modify color attributes. The odor shifted toward a more pronounced sour milk characteristic in samples with higher microbial loads, consistent with higher lactic acid production and related fermentation compounds. Texturally, most gels exhibited a firm, semi-solid consistency. The detailed results of the sensory analysis are provided in the
Supplementary Materials.
Overall, the formation of clots and the duration of gelation in bovine blood plasma strongly depended on both the species and concentration of the added microorganisms. Certain strains, especially Bifidobacterium lactis, Bifidobacterium longum, Staphylococcus xylosus, and Pediococcus pentosaseus, demonstrated pronounced gel-forming capacities at relatively low percentages, while others, such as Lactobacillus curvatus and Pediococcus acidilactici, required higher inoculum levels to elicit similar effects. Most notably, combining multiple strains drastically reduced gelation times and produced cohesive, firm gels.
3.3. Investigation of the Hydrogen Index (pH)
The hydrogen ion concentration (pH) is a critical factor influencing both the formation of the clot and its stability. Shifts in pH regulate the activity of enzymes responsible for establishing protein structures. An optimal pH range ensures uniform clot development, prevents clot disintegration, and preserves the functional properties of blood plasma. During strain selection, emphasis is placed on the microorganism’s ability to lower or maintain pH within the range necessary for stable clot formation [
41]. The pH values of the samples under investigation were measured immediately after inoculation with the strains and again after 24 h.
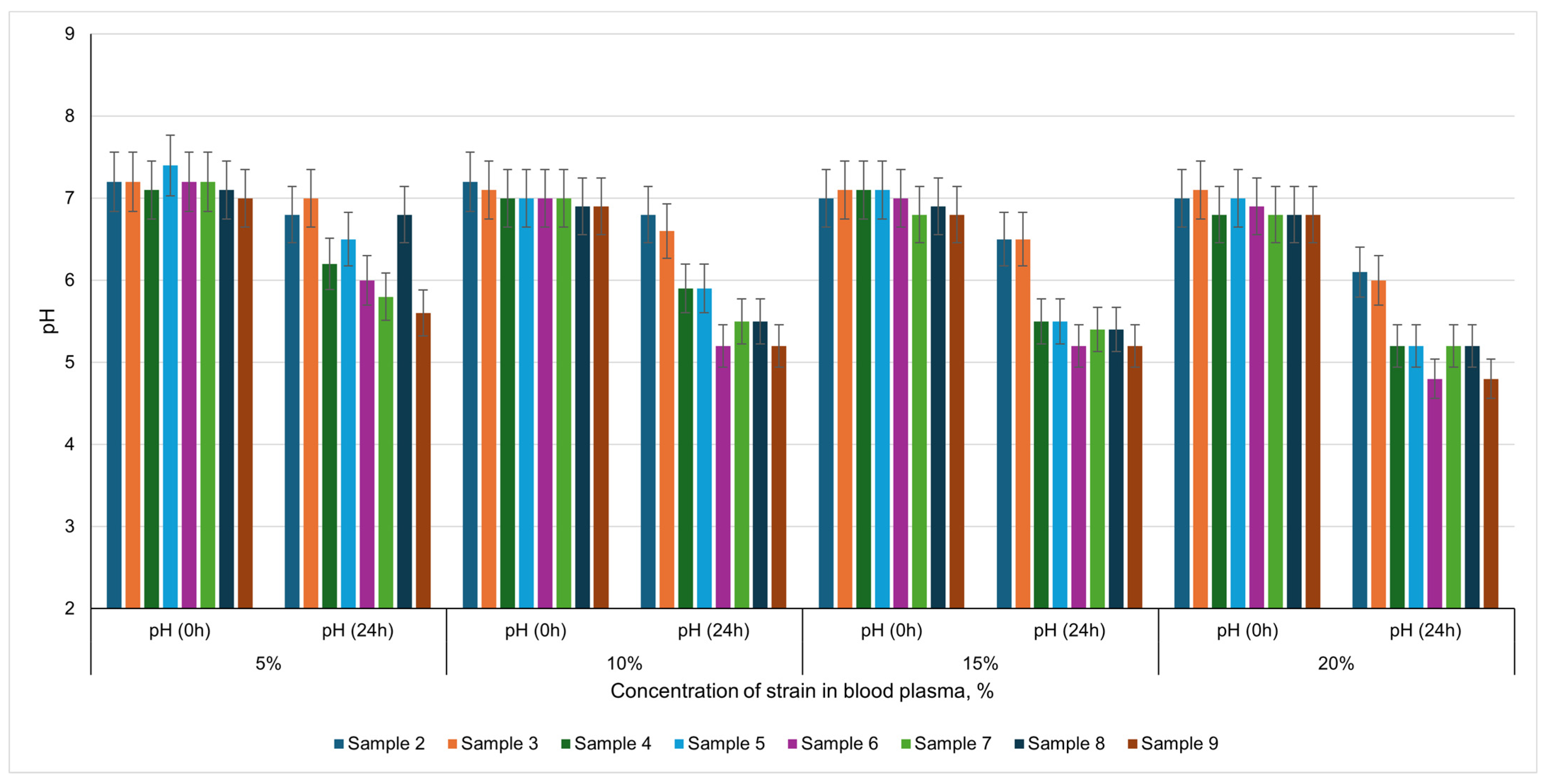
A key observation in this study is the decline in pH over a 24 h period following the introduction of different lactic acid bacteria into bovine blood plasma. Initially, the plasma maintains a near-neutral pH, a consequence of its inherent buffering capacity. Over time, however, each microbial strain metabolizes available substrates, primarily carbohydrates, generating lactic and other organic acids, which lower the pH. This pH decrease can critically influence the stability and structural properties of plasma proteins, as it approaches the isoelectric point at which protein coagulation is commonly expedited.
When 5% of each microorganism was introduced, relatively modest pH shifts were recorded in
Lactobacillus curvatus,
Pediococcus acidilactici, and
Bifidobacterium lactis cultures, indicating slower acid production and minimal decrease in pH (e.g., from pH 7.2 to approximately 6.5–6.8). In contrast,
Bifidobacterium longum,
Lactobacillus casei, and the multi-strain blend (
B. lactis + B. longum + Pediococcus pentosaseus + Staphylococcus xylosus) led to a more pronounced acidification (pH 5.6–5.8), suggesting greater lactic acid output (
Figure 2).
At 10% inoculum, the acidification patterns were similar. Cultures like Lactobacillus curvatus, Pediococcus acidilactici, and Pediococcus pentosaseus elicited only moderate shifts from near-neutral conditions (pH 7.0–7.2) to values of 5.9–6.6, indicating slower acid generation. Conversely, combinations that included both Bifidobacterium lactis and Bifidobacterium longum decrease the pH closer to 5.2, underscoring higher metabolic activity. Notably, this aligns with more rapid protein network formation, as plasma proteins coagulate more effectively when the pH nears their isoelectric point.
Raising the inoculum concentration to 15% and 20% amplified acid production further. In some treatments, the pH dropped to approximately 4.8, indicating robust lactic acid generation and potentially quicker clot formation. The multi-strain culture, in particular, showcased an intensive decrease from about pH 6.8 to 4.8, emphasizing the synergistic metabolic activity of these strains. Still, it is important to highlight that while a dramatic drop in pH can accelerate plasma structuring, other microbial factors (e.g., proteolysis, exopolysaccharide production) also play a role in gel formation and stability.
The findings underscore that higher inoculum levels of strains capable of rapid acid production (particularly
Bifidobacterium lactis,
Bifidobacterium longum, and certain multi-strain blends) produce more substantial pH declines, thus promoting faster and more extensive coagulation in blood plasma. Meanwhile, species like
Lactobacillus curvatus and
Pediococcus acidilactici effect a more gradual pH decrease, potentially leading to slower but still viable structuring processes. Optimal strain selection and concentration must be balanced to achieve the desired textural and functional properties, considering that excessive acidification may adversely affect flavor profiles or induce undesirable protein breakdown [
42].
3.4. Influence of Microbial Concentration on Yield Stress of Plasma Gels
Yield stress measurements provide valuable insight into the firmness or rigidity of blood plasma gels formed under various microbial treatments. By definition, yield stress is the minimum force per unit area required to initiate flow or deformation of the material [
43]. The untreated (control) plasma sample displayed a yield stress of 410 Pa, suggesting a stable and robust coagulated network. In the presence of microorganisms, the formation of gel structures was still evident, but yield stress values did not surpass those of the control. This indicates that while microbial action can induce or modify protein networks in plasma, it may also partially disrupt or remodel the clot matrix, preventing it from reaching the full rigidity achieved by the recalcified control sample.
A clear trend emerged when comparing yield stress at concentrations of 10%, 15%, and 20%. At 10% inoculation,
Lactobacillus curvatus and
Pediococcus acidilactici produced no yield stresses, indicating a liquid consistency with minimal network formation at this relatively low microbial load. Other strains, including
Lactobacillus casei,
Staphylococcus xylosus, and various
Bifidobacterium spp., yielded intermediate values between 184 Pa (
Bifidobacterium lactis) and 260 Pa (the multi-strain combination), demonstrating that moderate microbial levels can form semi-rigid gels (
Table 4).
Increasing the inoculum to 15% allowed Lactobacillus curvatus and Pediococcus acidilactici to achieve measurable yield stresses (198 Pa and 212 Pa, respectively). Single strains such as Lactobacillus casei (310 Pa) and Staphylococcus xylosus (318 Pa) provided even firmer gels, approaching the structural integrity of the control. The combined culture recorded 315 Pa, close to the strongest single-strain result, yet still below the control’s 410 Pa.
At 20%, all microbial treatments produced more robust gels, with yield stresses spanning 278 Pa (Lactobacillus curvatus) to 396 Pa (combined culture). Notable single-strain performers included Staphylococcus xylosus at 385 Pa and Lactobacillus casei at 372 Pa, reflecting strong structural development under higher doses. The combined inoculum reached 396 Pa, which closely approached the control and highlighted the potential of synergistic interactions to reinforce network formation.
Overall, yield stress systematically increased with microorganism concentration from 10% to 20%. This outcome likely results from higher microbial metabolism, increased acid production, and the possible generation of exopolysaccharides or proteolytic by-products at larger cell counts—all factors that contribute to strengthening or reorganizing the gel network.
Several strains demonstrated particular efficacy in building gel strength. Staphylococcus xylosus consistently produced higher yield stresses at every inoculum level, indicating a strong aptitude for forming or stabilizing protein networks. Likewise, Lactobacillus casei reached one of the highest yield stresses among single strains, particularly at 15% and 20%, likely due to robust acid production and proteolytic activity that contribute to a denser protein matrix. In many cases, mixed cultures (Sample 9) equaled or surpassed single-strain results, demonstrating microbial synergy; different species may release complementary enzymes and metabolites that encourage efficient protein cross-linking or partial hydrolysis, generating a more cohesive network.
By contrast, Lactobacillus curvatus and Pediococcus acidilactici showed weaker structuring capacities at 10%, resulting in minimal gel firmness. Their ability to form stable gels became evident only at higher inoculation levels (≥15%), suggesting these species rely on greater cell density or supportive co-cultures to achieve notable clot strength. The closest to the control value of the shear stress limit was observed when introducing into the plasma a starter containing strains of Bifidobacterium lactis, Bifidobacterium longum, Pediococcus pentosaseus, Staphylococcus xylosus.
Acidification plays a crucial role, since many lactic acid bacteria reduce the plasma’s pH, promoting protein denaturation and aggregation [
44]. The extent and speed of acid production closely correlate with better gel formation and higher yield stress. Moderate proteolysis can facilitate partial unfolding of plasma proteins, exposing reactive sites for cross-linking, although excessive proteolysis may degrade the network and weaken mechanical strength.
3.5. Evaluation of Plasma Coagulant Syneresis
Syneresis refers to the expulsion of liquid (serum) from a clot or gel matrix, and thus reflects the stability and water-holding capacity of a structured system. A high level of syneresis signals clot instability, which is undesirable for formulating structured compositions [
45]. In blood plasma gels, syneresis often arises from fibrinolytic processes that activate shortly after clotting, ultimately leading to phase separation into serum and coagulate over time [
46].
This investigation assessed how various lactic acid bacteria strains, alone or in combination, at inoculum levels of 10%, 15%, and 20%, affect syneresis in plasma gels. Freezing and thawing can significantly destabilize protein matrices, with tighter, denser networks retaining water more effectively. Hence, strains that promote robust cross-linking, acidification, and exopolysaccharide production generally yield firmer gels with lower serum separation. Here, Lactobacillus casei and Pediococcus pentosaseus repeatedly produced relatively stable networks, whereas Bifidobacterium longum yielded higher separation at lower inoculation levels, suggesting a weaker gel network unless higher cell densities were used.
An increase in microbial concentration (from 10% to 15% or 20%) typically reduces syneresis (
Table 5). This trend indicates that a greater population of lactic acid bacteria enhances enzyme production, acidification, and possibly exopolysaccharide synthesis—factors that reinforce the fibrin network and improve water retention. Notably, while multi-strain cultures can synergize to accelerate coagulation, imbalances in proteolysis or strain ratios sometimes led to elevated serum release upon thawing.
Freeze–thaw syneresis fell as inoculum density rose. At 10%, some cultures of Lactobacillus curvatus and Pediococcus acidilactici did not gel, confirming that a minimal cell mass is required to initiate coagulation. Where clots did form, Lactobacillus casei released only 23% serum, while Pediococcus pentosaseus and Staphylococcus xylosus gave ~27–28%. The poorest water retention was recorded for Bifidobacterium longum (45%), exceeding the recalcified control (39%), indicating a weak network at this dose. A mixed starter (BB 12 + BB 46 + T-SPX) showed intermediate performance (30%).
Raising the load to 15% improved almost every culture. P. pentosaseus fell to 19.5% serum, L. casei to 21.1%, and S. xylosus to 22.4%, while L. curvatus and P. acidilactici now matched these values (~23%). B. longum dropped but remained the weakest (31.5%). The mixed starter decreased to 27.4%, but still lagged the best single strains.
At 20% all clots became markedly more stable. P. pentosaseus again led (12.2% serum), followed by L. casei, P. acidilactici, and L. curvatus (16.7–18.4%). B. lactis and S. xylosus gave ~18–20%, while B. longum remained highest at 26.2%. The mixed culture (24.2%) did not outperform the best single strains, implying that excessive proteolysis or unbalanced acidification can offset the synergy expected from multi-strain systems.
Greater cell densities intensify acid and exopolysaccharide production and, in many strains, controlled proteolysis. These effects tighten the fibrin network and limit water channels generated by ice crystals, thereby reducing syneresis. Strains differ in their ability to generate stabilizing polysaccharides or to avoid over-degrading plasma proteins [
47].
P. pentosaseus and
L. casei provide an optimal balance, whereas
B. longum tends to destabilize the matrix unless supported by higher inocula or complementary species.
An inoculum of 15–20% is necessary to secure robust water-holding, with P. pentosaseus and L. casei giving the lowest serum loss. Multi-strain starters accelerate acidification but will not automatically minimize syneresis; precise strain ratios and fermentation control are required to prevent excess proteolysis. For plasma-based gel ingredients, formulating around these high-performing single strains, or well-balanced blends, offers the best compromise between rapid gelation, firmness, and freeze–thaw stability while maintaining acceptable sensory attributes.
Increasing the lactic acid starter above 20% accelerates gelation but reduces pH below 5.0, causing excessive proteolysis, a 15% reduction in yield stress, and 20% greater syneresis after seven days at 4 °C, with off-flavors. To maintain gel quality, inoculum should be limited to 20% and fermentation optimized. Buffering agents or flax polysaccharides may help control acidification and proteolysis.
3.6. Investigation of the Effect of the Flaxseed Oil Cake Flour on the Stability of the Coagulation Structure of Blood Plasma
In determining syneresis in plasma coagulants, it was observed that structured blood plasma constitutes an unstable coagulation system in which syneresis occurs over time. Consequently, the use of this gel in the meat industry would be challenging. One potential solution for preventing serum separation is the inclusion of an additional plant-derived component in the plasma. By occupying the fibrin matrix cells, this additive can help resist deformation. Moreover, it must not inhibit the growth of lactic acid bacteria or enzyme biosynthesis. From this standpoint, flaxseed oil cake flour holds considerable interest.
In an effort to address the syneresis observed in structured bovine blood plasma, flaxseed oil cake flour was introduced at various levels (2%, 5%, 8%, and 10%) to evaluate its impact on both gel formation and post-coagulation stability (
Figure 3). Previous experiments indicated that although blood plasma could be structured using a 20% mixed starter culture of
Bifidobacterium lactis,
Bifidobacterium longum,
Pediococcus pentosaseus, and
Staphylococcus xylosus, the resulting gel exhibited noticeable syneresis after freeze–thaw and even over time at ambient conditions. Such a phenomenon could compromise the texture and stability of plasma-based products in the meat industry, necessitating a means of enhancing water retention within the gel matrix.
The primary rationale for incorporating flaxseed oil cake flour lies in its ability to swell when hydrated and fill the interstitial spaces within the fibrin network. By integrating into this matrix, the flour particles can potentially prevent excessive serum expulsion [
48]. Moreover, flaxseed oil cake flour, if properly hydrated, should not interfere with the vital activities of lactic acid bacteria or enzyme production, making it an appealing choice for developing stabilized plasma gels.
Flaxseed oil cake proteins exhibit high biological value, reaching approximately 92% relative to casein protein [
49]. These oil cake fractions contain a complete set of essential amino acids, with methionine + cystine and tryptophan levels comparable to those in an ideal protein. However, lysine is the limiting amino acid [
50].
Flaxseed oil cake is a by-product derived from flaxseed oil production. When flaxseed oil cake flour undergoes hydrothermal treatment and is subsequently combined with lactic acid microorganisms (maintained at 20–22 °C for 4–20 h), it displays the ability to form elastic, resilient gels. During the fermentation of the flour’s mono- and disaccharides by lactic acid bacteria, organic acids such as lactic acid accumulate. This acid buildup reduces pH, altering the conformation of flaxseed protein, facilitating inter-chain contacts, and thus promoting gel network formation. Moreover, the dietary fibers present in flaxseed exhibit the capacity to form adsorption complexes with animal proteins [
51]. In blood plasma systems, the introduction of such polysaccharides appears to exert a stabilizing effect when lactic acid bacteria are used to structure blood plasma [
52].
3.7. Increasing Flour Content and Prolonged Structure Formation
As the concentration of flaxseed oil cake flour increased from 2% to 10%, the duration of structure formation progressively rose from about 1.1 h at 2% to more than four hours at 10%. This trend suggests that while flaxseed flour contributes to a more stable matrix, its presence may slow down or modify the overall gelation kinetics. One possible explanation is that the plant-based solids interfere with fibrin cross-linking or reduce the effective concentration of proteins and microorganisms in direct contact, thus delaying the onset of clot stabilization [
53]. Additionally, the viscosity imparted by the hydrated flour might alter mass transfer of microbial metabolites or ions, further impacting the gelation rate.
The marked reduction in syneresis was a major benefit of the flaxseed flour addition. With no flour present (control), syneresis measured 24.2%. Incorporating 2% of flaxseed flour dropped this value to 21.5%, and increased levels (8% or 10%) reduced syneresis more drastically, reaching as low as 9.8% (
Table 6). These results reinforce the hypothesis that the plant material effectively binds or traps water within the matrix, thus limiting serum separation. Given that syneresis is a key concern for product quality and shelf life, especially in meat applications where excessive water loss compromises texture, this reduction is highly advantageous.
Based on the
Table 6, we concluded that the positive effect of flaxseed cake flour on the stability of plasma coagulation. Increasing the concentration of hydrated flour from flaxseed cake increases the duration of structure formation in blood plasma. decreases the amount of separated serum.
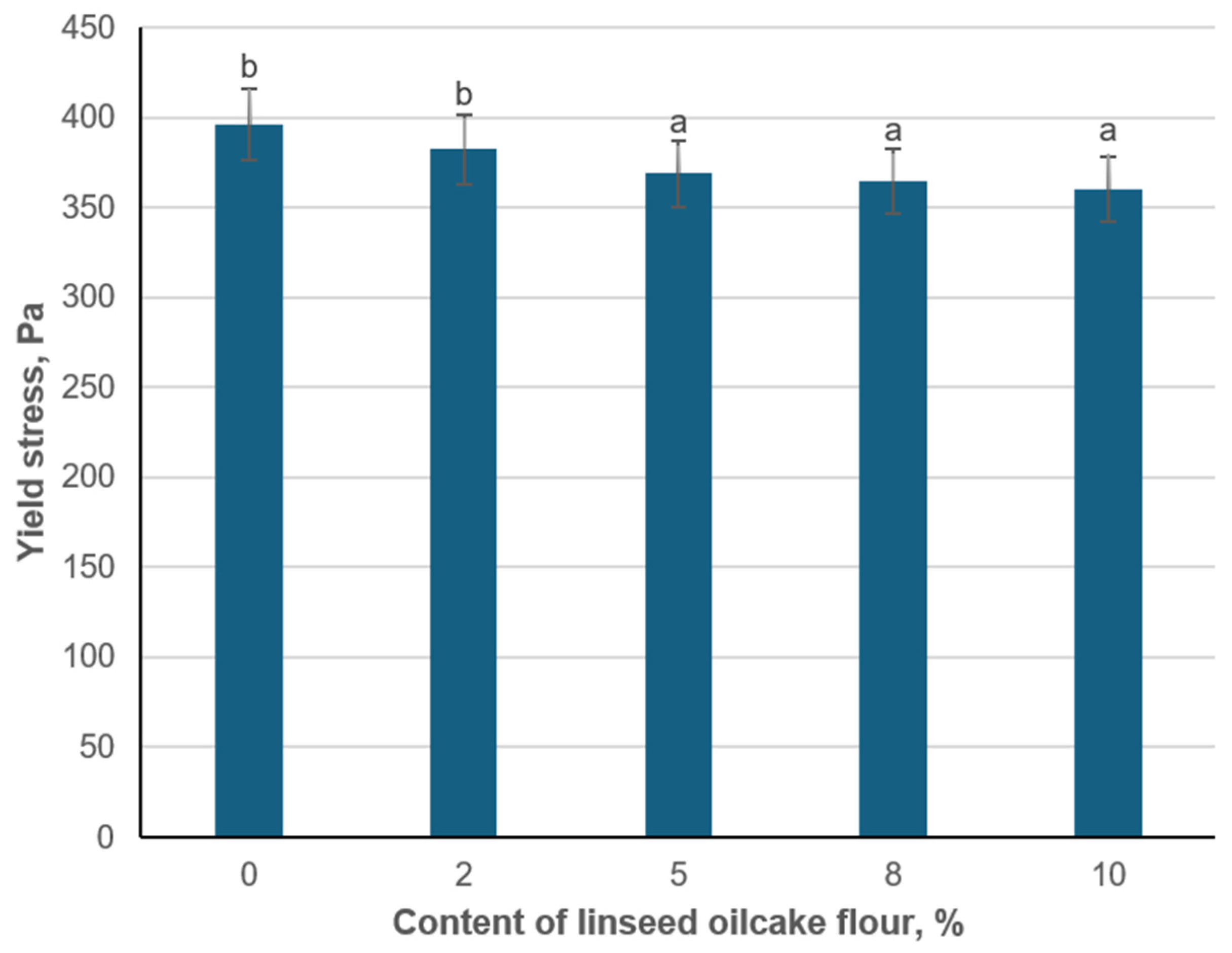
3.8. Effect of Flaxseed Flour Content on Yield Stress
The consistency of structured blood plasma at different concentrations of hydrated flaxseed cake flour was determined by the value of yield stress. Introducing a small amount of flaxseed meal (2%) resulted in a slight decrease in yield stress (382.2 Pa) from the control value (396 Pa). Increasing the flaxseed content further (5%, 8%, and 10%) caused progressively lower yield stresses, down to 360.4 Pa at 10% (
Figure 4). While these changes are relatively modest, they indicate that flaxseed flour partially reduces the rigidity of the plasma gel network.
One plausible explanation for this decline is that flaxseed flour acts as a filler within the fibrin matrix, potentially interfering with the continuous protein cross-linking that underpins higher yield stress values [
54]. Flaxseed particles occupy space and bind water. They may alter the effective concentration or spatial arrangement of plasma proteins and microbial cells, thus diminishing the cohesive forces responsible for gel rigidity. Moreover, any plant-derived enzymes or fibers (e.g., mucilage) might modify local pH or ionic strength, further influencing the protein–protein interactions that drive clot firmness [
55].
When examining the effect of hydrated flaxseed oil cake flour on the yield stress of blood plasma structured by lactic acid bacteria, it was found that increasing the flour concentration beyond 5% does not substantially reduce syneresis. Consequently, incorporating 5% hydrated flaxseed oil cake flour is advisable for the lactic-acid-bacteria-structured plasma. Moreover, adding more than 5% flaxseed flour may negatively affect the meat product’s sensory properties.
Hydrated flaxseed oil cake flour particles can physically occupy interstitial spaces between fibrin filaments, which increases the matrix’s density and decreases the pathways through which liquid escapes. In addition, flaxseed flour contains biopolymers such as proteins, fiber, and possibly mucilage-like polysaccharides, all of which can bind water and interact with plasma proteins to reinforce the gel and slow serum migration [
56]. The fact that the lactic acid bacteria remain capable of promoting gelation in the presence of flaxseed flour also suggests that this plant component does not significantly inhibit microbial metabolism. Instead, prolonged structure formation at higher flour levels likely reflects physicochemical interactions rather than a reduction in bacterial growth or enzyme activity.
Despite the drop in yield stress, all samples formed visible clots, indicating that flaxseed flour does not inhibit overall coagulation. The lactic acid bacteria strains (Bifidobacterium lactis, Bifidobacterium longum, Pediococcus pentosaseus, Staphylococcus xylosus) effectively reduce pH, promote proteolysis, and facilitate gelation in blood plasma. The addition of flaxseed meal does not appear to disrupt these microbial activities to the point of preventing clot formation; rather, its impact is subtler, reflecting changes in the mechanical properties of the gel.
3.9. Selection of the Optimal Plasma Gel Variant
Among the formulations tested, the inclusion of 5% hydrated flaxseed cake flour yielded the most favorable balance of functional and compositional properties and was therefore selected for further study. This gel exhibited a proximate composition of 75.5% water, 16.7% protein, 1.3% fat, 1.8% ash, and 4.7% carbohydrates. After 24 h fermentation with a 20% mixed lactic acid starter, the pH stabilized at 5.3—close to the isoelectric point of major plasma proteins, promoting optimal fibrin network formation without excessive acidity.
This formulation demonstrated a water-holding capacity (WHC) of 135%, indicating excellent moisture retention and minimal syneresis under freeze–thaw conditions. It maintained a fat-holding capacity (FHC) of 33%, ensuring adequate lipid entrapment for juiciness and oxidative stability. Compared to variants with higher flax levels, the 5% addition preserves sufficient protein binding sites for emulsification while benefiting from polysaccharide-mediated reinforcement of the fibrin network. These results demonstrate that a modest flaxseed flour content optimizes moisture retention without sacrificing fat stabilization or introducing off-flavors. The pH, rheological strength, and holding capacities observed confirm that the 5% flax—flour variant forms a coherent, resilient gel appropriate for meat matrices.
Building on these findings, incorporation of the plasma gel into minced meat formulations for sausage production will be pursued, with quantitative assessment of its effects on cooking yield, texture profile, and sensory acceptance to validate industrial applicability. These proposed experiments are grounded in, and extend, a growing body of research exploring the functional valorization of blood plasma in food systems.
For example, Fort (2010) demonstrated that structuring of blood plasma gels by endogenous transglutaminase and lactic acid bacteria can restore the heat-induced gel properties of plasma under acidic conditions, promoting fibrin–polymer network formation and reactivation of coagulation pathways [
57]. Oro et al. (2018) reported that substituting broiler blood plasma powder for soy proteins in chicken sausages achieved microbial safety, acceptable sensory properties, and minimal cooking loss (<5%) [
58]. Similarly, fermenting bovine plasma with a 20% lactic starter and 5% flaxseed cake flour produced gels with less than 10% syneresis, a 135% water-holding capacity, and a neutral flavor, illustrating the efficacy of combined microbial and plant-fiber approaches for plasma valorization.
Further supporting these findings, Fernández et al. (2021) showed that bovine plasma structured with flaxseed oil can produce oleogels with high gel strength (yield stress ≈ 372 Pa) and excellent oil-binding capacity, particularly when gelation occurs at elevated temperature [
59]. The addition of 5% hydrated flaxseed cake flour reduced syneresis below 10% without imparting off-flavors, underscoring the stabilizing function of flaxseed polysaccharides in plasma gels. Rodriguez Furlán et al. (2010) also demonstrated that bovine plasma proteins processed by ultrafiltration–diafiltration and freeze-drying exhibit enhanced solubility, emulsifying, and foaming properties across a broad pH range, and, when applied in minced meat formulations, improve moisture retention, emulsion stability, and textural consistency without adverse sensory effects [
60]. These studies provide a strong foundation for the continued development and industrial evaluation of plasma-based gel systems for innovative meat product applications.