Photoautotrophic Production of Eicosapentaenoic Acid (EPA) with Nannochloropsis oceanica Under Dynamic Climate Simulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Reaction Media
2.2. Seed Cultures and Pre-Cultures in Bubble Column Reactors
2.3. Cultivation in Flat-Plate Gas-Lift Photobioreactors
2.4. Climate Simulation and AlgaeTec Center
2.5. Cultivation in Thin-Layer Cascade Photobioreactors
2.6. Determination of Optical Density and Cell Dry Weight Concentration
2.7. Determination of Nitrate and Urea Concentrations, and Salinity
2.8. Determination of EPA Concentration and Microalgae Content
3. Results and Discussion
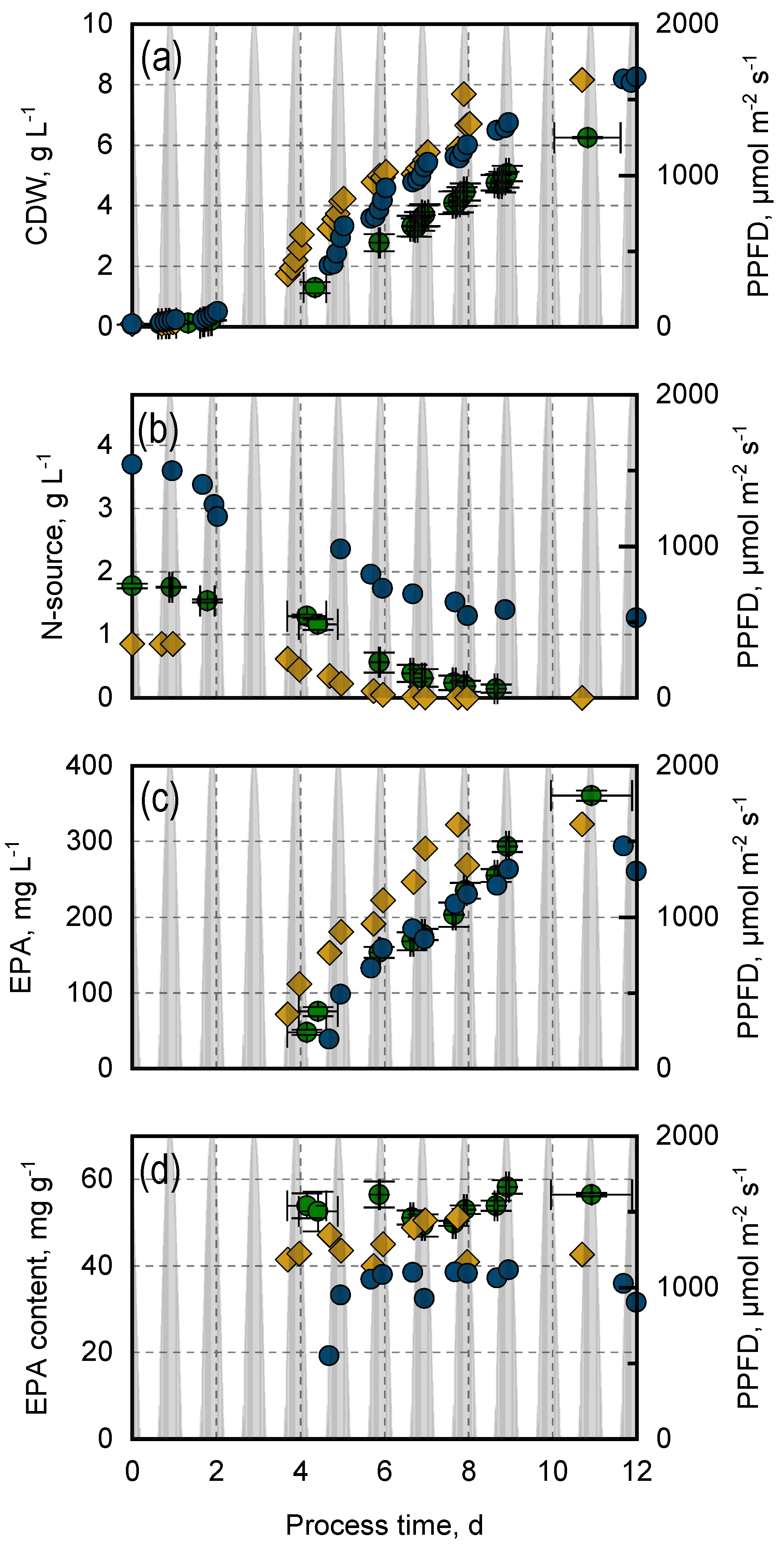
3.1. Photoautotrophic Biomass Growth and EPA Production in Closed Flat-Plate Gas-Lift Photobioreactors with N. oceanica Compared to M. salina


| t = 11 d | N. oceanica | M. salina |
|---|---|---|
| CDW, g L−1 | 6.3 ± 0.0 | 5.4 ± 0.5 |
| EPA, mg L−1 | 360 ± 7 | 255 ± 23 |
| EPA content, mg g−1 | 56 ± 0 | 48 ± 1 |
| STYEPA, mg L−1 | 33.0 ± 0.6 | 23.7 ± 0.1 |
3.2. Variation of Nitrogen Concentration and Nitrogen Source in Photoautotrophic Batch Processes with N. oceanica
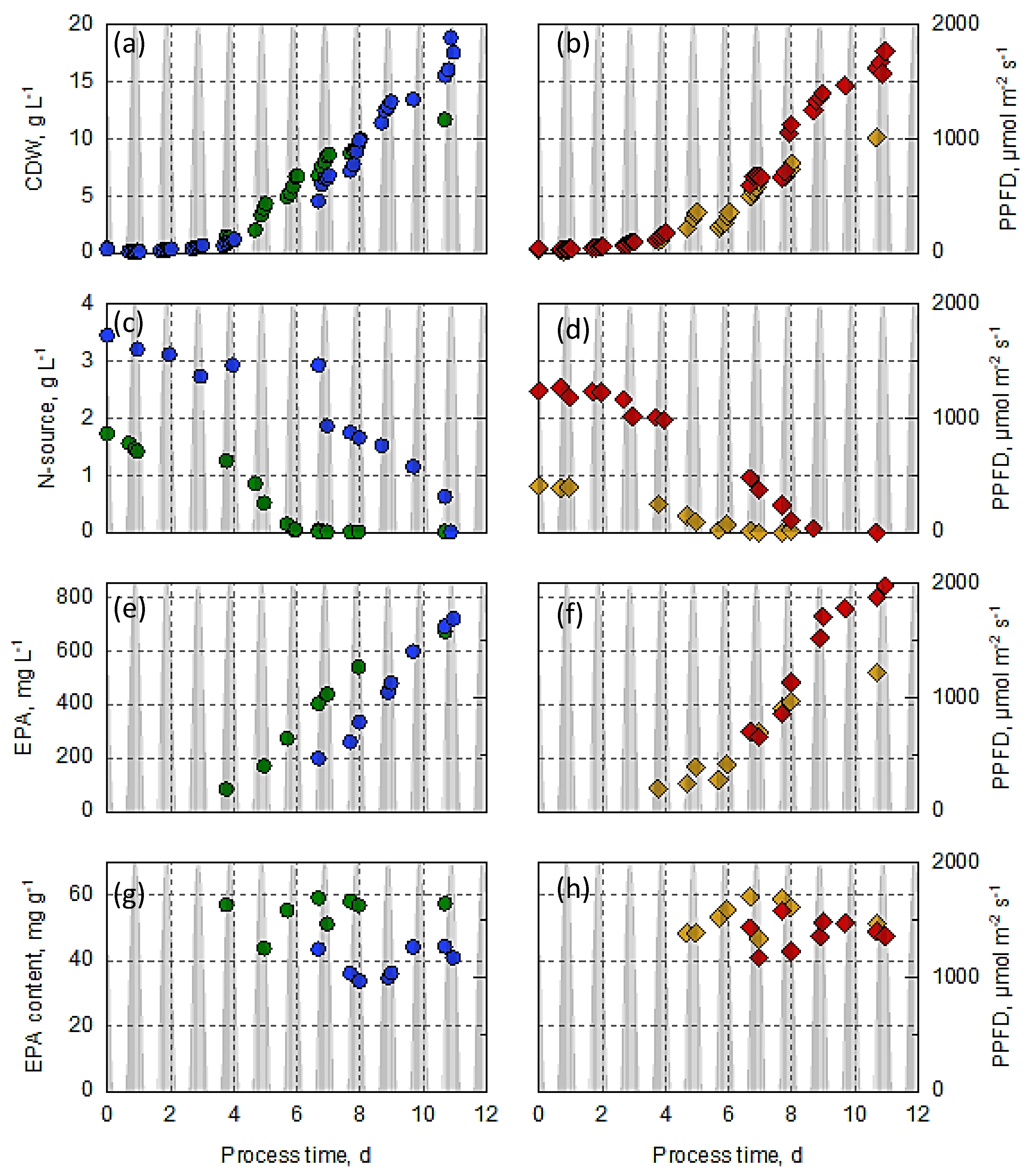
3.3. Production of EPA in Open Thin-Layer Cascade Reactors with N. oceanica, Varying the Nitrogen Source and Concentration
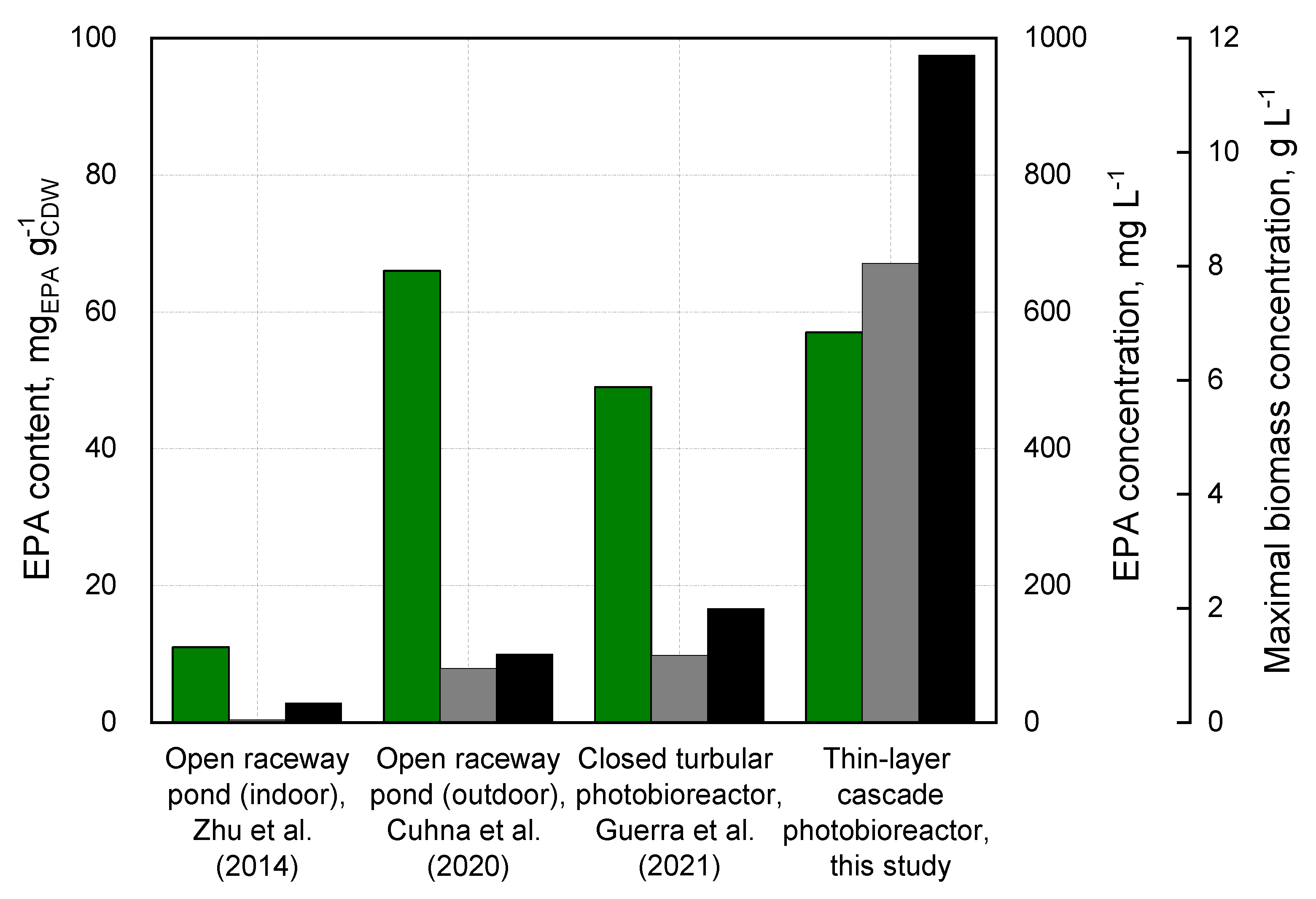
3.4. Comparison of Biomass and EPA Production in Open and Closed Photobioreactors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDW | Cell dry weight |
| EPA | Eicosapentaenoic acid |
| FPR | Flat-plate gas-lift photobioreactor |
| PPFD | PAR photon flux density |
| STY | Space-time yield |
| TLC | Thin-layer cascade photobioreactor |
References
- Calder, P.C.; Yaqoob, P. Marine omega-3 fatty acids and coronary heart disease. Curr. Opin. Cardiol. 2012, 27, 412–419. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Long chain omega-3 fatty acids and cardiovascular disease: A systematic review. Br. J. Nutr. 2012, 107 (Suppl. S2), S201–S213. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. (n-3) Fatty Acids and Cardiovascular Health: Are Effects of EPA and DHA Shared or Complementary?1–323. J. Nutr. 2012, 142, 614S–625S. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Hamilton, H.A.; Newton, R.; Auchterlonie, N.A.; Müller, D.B. Systems approach to quantify the global omega-3 fatty acid cycle. Nat. Food 2020, 1, 59–62. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Xu, L.; Weathers, P.J.; Xiong, X.-R.; Liu, C.-Z. Microalgal bioreactors: Challenges and opportunities. Eng. Life Sci. 2009, 9, 178–189. [Google Scholar] [CrossRef]
- Sharma, K.; Schenk, P.M. Rapid induction of omega-3 fatty acids (EPA) in Nannochloropsis sp. by UV-C radiation. Biotechnol. Bioeng. 2015, 112, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Nagarajan, D.; Cheah, W.Y. Eicosapentaenoic acid production from Nannochloropsis oceanica CY2 using deep sea water in outdoor plastic-bag type photobioreactors. Bioresour. Technol. 2018, 253, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.; Pereira, H.; Costa, M.; Pereira, J.; Silva, J.T.; Fernandes, N.; Varela, J.; Silva, J.; Simões, M. Nannochloropsis oceanica Cultivation in Pilot-Scale Raceway Ponds—From Design to Cultivation. Appl. Sci. 2020, 10, 1725. [Google Scholar] [CrossRef]
- Gharat, K.; Agarwal, A.; Pandit, R.A.; Lali, A.M. Development of fed batch strategies to improve the production of eicosapentaenoic acid from a marine microalga Nannochloropsis oculata. Bioresour. Technol. Rep. 2018, 4, 193–201. [Google Scholar] [CrossRef]
- de Vree, J.H.; Bosma, R.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H. Comparison of four outdoor pilot-scale photobioreactors. Biotechnol. Biofuels 2015, 8, 215. [Google Scholar] [CrossRef]
- San Pedro, A.; González-López, C.V.; Acién, F.G.; Molina-Grima, E. Marine microalgae selection and culture conditions optimization for biodiesel production. Bioresour. Technol. 2013, 134, 353–361. [Google Scholar] [CrossRef]
- Koruyucu, A.; Schädler, T.; Gniffke, A.; Mundt, K.; Krippendorf, S.; Urban, P.; Blums, K.; Halim, B.; Brück, T.; Weuster-Botz, D. Energy-Efficient Production of Microchloropsis salina Biomass with High CO2 Fixation Yield in Open Thin-Layer Cascade Photobioreactors. Processes 2024, 12, 1303. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I. Lipid Metabolism in Microalgae. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 413–484. [Google Scholar]
- Gurr, M.I.; Harwood, J.L. Fatty Acid Structure and Metabolism. In Lipid Biochemistry; Springer: Boston, MA, USA, 2002; pp. 13–92. [Google Scholar]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Harwood, J.L. Membrane Lipids in Algae. In Lipids in Photosynthesis: Structure, Function and Genetics; Paul-André, S., Norio, M., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 53–64. [Google Scholar]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Progress Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef]
- Meng, Y.; Jiang, J.; Wang, H.; Cao, X.; Xue, S.; Yang, Q.; Wang, W. The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes. Bioresour. Technol. 2015, 179, 483–489. [Google Scholar] [CrossRef]
- Sá, M.; Ferrer-Ledo, N.; Wijffels, R.; Crespo, J.G.; Barbosa, M.; Galinha, C.F. Monitoring of eicosapentaenoic acid (EPA) production in the microalgae Nannochloropsis oceanica. Algal Res. 2020, 45, 101766. [Google Scholar] [CrossRef]
- Schadler, T.; Caballero Cerbon, D.; de Oliveira, L.; Garbe, D.; Bruck, T.; Weuster-Botz, D. Production of lipids with Microchloropsis salina in open thin-layer cascade photobioreactors. Bioresour. Technol. 2019, 289, 121682. [Google Scholar] [CrossRef] [PubMed]
- Campos, H.; Boeing, W.J.; Dungan, B.N.; Schaub, T. Cultivating the marine microalga Nannochloropsis salina under various nitrogen sources: Effect on biovolume yields, lipid content and composition, and invasive organisms. Biomass Bioenergy 2014, 66, 301–307. [Google Scholar] [CrossRef]
- Thurn, A.-L.; Schobel, J.; Weuster-Botz, D. Photoautotrophic Production of Docosahexaenoic Acid- and Eicosapentaenoic Acid-Enriched Biomass by Co-Culturing Golden-Brown and Green Microalgae. Fermentation 2024, 10, 220. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef]
- Pfaffinger, C.E.; Schöne, D.; Trunz, S.; Löwe, H.; Weuster-Botz, D. Model-based optimization of microalgae areal productivity in flat-plate gas-lift photobioreactors. Algal Res. 2016, 20, 153–163. [Google Scholar] [CrossRef]
- Wolf, L.; Cummings, T.; Müller, K.; Reppke, M.; Volkmar, M.; Weuster-Botz, D. Production of β-carotene with Dunaliella salina CCAP19/18 at physically simulated outdoor conditions. Eng. Life Sci. 2021, 21, 115–125. [Google Scholar] [CrossRef]
- Benemann, J. Microalgae for Biofuels and Animal Feeds. Energies 2013, 6, 5869–5886. [Google Scholar] [CrossRef]
- Setlík, I.; Sust, V.; Malek, I. Dual purpose open circulation units for large scale culture of algae in temperate zones. I. Basic design considerations and scheme of pilot plant. Algol. Stud. 1970, 1, 111–164. [Google Scholar]
- Doucha, J.; Lívanský, K. Outdoor open thin-layer microalgal photobioreactor: Potential productivity. J. Appl. Phycol. 2009, 21, 111–117. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. J. Appl. Phycol. 2006, 18, 811–826. [Google Scholar] [CrossRef]
- Masojídek, J.; Kopecký, J.; Giannelli, L.; Torzillo, G. Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J. Ind. Microbiol. Biotechnol. 2011, 38, 307–317. [Google Scholar] [CrossRef]
- Apel, A.C.; Pfaffinger, C.E.; Basedahl, N.; Mittwollen, N.; Göbel, J.; Sauter, J.; Brück, T.; Weuster-Botz, D. Open thin-layer cascade reactors for saline microalgae production evaluated in a physically simulated Mediterranean summer climate. Algal Res. 2017, 25, 381–390. [Google Scholar] [CrossRef]
- Pfaffinger, C.E.; Severin, T.S.; Apel, A.C.; Göbel, J.; Sauter, J.; Weuster-Botz, D. Light-dependent growth kinetics enable scale-up of well-mixed phototrophic bioprocesses in different types of photobioreactors. J. Biotechnol. 2019, 297, 41–48. [Google Scholar] [CrossRef]
- Schädler, T.; Neumann-Cip, A.-C.; Wieland, K.; Glöckler, D.; Haisch, C.; Brück, T.; Weuster-Botz, D. High-Density Microalgae Cultivation in Open Thin-Layer Cascade Photobioreactors with Water Recycling. Appl. Sci. 2020, 10, 3883. [Google Scholar] [CrossRef]
- Havel, J.; Franco-Lara, E.; Weuster-Botz, D. A parallel bubble column system for the cultivation of phototrophic microorganisms. Biotechnol. Lett. 2008, 30, 1197–1200. [Google Scholar] [CrossRef]
- Thurn, A.-L.; Stock, A.; Gerwald, S.; Weuster-Botz, D. Simultaneous photoautotrophic production of DHA and EPA by Tisochrysis lutea and Microchloropsis salina in co-culture. Bioresour. Bioprocess. 2022, 9, 130. [Google Scholar] [CrossRef]
- Hulatt, C.J.; Wijffels, R.H.; Bolla, S.; Kiron, V. Production of Fatty Acids and Protein by Nannochloropsis in Flat-Plate Photobioreactors. PLoS ONE 2017, 12, e0170440. [Google Scholar] [CrossRef]
- Ren, M.; Ogden, K. Cultivation of Nannochloropsis gaditana on mixtures of nitrogen sources. Environ. Progress Sustain. Energy 2014, 33, 551–555. [Google Scholar] [CrossRef]
- Sassano, C.E.N.; Carvalho, J.C.M.; Gioielli, L.A.; Sato, S.; Torre, P.; Converti, A. Kinetics and bioenergetics of Spirulina platensis cultivation by fed-batch addition of urea as nitrogen source. Appl. Biochem. Biotechnol. 2004, 112, 143–150. [Google Scholar] [CrossRef]
- Guerra, I.; Pereira, H.; Costa, M.; Silva, J.T.; Santos, T.; Varela, J.; Mateus, M.; Silva, J. Operation Regimes: A Comparison Based on Nannochloropsis oceanica Biomass and Lipid Productivity. Energies 2021, 14, 1542. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, F.; Yang, M.; Lu, L.; Yang, G.; Pan, K. Large-scale biodiesel production using flue gas from coal-fired power plants with Nannochloropsis microalgal biomass in open raceway ponds. Bioresour. Technol. 2014, 174, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wan Razali, W.A.; Pandhal, J. Outdoor pilot-scale cultivation and techno economic assessment of a novel omega-3 eicosapentaenoic acid over-producing Nannochloropsis oculata strain. Bioresour. Technol. Rep. 2023, 24, 101682. [Google Scholar] [CrossRef]

| Flat-Plate Gas-Lift Photobioreactor | Thin-Layer Cascade Photobioreactor | |
|---|---|---|
| Working volume (VReactor, L) | 1.8 | 55 |
| (Illuminated) reactor surface (AReactor, m2) | 0.09 | 8 |
| Surface-to-volume ratio (AReactor VReactor−1, m−1) | 50 | 150 |
| Fluid layer depth (cm) | 2 | 0.5 |
| CO2 supply | pH-dependent Addition via aeration trough gassing pipe | pH-dependent Addition through perforated hoses in the retention tank |
| t = 11 d | Flat-Plate Gas-Lift Photobioreactor | Thin-Layer Cascade Photobioreactor |
|---|---|---|
| CDW, g L−1 | 6.3 ± 0.0 | 11.7 |
| EPA concentration, mg L−1 | 360 ± 7 | 671 |
| EPA content, mg g−1 | 56 ± 0 | 57 |
| EPA productivity, mg L−1, d−1 | 33.0 ± 0.6 | 65.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thurn, A.-L.; Gerwald, S.; Brück, T.; Weuster-Botz, D. Photoautotrophic Production of Eicosapentaenoic Acid (EPA) with Nannochloropsis oceanica Under Dynamic Climate Simulations. Processes 2025, 13, 1649. https://doi.org/10.3390/pr13061649
Thurn A-L, Gerwald S, Brück T, Weuster-Botz D. Photoautotrophic Production of Eicosapentaenoic Acid (EPA) with Nannochloropsis oceanica Under Dynamic Climate Simulations. Processes. 2025; 13(6):1649. https://doi.org/10.3390/pr13061649
Chicago/Turabian StyleThurn, Anna-Lena, Sebastian Gerwald, Thomas Brück, and Dirk Weuster-Botz. 2025. "Photoautotrophic Production of Eicosapentaenoic Acid (EPA) with Nannochloropsis oceanica Under Dynamic Climate Simulations" Processes 13, no. 6: 1649. https://doi.org/10.3390/pr13061649
APA StyleThurn, A.-L., Gerwald, S., Brück, T., & Weuster-Botz, D. (2025). Photoautotrophic Production of Eicosapentaenoic Acid (EPA) with Nannochloropsis oceanica Under Dynamic Climate Simulations. Processes, 13(6), 1649. https://doi.org/10.3390/pr13061649