A Simple Method to Determine Pheomelanin Content and Structure in FFPE Human Melanoma Specimens
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of FFPE for Analysis
2.3. Reagents and Compounds
2.4. Preparation of Reference Synthetic Melanins
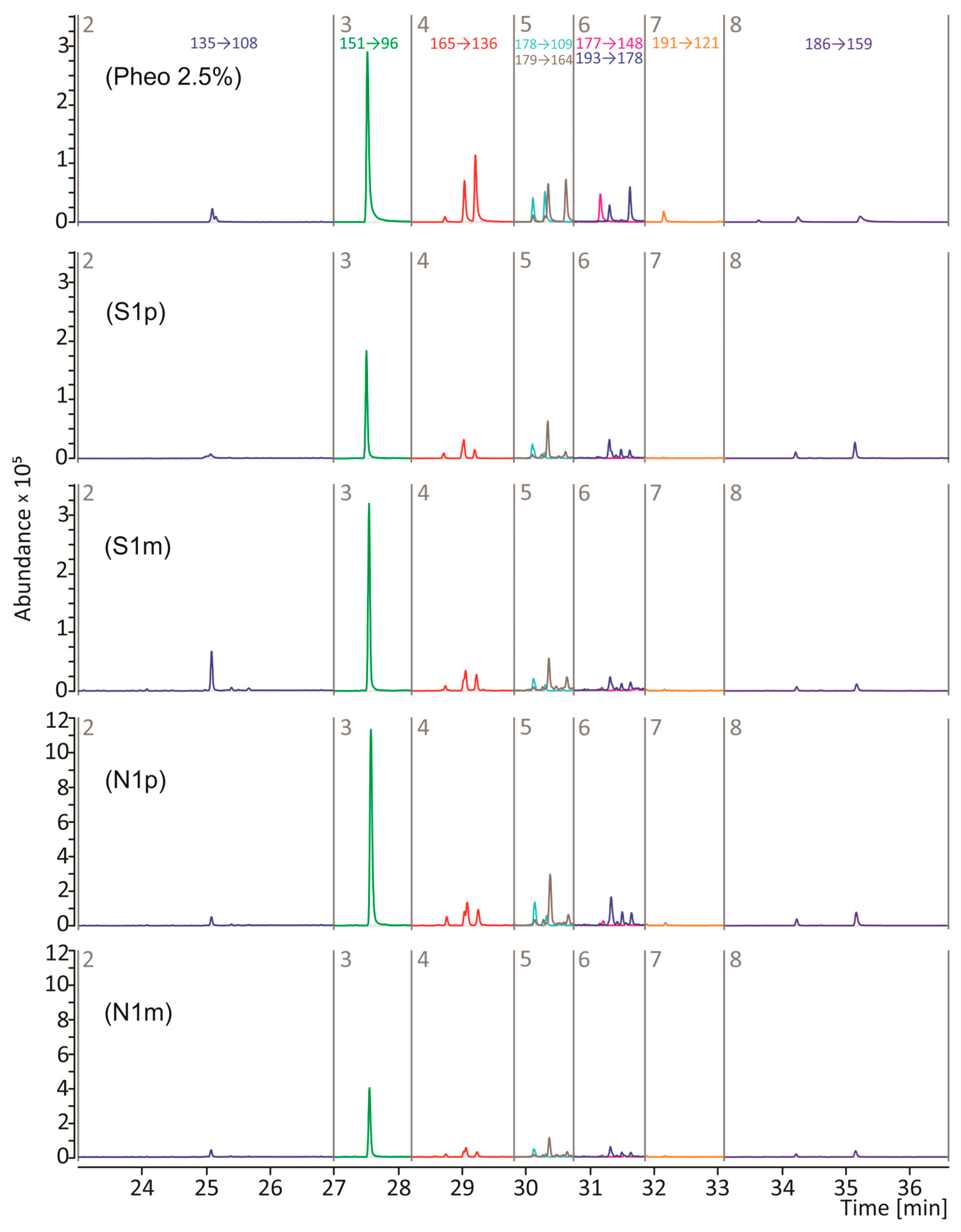
2.5. Py-GC/MS/MS Analysis
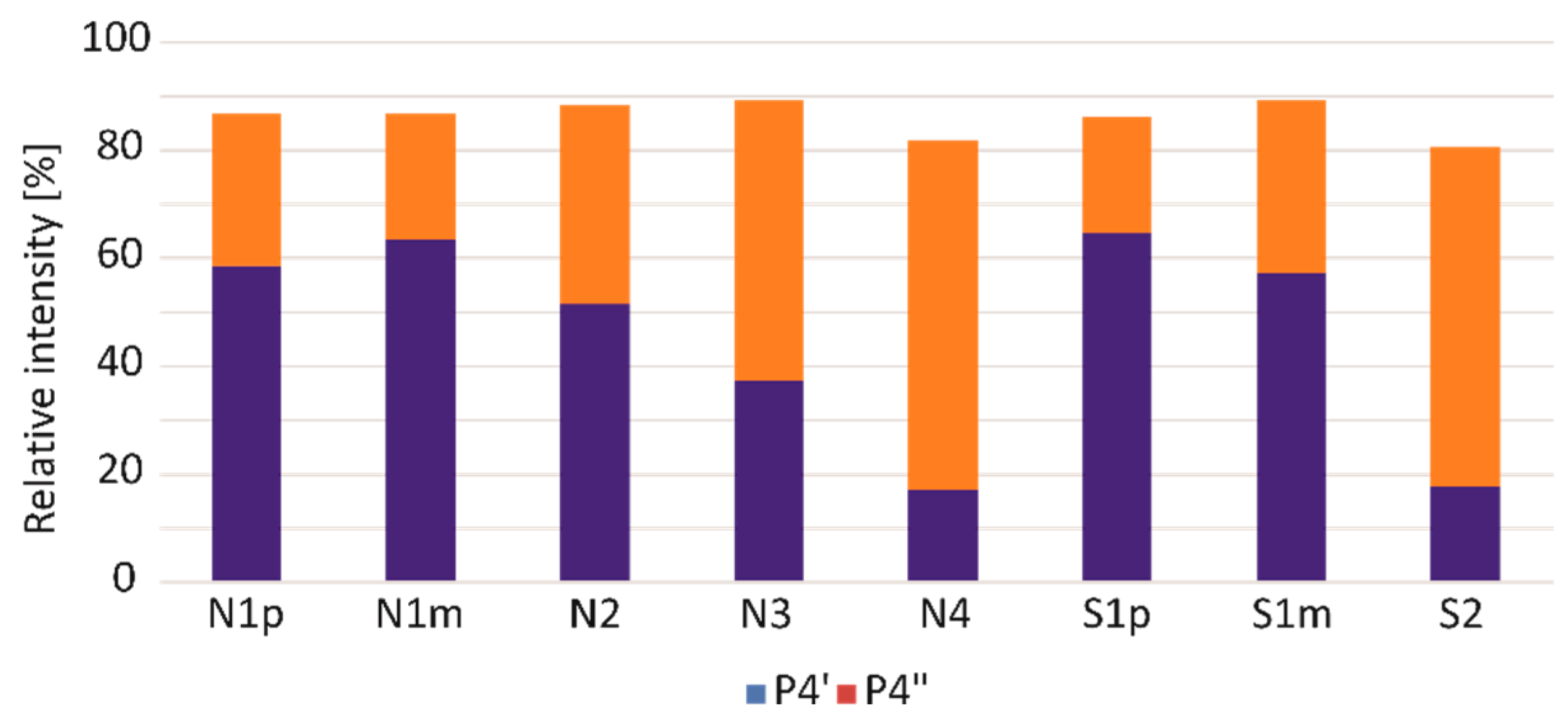
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef] [PubMed]
- Saud, A.; Sagineedu, S.; Ng, H.-S.; Stanslas, J.; Lim, J. Melanoma Metastasis: What Role Does Melanin Play? (Review). Oncol. Rep. 2022, 48, 217. [Google Scholar] [CrossRef] [PubMed]
- El Sharouni, M.-A.; Van Diest, P.J.; Witkamp, A.J.; Sigurdsson, V.; Van Gils, C.H. Subtyping Cutaneous Melanoma Matters. JNCI Cancer Spectr. 2020, 4, pkaa097. [Google Scholar] [CrossRef] [PubMed]
- Fortarezza, F.; Cazzato, G.; Ingravallo, G.; Dei Tos, A.P. The 2023 WHO Updates on Skin Tumors: Advances since the 2018 Edition. Pathologica 2024, 116, 193–206. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Szumera-Ciećkiewicz, A.; Nasierowska-Guttmejer, A. New Pathomorphological Classification of Melanomas. Nowotw. J. Oncol. 2019, 69, 103–107. [Google Scholar] [CrossRef]
- Rutkowski, P.; Wysocki, P.J.; Nasierowska-Guttmejer, A.; Jeziorski, A.; Wysocki, W.M.; Kalinka, E.; Świtaj, T.; Kozak, K.; Kamińska-Winciorek, G.; Czarnecka, A.M.; et al. Cutaneous Melanoma. Oncol. Clin. Pract. 2020, 16, 163–182. [Google Scholar] [CrossRef]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular Mechanisms Regulating Human Melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The Melanogenesis and Mechanisms of Skin-lightening Agents—Existing and New Approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Dzierżęga-Lęcznar, A.; Kurkiewicz, S.; Tam, I.; Marek, Ł.; Stępień, K. Pheomelanin Content of Cultured Human Melanocytes from Lightly and Darkly Pigmented Skin: A Pyrolysis-Gas Chromatography/Tandem Mass Spectrometry Study. J. Anal. Appl. Pyrolysis 2017, 124, 349–354. [Google Scholar] [CrossRef]
- Dall’Olmo, L.; Papa, N.; Surdo, N.C.; Marigo, I.; Mocellin, S. Alpha-Melanocyte Stimulating Hormone (α-MSH): Biology, Clinical Relevance and Implication in Melanoma. J. Transl. Med. 2023, 21, 562. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Boissy, R.E.; Hearing, V.J.; King, R.A.; Oetting, W.S.; Ortonne, J.P. The Pigmentary System: Physiology and Pathophysiology, 2nd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; ISBN 978-1-4051-2034-0. [Google Scholar]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current Challenges in Understanding Melanogenesis: Bridging Chemistry, Biological Control, Morphology, and Function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Nezirevic Dernroth, D.; Kågedal, B.; Hansson, C. Pheomelanin Markers in Melanoma with Reference to Their Excretion into Urine; Linköping University Electronic Press: Linköping, Sweden, 2009; ISBN 978-91-7393-566-1. [Google Scholar]
- Wakamatsu, K.; Fukushima, S.; Minagawa, A.; Omodaka, T.; Hida, T.; Hatta, N.; Takata, M.; Uhara, H.; Okuyama, R.; Ihn, H. Significance of 5-S-Cysteinyldopa as a Marker for Melanoma. Int. J. Mol. Sci. 2020, 21, 432. [Google Scholar] [CrossRef]
- Katoh, Y.; Hara, H.; Harada, T.; Hirai, S. Combination of Serum 5-S-Cysteinyldopa, Melanoma Inhibitory Activity and IL-8 Improves the Diagnostic Accuracy of Malignant Melanoma Compared with Individual Markers. Medicine 2022, 101, e30471. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Yokochi, M.; Naito, A.; Kageshita, T.; Ito, S. Comparison of Phaeomelanin and Its Precursor 5-S-Cysteinyldopa in the Serum of Melanoma Patients. Melanoma Res. 2003, 13, 357. [Google Scholar] [CrossRef]
- Umemura, H.; Yamasaki, O.; Kaji, T.; Otsuka, M.; Asagoe, K.; Takata, M.; Iwatsuki, K. Usefulness of Serum 5-S-Cysteinyl-Dopa as a Biomarker for Predicting Prognosis and Detecting Relapse in Patients with Advanced Stage Malignant Melanoma. J. Dermatol. 2017, 44, 449–454. [Google Scholar] [CrossRef]
- Salopek, T.G.; Yamada, K.; Ito, S.; Jimbow, K. Dysplastic Melanocytic Nevi Contain High Levels of Pheomelanin: Quantitative Comparison of Pheomelanin/Eumelanin Levels Between Normal Skin, Common Nevi, and Dysplastic Nevi. Pigment Cell Res. 1991, 4, 172–179. [Google Scholar] [CrossRef]
- Mitsui, H.; Kiecker, F.; Shemer, A.; Cannizzaro, M.V.; Wang, C.Q.F.; Gulati, N.; Ohmatsu, H.; Shah, K.R.; Gilleaudeau, P.; Sullivan-Whalen, M.; et al. Discrimination of Dysplastic Nevi from Common Melanocytic Nevi by Cellular and Molecular Criteria. J. Investig. Dermatol. 2016, 136, 2030–2040. [Google Scholar] [CrossRef]
- Wang, H.; Osseiran, S.; Roider, E.; Fisher, D.E.; Evans, C.L. New Imaging-Based Biomarkers for Melanoma Diagnosis Using Coherent Raman Scattering Microscopy (Conference Presentation). In Photonic Therapeutics and Diagnostics XII; SPIE: Bellingham, WA, USA, 2016; Volume 9689, p. 2-2. [Google Scholar]
- Pavel, S.; van Nieuwpoort, F.; van der Meulen, H.; Out, C.; Pizinger, K.; Cetkovská, P.; Smit, N.P.M.; Koerten, H.K. Disturbed Melanin Synthesis and Chronic Oxidative Stress in Dysplastic Naevi. Eur. J. Cancer 2004, 40, 1423–1430. [Google Scholar] [CrossRef]
- Mitra, D.; Luo, X.; Morgan, A.; Wang, J.; Hoang, M.P.; Lo, J.; Guerrero, C.R.; Lennerz, J.K.; Mihm, M.C.; Wargo, J.A.; et al. An Ultraviolet-Radiation-Independent Pathway to Melanoma Carcinogenesis in the Red Hair/Fair Skin Background. Nature 2012, 491, 449–453. [Google Scholar] [CrossRef]
- Morgan, A.M.; Lo, J.; Fisher, D.E. How Does Pheomelanin Synthesis Contribute to Melanomagenesis?: Two Distinct Mechanisms Could Explain the Carcinogenicity of Pheomelanin Synthesis. BioEssays 2013, 35, 672–676. [Google Scholar] [CrossRef]
- Nasti, T.H.; Timares, L. MC 1R, Eumelanin and Pheomelanin: Their Role in Determining the Susceptibility to Skin Cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, S.; Wakamatsu, K.; Galván, I. Increase of the Benzothiazole Moiety Content of Pheomelanin Pigment after Endogenous Free Radical Inducement. Dye. Pigment. 2020, 180, 108516. [Google Scholar] [CrossRef]
- Lembo, S.; Di Caprio, R.; Micillo, R.; Balato, A.; Monfrecola, G.; Panzella, L.; Napolitano, A. Light-independent Pro-inflammatory and Pro-oxidant Effects of Purified Human Hair Melanins on Keratinocyte Cell Cultures. Exp. Dermatol. 2017, 26, 592–594. [Google Scholar] [CrossRef]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative Stress Inhibits Distant Metastasis by Human Melanoma Cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Dzierzega-Lecznar, A.; Kurkiewicz, S.; Stepien, K.; Chodurek, E.; Wilczok, T.; Arzberger, T.; Riederer, P.; Gerlach, M. GC/MS Analysis of Thermally Degraded Neuromelanin from the Human Substantia Nigra. J. Am. Soc. Mass Spectrom. 2004, 15, 920–926. [Google Scholar] [CrossRef]
- Kurkiewicz, S.; Dzierżęga-Lęcznar, A.; Stanek-Widera, A.; Lange, D. Development of a Method for Isolation of Melanin from Archival FFPE Tissues of Human Melanoma for Structural Studies by Pyrolysis-Gas Chromatography-Tandem Mass Spectrometry. Adv. Hyg. Exp. Med. 2022, 76, 122–127. [Google Scholar] [CrossRef]
- Greco, G.; Wakamatsu, K.; Panzella, L.; Ito, S.; Napolitano, A.; D’Ischia, M. Isomeric Cysteinyldopas Provide a (Photo)Degradable Bulk Component and a Robust Structural Element in Red Human Hair Pheomelanin. Pigment Cell Melanoma Res. 2009, 22, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.R.; Roberts, J.C.; Wilkins, D.G.; Rollins, D.E. Relationship of Melanin Degradation Products to Actual Melanin Content: Application to Human Hair. Anal. Biochem. 2001, 290, 116–125. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
| Symbol * | Melanoma Subtype | Tumour Site | pTNM | Breslow Thickness (mm) | Clark Level | TILs | Vascular Invasion | Mitotic Rate (Number of Mitoses/mm²) | Lymph Node in Which Any Metastatic Tumour Cells Are Identified | Deparaffinised Sample Under Analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | nodular | auricle | pT4a | 18 | V | No | Yes | 5 | 13/27 | primary tumour (N1p) |
| lymph node metastases (N1m) | ||||||||||
| N2 | nodular | sole | pT4b | 5 | IV | Yes | No | 20 | 0 | primary tumour |
| N3 | nodular | face | pT4b | 7 | III | Yes | No | 32 | 0 | primary tumour |
| N4 | nodular | shoulder | pT3a | 4 | IV | Yes | No | 8 | 0 | primary tumour |
| S1 | superficial spreading | chest | pT4b | 15 | IV | Yes | No | 3 | 12/25 | primary tumour (S1p) |
| lymph node metastases (S1m) | ||||||||||
| S2 | superficial spreading | shank | pT3a | 3.5 | III | Yes | No | 17 | 0 | primary tumour |
| Time Segment | RT [min] | Compound (Symbol) | MRM Transition * (m/z → m/z) | Collision Energy [eV] |
|---|---|---|---|---|
| 1 | 9.7 | Thiazole (P1) | 85 → 58 | 15 |
| 2 | 25.1 | Benzothiazole (P2) | 135 → 108 | 18 |
| 3 | 27.5 | 4-Hydroxybenzothiazole (P3) | 151 → 96 | 23 |
| 4 | 28.7–29.3 | 2,3-Dihydro-5H-1,4-benzothiazin-5-one (P4) and its isomers (P4′ and P4″) | 165 → 136 | 22 |
| 5 | 30.1–30.3 | Methyl-2,3-dihydro-5H-1,4-benzothiazin -5-one (P5) and its isomer (P5′) | 178 → 109 | 20 |
| 5 | 30.3–30.6 | 4-Hydroxy-6-ethylbenzothiazole (P6) and its isomer (P6′) | 179 → 164 | 15 |
| 6 | 31.2 | 7-Methyl-5H-1,4-benzothiazin-5-one (P7) | 177 → 148 | 20 |
| 6 | 31.3–31.6 | 7-Ethyl-2,3-dihydro-5H-1,4-benzothiazin-5-one (P8) and its isomer (P8′) | 193 → 178 | 15 |
| 7 | 32.2 | 7-propyl-2H-1,4-benzothiazine (P9) | 191 → 121 | 26 |
| 8 | 33.6–35.2 | Thiazoloisoquinoline (P10) and its isomers (P10′, and P10″) | 186 → 159 | 19 |
| Symbol | Melanoma Subtype | Sample Under Analysis | Pheomelanin Content (%) |
|---|---|---|---|
| N1 | nodular | primary tumour (N1p) | 4.51 |
| lymph node metastases (N1m) | 1.53 | ||
| N2 | nodular | primary tumour | 1.50 |
| N3 | nodular | primary tumour | 0.46 |
| N4 | nodular | primary tumour | 1.07 |
| S1 | superficial spreading | primary tumour (S1p) | 0.60 |
| lymph node metastases (S1m) | 0.99 | ||
| S2 | superficial spreading | primary tumour | 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurkiewicz, S.; Marek, Ł.; Tam, I.; Stanek-Widera, A.; Lange, D.; Stojko, J. A Simple Method to Determine Pheomelanin Content and Structure in FFPE Human Melanoma Specimens. Processes 2025, 13, 1636. https://doi.org/10.3390/pr13061636
Kurkiewicz S, Marek Ł, Tam I, Stanek-Widera A, Lange D, Stojko J. A Simple Method to Determine Pheomelanin Content and Structure in FFPE Human Melanoma Specimens. Processes. 2025; 13(6):1636. https://doi.org/10.3390/pr13061636
Chicago/Turabian StyleKurkiewicz, Slawomir, Łukasz Marek, Irena Tam, Agata Stanek-Widera, Dariusz Lange, and Jerzy Stojko. 2025. "A Simple Method to Determine Pheomelanin Content and Structure in FFPE Human Melanoma Specimens" Processes 13, no. 6: 1636. https://doi.org/10.3390/pr13061636
APA StyleKurkiewicz, S., Marek, Ł., Tam, I., Stanek-Widera, A., Lange, D., & Stojko, J. (2025). A Simple Method to Determine Pheomelanin Content and Structure in FFPE Human Melanoma Specimens. Processes, 13(6), 1636. https://doi.org/10.3390/pr13061636







