Characterisation of Cork Volatile Organic Compounds Using TD-GC-MS: Effects of Origin, Washing Process, and Thermal Processing of Cork Stoppers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Thermal Desorption Extraction (TD)
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of the Thermally Desorbed Compounds
2.4. Statistical Data Analyses
3. Results
3.1. TD-GC-MS as a Methodology to Obtain the Volatile Aromatic Profile of Cork
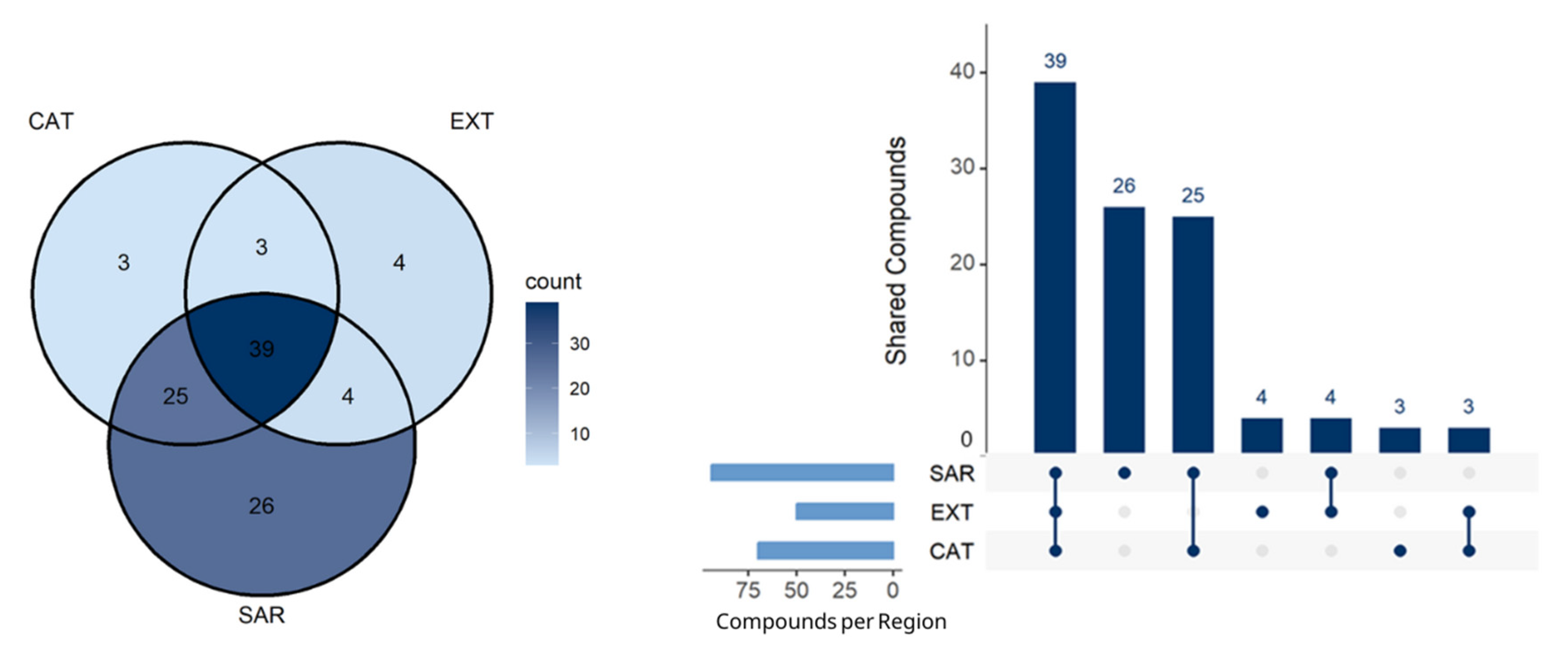
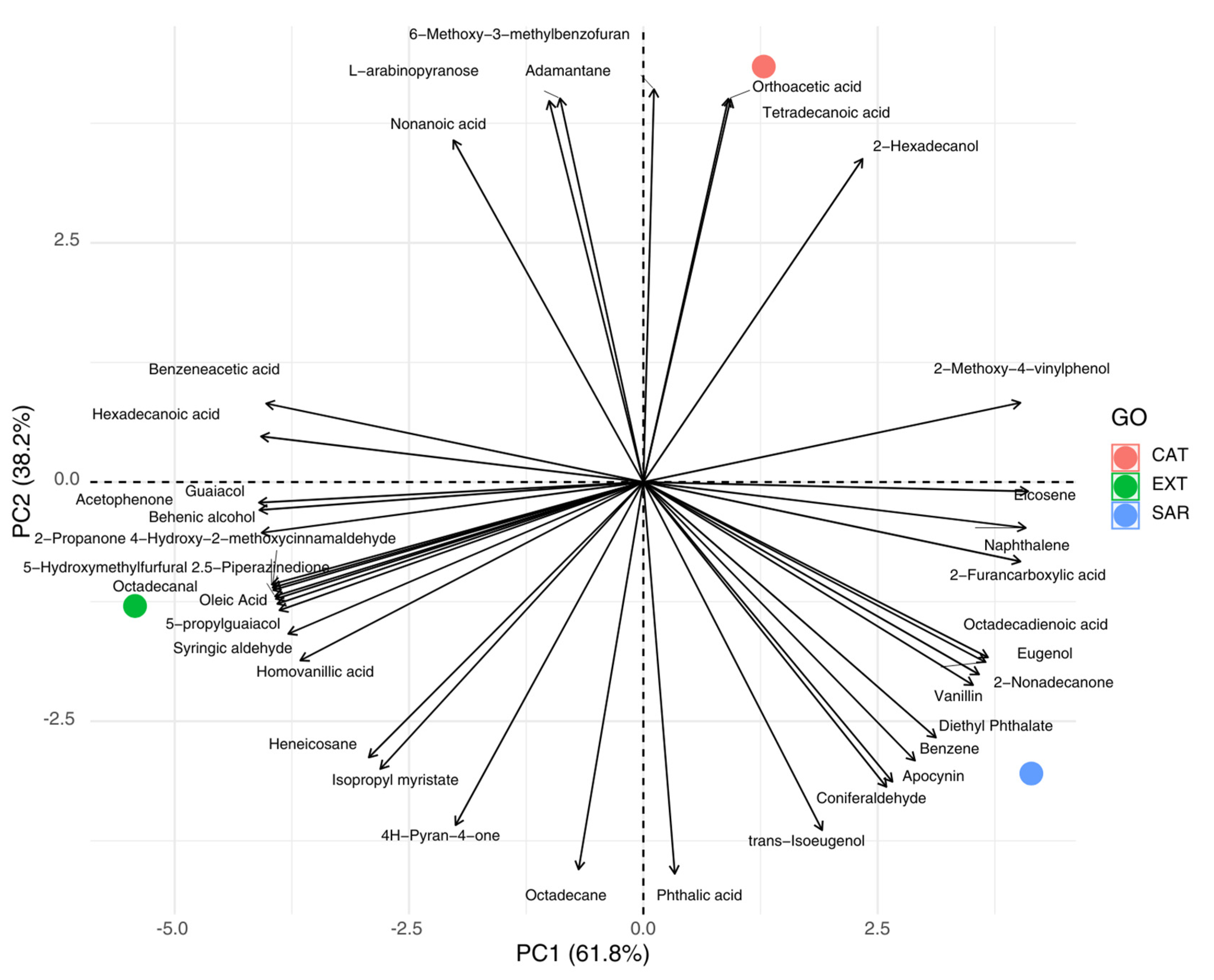
3.2. Volatile Aromatic Profile of Cork with Different Geographical Origins
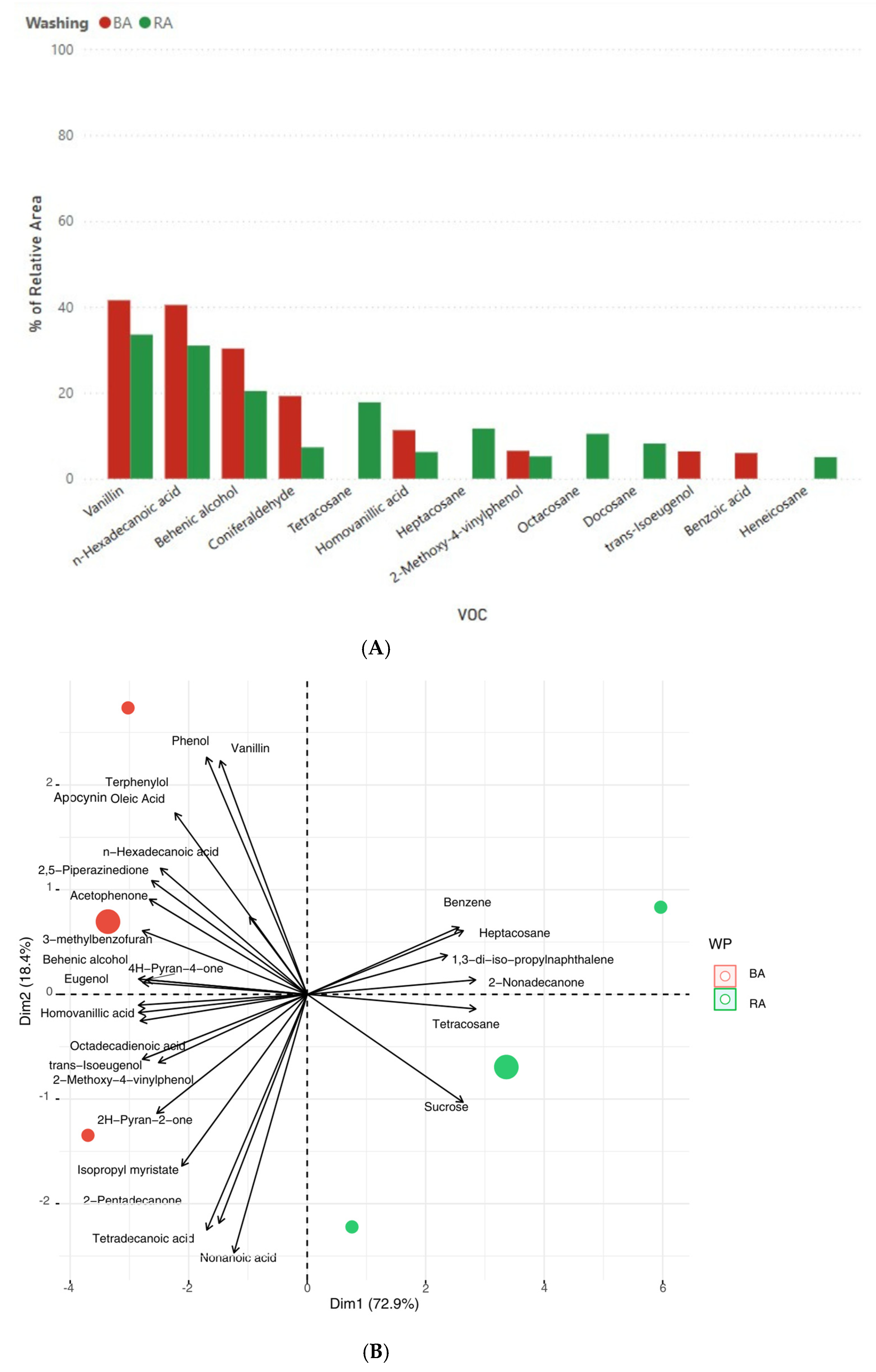
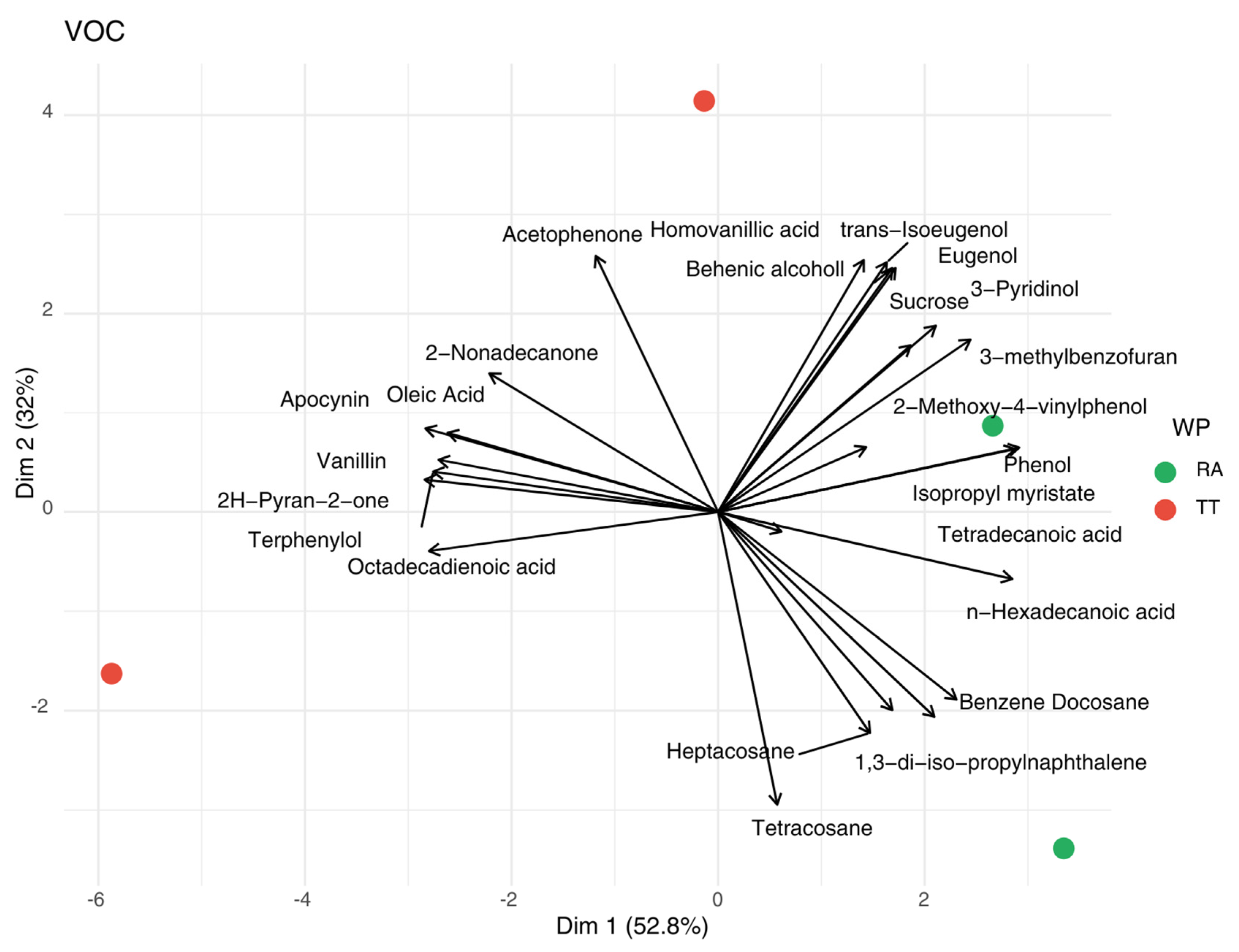
3.3. Volatile Aromatic Profile of Cork Stoppers During the Manufacturing Process
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TD-GC-MS | Thermal Desorption Coupled to Gas Chromatography-Mass Spectrometry |
| VOC | Volatile Organic Compound |
| PCA | Principal Component Analysis |
| CAT | Catalonia |
| EXT | Extremadura |
| SAR | Sardinia |
| BA | Immersion-Washed Cork Stoppers (from Baño or Bath) |
| RA | Spray-Washed Cork Stoppers (from Rociado or Aspersion) |
| TT | Thermally Treated Cork Stoppers |
| NIST | National Institute of Standards and Technology |
| CAGR | Compound Annual Growth Rate |
References
- Maga, J.A.; Puech, J.L. Cork and Alcoholic Beverages. Food Rev. Int. 2005, 21, 53–68. [Google Scholar] [CrossRef]
- Pereira, H. Chemical Composition and Variability of Cork from Quercus suber L. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Pinheiro, M.C.; Symochko, L.; Castro, L.M. Valorization of Cork Industry By-Products as Sustainable Natural Dyes for Textiles. ACS Sustain. Chem. Eng. 2023, 11, 10555–10565. [Google Scholar] [CrossRef]
- Pereira, H. The Chemical Composition of Cork. In Cork; Elsevier: Amsterdam, The Netherlands, 2007; pp. 55–99. [Google Scholar]
- Rocha, S.; Coimbra, M.; Delgadillo, I. Occurrence of Furfuraldehydes during the Processing of Quercus suber L. Cork: Simultaneous Determination of Furfural, 5-Hydroxymethylfurfural and 5-Methylfurfural and Their Relation with Cork Polysaccharides. Carbohydr. Polym. 2004, 56, 287–293. [Google Scholar] [CrossRef]
- Rocha, S.M.; Ganito, S.; Barros, A.; Carapuça, H.M.; Delgadillo, I. Study of Cork (from Quercus suber L.)–Wine Model Interactions Based on Voltammetric Multivariate Analysis. Anal. Chim. Acta 2005, 528, 147–156. [Google Scholar] [CrossRef]
- Sousa, A.F.; Pinto, P.C.; Silvestre, A.J.; Pascoal Neto, C. Triterpenic and Other Lipophilic Components from Industrial Cork Byproducts. J. Agric. Food Chem. 2006, 54, 6888–6893. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Lopes, P.; Cabral, M.; Guedes de Pinho, P. HS-SPME/GC-MS Methodologies for the Analysis of Volatile Compounds in Cork Material. Eur. Food Res. Technol. 2016, 242, 457–466. [Google Scholar] [CrossRef]
- Pinto, J.; Oliveira, A.S.; Lopes, P.; Roseira, I.; Cabral, M.; de Lourdes Bastos, M.; de Pinho, P.G. Characterization of Chemical Compounds Susceptible to Be Extracted from Cork by the Wine Using GC-MS and 1H NMR Metabolomic Approaches. Food Chem. 2019, 271, 639–649. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Furtado, I.; de Lourdes Bastos, M.; Guedes de Pinho, P.; Pinto, J. The Influence of Different Closures on Volatile Composition of a White Wine. Food Packag. Shelf Life 2020, 23, 100451. [Google Scholar] [CrossRef]
- Furtado, I.; Oliveira, A.S.; Amaro, F.; Lopes, P.; Cabral, M.; de Lourdes Bastos, M.; Pinto, J. Volatile Profile of Cork as a Tool for Classification of Natural Cork Stoppers. Talanta 2021, 223, 121698. [Google Scholar] [CrossRef]
- Fernandes, A.; Fernandes, I.; Cruz, L.; Mateus, N.; Cabral, M.; de Freitas, V. Antioxidant and Biological Properties of Bioactive Phenolic Compounds from Quercus suber L. J. Agric. Food Chem. 2009, 57, 11154–11160. [Google Scholar] [CrossRef]
- Mislata, A.M.; Puxeu, M.; Ferrer-Gallego, R. Aromatic Potential and Bioactivity of Cork Stoppers and Cork By-Products. Foods 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.; Pinto, C.; Cravo, S.; Rocha e Silva, J.; Afonso, C.; Sousa Lobo, J.M.; Tiritan, M.E.; Cidade, H.; Almeida, I.F. Quercus suber: A Promising Sustainable Raw Material for Cosmetic Application. Appl. Sci. 2022, 12, 4604. [Google Scholar] [CrossRef]
- Aroso, I.M.; Araujo, A.R.; Pires, R.A.; Reis, R.L. Cork: Current Technological Developments and Future Perspectives for This Natural, Renewable, and Sustainable Material. ACS Sustain. Chem. Eng. 2017, 5, 11130–11146. [Google Scholar] [CrossRef]
- Lavado, G.; Ladero, L.; Cava, R. Cork Oak (Quercus suber L.) Leaf Extracts Potential Use as Natural Antioxidants in Cooked Meat. Ind. Crops Prod. 2021, 160, 113086. [Google Scholar] [CrossRef]
- Touati, R.; Santos, S.A.; Rocha, S.M.; Belhamel, K.; Silvestre, A.J. The Potential of Cork from Quercus suber L. Grown in Algeria as a Source of Bioactive Lipophilic and Phenolic Compounds. Ind. Crops Prod. 2015, 76, 936–945. [Google Scholar] [CrossRef]
- Carriço, C.; Ribeiro, H.M.; Marto, J. Converting Cork By-Products to Ecofriendly Cork Bioactive Ingredients: Novel Pharmaceutical and Cosmetics Applications. Ind. Crops Prod. 2018, 125, 72–84. [Google Scholar] [CrossRef]
- Castola, V.; Marongiu, B.; Bighelli, A.; Floris, C.; Laï, A.; Casanova, J. Extractives of Cork (Quercus suber L.): Chemical Composition of Dichloromethane and Supercritical CO2 Extracts. Ind. Crops Prod. 2005, 21, 65–69. [Google Scholar] [CrossRef]
- Leffingwell & Associates. Flavour and Fragrance Industry Leaders. Leffingwell Report 2010. Available online: http://www.leffingwell.com/top_10.htm (accessed on 8 June 2012).
- Mishra, S.; Sachan, A.; Sachan, S.G. Production of Natural Value-Added Compounds: An Insight into the Eugenol Biotransformation Pathway. J. Ind. Microbiol. Biotechnol. 2013, 40, 545–550. [Google Scholar] [CrossRef]
- Mazzoleni, V.; Caldentey, P.; Careri, M.; Mangia, A.; Colagrande, O. Volatile Components of Cork Used for Production of Wine Stoppers. Am. J. Enol. Vitic. 1994, 45, 401–406. [Google Scholar] [CrossRef]
- Rocha, S.; Delgadillo, I.; Ferrer Correia, A.J. GC-MS Study of Volatiles of Normal and Microbiologically Attacked Cork from Quercus suber L. J. Agric. Food Chem. 1996, 44, 865–871. [Google Scholar] [CrossRef]
- Rocha, S.; Delgadillo, I.; Ferrer Correia, A.J.; Barros, A.; Wells, P. Application of an Electronic Aroma Sensing System to Cork Stopper Quality Control. J. Agric. Food Chem. 1998, 46, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Casado-Carmona, F.A.; Lasarte-Aragonés, G.; Lucena, R.; Cárdenas, S. Green Sample Preparation Techniques in Environmental Analysis. In Green Approaches for Chemical Analysis; Elsevier: Amsterdam, The Netherlands, 2023; pp. 241–276. [Google Scholar]
- Caldentey, P.; Fumi, M.D.; Mazzoleni, V.; Careri, M. Volatile Compounds Produced by Microorganisms Isolated from Cork. Flavour Fragr. J. 1998, 13, 185–188. [Google Scholar] [CrossRef]
- Freitas, D.S.; Rocha, D.; Castro, T.G.; Noro, J.; Castro, V.I.; Teixeira, M.A.; Silva, C. Green Extraction of Cork Bioactive Compounds Using Natural Deep Eutectic Mixtures. ACS Sustain. Chem. Eng. 2022, 10, 7974–7989. [Google Scholar] [CrossRef]
- Páscoa, R.N.; Pinto, C.; Rego, L.; Tiritan, M.E.; Cidade, H.; Almeida, I.F. Application of NIR Spectroscopy for the Valorisation of Cork By-Products: A Feasibility Study over the Screening and Discrimination of Chemical Compounds of Interest. Pharmaceuticals 2024, 17, 180. [Google Scholar] [CrossRef]
- Jové, P.; Vives-Mestres, M.; Nadal, R.D.; Verdum, M. Development, Optimization and Validation of a Sustainable and Quantifiable Methodology for the Determination of 2,4,6-Trichloroanisole, 2,3,4,6-Tetrachloroanisole, 2,4,6-Tribromoanisole, Pentachloroanisole, 2-Methylisoborneole and Geosmin in Air. Processes 2021, 9, 1571. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Nassar, M.I.; Mohamed, T.A.; Hegazy, M.E.F. Chemical and Biological Profile of Cespitularia Species: A Mini Review. J. Adv. Res. 2016, 7, 209–224. [Google Scholar] [CrossRef]
- Coquet, C.; Ferré, E.; Peyronel, D.; Dal Farra, C.; Farnet, A.M. Identification of New Molecules Extracted from Quercus suber L. Cork. C. R. Biol. 2008, 331, 853–858. [Google Scholar] [CrossRef]
- Azevedo, J.; Fernandes, I.; Lopes, P.; Roseira, I.; Cabral, M.; Mateus, N.; Freitas, V. Migration of Phenolic Compounds from Different Cork Stoppers to Wine Model Solutions: Antioxidant and Biological Relevance. Eur. Food Res. Technol. 2014, 239, 951–960. [Google Scholar] [CrossRef]
- Amaro, F.; Almeida, J.; Oliveira, A.S.; Furtado, I.; de Lourdes Bastos, M.; Guedes de Pinho, P.; Pinto, J. Impact of Cork Closures on the Volatile Profile of Sparkling Wines during Bottle Aging. Foods 2022, 11, 293. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Delgadillo, I.; Rocha, S.M.; Costa, A.M.; Legin, A. Quality Evaluation of Cork from Quercus suber L. by the Electronic Tongue. Anal. Chim. Acta 2006, 563, 315–318. [Google Scholar] [CrossRef]
- Lopes, M.; Pascoal Nero, C.; Evtuguin, D.; Silvestre, A.J.D.; Gil, A.; Cordeiro, N.; Gandini, A. Products of the Permanganate Oxidation of Cork, Desuberized Cork, Suberin and Lignin from Quercus suber L. Holzforschung 1998, 52, 146–148. [Google Scholar]
- Conde, E.; Garcia-Vallejo, M.C.; Cadahía, E. Waxes Composition of Reproduction Cork from Quercus suber and Its Variability throughout the Industrial Processing. Wood Sci. Technol. 1999, 33, 229–244. [Google Scholar] [CrossRef]
- Rabhi, F.; Narváez-Rivas, M.; Boukhchina, S.; León-Camacho, M. Authentication of Quercus Species According to Their n-Alkanes Profile by Off-Line Combination of High-Performance Liquid Chromatography and Gas Chromatography. Food Anal. Methods 2015, 8, 1710–1717. [Google Scholar] [CrossRef]
- Mazzoleni, V.; Caldentey, P.; Silva, A. Phenolic Compounds in Cork Used for Production of Wine Stoppers as Affected by Storage and Boiling of Cork Slabs. Am. J. Enol. Vitic. 1998, 49, 6–10. [Google Scholar] [CrossRef]
- Marques, A.V.; Pereira, H.; Meier, D.; Faix, O. Isolation and Characterization of a Guaiacyl Lignin from Saponified Cork of Quercus suber L. Holzforschung 1996, 50, 393–400. [Google Scholar] [CrossRef]
- Varea, S.; García-Vallejo, M.C.; Cadahía, E.; de Simón, F.B. Polyphenols Susceptible to Migrate from Cork Stoppers to Wine. Eur. Food Res. Technol. 2001, 213, 56–61. [Google Scholar]
- Boudaoud, N.; Eveleigh, L. A New Approach to the Characterization of Volatile Signatures of Cork Wine Stoppers. J. Agric. Food Chem. 2003, 51, 1530–1533. [Google Scholar] [CrossRef]
- Akim, L.G.; Cordeiro, N.; Pascoal Neto, C.; Gandini, A. Comparative Analysis of the Lignins of Cork from Quercus suber L. and Wood from Eucalyptus globulus L. by Dry Hydrogen Iodide Cleavage. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1999; Volume 742, pp. 291–302. [Google Scholar]
- Careri, M.; Mazzoleni, V.; Musci, M.; Molteni, R. Effects of Electron Beam Irradiation on Cork Volatile Compounds by Gas Chromatography-Mass Spectrometry. Chromatographia 1999, 49, 166–172. [Google Scholar] [CrossRef]
- Barreto, M.C.; Vilas Boas, L.; Carneiro, L.C.; San Romão, M.V. Volatile Compounds in Samples of Cork and Also Produced by Selected Fungi. J. Agric. Food Chem. 2011, 59, 6568–6574. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Villaverde, J.J.; Sousa, A.F.; Coelho, J.F.J.; Neto, C.P.; Silvestre, A.J.D. Phenolic Composition and Antioxidant Activity of Industrial Cork By-Products. Ind. Crops Prod. 2013, 47, 262–269. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Y.; Shi, J.; Mohamed, S.R.; Xu, J.; Liu, X. The Antioxidant Guaiacol Exerts Fungicidal Activity against Fungal Growth and Deoxynivalenol Production in Fusarium graminearum. Front. Microbiol. 2021, 12, 762844. [Google Scholar] [CrossRef] [PubMed]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A Comprehensive Review on Biological Activities of p-Hydroxy Benzoic Acid and Its Derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Ahmadpourmir, H.; Attar, H.; Asili, J.; Soheili, V.; Taghizadeh, S.F.; Shakeri, A. Natural-Derived Acetophenones: Chemistry and Pharmacological Activities. Nat. Prod. Bioprosp. 2024, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Jové Martín, P.; Nadal, R.D.; Verdum Virgos, M.; Fiol Santaló, N. Study of Cork from Quercus suber L. with and without Yellow Spot: Aromatic Fraction and Cellular Structure. J. Eng. Res. 2024, 4, 24. [Google Scholar]
- Cacho, J.I.; Nicolás, J.; Viñas, P.; Campillo, N.; Hernández-Córdoba, M. Direct Sample Introduction–Gas Chromatography-Mass Spectrometry for the Determination of Haloanisole Compounds in Cork Stoppers. J. Chromatogr. A 2016, 1475, 74–79. [Google Scholar] [CrossRef]



| Geographical Origin | Coordinates (Decimal Degrees) | Type of Sample | Manufacturing Processes Implemented | Code |
|---|---|---|---|---|
| Catalonia | 41.883° N, 2.975° E | Raw cork | None | CAT |
| Extremadura | 38.1127° N, −6.49561° W | Raw cork | None | EXT |
| Sardinia | 39.169° N, 8.654° E | Raw cork | None | SAR |
| Cork stoppers | Washing by immersion | BA | ||
| Washing by a spray without thermal treatment | RA | |||
| Washing by a spray with thermal treatment | TT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jové, P.; de Nadal, R.; Verdum, M.; Fiol, N. Characterisation of Cork Volatile Organic Compounds Using TD-GC-MS: Effects of Origin, Washing Process, and Thermal Processing of Cork Stoppers. Processes 2025, 13, 1505. https://doi.org/10.3390/pr13051505
Jové P, de Nadal R, Verdum M, Fiol N. Characterisation of Cork Volatile Organic Compounds Using TD-GC-MS: Effects of Origin, Washing Process, and Thermal Processing of Cork Stoppers. Processes. 2025; 13(5):1505. https://doi.org/10.3390/pr13051505
Chicago/Turabian StyleJové, Patricia, Raquel de Nadal, Maria Verdum, and Núria Fiol. 2025. "Characterisation of Cork Volatile Organic Compounds Using TD-GC-MS: Effects of Origin, Washing Process, and Thermal Processing of Cork Stoppers" Processes 13, no. 5: 1505. https://doi.org/10.3390/pr13051505
APA StyleJové, P., de Nadal, R., Verdum, M., & Fiol, N. (2025). Characterisation of Cork Volatile Organic Compounds Using TD-GC-MS: Effects of Origin, Washing Process, and Thermal Processing of Cork Stoppers. Processes, 13(5), 1505. https://doi.org/10.3390/pr13051505







