Electrocoagulation with Fe-SS Electrodes as a Fourth Stage of Tequila Vinasses Treatment for COD and Color Removal
Abstract
1. Introduction
1.1. Tequila
1.2. Tequila Vinasse Characterization
1.3. Environmental Normative Update and Tequila Industry Challenges
1.4. Tequila Vinasses First-Stage Treatment
1.5. Tequila Vinasses Second-Stage Treatment
1.6. Tequila Vinasses Third-Stage Treatment
1.7. Tequila Vinasses Fourth-Stage Treatment
1.8. Electrocoagulation as Fourth-Stage Treatment
1.9. Objective
2. Materials and Methods
2.1. Equipment
2.2. Pretreated Tequila Vinasses
2.3. Methods
2.3.1. Analytical Methods
2.3.2. Calculations
2.4. Experimental Design
3. Results
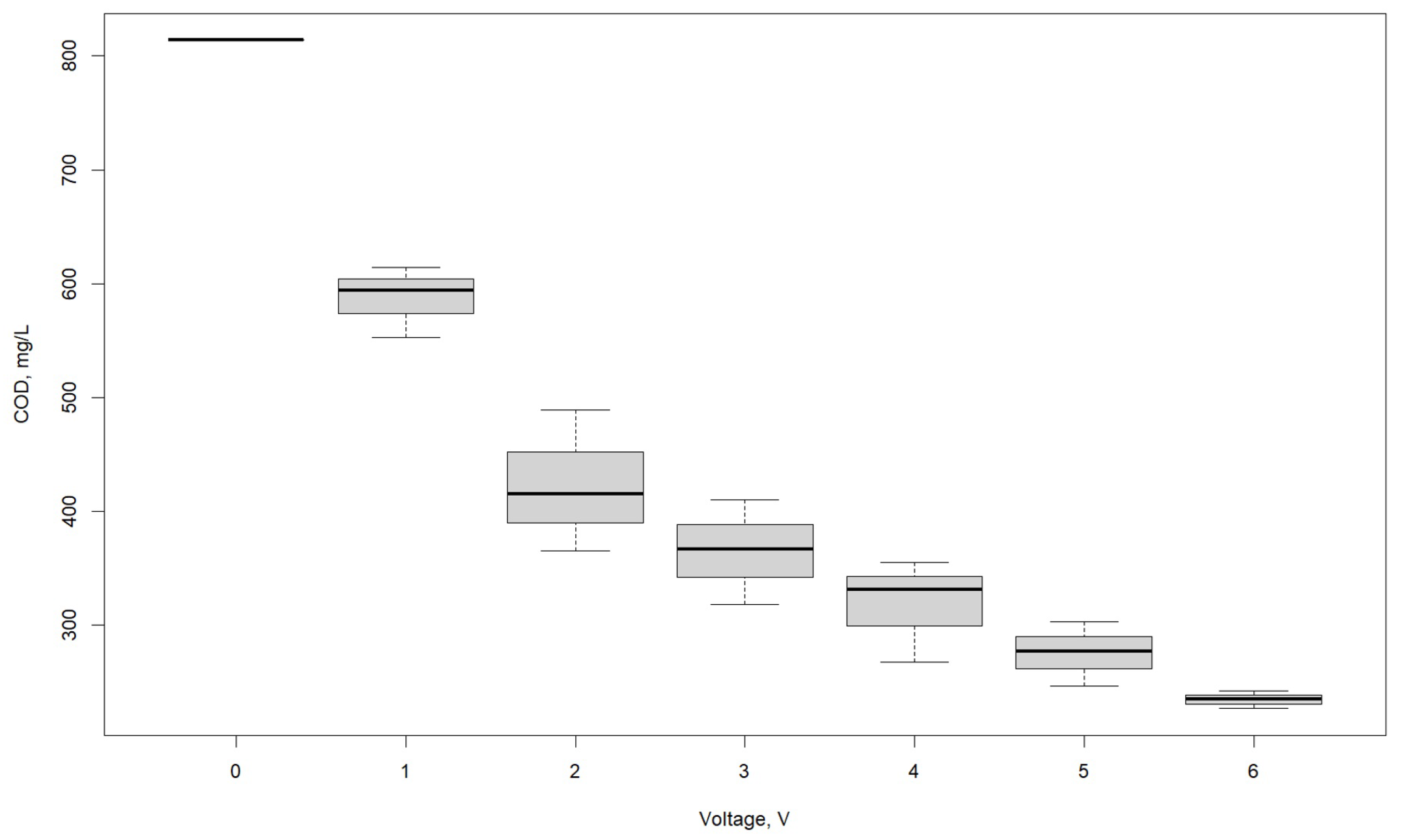
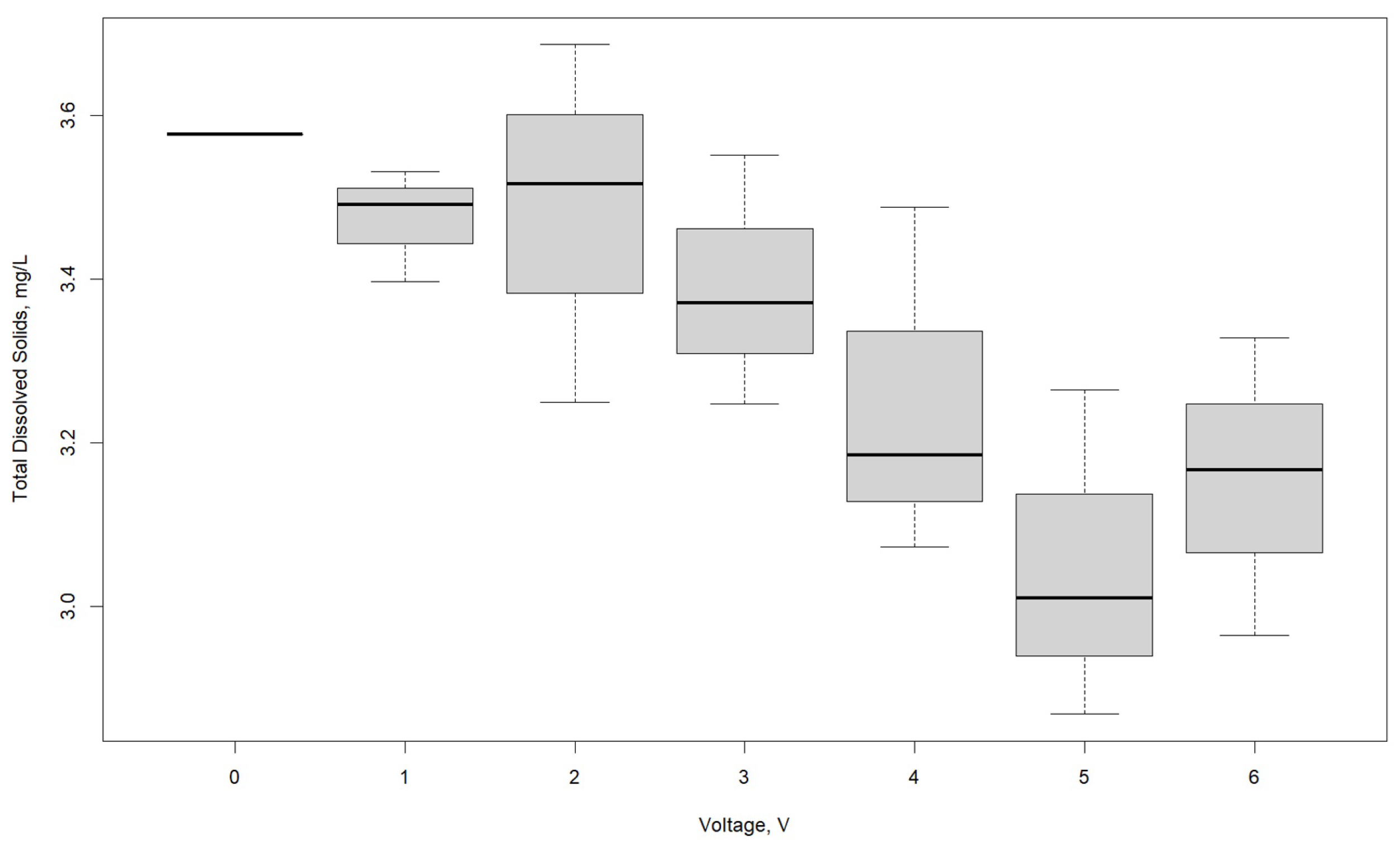
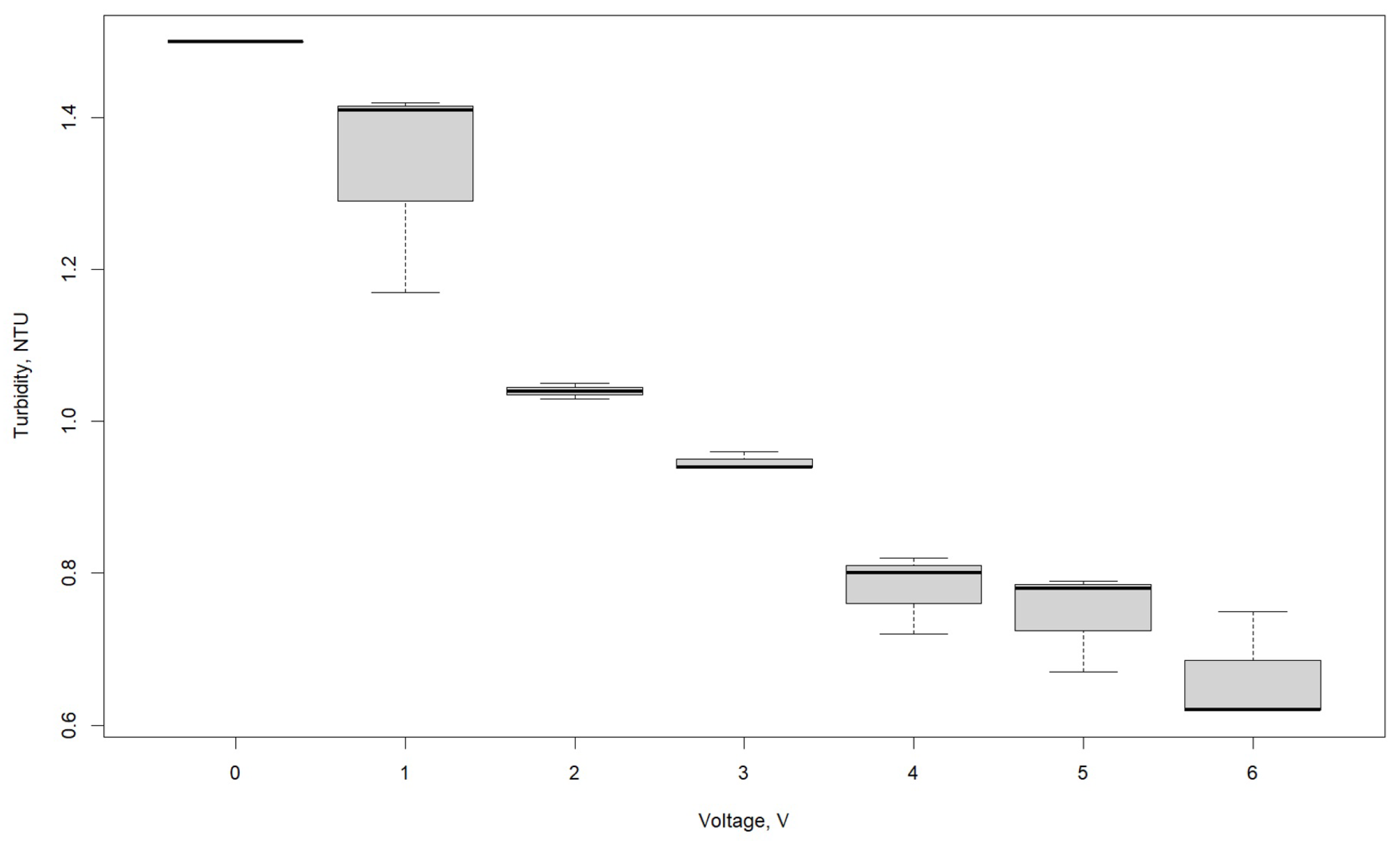
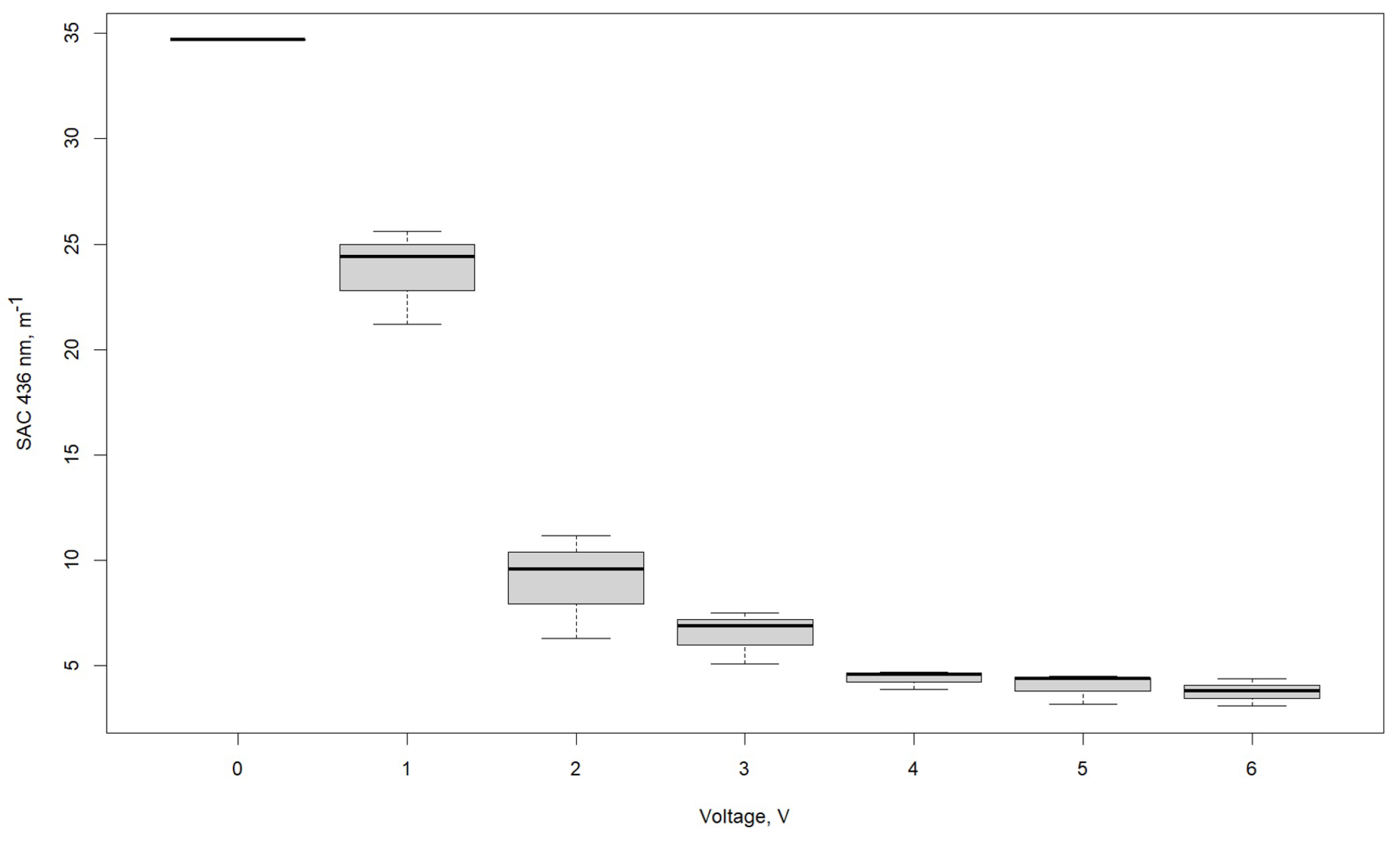
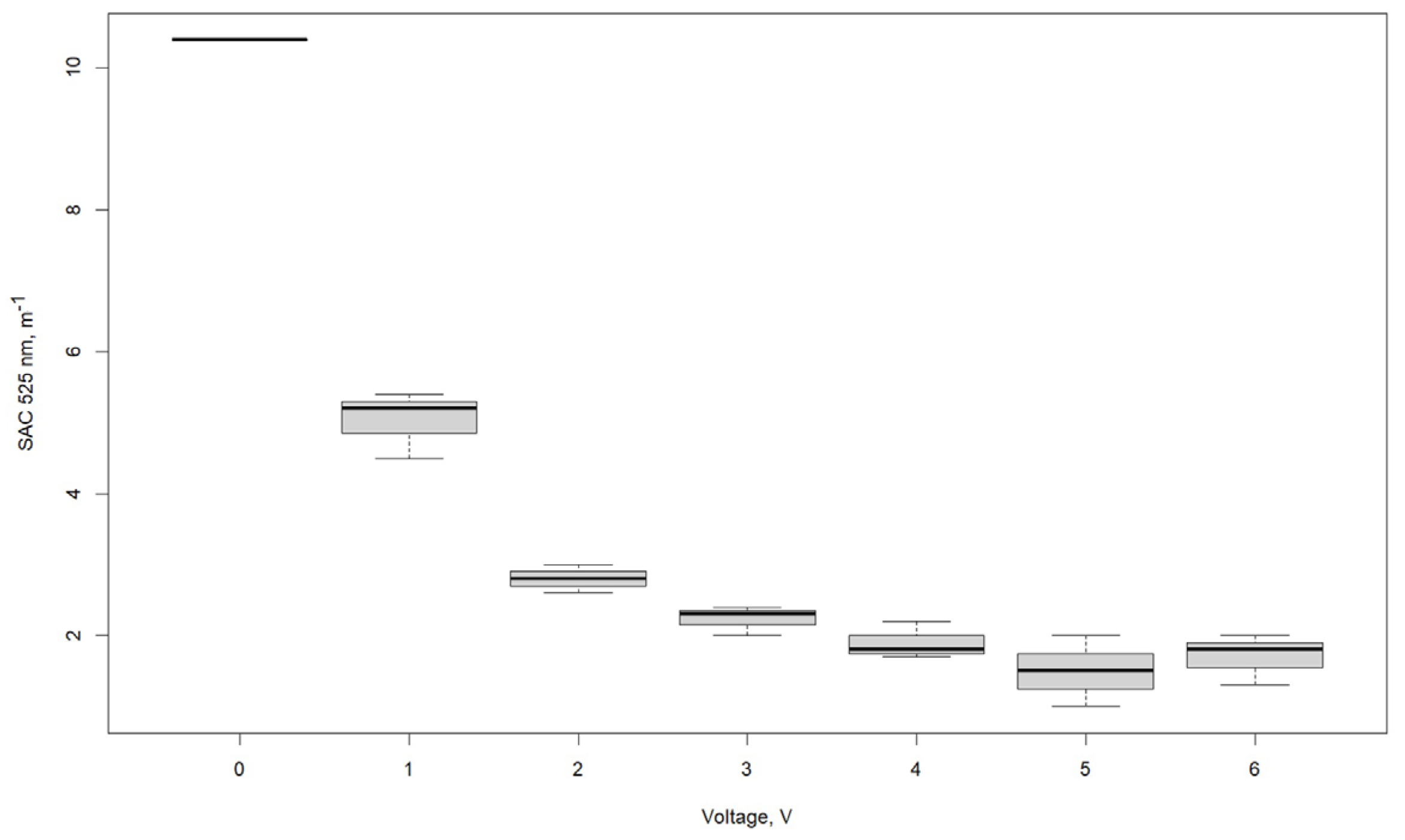
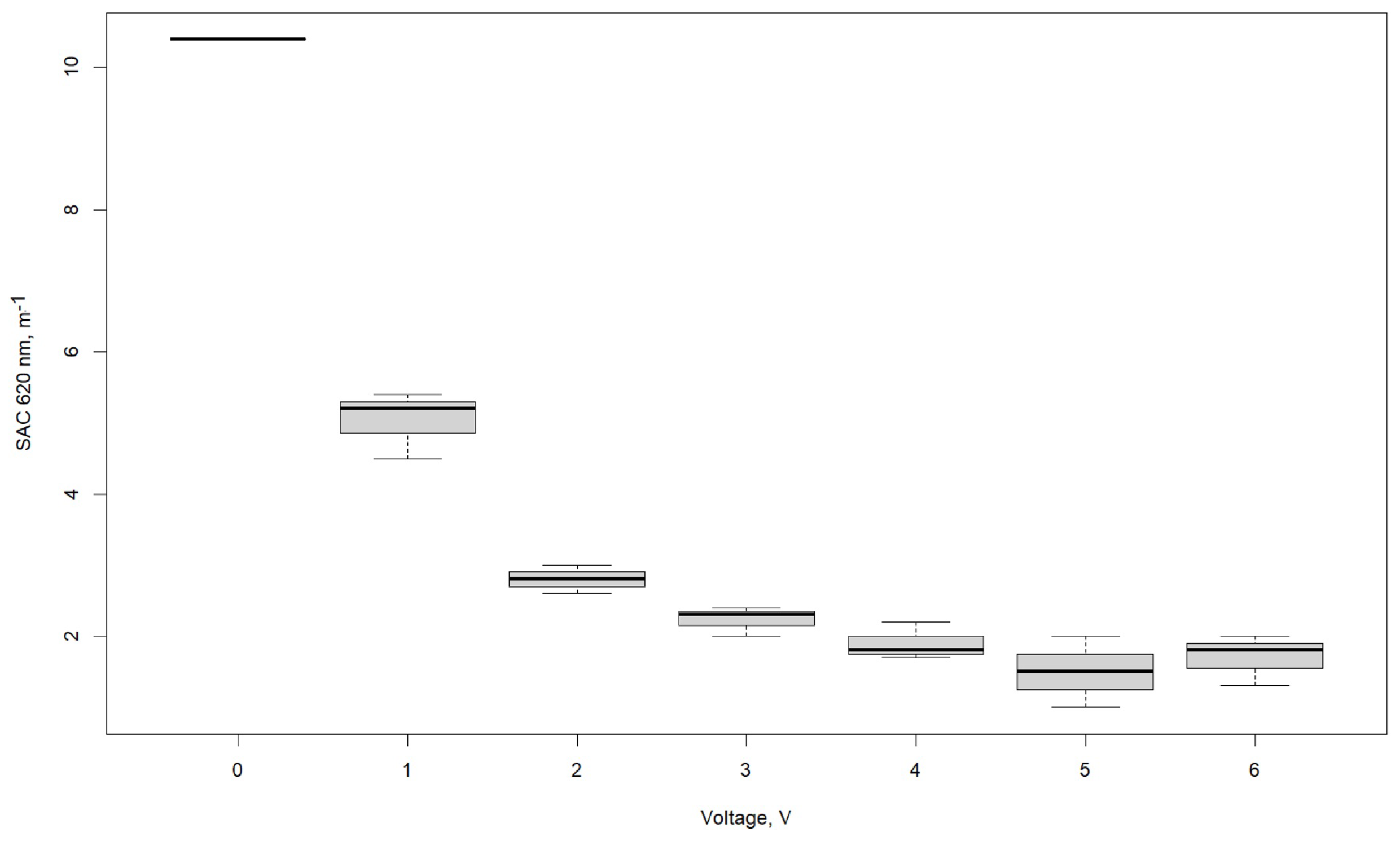
3.1. Effect of Voltage
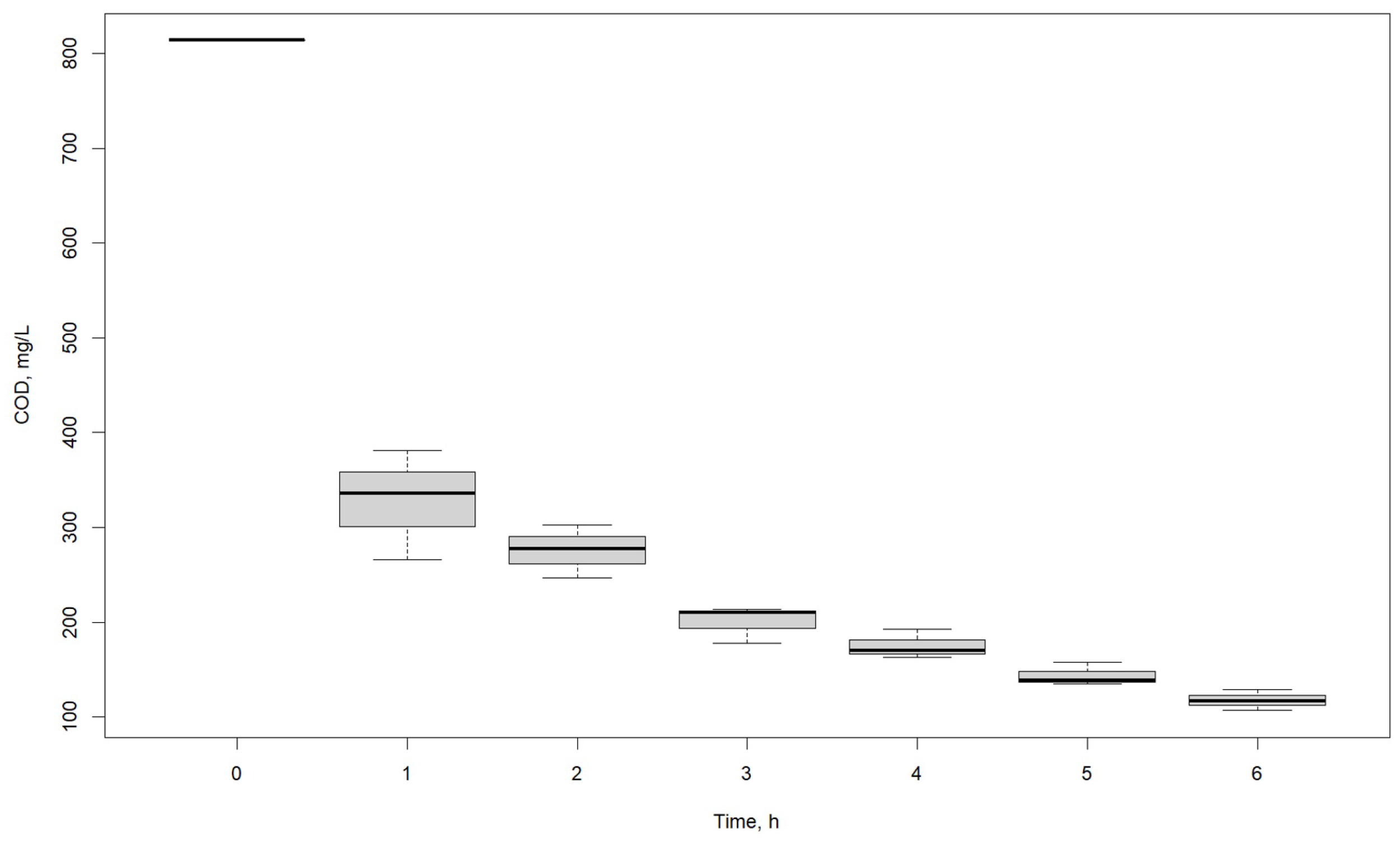
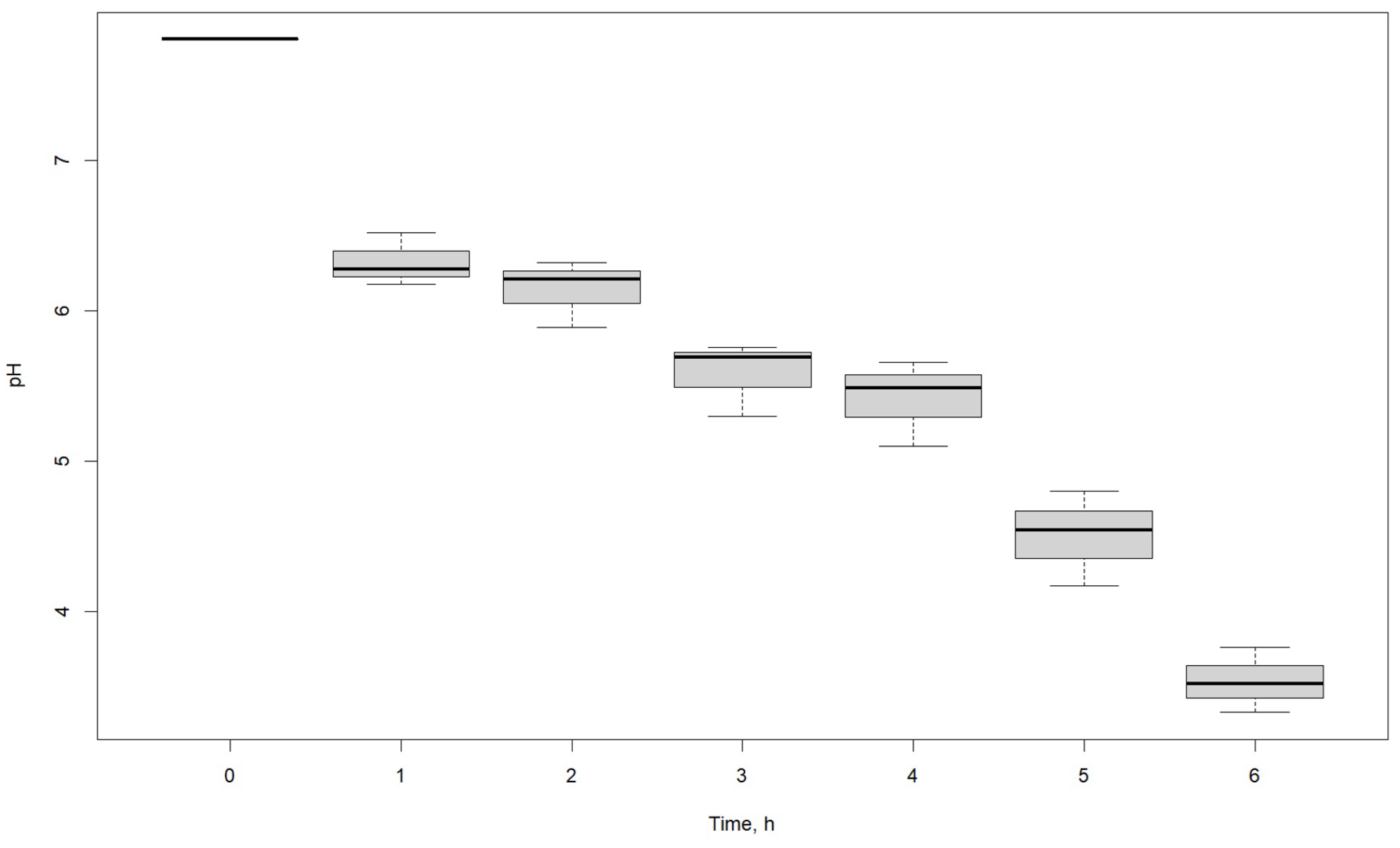
3.2. Effect of Time
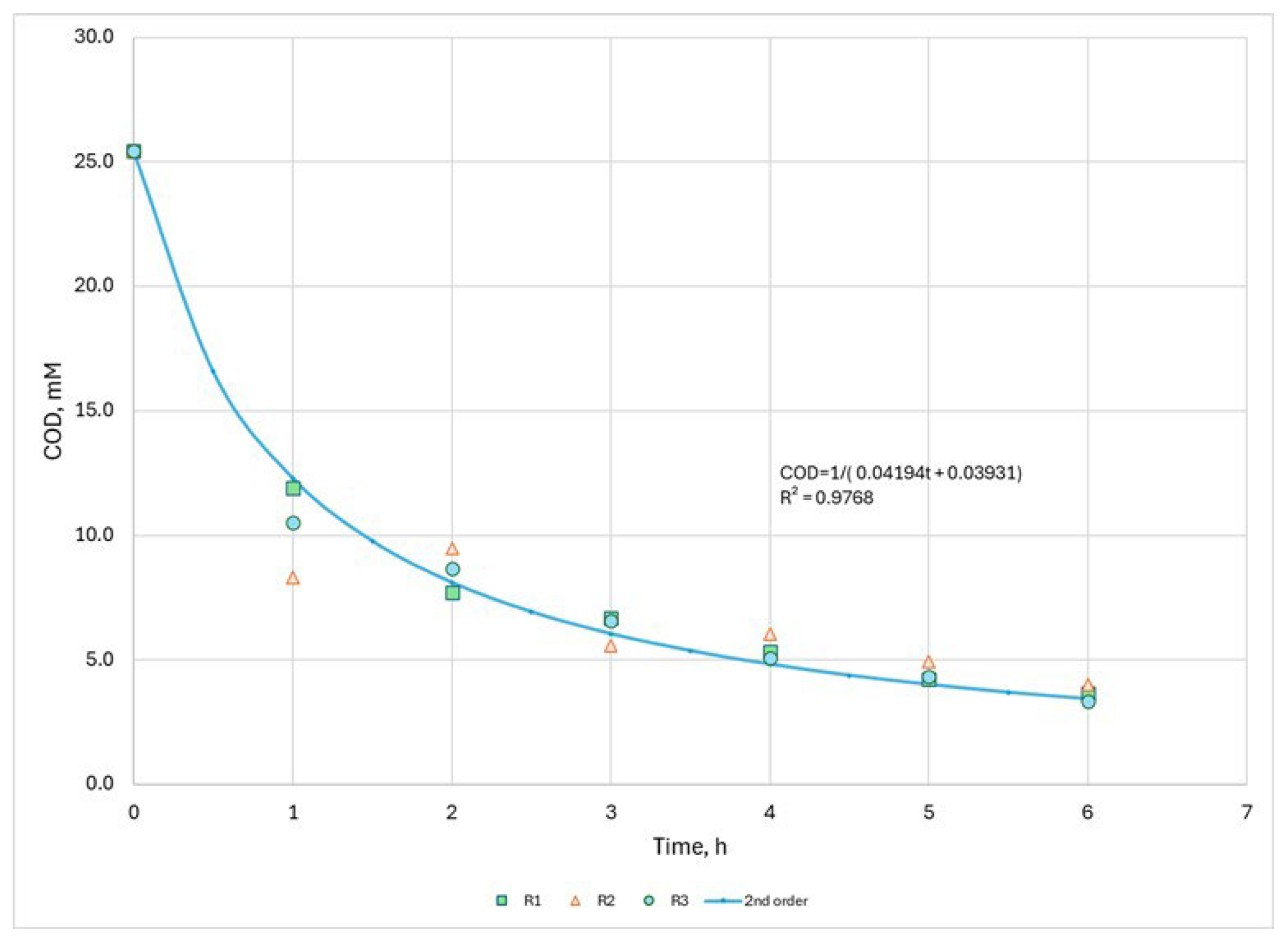
3.3. Kinetic Model
3.4. Economic Analysis
3.5. Sludge Generation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COD | Chemical Oxygen Demand |
| EC | Electrical Conductivity |
| SAC | Spectral Absorption Coefficient |
| TDS | Total Dissolved Solids |
| TSS | Total Suspended Solids |
References
- González-Pacheco, B.E.; Rojas-Martínez, R.I.; Ochoa-Martínez, D.L.; Silva-Rosales, L. First Report of a 16Sr IV Group Phytoplasma Associated with the Lethal Yellowing in Agave Tequilana. J. Plant Pathol. 2014, 96, 603. [Google Scholar] [CrossRef]
- Statista Tequila Sales Value in Mexico. Available online: https://www.statista.com/statistics/1097582/sales-value-tequila-mexico/ (accessed on 5 January 2025).
- Statista Tequila Sales Share by Type Mexico. 2023. Available online: https://www.statista.com/statistics/1097706/sales-share-tequila-type-mexico/ (accessed on 5 January 2025).
- Businesswire COVID-19 Recovery Analysis: Tequila Market in US. Increasing Number of Mergers and Acquisitions to Boost the Market Growth. Available online: https://www.businesswire.com/news/home/20200925005184/en/COVID-19-Recovery-Analysis-Tequila-Market-in-US-Increasing-Number-of-Mergers-and-Acquisitions-to-Boost-the-Market-Growth-Technavio (accessed on 5 January 2025).
- CRT. Available online: https://old.crt.org.mx/EstadisticasCRTweb/ (accessed on 6 January 2025).
- Martinez-Orozco, E.; Gortares-Moroyoqui, P.; Santiago-Olivares, N.; Napoles-Armenta, J.; Ulloa-Mercado, R.G.; De La Mora-Orozco, C.; Leyva-Soto, L.A.; Alvarez-Valencia, L.H.; Meza-Escalante, E.R. Tequila Still Distillation Fractioned Residual Streams for Use in Biorefinery. Energies 2020, 13, 6222. [Google Scholar] [CrossRef]
- López-López, A.; Davila-Vazquez, G.; León-Becerril, E.; Villegas-García, E.; Gallardo-Valdez, J. Tequila Vinasses: Generation and Full Scale Treatment Processes. Rev. Environ. Sci. Biotechnol. 2010, 9, 109–116. [Google Scholar] [CrossRef]
- SEMARNAT. Official Mexican Norm NOM-001-SEMARNAT-2021 Which Establishes the Permissible Limits of Contaminants in Wastewater Discharges into Receiving Bodies Owned by the Nation. Ministry of Environment and Natural Resources SEMARNAT, Mexico City, Mexico. 2022. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5645374&fecha=11/03/2022#gsc.tab=0 (accessed on 5 January 2025).
- López-López, A.; Contreras-Ramos, S.M. Tratamiento de Efluentes y Aprovechamiento de Residuos. In Ciencia y Tecnología del Tequila: Avances y Perspectivas; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. (CIATEJ): Guadalajara, Mexico, 2015; pp. 259–284. ISBN 9786079661984. [Google Scholar]
- Zurita, F.; Tejeda, A.; Montoya, A.; Carrillo, I.; Sulbarán-Rangel, B.; Carreón-álvarez, A. Generation of Tequila Vinasses, Characterization, Current Disposal Practices and Study Cases of Disposal Methods. Water 2022, 14, 1395. [Google Scholar] [CrossRef]
- Lorenzo-Santiago, M.A.; García-Hernández, E.; Rendón-Villalobos, R.; Rodriguez-Campos, J.; Tuesta-Popolizio, D.A.; Contreras-Ramos, S.M. Biodegradable Films Added with Conjugates from Residual Agave Vinasses: Chemical and Mechanical Characterization. J. Polym. Environ. 2024, 33, 497–511. [Google Scholar] [CrossRef]
- Gutiérrez Mora, A.; Rodríguez Garay, B.; Estarrón Espinosa, M.; Gschaedler Mathis, A.C.; Kirchmayr, M.R.; Moreno Terrazas, R.; Lappe, P.; Camacho Ruiz, R.M.; Ortiz Basurto, R.I.; Aguilar Uscanga, M.G.; et al. Integral and Sustainable Use of Agave, 1st ed.; Consejo Nacional de Ciencia y Tecnología CONACYT, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C. CIATEJ, Red temática mexicana aprovechamiento integral sustentable y biotecnología de los agaves Agared, Ed.; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C. CIATEJ: Zapopan, Mexico, 2019; ISBN 978-607-8734-03-0. [Google Scholar]
- Sánchez-Ureña, S.G.; Bolaños-Rosales, R.E.; Aguilar-Juárez, O.; Rosales-Colunga, L.M.; Contreras-Ramos, S.M.; Marino-Marmolejo, E.N. Tequila Production Process Influences on Vinasses Characteristics. A Comparative Study Between Traditional Process and Non-Cooked Agave Process. Waste Biomass Valorization 2022, 13, 3183–3195. [Google Scholar] [CrossRef]
- Espinoza-Escalante, F.M.; Pelayo-Ortíz, C.; Navarro-Corona, J.; González-García, Y.; Bories, A.; Gutiérrez-Pulido, H. Anaerobic Digestion of the Vinasses from the Fermentation of Agave Tequilana Weber to Tequila: The Effect of PH, Temperature and Hydraulic Retention Time on the Production of Hydrogen and Methane. Biomass Bioenergy 2009, 33, 14–20. [Google Scholar] [CrossRef]
- Yang, L.; Lu, M.; Carl, S.; Mayer, J.A.; Cushman, J.C.; Tian, E.; Lin, H. Biomass Characterization of Agave and Opuntia as Potential Biofuel Feedstocks. Biomass Bioenergy 2015, 76, 43–53. [Google Scholar] [CrossRef]
- Moran-Salazar, R.G.; Sanchez-Lizarraga, A.L.; Rodriguez-Campos, J.; Davila-Vazquez, G.; Marino-Marmolejo, E.N.; Dendooven, L.; Contreras-Ramos, S.M. Utilization of Vinasses as Soil Amendment: Consequences and Perspectives. SpringerPlus 2016, 5, 1007. [Google Scholar] [CrossRef]
- SEMARNAT. Official Mexican Norm NOM-001-ECOL-1996/NOM-001-SEMARNAT-1996 Which Establishes the Permissible Limits of Contaminants in Wastewater Discharges into Receiving Bodies Owned by the Nation. Ministry of Environment, Natural Resources and Fisheries SEMARNAT, Mexico City, Mexico. 1997. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5645374&fecha=11/03/2022#gsc.tab=0 (accessed on 18 May 2025).
- Haar, J.; Sánchez-Flores, R.B.; Courtemanche, P. The Status, Policies and Outlook for Green Supply Chains in North America|Wilson Center. Available online: https://www.wilsoncenter.org/article/status-policies-and-outlook-green-supply-chains-north-america (accessed on 5 January 2025).
- SCFI. Official Mexican Norm NOM-163-SEMARNAT-ENER-SCFI-2013 Carbon Dioxide (CO2) Emissions from Exhaust Gases and Their Equivalent in Terms of Fuel Efficiency, Applicable to New Motor Vehicles with a Gross Vehicle Weight of up to 3857 Kilograms. Secretariat of Commerce and Industrial Development, SCFI Mexico City, Mexico. 2013. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5303391&fecha=21/06/2013#gsc.tab=0 (accessed on 18 May 2025).
- Barry, M.; Tigre, M.A. February 2024 Updates to the Climate Case Charts|Sabin Center for Climate Change Law. Available online: https://climate.law.columbia.edu/news/february-2024-updates-climate-case-charts (accessed on 5 January 2025).
- International Trade Administration Mexico—Trade Standards. Available online: https://www.trade.gov/country-commercial-guides/mexico-trade-standards (accessed on 5 January 2025).
- Vela, C.; Villanueva, D.; Hurtado, M.; Dávila, C. Doing Business in Mexico 2024; Backer and Mackenzie Abogados: Mexico City, Mexico, 2024. [Google Scholar]
- International Trade Administration Mexico—Environmental Technologies. Available online: https://www.trade.gov/country-commercial-guides/mexico-environmental-technologies (accessed on 5 January 2025).
- ECIJA. Wastewater Discharges in Receiving Bodies of Mexico’s Federation: Analysis of the Legal Framework in Light of the New Obligations in 2023 and the International Context. Available online: https://ecija.com/en/sala-de-prensa/wastewater-discharges-in-receiving-bodies-of-mexicos-federation-analysis-of-the-legal-framework-in-light-of-the-new-obligations-in-2023-and-the-international-context/ (accessed on 5 January 2025).
- Von Wobeser y Sierra Mexican Official Standard Establishes New Permissible Limits for Pollutants in Discharges to Waters of National Jurisdiction. Available online: https://www.vonwobeser.com/index.php/publication?p_id=1712 (accessed on 5 January 2025).
- Foley & Lardner LLP Deadline to Comply with Requirements of Mexico’s Wastewater Disposal Standard Fast Approaching|Foley & Lardner LLP. Available online: https://www.foley.com/insights/publications/2023/02/deadline-requirements-mexico-wastewater-disposal/ (accessed on 5 January 2025).
- Aguilar-Aguilar, A.; de León-Martínez, L.D.; Forgionny, A.; Acelas Soto, N.Y.; Mendoza, S.R.; Zárate-Guzmán, A.I. A Systematic Review on the Current Situation of Emerging Pollutants in Mexico: A Perspective on Policies, Regulation, Detection, and Elimination in Water and Wastewater. Sci. Total Environ. 2023, 905, 167426. [Google Scholar] [CrossRef]
- eCFR ECFR: 40 CFR Part 133—Secondary Treatment Regulation. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-133 (accessed on 9 May 2025).
- EUR-Lex. Directive-91/271-EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/1991/271/oj/eng (accessed on 9 May 2025).
- Iñiguez-Covarrubias, G.; Peraza-Luna, F. Reduction of Solids and Organic Load Concentrations in Tequila Vinasses Using a Polyacrylamide (PAM) Polymer Flocculant. Rev. Int. Contam. Ambient. 2007, 23, 17–24. [Google Scholar]
- Carvajal-Zarrabal, O.; Nolasco-Hipólito, C.; Barradas-Dermitz, D.M.; Hayward-Jones, P.M.; Aguilar-Uscanga, M.G.; Bujang, K. Treatment of Vinasse from Tequila Production Using Polyglutamic Acid. J. Environ. Manag. 2012, 95, S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Valerio, C.; Peregrina-Lucano, A.A.; Manríquez-González, R.; Pérez-Fonseca, A.A.; Robledo-Ortíz, J.R.; Shenderovich, I.G.; Gómez-Salazar, S. Color Compounds Removal from Tequila Vinasses Using Silica Gel Adsorbents Functionalized with Thiol Moieties: Equilibrium and Kinetics Studies. Molecules 2024, 29, 5910. [Google Scholar] [CrossRef]
- Díaz-Vázquez, D.; Garibay, M.V.; Fernández del Castillo, A.; Orozco-Nunnelly, D.A.; Senés-Guerrero, C.; Gradilla-Hernández, M.S. Yeast Community Composition Impacts on Tequila Industry Waste Treatment for Pollution Control and Waste-to-Product Synthesis. Front. Chem. Eng. 2022, 4, 1013873. [Google Scholar] [CrossRef]
- Linerio-Gil, J.E.; Guzmán-Carrillo, A. Tratamiento de Efluentes y Aprovechamiento de Residuos. In Ciencia y Tecnología del Tequila; Avances y Perspectivas; CIATEJ, Ed.; Gschaedler-Mathis A.C.: Guadalajara, Mexico, 2004. [Google Scholar]
- Tejeda, A.; Montoya, A.; Sulbarán-Rangel, B.; Zurita, F.; Tejeda, A.; Montoya, A.; Sulbarán-Rangel, B.; Zurita, F. Possible Pollution of Surface Water Bodies with Tequila Vinasses. Water 2023, 15, 3773. [Google Scholar] [CrossRef]
- Ramírez-Ramírez, A.A.; Lozano-Álvarez, J.A.; Gutiérrez-Lomelí, M.; Zurita, F. Tequila Vinasse Treatment in Two Types of Vertical Downflow Treatment Wetlands (with Emergent Vegetation and Ligninolytic Fungi). Water 2024, 16, 1778. [Google Scholar] [CrossRef]
- Iñiguez, C.G.; Bernal, C.J.J.; Ramírez, M.W.; Villalvazo, N.J. Recycling Agave Bagasse of the Tequila Industry. Adv. Chem. Eng. Sci. 2014, 4, 135–142. [Google Scholar] [CrossRef][Green Version]
- Méndez-Acosta, H.O.; García-Sandoval, J.P.; González-Álvarez, V.; Alcaraz-González, V.; Jáuregui-Jáuregui, J.A. Regulation of the Organic Pollution Level in Anaerobic Digesters by Using Off-Line COD Measurements. Bioresour. Technol. 2011, 102, 7666–7672. [Google Scholar] [CrossRef]
- Dias, A.S.; Carneiro, P.A.; Boloy, R.A.M.; César, A.d.S.; de Oliveira, U.R. Advancements in Vinasse Application: An Integrated Analysis of Patents, Literature and Research Profile. Clean. Eng. Technol. 2024, 22, 100795. [Google Scholar] [CrossRef]
- Mendiola-Rodriguez, T.A.; Ricardez-Sandoval, L.A. Integration of Design and Control for Renewable Energy Systems with an Application to Anaerobic Digestion: A Deep Deterministic Policy Gradient Framework. Energy 2023, 274, 127212. [Google Scholar] [CrossRef]
- Estrada-Arriaga, E.B.; Reynoso-Deloya, M.G.; Guillén-Garcés, R.A.; Falcón-Rojas, A.; García-Sánchez, L. Enhanced Methane Production and Organic Matter Removal from Tequila Vinasses by Anaerobic Digestion Assisted via Bioelectrochemical Power-to-Gas. Bioresour. Technol. 2021, 320, 124344. [Google Scholar] [CrossRef] [PubMed]
- Daniel Silva-Martínez, R.; Sanches-Pereira, A.; Ortiz, W.; Fernanda, M.; Galindo, G.; Coelho, S.T. The State-of-the-Art of Organic Waste to Energy in Latin America and the Caribbean: Challenges and Opportunities. Renew. Energy 2020, 156, 509–525. [Google Scholar] [CrossRef]
- García-Depraect, O.; Diaz-Cruces, V.F.; León-Becerril, E. Upgrading of Anaerobic Digestion of Tequila Vinasse by Using an Innovative Two-Stage System with Dominant Lactate-Type Fermentation in Acidogenesis. Fuel 2020, 280, 118606. [Google Scholar] [CrossRef]
- García-Depraect, O.; Osuna-Laveaga, D.R.; León-Becerril, E. A Comprehensive Overview of the Potential of Tequila Industry By-Products for Biohydrogen and Biomethane Production: Current Status and Future Perspectives. In New Advances on Fermentation Processes; Intechopen: London, UK, 2020. [Google Scholar]
- Mahmoudabadi, Z.S.; Rashidi, A.; Maklavany, D.M. Optimizing Treatment of Alcohol Vinasse Using a Combination of Advanced Oxidation with Porous α-Fe2O3 Nanoparticles and Coagulation-Flocculation. Ecotoxicol. Environ. Saf. 2022, 234, 113354. [Google Scholar] [CrossRef]
- Rodríguez-Romero, J.d.J.; Aceves-Lara, C.A.; Silva, C.F.; Gschaedler, A.; Amaya-Delgado, L.; Arrizon, J. 2-Phenylethanol and 2-Phenylethylacetate Production by Nonconventional Yeasts Using Tequila Vinasses as a Substrate. Biotechnol. Rep. 2020, 25, e00420. [Google Scholar] [CrossRef]
- Sustainability—Consejo Regulador Del Tequila—Autenticidad y Calidad Certificada. Available online: https://www.crt.org.mx/en/sustainability/ (accessed on 5 January 2025).
- Rodriguez Arreola, A.; Sanchez Tizapa, M.; Zurita, F.; Morán-Lázaro, J.P.; Valderrama, R.C.; Rodríguez-López, J.L.; Carreon-Alvarez, A. Treatment of Tequila Vinasse and Elimination of Phenol by Coagulation–Flocculation Process Coupled with Heterogeneous Photocatalysis Using Titanium Dioxide Nanoparticles. Environ. Technol. 2018, 41, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Ferral-Pérez, H.; Torres Bustillos, L.G.; Méndez, H.; Rodríguez-Santillan, J.L.; Chairez, I. Sequential Treatment of Tequila Industry Vinasses by Biopolymer-Based Coagulation/Flocculation and Catalytic Ozonation. Ozone Sci. Eng. 2016, 38, 279–290. [Google Scholar] [CrossRef]
- Zurita, F.; Tejeda, A.; Ramirez-Ramirez, A.; Montoya, A. Integration of Coagulation–Flocculation(with Natural Coagulant) to Constructed Wetlands for Color Removal from Tequila Vinasses. Water 2024, 16, 3151. [Google Scholar] [CrossRef]
- Castillo-Monroy, J.; Godínez, L.A.; Robles, I.; Estrada-Vargas, A. Study of a Coupled Adsorption/Electro-Oxidation Process as a Tertiary Treatment for Tequila Industry Wastewater. Environ. Sci. Pollut. Res. 2020, 28, 23699–23706. [Google Scholar] [CrossRef]
- IIEG. Arandas Diagnóstico Del Municipio; Instituto de Información Estadística y Geográfica del Estado de Jalisco (IIEG): Zapopan, Mexico, 2019; pp. 1–37. [Google Scholar]
- EPA. How EPA Regulates Drinking Water Contaminants. Available online: https://www.epa.gov/sdwa/how-epa-regulates-drinking-water-contaminants (accessed on 23 February 2025).
- EPA. Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. Available online: https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals (accessed on 23 February 2025).
- NYC Environmental Protection. New York City 2018 Drinking Water Supply and Quality Report; New York City Environmental Protection: New York, NY, USA, 2018. [Google Scholar]
- FDA. FDA Regulation of Cosmetics and Personal Care Products; Food and Drug Administration: Silver Spring, MD, USA, 2012. [Google Scholar]
- Jiménez López, J.E. Remoción de Contaminantes Refractarios de Las Vinazas Tequileras Por Electrocoagulación. Master’s Thesis, Benemérita Universidad Autónoma de Puebla, Puebla, Mexico, 2016. [Google Scholar]
- Karmankar, S.B.; Sharma, A.; Ahirwar, R.C.; Mehra, S.; Pal, D.; Prajapati, A.K. Cost Cutting Approach of Distillery Effluent Treatment Using Solar Photovoltaic Cell Driven Electrocoagulation: Comparison with Conventional Electrocoagulation. J. Water Process Eng. 2023, 54, 103982. [Google Scholar] [CrossRef]
- Medina Collana, J.T.; Ayllon Ormeño, M.; Julca Meza, C.; Moreyra Cuadros, G.; Carrasco Venegas, L.A.; Ancieta Dextre, C.A.; Rodríguez Taranco, O.J.; Avelino Carhuaricra, C.; Diaz Bravo, P.; Montaño Pisfil, J.A. Processes Coupled to Electrocoagulation for the Treatment of Distillery Wastewaters. Sustainability 2024, 16, 6383. [Google Scholar] [CrossRef]
- Montaño Saavedra, M.D.; Cárdenas Concha, V.O.; Bastos, R.G. Electrocoagulation Treatment of Sugarcane Vinasse: Operating Parameters and Cost Analysis by Response Surface Methodology. J. Clean. Prod. 2024, 448, 141597. [Google Scholar] [CrossRef]
- Dubey, S.; Joshi, A.; Parmar, N.; Rekhate, C.; Amitesh; Prajapati, A.K. Process Optimization of Electrocoagulation Reactor for Treatment of Distillery Effluent Using Aluminium Electrode: Response Surface Methodology Approach. Chem. Data Collect. 2023, 45, 101023. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Chaudhari, P.K. Physicochemical Treatment of Distillery Wastewater—A Review. Chem. Eng. Commun. 2015, 202, 1098–1117. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrochemical Treatment of Distillery Spent Wash Using Aluminum and Iron Electrodes. Chin. J. Chem. Eng. 2012, 20, 439–443. [Google Scholar] [CrossRef]
- Alizadeh, R.; Farhadi, K.; Ghaneian, M.T.; Ehrampoush, M.H.; Jambarsang, S.; Salmani, M.H.; Kokya, T.A. Development of Circulating Electrocoagulation as a Novel Technique for the Treatment of Raw Vinasse Effluents of Ethanol Production Industries. Anal. Bioanal. Chem. Res. 2023, 10, 319–327. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Mechanistic Models of Electrocoagulation Kinetics of Pollutant Removal in Vinasse Waste: Effect of Voltage. J. Water Process Eng. 2020, 36, 101312. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Experiment and Kinetic Analysis of the Effect of Agitation Speed on Electrocoagulation Process for the Treatment of Vinasse. J. Water Process Eng. 2022, 50, 103144. [Google Scholar] [CrossRef]
- David, C.; Thangavelu, A. Degradation of Distillery Effluent by Twisted-Type Iron Electrodes: Experimental with ANN Approach. Int. J. Environ. Anal. Chem. 2022, 102, 6121–6133. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Chaudhari, P.K. Electrochemical Treatment of Rice Grain-Based Distillery Effluent: Chemical Oxygen Demand and Colour Removal. Environ. Technol. 2014, 35, 242–249. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Mechanistic Model of Electrocoagulation Process for Treating Vinasse Waste: Effect of Initial PH. J. Environ. Chem. Eng. 2020, 8, 103756. [Google Scholar] [CrossRef]
- AWWA. 4500-H+ PH—Standard Methods For the Examination of Water and Wastewater; American Water Works Association AWWA: Washington, DC, USA; Available online: https://www.standardmethods.org/doi/10.2105/SMWW.2882.082 (accessed on 17 May 2025).
- EPA. Method 410.4, Revision 2.0: The Determination of Chemical Oxygen Demand by SemiAutomated Colorimetry; Environmental Protection Agency EPA: Cincinnati, OH, USA, 1993. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_410-4_1993.pdf (accessed on 17 May 2025).
- AWWA. Total Dissolved Solids and Electrical Conductivity: Standard Method for the Examination of Water and Wastewater 2510 Conductivity 2510 Conductivity In: Standard Methods For the Examination of Water and Wastewater; American Water Works Association AWWA: Washington, DC, USA, 2017; Available online: https://www.standardmethods.org/doi/10.2105/SMWW.2882.027 (accessed on 17 May 2025).
- ISO 7887:2011; Water Quality—Examination and Determination of Colour. International Standard Organization ISO, Vernier: Geneva, Switzerland, 2011. Available online: https://www.iso.org/standard/46425.html (accessed on 17 May 2025).
- AWWA. Turbidity: Standard Method for the Examination of Water and Wastewater 2130 Turbidity; American Water Works Association AWWA: Washington, DC, USA, 2017; Available online: https://www.standardmethods.org/doi/10.2105/SMWW.2882.018 (accessed on 17 May 2025).









| Parameter | Value 1 [7] | Value 2 [9] |
|---|---|---|
| pH | 3.4–4.5 | 3.6–4.6 |
| Total COD (mg/L) | 60,000–100,000 | 41,740–67,970 |
| Total BOD (mg/L) | 35,000–60,000 | 16,162–35,102 |
| Total dissolved solids (mg/L) | 23,000–42,000 | 4550–6750 |
| Parameters | Unit | NOM-001-SEMARNAT-1996 MPL | NOM-001-SEMARNAT-2021 MPL |
|---|---|---|---|
| Temperature | 40 °C | 35 °C | |
| Biological Oxygen Demand (BOD5) | mg/L | 30–150 | Deletion |
| Oils and Fats | mg/L | 15 | 15 |
| Settleable Solids | mL/L | 1 | Deletion |
| Total Suspended Solids | mg/L | 40–150 | 20–60 |
| Floating Matter | Absent | Deletion | |
| Chemical Oxygen Demand (COD) | mg/L | Not required | 100–150 |
| Total Organic Carbon | mg/L | Not required | 25–38 |
| Total Nitrogen | mg/L | 15–40 | 15–25 |
| Total Phosphorous | mg/L | 5–20 | 5–15 |
| pH | 5–10 | 6–9 | |
| True Color | SAC | Not required | 436 nm/7.0 m−1 max 525 nm/5.0 m−1 max 620 nm/3.0 m−1 max |
| Acute Toxicity | UT | Not required | 2 (15 min exposure) |
| Arsenic | mg/L | 0.1–0.2 | 0.1–0.2 |
| Cadmium | mg/L | 0.1–0.2 | 0.1–0.2 |
| Cyanides | mg/L | 1.0–2.0 | 1.0 |
| Copper | mg/L | 4.0 | 4.0 |
| Chrome | mg/L | 0.5–1.0 | 0.5–1.0 |
| Mercury | mg/L | 0.005–0.01 | 0.005–0.01 |
| Nickel | mg/L | 2.0 | 2.0 |
| Lead | mg/L | 0.2–0.5 | 0.2 |
| Zinc | mg/L | 10.0 | 10.0 |
| Treatment | Advantages | Disadvantages |
|---|---|---|
| Neutralization | 4–6 g/L CaCO3 Complies with requirement of pH levels of 6.5–8.0 | Neutralization or pH adjustment must be performed after each treatment phase |
| Sedimentation Ponds | >80% sedimented solids removal | Organic matter concentration remains above 90%. Improper preparation leads to soil and subsoil pollution |
| Sedimentation Pools | >80% sedimented solids removal | Organic matter concentration remains above 90% |
| Dissolved Air Flocculation (DAF) | >80% sedimented solids removal | Dissolved solids and biological oxygen demand have not decreased significantly |
| Coagulation–Flocculation | 5 mg/L Al2(SO)4 + 1.5 mg polymer 20–30% suspended solids and colloidal solids removal | Low chemical and biological oxygen demands reached |
| Treatment | Type of Reactor | COD Removal |
|---|---|---|
| Anaerobic | UASB reactor | 70–85% |
| Anaerobic filter | 65% | |
| UASB reactor + anaerobic filter | 50–70% | |
| UASB without recirculation | 36% | |
| UASB with recirculation | 74% | |
| Fluidized bed | 70% | |
| CSTR (batch or continuous) | 90–95% | |
| Aerobic | Activated sludge | 5–10% |
| Extended aeration | 5–10% | |
| Aeration pools/ponds | 5–10% | |
| SBR | 5–10% |
| Treatment | Electrode Material | Initial Condition | Highest Removal | Surface Electric Current A/m2 | Specific Energy Consumption kWh/m3 | Ref. |
|---|---|---|---|---|---|---|
| Pretreated tequila vinasses pH: 5 40 °C 180 min No stirring | Al | COD 304 mg/L | 94.41% | 148.57 | 18.75 | [57] |
| Sugarcane vinasse 3 h 430 rpm | Al | TDS: 6810 ± 840 mg/L; TSS: 5200 ± 300 mg/L | TDS: 20% TSS: 96% | 61 | 13.5 | [60] |
| Distillery effluent 93 min pH: 3.5–9.5 | Al | COD: 5.15 g/L | COD: 85.1% | 135 | NA | [61] |
| Distillery wastewater 0–30 V 1.5 L 2 h 0–5 A | Al | COD: 13.8 g/L | COD: 93% | 89.3 | 31.4 | [68] |
| Distillery spent wash 300 mL 2 h pH: 3 500 rpm | Al-Al Al-Fe Fe-Fe | COD: 120 g/L | COD: Al-Al: 73.3% Al-Fe: 60% Fe-Fe: 46.6% | 1870 | NA | [63] |
| Vinasse effluents 1 A pH: 7 45 min | Al-Fe | COD: 46,550 mg/L | COD: 80.8% | 167 | NA | [64] |
| Vinasse waste from the bioethanol industry 0, 250, 500 rpm T 301.65°K pH: 4.1 12.6, 12.3, 12.4 V Volume: 1 L | Fe | COD: 113.70 g/L | 67.62% | NA | NA | [66] |
| Vinasse residues 12.5 V 200 rpm pH: 6 105–110 °C 6 h | Fe | COD: 100.16 g/L | 51.67% | 1021.41 | 264.75 | [65] |
| Distillery wastewater pH: 6 2.98 A 10 V Time: 1 h 200 rpm | Fe | COD: 100.16 g/L | COD: 13.96% | 1045.6 | 29.8 | [69] |
| Distillery Effluent 100 rpm; Time: 2 h pH: 3–9 0.5, 1, 1.5, 1.9 A | Fe | COD: 140 g/L | Color: 83.75% | NA | NA | [67] |
| Parameter | Units | Norm | Result |
|---|---|---|---|
| pH | [-] | 6.5–8.0 | 7.81 |
| COD | mg/L | <150 | 814 |
| TDS | mg/L | 3.577 | |
| Electrical conductivity | mS/cm | 7.299 | |
| Color | |||
| Abs 436 nm | m−1 | 7.0 | 34.7 |
| Abs 525 nm | m−1 | 5.0 | 10.4 |
| Abs 620 nm | m−1 | 3.0 | 3.7 |
| Voltage | Initial Electrical Current, Amp | Final Electrical Current, Amp | Electrical Current Density, Amp/m2 |
|---|---|---|---|
| V | I1 | I2 | ECD |
| 1 | 0.02 | 0.02 | 10.2 |
| 2 | 0.06 | 0.06 | 30.6 |
| 3 | 0.08 | 0.08 | 40.9 |
| 4 | 0.14 | 0.14 | 71.5 |
| 5 | 0.16 | 0.15 | 79.2 |
| 6 | 0.18 | 0.17 | 89.4 |
| COD, mg/L | |||
|---|---|---|---|
| Voltage, V | R1 | R2 | R3 |
| 0 | 814 | 814 | 814 |
| 1 | 594 | 553 | 614 |
| 2 | 489 | 415 | 365 |
| 3 | 367 | 410 | 318 |
| 4 | 331 | 268 | 355 |
| 5 | 247 | 303 | 277 |
| 6 | 227 | 242 | 235 |
| pH | |||
|---|---|---|---|
| Voltage, V | R1 | R2 | R3 |
| 0 | 7.81 | 7.81 | 7.81 |
| 1 | 7.35 | 7.30 | 7.10 |
| 2 | 7.43 | 6.52 | 7.29 |
| 3 | 6.54 | 6.61 | 6.49 |
| 4 | 6.32 | 6.21 | 5.89 |
| 5 | 4.99 | 5.28 | 5.3 |
| 6 | 4.46 | 4.68 | 5.32 |
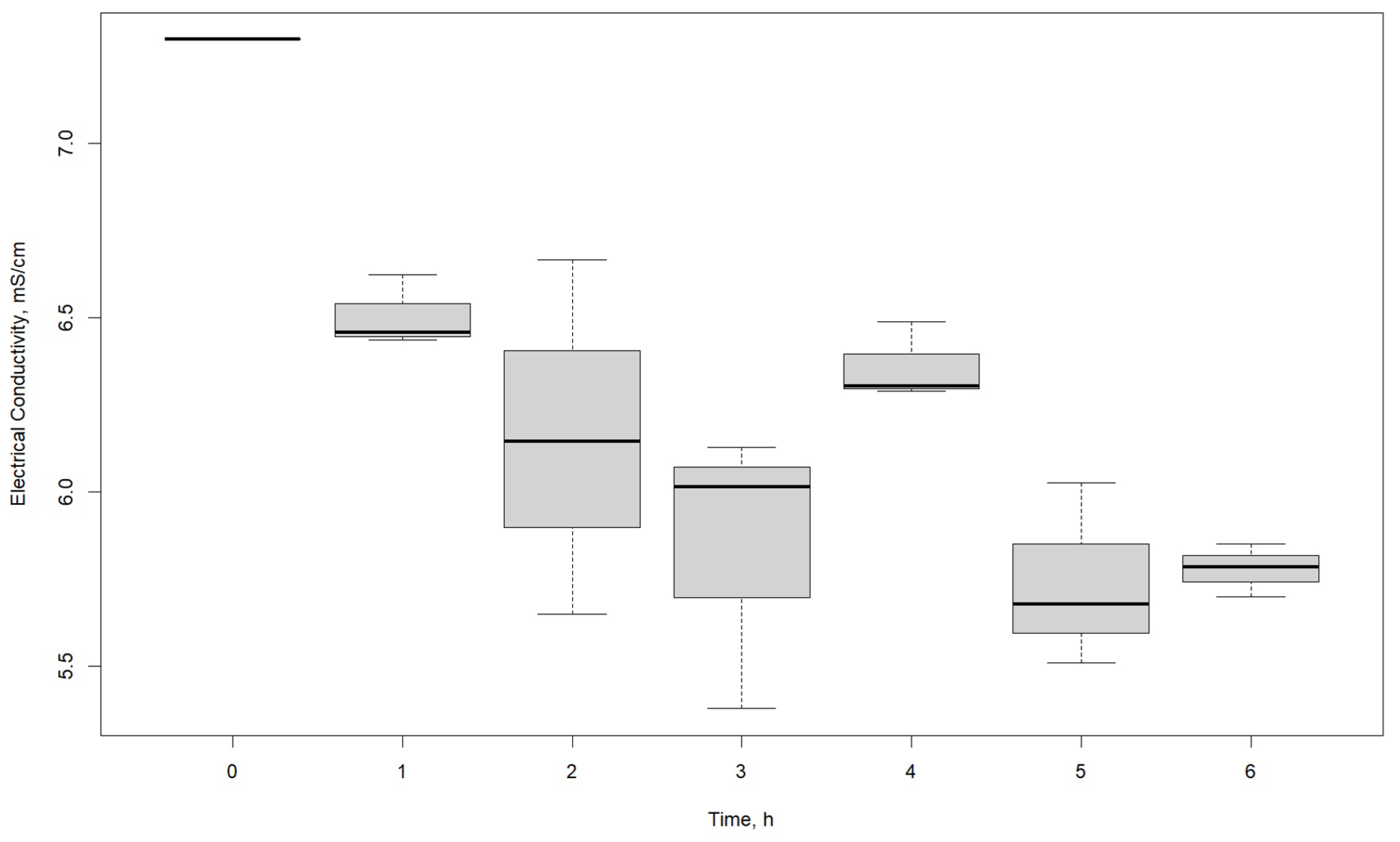
| EC, mS/cm | |||
|---|---|---|---|
| Voltage, V | R1 | R2 | R3 |
| 0 | 7.299 | 7.299 | 7.299 |
| 1 | 7.130 | 6.933 | 7.208 |
| 2 | 7.459 | 7.174 | 6.631 |
| 3 | 6.628 | 7.248 | 6.879 |
| 4 | 7.117 | 6.258 | 6.498 |
| 5 | 5.649 | 6.666 | 6.145 |
| 6 | 6.463 | 6.793 | 6.051 |
| TDS, mg/L | |||
|---|---|---|---|
| Voltage, V | R1 | R2 | R3 |
| 0 | 3.577 | 3.577 | 3.577 |
| 1 | 3.491 | 3.397 | 3.532 |
| 2 | 3.687 | 3.516 | 3.25 |
| 3 | 3.248 | 3.552 | 3.371 |
| 4 | 3.488 | 3.073 | 3.185 |
| 5 | 2.869 | 3.265 | 3.011 |
| 6 | 3.167 | 3.329 | 2.965 |
| Turbidity, NTU | |||
|---|---|---|---|
| Voltage, V | R1 | R2 | R3 |
| 0 | 1.50 | 1.50 | 1.50 |
| 1 | 1.41 | 1.17 | 1.42 |
| 2 | 1.03 | 1.05 | 1.04 |
| 3 | 0.94 | 0.96 | 0.94 |
| 4 | 0.80 | 0.72 | 0.82 |
| 5 | 0.79 | 0.67 | 0.78 |
| 6 | 0.75 | 0.62 | 0.62 |
| SAC λ 436 nm, m−1 | |||
|---|---|---|---|
| Voltage, V | R1 | R2 | R3 |
| 0 | 34.7 | 34.7 | 34.7 |
| 1 | 24.4 | 25.6 | 21.2 |
| 2 | 11.2 | 9.6 | 6.3 |
| 3 | 6.9 | 7.5 | 5.1 |
| 4 | 4.6 | 4.7 | 3.9 |
| 5 | 4.4 | 3.2 | 4.5 |
| 6 | 4.4 | 3.1 | 3.8 |
| SAC λ 525 nm, m−1 | |||
|---|---|---|---|
| Voltage, V | R1 | R2 | R3 |
| 0 | 10.4 | 10.4 | 10.4 |
| 1 | 5.4 | 4.5 | 5.2 |
| 2 | 2.8 | 2.6 | 3.0 |
| 3 | 2.3 | 2.4 | 2.0 |
| 4 | 1.8 | 1.7 | 2.2 |
| 5 | 1.0 | 1.5 | 2.0 |
| 6 | 2.0 | 1.8 | 1.3 |
| SAC λ 620 nm, m−1 | |||
| Voltage, V | R1 | R2 | R3 |
| 0 | 10.4 | 10.4 | 10.4 |
| 1 | 5.4 | 4.5 | 5.2 |
| 2 | 2.8 | 2.6 | 3 |
| 3 | 2.3 | 2.4 | 2 |
| 4 | 1.8 | 1.7 | 2.2 |
| 5 | 1 | 1.5 | 2 |
| 6 | 2 | 1.8 | 1.3 |
| COD, mg/L | |||
|---|---|---|---|
| Time, h | R1 | R2 | R3 |
| 0 | 814 | 814 | 814 |
| 1 | 381 | 266 | 336 |
| 2 | 247 | 303 | 277 |
| 3 | 214 | 178 | 210 |
| 4 | 170 | 193 | 163 |
| 5 | 135 | 158 | 139 |
| 6 | 117 | 129 | 107 |
| pH | |||
|---|---|---|---|
| Time, h | R1 | R2 | R3 |
| 0 | 7.81 | 7.81 | 7.81 |
| 1 | 6.52 | 6.18 | 6.28 |
| 2 | 6.32 | 6.21 | 5.89 |
| 3 | 5.69 | 5.76 | 5.30 |
| 4 | 5.10 | 5.66 | 5.49 |
| 5 | 4.54 | 4.17 | 4.80 |
| 6 | 3.76 | 3.52 | 3.33 |
| EC, mS/cm | |||
|---|---|---|---|
| Time, h | R1 | R2 | R3 |
| 0 | 7.299 | 7.299 | 7.299 |
| 1 | 6.436 | 6.457 | 6.623 |
| 2 | 5.649 | 6.666 | 6.145 |
| 3 | 5.378 | 6.128 | 6.014 |
| 4 | 6.489 | 6.290 | 6.302 |
| 5 | 5.678 | 6.025 | 5.509 |
| 6 | 5.783 | 5.850 | 5.698 |
| TDS, mg/L | |||
|---|---|---|---|
| Time, h | R1 | R2 | R3 |
| 0 | 3.577 | 3.577 | 3.577 |
| 1 | 3.174 | 3.206 | 3.246 |
| 2 | 2.869 | 3.265 | 3.011 |
| 3 | 2.658 | 3.003 | 2.971 |
| 4 | 3.180 | 3.083 | 3.089 |
| 5 | 2.787 | 2.953 | 2.734 |
| 6 | 2.878 | 2.867 | 2.793 |
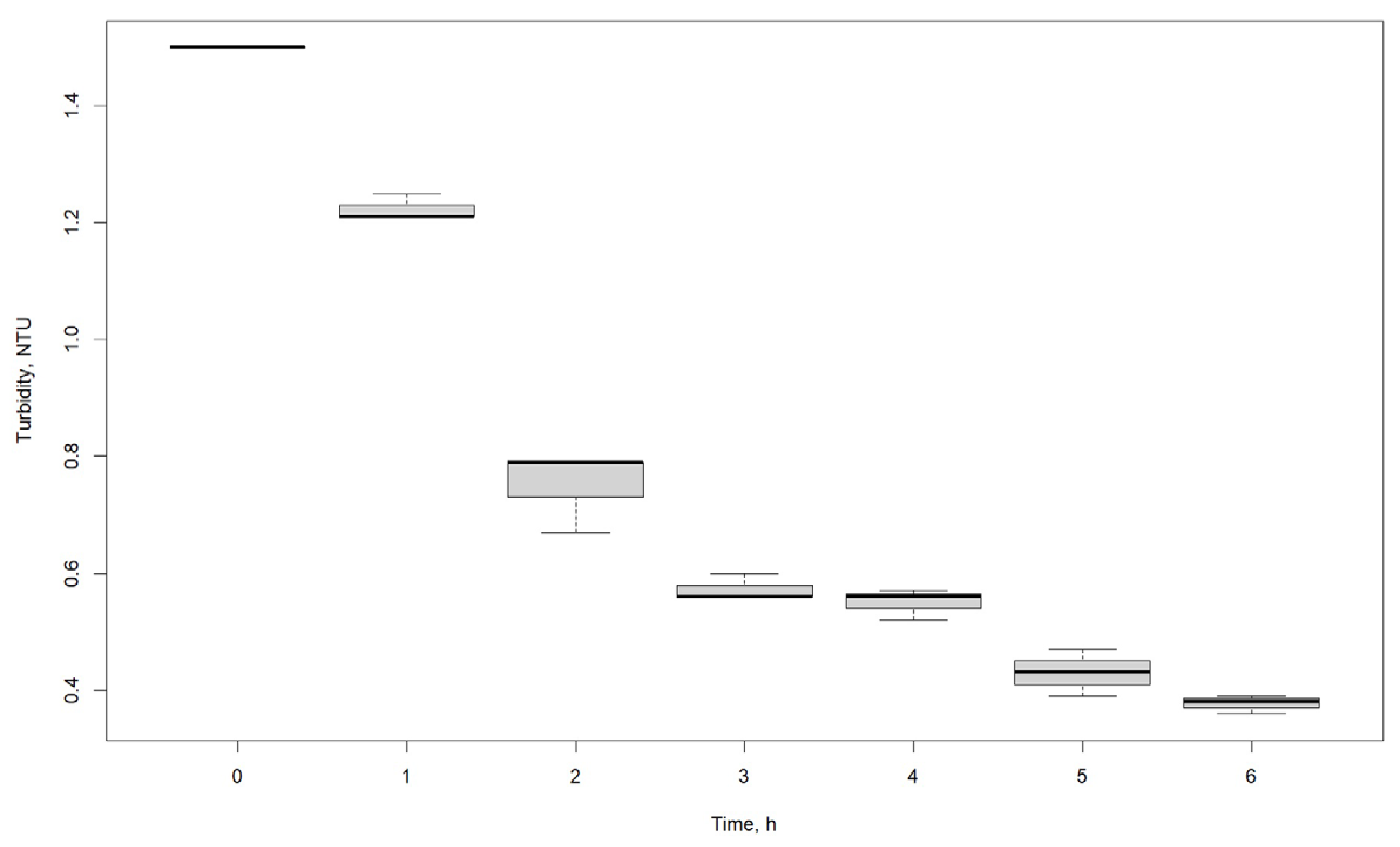
| Turbidity, NTU | |||
|---|---|---|---|
| Time, h | R1 | R2 | R3 |
| 0 | 1.50 | 1.50 | 1.50 |
| 1 | 1.21 | 1.21 | 1.25 |
| 2 | 0.79 | 0.67 | 0.79 |
| 3 | 0.60 | 0.56 | 0.56 |
| 4 | 0.56 | 0.57 | 0.52 |
| 5 | 0.47 | 0.39 | 0.43 |
| 6 | 0.36 | 0.39 | 0.38 |
| SAC λ 436 nm, m−1 | |||
|---|---|---|---|
| Time, h | R1 | R2 | R3 |
| 0 | 34.7 | 34.7 | 34.7 |
| 1 | 4.7 | 4.6 | 5.4 |
| 2 | 4.4 | 3.2 | 4.5 |
| 3 | 3.8 | 4.0 | 4.1 |
| 4 | 3.6 | 4.2 | 3.9 |
| 5 | 2.7 | 2.5 | 3.3 |
| 6 | 2.3 | 1.9 | 2.7 |
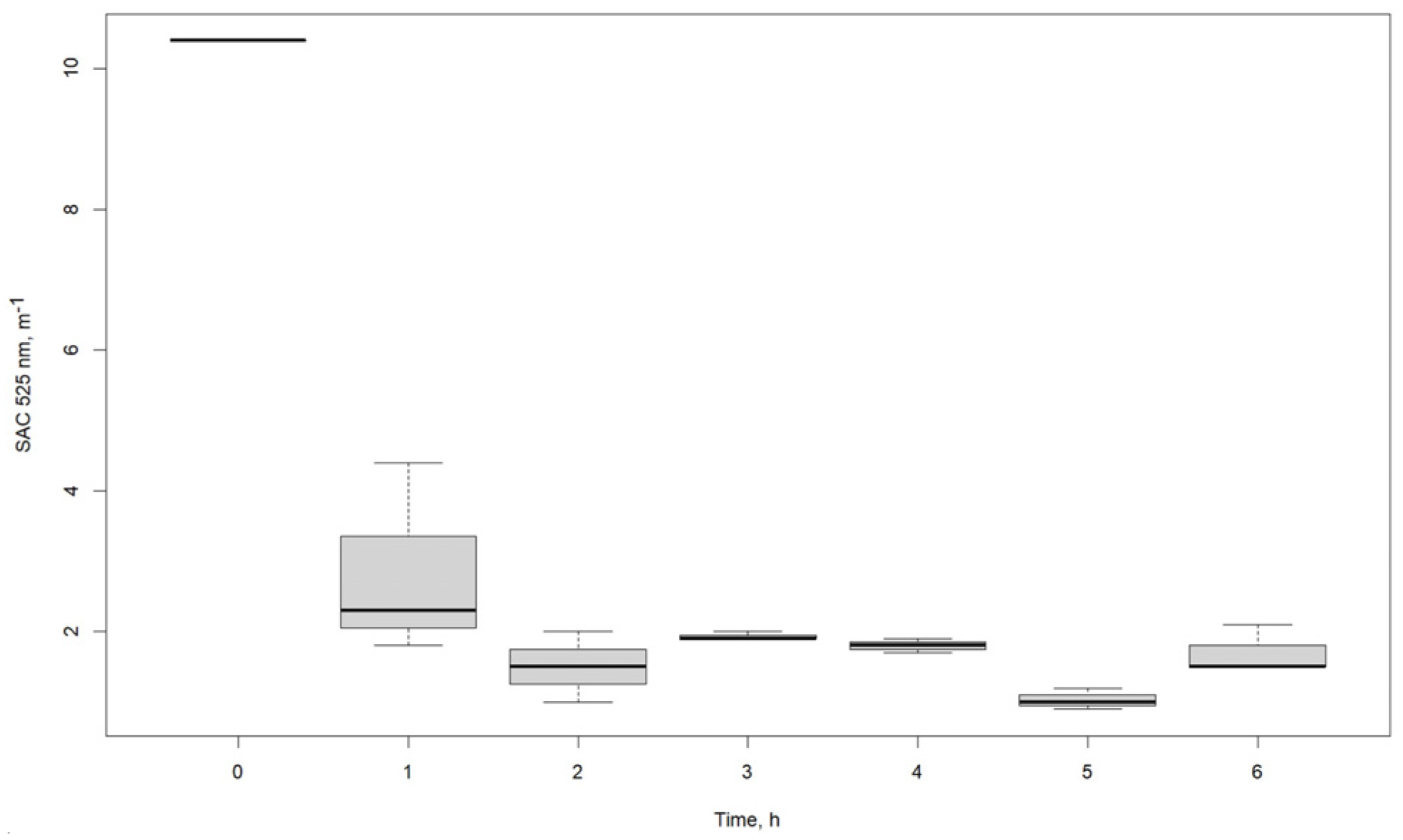
| SAC λ 525 nm, m−1 | |||
|---|---|---|---|
| Time, h | R1 | R2 | R3 |
| 0 | 10.4 | 10.4 | 10.4 |
| 1 | 2.3 | 1.8 | 4.4 |
| 2 | 1.0 | 1.5 | 2.0 |
| 3 | 1.9 | 2.0 | 1.9 |
| 4 | 1.7 | 1.9 | 1.8 |
| 5 | 1.2 | 0.9 | 1.0 |
| 6 | 1.5 | 1.5 | 2.1 |
| SAC λ 620 nm, m−1 | |||
|---|---|---|---|
| Time, h | R1 | R2 | R3 |
| 0 | 3.7 | 3.7 | 3.7 |
| 1 | 1.2 | 1.1 | 1.6 |
| 2 | 1.1 | 0.9 | 1.0 |
| 3 | 1.2 | 1.1 | 1.1 |
| 4 | 0.9 | 1.3 | 1.0 |
| 5 | 1.2 | 1.4 | 1.4 |
| 6 | 1.3 | 0.8 | 1.2 |
| Parameter | Data |
|---|---|
| Optimal laboratory conditions | |
| Sample volume | 0.2 L |
| Average electric current | 0.155 A |
| Voltage | 5 V |
| Treatment time | 6 h |
| Calculated power | 0.775 W |
| Electrode surface | 19.58 cm2 |
| Initial COD | 814 mg/L |
| Average final COD | 118 mg/L |
| Anode consumption | 0.1118 g/h |
| 0.1442 kg/kWh | |
| Data for industrial applications | |
| Surface electric current | 79.16 A/m2 |
| Unitary energy consumption | 3.775 kWh/m3 |
| Specific energy consumption | 33.31 kWh/kg COD |
| Theoretical electrode consumption | 4.84 kg/m3 |
| Faraday efficiency | 0.51 |
| Energy cost | 0.21 USD/KWh 0.79 USD/m3 |
| Anode consumption | 0.1442 kg/KWh |
| 0.544 kg/m3 | |
| Anode cost | 1.05 USD/kg 0.57 USD/m3 |
| Treatment cost | 1.36 USD/m3 |
| Voltage | Replicate | Filtered Volume, mL | Sludge Percentage |
|---|---|---|---|
| 1 | R1 | 183.72 | 8.86% |
| R2 | 188.68 | 6.00% | |
| R3 | 189.90 | 5.32% | |
| 2 | R1 | 178.83 | 11.84% |
| R2 | 176.23 | 13.49% | |
| R3 | 174.56 | 14.57% | |
| 3 | R1 | 162.64 | 22.97% |
| R2 | 169.12 | 18.26% | |
| R3 | 170.08 | 17.59% | |
| 4 | R1 | 157.19 | 27.23% |
| R2 | 155.31 | 28.77% | |
| R3 | 155.69 | 28.46% | |
| 5 | R1 | 153.17 | 30.57% |
| R2 | 151.11 | 32.35% | |
| R3 | 155.01 | 29.02% | |
| 6 | R1 | 146.68 | 36.35% |
| R2 | 149.29 | 33.97% | |
| R3 | 150.16 | 33.19% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Pérez, R.; Fajardo Montiel, A.L.; Martínez Orozco, E.; Santiago Olivares, N.; Nápoles Armenta, J.; García Gómez, C. Electrocoagulation with Fe-SS Electrodes as a Fourth Stage of Tequila Vinasses Treatment for COD and Color Removal. Processes 2025, 13, 1637. https://doi.org/10.3390/pr13061637
González Pérez R, Fajardo Montiel AL, Martínez Orozco E, Santiago Olivares N, Nápoles Armenta J, García Gómez C. Electrocoagulation with Fe-SS Electrodes as a Fourth Stage of Tequila Vinasses Treatment for COD and Color Removal. Processes. 2025; 13(6):1637. https://doi.org/10.3390/pr13061637
Chicago/Turabian StyleGonzález Pérez, Rafael, Aída Lucía Fajardo Montiel, Edgardo Martínez Orozco, Norberto Santiago Olivares, Juan Nápoles Armenta, and Celestino García Gómez. 2025. "Electrocoagulation with Fe-SS Electrodes as a Fourth Stage of Tequila Vinasses Treatment for COD and Color Removal" Processes 13, no. 6: 1637. https://doi.org/10.3390/pr13061637
APA StyleGonzález Pérez, R., Fajardo Montiel, A. L., Martínez Orozco, E., Santiago Olivares, N., Nápoles Armenta, J., & García Gómez, C. (2025). Electrocoagulation with Fe-SS Electrodes as a Fourth Stage of Tequila Vinasses Treatment for COD and Color Removal. Processes, 13(6), 1637. https://doi.org/10.3390/pr13061637








