Abstract
To enhance the nutritional value of food products, new functional extruded products have been developed based on combinations of corn and lentil flour (70:30), with added salt (1.25%), sugar (5%), and resistant starch V (5–20%), and fortified winemaking by-products (fermented and unfermented pomace/pomace seeds) (5–20%). The formulations were processed through a 32 mm twin screw extruder. The developed extrudates were analyzed for bioactive content. The findings show that among the experimental formulations, those with the highest concentration (20%) presented the greatest amounts of the following functional compound total dietary fiber, total arabinoxylans, resistant starch, total phenols, total flavonols, and total anthocyanins, and the lowest content of raffinose and stachyose. These study results indicate that extrusion is an effective method for adding value to underutilized commodities, such as winemaking by-products. A future sensory evaluation study will be conducted on the extruded products with the highest amount of winemaking by-products of 20%.
1. Introduction
In upcoming years, there is expected to be a high demand for food, especially healthy options, including a wide variety of processed foods which should meet consumer expectations of being both tasty and nutritious [1]. The food industry is under pressure to produce affordable, abundant, nutritious, and sustainable foods [2], and to address health problems associated with the consumption of processed foods. The rise in food demand leads to an increased production of waste and by-products, which has a negative environmental effect. Thus, the reutilization of plant waste to create high-value-added food products has become one of the most significant challenges of the 21st century [3].
The wine industry generates by-products such as skins, seeds, and stems, accounting for 10 to 30% of the weight of processed grapes [4]. In 2020, global grape production reached 73.5 million tonnes, with wine production totaling 26 million liters in the same year [5]. Grape skins are highly complex and dynamic, consisting of polysaccharides and dietary fiber rich in bioactive compounds [6,7]. The degradation of these compounds to release desired components is directly related to the composition of the skin cell wall, its morphology, origin, and the grape cultivar [8]. On the other hand, grape seeds contain dietary fiber, polyphenols and fatty acids, which remain substantially intact during the vinification processing, thanks to the lignification process that occurs during grape ripening [9]. As a result, vinification by-products are excellent candidates for food applications due to their bioactive properties [10]. Wine by-products have been explored to enhance the nutritional and functional value of various foods, including baked goods, pastries, and pasta, by increasing their dietary fiber and antioxidant content [11].
Among the foods experiencing increasing demand, gluten-free [12], functional and nutraceutical foods have attracted special attention. Lentils (Lens culinaris Medik) are in high demand as a gluten-free, nutritious, and healthy functional food due to their content of fiber, resistant starch, and phytochemicals with health-promoting properties [13], which can help to reduce the risk of cardiovascular diseases, cancer, diabetes, and cholesterol levels [14]. The gut health-promoting effects of lentils can be attributed to short-chain fatty acids, resulting from the microbial fermentation of non-digestible lentil carbohydrate fractions (soluble fiber, resistant starch, and oligosaccharides) [15]. Moreover, lentils are rich in protein with a favorable amino acid profile being only deficient in methionine. The soluble fiber in lentils can contribute to lower blood cholesterol levels, and the starch they contain has a low glycemic index, which can help in maintaining blood glucose levels [14].
Extrusion technology has gained significant interest and attention from the scientific community, due to its continuous, highly productive process, minimum processing times, significant retention of nutritional quality, and versatility [16]. It also offers a great opportunity to modify the functionality of food ingredients, such as the solubilization of dietary fiber, the inactivation of thermolabile toxins and anti-nutritional factors, and the preservation of α-galactosides, among others [13,17,18]. As a result, it can be used to produce a variety of directly expanded fortified food products including vegetable by-products rich in insoluble fiber [19].
This study aimed to compare the flours of different extruded products made from corn and lentil formulations with the addition of winemaking by-products and resistant starch. The study assessed the products in terms of dietary fiber, oligosaccharides, arabinoxylans, total available carbohydrates, total starch (amylose and amylopectin), and resistant starch. This study aims to bring value to winemaking by-products and promote the consumption of lentils through diverse products with reduced production costs through flexible and versatile extrusion technology, to create sustainable and functional new processed foods.
2. Materials and Methods
2.1. Food Formulations
The control formulation consisted of a mixture of 93.75 g/100 g of corn and lentils (70:30), 1.25 g/100 g of salt, and 5 g/100 g of sugar. Different experimental formulations were developed from a corn/lentil mixture (70:30) with different percentages of winemaking by-products (fermented Cabernet Sauvignon skin/seed and non-fermented Chardonnay seed), provided by the Kendall–Jackson winery (Santa Rosa, CA, USA), Hylon® V starch (Ingredion, Westchester, IL, USA), and fixed proportions of salt (1.25%) and sugar (5%) [20]. Salt and sugar were obtained at a local market in Albany, CA, USA. The code for each sample is shown in Table 1, where the first letters are related to the grape variety: Cabernet Sauvignon (CS) and Chardonnay (Ch). Secondly, the type of winemaking by-products were as follows: fermented Cabernet Sauvignon skin/seed, Sk, and non-fermented Chardonnay seed, Sd. The text then refers to the various extruded formulations, labeled E1 through E4.

Table 1.
Formulation of extruded products from corn/lentils fortified with winemaking by-products: fermented Cabernet Sauvignon skin/seed or non-fermented Chardonnay seed.
2.2. Extrusion Conditions and Sample Obtaining
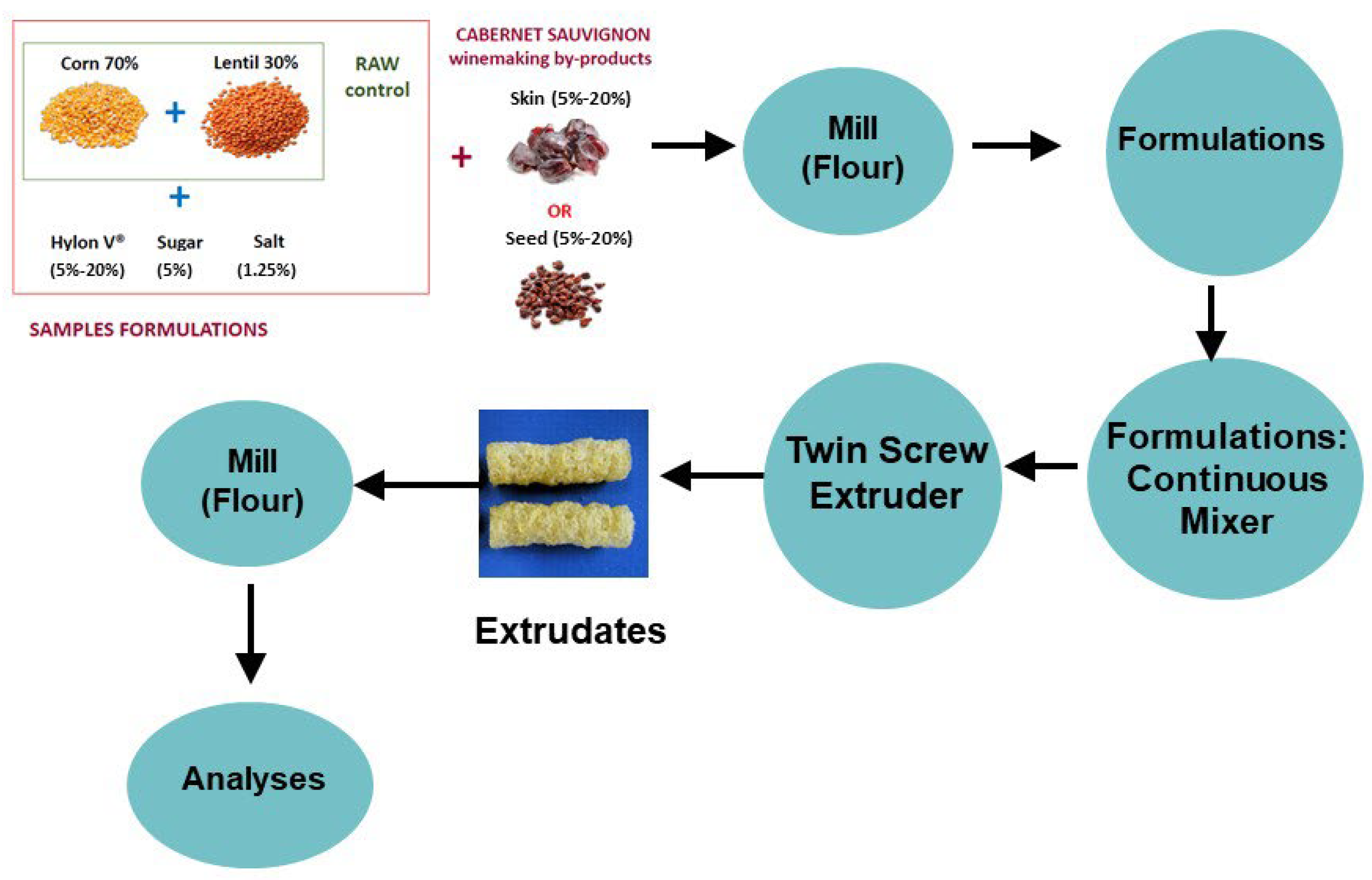
The extrusion process was carried out in a Clextral EV32B105 twin screw extruder (Clextral, Inc., Tampa, FL, USA) with co-rotating and closely intermeshing screws. The extruder was equipped with six-barrel sections, each 128 mm in length. The screw diameter (D) was 32 mm and the total configured screw length (L) was 768 mm, which gave an overall L/D ratio of 24. The different formulations were conditioned at a humidity of 17% and the operating conditions of the extruder were established at a feed rate of 50 kg/h, feed moisture of 17%, screw speed 500 rpm, and die temperature 160 °C. The mixture was extruded through two circular dyes each with a 3.5 mm diameter opening. The extrudates (snacks samples) in the form of cylindrical rods or pellets obtained from the different formulations were reduced to uniform powders using a Cyclone mill (Udy Corp., Fort Collins, CO, USA) equipped with a 0.5 mm sieve. The milled samples were transferred into polyethylene bags (50 g) and stored in a desiccator at room temperature until further analysis. Figure 1 provides a schematic view of the process.
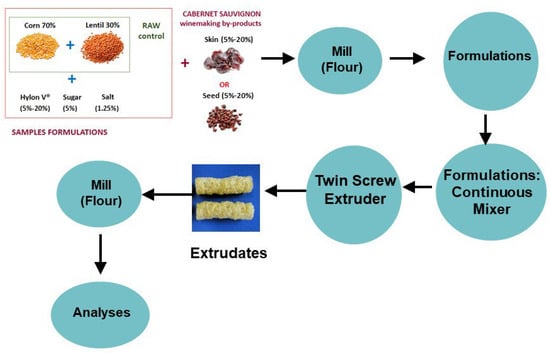
Figure 1.
Schematic view of the process to obtain the extrudates that were further milled into powder for subsequent analysis.
2.3. Analysis of the Hydrocarbon Fraction
2.3.1. Dietary Fiber
Insoluble (IF), soluble (SF), and total (TF) dietary fiber were determined using a combination of enzymatic–gravimetric methods AOAC 993.19 and 991.42 [21]. Enzymatic digestion with α-amylase, protease, and amyloglucosidase (Sigma-Aldrich, St. Louis, MO, USA) was performed in quadruplicate. First, it was filtered for IF, and the liquid was reserved for 24 h ethanol precipitation, after which it was filtered for SF, and the TF was calculated by summing the insoluble and soluble fibers.
2.3.2. Soluble Sugars: Ciceritol and α-Galactosides
Soluble sugars were determined according to Pedrosa et al. [22] using the Beckman HPLC System Gold (Los Angeles, CA, USA) equipped with a refractive index detector. A Spherisorb-5-NH2 column (250 × 4.6 mm i.d., Waters, Milford, MA, USA), equilibrated with acetonitrile/water 60:40 (v/v), was used at a flow rate of 1 mL/min. Extraction was performed in aqueous ethanol, and the resulting supernatant was filtered through Sep-Pak C18 columns (500 mg, Waters, Milford, MA, USA). The extracts were evaporated (UNIVAPO 100H). The residue was redissolved in milli-Q water and filtered into glass microvials using 25 mm Puradisc syringe filters. Then, 20 µL aliquots were injected into the HPLC. Sugars were quantified by comparing them to a calibration curve for ciceritol, raffinose, and stachyose (Sigma, St. Louis, MO, USA). A linear response was observed in the range of 0–500 mg/100 mL, with an R2 of 0.99. The tests were performed in triplicate.
2.3.3. Arabinoxylans
Total arabinoxylans (AX-T) and extractable arabinoxylans (AX-E) were determined according to Douglas [23]. AX-T and AX-E were extracted from a suspension under constant stirring and from the supernatant after centrifugation, respectively. The dye reagent was added to the extractions and the absorbances (λ of 448 and 508 nm) were measured in a plate reader (BioTek Synergy HTX, Santa Clara, CA, USA). The xylose calibration curve was fitted to a third-degree polynomial equation (R2 = 0.99). All analyses were performed in triplicate.
2.3.4. Total Available Carbohydrates
Total available carbohydrates were determined according to Osborne et al. [24]. The samples were hydrolyzed for 24 h in distilled water plus 52% perchloric acid. The hydrolyzed liquid was diluted with distilled water to 250 mL, from which 10 mL was removed and the volume was adjusted to 100 mL. Then, 1 mL of the dissolved solution was removed, and 5 mL of anthrone reagent was added for the reaction at 100 °C for 12 min. Subsequently, the absorbance reading was performed at a wavelength of 630 nm in a plate reader (Agilant BioTek Synergy HTX, Santa Clara, CA, USA). The calculations were made based on a concentration of 200 µg/mL of glucose.
2.3.5. Total and Resistant Starch
Total starch was determined by spectrophotometry according to Arribas et al. [22], using a Megazyme kit total starch K-TSTA-100A kit (Wicklow, Ireland) based on a combination of the AOAC method 996.11 and AACC Method 76-13.01. Likewise, the resistant starch content was determined using the Megazyme kit based on the AOAC 2002.02/32-40.01 method. For total starch, the sample was treated with ethanol, potassium hydroxide, and sodium acetate buffer, and α-amylase and amyloglucosidase were added, then incubated in a water bath at 50 °C for 45 min. For resistant starch, the sample was treated with pancreatic α-amylase containing amyloglucosidase and stirred for 16 h in a water bath at 37 °C. The pellet obtained by centrifugation was washed with ethanol. The residue was redissolved with potassium hydroxide, sodium acetate buffer, and amyloglucosidase, and heated in a water bath at 50 °C for 30 min. After the sample treatments for total starch and resistant starch, common procedures were followed. One mL of the final solution was removed and dissolved in a 1/10 ratio with distilled water. A 0.1 mL aliquot was then removed for the final glucose oxidase–peroxidase reaction, and incubated at 50 °C for 50 min. Finally, the absorbance of both total and resistant starch was measured at λ of 510 nm using an Eppendorf BioSpectrometer basic spectrophotometer. All tests were performed in triplicate.
2.3.6. Amylose and Amylopectin
The amylose content was determined according to Guzmán et al. [25]. The sample was mixed with ethanol and sodium hydroxide and stirred for 10 min. It was then diluted to 100 mL with distilled water. Furthermore, 5 mL of the resulting solution was extracted and combined with 2 mL of acetic acid and 2 mL of iodine solution. The solution was adjusted to 100 mL with distilled water and allowed to stand for 10 min. After this time, the absorbance was read at λ of 620 and 510 nm using a spectrophotometer (Thomas Science, Eppendorf BioSpectrometer Basic, Swedesboro, NJ, USA). The calibration curve was prepared from 66% (w/w) amylose with a fixed concentration of iodine solution and different concentrations of acetic acid. The percentage of amylose was calculated based on the amylose calibration curve, and the percentage of amylopectin was the difference (100-amylose).
2.4. Spectrophotometric Analysis of Total Phenolic Compounds, Total Flavonols, and Total Monomeric Anthocyanins
Extraction was performed according to Drevelegka and Goula [26]. The sample was mixed with methanol/water (1:1, v/v; pH 2) and stirred for 10 min, then centrifuged, and the supernatant removed (methanolic extract). The methanol extraction residue was mixed with acetone/water (70:30 v/v), and the methanolic extraction procedures were repeated until the supernatant was obtained (acetonic extract). Both extractions were stored at −40 °C. Total phenolic compounds were determined according to Macavilca and Condezo-Hoyos [27]. The methanolic or acetonic extracts were mixed with Folin–Ciocalteu, and sodium hydroxide (0.3 M) was added for the reaction for 15 min. Next, the absorbance readings were carried out at λ of 760 nm. The results were obtained using a standard gallic acid standard curve (R2 of 0.99). Total flavonols were determined according to Abderrahim et al. [28]. The methanolic or acetonic extracts were mixed with 2-aminoethyl-diphenylborinate (2-APB), and the absorbance was read at λ of 405 nm. The results were obtained using a quercetin standard curve (R2 of 0.9988). Anthocyanins were determined by the method of Lee et al. [29]. The methanolic or acetonic extract was mixed with a pH 1 buffer solution. In parallel, the methanolic or acetonic extract was mixed with a pH 4.5 buffer. The absorbance were readings at λ of 520 and 700 nm. The final results of total phenolic compounds, flavonols and anthocyanins were obtained by adding the results of the methanolic and acetone extracts. All absorbance readings were taken using a Synergy HTX Multi-Mode microplate reader. In all cases, the tests were performed in triplicate.
2.5. Statistical Analysis
Statgraphics Plus 5.1 software was used to analyze the data. An analysis of variance was applied using Duncan’s test to evaluate the effect of the extrusion process by comparing differences between samples (extruded and unprocessed), considering the p value (p < 0.05).
3. Results and Discussion
3.1. Hydrocarbon Fraction
3.1.1. Dietary Fiber
The control flour had a TF content of 13.25 g/100 g with an IF:SF ratio of 81:18. The extruded samples CS-Sk-E3 and CS-Sk-E4 had the highest amounts of TF of 25.79 g/100 g and 24.44 g/100 g, respectively, mainly from the IF content of 23.91 g/100 g and 22.39 g/100 g, respectively, which shows the significant contribution of fermented grape skins (20% inclusion in both cases) to the TF content (Table 2).

Table 2.
Insoluble fiber, soluble fiber, and total fiber content in extruded products based on corn/lentil flour (70:30) formulations fortified with different winemaking by-products.
These results indicate that the different formulations have a significant effect (p < 0.05) on the dietary fiber content. The Cabernet Sauvignon fermented grape skin had the highest contribution to the TF content, and both soluble and insoluble fractions compared to its raw counterparts. This is likely due to water evaporation inside the flour, induced by the exposure of the material to high-saturated steam pressure, known as “instant controlled pressure drop”. Other studies have also reported an increase in total fiber after processing [6] and also the predominance of 20% grape skin in the flour. Previous studies have reported that Cabernet Sauvignon grapes have a high content of IF, between 52.4 and 53.2 g/100 g [10,30].
Regarding products containing fermented Cabernet Sauvignon seed, a significant difference (p < 0.05) was also observed in the TF and IF content of the different formulations with respect to the control. The TF content ranged from 16.59 to 31.44 g TF/100 g in samples CS-Sd-E2 and CS-Sd-E4, respectively, while the IF content varied from 13.98 to 27.85 g IF/100 g in samples CS-Sd-E2 and CS-Sd-E3, respectively. The FS content values ranged between 2.58 and 3.98 g FS/100 g for samples CS-Sd-E1 and CS-Sd-E4, respectively. There was a statistical difference observed in the CS-Sd-E3 and CS-Sd-E4 formulations, but not in the CS-Sd-E1 and CS-Sd-E2 formulations possibly due to the higher content of winemaking by-products.
In extruded products made with non-fermented Chardonnay grape seed, the IF and TF levels significantly differed (p < 0.05) between the control extruded flour and the various extruded formulations. Products containing 20% of unfermented grape seed showed statistically similar results. Regarding the SF, most formulations exhibited no statistical difference, except for the Ch-Sd-E4 formulation, compared to the control. The content of TF values in the samples formulated with Ch-Sd ranged from 15.50 and 24.58 g TF/100 g. The extrusion process led to a redistribution of insoluble and soluble fractions due to modifications in the physical–chemical and biochemical properties of the fiber. This is attributed to the degradation of macromolecules, such as glycosidic bonds, leading to the conversion of IF to SF [31]. Moreover, Guo et al. [32] have previously indicated that a lower humidity in samples increases pressure, friction force, and shear force in the screw chamber, which promotes the redistribution of IF to SF. Furthermore, a distinct trend in the amount of SF was observed in formulations with 5% and 20% Hylon® V, with higher values in formulations with 20% Hylon® V. These variations suggest that higher amounts of Hylon® V (20%) result in increased SF levels, while a lower proportion of Hylon® V may cause slight decreases or minimal changes in SF content, possibly due to the low capacity of amylose to form inclusion complexes for dietary fiber when amylose is present in lower amounts [33]. Extrusion conditions, particularly feed moisture followed by barrel temperature, have significant effects on promoting the conversion of IF to SF. The interactive effects of screw speed and total barrel humidity further intensify the significance of these conditions [34].
Among different formulations, those containing 20% of fermented Cabernet Sauvignon seed had the highest fiber content, while adding Hylon® V starch did not have a significant effect on the fiber content.
All the extruded products in the study contained more than 3 g of fiber per 100 g, and can thus be considered a source of fiber based on current European Regulation [35]. Extruded flours containing 20% of fermented Cabernet Sauvignon seed had a fiber content exceeding 25 g/100 g, regardless of the Hylon® V percentage. Thus, consuming 100 g of this product would fulfil the daily fiber requirement recommended by the European Food Safety Authority [36].
3.1.2. α-Galactosides, Ciceritol, and Arabinoxylans
The different extruded products considered in this study had a lower total carbohydrate content than the control product. The decrease in TAC content led to a beneficial effect on the reduction of the caloric value of the extruded flours.
Stachyose was the most predominant α-galactoside oligosaccharide, followed by ciceritol and a smaller amount of raffinose. Despite these compounds being reported to have some prebiotic benefits, these oligosaccharides play an important role on product acceptability because these sugars are associated with undesirable flatulence factor, while reported in the literature. The extrusion process is a high temperature and pressure cooking technology that reduce the content of oligosaccharides by partially hydrolyzing them into monosaccharides due to the application of heat, moisture, and mechanical force. The sample with the lowest oligosaccharide content in this study was sample Ch-Sd-E4, with the highest content of winemaking by-products.
The ciceritol content in the different extruded products formulated with fermented Cabernet Sauvignon skin/seed showed significant (p < 0.05) differences between the formulations, except for samples Ch-Sd-E2 and Ch-Sd-E3 formulated with Chardonnay seeds, which showed no significant variation (Table 3).

Table 3.
α-galactosides, ciceritol, and arabinoxylans (extractable and total) in extruded products based on corn/lentil flours (70:30) fortified with different winemaking by-products.
Stachyose was the main α-galactoside, with higher proportions than raffinose, in which the formulations with fermented Cabernet Sauvignon skin/seed were significantly (p < 0.05) different except for the formulations: CS-Sk-E2 and CS-Sk-E3/CS-Sd-E2 and CS-Sd-E3. On the other hand, the extruded products formulated with non-fermented Chardonnay seeds did not show a significant (p < 0.05) difference in most formulations for raffinose. However, these samples present a significant (p < 0.05) difference for stachyose content among all formulations. The soluble sugar contents in the different samples fortified with winemaking by-products were significantly (p < 0.05) lower compared to their corresponding controls. Moreover, the formulations with the smallest amount of the corn/lentil mixture (53.75%) had the lowest values of soluble sugars compared to the rest of the formulations (Table 3). This indicates that the reduction in the content of different soluble sugars is likely associated with the corn/lentil mixture, which contain more soluble sugars than those formulations containing winemaking by-products (fermented Cabernet Sauvignon skin and seed, and unfermented Chardonnay seed).
The observed decrease in soluble sugars may be further influenced by hydrothermal processing, resulting in the formation of simple disaccharides and monosaccharides [37]. Additionally, the decrease in the content of soluble sugars could be also associated with the Maillard reaction resulting from the breakdown of starch and other polysaccharides into lower molecular weight components, such as glucose and fructose, during the extrusion process [17].
In this study, a significant (p < 0.05) decrease in the content of extractable arabinoxylans in the different formulations with fermented Cabernet Sauvignon skin/seed or non-fermented Chardonnay seed compared to the control was observed. Formulations CS-Sk-E1, CS-Sd-E1, and Ch-Sd-E1 showed the highest proportion of extractable arabinoxylans, while formulations CS-Sk-E4, CS-Sd-E4, and Ch-Sd-E4 had the lowest proportion. However, the differences were not significant in some comparisons between the same formulations. Additionally, Ch-Sd-E4 showed the maximum values of total arabinoxylans due to the high percentage of Hylon® V (20%) present in formulation (Table 3). The samples with the highest content of Hylon® V (E2 and E4) presented the highest concentration in total arabinoxylans. This indicates that Hylon® V is the ingredient that contributed the most to the total arabinoxylan content, regardless of the specific winemaking by-product added (skin or seed) or the grape variety considered (Cabernet Sauvignon or Chardonnay).
The findings of this study align with other cereal and legume formulations as those based on wheat and rye, lentil, and chickpea and rice fortified with passion fruit [12,38]. In all instances, the different levels of arabinoxylans obtained are likely due to the increased cell porosity caused by the high temperature and mechanical shear during extrusion, leading to the extractability of arabinoxylans [38,39,40].
3.1.3. Total Available Carbohydrates (TAC), and Total and Resistant Starch
In the samples containing fermented Cabernet Sauvignon skin, the TAC content in the extruded products varied between 57.65 and 68.89 g glucose/100 g in formulations CS-Sk-E3 and CS-Sk-E2, respectively. The addition of Hylon® V had more impact on TAC content than grape skins. The extrusion process did not result in a significant difference on TAC content between the products formulated with grape skin and Hylon® V. This is due to carbohydrate fermentation by yeast during the winemaking process and Hylon® V mainly serves as a source of type 2 resistant starch [41].
The contribution to TAC from grape seed was not significant. It was observed that the TAC amount increased when samples contained 20% of Hylon® V compared to those formulated with 5% of Hylon® V.
This behavior may be influenced by the amount of amylose present in Hylon® V (55%), as indicated by previous studies, which showed a correlation between lower amounts of resistant starch and starches with more than 70% amylose [42]. The presence of TAC in all formulations could be attributed to the Chardonnay seed in its non-fermented state, as the thermomechanical effects of extrusion could contribute to the TAC content. According to Corbin et al. [43], the mass balance of carbohydrates in unfermented Cabernet Sauvignon winemaking by-products reached, between soluble carbohydrates (20.7% w/w) and other insoluble components (63.9% w/w), a total of 84.6% w/w and total carbohydrates 31.4% w/w.
The resistant starch fraction was the lowest one determined in the samples under study. Nevertheless, the resistant starch content in all the experimental samples was higher than in the control sample. Moreover, as observed from Table 4, those samples with the highest content of Hylon® V (E2 and E4) also presented the highest content of resistant starch. In general, food processing involving heat and moisture, as extrusion, largely destroys resistant starch type 1 and 2 (physically inaccessible starch and native starch, respectively), forming resistant/retrograded starch type 3. This type of starch can potentially be used as food ingredient, given that it is generally stable under heat processes and remains stable after most food processes [44].

Table 4.
Total available carbohydrates, total starch, and resistant starch in extruded formulations based on corn/lentil flours (70:30) fortified with different winemaking by-products.
The result of the analysis of the total starch content of extruded products containing fermented Cabernet Sauvignon skin, their content was found in the low range of 43.81 to 59.31 g/100 g for samples CS-Sk-E3 and CS-Sk-E2, respectively, when 20% of fermented grape skin. It is desirable that formulations include an adequate starch (amylose and amylopectin) content in order to obtain a crispy texture and good sensory attributes in the extruded products [45,46,47,48,49]. The percentage of amylose content in the extruded products formulated with fermented CS skin and Hylon® V ranged from 22.34 to 26.38% (CS-Sk-E1 and CS-Sk-E2, respectively), which was higher than in the extruded control sample that contained 20.32% amylose. Moreover, in samples with a higher proportion of fermented CS skin (CS-Sk-E3 and CS-Sk-E4), the amylose content was lower compared that the other samples containing less content of fermented CS skin in their formulations. Sample CS-Sk-E4 with a higher proportion of Hylon® V presented low values of amylose due to the lower content of corn/lentil mixture in the formulations.
The percentage of amylopectin content ranged from 73.62 to 77.66% (CS-Sk-E2 and CS-Sk-E1, respectively). These values were significantly (p < 0.05) lower than the value of the control sample (79.68%). This indicates that content Hylon® V had a lower influence on amylopectin content, since Hylon® V produced a significant increase in amylose (56.93%). and, as a counterpart, amylopectin decreased (43.07%). Other authors have also observed the effect of Hylon® V on amylose and amylopectin content [47,48]. In order to achieve extruded products with satisfactory expansion and texture, a desirable amylose-to-amylopectin ratio is 1:3 or 1:4 [45]. The analyzed formulations have appropriate technological properties with an amylose-to-amylopectin ratio of 1:3.2, since amylose primarily affects mechanical properties, solubility, cooking time, oxygen permeability, water retention capacity, gelatinization, viscosity, and the shape and volume of the pores [49,50]. Therefore, this could suggest that amylose forms inclusion complexes with hydrophobic molecules, making it resistant to enzymatic hydrolysis and allowing it to act as dietary fiber [33].
Extruded products fortified with fermented CS seed, presented total starch values that varied between 41.83 and 56.21 g/100 g (samples CS-Sk-E3 and CS-Sk-E2, respectively). Hylon® V with a high content of total starch (85.75%) and the corn–lentil proportion maintain the total starch balance for the extrusion process. However, a higher proportion of fermented grape seed would affect the total starch balance. In contrast to these findings, other authors have indicated that the reduction in total starch content is the result of adding certain legumes, such as beans and carob pods. The variations in total starch are because Hylon® V contributes 56.93% amylose and amylopectin decreases by 43.07%, as the addition of fermented CS seed negatively impacts the amylose-to-amylopectin ratio, which is a technological factor for successful extrusion process [45,46].
Regarding extruded samples fortified with non-fermented Chardonnay seed (Ch-Sd), values followed a similar trend. However, the total starch of samples Ch-Sd-E1 and Ch-Sd-E2 presented statistically non-significant values compared to the control. (Table 4), while the amylose and amylopectin components varied significantly (p < 0.05) in most formulations, except for the Ch-Sd-E3 formulation in comparison to their, respective, controls. In contrast, the values for samples Ch-Sd-E3 and Ch-Sd-E4 were significantly (p < 0.05) lower compared to the control (Table 4). The amylose and amylopectin components varied significantly (p < 0.05) in most of the samples, except for sample Ch-Sd-E3, with respect to their controls. The amylopectin values are linked to the amylose results because they have been calculated by subtracting the amylose percentage from 100. This explains why both components exhibit similar statistical behavior. Some researchers have reported that adequate amylose-to-amylopectin ratio and the proportion of the processed material are important to obtain extrudates with crispy textures and adequate sensory attributes [45,46]. Amylose contributes to a rough layered gel structure, while amylopectin promotes the formation of an anisotropic fibrous structure in the extrudate [51].
The amount of resistant starch in samples with fermented CS skin ranged from 0.46 to 1.38 g/100 g (CS-Sk-E3 and CS-Sk-E2, respectively) after extrusion. The control sample had a significantly lower amount of resistant starch compared to the samples containing CS skin, except the CS-Sk-E3 sample. These results are in line with other studies on lentil samples [52] where extrusion–cooking led to the disintegration of the starch structure due to high shear forces and temperatures of the process, promoting an almost complete gelatinization of the starch, which is detrimental to the stability of resistant starch. Other authors have reported the absence of resistant starch after the extrusion process of samples formulated with a mixture of rice, carob and beans. On the other hand, extrusion can break down resistant starch type 1 and 2 (physically trapped inaccessible starch and native starch, respectively) and create resistant starch type 3 (retrograded starch). This suggests the presence of resistant starch type 3 at a consequence of the extrusion process, particularly in samples with the highest amount of Hylon® V (20%) [44]. In samples with fermented CS seed, the resistant starch content in the extruded samples varied from 1.11 to 2.70 g/100 g in formulations CS-Sd-E3 and CS-Sd-E4, respectively. All the samples contained a higher (p < 0.05) resistant starch content compared to the control. These results are supported by the presence of amylose-rich Hylon® V, as amylose is reported to be associated with increased resistant starch [42]. Regarding the extruded products formulated with unfermented Ch seed, the resistant starch content fluctuated between 1.34 and 1.81 g/100 g in samples Ch-Sd-E1 and Ch-Sd-E2, respectively (Table 4). The trend of resistant starch content of these samples was similar to the samples previously mentioned, highlighting the presence of Hylon® V starch as the additive that promotes the increase level of resistant starch.
As a conclusion, in terms of total starch content, samples with code 2 (5% of by-product and 20% of Hylon® V) and, specifically, sample CS-Sk-E2, stand out among other samples containing winemaking by-products. These results suggest that the based ingredient (corn and lentil) was the main factor contributing to the starch content. In the control extruded sample (corn and lentil, 70:30), the amylose-to-amylopectin ratio was 20:80, highlighting corn’s significant starch contribution to this starch fractions. This ratio remained similar in the various evaluated extruded samples. The samples with code E2 (5% of by-product and 20% of Hylon® V) again stood out, with an amylose-to-amylopectin ratio of 27:73, regardless of the winemaking by-product added. This means that a higher amylopectin content was influenced by the higher percentage of the corn and lentil flour in the formulated samples under study.
3.2. Total Phenolics, Flavonols, and Anthocyanins
The total phenolic content was higher in all the experimental extruded samples, compared to the unprocessed sample. Moreover, the formulations with 20% of winemaking by-products (codes E3 and E4) had the highest content of total phenolics. The samples containing unfermented Chardonnay seed stood out, with values six times higher of total phenolics compared to the extruded control sample. This means that the fortification with winemaking by-products had a positive effect on the functional characteristics of the developed extruded samples, due to their improved quality.
The total phenolic amount in extruded products containing fermented CS skin ranged from 368.04 to 767.72 mg GAE/100 g, with samples CS-Sk-E1 and CS-Sk-E4 as the lowest and highest values, respectively (Table 5).

Table 5.
Total phenolics, total flavonols, and total anthocyanins in extruded products based on corn/lentil flours (70:30) fortified with different winemaking by-products [18].
In samples with fermented CS seed, the total phenolics amount in the extruded samples ranged from 340.29 to 732.36 mg GAE/100 g, with sample CS-Sd-E2 with the lowest, and sample CS-Sd-E3 the highest value. Similarly, in samples with unfermented Ch seed, the total phenolics amount in the extruded samples ranged from 550.91 to 1200.10 mg GAE/100 g in samples Ch-Sd-E2 (68.75% of corn and lentil, 5% of fermented grape seed and 20% of Hylon® V) and Ch-Sd-E3 (68.75% of corn and lentil, 20% of fermented grape seed and 5% of Hylon® V), respectively. As expected, it was observed that the extruded products with a higher proportion (20%) of winemaking by-products had higher concentrations in total phenolics compared to those with a lower proportion (5%). This result indicates that the fortification of formulations with the highest content of winemaking by-products have a positive effect of a higher concentrations in total phenolics in the extrudates. Other studies on legume-based products have reported that high extrusion temperatures can decrease the content and stability of these compounds [52], whereas Brennan et al. [53] and Leonard et al. [54] have indicated that the effect of extrusion on the total phenol content does not follow a constant trend, as the difference in the intrinsic nature of the raw materials and the extrusion process conditions, along with the characteristics of the food matrix.
Regarding total flavonol content, the extruded samples made with unfermented Chardonnay seed had the highest flavonol content, especially those with 5% and 20% of winemaking by-products. This indicates that the antioxidant potential of the yellow-pigmented Chardonnay variety is higher than that of the red-pigmented Cabernet Sauvignon varieties. The total flavonol content was also notably high in all samples fortified with skins and seeds of Cabernet Sauvignon.
The total flavonol values in samples containing fermented CS skin ranged from 34.07 and 57.59 mg EQ/100 g. These values corresponded to the CS-Sk-E2 and CS-Sk-E4 samples, respectively. Similarly, samples containing fermented CS seed, had values ranging from 31.83 to 44.61 mg QE/100 g in samples CS-Sd-E2 and CS-Sd-E3, respectively. In products containing unfermented Chardonnay seed, the total flavonol content varied between 49.63 to 63.98 mg QE/100 g in samples Ch-Sd-E2 and Ch-Sd-E4, respectively. It is noteworthy that samples with 5% of winemaking by-products tended to have the lowest total flavonol values, while samples with 20% of winemaking by-products had the highest values (Table 5).
In the extruded products containing fermented CS skin, the total anthocyanin content ranged from 0.17 mg cyanidin-3-glucoside/100 g (CS-Sd-E2) to 0.64 mg cyanidin-3-glucoside/100 g (CS-Sd-E3) (Table 5). These values were significantly higher than the control sample. For samples formulated with fermented CS seed, the total anthocyanin content varied from 0.08 mg cyanidin-3-glucoside/100 g (CS-Sd-E1 and CS-Sd-E2) to 0.59 mg cyanidin-3-glucoside/100 g (CS-Sd-E4). Only the formulations with a higher proportion of fermented CS seed (20%) were significantly (p < 0.05) greater than the control. In the samples formulated with non-fermented Chardonnay seed, the total anthocyanin content ranged from 0.17 mg cyanidin-3-glucoside/100 g to 0.63 mg cyanidin-3-glucoside/100 g (Ch-Sd-E1 and Ch-Sd-E4, respectively), with all samples containing winemaking by-products being significantly higher than the control. As it was observed from the results of the previous phenolic components analyzed, the samples containing 5% of winemaking by-products had significantly lower total anthocyanin values compared to those containing 20% of winemaking by-products. This indicates that the final extruded product greatly benefitted by maintaining a much higher content of total anthocyanin in formulation containing 20% of winemaking by-products. Neder-Suárez et al. [55] have previously reported that winemaking by-products could potentially increase the amount of anthocyanins in the final product. Extrusion is a high-temperature but short-time processing, which minimizes excessive thermal damage to labile antioxidant compounds such as anthocyanins.
4. Conclusions
The fortification of corn–lentil-based flours with 20% of winemaking by-products increased the total fiber content in the developed extruded samples. All the extruded products in the study contained more than 3 g of fiber per 100 g, meeting the requirements of current European Regulation [35] as a source of fiber, making them valuable as functional food and/or food ingredient. Conversely, the total carbohydrate content in the extruded samples decreased, which has a beneficial effect on reducing the caloric value of the product. Also, the findings show that among the experimental formulations containing various concentrations of winemaking by-products, those with the highest concentration (20%) presented the greatest amounts of functional compounds: total arabinoxylans, resistant starch, total phenols, total flavonols, and total anthocyanins, leading to a positive impact on the functional quality of the extruded samples due to their potential antioxidant capacity. Additionally, those formulations displayed the lowest content of raffinose and stachyose, which are compounds considered undesirable due to their flatulence effect. The results of this study indicate that extrusion is an effective method for adding value to underutilized commodities, such as winemaking by-products. To our knowledge, this is the first instance of using winemaking by-products as a functional ingredient in extruded products. Based on the results of this study, a future sensory evaluation study will be conducted on the extruded products with the highest amount of winemaking by-products of 20%.
Author Contributions
Conceptualization, M.C., P.M. and J.d.J.B.; methodology, M.C.-S., M.C., P.M., M.M.P. and P.A.; validation, M.C., P.M. and M.M.P.; investigation, M.C.-S. and C.A.; resources, J.d.J.B., J.P. and M.C.; data curation, M.C., P.M. and M.M.P.; formal analysis, P.A.; writing—original draft preparation, M.C.-S.; writing—review and editing, M.C., M.M.P., C.A., J.d.J.B. and J.P.; supervision, M.C. and P.M.; funding acquisition, M.C. and J.d.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by UCM-ALIMNOVA Research Group (grant no. GR29/20; GFRN-17/21; GRFN14-22) and Project USDA-ARS-WRRC, California, USA (ref. 2030-41000-510-001). Mario Cotapallapa-Sucapuca is grateful for his PhD grant PRONABEC (Peru) nº 307541.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Domínguez-Viera, M.E.; Van den Berg, M.; Donovan, J.; Pérez-Luna, M.E.; Ospina-Rojas, D.; Handgraaf, M. Demand for healthier and higher-priced processed foods in low-income communities: Experimental evidence from Mexico City. Food Qual. Prefer. 2022, 95, 104362. [Google Scholar] [CrossRef]
- Rosenthal, A.; Maciel Guedes, A.M.; Dos Santos, K.M.O.; Deliza, R. Healthy food innovation in sustainable food system 4.0: Integration of entrepreneurship, research, and education. Curr. Opin. Food Sci. 2021, 42, 215–223. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; Da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; Carvalho Junior, R.N.d. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recyc. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Sinrod, A.J.G.; Shah, I.M.; Surek, E.; Barile, D. Uncovering the promising role of grape pomace as a modulator of the gut microbiome: An in-depth review. Heliyon 2023, 9, e20499. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, L.; Ding, Y.; Zhang, L.; Gao, F.; Chen, N.; Yinghui, S.; Hua, L.; Wang, H. Natural and sustainable wine: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 8249–8260. [Google Scholar] [CrossRef]
- Martínez-Meza, Y.; Pérez-Jiménez, J.; Rocha-Guzmán, N.E.; Rodríguez-García, M.E.; Alonzo-Macías, M.; Reynoso-Camacho, R. Modification on the polyphenols and dietary fiber content of grape pomace by instant controlled pressure drop. Food Chem. 2021, 360, 130035. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gil-Muñoz, R. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Gómez-Plaza, E.; Terrier, N.; Doco, T.; Ros-García, J.M. The composition of cell walls from grape skin in Vitis vinifera intraspecific hybrids. J. Sci. Food Agric. 2017, 97, 4029–4035. [Google Scholar] [CrossRef]
- Imperio, D.; Bordiga, M.; Passos, C.P.; Silva, S.P.; Coimbra, M.A.; Travaglia, F.; Arlorio, M.; Coïsson, J.D.; Panza, L. Gentianose: Purification and structural determination of an unknown oligosaccharide in grape seeds. Food Chem. 2021, 344, 128588. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. Potential of Grape Byproducts as Functional Ingredients in Baked Goods and Pasta. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2473–2505. [Google Scholar] [CrossRef] [PubMed]
- Ciudad-Mulero, M.; Barros, L.; Fernandes, Â.; Berrios, J.J.; Cámara, M.; Morales, P.; Fernández-Ruiz, V.; Ferreira, I.C.F.R. Bioactive compounds and antioxidant capacity of extruded snack-type products developed from novel formulations of lentil and nutritional yeast flours. Food Funct. 2018, 9, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Cotacallapa Sucapuca, M.R.; Vega, E.N.; Maieves, H.A.; Berrios, J.d.J.; Morales, P.; Fernández-Ruiz, V.; Cámara, M. Extrusion process as an alternative to improve pulses products consumption. A review. Foods 2021, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Samaranayaka, A. Chapter 11—Lentil: Revival of Poor Man’s Meat. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 185–196. [Google Scholar]
- Cámara, M.; Fernández-Ruiz, V.; Morales, P.; Sánchez-Mata, M.C. Fiber compounds and human health. Curr. Pharm. Des. 2017, 23, 2835–2849. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios, J.D.J.; Powers, J.R.; Tang, J. Effect of extrusion on the antioxidant capacity and color attributes of expanded extrudates prepared from purple potato and yellow pea flour mixes. J. Food Sci. 2011, 76, C874–C883. [Google Scholar] [CrossRef]
- Berrios, J.D.J.; Morales, P.; Cámara, M.; Sánchez-Mata, M.C. Carbohydrate composition of raw and extruded pulse flours. Food Res. Int. 2010, 43, 531–536. [Google Scholar] [CrossRef]
- Cotacallapa-Sucapuca, M.R. Nutritional and Functional Quality of Extruded Flours Based on Corn and Lentil with Winemaking by-Products. Ph.D. Thesis, Complutense University of Madrid, Madrid, Spain, 2025. [Google Scholar]
- Dey, D.; Richter, J.K.; Ek, P.; Gu, B.-J.; Ganjyal, G.M. Utilization of Food Processing By-products in Extrusion Processing: A Review. Front. Sustain. Food Syst. 2021, 4, 603751. [Google Scholar] [CrossRef]
- Cotacallapa-Sucapuca, M.; Berrios, J.D.J.; Pan, J.; Arribas, C.; Pedrosa, M.M.; Morales, P.; Cámara, M. Winemaking by-products fortification of flour formulations based on corn and lentil. Int. J. Food Sci. Nutr. 2025, 76, 290–303. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Rockville, MD, USA, 2012. [Google Scholar]
- Arribas, C.; Cabellos, B.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. The effect of extrusion on the bioactive compounds and antioxidant capacity of novel gluten-free expanded products based on carob fruit, pea and rice blends. Innov. Food Sci. Emerg. 2019, 52, 100–107. [Google Scholar] [CrossRef]
- Douglas, S.G. A rapid method for the determination of pentosans in wheat flour. Food Chem. 1981, 7, 139–145. [Google Scholar] [CrossRef]
- Osborne, D.R.; Voogt, P.; Barrado, A.M. Análisis de los Nutrientes de los Alimentos; Editorial Acribia: Zaragoza, Spain, 1985. [Google Scholar]
- Guzmán, C.; Caballero, L.; Álvarez, J.B.; Yamamori, M. Amylose content and starch properties in emmer and durum wheat lines with different waxy proteins composition. J. Sci. Food Agric. 2011, 91, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process. Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Macavilca, E.A.; Condezo-Hoyos, L. Assessment of total antioxidant capacity of altiplano colored quinoa (Chenopodium quinoa willd) by visible and near-infrared diffuse reflectance spectroscopy and chemometrics. LWT 2020, 134, 110182. [Google Scholar] [CrossRef]
- Abderrahim, F.; Huanatico, E.; Segura, R.; Arribas, S.; Gonzalez, M.C.; Condezo-Hoyos, L. Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2015, 183, 83–90. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Chapter 6—Grapes. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: London, UK, 2019; pp. 133–163. [Google Scholar]
- Khanpit, V.V.; Tajane, S.P.; Mandavgane, S.A. Extrusion for soluble dietary fiber concentrate: Critical overview on effect of process parameters on physicochemical, nutritional, and biological properties. Food Rev. Int. 2023, 39, 6250–6271. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wu, B.; Wu, P.; Duan, Y.; Yang, Q.; Ma, H. Modification of garlic skin dietary fiber with twin-screw extrusion process and in vivo evaluation of Pb binding. Food Chem. 2018, 268, 550–557. [Google Scholar] [CrossRef]
- Di Marco, A.E.; Ixtaina, V.Y.; Tomás, M.C. Analytical and technological aspects of amylose inclusion complexes for potential applications in functional foods. Food Biosci. 2022, 47, 101625. [Google Scholar] [CrossRef]
- Zhong, L.; Fang, Z.; Wahlqvist, M.L.; Hodgson, J.M.; Johnson, S.K. Multi-response surface optimisation of extrusion cooking to increase soluble dietary fibre and polyphenols in lupin seed coat. LWT 2021, 140, 110767. [Google Scholar] [CrossRef]
- Regulation (EC), No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/eli/reg/2006/1924/oj/eng (accessed on 14 May 2025).
- EFSA. Dietary Reference Values for nutrients Summary report. Eur. Food Saf. Auth. 2017, 14, e15121E. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Guillamón, E.; Arribas, C. Autoclaved and Extruded Legumes as a Source of Bioactive Phytochemicals: A Review. Foods 2011, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Demuth, T.; Betschart, J.; Nyström, L. Structural modifications to water-soluble wheat bran arabinoxylan through milling and extrusion. Carbohydr. Polym. 2020, 240, 116328. [Google Scholar] [CrossRef]
- Fadel, A.; Ashworth, J.; Plunkett, A.; Mahmoud, A.M.; Ranneh, Y.; Li, W. Improving the extractability of arabinoxylans and the molecular weight of wheat endosperm using extrusion processing. J. Cereal Sci. 2018, 84, 55–61. [Google Scholar] [CrossRef]
- Nishitsuji, Y.; Whitney, K.; Nakamura, K.; Hayakawa, K.; Simsek, S. Analysis of molecular weight and structural changes in water-extractable arabinoxylans during the breadmaking process. Food Chem. 2022, 386, 132772. [Google Scholar] [CrossRef]
- Asp, N.G.; Van Amelsvoort, J.M.M.; Hautvast, J.G.A.J. Nutritional implications of resistant starch. Nutr. Res. Rev. 1996, 9, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, J.; Jin, Z.; Jiao, A. Development and characterization of resistant starch produced by an extrusion–debranching strategy with a high starch concentration. Food Hydrocoll. 2023, 136, 108276. [Google Scholar] [CrossRef]
- Corbin, K.R.; Hsieh, Y.S.Y.; Betts, N.S.; Byrt, C.S.; Henderson, M.; Stork, J.; DeBolt, S.; Fincher, G.B.; Burton, R.A. Grape marc as a source of carbohydrates for bioethanol: Chemical composition, pre-treatment and saccharification. Bioresour. Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef]
- Masatcioglu, T.M.; Sumer, Z.; Koksel, H. An innovative approach for significantly increasing enzyme resistant starch type 3 content in high amylose starches by using extrusion cooking. J. Cereal Sci. 2017, 74, 95–102. [Google Scholar] [CrossRef]
- Ek, P.; Gu, B.-J.; Saunders, S.R.; Huber, K.; Ganjyal, G.M. Exploration of physicochemical properties and molecular interactions between cellulose and high-amylose cornstarch during extrusion processing. Curr. Res. Food Sci. 2021, 4, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, L.; Mitrus, M.; Oniszczuk, T.; Rejak, A.; Wójtowicz, A. Extrusion-Cooking of Starch. In Advances in Agrophysical Research; Magnum Publishing LLC: New York, NY, USA, 2013; pp. 319–346. [Google Scholar]
- Błaszczak, W.; Fornal, J.; Kiseleva, V.I.; Yuryev, V.P.; Sergeev, A.I.; Sadowska, J. Effect of high pressure on thermal, structural and osmotic properties of waxy maize and Hylon VII starch blends. Carbohydr. Polym. 2007, 68, 387–396. [Google Scholar] [CrossRef]
- Khachatryan, G.; Krzeminska-Fiedorowicz, L.; Nowak, E.; Fiedorowicz, M. Molecular structure and physicochemical properties of Hylon V and Hylon VII starches illuminated with linearly polarised visible light. LWT 2014, 58, 256–262. [Google Scholar] [CrossRef]
- Khlestkin, V.K.; Peltek, S.E.; Kolchanov, N.A. Review of direct chemical and biochemical transformations of starch. Carbohydr. Polym. 2018, 181, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, S.; Zhang, B.; Qiao, D.; Pu, H.; Liu, S.; Li, L. Structural features and thermal property of propionylated starches with different amylose/amylopectin ratio. Int. J. Biol. Macromol. 2017, 97, 123–130. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Kaplan, D.L.; Wang, Q. Protein-amylose/amylopectin molecular interactions during high-moisture extruded texturization toward plant-based meat substitutes applications. Food Hydrocoll. 2022, 127, 107559. [Google Scholar] [CrossRef]
- Blandino, M.; Bresciani, A.; Locatelli, M.; Loscalzo, M.; Travaglia, F.; Vanara, F.; Marti, A. Pulse type and extrusion conditions affect phenolic profile and physical properties of extruded products. Food Chem. 2023, 403, 134369. [Google Scholar] [CrossRef]
- Brennan, C.; Brennan, M.; Derbyshire, E.; Tiwari, B.K. Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends Food Sci. Technol. 2011, 22, 570–575. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Xiong, Y.; Fang, Z. Extrusion improves the phenolic profile and biological activities of hempseed (Cannabis sativa L.) hull. Food Chem. 2021, 346, 128606. [Google Scholar] [CrossRef]
- Neder-Suárez, D.; Quintero-Ramos, A.; Meléndez-Pizarro, C.O.; de Jesús Zazueta-Morales, J.; Paraguay-Delgado, F.; Ruiz-Gutiérrez, M.G. Evaluation of the physicochemical properties of third-generation snacks made from blue corn, black beans, and sweet chard produced by extrusion. LWT 2021, 146, 111414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).