Abstract
Human cutaneous malignant melanoma is a skin cancer that develops from melanocytes, the cells specialised in the production of eu- and pheomelanin. A growing body of evidence suggests that pheomelanin in particular is involved in melanoma development. The aim of this study was to develop a new method enabling the determination of the pheomelanin in formalin-fixed paraffin-embedded (FFPE) tissue specimens of human nodular (NM) and superficial spreading (SSM) melanomas. The pheomelanin level was evaluated in a small amount of material obtained from FFPE melanoma samples (less than 1 mg), using a multi-step procedure of paraffin removal, tissue rehydration, and homogenisation, omitting the melanin isolation step. The obtained product was studied for pheomelanin content using the Py-GC/MS/MS method operating in a multiple reaction monitoring (MRM) mode. The results of our research confirmed the presence of all the pheomelanin markers in the FFPE human melanoma specimens and showed that the tissues analysed contained different amounts of pheomelanin isomers (5-S-cysteinylDOPA and 2-S-cysteinylDOPA). The developed Py-GC/MS/MS procedure enables sensitive quantification of pheomelanin in FFPE human melanoma samples, facilitating broader studies on its role in melanoma development and progression. This method opens new avenues for investigating pheomelanin’s involvement in melanoma malignancy.
1. Introduction
Human cutaneous malignant melanoma originates from epidermal melanocytes and has traditionally been histopathologically classified into four main subtypes: superficial spreading melanoma (SSM), nodular melanoma (NM), lentigo maligna melanoma (LMM), and acral lentiginous melanoma (ALM) [1,2]. SSM is the most common subtype, accounting for approximately 70% of cases, and typically presents as a slowly growing or changing flat patch. NM accounts for about 20% of all melanomas and presents as a rapidly enlarging nodule [3]. In the fifth edition of the WHO Classification of Skin Tumours, a more nuanced approach has been adopted, incorporating molecular characteristics and cumulative solar damage (CSD) into the classification of melanocytic tumours. For instance, melanomas arising on sun-exposed skin are further subdivided into low-CSD and high-CSD types, with SSM typically falling into the low-CSD category and LMM into the high-CSD category. Nodular melanoma (NM) is not limited to a single pathway and may arise in diverse molecular and etiological contexts. It is defined mainly by its vertical growth pattern and supported by molecular and anatomical assessment [4]. In this study, the traditional classification of four subtypes was used in order to maintain consistency with the previous histopathological classification of the examined material. The melanomas differ in their clinical signs, mechanism of oncogenesis, genetic changes, and prognosis. Nodular melanoma tends to grow faster vertically, penetrate deeper layers of the skin, and metastasise earlier to other parts of the body. Therefore, nodular melanoma is considered more aggressive, and its prognosis is worse compared to other melanoma subtypes [5,6]. LMM represents 5–10% of all melanomas and most often appears as large, flat macules on the face in older patients. ALM is the most common variant of melanoma in people with dark skin, mostly diagnosed on the palms and soles [3].
Melanocytes produce two chemically different melanins, black to brown eumelanin and yellow to reddish-brown pheomelanin. Eumelanins are heterogeneous polymers comprising 5,6-dihydroxyindole (DHI) and/or 5,6-dihydroxyindole-2-carboxylic acid (DHICA) units, found in greater amounts in people with dark skin and with dark hair. Pheomelanins, which consist of sulphur-containing benzothiazine and benzothiazole derivatives, predominate in people with light skin and with red hair [2,7]. Epidermal eumelanin confers photoprotection to the skin by functioning as a natural sunscreen, neutralising free radicals, and exhibiting antioxidant properties. In turn, pheomelanin is photosensitive, and when exposed to UV radiation, it may be a source of free radical production and may promote carcinogenesis. Therefore, the risk of skin cancer in people with light skin is higher than in people with dark skin [8,9].
Melanin is produced in the unique organelles of melanocytes, the melanosomes. The most potent stimulator of the production of melanin is ultraviolet B (UVB) radiation. Upon UV exposure, keratinocytes release signalling molecules like alpha-melanocyte-stimulating hormone (α-MSH), which binds to melanocortin 1 receptors (MC1R) on melanocytes and activates intracellular pathways that enhance melanin production. The newly produced melanin, primarily eumelanin, is then transferred into surrounding keratinocytes where it accumulates above the nucleus, absorbing further UV radiation and protecting DNA from mutagenic damage [10]. The substrate for the production of both eumelanin and pheomelanin is the cyclic amino acid tyrosine. In the presence of the enzyme tyrosinase, tyrosine is oxidised, resulting in the formation of dopaquinone. The amount of pheomelanin produced depends on the availability of cysteine [11,12]. In the presence of cysteine, dopaquinone reacts with it to form mainly 5-S-cysteinylDOPA (74%) and, in smaller amounts, the isomers 2-S-cysteinylDOPA (14%), 2,5-S,S′-dicysteinylDOPA (5%), and 6-S-cysteinylDOPA (~1%) [13]. 5-S-cysteinyl-DOPA (5-S-CD) levels have been shown to be significantly elevated in the serum and urine of patients with melanoma. Currently, 5-S-CD is a widely used biomarker for melanoma in Japan, and monitoring its levels in patients is particularly useful for predicting the presence of distant melanoma metastases and monitoring response to treatment [14,15,16,17]. Numerous studies indicate that pheomelanin contributes to melanoma development through mechanisms that may occur independently of ultraviolet (UV) radiation exposure. Salopek et al. [18] found that dysplastic melanocytic nevi (DNs) contain significantly higher amounts of pheomelanin than either common melanocytic nevi (CMNs) or normal skin. DNs are clinically relevant as their presence is an important risk factor for melanoma [19]. The presence of pheomelanin has also been demonstrated in amelanotic melanoma cells, considered to be the type of cancer with the highest clinical malignancy [20]. A significantly higher sulphur content (an indicator of pheomelanin) was found in melanosomes from DNs and melanoma cells in comparison to normal melanocytes and cells from banal nevi [21]. Mitra et al. [22] introduced a mostly mutated melanoma oncogene, BRAF V600E, into mice with different pigmentation phenotypes and found that the melanoma risk was higher in mice with red hair/fair skin. The red-haired mice had more oxidative DNA and lipid damage in comparison to the albino mice and the black coat colour mice and formed melanoma tumours spontaneously in the absence of UV exposure [22]. Morgan et al. [23] proposed two pathways of pheomelanin action that increase the risk of developing melanoma. The authors suggest that pheomelanin might generate ROS that lead indirectly or directly to DNA damage. In addition, pheomelanin synthesis requires high amounts of antioxidants, which in turn depletes the scavengers of ROS such as glutathione (GSH) and NADH, potentially exposing cells to free radical attack [2,23,24]. Additionally, pheomelanin may assist in the conversion of benzothiazine to benzothiazole, and benzothiazole is thought to promote greater GSH depletion and ROS formation under UV light than benzothiazine [25]. Moreover, Lembo et al. (2017) indicate that pheomelanin isolated from red hair and, to a lesser extent, eumelanin from black hair promoted the expression of pro-inflammatory interleukins and reduced cell viability in the in vitro model [26]. On the other hand, ROS generated by oxidative stress are necessary and essential to control melanoma cells in the early stages of cancer initiation and development. It has been suggested that metastatic cells have successfully undergone a metabolic change to survive oxidative stress. This is carried out by increasing the production of NADPH, which helps to regenerate GSH in order to cope with oxidative stress [27]. Taken together, these observations suggest that pheomelanin-induced oxidative stress may prevent metastasis in early-stage melanoma, but increased oxidative stress may promote metastasis in late-stage melanoma.
The aim of this study was to develop a new method enabling the determination of the pheomelanin in a small amount of material obtained from FFPE melanoma samples (less than 1 mg), bypassing the stage of melanin isolation, during which the greatest loss of material occurs. The usefulness of the developed method for examining the structure of pheomelanin was assessed in the two most common subtypes of melanoma, nodular (NM) and superficial spreading (SSM).
2. Materials and Methods
2.1. Material
Tumour specimens taken from primary or metastatic lesions were fixed in formalin and embedded in paraffin blocks (FFPE; formalin-fixed, paraffin-embedded tissues). The blocks were cut into thin sections using a microtome (Microm HM 355S, Thermo Fisher Scientific, Waltham, MA, USA). The neoplastic lesions were classified as nodular melanoma (NM) or superficial spreading melanoma (SSM). FFPE tissue specimens of six melanoma patients (characterised in Table 1) were obtained from the Department of Tumor Pathology at the Centre of Oncology-Maria Skłodowska-Curie Institute in Gliwice, Poland.

Table 1.
Characteristics of FFPE specimens.
2.2. Preparation of FFPE for Analysis
Microtome-cut sections of each FFPE tissue sample underwent deparaffinisation by heating in xylene at 70 °C for 20 min in an ultrasonic bath (F5 Minor, Decon Laboratories Ltd., Hove, East Sussex, UK). After this step, the samples were subjected to ultrasonic disintegration for 30 s using an ultrasonic homogeniser (Sonopuls mini 20, Bandelin Electronic, Berlin, Germany). Heating was then repeated in fresh xylene at 70 °C for 20 min in an ultrasonic bath to ensure complete wax removal. Following centrifugation at 21,000× g for 10 min (centrifuge 5804 R, Eppendorf AG, Hamburg Germany), the resulting tissue pellets were sequentially washed in an ultrasonic bath using isopropanol, then 96%, 70%, and 50% ethanol, and finally water, to effectively eliminate residual xylene. The obtained pellets were then dried in a thermostat (CLN 53 STD, POL-EKO, Wodzisław Śląski, Poland) at 37 °C for 48 h.
2.3. Reagents and Compounds
All the reagents employed in the study were of the highest available analytical grade. 3-Hydroxytyramine (dopamine hydrochloride), L-cysteine, and mushroom tyrosinase (EC 1.14.18.1; 6680 U/mg of solid) were obtained from Sigma-Aldrich (Poznan, Poland).
2.4. Preparation of Reference Synthetic Melanins
Standard melanin pigments were synthesised according to a previously published procedure with some modification [28]. Eumelanin (DA-melanin) was generated through spontaneous air-mediated polymerisation of dopamine, while pheomelanin (CDA-melanin) was produced by enzymatic oxidation of a dopamine and L-cysteine mixture (molar ratio 1:2), catalysed by 500 U/mL of mushroom tyrosinase. For both protocols, substrates were prepared in 50 mM sodium phosphate buffer (pH 6.8) to a final concentration of 0.5 mM. Reactions were carried out at 37 °C for 24 h with continuous stirring under light-protected conditions. The resulting pigments were harvested by centrifugation, extensively washed with deionised water to remove residual reactants, and then dried at 37 °C until a constant mass was reached.
Then, by mixing DA-melanin and CDA-melanin in a mortar, appropriate concentrations of pheomelanin were prepared: 0.05%; 0.1%; 0.25%; 0.5%; 1.0%; 2.5%; and 5.0% (w/w). A series of these synthetic mixtures was used to prepare a standard curve.
2.5. Py-GC/MS/MS Analysis
We designed a highly sensitive technique that facilitates the fast and accurate identification, measurement, and distinction of pheomelanin from other constituents within biological matrices. This approach employs gas chromatography coupled with tandem mass spectrometry (GC-MS/MS) to detect specific marker compounds produced during the pyrolytic decomposition of melanin. The markers indicative of pheomelanin are detected in the pyrolysate through the concurrent monitoring of distinct precursor/product ion transitions, using a triple quadrupole mass spectrometer operating in multiple reaction monitoring (MRM) mode [9,29]. The dried tissue pellet (0.2–0.5 mg) was placed into a pyrolysis device (Pyrojector II, SGE Analytical Science, Melbourne, Australia) and thermally degraded at 500 °C. Pyrolysis products were transferred with a stream of helium directly to the split/splitless injector of the Agilent Technologies 7890A gas chromatograph (Agilent Technologies, Palo Alto, CA, USA). The GC separations were performed on an Agilent HP-5ms capillary column (5% diphenyl, 95% dimethyl polysiloxane, 60 m × 0.32 mm i.d. × 0.5 µm film thickness) with helium as the carrier gas.
The gas chromatograph oven temperature was initially held at 35 °C for 5 min, then increased to 100 °C at a rate of 5 °C per minute, followed by a ramp to 260 °C at 10 °C per minute, where it was maintained for 16 min. The outlet of the GC column was directly interfaced with the electron ionisation (EI) source of an Agilent Technologies 7000 Triple Quadrupole GC/MS system (Agilent Technologies, Palo Alto, CA, USA). Operating temperatures were set at 240 °C for the GC/MS interface, 230 °C for the ion source, and 150 °C for both quadrupoles. Electron ionisation was carried out at 70 eV. The instrument was configured in multiple reaction monitoring (MRM) mode, utilising nitrogen as the collision gas and helium as the quenching agent. Data acquisition and spectral analysis were performed using Agilent’s MassHunter GC/MS Acquisition B.07.01 and Qualitative Analysis B.07.00 software. The most intense MRM transitions, from which the chromatograms were reconstructed and quantified, are shown in Table 2. Details on the thermal degradation products analysed in melanoma pyrolysates via tandem mass spectrometry, including the optimised MRM parameters and their corresponding Kovats retention indexes, are provided in Table S1 of the Supplementary Information.

Table 2.
The most intense MRM transitions used in the MS/MS analysis of the pheomelanin markers, according to which the chromatograms of the melanoma pyrolysate have been reconstructed and quantified.
3. Results and Discussion
Previously, Kurkiewicz et al. (2022) developed a method for isolating and purifying melanin from FFPE melanoma specimens [29]. Rehydration, homogenisation, and proteolytic enzyme digestion were used to purify the tested material from paraffin. The sample was then pyrolysed, and the obtained products were analysed by GC/MS/MS. This method works well if an appropriate amount of tissue material is available (30–50 mg). In the case of smaller neoplastic lesions (4–6 thickness on Breslow’s scale), the amount of material available for melanin testing is much smaller. The weight of FFPE obtained for testing ranged from 2 to 4 mg. After deparaffinisation, the amount of tissue material was only 0.2 to 0.5 mg. Thus, it was necessary to develop a new method taking into account the very small amounts of material available for testing.
Considering that protein pyrolysis does not produce pheomelanin markers, which has been confirmed in our many tests (data not published), we decided not to determine the pheomelanin level in melanin isolates but in whole cells of FFPE specimens. This approach seems to be the optimal solution. By omitting the pigment isolation step, which involves repeated use of digestion enzymes and washing the sample with buffers/water, we do not expose the melanin polymer to the inevitable losses of pheomelanin, which is particularly unstable under such conditions. Additionally, thanks to the proposed approach, the sample preparation time has been significantly shortened, and the consumption of reagents has been limited. However, it is necessary to remember that the final result does not refer to the pheomelanin content in melanin isolates, but in whole cells.
Using the Py-GC/MS/MS method operating in a multiple reaction monitoring (MRM) mode, pyrograms of the material from primary and metastatic lesions of NM and SSM were obtained, on which the presence of all pheomelanin markers was confirmed (Figure 1 and Figure S1). The most abundant compounds were thiazole, benzothiazole, hydroxybenzothiazole, and hydroxyethylenebenzothiazole. The remaining markers, such as dihydrobenzothiazole, methyldihydroxybenzothiazole, methylbenzothiazole, and ethylenedihydroxybenzothiazole, were also present, although in smaller amounts. A similar MRM pyrolytic profile in the distribution of pheomelanin markers was obtained for the synthetic pheomelanin standard at a concentration of 2.5% (Figure 1).
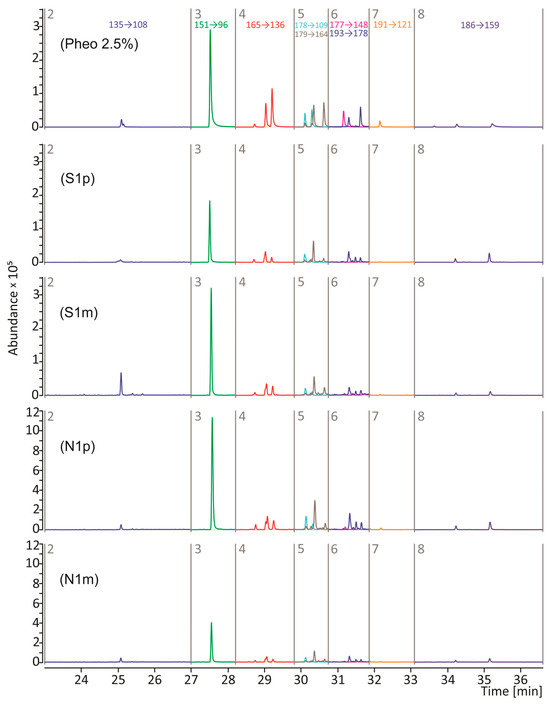
Figure 1.
Reconstructed MRM chromatograms of the pyrolysate of the 2.5% synthetic pheomelanin standard (Pheo 2.5%) and tissue pellets obtained from primary (p) and metastatic (m) lesions of nodular (N) and superficially spreading (S) melanoma. Peak designations are given in Table 2. Sample characteristics are shown in Table 1.
Using the standard curve method, the amount of pheomelanin in the tissue material was determined. The presence of pheomelanin was detected in all tumour tissue samples analysed after deparaffinisation. However, higher levels of pigment were detected in the nodular melanoma (NM) samples, both in primary and metastatic lesions, compared to the SSM samples (Table 3). The exception to this relationship was sample N3. The cells of this primary NM were characterised by a high mitotic potential (32 mitoses/mm2) in which cell division processes dominate melanogenesis. This is probably the reason for the very low concentration of pheomelanin (0.46%) in sample N3. The highest amount of pheomelanin was detected in a sample of NM from the primary site N1p (4.5%), while in the metastatic lesion (N1m) it was 1.5%. In the sample of superficial spreading melanoma that had metastasised, 0.6% pheomelanin was detected in the primary tumour (S1p) and 0.99% in the metastatic lesion (S1m). This observation may confirm the hypotheses that higher levels of pheomelanin can lead to excessive oxidative stress, which increases the ability of melanoma cells to spread, and that nodular melanoma has a greater ability to metastasise than superficial melanoma [2]. As suggested by other authors, a higher pheomelanin content may be associated with a worse prognosis for the patient [18,21]. The observed variation in pheomelanin levels between different melanoma types may be due to different melanogenesis pathways and their disruption during tumour transformation.

Table 3.
Percentage of pheomelanin in melanoma tissue after deparaffinisation.
In Figure 1 and Figure S1, three peaks were observed in the fourth time window. These are isomers of 2,3-dihydro-5H-1,4-benzothiazin-5-one (P4, P4′, and P4″), for which the 165 -> 136 transition is characteristic. As we reported earlier, the P4′isomer is formed as a result of pyrolytic decomposition of pheomelanin synthesised from 2-S-cysteinylDOPA (2-S-CD), while the P4″ isomer is characteristic of pheomelanin synthesised from 5-S-cysteinylDOPA (5-S-CD) [29]. The amount of the P4 isomer does not depend on the precursor from which the pheomelanin was synthesised. Oxidation, cyclisation, and dimerization of 5-S-CD and 2-S-CD lead to the formation of mixed-type melanin and trichochromes. A 5-S-CD/2-S-CD ratio of 5:1 or more has been determined in the urine and serum of melanoma patients [13,30], but these proportions do not necessarily have to be maintained in the naturally formed melanin polymer. Previous models of pheomelanogenesis assumed the participation of mainly the 5-S-CD isomer in the formation of this pigment, while the 2-S-CD isomer was treated as a secondary component with a smaller impact on the structure and function of the pheomelanin polymer [13]. Borges et al. (2001) showed that the pheomelanin component of human hair consists of 80% 2-S-cysteinylDOPA and 20% 5-S-cysteinyl-DOPA-derived units [31]. The properties, structure, and durability of pheomelanin depend largely on the proportion of CD isomers that form the pigment. Modulation of the intracellular content of 5-S-CD and 2-S-CD is most likely associated with structural remodelling and altered physicochemical features of the resulting pheomelanin polymer. The content of the P4′and P4″ isomers in pyrolysates of samples obtained from FFPE was different compared to the pyrolysate of the synthetic pheomelanin (Figure 1 and Figure S1). Figure 2 displays a comparison of relative content of P4′ and P4″ isomers in the tested samples. We found that the P4′ isomer (2-S-CD) predominates in nodular melanoma and superficial spreading melanoma that has metastasised. In melanoma cells, the melanogenesis can be dysregulated with intermediates of melanogenesis leaking outside melanosomes [32]. It is possible that the penetration of 5-S-CD into the blood, and subsequently into the urine of patients, increases the cellular pool of 2-S-CD and may influence the stability of the pigment within the tissue, thereby reducing the visibility of melanoma to the immune response. The change in the ratio of these two isomers in favour of 2-S-CD becomes more pronounced as the tumour progresses. This phenomenon may be attributable to an adaptive cellular mechanism that facilitates the survival and proliferation of melanoma cells within a microenvironment characterised by elevated levels of ROS. Analysing human hair, Greco et al. (2009) showed that pheomelanin subunits, the precursor of which was 2-S-CD, show higher resistance to ROS attack or degradation induced by UV radiation, unlike components derived from 5-S-CD, which are degraded in hair and are an indicator of pigment aging [30].
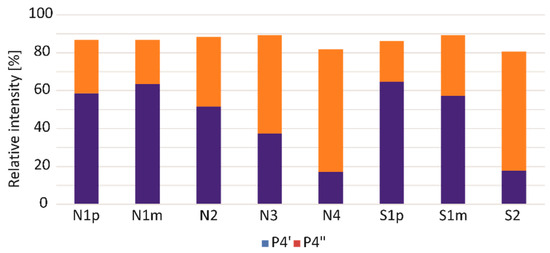
Figure 2.
Relative content of P4′ (2-S-CD) and P4″ (5-S-CD) isomers in pyrolysates of samples obtained from primary (p) and metastatic (m) lesions of nodular (N) and superficial (S) melanoma. The amount of P4′ and P4″ was estimated in relation to the sum of the areas of all the P4 isomers (100%). Sample characteristics are shown in Table 1.
The number of samples tested is not large enough to allow statistically significant conclusions to be drawn; so, the research should be continued on a larger, representative group of patients. Since there are very few data linking the amount of pheomelanin in neoplastic lesions with their malignancy and potential for metastasis, this research should be developed as it may contribute to a better understanding of the role of pheomelanin in melanomagenesis and, consequently, could be useful in developing new treatment strategies aimed at modulating melanogenesis pathways, e.g., through the inhibition of pheomelanin synthesis or the induction of melanogenesis to prevent melanoma progression [2]. The analysis of P4′ and P4″ isomers offers valuable information about the dynamic changes in pheomelanin. Their relative levels may reflect the metabolic profile of the melanoma cell and its responsiveness to environmental factors such as UV radiation and reactive oxygen species.
4. Conclusions
The developed procedure of paraffin removal, tissue rehydration, homogenisation, and analysis of whole tissue isolates using the Py-GC/MS/MS method allows the quantitative determination of pheomelanin from FFPE human melanoma specimens. Due to the small amount of tissue material provided for testing, the enzymatic digestion step used in the previous method was omitted. Thus, we were able to determine pheomelanin in smaller FFPE tissues from patients with various types of melanomas. The results of our research show that archival FFPE tissues contain different amounts of pheomelanin and pheomelanin isomers (5-S-cysteinylDOPA and 2-S-cysteinylDOPA) and can be used for broader studies aimed at explaining the role of pheomelanin in the development and progression of melanoma.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13061636/s1, Figure S1: Reconstructed MRM chromatogram of the pyrolysate of melanin obtained from FFPE of nodular melanomas from the primary sites (N2, N3 and N4) and of superficial spreading melanoma from the primary site (S2). Each peak corresponds to the most intense MRM transition. Peak designations are given in Table 2. Sample characteristics are shown in Table 1; Table S1: Kovats retention indexes and multiple reaction monitoring (MRM) settings for the pheomelanin markers in pyrolysate analyzed by GC/MS/MS.
Author Contributions
Conceptualisation, S.K., Ł.M., I.T., A.S.-W., D.L. and J.S.; methodology, S.K.; formal analysis, S.K.; investigation, S.K., Ł.M., I.T., A.S.-W., D.L. and J.S.; writing—original draft, S.K., Ł.M. and I.T.; writing—review and editing, Ł.M., I.T., A.S.-W., D.L. and J.S.; visualisation, S.K.; supervision, J.S.; project administration, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Medical University of Silesia in Katowice, Poland (grant no. PCN-1-020/K/2/F).
Institutional Review Board Statement
The study was approved by the Bioethical Committee of the Centre of Oncology-Maria Skłodowska-Curie Institute in Gliwice, Poland (approval code: KB/430-50/19) on [14 May 2019].
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors have no potential conflicts of interest to declare.
References
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef] [PubMed]
- Saud, A.; Sagineedu, S.; Ng, H.-S.; Stanslas, J.; Lim, J. Melanoma Metastasis: What Role Does Melanin Play? (Review). Oncol. Rep. 2022, 48, 217. [Google Scholar] [CrossRef] [PubMed]
- El Sharouni, M.-A.; Van Diest, P.J.; Witkamp, A.J.; Sigurdsson, V.; Van Gils, C.H. Subtyping Cutaneous Melanoma Matters. JNCI Cancer Spectr. 2020, 4, pkaa097. [Google Scholar] [CrossRef] [PubMed]
- Fortarezza, F.; Cazzato, G.; Ingravallo, G.; Dei Tos, A.P. The 2023 WHO Updates on Skin Tumors: Advances since the 2018 Edition. Pathologica 2024, 116, 193–206. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Szumera-Ciećkiewicz, A.; Nasierowska-Guttmejer, A. New Pathomorphological Classification of Melanomas. Nowotw. J. Oncol. 2019, 69, 103–107. [Google Scholar] [CrossRef]
- Rutkowski, P.; Wysocki, P.J.; Nasierowska-Guttmejer, A.; Jeziorski, A.; Wysocki, W.M.; Kalinka, E.; Świtaj, T.; Kozak, K.; Kamińska-Winciorek, G.; Czarnecka, A.M.; et al. Cutaneous Melanoma. Oncol. Clin. Pract. 2020, 16, 163–182. [Google Scholar] [CrossRef]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular Mechanisms Regulating Human Melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The Melanogenesis and Mechanisms of Skin-lightening Agents—Existing and New Approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Dzierżęga-Lęcznar, A.; Kurkiewicz, S.; Tam, I.; Marek, Ł.; Stępień, K. Pheomelanin Content of Cultured Human Melanocytes from Lightly and Darkly Pigmented Skin: A Pyrolysis-Gas Chromatography/Tandem Mass Spectrometry Study. J. Anal. Appl. Pyrolysis 2017, 124, 349–354. [Google Scholar] [CrossRef]
- Dall’Olmo, L.; Papa, N.; Surdo, N.C.; Marigo, I.; Mocellin, S. Alpha-Melanocyte Stimulating Hormone (α-MSH): Biology, Clinical Relevance and Implication in Melanoma. J. Transl. Med. 2023, 21, 562. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Boissy, R.E.; Hearing, V.J.; King, R.A.; Oetting, W.S.; Ortonne, J.P. The Pigmentary System: Physiology and Pathophysiology, 2nd ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; ISBN 978-1-4051-2034-0. [Google Scholar]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current Challenges in Understanding Melanogenesis: Bridging Chemistry, Biological Control, Morphology, and Function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Nezirevic Dernroth, D.; Kågedal, B.; Hansson, C. Pheomelanin Markers in Melanoma with Reference to Their Excretion into Urine; Linköping University Electronic Press: Linköping, Sweden, 2009; ISBN 978-91-7393-566-1. [Google Scholar]
- Wakamatsu, K.; Fukushima, S.; Minagawa, A.; Omodaka, T.; Hida, T.; Hatta, N.; Takata, M.; Uhara, H.; Okuyama, R.; Ihn, H. Significance of 5-S-Cysteinyldopa as a Marker for Melanoma. Int. J. Mol. Sci. 2020, 21, 432. [Google Scholar] [CrossRef]
- Katoh, Y.; Hara, H.; Harada, T.; Hirai, S. Combination of Serum 5-S-Cysteinyldopa, Melanoma Inhibitory Activity and IL-8 Improves the Diagnostic Accuracy of Malignant Melanoma Compared with Individual Markers. Medicine 2022, 101, e30471. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Yokochi, M.; Naito, A.; Kageshita, T.; Ito, S. Comparison of Phaeomelanin and Its Precursor 5-S-Cysteinyldopa in the Serum of Melanoma Patients. Melanoma Res. 2003, 13, 357. [Google Scholar] [CrossRef]
- Umemura, H.; Yamasaki, O.; Kaji, T.; Otsuka, M.; Asagoe, K.; Takata, M.; Iwatsuki, K. Usefulness of Serum 5-S-Cysteinyl-Dopa as a Biomarker for Predicting Prognosis and Detecting Relapse in Patients with Advanced Stage Malignant Melanoma. J. Dermatol. 2017, 44, 449–454. [Google Scholar] [CrossRef]
- Salopek, T.G.; Yamada, K.; Ito, S.; Jimbow, K. Dysplastic Melanocytic Nevi Contain High Levels of Pheomelanin: Quantitative Comparison of Pheomelanin/Eumelanin Levels Between Normal Skin, Common Nevi, and Dysplastic Nevi. Pigment Cell Res. 1991, 4, 172–179. [Google Scholar] [CrossRef]
- Mitsui, H.; Kiecker, F.; Shemer, A.; Cannizzaro, M.V.; Wang, C.Q.F.; Gulati, N.; Ohmatsu, H.; Shah, K.R.; Gilleaudeau, P.; Sullivan-Whalen, M.; et al. Discrimination of Dysplastic Nevi from Common Melanocytic Nevi by Cellular and Molecular Criteria. J. Investig. Dermatol. 2016, 136, 2030–2040. [Google Scholar] [CrossRef]
- Wang, H.; Osseiran, S.; Roider, E.; Fisher, D.E.; Evans, C.L. New Imaging-Based Biomarkers for Melanoma Diagnosis Using Coherent Raman Scattering Microscopy (Conference Presentation). In Photonic Therapeutics and Diagnostics XII; SPIE: Bellingham, WA, USA, 2016; Volume 9689, p. 2-2. [Google Scholar]
- Pavel, S.; van Nieuwpoort, F.; van der Meulen, H.; Out, C.; Pizinger, K.; Cetkovská, P.; Smit, N.P.M.; Koerten, H.K. Disturbed Melanin Synthesis and Chronic Oxidative Stress in Dysplastic Naevi. Eur. J. Cancer 2004, 40, 1423–1430. [Google Scholar] [CrossRef]
- Mitra, D.; Luo, X.; Morgan, A.; Wang, J.; Hoang, M.P.; Lo, J.; Guerrero, C.R.; Lennerz, J.K.; Mihm, M.C.; Wargo, J.A.; et al. An Ultraviolet-Radiation-Independent Pathway to Melanoma Carcinogenesis in the Red Hair/Fair Skin Background. Nature 2012, 491, 449–453. [Google Scholar] [CrossRef]
- Morgan, A.M.; Lo, J.; Fisher, D.E. How Does Pheomelanin Synthesis Contribute to Melanomagenesis?: Two Distinct Mechanisms Could Explain the Carcinogenicity of Pheomelanin Synthesis. BioEssays 2013, 35, 672–676. [Google Scholar] [CrossRef]
- Nasti, T.H.; Timares, L. MC 1R, Eumelanin and Pheomelanin: Their Role in Determining the Susceptibility to Skin Cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, S.; Wakamatsu, K.; Galván, I. Increase of the Benzothiazole Moiety Content of Pheomelanin Pigment after Endogenous Free Radical Inducement. Dye. Pigment. 2020, 180, 108516. [Google Scholar] [CrossRef]
- Lembo, S.; Di Caprio, R.; Micillo, R.; Balato, A.; Monfrecola, G.; Panzella, L.; Napolitano, A. Light-independent Pro-inflammatory and Pro-oxidant Effects of Purified Human Hair Melanins on Keratinocyte Cell Cultures. Exp. Dermatol. 2017, 26, 592–594. [Google Scholar] [CrossRef]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative Stress Inhibits Distant Metastasis by Human Melanoma Cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Dzierzega-Lecznar, A.; Kurkiewicz, S.; Stepien, K.; Chodurek, E.; Wilczok, T.; Arzberger, T.; Riederer, P.; Gerlach, M. GC/MS Analysis of Thermally Degraded Neuromelanin from the Human Substantia Nigra. J. Am. Soc. Mass Spectrom. 2004, 15, 920–926. [Google Scholar] [CrossRef]
- Kurkiewicz, S.; Dzierżęga-Lęcznar, A.; Stanek-Widera, A.; Lange, D. Development of a Method for Isolation of Melanin from Archival FFPE Tissues of Human Melanoma for Structural Studies by Pyrolysis-Gas Chromatography-Tandem Mass Spectrometry. Adv. Hyg. Exp. Med. 2022, 76, 122–127. [Google Scholar] [CrossRef]
- Greco, G.; Wakamatsu, K.; Panzella, L.; Ito, S.; Napolitano, A.; D’Ischia, M. Isomeric Cysteinyldopas Provide a (Photo)Degradable Bulk Component and a Robust Structural Element in Red Human Hair Pheomelanin. Pigment Cell Melanoma Res. 2009, 22, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.R.; Roberts, J.C.; Wilkins, D.G.; Rollins, D.E. Relationship of Melanin Degradation Products to Actual Melanin Content: Application to Human Hair. Anal. Biochem. 2001, 290, 116–125. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).