Abstract
The aim of this article was to present the biological activity of milk components, particularly lactoferrin (LF), and techniques for its extraction and purification. Dairy products have long been recognized for their significant contributions to human health and nutrition. Recent studies indicate that dairy consumption offers various health benefits, particularly concerning bone health, metabolic wellness, and cardiovascular health. LF, abundantly present in milk, exhibits a range of health-promoting properties that are increasingly recognized for their significance in nutrition and disease prevention. The production of LF can be approached through two main avenues: extraction from milk and recombinant expression systems. Both methods present unique advantages and challenges that influence the efficiency of LF production on an industrial scale. Moreover, advances in purification and drying techniques are crucial to enhance the overall efficiency of LF production. Recent studies have focused on methods such as monolithic ion-exchange chromatography and membrane technologies to improve yield and reduce costs of LF extraction. These innovations not only facilitate the extraction but also preserve the structural integrity and the functional properties of LF. The article presents the discussion of the applications of the LF in the dairy industry, indicating its growing importance as a functional ingredient in health products.
1. Introduction
Milk consumption could range from 10 to 212 kg per person per year [1]. The global market for functional dairy products reached USD 44 billion in 2023, and according to forecasts, its value will increase by 4.5% in the years 2023–2033, reaching USD 67.1 billion. This growth is a result of growing consumer awareness as well as increasing consumption of dairy products. The consumers observed an improvement in the functioning of the digestive tract, stimulation of the immune system, and potentially preventive effect on many chronic diseases [2]. An increasing amount of precise scientific evidence, cohort studies, and meta-analyses indicated the beneficial effects of dairy products on consumer health (Table 1). However, it is difficult to draw clear conclusions because practically all analyses began with formulations indicating problems with interpretation of the previous experiments, uncertainty over the analyzed data, and finally an indication of the need for further work.
Meta-analysis indicated a negative association between the consumption of fat-rich dairy products and the occurrence of cardiometabolic syndrome (risk of developing cardiovascular disease and/or type 2 diabetes). However, regular consumption of dairy products and their inclusion in general healthy eating patterns is recommended. Some analyses suggested that the consumption of fat-rich milk and yogurt may have a beneficial effect on body weight and regular consumption of ripened cheese on blood lipid profiles [3]. Milk fat is an important source of branched-chain fatty acids (BCFA). Studies suggested that the BCFAs help maintain a healthy body weight and metabolism. The limited data on this topic require further advanced analysis [4]. Conjugated linoleic acid (CLA) has anti-cancer and anti-atherosclerotic properties and stimulates weight loss [5,6,7]. A high level of trans-palmitoleic acid (TPA, 16:1, n-7) in the blood is associated with a lower risk of developing type 2 diabetes. TPA reduces the risk of sudden cardiac death (SCD) and modifies the profile of the microbiota and short-chain fatty acids in the gastrointestinal tract [8,9,10,11]. Further work is needed to determine the origin and role of TPA in human nutrition. Milk phospholipids support the integrity and function of neuronal cells. Milk fat globule membrane (MFGM) components reduce stress symptoms in healthy adults and also reduce anxiety symptoms. The MFGM supplementation may improve mental health by reducing inflammation and supporting neurotransmitter synthesis. The MFGM-fortified infant formulas mimic the composition of mother’s milk and stimulate gut and central nervous system development [12,13,14].
Milk proteins are characterized by high nutritional quality and biological activity resulting from their amino acid composition. Currently, milk and dairy products provide 15% to 20% of the daily protein intake in the United States. Whey proteins have become popular as supplements supporting health and muscle mass development due to their high concentration of leucine (12% w/w). Whey proteins, e.g., α-lactalbumin (α-lac), are used in the production of infant formulas, and glycomacropeptide is used in the diets of people suffering from phenylketonuria. The α-lac is a rich source of tryptophan—a precursor of serotonin, a neurotransmitter produced in the brain, kidneys, lungs, and intestinal epithelial cells. Serotonin improves sleep and mood and regulates food intake. Cysteine present in α-lac acts similarly to prebiotics, and together with other whey proteins, they affect the functioning of the digestive tract and exhibit antimicrobial activity [15,16]. Peptides that are released during digestion or because of fermentation are considered biologically active peptides (BAP). They are angiotensin-converting enzyme (ACE I) inhibitors, which lower blood pressure to a similar extent as limiting sodium in the diet or applying a diet that lowers hypertension (Dietary Approaches to Stop Hypertension, DASH). BAPs support the treatment of type 2 diabetes. They have hypocholesterolemic, anticancer, antithrombotic, antioxidant, and antimicrobial effects.
The structural homology of the milk oligosaccharides to glucans found on the surface of intestinal epithelial cells allows them to act as receptors to which pathogens can bind instead of to the host epithelial cells. This results in the removal of undesirable microorganisms from the gut. Milk oligosaccharides also have anti-inflammatory and immunomodulatory effects and have been shown to reduce intestinal permeability [17,18]. Sialic acid found in milk oligosaccharides is associated with neonatal brain development and contributes to the development of learning abilities [19].
Growth factors isolated from milk, colostrum, or whey, despite low concentrations, show pleiotropy and are used as preparations for wound healing and in the treatment of inflammatory disorders of the digestive system [20]. They show anti-cancer activity, and newer applications are related to insulin-like activity, tissue regeneration, protection against allergies, and treatment of inflammatory skin diseases such as psoriasis [21].
The data presented do not close the topic of scientific discussion on the beneficial or negative impact of milk components in the complex matrix of dairy products on human health [22]. There are still concerns about the methodology and quality of the experiments conducted, and modern analytical techniques open up new possibilities for carrying out scientific work. Attention should also be paid to the problem of the effective concentration of biologically active components of milk and their bioavailability, as well as to the complex problem of the impact of milk components on the microbiota of the products and the digestive tract, and consequently on human health [23].

Table 1.
Summary of studies on the impact of dairy products on human health (ScienceDirect Database, keywords: dairy and cohort or metanalyses, 2020–2025).
Table 1.
Summary of studies on the impact of dairy products on human health (ScienceDirect Database, keywords: dairy and cohort or metanalyses, 2020–2025).
| Scope of Analysis/Dairy Products | Impact on Human Health | Literature |
|---|---|---|
| Cohort analysis of data on dairy product consumption and its impact on overweight or obesity, hypertension, and type 2 diabetes. | Dairy consumption was associated with a low risk of overweight or obesity (milk and yogurt), hypertension (low-fat dairy and milk), and type 2 diabetes (yogurt). | [24] |
| Analysis of 13 cohort studies, including the Epidemiology and Nutrition Dietary Determinants (BLEND). | No evidence linking dairy consumption to bladder cancer risk. Yogurt consumption may be associated with a reduced risk of developing bladder cancer. | [25] |
| Analysis of data from 1334 healthy patients (median age 67 years at baseline) with a mean follow-up of 5.6 years from the CoLaus|PsyColaus cohort in Lausanne, Switzerland. | Adding dairy to the diet or replacing meat, vegetables, or fruit with milk did not affect cognitive function in a cohort study of the older adults. Replacing fish and eggs with dairy may have a negative effect on some outcomes, but more studies examining food substitutions are needed to confirm these results. | [26] |
| Analysis of milk consumption from adolescence to adulthood in relation to breast cancer incidence, menopausal status, and molecular subtypes of cancer in the Nurses’ Health Study (NHS) cohort. | Overall dairy intake was not associated with breast cancer risk. However, heterogeneity was observed for dairy food type, life span, and cancer subtypes. | [27] |
| Examination of the association between dairy product consumption and renal dysfunction in patients after myocardial infarction (MI). | Consumption of milk, cheese, or dairy desserts was not associated with worsening renal function after MI. The adverse association with yogurt consumption should be verified in other cohort studies. | [28] |
| Evaluation of the effect of total dairy products, yogurt, milk, and cheese on the bone health of women in the Nurses’ Health Study (NHS) conducted in the United States. | Higher total milk, milk, and cheese intake is associated with a lower risk of fractures in women in the NHS. | [29] |
| Meta-analysis of prospective cohort studies to determine the association between dairy consumption and cancer incidence and mortality. | High milk consumption, particularly high-fat milk, was associated with increased cancer mortality compared with low milk consumption. High consumption of fermented dairy products was associated with reduced cancer mortality, and this association was particularly notable in women. High milk consumption was associated with increased mortality from liver, ovarian, and prostate cancers. | [30] |
| Effect of dairy consumption on mortality from ischemic heart disease (IHD), cardiovascular disease (CVD), stroke, and survival after myocardial infarction (MI). | In post-MI patients, yogurt consumption reduced CVD mortality and all-cause mortality. Associations for milk and other dairy products were neutral or inconsistent. | [31] |
| Analysis of the relationship between dairy consumption and the initial development of type 2 diabetes. | A positive relationship is suggested between moderate milk and cheese consumption and the prevention of diabetes development. Further comprehensive analyses are necessary. | [32] |
| The assessment of the relationship between long-term consumption of milk and fermented milk products and the risk of breast cancer. | In postmenopausal women, long-term milk consumption was associated with an increased risk of breast cancer, whereas long-term consumption of fermented milk products was associated with a reduced risk of breast cancer. | [33] |
The COVID-19 pandemic in 2020–2022 certainly had an impact on consumer requirements for food products, but also on the revival of preferences for food as a pharmaceutical [34]. At that time, attention was paid to food that strengthens immunity and helps to fight bacterial and viral infections. Particular attention was paid to LF and products supplemented with LF [35]. This interest is also confirmed by the scope of research work and bibliometric analysis, which indicates experimental work with LF focused on 5 areas: medicine, pharmacy, nutrition, chemistry, biochemistry, and microbiology (Figure 1).
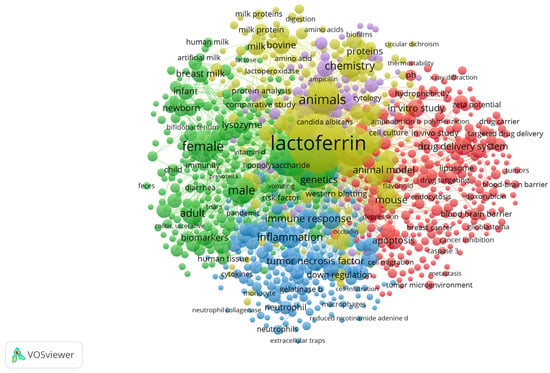
Figure 1.
Bibliometric map generated in VOSviewer (version 1.6.20) [36]. Scopus database analysis using the keywords used by the authors, “lactoferrin and bovine”.
The global bovine lactoferrin (bLF bovine lactoferrin) market was valued at USD 659.0 million in 2023 and is projected to grow from USD 669.9 million in 2024 to USD 969.9 million by 2032, with a CAGR of 4.7% during 2024–2032 [37]. The main market share of LF in 2023 was infant formula milk (approximately 48%), followed by food supplements containing LF (approximately 40%). The use of LF in food production has been accepted by EFSA (European Food Safety Authority). At the request of the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens, Nutrition and Allergies (NDA) was asked to conduct an additional assessment of LF as a food ingredient in the context of Regulation (EC) No 258/97, considering the scientific comments and concerns raised by the Member States. The purpose of the application was to place bLF on the market as an ingredient in food supplements, infant formulas, dietary foods for special medical purposes, and sports nutrition, as well as various types of food. For infants aged 0 to 6 months, the applicant estimated the intake of approximately 200 mg per kg body weight and 1.2 g bLF per day. For adults, the intake of LF was estimated to be approximately 1.4 g to 3.4 g per day. Toxicological information, including in vitro genotoxicity data, indicates no adverse effects of LF when consumed at the proposed doses. The panel concluded that the bLF food ingredient is safe within the proposed uses and the use levels [7]. The literature on various aspects of obtaining, modifying, using, and properties of the LF is very extensive, and additional information can be obtained from other publications [1,11,17,20,22,38]. The presented material does not present comprehensive information on medical and nutritional aspects of LF activity. Selected information is presented on this topic, confirming the currently defined nutritional role of milk components, including primarily lactoferrin.
The purpose of this literature scope review was to present current information on the technology for obtaining and using bovine lactoferrin, as well as its stability and multidirectional activity in the complex matrix of milk active compounds. Although there are several reviews on LF activity and technology, recent advances in LF separation and stabilization require an update. Unlike previous reviews, this one considers the latest discoveries on LF’s biological activity and technology for its separation and stabilization and identifies application issues in the dairy industry.
2. Characteristics and Biological Activity of Lactoferrin
LF is a multifunctional protein from the transferrin family, present in many biological secretions, e.g., milk, saliva, tears, or in the secretions of the glands of the nasal cavity [39]. The highest levels of LF are found in colostrum (8 mg/cm3), milk (1.4–4 mg/cm3), tears (2 mg/cm3), seminal fluid (0.112 mg/cm3) and saliva (0.008 mg/cm3) [40]. Data on LF concentration vary, depending on the physiological state of the organism and the analytical method used. Moreover, the occurrence of this glycoprotein in the granules of neutrophils has been found, which are associated with their key immune function [41]. Although there are various methods available for determination of the LF concentration, mainly chromatographic ones [42,43], different concentration results are still obtained depending on the analytical method used (Table 2). Interesting are the analyses of the LF concentration changes at different stages of cheese curd production, in milk, milk after pasteurization, acid whey, and cheese curd: 140 mg/kg, 128 mg/kg, 91 mg/kg, and 266 g/kg, respectively [44]. Increasingly, techniques involve the use of biosensors due to the ease of analysis and their sensitivity and accuracy. Liu et al. [45] presented a technique for determining the concentration of LF using a 6-carboxyflurescein (6-FAM)-labeled aptamer. The method was based on the use of the Förster resonance energy transfer (FRET), which causes a decrease in the fluorescence of the aptamer-LF complex. A method for determining the concentration of LF in infant formulas, consisting of extraction of ingredients from powder, isolation of LF using a solid-phase extraction technique (HiTrap heparin), and determination of its concentration using liquid chromatography with a spectrophotometric detector, was also demonstrated to be suitable according to the requirements of AOAC International Standard Method Performance Requirements (SMPR®) 2020.005 [46].
The molecular weight of LF varies depending on the origin, i.e., the molecular weight of the LF from milk and colostrum is 83–87 kDa, whereas the LF from neutrophils is characterized by a mass in the range of 87–91 kDa [44]. The amino acid sequence similarity of the LF from cow’s milk (PDB 1BLF) and human milk (PDB 1LFG) is 69.52% (BLAST). At least 60 LF genes have been characterized, and the bLF gene is located on chromosomes 22 and U12 and is 2351 bp long [45]. The regulation of the LF genes is diverse and complex, which allows the control of its biological activity. The LF from cow’s milk and recombinant human lactoferrin (talactoferrin, hLF) are characterized by similar proliferative activity, but differences in their functional properties have also been demonstrated [46]. The profile of released peptides from the human and recombinant hLF was slightly different from the peptide profile after the bLF digestion [47]. LF has anti-inflammatory, antimicrobial, antiviral, immunomodulatory, antioxidant, probiotic, and anticancer properties (Figure 2) [39]. LF has a particular effect on the development of the fetus and infants, ensuring the proper functioning of the digestive, nervous, and skeletal systems, increasing calcium deposition in osteoblasts [48,49,50,51], and also shows neuroprotective effects in Parkinson’s and Alzheimer’s diseases [52,53].
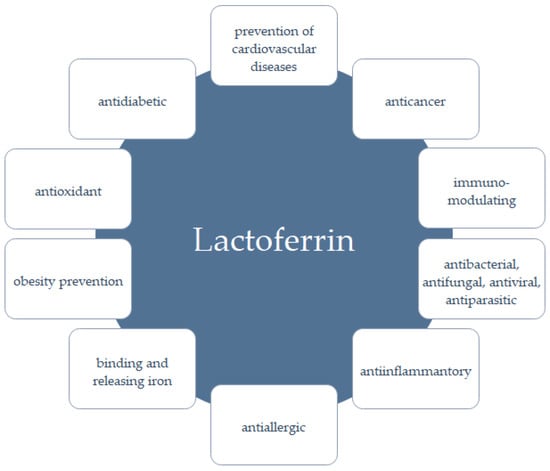
Figure 2.
Examples of biological activities of LF (based on [39]).
The application of LF is not limited to human nutrition but also includes animal breeding [54,55] and the cultivation of transgenic plants [56], mainly due to the impact of LF on pathogens causing various diseases. Transfer of the LF gene into plant genomes and its expression can enhance the natural immune defense of plants by sequestering iron and directly destroying pathogen cells [57].
However, one of the most important functions of LF is its participation in iron metabolism [51]. This protein has the ability to bind iron strongly and reversibly with high affinity in an acidic environment. Reversible binding of iron ions causes it to occur in two forms: apo (iron-free) and holo (with bound iron). The structure of the LF is formed by two lobes; each of them can reversibly chelate two ferric ions. Each lobe contains two domains, referred to as N1 and N2 and C1 and C2, between which there is a space allowing both lobes to bind iron (Figure 3) [39]. The Apo-LF is more compact and more resistant to thermal and enzymatic degradation. It is worth noting the duality of the form of the LF molecules, i.e., mono- and diferric, due to the binding of iron ions [58]. In general, the natural form of the LF powder (apo- and holo-LF mixture) is salmon-pink in color and is characterized by 15–20% iron saturation. Four amino acid residues participate in iron ion binding: Asp, Tyr, Tyr, and His, while two Arg residues participate in bicarbonate or hydrocarbonate ion binding. The binding site in LF has the highest affinity for iron ions; however, other metal ions, e.g., Ga3+, Al3+, VO2+, Mn2+, Co3+, Cu2+, and Zn2+, can be bound after removal of Fe3+. However, little is known about the biological consequences of LF binding other metals.
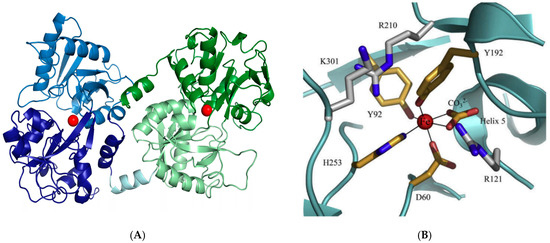
Figure 3.
(A) Presentation of the bovine lactoferrin structure. The N-lobe is blue (N1 pale blue and N2 dark blue), and the C-lobe is green (C1 dark green and C2 pale green). The hinge helix is represented in a pale cyan. Iron ions are reported as red spheres [39], (B) iron binding site in the lactoferrin molecule [13].

Table 2.
Lactoferrin concentration in raw materials and dairy products depending on the analytical method used.
Table 2.
Lactoferrin concentration in raw materials and dairy products depending on the analytical method used.
| Raw Material/Product | Lactoferrin Concentration (mg/cm3 or mg/g) | Analytical Technique Used | Literature |
|---|---|---|---|
| Raw milk | 0.020–0.200 | Radioimmunoassay | [59] |
| 0.157 ± 0.007 | ELISA * | ||
| 0.182 | Immunosensor | ||
| 188.4 ± 13.2 | HPLC (reverse phase system) | ||
| Raw milk (Holstein Friesian) | 13.06 | RID ** | [60] |
| Raw milk (Simmental cows) | 13.64 | ||
| Raw milk crossbreed (Lithuanian Black-and-White and Holstein dairy cows) | 0.08–0.12 | ELISA * | [61] |
| Raw milk (Chinese Holstein cows) | 0.031 and 0.485 | ||
| Raw milk (Gyr cows) | 16.80 ± 12.41 μg/cm3 | Immunoenzymatic kit | [62] |
| Raw milk (Holstein and Simmental cows) | 0.128–0.179 | RID ** | [60] |
| Whey from Feta cheese production | 0.272 ± 0.024 | HPLC/UV | [63] |
| Pasteurized milk | 0.020–0.032 | Immunoaffinity magnetic purification coupled with HPLC-FLD *** | [64] |
| Baby food | 0.079–0.773 | ||
| Whey protein concentrate | 0.590–0.623 | ||
| Pasteurized milk | 0.174 ± 0.017 | ELISA * | [59] |
| UHT milk | 0.018 | Immunosensor | |
| Swiss-type cheese | 1.112 ± 0.111 | ELISA * | [65] |
| Semi-hard cheese | 1.143 ± 0.118 | ||
| Soft cheese | 0.680 ± 0.015 |
* ELISA—immunoenzymatic test (enzyme-linked immunosorbent assay), ** RID—radial immunodiffusion, *** FLD—fluorescent detector.
The biological activity of LF allows it to be used in medicine, the production of functional food, pharmaceuticals, and cosmetics, but also in the production of packaging [38]. It is impossible to discuss all biological activities of LF in this paper, but the most important one is undoubtedly the strong antimicrobial and antiviral effect, a mechanism of which is diverse. First of all, the LF chelates free iron (III) ions, which prevents bacteria from acquiring this key element, necessary for their growth and development. The LF works by directly binding to receptors on the surface of bacterial cells and participates in the reduction of iron ions, which does not allow microorganisms to assimilate them [66]. Until now, it was believed that only its affinity for iron makes LF part of the immune system. However, recent studies have shown a more direct way in which this protein works. Because the N-terminal fragment of the LF has a strong positive charge, it can interact with the bacterial cell wall and damage it [67]. The release of bacterial lipopolysaccharides is also possible as a result of Ca2+ ions binding by LF [68,69]. Biological activity is also demonstrated by peptides, e.g., lactoferricin, which is formed by hydrolysis of the LF catalyzed by pepsin. It is an N-terminal fragment of the LF, and analyses suggest that this peptide exhibits even greater bioactivity than the LF. LF has been found to have both bacteriostatic and bactericidal effects, effective against pathogens such as Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Salmonella, Enterobacter, Helicobacter pylori, Yersinia, Klebsiella pneumoniae, and Porphyromonas gingivalis) and Gram-positive bacteria (Bacillus, Listeria monocytogenes, and Staphylococcus aureus), making it a valuable support for modern antibiotic therapy. In addition, its presence can increase the effectiveness of some antibiotics, and this synergistic effect can help treat infections [70,71,72].
LF also supports the intestinal microbiota through its prebiotic effect [73]. Its activity is based on supporting the growth of beneficial probiotic bacteria, such as Lactobacillus and Bifidobacterium [74]. Studies have shown that the LF can modulate molecular pathways in these microorganisms, contributing to their proliferation and beneficial health effects in the host [75,76,77,78]. Additionally, it has antifungal activity against yeasts such as Candida albicans and Candida krusei [79,80] and antiparasitic activity against Babesia caballi and Babesia equi, with this effect being more pronounced for B. caballi and occurring only in the presence of the apoform of the LF [81,82]. Additionally, peptides derived from LF, both human and bovine, as well as LF itself, exhibit antiparasitic activity against Giardia lamblia and Giardia intestinalis [83,84].
The antiviral activity of LF depends on its origin and the degree of saturation with iron ions. A possible mechanism of LF antiviral action is its binding to heparan sulfate proteoglycans (HSPGs) on the cell surface, which reduces virus adsorption and its penetration into the cell. A similar mechanism of the LF antiviral action is the disruption of virus-cell interaction through its binding to the low-density lipoprotein receptor (LDLR). It is also possible for the LF to bind directly to viral proteins and inhibit its adsorption on cells, as well as to disrupt intracellular transport of the virus and the viral genome into the cytoplasm [69]. The LF can also induce immune cell activity to modulate cytokine release and enhance the host immune response against viral infections [85]. The LF has antiviral activity, although data indicate greater antiviral activity of the LF hydrolysis products [86] and the LF-functionalized nanoparticles [87]. The LF effectively inhibits the replication of many viruses, such as influenza viruses, HIV, hepatitis C and B viruses, herpes simplex viruses, coronaviruses, adenoviruses, rotaviruses, echoviruses, and noroviruses [34,88,89,90,91,92,93,94,95,96]. The LF acts synergistically with some antiviral drugs, e.g., acyclovir and ribavirin [67,97].
No less important is the antioxidant and anti-inflammatory activity of LF. This is related to the binding of metal ions, such as Fe2⁺ and Cu2⁺ by LF. Thanks to that, this protein reduces the pro-oxidant effect of these ions and also activates genes responsible for the production of antioxidant enzymes in vitro [98,99,100]. Moreover, LF affects immune homeostasis and inflammatory responses of the organism by reducing oxidative stress induced by reactive oxygen species (ROS) [97]. Since iron is crucial in modulating ROS production, the LF, as a protein binding this element, can reduce oxidative stress and thus control excessive inflammatory response. The LF also supports maturation and functioning of immune cells, and its interactions with surface receptors are crucial for modulation of immune responses [100,101]. The LF has a strong modulatory effect on innate and adaptive immune responses by accelerating T lymphocyte maturation and immature B cell differentiation. Additionally, during inflammation, the LF shows anti-inflammatory activity against interleukin 6 (IL-6). Immunomodulatory and anti-inflammatory in vitro and in vivo activity of the LF is related to its interaction with specific cell surface receptors, e.g., PAMPs (pathogen-associated molecular patterns), which are mainly recognized by Toll-like receptors (TLRs). The mechanisms of LF interaction with different receptors are closely related to its glycan conformation. Observations showed that there is an interaction between some TLRs and LF, which is mediated by its glycans, and this in turn leads to immunomodulatory effects [102,103,104]. In vitro and in vivo studies have shown that macrophages and dendritic cells can bind to LF through interaction with its surface receptors, which induces the maturation and functional activity of monocytes/macrophages, contributing to a reduced pro-inflammatory profile [92,105,106].
LF is successfully used in the treatment of cancer, apoptosis, and angiogenesis [40,107,108]. LF has been shown to inhibit the growth and proliferation of various cancer cell lines, including breast [107,109], lung [110], stomach [111], oral cavity [112], and prostate [113]. Contrary results were found in studies with hLF, which linked its high expression level with the promotion of proliferation and invasiveness of cancer cells, as well as deterioration of the malignant phenotype of some cancer cells [114]. Anticancer activity has been found for both hLF and bLF, but the role of these proteins in the treatment of cancer diseases and the mechanisms of their action are still not fully understood. It is assumed that the mechanism of the LF’s effect on cancer cells is iron ion chelation. LF supports anticancer activity by regulating the level of this element in the body [70,107,111,114,115,116] and by activating cellular signals, which may lead to cell cycle arrest and structural damage [117]. It has been shown that many cancer cells are characterized by a high content of proteoglycans, glycosaminoglycans, and sialic acid, which can interact with LF, possibly leading to the activation of additional signaling pathways, causing harmful effects on cancer cells. Novel cancer treatment involves the design of intelligent carrier systems that increase the bioavailability of anticancer agents while increasing their stability and reducing cytotoxic properties. A peptide derivative of the bovine LF, L12, has shown anticancer activity in the various cell lines. Undesirable side effects in healthy tissues and inappropriate release kinetics limit the possibilities of effective delivery of this peptide. In order to overcome these difficulties, a carrier that resembles elastin (ELP) and has the ability to thermally direct therapeutic peptides and chemotherapeutics to the tumor site was designed [118].
3. Isolation and Purification of Lactoferrin
LF is isolated from skimmed milk or, most often, from sweet or sour whey. The concentration of LF in the raw material varies, depending on the technological parameters used in dairy plants. The composition of the raw material is also significantly influenced by the breed of cows, the region of breeding, and the season, which determines the composition of milk and, consequently, whey. Whey may contain small amounts of LF, e.g., 50 mg/dm3, while in skimmed milk the concentration of LF may be 485 mg/dm3 [47]. The challenge for LF isolation is the fact that a large volume of whey with a very low LF concentration (approximately 1% of all proteins) must be processed. Membrane techniques play a key role at the initial stage of LF isolation, as well as other milk components [35]. Many techniques and unit operations are available, which determine the selectivity and efficiency of the technologies used. The selection of methods is determined by the properties of LF and other milk proteins. The isoelectric point (IEP) of LF is 9.3–9.5 and is much higher than that of other milk protein fractions: casein 4.9–5.6; β-lactoglobulin 4.2; α-lactalbumin 5.4; serum albumin 4.7; and immunoglobulins 4.8–7.8. This feature makes cation exchange possible to selectively isolate LF from milk or whey, both using ion exchange columns and membrane chromatography. However, the problem is the similar IEP and molecular weight values of the bLF and the lactoperoxidase (LPO, 77.5 kDa, IEP 9.8), which significantly complicates the separation of these components.
Little detailed information is available on the industrial processes of LF isolation and purification. It is known that in order to avoid LF denaturation, skimmed milk or whey is used as a raw material, which was not subjected to high heat treatment due to the problem of the LF stability (Table 3). Pasteurization of human milk (62.5 °C, 30 min), commonly used in human milk banks (HMBANA), caused a 61% decrease in the LF concentration. However, it has been shown that iron ions stabilize thermolabile LF [119]. Usually, after filtration, the raw material is passed through a cation exchange column (Figure 4). Contaminants are eluted from the column using a low-salt buffer, while the LF is eluted using a high-salt buffer. Obtaining a fixed LF preparation is preceded by concentration by ultrafiltration and desalting by diafiltration. Drying is required to transform the LF concentrate into a form suitable for transport and sale. Two industrially used LF drying methods are freeze-drying and spray-drying. Previous analyses indicated that spray-drying (temperatures: inlet 180 °C, outlet 95 °C) can produce a product with excellent physicochemical properties and antioxidant activity (in vitro), even better than the product obtained after freeze-drying [19]. In a recently presented work using new, available analytical techniques, it was shown that the spray-dried LF showed a much greater range of denaturation and lower iron-binding capacity compared to the liquid concentrate or freeze-dried product [13]. The semi-finished product and the final product showed bacteriostatic activity even at low LF concentrations of 0.01 mg/cm3. However, no bactericidal effect was observed for any of the LF concentrations, regardless of the technology used.
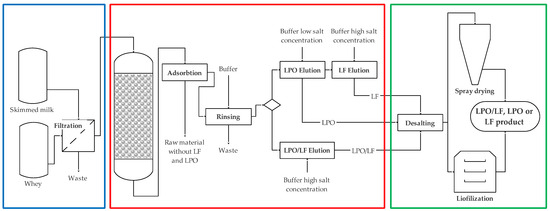
Figure 4.
General scheme of obtaining LF, LPO, and a mixture of LF and LPO. The technological process consists of 3 main stages: preparation of raw material (blue rectangle), isolation of the product by chromatography (red rectangle), and formulation of the product (green rectangle) (based on [98]).

Table 3.
The influence of processes and unit operations on lactoferrin.
Table 3.
The influence of processes and unit operations on lactoferrin.
| Process or Unit Operation | Parameters | Origin/Type Lactoferrin | Effect on Lactoferrin | Literature |
|---|---|---|---|---|
| Heat treatment | 65–121 °C, 2–300 s | bLF | Denaturation rates up to 80 °C likely reflected the greater heat stability of the more iron-saturated LF | [120] |
| Heat treatment | 180 °C | bLF | Apo-LF stabilized by whey protein | [121] |
| Heat treatment | 72–95 °C | bLF | Irreversible changes of the structure and physicochemical properties | [122] |
| Heat treatment | pH 2–5, >100 °C | bLF | No effect | [123] |
| pH > 6 | bLF | Denaturation | ||
| Heat treatment | 72–64 °C, 50 min | bLF | No effect | [124] |
| Heat treatment | 60 and 90 °C, 20 min | bLF | Denaturation, formation of nanoparticles | [125] |
| Heat treatment | pH 3–7 | bLF, caprine lactoferrin (cLF) | A gradual reduction in the denaturation temperature of LF when decreasing the pH | [126] |
| Heat treatment | 60–100 °C, 20 min, pH 3–9 | bLF | Antimicrobial activity: inactivated at >75 °C, stable at pH 6–9, but no inhibition at pH 3–5 | [127] |
| Heat treatment | 72 °C, 15 s | bLF | No effect | [128] |
| Heat treatment | 72–95 °C | bLF | Irreversible changes | [122] |
| Heat treatment | 72 °C, 20 s; 85 °C, 20 min; 135 °C, 8 s | hLF | No effect | [129] |
| Heat treatment | 90–100 °C, 5 min, pH 4.0 | Apo-LF | No effect | [130] |
| Heat treatment | 65 °C for 30 min | bLF | Stimulated the growth of Lactococcus lactis subsp. cremoris | [131] |
| High hydrostatic pressure (HPP) | 300–700 MPa, 30 or 60 min | bLF | Significant modification with increased intensity of HPP, indicating partial denaturation and aggregation; improved solubility, foaming, and emulsifying properties; denaturation increases with increasing pressure | [132] |
| HPP | 450–700 MPa, 20 °C | bLF | Denaturation increased with the increase in pressure and holding time | [133] |
| HPP | 800 MPa, 30 min, | bLF | Aggregation | [134] |
| High-pressure homogenization (HPH) | 100 MPa | bLF | Enhanced antimicrobial activity against Listeria monocytogenes | [135] |
| Ultra-high-pressure homogenization (UHPH) | 200–300 MPa, 5–30 min, 24 °C | bLF | No effect | [136] |
| Pulsed electric field (PEF) | 35 kV/cm, 19.2 μs using bipolar 2 μs pulses, 0.17 to 1.04 S/m | bLF | Iron depletion, no conformational change | [137] |
| Fermentation (yogurt) | pH 4.5; 28 days | bLF | No effect | [138] |
| Fermentation | pH 4.6; 21 days | bLF | No effect | [139] |
| Drying | Tinlet 190 °C, Toutlet 75 or 95 °C | bLF | Spray-dried LF showed a significantly larger extent of denaturation and lower iron-binding capacity when compared with fresh or freeze-dried LF | [140] |
| Spray drying | 80, 100 and 120 °C, 4 and 7 μm | LF-glycomacropeptide nanohydrogels | No effect | [141] |
| Freeze drying | −40 °C, 24 h | No effect (high stability) | ||
| Convective air-drying | 75 and 90 °C | bLF (native LF, apo-LF, holo-LF) | Holo-LF more stable than apo-LF | [142] |
| Spray drying | Tinlet 180 °C, Toutlet 95 °C | bLF | No effect | [143] |
| Freeze drying | −80 °C, vacuum 16 Pa | bLF | No effect | |
| Freeze drying | −80 °C, 72 h | hLF | No effect | [144] |
| Droplet atomization via two-fluid nozzle | Two-fluid pneumatic nozzle: 0.7 and 1.5 mm, atomizing gas flow rate 0–40 dm3/min | bLF | Denaturation, aggregation | [145] |
| Cross-linking complex LF and α-lactoalbumin | Reaction catalyzed by transglutaminase | bLF | Increased thermal stability | [146] |
Conventional purification methods such as cryogel separation [147], batch fractionation with foam formation [148], ultrafiltration and cation exchange membranes [149], membrane filtration and electrodialysis [150], as well as non-conventional two-phase or three-phase extraction and reverse micelle extraction [151] (Table 4), were used for LF isolation. The most commonly used purification method for LF, after the use of membrane techniques, mainly ultrafiltration [152,153], is cation exchange chromatography (Figure 4). This technique uses the positive charge of LF at physiological pH, enabling it to bind to negatively charged ion exchanger particles. It has been shown that cation exchange chromatography can effectively isolate LF from bovine colostrum and whey, achieving purity of the preparation over 90% [154,155,156]. This process is relatively simple and can be scaled up to industrial applications, making it the preferred choice for large-scale LF production [157,158]. Methods for creating specific cation exchange resins, such as SPEC 70 SLS, are being developed. They had enabled increased efficiency of LF isolation and simultaneous extraction of LPO [47]. An example of classical technology is, for example, the use of sweet whey containing 0.12 mg/cm3 of LF, subjected to microfiltration, followed by ultrafiltration and diafiltration, which allows for a 10-fold reduction in liquid volume and obtaining a solution with the LF concentration of 1.1 mg/cm3 [159]. In the second stage, the protein is purified using preparative chromatography based on cationic exchange expanded bed chromatography (EBC). The EBC technique scheme is shown in Figure 5. The method enables separation of larger particles and the LF sorption at the first stage and then its elution at the second. The final concentration of the purified LF was 17.4 mg/cm3, purity was 92.7%, and process efficiency was 87.0%. The EBC technique also enables direct purification of LF from raw whey without the need for its prior concentration [155].
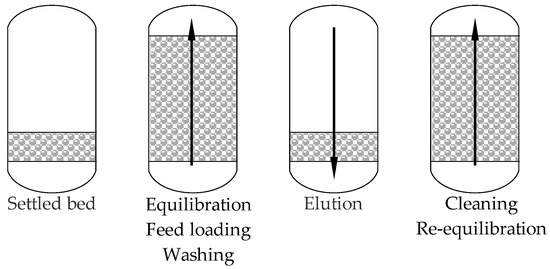
Figure 5.
ECB process flowchart (based on [160]).
In addition to cation exchange chromatography, affinity chromatography has become an effective technique for purifying LF. This method uses specific ligands that selectively bind to the LF, e.g., anionic heparin [161,162]. Affinity chromatography, on an industrial scale, using heparin may be limited due to high costs [157,161]. Therefore, alternative ligands are used in affinity chromatography, such as Yellow HE-4R, which was used to efficiently isolate LF [163].
Gel filtration is another technique used for the isolation of LF, enabling its preliminary separation [164,165]. Most often, however, gel filtration is used in combination with cation exchange chromatography [153,155].
An alternative isolation of the LF after applying controlled changes of acidity and salting out with ammonium sulfate [166] made it possible to obtain LF from camel milk. An important aspect of this method was that in the process, LF activity is not lost. This method is characterized by simplicity and the possibility of scaling up production.

Table 4.
Examples of the influence of the extraction techniques on the efficiency of bLF acquisition.
Table 4.
Examples of the influence of the extraction techniques on the efficiency of bLF acquisition.
| Separation Technique | Method Description | Extraction Efficiency | Literature |
|---|---|---|---|
| Dye-affinity chromatography | Cross-linked chitosan mini-spheres with immobilized Yellow HE-4R dye | 77% | [156] |
| Cation-exchange chromatography | Carboxymethyl-Toyopearl® column chromatography | Lactoferrin-a 31.3%; lactoferrin-b 68.7% | [167] |
| Ion exchange Chromatography | XK16 cation exchanger | Absorption efficiency: 48.6 mg/cm3 | [168] |
| Membrane absorption | Cationic membrane | bLF purity > 94% | [169] |
| Foam separation | Surfactant stabilized microbubbles. Anionic surfactant | 90% | [170] |
| Cationic membrane | SP cation exchanger built into the membrane | Efficiency > 95% | [171] |
| Magnetic nanoparticles | Magnetic nanoparticles with heparin | bLF absorption efficiency 164 mg/g, purity higher than commercial standard | [172] |
| Two-phase system | Poly(ethylene glycol) and sodium citrate | >94% | [173] |
| Two-phase system | 1-butyl-3-methylimidazole bisimide: water | Extraction efficiency 80 mg/dm3 | [174] |
| Membrane technique and magnetic field separation | Microfiltration, ultrafiltration and bLF absorption on iron oxide | Efficiency 60%, bLF purity 78% | [175] |
| Ion exchange chromatography | Strong cation exchange membrane with sulfonic acid ligand | 70% apo-LF; >85% holo-LF | [176] |
A promising method for LF separation is the use of aqueous two-phase systems (ATPS). This method involves separation of proteins between two immiscible liquid phases, usually formed by a polymer and a salt solution. Over 94% the LF extraction efficiency has been demonstrated in ATPS consisting of 14% (w/w) polyethylene glycol (PEG) and 10% (w/w) sodium citrate, pH 5.5 and at 25 °C [168]. The efficiency of the LF extraction using ATPS can be influenced by many factors, e.g., pH value, temperature and concentration of phase-forming components. This can cause many problems but also creates opportunities for selecting process parameters to optimize protein yield and purity [150].
Three-phase system (TPP) is another innovative technique that has been used for LF separation. The TPP system using ionic liquid (ILTPP, ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate (BmimBF4) in combination with sodium dihydrogen phosphate (NaH2PO4) enabled separation of LF at the interface with an efficiency of 80% [174].
The possibilities of synthesis of recombinant human and bovine LF by modified bacterial strains [177], fungi [70], transgenic cows [178], and cell cultures [179] are presented in Table 5. These biotechnologies not only increase the availability of LF but also allow to produce the LF with properties adapted to the specific applications. The development of synthetic systems for the synthesis of LF under specific and controlled environmental conditions helps to eliminate limitations associated with its extraction from traditional raw materials [157,165].

Table 5.
Examples of lactoferrin synthesis efficiency using bacterial and fungal expression systems.
Table 5.
Examples of lactoferrin synthesis efficiency using bacterial and fungal expression systems.
| Type of Organism | Origin of Lactoferrin | Efficiency of Synthesis (mg/dm3) | Biological Activity of Lactoferrin (In Vitro) | Literature |
|---|---|---|---|---|
| Nicotiana tabacum hairy roots | LFchimera | 4.8 μg/g fresh weight | Antimicrobial | [179] |
| Pichia pastoris Glucose-Inducible Expression System | Porcine | 87 | Antimicrobial, anticancer | [180] |
| Javanica rice cv. Rojolele | Human | 2 mg/g dry wet dehusked seeds | Antibacterial | [181] |
| Pichia pastoris | Human | 1200 | Glycosylation | [182] |
| Pichia pastoris | Bovine | 3500 | Antimicrobial activity | [70] |
| Chinese hamster ovary cells | Human | >200 | Protective effect against oxidative stress | [66] |
| Komagataella phaffii | Helaina recombinant hLF | No data. Industrial scale production. | 100% identical amino acid sequence to hLF | [183] |
| Transgenic mice | Porcine | 40–106 µL/cm3 | Similar properties to those of native LF | [184] |
| Bacillus subtilis | Bovine N-lobe | 16.5 | Antibacterial | [185] |
| Transgenic cow | Human | 2900 | No protection against intrauterine infection | [186] |
| Transgenic cow | Human | 4500–13,600 | Chelation and iron ion release like hLF | [178] |
| Saccharomyces cerevisiae | Human | 1.5–2.0 | Iron- and copper-binding activity | [187] |
| Escherichia coli | Bovine | 15.3 | Antibacterial | [177] |
| Lactobacillus casei | Human | 10.6 | Antibacterial | [188] |
| Bacillus subtilis | Human | 16.5 | Antibacterial | [185] |
| Pichia pastoris | Human | 115 | Antibacterial, Antiviral | [189] |
| Pichia pastoris | Bovine | 3500 | Antibacterial | [70] |
| Pichia pastoris | Bovine | 824.93 | Antibacterial | [190] |
| Aspergillus nidulans | Human | 5 | Iron ion chelation | [191] |
| Aspergillus awamori | Human | 2000 | Antibacterial, Iron ion chelation | [192] |
The choice of LF purification method often depends on its origin, intended use, and required level of purity. For example, the LF derived from goat milk may require different purification strategies than that derived from cow milk due to differences in protein composition and degree of glycosylation [152,153,154]. Understanding these differences is crucial to optimizing purification protocols and to ensuring the functional integrity of isolated LF. Due to the variable chemical structure of LF and the complex arrangement of its components in the raw materials used, it is always necessary to optimize the process of its separation and purification. Salting-out, or precipitation of LF, is an easy-to-implement, low-cost technique, although intermittent and inefficient (40–60%). Chromatographic techniques, cation exchange, and membrane techniques, including ion-exchange membranes, provide higher process yields (90–95%) and less protein denaturation, and their implementation in a continuous system under industrial conditions. Chromatographic techniques using hydroxyapatite, magnetic nanoparticles, or extraction with ionic liquids are efficient and provide mild process execution conditions [157].
4. Obtaining Products Containing Lactoferrin
LF is used primarily in dietary supplements with immunomodulatory effects [40]. In addition, it is often used as an auxiliary ingredient in preparations used in the case of upper respiratory tract infections, especially in the case of throat inflammation [98]. The ability of LF to strengthen the immune system as well as its prebiotic properties may be particularly important in preparations used for infants and for people after antibiotic therapy, as well as in the autumn-winter season during the periodic decrease in the immunity of the society [75].
The main problem of delivering LF to the body and its effective action is its sensitivity to environmental conditions and conditions in the gastrointestinal tract. Direct absorption of the LF in the gastrointestinal tract is limited due to the susceptibility of this protein to hydrolysis by pepsin, which also leads to the loss of its bioactivity. The biological activity of LF depends on its structural integrity, and it should be assumed that only trace amounts of the LF in its native state reach the small intestine [193]. In order to prevent degradation and increase the bioavailability of the LF, many techniques have been developed to prepare biopreparations in the form of carrier systems. Hydrogel carriers based on sodium alginate, gellan gum, and sodium carboxymethylcellulose have been used [194]. Among the various variants of hydrogels, intelligent hydrogels that change properties and are sensitive to changes in acidity (pH) and consist of anionic polymers seem to be particularly interesting. They are characterized by the ability to undergo structural changes in response to small changes in the pH value. Hydrogels can be designed not only to protect bioactive compounds but also to enable their release in specific locations, depending on the acidity of the environment. Composite hydrogels of the LF and chitosan (CS) with good binding efficiency and protection of thermosensitive bioactive substances have also been obtained through cross-linking catalyzed by transglutaminase [195]. The digestion in vitro results confirmed that LF in complex with CS is stable after digestion in the stomach and is released in the small intestine. Another example of an intelligent LF delivery system is liposomes based on rapeseed phospholipids [196]. After in vitro digestion in the stomach and intestines, 67–80% and 16–35% of the native LF remained, respectively. The possibility of obtaining microparticles of holo-LF with a polymer belonging to the family of methacrylate copolymers with the trade name Eudragit was also tested. It was shown that after in vitro digestion in the gastric fluid environment, 40% of the LF was released in the native state and the level of iron ion saturation was 33% [197].
LF has also been added to the matrix of dairy products, e.g., yogurt and cheese, but only a few examples were described in the literature. Probably due to the high price of LF, such products are usually not attractive. Due to the small doses of the added LF, no major changes in the nutritional quality of the products are expected. Many researchers reported substantial reductions in iron availability when dairy products were consumed. Yet other studies indicate that dairy products have little effect on iron availability when added to complex meals. The conflicting data may be due to differences in the technique used to measure availability, species of animal used, form of iron in the diet, and meal composition.
Studies indicate that the bioavailability of iron can decrease when dairy products are consumed alongside iron-rich meals. Research has explored the impact of various iron compounds on absorption levels, discovering that iron present in ferric pyrophosphate, commonly used to fortify bouillon cubes, exhibits notably low bioavailability, particularly when sodium pyrophosphate is present [198,199]. In these contexts, calcium from dairy might contribute to the inhibition of iron absorption due to its competitive relationship during the uptake process, further complicating the existing evidence on dairy-iron interactions.
LF plays a pivotal role in the modulation of iron metabolism and the immune response, primarily through its iron-binding properties. Research has established that LF not only sequesters non-heme iron but also influences the dynamics of iron availability during inflammatory states. For instance, during acute inflammation, the release of apo-LF by neutrophilic leukocytes leads to an accumulation of iron within the reticuloendothelial system, significantly impacting iron availability for hemoglobin synthesis. This diversion towards the iron sequestered in macrophages often results in hypochromic anemia, where the body predominantly utilizes iron from lysed erythrocytes rather than its stored reserves [200]. Such mechanisms underscore the complexity of LF interaction with iron metabolism, particularly in conditions of systemic inflammation.
The dual role of lLF becomes even more prominent in the context of infections, such as those caused by Helicobacter pylori. Elevated concentrations of LF in gastric mucosa correlate with increased inflammation and the resultant iron deficiency associated with these infections. The presence of LF not only aids in iron binding but paradoxically can enhance bacterial growth by providing the necessary iron substrates to pathogens like Helicobacter pylori, which utilizes this iron for proliferation [200]. This intricate interplay can further complicate the management of anemia in patients suffering from chronic infections. As a result, LF may serve both as a mediator in inflammation-related anemia and as a potential target for therapeutic interventions aimed at ameliorating iron deficiency.
Recent clinical investigations have championed LF as a viable treatment option for nutritional iron deficiency anemia (IDA), particularly among high-risk populations such as pregnant women. A comparative analysis of oral LF supplementation versus traditional ferrous sulfate has shown promising results, indicating that LF increases hemoglobin levels significantly without the gastrointestinal side effects often linked to iron salts [201]. These findings are further supported by meta-analyses confirming that LF not only enhances the overall iron profile—boosting serum iron and ferritin levels—but does so while being well tolerated by patients [202,203]. Importantly, the absence of notable side effects reinforces the position of LF as a superior alternative in managing iron deficiency, especially during pregnancy when the demand for iron is critical.
LF beneficial effects extend to pediatric populations. For example, studies have documented that the administration of bLF can significantly enhance hemoglobin levels in children undergoing hemodialysis [204]. This broad applicability further accentuates LF’s therapeutic potential and necessitates further exploration into its mechanisms, especially regarding its ability to increase iron bioavailability through the modulation of intestinal absorption pathways.
Additionally, evidence suggests that LF influences immune responses, which, when coordinated alongside its iron-modulating effects, leads to an improved iron status ultimately helpful in combating anemia. This immune modulation has been particularly noted in the context of chronic inflammatory conditions, which often accompany IDA. LF can down-regulate the production of pro-inflammatory cytokines, leading to decreased levels of hepcidin, a hormone responsible for suppressing iron absorption and releasing iron stores from macrophages [203,205]. By mitigating the inflammatory response, LF not only supports increased bioavailability of iron but also addresses one of the underlying factors contributing to anemia.
Thus, the convergence of LF roles in iron metabolism, immune modulation, and clinical efficacy substantiates its significance as both a therapeutic and preventive agent against IDA. The evidence leads to a growing consensus that LF should be integrated into dietary interventions and supplementation plans aimed at mitigating anemia in at-risk groups, thereby improving overall health outcomes during periods of increased physiological demand.
Supplementation of milk with the LF did not significantly affect the physical properties of the yogurt, although apo-LF slightly reduced the acidification dynamics due to its effect on Streptococcus thermophilus [138]. Nadi et al. [206] demonstrated no effect of the LF on yogurt starter strains and, at the same time, demonstrated its high antimicrobial activity against E. coli and S. typhimurium (MIC, 0.0001 and 0.01 mg/cm3, respectively). Janczuk et al. [207] demonstrated that enrichment of yogurt with LF in the amount of 80 mg/100 g did not cause any change in its physicochemical, microbiological, and organoleptic parameters. The LF retained its biological activity during yogurt storage. After 90 days of ripening of cheddar cheese, no significant changes in the concentration of the added LF were observed. The LF added to the cheddar cheese in amounts from 5 to 20 mg/100 g did not significantly change the physicochemical and microbiological parameters of the cheese, fatty acid composition, color, texture, taste, or peroxide value. The addition of the LF increased the antioxidant potential of cheeses (in vitro) produced with the addition of LF [208].
High stability of LF was demonstrated during attempts to develop a form of the LF preparation for dairy products. It was shown that homogenization and pasteurization increased the antimicrobial activity of the LF, also after in vitro digestion in the gastrointestinal tract [209].
The main problem in the use of LF seems to be its stability and control of changes occurring in the production process and the human digestive tract. The availability of LF is still limited, and the prospects for its use are probably related to recombinant products, including those obtained in cell-free protein systems. The biological activity of LF depends on its origin. Currently, much attention is being paid to LF from camels, which can replace other preparations due to its much higher activity than bLF. The use of LF in products requires the development of analytical methods for product control.
5. Conclusions
LF is a multifunctional glycoprotein with very diverse biological activities and, consequently, wide applications. The production of food supplements and food products enriched with the LF requires analysis of its interactions with the components of the product matrix. Many technologies and techniques related to the production and preparation of preparations containing LF still require improvement, and current applications require control of the production process. Not only is the amount of the LF important, but also the functional characteristics of the isolated protein at each stage of the implementation of the processes and unit operations. One of the first and basic properties of the LF is its antimicrobial activity. Due to the emergence of antibiotic resistance, the use of nutraceuticals is an alternative strategy for different biomedical applications. LF is one of the key immunomodulatory substances naturally occurring in body fluids, and obtaining the LF from milk and whey is particularly justified. The development in the field of obtaining recombinant LF is significant. The administration of the LF is effective in reducing the risk of respiratory tract infections [210]. LF may also play a beneficial role in the treatment of symptoms and recovery of patients suffering from RTI and support the treatment of COVID-19, but this requires further evidence and analysis from large groups of patients [211]. The results of studies indicate the validity of fortifying infant formulas with LF [212]. It is also worth paying attention to the changes in the market of the LF producers, consolidation of the production, and evolution of legal regulations regarding the assessment of milk and whey after separation of LF and other proteins.
Author Contributions
Conceptualization, M.A.; resources, M.O. and M.A.; writing—original draft preparation, M.O. and M.A.; writing—review and editing, M.O., B.B., A.B. and M.A.; visualization, M.O., B.B. and M.A.; supervision, A.B. and M.A.; project administration, M.A.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
Project funded under the designated subsidy of the Minister of Science and Higher Education Republic of Poland, task entitled ‘The Research Network of Life Sciences Universities for the Development of the Polish Dairy Industry—Research Project’ (MEiN/2023/DPI/2875).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Authors Monika Ostrowska and Andrzej Babuchowski were employed by the Institute of Dairy Industry Innovation Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The Institute of Dairy Industry Innovation Ltd. had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/4/ac911e/ac911e05.htm (accessed on 24 February 2025).
- Future Market Insights Inc. Available online: https://www.futuremarketinsights.com/reports/functional-dairy-products-market (accessed on 24 February 2025).
- Taormina, V.M.; Unger, A.L.; Kraft, J. Full-fat dairy products and cardiometabolic health outcomes: Does the dairy-fat matrix matter? Front. Nutr. 2024, 11, 1386257. [Google Scholar] [CrossRef] [PubMed]
- Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-chain fatty acids—An underexplored class of dairy-derived fatty acids. Nutrients 2020, 12, 2875. [Google Scholar] [CrossRef] [PubMed]
- McCrorie, T.A.; Keaveney, E.M.; Wallace, J.M.W.; Binns, N.; Livingstone, M.B.E. Human health effects of conjugated linoleic acid from milk and supplements. Nutr. Res. Rev. 2011, 24, 206–227. [Google Scholar] [CrossRef]
- Wang, K.; Xin, Z.; Chen, Z.; Li, H.; Wang, D.; Yuan, Y. Progress of conjugated linoleic acid on milk fat metabolism in ruminants and humans. Animals 2023, 13, 3429. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.; Kim, Y.J.; Park, Y. Conjugated linoleic acid: Potential health benefits as a functional food ingredient. Ann. Rev. Food Sci. Technol. 2016, 7, 221–244. [Google Scholar] [CrossRef]
- Kleber, M.E.; Delgado, G.E.; Lorkowski, S.; März, W.; von Schacky, C. Trans fatty acids and mortality in patients referred for coronary angiography: The Ludwigshafen Risk and Cardiovascular Health Study. Eur. Heart J. 2016, 37, 1072–1078. [Google Scholar] [CrossRef]
- Guillocheau, E.; Penhoat, C.; Drouin, G.; Godet, A.; Catheline, D.; Legrand, P.; Rioux, V. Current intakes of trans-palmitoleic (trans-C16:1 n-7) and trans-vaccenic (trans-C18:1 n-7) acids in France are exclusively ensured by ruminant milk and ruminant meat: A market basket investigation. Food Chem. X 2020, 5, 100081. [Google Scholar] [CrossRef]
- Korkus, E.; Szustak, M.; Madaj, R.; Chworos, A.; Drzazga, A.; Koziołkiewicz, M.; Dąbrowski, G.; Czaplicki, S.; Konopka, I.; Gendaszewska-Darmach, E. Trans-palmitoleic acid, a dairy fat biomarker, stimulates insulin secretion and activates G protein-coupled receptors with a different mechanism from the cis isomer. Food Funct. 2023, 14, 6496–6512. [Google Scholar] [CrossRef]
- Szustak, M.; Korkus, E.; Madaj, R.; Chworos, A.; Dąbrowski, G.; Czaplicki, S.; Tabandeh, E.; Maciejewska, G.; Koziołkiewicz, M.; Konopka, I.; et al. Lysophosphatidylcholines enriched with cis and trans palmitoleic acid regulate insulin secretion via GPR119 receptor. ACS Med. Chem. Lett. 2024, 15, 197–204. [Google Scholar] [CrossRef]
- Romo Ventura, E.; Konigorski, S.; Rohrmann, S.; Schneider, H.; Stalla, G.K.; Pischon, T.; Linseisen, J.; Nimptsch, K. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in Bavarian adults. Eur. J. Nutr. 2020, 59, 1413–1420. [Google Scholar] [CrossRef]
- Davies, N.; Frampton, C.; Fuad, M.; Slykerman, R. The effect of supplementation with milk fat globule membranes on psychological health: A randomized clinical trial in healthy adults with moderate stress. J. Funct. Foods 2023, 105, 105585. [Google Scholar] [CrossRef]
- Slykerman, R.; Davies, N.; Fuad, M.; Dekker, J. Milk fat globule membranes for mental health across the human lifespan. Foods 2024, 13, 1631. [Google Scholar] [CrossRef] [PubMed]
- Auestad, N.; Layman, D.K. Dairy bioactive proteins and peptides: A narrative review. Nutr. Rev. 2021, 79, 36–47. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Fiori, L.; Li, J.; Fanzaga, M.; d’Adduzio, L.; Tosi, M.; Burlina, A.; Zuccotti, G.; Verduci, E. Glycomacropeptide (GMP) rescued the oxidative and inflammatory activity of free L-AAs in human Caco-2 cells: New insights that support GMP as a valid and health-promoting product for the dietary management of phenylketonuria (PKU) patients. Food Res. Int. 2023, 173, 113258. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.M.; Barile, D. Bovine milk as a source of functional oligosaccharides for improving human health. Adv. Nutr. 2011, 2, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.C. Structures and metabolic properties of bovine milk oligosaccharides and their potential in the development of novel therapeutics. Front. Nutr. 2019, 6, 50. [Google Scholar] [CrossRef]
- Kurakevich, E.; Hennet, T.; Hausmann, M.; Rogler, G.; Borsig. Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc. Natl Acad. Sci. USA 2013, 110, 17444–17449. [Google Scholar] [CrossRef]
- Pouliot, Y.; Gauthier, S.F. Milk growth factors as health products: Some technological aspects. Int. Dairy J. 2006, 16, 1415–1420. [Google Scholar] [CrossRef]
- Watling, C.Z.; Kelly, R.K.; Dunneram, Y.; Knuppel, A.; Piernas, C.; Schmidt, J.A.; Travis, R.C.; Key, T.J.; Perez-Cornago, A. Associations of intakes of total protein, protein from dairy sources, and dietary calcium with risks of colorectal, breast, and prostate cancer: A prospective analysis in UK Biobank. Br. J. Cancer 2023, 129, 636–647. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Torres-Gonzalez, M.; Geurts, J.; Rosales, A.; Farhang, B.; Marmonier, C.; Ulleberg, E.K.; Hocking, E.; Neiderer, I.; Gandolfi, I.; et al. The dairy matrix: Its importance, definition, and current application in the context of nutrition and health. Nutrients 2024, 16, 2908. [Google Scholar] [CrossRef]
- Gallo, V.; Arienzo, A.; Tomassetti, F.; Antonini, G. Milk bioactive compounds and gut microbiota modulation: The role of whey proteins and milk oligosaccharides. Foods 2024, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, Y.; Liu, J.; Huang, Z.; Yang, X.; Qin, P.; Chen, C.; Luo, X.; Li, Y.; Wu, Y.; et al. Consumption of dairy products and the risk of overweight or obesity, hypertension, and type 2 diabetes mellitus: A dose–response meta-analysis and systematic review of cohort studies. Adv. Nutr. 2022, 13, 2165–2179. [Google Scholar] [CrossRef]
- Acham, M.; Wesselius, A.; van Osch, F.H.M.; Yu, E.Y.-W.; van den Brandt, P.A.; White, E.; Adami, H.-O.; Weiderpass, E.; Brinkman, M.; Giles, G.G.; et al. Intake of milk and other dairy products and the risk of bladder cancer: A pooled analysis of 13 cohort studies. Eur. J. Clin. Nutr. 2020, 74, 28–35. [Google Scholar] [CrossRef]
- Ortega, N.; Carmeli, C.; Efthimiou, O.; Beer, J.-H.; Gunten, A.v.; Preisig, M.; Zullo, L.; Vaucher, J.; Vollenweider, P.; Marques-Vidal, P.; et al. Effect of dairy consumption on cognition in older adults: A population-based cohort study. J. Nutr. Health Aging 2024, 28, 100031. [Google Scholar] [CrossRef] [PubMed]
- Riseberg, E.; Wu, Y.; Lam, W.C.; Eliassen, A.H.; Wang, M.; Zhang, X.; Willett, W.C.; Smith-Warner, S.A. Lifetime dairy product consumption and breast cancer risk: A prospective cohort study by tumor subtypes. Am. J. Clin. Nutr. 2024, 119, 302–313. [Google Scholar] [CrossRef]
- van Westing, A.C.; Cruijsen, E.; Voortman, T.; Geleijnse, J.M. Dairy products and kidney function decline after myocardial infarction: A prospective analysis in the Alpha Omega Cohort. Clin. Nutr. 2023, 42, 1501–1509. [Google Scholar] [CrossRef]
- Yuan, M.; Hu, F.B.; Li, Y.; Cabral, H.J.; Das, S.K.; Deeney, J.T.; Zhou, X.; Paik, J.M.; Moore, L.L. Types of dairy foods and risk of fragility fracture in the prospective Nurses’ Health Study cohort. Am. J. Clin. Nutr. 2023, 118, 1172–1181. [Google Scholar] [CrossRef]
- Jin, S.; Je, Y. Dairy consumption and total cancer and cancer-specific mortality: A meta-analysis of prospective cohort studies. Adv. Nutr. 2022, 13, 1063–1082. [Google Scholar] [CrossRef] [PubMed]
- Cruijsen, E.; Jacobo Cejudo, M.G.; Küpers, L.K.; Busstra, M.C.; Geleijnse, J.M. Dairy consumption and mortality after myocardial infarction: A prospective analysis in the Alpha Omega Cohort. Am. J. Clin. Nutr. 2021, 114, 59–69. [Google Scholar] [CrossRef]
- Slurink, I.A.L.; Vogtschmidt, Y.D.; Brummel, B.; Smeets, T.; Kupper, N.; Soedamah-Muthu, S.S. Dairy intake in relation to prediabetes and continuous glycemic outcomes: A systematic review and dose-response meta-analysis of prospective cohort studies. Curr. Dev. Nutr. 2024, 8, 104470. [Google Scholar] [CrossRef]
- Kaluza, J.; Komatsu, S.; Lauriola, M.; Harris, H.R.; Bergkvist, L.; Michaëlsson, K.; Wolk, A. Long-term consumption of non-fermented and fermented dairy products and risk of breast cancer by estrogen receptor status—Population-based prospective cohort study. Clin. Nutr. 2021, 40, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Actor, J.K.; Kruzel, M.L. The potential for lactoferrin to reduce SARS-CoV-2 induced cytokine storm. Int. Immunopharmacol. 2021, 95, 107571. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Bioactives in bovine milk: Chemistry, technology, and applications. Nutr. Rev. 2021, 79, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Eck, N.; Waltman, L. Manual for VOSviewer Version, 1 6 18. 2022. Available online: https://www.vosviewer.com/ (accessed on 19 May 2025).
- Fortune Buisness Insights. Available online: www.fortunebusinessinsight.com (accessed on 24 February 2025).
- Artym, J. Lactoferrin-An Unusual Protein; Borgis Sp. z o.o.: Warszawa, Poland, 2012. (In Polish) [Google Scholar]
- Superti, F. Lactoferrin from bovine milk: A protective companion for life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Lomax, K.J.; Gallin, J.I.; Rotrosen, D.; Raphael, G.D.; Kaliner, M.A.; Benz, E.J., Jr.; Boxer, L.A.; Malech, H.L. Selective defect in myeloid cell lactoferrin gene expression in neutrophil specific granule deficiency. J. Clin. Investig. 1989, 83, 514–519. [Google Scholar] [CrossRef]
- Chen, M.; Wen, F.; Zhang, Y.; Li, P.; Zheng, N.; Wang, J. Determination of native lactoferrin in milk by HPLC on HiTrapTM Heparin HP column. Food Anal. Methods 2019, 12, 2518–2526. [Google Scholar] [CrossRef]
- Tsakali, E.; Aggarwal, R.K.; Houhoula, D.; Konteles, S.; Batrinou, A.; Verheyen, D.; Van Impe, J.F.M.; Chatzilazarou, A. Lactoferrin in breast milk-based powders. J. Dairy Res. 2023, 90, 409–412. [Google Scholar] [CrossRef]
- Ostertag, F.; Sommer, D.; Berensmeier, S.; Hinrichs, J. Development and validation of an enzyme-linked immunosorbent assay for the determination of bovine lactoferrin in various milk products. Int. Dairy J. 2022, 125, 105246. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Qin, H.; Yan, M.; Zhu, C.; Li, L.; Qu, F. Self-responsive fluorescence aptasensor for lactoferrin determination in dairy products. Molecules 2024, 29, 3013. [Google Scholar] [CrossRef]
- Frueh, J.L.; Shu, P.; Vennard, T.R.; Gray, M.A.; Phillips, S.C. Determination of bovine lactoferrin in powdered infant formula and adult nutritionals by heparin affinity extraction and reverse-phase high-performance liquid chromatography/ultraviolet detection (HPLC/UV): Single-laboratory validation, First Action 2021.10. J. AOAC Internat. 2024, 107, 693–704. [Google Scholar]
- Dyrda-Terniuk, T.; Pomastowski, P. The multifaceted roles of bovine lactoferrin: Molecular structure, isolation methods, analytical characteristics, and biological properties. J. Agric. Food Chem. 2023, 71, 20500–20531. [Google Scholar] [CrossRef] [PubMed]
- Schwerin, M.; Solinas Toldo, S.; Eggen, A.; Brunner, R.; Seyfert, H.M.; Fries, R. The bovine lactoferrin gene (LTF) maps to chromosome 22 and syntenic group U12. Mamm. Genome 1994, 5, 486–489. [Google Scholar] [CrossRef]
- Jiang, R.; Du, X.; Lönnerdal, B. Comparison of bioactivities of talactoferrin and lactoferrins from human and bovine milk. J. Pediatr. Gastroenterol. Nutrit. 2014, 59, 642–652. [Google Scholar] [CrossRef]
- Kim, B.J.; Kuhfeld, R.F.; Haas, J.L.; Anaya, Y.M.; Martinez, R.R.; Sah, B.N.P.; Breen, B.; Newsham, K.; Malinczak, C.-A.; Dallas, D.C. Digestive profiles of human milk, recombinant human and bovine lactoferrin: Comparing the retained intact protein and peptide release. Nutrients 2024, 16, 2360. [Google Scholar] [CrossRef]
- Takayama, Y.; Mizumachi, K. Effect of bovine lactoferrin on extracellular matrix calcification by human osteoblast-like cells. Biosci. Biotechnol. Biochem. 2008, 72, 226–230. [Google Scholar] [CrossRef]
- Eker, F.; Bolat, E.; Pekdemir, B.; Duman, H.; Karav, S. Lactoferrin: Neuroprotection against Parkinson’s disease and secondary molecule for potential treatment. Front. Aging Neurosci. 2023, 15, 1204149. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Veerakumarasivam, A.; Lim, W.L.; Chew, J. Neuroprotective effects of lactoferrin in Alzheimer’s and Parkinson’s diseases: A narrative review. ACS Chem. Neurosci. 2023, 14, 1342–1355. [Google Scholar] [CrossRef]
- Ashraf, M.F.; Zubair, D.; Bashir, M.N.; Alagawany, M.; Ahmed, S.; Shah, Q.A.; Buzdar, J.A.; Arain, M.A. Nutraceutical and health-promoting potential of lactoferrin, an iron-binding protein in human and animal: Current knowledge. Biol. Trace Elem. Res. 2024, 202, 56–72. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Ghazanfar, S.; Abdel-Hamid, M.; Abdel-Latif, H.M.R.; Zhang, Z.; Naiel, M.A.E. Therapeutic uses and applications of bovine lactoferrin in aquatic animal medicine: An overview. Vet. Res. Commun. 2023, 47, 1015–1029. [Google Scholar] [CrossRef]
- Lakshman, D.K.; Natarajan, S.; Mandal, S.; Mitra, A. Lactoferrin-derived resistance against plant pathogens in transgenic plants. J. Agric. Food Chem. 2013, 61, 11730–11735. [Google Scholar] [CrossRef]
- Buziashvili, A.; Yemets, A. Lactoferrin and its role in biotechnological strategies for plant defense against pathogens. Transgenic Res. 2023, 32, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Artym, J. Lactoferrrin—A sensor and regulator of iron absorption. Adv. Cell Biol. 2015, 42, 283–308. (In Polish) [Google Scholar]
- Cutone, A.; Ianiro, G.; Lepanto, M.S.; Rosa, L.; Valenti, P.; Bonaccorsi di Patti, M.C.; Musci, G. Lactoferrin in the prevention and treatment of intestinal inflammatory pathologies associated with colorectal cancer development. Cancers 2020, 12, 3806. [Google Scholar] [CrossRef] [PubMed]
- Niero, G.; Thomas, S.A.; Mouratidou, K.; Visentin, G.; De Marchi, M.; Penasa, M.; Cassandro, M. Lactoferrin concentration in bovine milk: Validation of radial immunodiffusion technique, sources of variation, and association to udder health status. Ital. J. Anim. Sci. 2023, 22, 230–238. [Google Scholar] [CrossRef]
- Musayeva, K.; Sederevičius, A.; Želvytė, R.; Monkevičienė, I.; Beliavska-Aleksiejūnė, D.; Kerzienė, S. Concentration of lactoferrin and immunoglobulin G in cows’ milk in relation to health status of the udder, lactation and season. Pol. J. Vet. Sci. 2016, 19, 737–744. [Google Scholar] [CrossRef]
- Ujita, A.; Negrão, J.A.; Filho, A.E.V.; Fernandes, A.R.; Faro, L.E. Milk lactoferrin and milk constituents in dairy Gyr heifers. Livest. Sci. 2019, 226, 87–92. [Google Scholar] [CrossRef]
- Tsakali, E.; Chatzilazarou, A.; Houhoula, D.P.; Koulouris, S.; Tsaknis, J.; Van Impe, J.F.M. A rapid HPLC method for the determination of lactoferrin in milk of various species. J. Dairy Res. 2019, 86, 238–241. [Google Scholar] [CrossRef]
- Pang, J.; Xiao, Q.; Yan, H.; Cao, Y.; Miao, J.; Wang, S.; Li, X.; Li, H.; Cheng, Z. Bovine lactoferrin quantification in dairy products by a simple immunoaffinity magnetic purification method coupled with high-performance liquid chromatography with fluorescence detection. J. Agric. Food Chem. 2020, 68, 892–898. [Google Scholar] [CrossRef]
- Dupont, D.; Arnould, C.; Rolet-Repecaud, O.; Duboz, G.; Faurie, F.; Martin, B.; Beuvier, E. Determination of bovine lactoferrin concentrations in cheese with specific monoclonal antibodies. Int. Dairy J. 2006, 16, 1081–1087. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Actor, J.K.; Zimecki, M.; Wise, J.; Płoszaj, P.; Mirza, S.; Hwang, S.-A.; Ba, X.; Boldogh, I. Novel recombinant human lactoferrin: Differential activation of oxidative stress related gene expression. J. Biotechnol. 2013, 168, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Gruden, Š.; Poklar Ulrih, N. Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrin-derived peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Giansanti, F.; Boffi, A.; Ajello, M.; Valenti, P.; Chiancone, E.; Antonini, G. Ca2+ binding to bovine lactoferrin enhances protein stability and influences the release of bacterial lipopolysaccharide. Biochem. Cell Biol. 2002, 80, 41–48. [Google Scholar] [CrossRef]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.-s.; Yang, Z.; Chen, Z. Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr. 2023, 9, 1018336. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Figueroa, B.; Valdiviezo-Godina, N.; Siqueiros-Cendón, T.; Sinagawa-García, S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. High-level expression of recombinant bovine lactoferrin in Pichia pastoris with antimicrobial activity. Int. J. Mol. Sci. 2016, 17, 902. [Google Scholar] [CrossRef]
- García-Borjas, K.A.; Ceballos-Olvera, I.; Luna-Castro, S.; Peña-Avelino, Y. Bovine lactoferrin can decrease the in vitro biofilm production and show synergy with antibiotics against Listeria and Escherichia coli isolates. Protein Pept. Lett. 2021, 28, 101–107. [Google Scholar] [CrossRef]
- Ciccaglione, A.F.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Cellini, L.; Marzio, L. Bovine lactoferrin enhances the efficacy of levofloxacin-based triple therapy as first-line treatment of Helicobacter pylori infection: An in vitro and in vivo study. J. Antimicrob. Chemother. 2019, 74, 1069–1077. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Prebiotic and modulatory evidence of lactoferrin on gut health and function. J. Funct. Foods 2023, 108, 105741. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Chen, P.-W. Featured prebiotic agent: The roles and mechanisms of direct and indirect prebiotic activities of lactoferrin and its application in disease control. Nutrients 2023, 15, 2759. [Google Scholar] [CrossRef]
- Chen, P.W.; Jheng, T.T.; Shyu, C.L.; Mao, F.C. Antimicrobial potential for the combination of bovine lactoferrin or its hydrolysate with lactoferrin-resistant probiotics against foodborne pathogens. J. Dairy Sci. 2013, 96, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Rahman, M.M.; Kumura, H.; Shimazaki, K.-I. Comparison of growth promoting effects on Bifidobacterium spp. by bovine lactoferrin hydrolysates. Biosci. Microflora 2005, 24, 119–123. [Google Scholar] [CrossRef][Green Version]
- Chen, P.-W.; Liu, Z.-S.; Kuo, T.-C.; Hsieh, M.-C.; Li, Z.-W. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. BioMetals 2017, 30, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.H.; Green, I.; Rich, R.R.; Schade, A.L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: Relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infec. Dis. 1971, 124, 539–544. [Google Scholar] [CrossRef]
- Nikawa, H.; Samaranayake, L.P.; Tenovuo, J.; Pang, K.M.; Hamada, T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch. Oral Biol. 1993, 38, 1057–1063. [Google Scholar] [CrossRef]
- Ikadai, H.; Tanaka, T.; Shibahara, N.; Tanaka, H.; Matsuu, A.; Kudo, N.; Shimazaki, K.; Igarashi, I.; Oyamada, T. Inhibitory effect of lactoferrin on in vitro growth of Babesia caballi. Am. J. Trop. Med. Hyg. 2005, 73, 710–712. [Google Scholar] [CrossRef]
- Wilson, M.E.; Britigan, B.E. Iron acquisition by parasitic protozoa. Parasitol. Today 1998, 14, 348–353. [Google Scholar] [CrossRef]
- Frontera, L.S.; Moyano, S.; Quassollo, G.; Lanfredi-Rangel, A.; Rópolo, A.S.; Touz, M.C. Lactoferrin and lactoferricin endocytosis halt Giardia cell growth and prevent infective cyst production. Sci. Rep. 2018, 8, 18020. [Google Scholar] [CrossRef]
- Aguilar-Diaz, H.; Canizalez-Roman, A.; Nepomuceno-Mejia, T.; Gallardo-Vera, F.; Hornelas-Orozco, Y.; Nazmi, K.; Bolscher, J.G.M.; Carrero, J.C.; Leon-Sicairos, C.; Leon-Sicairos, N. Parasiticidal effect of synthetic bovine lactoferrin peptides on the enteric parasite Giardia intestinalis. Biochem. Cell Biol. 2017, 95, 82–90. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Ertürk, M.; Karav, S. The potential of lactoferrin as antiviral and immune-modulating agent in viral infectious diseases. Front. Immunol. 2024, 15, 1402135. [Google Scholar] [CrossRef]
- Shestakov, A.; Jenssen, H.; Nordström, I.; Eriksson, K.M. Lactoferricin but not lactoferrin inhibit herpes simplex virus type 2 infection in mice. Antivir. Res. 2012, 93, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Krzyzowska, M.; Chodkowski, M.; Janicka, M.; Dmowska, D.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Bednarczyk, K.; Celichowski, G.; Grobelny, J. Lactoferrin-functionalized noble metal nanoparticles as new antivirals for HSV-2 infection. Microorganisms 2022, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, M.G.; Agamennone, M.; Pietrantoni, A.; Lannutti, F.; Siciliano, R.A.; De Giulio, B.; Amici, C.; Superti, F. Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus. Pathog. Glob. Health 2012, 106, 12–19. [Google Scholar] [CrossRef]
- Manns, M.P.; Buti, M.; Gane, E.; Pawlotsky, J.-M.; Razavi, H.; Terrault, N.; Younossi, Z. Hepatitis C virus infection. Nat. Rev. Dis. Primers 2017, 3, 17006. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Ikeda, M.; Saito, S.; Matsumoto, S.; Numata, K.; Kato, N.; Tanaka, K.; Sekihara, H. Lactoferrin inhibits hepatitis B virus infection in cultured human hepatocytes. Hepatol. Res. 2002, 24, 228–235. [Google Scholar] [CrossRef]
- Siciliano, R.; Rega, B.; Marchetti, M.; Seganti, L.; Antonini, G.; Valenti, P. Bovine lactoferrin peptidic fragments involved in inhibition of herpes simplex virus type 1 infection. Biochem. Biophys. Res. Commun. 1999, 264, 19–23. [Google Scholar] [CrossRef]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef]
- Arnold, D.; Di Biase, A.M.; Marchetti, M.; Pietrantoni, A.; Valenti, P.; Seganti, L.; Superti, F. Antiadenovirus activity of milk proteins: Lactoferrin prevents viral infection. Antivir. Res. 2002, 53, 153–158. [Google Scholar] [CrossRef]
- Superti, F.; Ammendolia, M.G.; Valenti, P.; Seganti, L. Antirotaviral activity of milk proteins: Lactoferrin prevents rotavirus infection in the enterocyte-like cell line HT-29. Med. Microb. Immunol. 1997, 186, 83–91. [Google Scholar] [CrossRef]
- Tinari, A.; Pietrantoni, A.; Ammendolia, M.G.; Valenti, P.; Superti, F. Inhibitory activity of bovine lactoferrin against echovirus induced programmed cell death in vitro. Int. J. Antimicrob. Agents 2005, 25, 433–438. [Google Scholar] [CrossRef]
- Oda, H.; Kolawole, A.O.; Mirabelli, C.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Wobus, C.E. Antiviral effects of bovine lactoferrin on human norovirus. Biochem. Cell Biol. 2020, 99, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The lactoferrin phenomenon—A miracle molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef]
- Krolitzki, E.; Schwaminger, S.P.; Pagel, M.; Ostertag, F.; Hinrichs, J.; Berensmeier, S. Current practices with commercial scale bovine lactoferrin production and alternative approaches. Int. Dairy J. 2022, 126, 105263. [Google Scholar] [CrossRef]
- Narmuratova, Z.; Hentati, F.; Girardet, J.-M.; Narmuratova, M.; Cakir-Kiefer, C. Equine lactoferrin: Antioxidant properties related to divalent metal chelation. LWT Food Sci. Technol. 2022, 161, 113426. [Google Scholar] [CrossRef]
- Burrow, H.; Kanwar, R.K.; Mahidhara, G.; Kanwar, J.R. Effect of selenium-saturated bovine lactoferrin (Se-bLF) on antioxidant enzyme activities in human gut epithelial cells under oxidative stress. Anti-Cancer Agents Med. Chem. 2011, 11, 762–771. [Google Scholar] [CrossRef]
- Rascón-Cruz, Q.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Nakamura-Bencomo, S.I.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A glycoprotein involved in immunomodulation, anticancer, and antimicrobial processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef]
- Figueroa-Lozano, S.; Valk-Weeber, R.L.; Akkerman, R.; Abdulahad, W.; Van Leeuwen, S.S.; Dijkhuizen, L.; De Vos, P. Inhibitory effects of dietary N-glycans from bovine lactoferrin on toll-like receptor 8; comparing efficacy with chloroquine. Front. Immunol. 2020, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Valk-Weeber, R.L.; Eshuis-De Ruiter, T.; Dijkhuizen, L.; Van Leeuwen, S.S. Dynamic temporal variations in bovine lactoferrin glycan structures. J. Agric. Food Chem. 2020, 68, 549–560. [Google Scholar] [CrossRef]
- Legrand, D. Overview of lactoferrin as a natural immune modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a context of inflammation-induced pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Hu, L.; Hu, X.; Long, K.; Gao, C.; Dong, H.L.; Zhong, Q.; Gao, X.M.; Gong, F.Y. Extraordinarily potent proinflammatory properties of lactoferrin-containing immunocomplexes against human monocytes and macrophages. Sci. Rep. 2017, 7, 4230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lima, C.F.; Rodrigues, L.R. Anticancer effects of lactoferrin: Underlying mechanisms and future trends in cancer therapy. Nutr. Rev. 2014, 72, 763–773. [Google Scholar] [CrossRef]
- Ramírez-Rico, G.; Drago-Serrano, M.E.; León-Sicairos, N.; de la Garza, M. Lactoferrin: A nutraceutical with activity against colorectal cancer. Front. Pharmacol. 2022, 13, 855852. [Google Scholar] [CrossRef]
- Duarte, D.C.; Nicolau, A.; Teixeira, J.A.; Rodrigues, L.R. The effect of bovine milk lactoferrin on human breast cancer cell lines. J. Dairy Sci. 2011, 94, 66–76. [Google Scholar] [CrossRef]
- Yu-Tang, T.; Chen, H.C.; Yen, C.C.; Lee, P.Y.; Tsai, H.C.; Lin, M.F.; Chen, M.C. Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor. J. Dairy Sci. 2013, 96, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Jiang, H.R.; Li, H.B.; Zhang, T.N.; Zhou, Q.; Liu, N. Apoptosis of stomach cancer cell SGC-7901 and regulation of Akt signaling way induced by bovine lactoferrin. J. Dairy Sci. 2010, 93, 2344–2350. [Google Scholar] [CrossRef]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Febriyanti Ayuningtyas, N.; Subarnbhesaj, A.; Nguyen, P.T.; Shrestha, M.; Haing, S.; Ohta, K.; Takata, T. Molecular mechanism of inhibitory effects of bovine lactoferrin on the growth of oral squamous cell carcinoma. PLoS ONE 2018, 13, e0191683. [Google Scholar] [CrossRef]
- Guedes, J.P.; Pereira, C.S.; Rodrigues, L.R.; Côrte-Real, M. Bovine milk lactoferrin selectively kills highly metastatic prostate cancer PC-3 and osteosarcoma MG-63 cells in vitro. Front. Oncol. 2018, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Antonini, G. Controversial role of lactoferrin in cancer: A narrative review. Biomed. Pharmacother. 2024, 181, 117743. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi Di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s anti-cancer properties: Safety, selectivity, and wide range of action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Nakamura-Bencomo, S.; Gutierrez, D.A.; Robles-Escajeda, E.; Iglesias-Figueroa, B.; Siqueiros-Cendón, T.S.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Aguilera, R.J.; Rascón-Cruz, Q.; Varela-Ramirez, A. Recombinant human lactoferrin carrying humanized glycosylation exhibits antileukemia selective cytotoxicity, microfilament disruption, cell cycle arrest, and apoptosis activities. Investig. New Drugs 2021, 39, 400–415. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, L.M.; Guzman, M.L.; Rivella, S. Iron and reactive oxygen species: Friends or foes of cancer cells? Antiox. Redox Signal. 2014, 20, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Massodi, I.; Thomas, E.; Raucher, D. Application of thermally responsive elastin-like polypeptide fused to a lactoferrin-derived peptide for treatment of pancreatic cancer. Molecules 2009, 14, 1999–2015. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Menéndez, S.; Peixoto, R.R.A.; Fernández-Colomer, B.; Suarez-Rodríguez, M.; Sanz-Medel, A.; Fernández-Sánchez, M.L. Effect of holder pasteurisation on total concentrations and iron-binding profiles of holo-lactoferrin used as fortifier in donor human milk. Int. Dairy J. 2020, 100, 104564. [Google Scholar] [CrossRef]
- Liu, H.; Boggs, I.; Weeks, M.; Li, Q.; Wu, H.; Harris, P.; Ma, Y.; Day, L. Kinetic modelling of the heat stability of bovine lactoferrin in raw whole milk. J. Food Eng. 2020, 280, 109977. [Google Scholar] [CrossRef]
- Darmawan, K.K.; Karagiannis, T.C.; Hughes, J.G.; Small, D.M.; Hung, A. High temperature induced structural changes of apo-lactoferrin and interactions with β-lactoglobulin and α-lactalbumin for potential encapsulation strategies. Food Hydrocoll. 2020, 105, 105817. [Google Scholar] [CrossRef]
- Goulding, D.A.; O’Regan, J.; Bovetto, L.; O’Brien, N.M.; O’Mahony, J.A. Influence of thermal processing on the physicochemical properties of bovine lactoferrin. Int. Dairy J. 2021, 119, 105001. [Google Scholar] [CrossRef]
- Saito, H.; Takase, M.; Tamura, Y.; Shimamura, S.; Tomita, M. Physicochemical and antibacterial properties of lactoferrin and its hydrolysate produced by heat treatment at acidic pH. Adv. Exp. Med. Biol. 1994, 357, 219–226. [Google Scholar]
- Sanchez, L.; Peiro, J.M.; Castillo, H.; Perez, M.D.; Ena, J.M.; Calvo, M. Kinetic parameters for denaturation of bovine milk lactoferrin. J. Food Sci. 1992, 57, 873–879. [Google Scholar] [CrossRef]
- Bengoechea, C.; Peinado, I.; McClements, D.J. Formation of protein nanoparticles by controlled heat treatment of lactoferrin: Factors affecting particle characteristics. Food Hydrocoll. 2011, 25, 1354–1360. [Google Scholar] [CrossRef]
- Sreedhara, A.; Flengsrud, R.; Prakash, V.; Krowarsch, D.; Langsrud, T.; Kaul, P.; Devold, T.G.; Vegarud, G.E. A comparison of effects of pH on the thermal stability and conformation of caprine and bovine lactoferrin. Int. Dairy J. 2010, 20, 487–494. [Google Scholar] [CrossRef]
- Khatoon, A.; Khan, U.M.; Kaur, M. Exploring the antimicrobial potentials and thermal stability of bovine lactoferrin against foodborne pathogens. Int. J. Food Sci. Technol. 2025, 60, vvae019. [Google Scholar] [CrossRef]
- Navarro, F.; Harouna, S.; Calvo, M.; Pérez, M.D.; Sánchez, L. Kinetic and thermodynamic parameters for thermal denaturation of ovine milk lactoferrin determined by its loss of immunoreactivity. J. Dairy Sci. 2015, 98, 4328–4337. [Google Scholar] [CrossRef] [PubMed]
- Mata, L.; Sánchez, L.; Headon, D.R.; Calvo, M. Thermal denaturation of human lactoferrin and its effect on the ability to bind iron. J. Agric. Food Chem. 1998, 46, 3964–3970. [Google Scholar] [CrossRef]
- Abe, H.; Saito, H.; Miyakawa, H.; Tamura, Y.; Shimamura, S.; Nagao, E.; Tomita, M. Heat stability of bovine lactoferrin at acidic pH. J. Dairy Sci. 1991, 74, 65–71. [Google Scholar] [CrossRef]
- Kim, W.-S. Effects of heat-treated bovine lactoferrin on the growth of Lactococcus lactis subsp. cremoris JCM 20076. J. Milk Sci. Biotechnol. 2019, 37, 129–135. [Google Scholar] [CrossRef]
- He, X.; Mao, L.; Gao, Y.; Yuan, F. Effects of high pressure processing on the structural and functional properties of bovine lactoferrin. Innov. Food Sci. Emerg. Technol. 2016, 38, 221–230. [Google Scholar] [CrossRef]
- Mazri, C.; Sánchez, L.; Ramos, S.J.; Calvo, M.; Pérez, M.D. Effect of high-pressure treatment on denaturation of bovine lactoferrin and lactoperoxidase. J. Dairy Sci. 2012, 95, 549–557. [Google Scholar] [CrossRef]
- Patel, H.A.; Singh, H.; Anema, S.G.; Creamer, L.K. Effects of heat and high-hydrostatic pressure treatments on the aggregation of whey proteins in whey protein concentrate solutions. Food New Zealand 2004, 4, 29–35. [Google Scholar]
- Iucci, L.; Patrignani, F.; Vallicelli, M.; Guerzoni, M.E.; Lanciotti, R. Effects of high pressure homogenization on the activity of lysozyme and lactoferrin against Listeria monocytogenes. Food Control 2007, 18, 558–565. [Google Scholar] [CrossRef]
- Grácia-Juliá, A.; René, M.; Cortés-Muñoz, M.; Picart, L.; López-Pedemonte, T.; Chevalier, D.; Dumay, E. Effect of dynamic high pressure on whey protein aggregation: A comparison with the effect of continuous short-time thermal treatments. Food Hydrocoll. 2008, 22, 1014–1032. [Google Scholar] [CrossRef]
- Sui, Q.; Roginski, H.; Williams, R.P.; Wooster, T.J.; Versteeg, C.; Wan, J. Effect of the ionic strength of pulsed electric field treatment medium on the physicochemical and structural characteristics of lactoferrin. J. Agric. Food Chem. 2010, 58, 11725–11731. [Google Scholar] [CrossRef] [PubMed]
- Franco, I.; Castillo, E.; Pérez, M.D.; Calvo, M.; Sánchez, L. Effect of bovine lactoferrin addition to milk in yogurt manufacturing. J. Dairy Sci. 2010, 93, 4480–4489. [Google Scholar] [CrossRef]
- Palmano, K.P.; Ramos, R.; Watson, M.; Callon, K.E.; Cornish, J. Survival and bone-active properties of bovine lactoferrin supplemented into stirred yoghurt. Int. Dairy J. 2011, 21, 477–483. [Google Scholar] [CrossRef]
- Morel, J.; Md Zain, S.N.; Archer, R. Comparison of drying techniques for bovine lactoferrin: Iron binding and antimicrobial properties of dried lactoferrin. Int. Dairy J. 2021, 124, 105142. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Barbosa-Pereira, L.; Vicente, A.A.; Cerqueira, M.A.; Pastrana, L. Dehydration of protein lactoferrin-glycomacropeptide nanohydrogels. Food Hydrocoll. 2020, 101, 105550. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Mild thermal treatment and in-vitro digestion of three forms of bovine lactoferrin: Effects on functional properties. Int. Dairy J. 2017, 64, 22–30. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Characteristics of bovine lactoferrin powders produced through spray and freeze drying processes. Int. J. Biol. Macromol. 2017, 95, 985–994. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Rożek, P.; Puta, M. The effect of freeze-drying and storage on lysozyme activity, lactoferrin content, superoxide dismutase activity, total antioxidant capacity and fatty acid profile of freeze-dried human milk. Dry. Technol. 2022, 40, 615–625. [Google Scholar] [CrossRef]
- Dao, H.M.; Sahakijpijarn, S.; Chrostowski, R.R.; Moon, C.; Mangolini, F.; Cui, Z.; Williams, R.O., 3rd. Aggregation of lactoferrin caused by droplet atomization process via a two-fluid nozzle: The detrimental effect of air-water interfaces. Mol. Pharm. J. 2022, 19, 2662–2675. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, T.; Dadmohammadi, Y.; Li, P.; Dong, H.; Yang, L.; He, Y.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Using transglutaminase to cross-link complexes of lactoferrin and α-lactalbumin to increase thermal stability. J. Food Sci. 2024, 89, 5488–5502. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.A.; Da Silva, S.L.; Da Silva, L.H.M.; Minim, V.P.R.; Da Silva, M.C.H.; Carvalho, L.M.; Minim, L.A. Cryogel poly(acrylamide): Synthesis, structure and applications. Sep. Purif. Rev. 2014, 43, 241–262. [Google Scholar] [CrossRef]
- Saleh, Z.S.; Hossain, M.M. A study of the separation of proteins from multicomponent mixtures by a semi-batch foaming process. Chem. Eng. Process. Process Intensif. 2001, 40, 371–378. [Google Scholar] [CrossRef]
- Lu, R.R.; Xu, S.Y.; Wang, Z.; Yang, R.J. Isolation of lactoferrin from bovine colostrum by ultrafiltration coupled with strong cation exchange chromatography on a production scale. J. Membr. Sci. 2007, 297, 152–161. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.Q.; Kentish, S.E. Isolation of lactoferrin and immunoglobulins from dairy whey by an electrodialysis with filtration membrane process. Sep. Purif. Technol. 2020, 233, 115987. [Google Scholar] [CrossRef]
- Pawar, S.S.; Iyyaswami, R.; Belur, P.D. Selective extraction of lactoferrin from acidic whey using CTAB/n-heptanol reverse micellar system. J. Food Sci. Technol. 2019, 56, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, S.; Radhakrishnan, U.; Eldho, L.; Kk, J. Isolation and purification of lactoferrin from colostrum of malabari goats. J. Exp. Biol. Agric. Sci. 2017, 5, 550–555. [Google Scholar] [CrossRef]
- Parc, A.L.; Dallas, D.C.; Duaut, S.; Léonil, J.; Martin, P.; Barile, D. Characterization of goat milk lactoferrin N-glycans and comparison with the N-glycomes of human and bovine milk. Electrophoresis 2014, 35, 1560–1570. [Google Scholar] [CrossRef]
- Abbas, Z.H.; Doosh, K.S.; Yaseen, N.Y. Isolation, Purification and characterization of lactoferrin from goat colostrum whey. Pak. J. Nutrit. 2015, 14, 517–523. [Google Scholar] [CrossRef][Green Version]
- Cheng, J.; Zhang, Z.; Yong-jiu, Z.; Zhao, J.; Li, J. Current advances in production and drying technologies of lactoferrin. J. Biobased Mater. Bioenergy 2024, 18, 339–358. [Google Scholar] [CrossRef]
- Baieli, M.F.; Urtasun, N.; Miranda, M.V.; Cascone, O.; Wolman, F.J. Bovine lactoferrin purification from whey using yellow HE-4R as the chromatographic affinity ligand. J. Sep Sci. 2014, 37, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Lv, X.; Sun, G.; Wu, W.Y.; Xu, H.; Li, Y.; Liu, Y.; Li, J.; Du, G.; Wang, M.; et al. Recent advances and prospects in purification and heterologous expression of lactoferrin. Food Bioeng. 2022, 1, 58–67. [Google Scholar] [CrossRef]
- Du, Q.-Y.; Lin, D.Q.; Xiong, Z.-S.; Yao, S.J. One-step purification of lactoferrin from crude sweet whey using cation-exchange expanded bed adsorption. Ind. Eng. Chem. Res. 2013, 52, 2693–2699. [Google Scholar] [CrossRef]
- Pilbrow, J.; Bekhit, A.E.A.; Carne, A. Fractionation of sheep cheese whey by a scalable method to sequentially isolate bioactive proteins. Food Chem. 2016, 203, 165–174. [Google Scholar] [CrossRef]
- Jin, Z. Expanded bed adsorption expanded bed adsorption—Challenges and advances in column and process design. Pharm. Eng. 2015, 35, 1–12. [Google Scholar]
- Kong, Y.; Liu, M.; Wei, D.; Wang, C.; Du, M.; Zhang, L. Purification and identification of lactoferrin from bovine milk. Adv. Mater. Res. 2012, 524–527, 2290–2293. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Xu, E.; Chen, L.; Feng, H.; Lu, C.; Deng, L.; Guo, D. Determination of lactoferrin in camel milk by ultrahigh-performance liquid chromatography-tandem mass spectrometry using an isotope-labeled winged peptide as internal standard. Molecules 2019, 24, 4199. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, X.; Wu, M.; Zhu, W. Simultaneous isolation of lactoferrin and lactoperoxidase from bovine colostrum by SPEC 70 SLS cation exchange resin. Int. J. Environ. Res. Public Health 2011, 8, 3764–3776. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tong, Z.; Zhang, X.; Dahmani, M.; Zhao, M.; Hu, M.; Li, X.; Xue, Z. A review: Development of a synthetic lactoferrin biological system. Biodes. Res. 2024, 6, 0040. [Google Scholar] [CrossRef]
- Narmuratova, M.; Cakir-Kiefer, C.; Narmuratova, Z. Isolation and purification of lactoferrin from Kazakhstan mare milk. Int. J. Biol. Chem. 2019, 12, 64–69. [Google Scholar] [CrossRef]
- Mahala, N.; Mittal, A.; Lal, M.; Dubey, U.S. Isolation and characterization of bioactive lactoferrin from camel milk by novel pH-dependent method for large scale production. Biotechnol. Rep. 2022, 36, e00765. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Wei, Z.; Shinmura, Y.; Fukunaga, N. Separation of lactoferrin-a and -b from bovine colostrum. J. Dairy Sci. 2000, 83, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Fee, C.J.; Chand, A. Capture of lactoferrin and lactoperoxidase from raw whole milk by cation exchange chromatography. Sep. Purif. Technol. 2006, 48, 143–149. [Google Scholar] [CrossRef]
- Wolman, F.J.; González Maglio, D.; Grasselli, M.; Cascone, O. One-step lactoferrin purification from bovine whey and colostrum by affinity membrane chromatography. J. Membr. Sci. 2007, 288, 132–138. [Google Scholar] [CrossRef]
- Fuda, E.; Jauregi, P.; Pyle, D.L. Recovery of lactoferrin and lactoperoxidase from sweet whey using colloidal gas aphrons (CGAs) generated from an anionic surfactant, AOT. Biotechnol. Prog. 2004, 20, 514–525. [Google Scholar] [CrossRef]
- Saufi, S.M.; Fee, C.J. Recovery of lactoferrin from whey using cross-flow cation exchange mixed matrix membrane chromatography. Sep. Purif. Technol. 2011, 77, 68–75. [Google Scholar] [CrossRef]
- Chen, L.; Guo, C.; Guan, Y.; Liu, H. Isolation of lactoferrin from acid whey by magnetic affinity separation. Sep. Purif. Technol. 2007, 56, 168–174. [Google Scholar] [CrossRef]
- Costa, A.R.d.; Jane Sélia dos Reis, C.; Ferreira, L.A.; Marcos, J.C.; Santos, I.J.B.; Saldaña, M.D.; Teixeira, J.A. Partitioning of bovine lactoferrin in aqueous two-phase system containing poly(ethylene glycol) and sodium citrate. Food Bioprod. Process. 2015, 95, 118–124. [Google Scholar] [CrossRef]
- Alvarez-Guerra, E.; Irabien, A. Extraction of lactoferrin with hydrophobic ionic liquids. Sep. Purif. Technol. 2012, 98, 432–440. [Google Scholar] [CrossRef]
- Krolitzki, E.; Ostertag, F.; Schwaminger, S.P.; Hinrichs, J.; Berensmeier, S. Purification of lactoferrin through a sequential filtration and magnetic separation process: A processing example for acid whey valorization. Future Foods 2024, 9, 100342. [Google Scholar] [CrossRef]
- Voswinkel, L.; Vogel, T.; Kulozik, U. Impact of the iron saturation of bovine lactoferrin on adsorption to a strong cation exchanger membrane. Int. Dairy J. 2016, 56, 134–140. [Google Scholar] [CrossRef]
- García-Montoya, I.; Salazar-Martínez, J.; Arévalo-Gallegos, S.; Sinagawa-García, S.; Rascón-Cruz, Q. Expression and characterization of recombinant bovine lactoferrin in E. coli. BioMetals 2013, 26, 113–122. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Z.; Yu, T.; Ding, F.; Li, L.; Wang, X.; Fu, M.; Wang, H.; Huang, J.; Li, N.; et al. Large-scale production of recombinant human lactoferrin from high-expression, marker-free transgenic cloned cows. Sci. Rep. 2017, 7, 10733. [Google Scholar] [CrossRef] [PubMed]
- Chahardoli, M.; Fazeli, A.; Ghabooli, M. Recombinant production of bovine lactoferrin-derived antimicrobial peptide in tobacco hairy roots expression system. Plant Physiol. Biochem. 2018, 123, 414–421. [Google Scholar] [CrossRef]
- Yen, C.-C.; Wu, P.-Y.; Ou-Yang, H.; Chen, H.-L.; Chong, K.-Y.; Chang, R.-L.; Chen, C.-M. Production of bioactive porcine lactoferrin through a novel glucose-inducible expression system in Pichia pastoris: Unveiling antimicrobial and anticancer functionalities. Int. J. Mol. Sci. 2024, 25, 1818. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, D.; Mori, T.; Hosaka, T.; Takaiwa, F.; Inoue, E.; Anzai, H. Production and characterization of recombinant human lactoferrin in transgenic Javanica rice. Breed. Sci. 2005, 55, 213–222. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, L.; Jia, S.; Chen, L.; Ma, Y. High-level expression and production of human lactoferrin in Pichia pastoris. Dairy Sci. Technol. 2008, 88, 173–181. [Google Scholar] [CrossRef]
- Lu, X.; Cummings, C.; Osuala, U.A.; Yennawar, N.H.; Namitz, K.E.W.; Hellner, B.; Besada-Lombana, P.B.; Peterson, R.D.; Clark, A.J. Characterization of recombinant human lactoferrin expressed in Komagataella phaffii. Analyst 2024, 149, 3636–3650. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chen, H.-L.; Yen, C.-C.; Kuo, M.-F.; Yang, T.-S.; Wang, S.-R.; Weng, C.-N.; Chen, C.-M.; Cheng, W.T.K. Recombinant porcine lactoferrin expressed in the milk of transgenic mice enhances offspring growth performance. J. Agric. Food Chem. 2007, 55, 4670–4677. [Google Scholar] [CrossRef]
- Jin, L.; Li, L.; Zhou, L.; Zhang, R.; Xu, Y.; Li, J. Improving expression of bovine lactoferrin N-lobe by promoter optimization and codon engineering in Bacillus subtilis and its antibacterial activity. J. Agric. Food Chem. 2019, 67, 9749–9756. [Google Scholar] [CrossRef]
- Hyvönen, P.; Suojala, L.; Orro, T.; Haaranen, J.; Simola, O.; Røntved, C.; Pyörälä, S. Transgenic cows that produce recombinant human lactoferrin in milk are not protected from experimental Escherichia coli intramammary infection. Infect. Immun. 2006, 74, 6206–6212. [Google Scholar] [CrossRef]
- Liang, Q.; Richardson, T. Expression and characterization of human lactoferrin in yeast Saccharomyces cerevisiae. J. Agric. Food Chem. 1993, 41, 1800–1807. [Google Scholar] [CrossRef]
- Chen, H.-L.; Lai, Y.-W.; Chen, C.-S.; Chu, T.-W.; Lin, W.; Yen, C.-C.; Lin, M.-F.; Tu, M.-Y.; Chen, C.-M. Probiotic Lactobacillus casei expressing human lactoferrin elevates antibacterial activity in the gastrointestinal tract. BioMetals 2010, 23, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.; Wu, S.; Wang, J.; Zhao, X.; Chen, J.; Zhang, X.; Hou, Y. Producing human lactoferrin by high-density fermentation recombinant Pichia pastoris. Chin. J. Exp. Clin. Virol. 2004, 18, 181–185. [Google Scholar]
- Zhang, X.; Xi, Z.; Zhao, H.; Zhang, W.; Xu, Y.; Zhang, R. Efficient heterologous expression of bovine lactoferrin in Pichia pastoris and characterization of antibacterial activity. Syst. Microbiol. Biomanufacturing 2025, 5, 237–248. [Google Scholar] [CrossRef]
- Ward, P.P.; May, G.S.; Headon, D.R.; Conneely, O.M. An inducible expression system for the production of human lactoferrin in Aspergillus nidulans. Gene 1992, 122, 219–223. [Google Scholar] [CrossRef]
- Sun, X.-L.; Baker, H.M.; Shewry, S.C.; Jameson, G.B.; Baker, E.N. Structure of recombinant human lactoferrin expressed in Aspergillus awamori. Acta Crystallogr. D 1999, 55, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Gajda-Morszewski, P.; Śpiewak-Wojtyła, K.; Oszajca, M.; Brindell, M. Strategies for oral delivery of metal-saturated lactoferrin. Curr. Protein Pept. Sci. 2019, 20, 1046–1051. [Google Scholar] [CrossRef]
- Cao, L.; Van de Walle, D.; Hirmz, H.; Wynendaele, E.; Dewettinck, K.; Parakhonskiy, B.V.; Skirtach, A.G. Food-based biomaterials: pH-responsive alginate/gellan gum/carboxymethyl cellulose hydrogel beads for lactoferrin delivery. Biomater. Adv. 2024, 165, 213999. [Google Scholar] [CrossRef]
- Wang, K.; Sun, H.; Cui, Z.; Wang, J.; Hou, J.; Lu, F.; Liu, Y. Lactoferrin–chitosan composite hydrogels induced by microbial transglutaminase: Potential delivery systems for thermosensitive bioactive substances. J. Agric. Food Chem. 2024, 72, 14302–14314. [Google Scholar] [CrossRef]
- Vergara, D.; López, O.; Bustamante, M.; Shene, C. An in vitro digestion study of encapsulated lactoferrin in rapeseed phospholipid–based liposomes. Food Chem. 2020, 321, 126717. [Google Scholar] [CrossRef] [PubMed]
- Gajda-Morszewski, P.; Poznańska, A.; Yus, C.; Arruebo, M.; Brindell, M. Encapsulation of iron-saturated lactoferrin for proteolysis protection with preserving iron coordination and sustained release. Nanomaterials 2023, 13, 2524. [Google Scholar] [CrossRef] [PubMed]
- Eilander, A.; Olumakaiye, M.; Moretti, D.; Zimmermann, M.; Owojuyigbe, T.; Blonk, C.; Duchateau, G. High bioavailability from ferric pyrophosphate-fortified bouillon cubes in meals is not increased by sodium pyrophosphate: A stable iron isotope study in young nigerian women. J. Nutr. 2019, 149, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Cercamondi, C.; Duchateau, G.; Harika, R.; Berg, R.; Murray, P.; Koppenol, W.; Moretti, D. Sodium pyrophosphate enhances iron bioavailability from bouillon cubes fortified with ferric pyrophosphate. Br. J. Nutr. 2016, 116, 496–503. [Google Scholar] [CrossRef]
- Choe, Y.; Oh, Y.; Lee, N.; Imoto, I.; Adachi, Y.; Toyoda, N.; Gabazza, E. Lactoferrin sequestration and its contribution to iron-deficiency anemia in helicobacter pylori-infected gastric mucosa. J. Gastroenterol. Hepatol. 2003, 18, 980–985. [Google Scholar] [CrossRef]
- Bayoumy, K.; Ragab, S.; Elghareeb, N. Compliance and efficacy of oral lactoferrin versus ferrous sulfate in treatment of nutritional iron deficiency anemia during second trimester among egyptian ladies. Egypt. J. Hosp. Med. 2021, 83, 903–909. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Teng, X.; Luo, J.; Luo, Y.; An, P. Comparative effects between oral lactoferrin and ferrous sulfate supplementation on iron-deficiency anemia: A comprehensive review and meta-analysis of clinical trials. Nutrients 2022, 14, 543. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M.; Kruzel, M. Lactoferrin for prevention and treatment of anemia and inflammation in pregnant women: A comprehensive review. Biomedicines 2021, 9, 898. [Google Scholar] [CrossRef]
- Farsy, M.; El-Hakim, I.; Rabie, H. Oral lactoferrin as a source of iron supplementation in pediatric hemodialysis patients. Geget 2021, 16, 23–30. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.; García-Montoya, I.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Nadi, W.G.; Taher, E.M.; Awad, A.A.N.; Ahmed, L.I. Lactoferrin’s potential application in enhancing yoghurt’s microbial and sensory qualities, with emphasis on the starter culture activity. J. Dairy Res. 2023, 90, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Jańczuk, A.; Brodziak, A.; Król, J.; Czernecki, T. Properties of yoghurt fortified in lactoferrin with effect of storage time. Animals 2023, 13, 1610. [Google Scholar] [CrossRef] [PubMed]
- Adnan, A.; Nadeem, M.; Ahmad, M.H.; Tayyab, M.; Kamran Khan, M.; Imran, M.; Iqbal, A.; Rahim, M.A.; Awuchi, C.G. Effect of lactoferrin supplementation on composition, fatty acids composition, lipolysis and sensory characteristics of cheddar cheese. Int. J. Food Prop. 2023, 26, 437–452. [Google Scholar] [CrossRef]
- Abad, I.; Pemán, L.; Pérez, M.D.; Grasa, L.; Sánchez, L. Does lactoferrin, free, encapsulated or in dairy matrices, maintain its antibacterial activity after in vitro digestion? J. Funct. Foods 2024, 112, 105936. [Google Scholar] [CrossRef]
- Ali, A.S.; Hasan, S.S.; Kow, C.S.; Merchant, H.A. Lactoferrin reduces the risk of respiratory tract infections: A meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 26–32. [Google Scholar] [CrossRef]
- Navarro, R.M.; Paredes, J.L.; Tucto, L.; Medina, C.; Angles-Yanqui, E.; Nario, J.C.; Ruiz-Cabrejos, J.; Quintana, J.L.; Turpo-Espinoza, K.; Mejia-Cordero, F.; et al. Bovine lactoferrin for the prevention of COVID-19 infection in health care personnel: A double-blinded randomized clinical trial (LF-COVID). BioMetals 2023, 36, 463–472. [Google Scholar] [CrossRef]
- Wang, W.; An, Q.; Huang, K.; Dai, Y.; Meng, Q.; Zhang, Y. Unlocking the power of lactoferrin: Exploring its role in early life and its preventive potential for adult chronic diseases. Food Res. Int. 2024, 182, 114143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).