Valorization of Grape Seed Cake by Subcritical Water Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Seed Cake
2.2. Methods
2.2.1. Analytical Methods
Proximate Composition of the Grape Seed Cake
Total Phenolics Content (TPC)
Total Flavonoids Content (TFC)
Total Antioxidant Capacity
DPPH Radical Scavenging Activity
Polyphenol Profile
Amino Acid Profile
Sugars
2.2.2. Subcritical Water Extraction
2.3. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of the Grape Seed Cake
3.2. Characterization of the Subcritical Water Extracts
3.2.1. Total Polyphenols, Total Flavonoids and Antioxidative Activity
3.2.2. Polyphenol Profiles
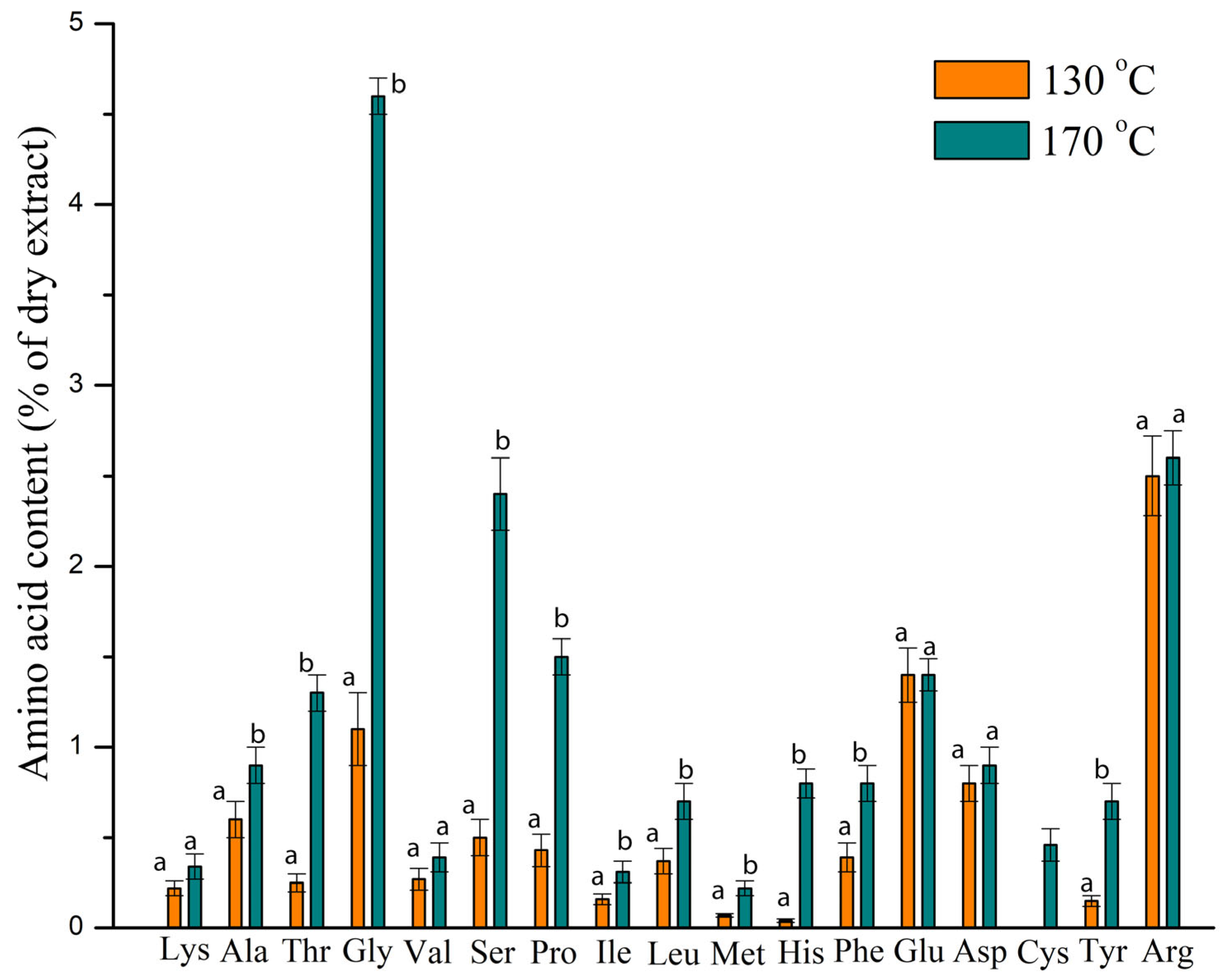
3.2.3. Total Protein and Amino Acid Profile
3.2.4. Sugars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baca-Bocanegra, B.; Nogales-Bueno, J.; Hernández-Hierro, J.M.; Heredia, F.J. Optimization of Protein Extraction of Oenological Interest from Grape Seed Meal Using Design of Experiments and Response Surface Methodology. Foods 2021, 10, 79. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shang, K.; Lin, C.; Wang, C.; Shi, X.; Wang, H.; Li, H. Processing technologies, phytochemical constituents, and biological activities of grape seed oil (GSO): A review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, C.; Rapinel, V.; Nguyen-Thanh, B.; Bonet-García, R.; Bartier, M.; Claux, O.; Jacques, L.; Tabasso, S.; Barrajón-Catalán, E.; Fabiano-Tixier, A.-S. Sustainable grape seed oil processing: Green solvent extraction and byproduct valorization. Food Bioprod. Process. 2025, 149, 428–438. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Malićanin, M.; Rac, V.; Antić, V.; Antić, M.; Palade, L.M.; Kefalas, P.; Rakić, V. Content of Antioxidants, Antioxidant Capacity and Oxidative Stability of Grape Seed Oil Obtained by Ultra Sound Assisted Extraction. J. Am. Oil Chem. Soc. 2014, 91, 989–999. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.; Cabezudo, I.; da Silva-James, N.; Teles, A.; Cruz, A.; Mellinger-Silva, C.; Tonon, R.; Cabral, L.; Freitas, S. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Muhlack, R.; Potumarthi, R.; Jeffery, D. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. TrAC—Trends Anal. Chem. 2023, 166, 117201. [Google Scholar] [CrossRef]
- Li, J.; Pettinato, M.; Campardelli, R.; De Marco, I.; Perego, P. High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Appl. Sci. 2022, 12, 3642. [Google Scholar] [CrossRef]
- Majeed, T.; Shabir, I.; Srivastava, S.; Maqbool, N.; Dar, A.H.; Jan, K.; Pandey, V.K.; Shams, R.; Bashir, I.; Dash, K.K. Valorization of food wastes by implementation of subcritical water extraction: A comprehensive review. Trends Food Sci. Technol. 2024, 143, 104316. [Google Scholar] [CrossRef]
- Gomez-Contreras, P.A.; Obando, C.; Freitas, P.A.V.d.; Martin-Perez, L.; Chiralt, A.; Gonzalez-Martinez, C. Applying Subcritical Water Extraction to Obtain Bioactive Compounds and Cellulose Fibers from Brewer Spent Grains. Molecules 2024, 29, 4897. [Google Scholar] [CrossRef]
- Trigueros, E.; Ramos, C.; Alonso-Riaño, P.; Beltrán, S.; Sanz, M.T. Subcritical Water Treatment for Valorization of the Red Algae Residue after Agar Extraction: Scale-Up from Laboratory to Pilot Plant. Ind. Eng. Chem. Res. 2023, 62, 3503–3514. [Google Scholar] [CrossRef]
- Santos, P.H.; Kammers, J.C.; Silva, A.P.; Oliveira, J.V.; Hense, H. Antioxidant and antibacterial compounds from feijoa leaf extracts obtained by pressurized liquid extraction and supercritical fluid extraction. Food Chem. 2021, 344, 128620. [Google Scholar] [CrossRef] [PubMed]
- Sumere, B.R.; de Souza, M.C.; Dos Santos, M.P.; Bezerra, R.M.N.; da Cunha, D.T.; Martinez, J.; Rostagno, M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem 2018, 48, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Domínguez, M.C.; Fuentes, J.L.; Mendiola, J.A.; Cerezal-Mezquita, P.; Morales, J.; Vílchez, C.; Ibáñez, E. Bioprospecting of cyanobacterium in Chilean coastal desert, Geitlerinema sp. molecular identification and pressurized liquid extraction of bioactive compounds. Food Bioprod. Process 2021, 128, 227–239. [Google Scholar] [CrossRef]
- Dobrincic, A.; Pedisic, S.; Zoric, Z.; Jurin, M.; Roje, M.; Coz-Rakovac, R.; Dragovic-Uzelac, V. Microwave Assisted Extraction and Pressurized Liquid Extraction of Sulfated Polysaccharides from Fucus virsoides and Cystoseira barbata. Foods 2021, 10, 1481. [Google Scholar] [CrossRef]
- Campos Mesquita, P.; Rodrigues, L.G.G.; Mazzutti, S.; Vasconcelos Ribeiro, P.R.; Sousa de Brito, E.; Lanza, M. Untargeted metabolomic profile of recovered bioactive compounds by subcritical water extraction of acerola (Malpighia emarginata DC.) pomace. Food Chem. 2022, 397, 133718. [Google Scholar] [CrossRef]
- Herbst, G.; Hamerski, F.; Errico, M.; Corazza, M.L. Pressurized liquid extraction of brewer’s spent grain: Kinetics and crude extracts characterization. J. Ind. Eng. Chem. 2021, 102, 370–383. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhang, J.; Yan, L.; Liu, S.; Aboueloyoun Taha, A.; Wang, J.; Ma, C. Subcritical water extraction of phenolic antioxidants with improved α-amylase and α-glucosidase inhibitory activities from exocarps of Castanea mollissima Blume. J. Supercrit. Fluids 2020, 158, 104747. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alanon, M.E. Implementation of subcritical water extraction with natural deep eutectic solvents for sustainable extraction of phenolic compounds from winemaking by-products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef]
- Sarfarazi, M.; Jafari, S.M.; Rajabzadeh, G.; Feizi, J. Development of an environmentally-friendly solvent-free extraction of saffron bioactives using subcritical water. LWT—Food Sci. Technol. 2019, 114, 108428. [Google Scholar] [CrossRef]
- Wu, G.; Dong, H.; Li, J.; Guo, L.; Cheng, Y.; Geng, Y.; Wang, X. Extraction of parishin B and parishin C from Gastrodiae Rhizoma by subcritical water technology. J. Ind. Eng. Chem. 2022, 108, 280–287. [Google Scholar] [CrossRef]
- Fan, X.; Ha, S.; Wang, K.; Yang, R.; Zhang, X. Coupling of ultrasound and subcritical water for peptides production from Spirulina platensis. Food Bioprod. Process. 2020, 121, 105–112. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielinska, M.; Bulkowska, K.; Witonska, I. Subcritical water extraction of bioactive phenolic compounds from distillery stillage. J. Environ. Manag. 2022, 318, 115548. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.C.; Tan, S.N.; Hong Yong, J.W.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Luong, D.; Sephton, M.; Watson, J. Subcritical water extraction of organic matter from sedimentary rocks. Anal. Chim. Acta 2015, 879, 48–57. [Google Scholar] [CrossRef]
- Ferreira, C.; Moreira, M.M.; Delerue-Matos, C.; Sarraguça, M. Subcritical Water Extraction to Valorize Grape Biomass—A Step Closer to Circular Economy. Molecules 2023, 28, 7538. [Google Scholar] [CrossRef]
- Silva, A.M.; Luís, A.S.; Moreira, M.M.; Ricardo Ferraz, R.; Brezo-Borjan, T.; Švarc-Gajić, J.; Paulo, C.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Influence of temperature on the subcritical water extraction of Actinidia arguta leaves: A screening of pro-healthy compounds. Sustain. Chem. Pharm. 2022, 25, 100593. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Rodrigues, F.; Moreira, M.; Delerue-Matos, C.; Morais, S.; Dorosh, O.; Silva, A.M.; Bassani, A.; Dzedik, V.; Spigno, G. Chemical composition and bioactivity of oilseed cake extracts obtained by subcritical and modified subcritical water. Bioresour. Bioprocess. 2022, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.; Bowra, S.; Cooper, H. Subcritical Water Processing of Proteins: An Alternative to Enzymatic Digestion? Anal. Chem. 2016, 88, 6425–6432. [Google Scholar] [CrossRef]
- Lazic, V.; Klaus, A.; Kozarski, M.; Doroški, A.; Tosti, T.; Simic, S.; Vunduk, J. The Effect of Green Extraction Technologies on the Chemical Composition of Medicinal Chaga Mushroom Extracts. J. Fungi 2024, 10, 225. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delure-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef]
- NMKL 169:2002; Dry Matter in Foodstuffs. The Vacuum Method. Nordic Committee on Food Analysis: Bergen, Norway, 2002.
- NMKL 173, 2nd Ed.:2005; Ash, Gravimetric Determination in Foods. Nordic Committee on Food Analysis: Bergen, Norway, 2005.
- ISO 16634-1:2008; Food Products—Determination of the Total Nitrogen Content by Combustion According to the Dumas Principle and Calculation of the Crude Protein Content. International Organization for Standardization: Geneva, Switzerland, 2008.
- NMKL 160:1998; Fat. Determination in Foods. Nordic Committee on Food Analysis: Bergen, Norway, 1998.
- AOAC 991.43:2000; Total, Soluble, and Insoluble Dietary Fiber in Foods. AOAC International: Rockville, MD, USA, 2000.
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Nicolescu, A.; Bunea, C.I.; Mocan, A. Total flavonoid content revised: An overview of past, present, and future determinations in phytochemical analysis. Anal. Biochem. 2025, 700, 115794. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Kostic, A.; Milincic, D.; Špirovic Trifunovic, B.; Nedic, N.; Gašic, U.; Tešic, Ž.; Stanojevic, S.; Pešic, M. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants 2023, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.; Stanisavljević, N.; Kostić, A.; Soković Bajić, S.; Kojić, M.; Gašić, U.; Barać, M.; Stanojević, S.; Tešić, Z.; Pešić, M. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Švarc-Gajic, J.; Cvetanovic, A.; Segura-Carretero, A.; Linares, I.B.; Maškovic, P. Characterisation of ginger extracts obtained by subcritical water. J. Supercrit. Fluids 2017, 123, 92–100. [Google Scholar] [CrossRef]
- Yalcin, H.; Kavuncuoglu, H.; Ekici, L.; Sagdic, O. Determination of Fatty Acid Composition, Volatile Components, Physico-Chemical and Bioactive Properties of Grape (Vitis vinifera) Seed and Seed Oil. J. Food Process. Preserv. 2017, 41, e12854. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ossorio, C.; Orive, M.; Sanmartín, E.; Alvarez-Sabatel, S.; Labidi, J.; Zufia, J.; Bald, C. Composition and Techno-functional Properties of Grape Seed Flour Protein Extracts. ACS Food Sci. Technol. 2022, 2, 125–135. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Malferrari, D.; Marchetti, A.; Roncaglia, F.; Tassi, L. Waste By-Product of Grape Seed Oil Production: Chemical Characterization for Use as a Food and Feed Supplement. Life 2023, 13, 326. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, W.; Shao, P.; Wu, W.; Chen, H.; Fang, X.; Mu, H.; Xiao, J.; Gao, H. Impact of thermal processing on dietary flavonoids. Curr. Opin. Food Sci. 2022, 48, 100915. [Google Scholar] [CrossRef]
- Ross, C.F.; Hoye, C.; Fernandez-Plotka, V.C. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci. 2011, 76, 884–890. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Kakuda, Y. Polyphenolics in Grape Seeds—Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Di Stefano, V.; Buzzanca, C.; Melilli, M.G.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol Characterization and Antioxidant Activity of Grape Seeds and Skins from Sicily: A Preliminary Study. Sustainability 2022, 14, 6702. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin–Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Ajuwon, K.M.; Zhong, R.; Li, T.; Chen, L.; Zhang, H.; Beckers, Y.; Everaert, N. Xylo-Oligosaccharides, Preparation and Application to Human and Animal Health: A Review. Front. Nutr. 2021, 8, 731930. [Google Scholar] [CrossRef] [PubMed]
- Pol, K.; Mars, M. L-arabinose and D-xylose: Sweet pentoses that may reduce postprandial glucose and insulin responses. Food Nutr. Res. 2021, 65, 6254. [Google Scholar] [CrossRef] [PubMed]
| Moisture | 8.8 ± 0.1 |
| Ash | 3.8 ± 0.1 |
| Lipids | 5.1 ± 0.1 |
| Proteins | 13.9 ± 0.2 |
| Carbohydrates | 68.2 ± 0.1 |
| Insoluble fiber | 57.0 ± 2.0 |
| Soluble fiber | 3.9 ± 0.4 |
| Sample | TPC (mg GAE/g) | TFC (mg RE/g) | TAA (mg AAE/g) | DPPH (mg AAE/g) |
|---|---|---|---|---|
| 130 °C | 35.5 ± 0.3 a | 1.52 ± 0.01 a | 69.1 ± 0.4 a | 0.915 ± 0.004 a |
| 170 °C | 33.0 ± 0.4 b | 1.56 ± 0.01 b | 55.4 ± 0.9 b | 0.979 ± 0.015 b |
| Compound Name | RT | Formula | m/z Exact Mass | Main MS Fragment | Samples | |
|---|---|---|---|---|---|---|
| 130 °C | 170 °C | |||||
| Phenolic acids and derivatives | ||||||
| Hydroxybenzoic acid and derivatives | ||||||
| Gallic acid b | 1.14 | C7H5O5− | 169.0162 | 125.02568 | 42 ± 2 A | 57 ± 4 B |
| Gallic acid hexoside is. I b | 1.75 | C13H15O10− | 331.0676 | 169.01615 | 2.88 ± 0.02 | - |
| Dihydroxybenzoic acid b | 2.83 | C7H5O4− | 153.0215 | 108.02274 | 99 ± 5 A | 99 ± 12 A |
| Gallic acid hexoside is. II b | 3.13 | C13H15O10− | 331.0676 | 125.02549 | 14.6 ± 0.2 | <LOQ |
| p-Hydroxybenzoic acid b | 4.91 | C7H5O3− | 137.0248 | / | 318 ± 2 A | 464 ± 1 B |
| Vanillic acid b | 5.86 | C8H7O4− | 167.0372 | 123.04583 | <LOQ | 4.9 ± 0.2 |
| p-Hydroxyphenylacetic acid b | 6.33 | C8H7O3− | 151.0410 | 108.02286 | <LOQ | 6.35 ± 0.05 |
| Homovanillic acid b | 7.14 | C9H9O4− | 181.0513 | 137.06204 | <LOQ | 7.12 ± 0.01 |
| Ethyl gallate b | 7.48 | C9H9O5− | 197.0467 | 124.01829 | 5.8 ± 0.2 A | 11.2 ± 0.5 B |
| Ellagic acid a | 7.88 | C14H5O8− | 301.0021 | 301.00206 | 17 ± 1 | - |
| ∑ | 499.28 | 649.57 | ||||
| Hydroxycinnamic acid and derivatives | ||||||
| Esculetin c | 4.14 | C9H5O4− | 177.0216 | 121.03093 | 30 ± 13 A | 38 ± 5 A |
| Caffeic acid c | 4.44 | C9H7O4− | 179.0363 | 179.03632 | 11.0 ± 0.3 A | 44.5 ± 0.9 B |
| Ferulic acid c | 5.39 | C10H9O4− | 193.0523 | 137.06222 | 10.99 ± 0.01 A | 97.3 ± 0.8 B |
| p-Coumaroyl tartaric acid c | 6.12 | C13H11O8− | 295.0466 | 119.05145 | <LOQ | - |
| p-Coumaric acid a | 6.60 | C9H7O3− | 163.0417 | 163.04169 | 1.76 ± 0.09 A | 11.4 ± 0.2 B |
| Methoxycinnamate c | 7.65 | C10H9O3− | 177.0576 | 133.03096 | 1.80 ± 0.09 A | 38.8 ± 0.7 B |
| p-Coumaric acid methyl ester c | 8.42 | C10H9O3− | 177.0568 | 162.02965 | 1.44 ± 0.02 A | 3.46 ± 0.04 B |
| Caffeic acid ethyl ester c | 9.74 | C11H11O4− | 207.0669 | 133.03073 | 1.11 ± 0.02 | <LOQ |
| ∑ | 58.1 | 233.46 | ||||
| Flavonoids and derivatives | ||||||
| Flavan-3-ols and derivatives | ||||||
| Gallocatechin d | 1.68 | C15H13O7− | 305.0683 | 109.03057 | 112 ± 3 | - |
| (Epi)catechin hexoside d | 5.18 | C21H23O11− | 451.1269 | 289.07553 | 24 ± 3 | - |
| Catechin a | 6.40 | C15H13O6− | 289.0739 | 123.04661 | 327 ± 5 A | 18.6 ± 0.9 B |
| Epicatechin 3-O-gallate d | 7.94 | C22H17O10− | 441.0843 | 169.01612 | 71.99 ± 0.09 | - |
| Epigallocatechin d | 8.49 | C15H13O7− | 305.0709 | 137.02601 | - | 163 ± 2 |
| Amurensisin d | 8.62 | C22H15O10− | 439.0721 | 287.02318 | 2.2 ± 0.4 | - |
| (Epi)catechin-(epi)afzelechin A-type d | 8.69 | C30H23O11− | 559.1270 | 269.04948 | 5.5 ± 0.2 | - |
| (Epi)gallocatechin ferulate is. I d | 8.73 | C25H21O10− | 481.1186 | 289.07584 | 2.92 ± 0.03 | - |
| Ethyl (epi)catechin-(epi)catechin d | 9.50 | C32H29O12− | 605.1703 | 315.09111 | 2.38 ± 0.01 | - |
| Afzelechin d | 10.51 | C15H13O5− | 273.0808 | 124.01742 | 7.3 ± 1.8 A | 3 ± 1 B |
| ∑ | 555.29 | 184.6 | ||||
| Pro(antho)cyanidins and derivatives | ||||||
| Procyanidin dimer B type is. I e | 6.07 | C30H25O12− | 577.1404 | 289.07544 | 12.9 ± 0.5 | - |
| Gambiriin A e | 6.34 | C30H27O12− | 579.1546 | 289.07568 | 18.73 ± 0.25 | - |
| Procyanidin dimer B type is. II e | 7.34 | C30H25O12− | 577.1403 | 289.07610 | 6.0 ± 0.6 | - |
| Procyanidin dimer B type gallate is. I e | 7.35 | C37H29O16− | 729.1512 | 407.08355 | 3.39 ± 0.06 | - |
| Procyanidin dimer B type gallate is. II e | 8.01 | C37H29O16− | 729.1521 | 729.1692 | <LOQ | - |
| ∑ | 41.02 | - | ||||
| ∑∑ total polyphenols | 1153.69 | 1067.63 | ||||
| 130 °C | 170 °C | |
| glucose | 1.8 ± 0.1 a | 1.04 ± 0.04 b |
| galactose/xylose + mannose | 0.58 ± 0.03 a | 1.76 ± 0.04 b |
| arabinose | 2.1 ± 0.1 a | 1.62 ± 0.02 b |
| xylobiose | 0 a | 3.16 ± 0.04 b |
| xylotriose | 2.88 ± 0.06 a | 4.7 ± 0.1 b |
| sucrose | 2.7 ± 0.2 a | 1.72 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malićanin, M.V.; Švarc-Gajić, J.; Lević, S.M.; Rac, V.A.; Salević-Jelić, A.S.; Pešić, M.B.; Milinčić, D.D.; Pasarin, D.; Rakić, V.M. Valorization of Grape Seed Cake by Subcritical Water Extraction. Processes 2025, 13, 1597. https://doi.org/10.3390/pr13051597
Malićanin MV, Švarc-Gajić J, Lević SM, Rac VA, Salević-Jelić AS, Pešić MB, Milinčić DD, Pasarin D, Rakić VM. Valorization of Grape Seed Cake by Subcritical Water Extraction. Processes. 2025; 13(5):1597. https://doi.org/10.3390/pr13051597
Chicago/Turabian StyleMalićanin, Marko V., Jaroslava Švarc-Gajić, Steva M. Lević, Vladislav A. Rac, Ana S. Salević-Jelić, Mirjana B. Pešić, Danijel D. Milinčić, Diana Pasarin, and Vesna M. Rakić. 2025. "Valorization of Grape Seed Cake by Subcritical Water Extraction" Processes 13, no. 5: 1597. https://doi.org/10.3390/pr13051597
APA StyleMalićanin, M. V., Švarc-Gajić, J., Lević, S. M., Rac, V. A., Salević-Jelić, A. S., Pešić, M. B., Milinčić, D. D., Pasarin, D., & Rakić, V. M. (2025). Valorization of Grape Seed Cake by Subcritical Water Extraction. Processes, 13(5), 1597. https://doi.org/10.3390/pr13051597












