Lignin Extracted from Green Coconut Waste Impregnated with Sodium Octanoate for Removal of Cu2+ in Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. GCF Collection and Processing
2.2. Alkaline Pre-Treatment of GCF
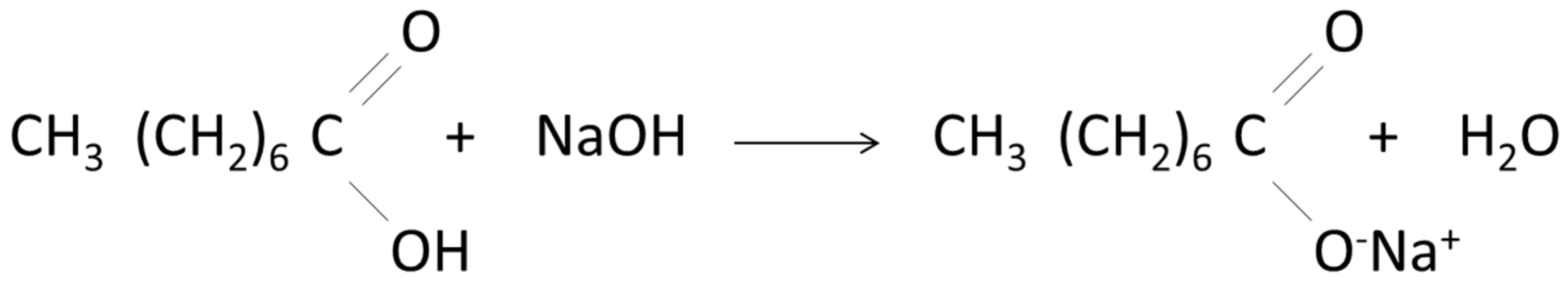
2.3. Synthesis of Sodium Octanoate
2.4. Impregnation of Sodium Octanoate in Lignin Surface
2.5. Study of Heavy Metal Adsorption
2.5.1. Batch Adsorption
2.5.2. Adsorption Kinetics
2.5.3. Adsorption Equilibrium Study
2.6. Bioadsorbent Characterization
3. Results and Discussion
3.1. Bioadsorbent Characteristics
3.1.1. Chemical Analysis by XRF
3.1.2. Adsorbent Surface Analysis by SEM
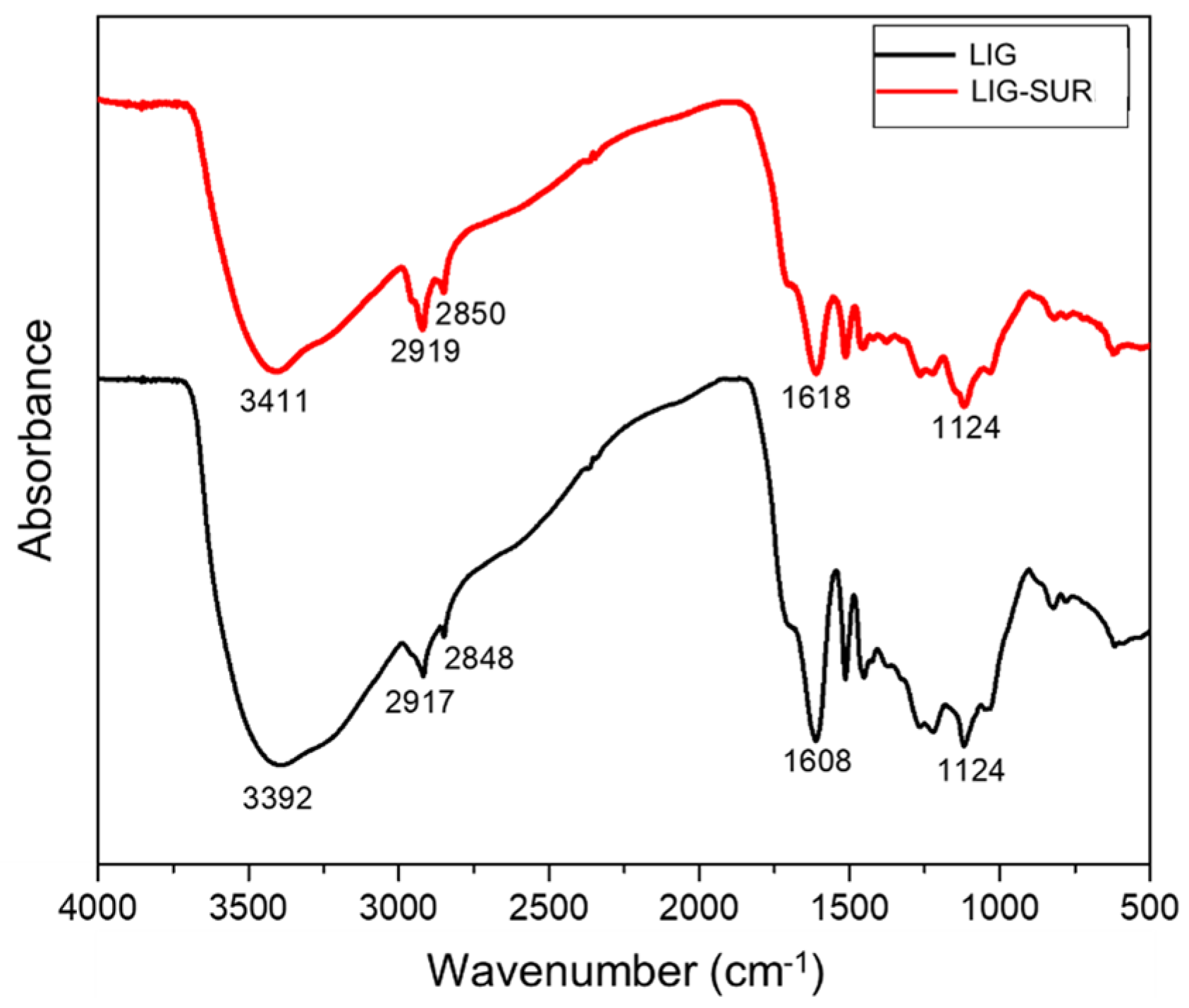
3.1.3. Molecular Structure Analysis by FTIR
3.2. Adsorption Parameter Effects
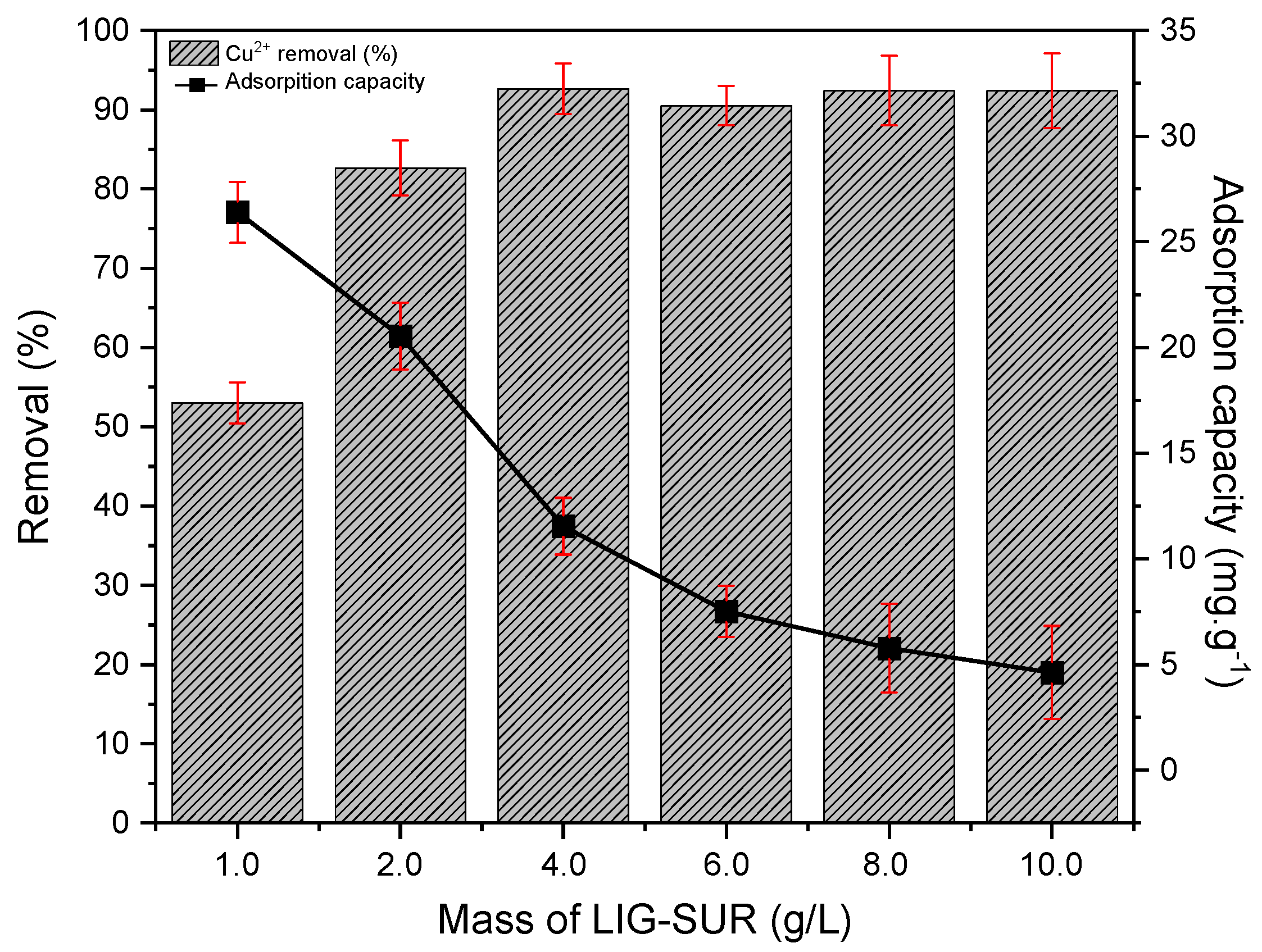
3.2.1. Bioadsorbent Mass
3.2.2. pH of Solution
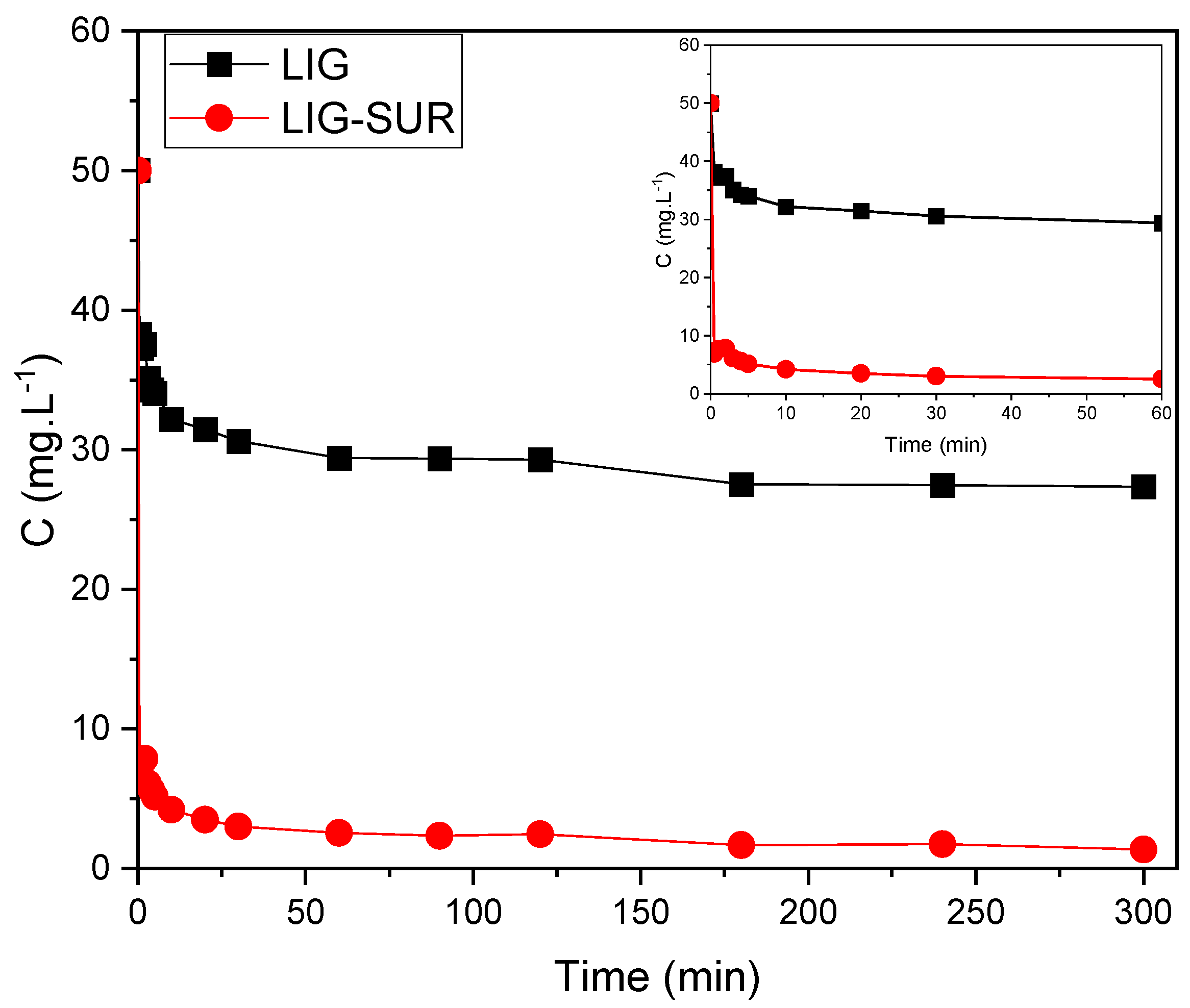
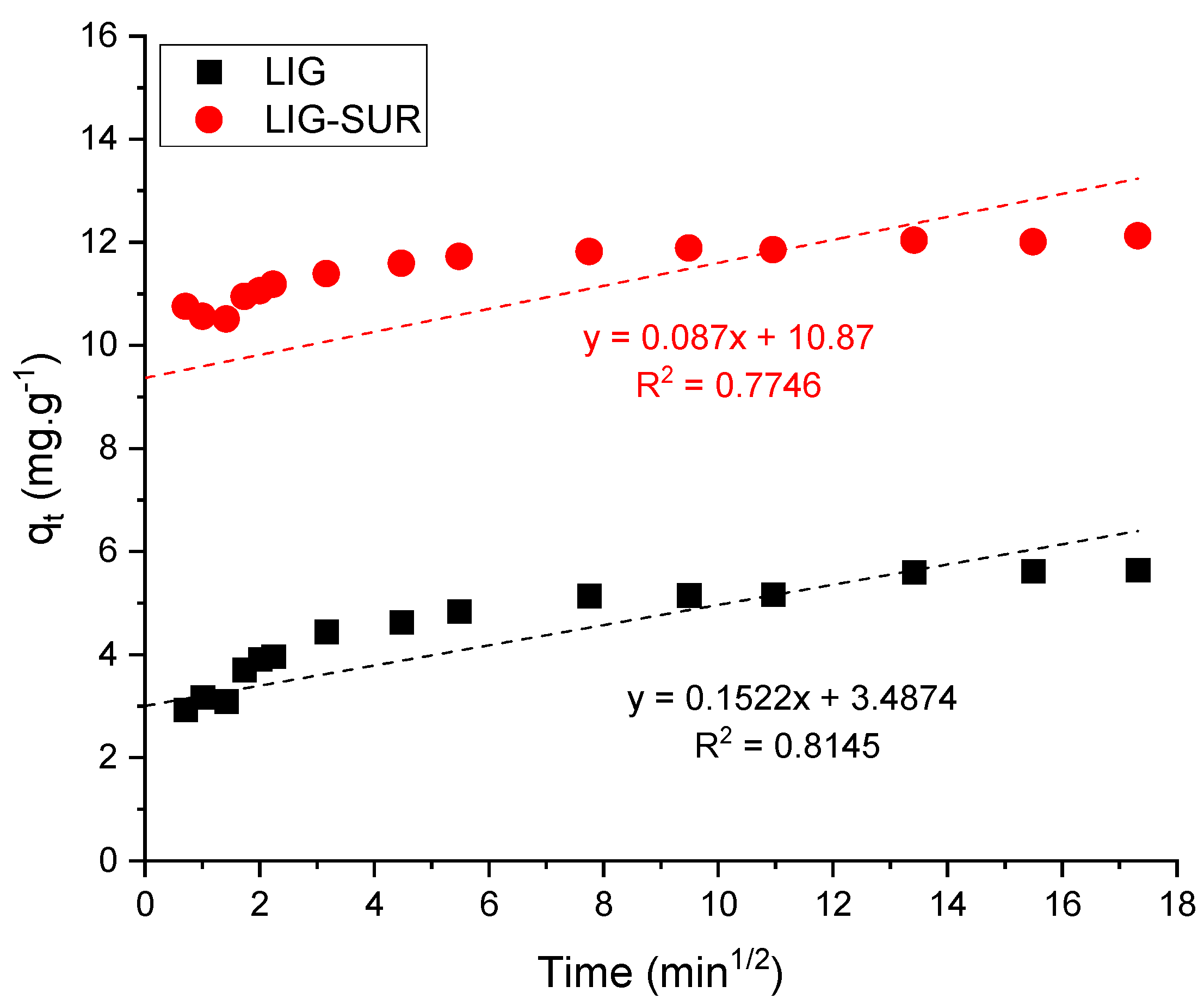
3.2.3. Kinetics
3.2.4. Adsorption Isotherms
4. Conclusions
- Chemical analysis by XRF and morphological analysis by SEM found that the impregnation of the surfactant, evidenced by the presence of Na2O, was partially replaced by CuO after adsorption.
- Microstructural analysis by FTIR indicated a slight shift and reduction in the intensity of the vibration bands of various chemical bonds in the lignin, such as O-H, C-H, C=O, and C=C, suggesting electrostatic interaction between the surfactant and the constituents of the lignin extracted from the green coconut fiber (GCF).
- The adsorption kinetics followed the pseudo-second-order model, indicating chemisorption. In addition, equilibrium was reached in around 10 and 60 min for LIG-SUR and LIG, respectively.
- The Langmuir model best described the isotherm data, suggesting monolayer adsorption with equivalent sites.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almomani, F.; Bhosale, R.R. Bio-Sorption of Toxic Metals from Industrial Wastewater by Algae Strains Spirulina Platensis and Chlorella Vulgaris: Application of Isotherm, Kinetic Models and Process Optimization. Sci. Total Environ. 2021, 755, 142654. [Google Scholar] [CrossRef] [PubMed]
- Malamis, S.; Katsou, E.; Haralambous, K.J. Study of Ni(II), Cu(II), Pb(II), and Zn(II) Removal Using Sludge and Minerals Followed by MF/UF. Water Air Soil. Pollut. 2011, 218, 81–92. [Google Scholar] [CrossRef]
- Sajadi, S.M.; Kolo, K.; Pirouei, M.; Mahmud, S.A.; Ali, J.A.; Hamad, S.M. Natural Iron Ore as a Novel Substrate for the Biosynthesis of Bioactive-Stable ZnO@CuO@iron Ore NCs: A Magnetically Recyclable and Reusable Superior Nanocatalyst for the Degradation of Organic Dyes, Reduction of Cr(VI) and Adsorption of Crude. RSC Adv. 2018, 8, 35557–35570. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- James, A.; Yadav, D. Valorization of Coconut Waste for Facile Treatment of Contaminated Water: A Comprehensive Review (2010–2021). Environ. Technol. Innov. 2021, 24, 102075. [Google Scholar] [CrossRef]
- de Araújo Padilha, C.E.; da Costa Nogueira, C.; de Santana Souza, D.F.; de Oliveira, J.A.; dos Santos, E.S. Valorization of Green Coconut Fibre: Use of the Black Liquor of Organolsolv Pretreatment for Ethanol Production and the Washing Water for Production of Rhamnolipids by Pseudomonas Aeruginosa ATCC 27583. Ind. Crops Prod. 2019, 140, 111604. [Google Scholar] [CrossRef]
- Ge, X.; Chang, C.; Zhang, L.; Cui, S.; Luo, X.; Hu, S.; Qin, Y.; Li, Y. Conversion of Lignocellulosic Biomass into Platform Chemicals for Biobased Polyurethane Application. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 161–213. [Google Scholar]
- Qian, E.W. Pretreatment and Saccharification of Lignocellulosic Biomass. In Research Approaches to Sustainable Biomass Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–204. [Google Scholar]
- Zhang, M.; Jia, L.; Li, M.; Peng, H.; Tan, Y.; Arvelli, S.; Huang, Y.; Neves, A.C.; Oh, E.J.; Zhao, J. One-Pot Biomass Pretreatment for Ethanol Production by Engineered Saccharomyces cerevisiae. ACS Sustain. Chem. Eng. 2025, 13, 5201–5209. [Google Scholar] [CrossRef]
- Roa, K.; Oyarce, E.; Boulett, A.; ALSamman, M.; Oyarzún, D.; Pizarro, G.D.C.; Sánchez, J. Lignocellulose-Based Materials and Their Application in the Removal of Dyes from Water: A Review. Sustain. Mater. Technol. 2021, 29, e00320. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Singh, J.; Rezki, B.; Kumar, K.; Moholkar, V.S.; Kutlu, O.; Aboulmagd, A.; Khamees Thabet, H.; El-Bahy, Z.M.; et al. Converting Lignocellulosic Biomass into Valuable End Products for Decentralized Energy Solutions: A Comprehensive Overview. Sustain. Energy Technol. Assess. 2024, 72, 104065. [Google Scholar] [CrossRef]
- STATISTA Produção Mundial de Coco de 2000 a 2021. Available online: https://www.statista.com/statistics/577497/world-coconut-production (accessed on 27 December 2023).
- da Costa Nogueira, C.; de Araújo Padilha, C.E.; de Sá Leitão, A.L.; Rocha, P.M.; de Macedo, G.R.; dos Santos, E.S. Enhancing Enzymatic Hydrolysis of Green Coconut Fiber—Pretreatment Assisted by Tween 80 and Water Effect on the Post-Washing. Ind. Crops Prod. 2018, 112, 734–740. [Google Scholar] [CrossRef]
- Nunes, L.A.; Silva, M.L.S.; Gerber, J.Z.; Kalid, R.d.A. Waste Green Coconut Shells: Diagnosis of the Disposal and Applications for Use in Other Products. J. Clean. Prod. 2020, 255, 120169. [Google Scholar] [CrossRef]
- da Câmara Rocha, J.; Ribeiro, V.T.; da Costa Filho, J.D.B.; de Sá Leitão, A.L.; Cavalcante, J.D.N.; de Araújo Padilha, C.E.; dos Santos, E.S. The Use of Ionic Liquid Pretreatment Aims to Enhance the Enzymatic Hydrolysis of Green Coconut Fiber and Produce Lignin. Biomass Convers. Biorefinery 2023, 15, 2495–2510. [Google Scholar] [CrossRef]
- Anuchi, S.O.; Campbell, K.L.S.; Hallett, J.P. Effective Pretreatment of Lignin-Rich Coconut Wastes Using a Low-Cost Ionic Liquid. Sci. Rep. 2022, 12, 6108. [Google Scholar] [CrossRef] [PubMed]
- Endut, A.; Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Hamid, S.H.A.; Lananan, F.; Kamarudin, M.K.A.; Umar, R.; Juahir, H.; Khatoon, H. Optimization of Biodiesel Production by Solid Acid Catalyst Derived from Coconut Shell via Response Surface Methodology. Int. Biodeterior. Biodegrad. 2017, 124, 250–257. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Ruiz, H.A.; dos Santos, E.S.; Teixeira, J.A.; de Macedo, G.R. Bioethanol Production from Coconuts and Cactus Pretreated by Autohydrolysis. Ind. Crops Prod. 2015, 77, 1–12. [Google Scholar] [CrossRef]
- da Costa Nogueira, C.; de Araújo Padilha, C.E.; de Jesus, A.A.; de Santana Souza, D.F.; de Assis, C.F.; de Sousa Junior, F.C.; dos Santos, E.S. Pressurized Pretreatment and Simultaneous Saccharification and Fermentation with in Situ Detoxification to Increase Bioethanol Production from Green Coconut Fibers. Ind. Crops Prod. 2019, 130, 259–266. [Google Scholar] [CrossRef]
- Ribeiro, V.T.; Campolina, A.C.; da Costa, W.A.; de Araújo Padilha, C.E.; da Costa Filho, J.D.B.; de Sá Leitão, A.L.O.; da Câmara Rocha, J.; dos Santos, E.S. Ethanol Production from Green Coconut Fiber Using a Sequential Steam Explosion and Alkaline Pretreatment. Biomass Convers. Biorefinery 2022, 14, 8579–8589. [Google Scholar] [CrossRef]
- Ribeiro, V.T.; da Costa Filho, J.D.B.; de Araújo Padilha, C.E.; dos Santos, E.S. Using Tween 80 in Pretreatment, Enzymatic Hydrolysis, and Fermentation Processes for Enhancing Ethanol Production from Green Coconut Fiber. Biomass Convers. Biorefin 2023, 14, 17955–17970. [Google Scholar] [CrossRef]
- de Araújo Padilha, C.E.; da Costa Nogueira, C.; de Santana Souza, D.F.; de Oliveira, J.A.; dos Santos, E.S. Organosolv Lignin/Fe3O4 Nanoparticles Applied as a β-Glucosidase Immobilization Support and Adsorbent for Textile Dye Removal. Ind. Crops Prod. 2020, 146, 112167. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V. Coconut Coir Derived Nanolignin for the Removal of Chromium (VI) from Aqueous Solution: Adsorption Characteristic and Mechanism. Chem. Afr. 2023, 7, 953–968. [Google Scholar] [CrossRef]
- Pinheiro Nascimento, P.F.; Barros Neto, E.L.; Fernandes Bezerra, D.V.; Ferreira da Silva, A.J. Anionic Surfactant Impregnation in Solid Waste for Cu2+ Adsorption: Study of Kinetics, Equilibrium Isotherms, and Thermodynamic Parameters. J. Surfactants Deterg. 2020, 23, 781–795. [Google Scholar] [CrossRef]
- Nascimento, P.F.P.; Neto, E.L.B.; Pereira, J.E.S.; Silva, A.J.F. Cu2+ and Cd2+ Adsorption Mechanism by Coconut Husk Powder with and without Amine Modification. J. Environ. Eng. 2020, 146, 04020076. [Google Scholar] [CrossRef]
- Pinheiro Nascimento, P.F.; Barros Neto, E.L. Steam Explosion: Hydrothermal Pretreatment in the Production of an Adsorbent Material Using Coconut Husk. Bioenergy Res. 2021, 14, 153–162. [Google Scholar] [CrossRef]
- de Araújo Padilha, C.E.; da Costa Nogueira, N.; Matias, S.C.B.; da Costa Filho, J.D.B.; de Santana Souza, D.F.; de Oliveira, J.A.; dos Santos, E.S. Fabrication of Hollow Polymer Microcapsules and Removal of Emulsified Oil from Aqueous Environment Using Soda Lignin Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125260. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Ahmad, D.; Jouhara, H. Removal of Copper Ions from Aqueous Solution Using Low Temperature Biochar Derived from the Pyrolysis of Municipal Solid Waste. Sci. Total Environ. 2019, 673, 777–789. [Google Scholar] [CrossRef]
- Semerjian, L. Removal of Heavy Metals (Cu, Pb) from Aqueous Solutions Using Pine (Pinus Halepensis) Sawdust: Equilibrium, Kinetic, and Thermodynamic Studies. Environ. Technol. Innov. 2018, 12, 91–103. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Saratale, R.G.; Kadam, A.A.; Saratale, G.D.; Syed, A.; Ameen, F.; Kim, D.-Y. Colorimetric Detection of Cu2+ Based on the Formation of Peptide–Copper Complexes on Silver Nanoparticle Surfaces. Beilstein J. Nanotechnol. 2018, 9, 1414–1422. [Google Scholar] [CrossRef]
- Oishi, T.; Yaguchi, M.; Takai, Y. Hydrometallurgical Recovery of High-Purity Copper Cathode from Highly Impure Crude Copper. Resour. Conserv. Recycl. 2021, 167, 105382. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, W.; Sun, M.; Liu, X.; Zhang, Q.; Zong, C.; Qu, J.; Gai, H. Colorimetric Detection of Cu2+ by Surface Coordination Complexes of Polyethyleneimine-Capped Au Nanoparticles. Sens. Actuators B Chem. 2016, 223, 411–416. [Google Scholar] [CrossRef]
- Manzl, C.; Enrich, J.; Ebner, H.; Dallinger, R.; Krumschnabel, G. Copper-Induced Formation of Reactive Oxygen Species Causes Cell Death and Disruption of Calcium Homeostasis in Trout Hepatocytes. Toxicology 2004, 196, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R. Copper and Prion Disease. Brain Res. Bull. 2001, 55, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Aydın, H.; Bulut, Y.; Yerlikaya, Ç. Removal of Copper (II) from Aqueous Solution by Adsorption onto Low-Cost Adsorbents. J. Environ. Manag. 2008, 87, 37–45. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, Z.; Cao, M. Surface Modification of Graphene Oxide by Pyridine Derivatives for Copper(II) Adsorption from Aqueous Solutions. J. Ind. Eng. Chem. 2017, 53, 325–332. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization’s (WHO): Geneva, Switzerland, 2017.
- CONAMA Resolução CONAMA No 430 DE 13/05/2011. 2011. Available online: https://www.suape.pe.gov.br/images/publicacoes/CONAMA_n.430.2011.pdf (accessed on 10 January 2025).
- Duarah, P.; Haldar, D.; Purkait, M.K. Technological Advancement in the Synthesis and Applications of Lignin-Based Nanoparticles Derived from Agro-Industrial Waste Residues: A Review. Int. J. Biol. Macromol. 2020, 163, 1828–1843. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, Y.; Wang, T.; Niu, N.; Chen, L. Hybrid Lignin Particles via Ion-Crosslinked for Selective Removal of Anionic Dyes from Water. Int. J. Biol. Macromol. 2023, 238, 124080. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, S.; Moghadas, B.K.; Esmaeili, H.; Shakerian Khoo, F.; Gholami, G.; Ghasemi, M. Improving the Surface Properties of Adsorbents by Surfactants and Their Role in the Removal of Toxic Metals from Wastewater: A Review Study. Process Saf. Environ. Prot. 2021, 148, 775–795. [Google Scholar] [CrossRef]
- Ahn, C.K.; Park, D.; Woo, S.H.; Park, J.M. Removal of Cationic Heavy Metal from Aqueous Solution by Activated Carbon Impregnated with Anionic Surfactants. J. Hazard. Mater. 2009, 164, 1130–1136. [Google Scholar] [CrossRef]
- Zhan, Y.; Lin, J.; Zhu, Z. Removal of Nitrate from Aqueous Solution Using Cetylpyridinium Bromide (CPB) Modified Zeolite as Adsorbent. J. Hazard. Mater. 2011, 186, 1972–1978. [Google Scholar] [CrossRef]
- Carvalho, G.K.G. Remoção de Metais Utilizando o Hexadecanoato de Sódio. Master’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2014. [Google Scholar]
- Santos, F.K.G.; Neto, E.L.B.; Moura, M.C.P.A.; Dantas, T.N.C.; Neto, A.A.D. Molecular Behavior of Ionic and Nonionic Surfactants in Saline Medium. Colloids Surf. A Physicochem. Eng. Asp. 2009, 333, 156–162. [Google Scholar] [CrossRef]
- De Castro Dantas, T.N.; Neto, A.A.D.; De Alencar Moura, M.C.P. Removal of Chromium from Aqueous Solutions by Diatomite Treated with Microemulsion. Water Res. 2001, 35, 2219–2224. [Google Scholar] [CrossRef]
- Silva, A.J.F.; Paiva de Alencar Moura, M.C.; da Silva Santos, E.; Saraiva Pereira, J.E.; Lins de Barros Neto, E. Copper Removal Using Carnauba Straw Powder: Equilibrium, Kinetics, and Thermodynamic Studies. J. Environ. Chem. Eng. 2018, 6, 6828–6835. [Google Scholar] [CrossRef]
- Iqbal, M.; Schiewer, S.; Cameron, R. Mechanistic Elucidation and Evaluation of Biosorption of Metal Ions by Grapefruit Peel Using FTIR Spectroscopy, Kinetics and Isotherms Modeling, Cations Displacement and EDX Analysis. J. Chem. Technol. Biotechnol. 2009, 84, 1516–1526. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z.; Kong, Y.; Song, Q.; Wang, K. Heavy Metal Ions Retention by Bi-Functionalized Lignin: Synthesis, Applications, and Adsorption Mechanisms. J. Ind. Eng. Chem. 2014, 20, 4429–4436. [Google Scholar] [CrossRef]
- Nchoe, O.B.; Sanni, S.O.; Viljoen, E.L.; Pholosi, A.; Pakade, V.E. Surfactant-Modified Macadamia Nutshell for Enhancement of Methylene Blue Dye Adsorption from Aqueous Media. Case Stud. Chem. Environ. Eng. 2023, 8, 100357. [Google Scholar] [CrossRef]
- Kannamba, B.; Reddy, K.L.; AppaRao, B.V. Removal of Cu(II) from Aqueous Solutions Using Chemically Modified Chitosan. J. Hazard. Mater. 2010, 175, 939–948. [Google Scholar] [CrossRef]
- Silva, A.; Nascimento, P.; Barros Neto, E.; Duarte, L.; Melo, R.; Lopes, F. Copper Removal from Synthetic Electroplating Wastewater Applying Anionic Surfactants Derived from Vegetable Oils. Environ. Eng. Manag. J. 2022, 21, 793–804. [Google Scholar]
- Puasa, S.W.; Ismail, K.N.; Khairi, N.A.I.A. Cleavable Surfactant-Impregnated Activated Carbon for Enhanced Adsorptive Removal of Reactive Dye from an Aqueous Solution. Mater. Today Proc. 2018, 5, 22020–22028. [Google Scholar] [CrossRef]
- Khokhar, A.; Siddique, Z. Misbah Removal of Heavy Metal Ions by Chemically Treated Melia azedarach L. Leaves. J. Environ. Chem. Eng. 2015, 3, 944–952. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Cellulose Based Grafted Biosorbents-Journey from Lignocellulose Biomass to Toxic Metal Ions Sorption Applications-A Review. J. Mol. Liq. 2017, 232, 62–93. [Google Scholar] [CrossRef]
- Nair, V.; Panigrahy, A.; Vinu, R. Development of Novel Chitosan–Lignin Composites for Adsorption of Dyes and Metal Ions from Wastewater. Chem. Eng. J. 2014, 254, 491–502. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; Chintalpudi, V.K.; Muddada, S. Application of Biosorption for Removal of Heavy Metals from Wastewater. In Biosorption; InTech: London, UK, 2018. [Google Scholar]
- Tohdee, K.; Kaewsichan, L. Asadullah Enhancement of Adsorption Efficiency of Heavy Metal Cu(II) and Zn(II) onto Cationic Surfactant Modified Bentonite. J. Environ. Chem. Eng. 2018, 6, 2821–2828. [Google Scholar] [CrossRef]
- Khobragade, M.U.; Pal, A. Adsorptive Removal of Mn(II) from Water and Wastewater by Surfactant-Modified Alumina. Desalination Water Treat. 2016, 57, 2775–2786. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I.; Martínez, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from Aqueous Solution by Olive Stone Waste. Sep. Purif. Technol. 2006, 50, 132–140. [Google Scholar] [CrossRef]
- Nascimento, R.F.; Lima, A.C.A.d.; Vidal, C.B.; Diego de Quadros, M.; Raulino, G.S.C. Adsorção Aspectos Teóricos e Aplicações Ambientais; Imprensa Universitária: Fortaleza, Brazil, 2014; 256p, ISBN 978-85-7485-186-0. [Google Scholar]
- Nezamzadeh-Ejhieh, A.; Kabiri-Samani, M. Effective Removal of Ni(II) from Aqueous Solutions by Modification of Nano Particles of Clinoptilolite with Dimethylglyoxime. J. Hazard. Mater. 2013, 260, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Kaveeshwar, A.R.; Kumar, P.S.; Revellame, E.D.; Gang, D.D.; Zappi, M.E.; Subramaniam, R. Adsorption Properties and Mechanism of Barium (II) and Strontium (II) Removal from Fracking Wastewater Using Pecan Shell Based Activated Carbon. J. Clean. Prod. 2018, 193, 1–13. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Melo, D.Q.; Vidal, C.B.; da Silva, A.L.; Teixeira, R.N.P.; Raulino, G.S.C.; Medeiros, T.C.; Fechine, P.B.A.; Mazzeto, S.E.; De Keukeleire, D.; Nascimento, R.F. Removal of Cd2+, Cu2+, Ni2+, and Pb2+ Ions from Aqueous Solutions Using Tururi Fibers as an Adsorbent. J. Appl. Polym. Sci. 2014, 131, 40883. [Google Scholar] [CrossRef]
- do Carmo Ramos, S.N.; Xavier, A.L.P.; Teodoro, F.S.; Elias, M.M.C.; Gonçalves, F.J.; Gil, L.F.; de Freitas, R.P.; Gurgel, L.V.A. Modeling Mono- and Multi-Component Adsorption of Cobalt(II), Copper(II), and Nickel(II) Metal Ions from Aqueous Solution onto a New Carboxylated Sugarcane Bagasse. Part I: Batch Adsorption Study. Ind. Crops Prod. 2015, 74, 357–371. [Google Scholar] [CrossRef]
- Kushwaha, J.; Singh, R. Cellulose Hydrogel and Its Derivatives: A Review of Application in Heavy Metal Adsorption. Inorg. Chem. Commun. 2023, 152, 110721. [Google Scholar] [CrossRef]


| Lignins | CuO | K2O | Na2O | SO3 | NiO | Fe2O3 | SiO2 | CaO | Cr2O3 | ZrO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LIG | 0.40 | 11.44 | - | 76.04 | 0.41 | 4.02 | 3.52 | 1.69 | 0.46 | 0.08 | 1.94 |
| LIG-SUR | 0.24 | 11.01 | 22.30 | 61.25 | 0.27 | 2.23 | 2.70 | - | |||
| LIG-SUR-Cu2+ | 68.14 | 3.32 | - | 15.10 | 0.21 | 1.48 | 7.17 | 4.36 | 0.22 |
| Lignins | O | C | Na | Cu | Ca | Fe | Cr | Ni |
|---|---|---|---|---|---|---|---|---|
| LIG | 37.0% | 61.9% | 1.0% | - | - | - | - | - |
| LIG-SUR | 35.8% | 61.2% | 3.0% | - | - | - | - | - |
| LIG-SUR-Cu2+ | 81.9% | - | 5.5% | 12.1% | 0.2% | 0.2% | 0.1% | 0.1% |
| Kinetic Parameters | LIG | LIG-SUR |
|---|---|---|
| Pseudo-first-order | ||
| (min−1) | 0.083 | 0.059 |
| (mg·g−1) | 5.152 | 11.889 |
| (mg·g−1) | 2.349 | 1.469 |
| R2 | 0.949 | 0.673 |
| Pseudo-second-order | ||
| (mg·g−1·min) | 0.059 | 0.134 |
| (mg·g−1) | 5.152 | 11.889 |
| (mg·g−1) | 5.640 | 12.077 |
| R2 | 0.999 | 0.999 |
| Intraparticle diffusion | ||
| (mg·g−1·min−1/2) | 0.195 | 0.223 |
| (mg·g−1) | 3.006 | 9.369 |
| R2 | 0.591 | 0.189 |
| Temperature | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| (mg·g−1) | (L·mg−1) | R2 | (L·mg−1) | R2 | ||
| 298.15 | 30.675 | 0.0159 | 0.9897 | 0.362 | 4.365 | 0.6579 |
| 303.15 | 25.445 | 0.0811 | 0.9749 | 0.400 | 3.495 | 0.5767 |
| 313.15 | 26.248 | 0.0504 | 0.9324 | 0.433 | 2.961 | 0.5269 |
| 323.15 | 30.581 | 0.0202 | 0.7531 | 0.539 | 1.773 | 0.5284 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.E.S.; Neto, E.L.B.; Duarte, L.J.N.; Ferreira, R.L.S.; Melo, R.P.F.; Nascimento, P.F.P. Lignin Extracted from Green Coconut Waste Impregnated with Sodium Octanoate for Removal of Cu2+ in Aqueous Solution. Processes 2025, 13, 1590. https://doi.org/10.3390/pr13051590
Pereira JES, Neto ELB, Duarte LJN, Ferreira RLS, Melo RPF, Nascimento PFP. Lignin Extracted from Green Coconut Waste Impregnated with Sodium Octanoate for Removal of Cu2+ in Aqueous Solution. Processes. 2025; 13(5):1590. https://doi.org/10.3390/pr13051590
Chicago/Turabian StylePereira, Jéssyca E. S., Eduardo L. Barros Neto, Lindemberg J. N. Duarte, Ruan L. S. Ferreira, Ricardo P. F. Melo, and Paula F. P. Nascimento. 2025. "Lignin Extracted from Green Coconut Waste Impregnated with Sodium Octanoate for Removal of Cu2+ in Aqueous Solution" Processes 13, no. 5: 1590. https://doi.org/10.3390/pr13051590
APA StylePereira, J. E. S., Neto, E. L. B., Duarte, L. J. N., Ferreira, R. L. S., Melo, R. P. F., & Nascimento, P. F. P. (2025). Lignin Extracted from Green Coconut Waste Impregnated with Sodium Octanoate for Removal of Cu2+ in Aqueous Solution. Processes, 13(5), 1590. https://doi.org/10.3390/pr13051590






