Abstract
Heap leaching of copper is faced with a complex set of challenges, including mineral heterogeneity, the formation of passivating species, and the need to regulate critical variables such as pH, redox potential (Eh), oxidant concentration, and irrigation rate. If these factors are not properly managed, copper recovery is reduced, and significant environmental impacts may be generated, highlighting the urgency for systematic and sustainable approaches. To address this challenge, a systematic literature review (SLR) was conducted, screening 2344 documents and selecting 106 primary sources to analyze operational drivers and environmental considerations. Statistical methodologies (factorial designs, response surface methodology), multiscale modeling, and laboratory column tests were used to validate key variables, including pH (1.5–2.0), Eh (600–), temperature (25–55 °C), irrigation rate (5–), acid concentration (0.5–), and emerging “green” reagents (e.g., glycine, organic surfactants). Precise control of these factors was found to reduce passivation, minimize fine-particle migration, and improve copper extraction up to 90%. The incorporation of oxidizing agents (e.g., Fe3+, H2O2) further accelerated mineral dissolution while preventing unwanted precipitates. In parallel, bioleaching strategies maintained high recoveries with lower chemical demand. Reviews of pilot studies confirmed the scalability of these optimized conditions, emphasizing both sustainability and cost-effectiveness.
1. Introduction
Copper heap leaching is a widespread and significant mining practice due to its capacity to process large volumes of ore at relatively low cost and with a smaller environmental footprint compared to other hydrometallurgical or pyrometallurgical methods [1,2]. Industrial-scale heap leaching plants are now operating in every major copper region—South America (Chile and Peru), North America (United States and Mexico), East Asia (China), Central Asia (Kazakhstan), and Oceania (Australia). Together, they supply roughly one-fifth of the world’s refined copper, or about 4 Mt in 2023, and are a key source of export income and employment for producing countries (e.g., heap-leached cathodes account for 40% of Chilean copper exports). Forecasts by the International Energy Agency indicate that the global roll-out of electric vehicles, solar photovoltaics, and wind power could raise annual copper demand by 4–5 Mt by 2030. Because it combines low capital intensity with a relatively small environmental footprint, heap leaching is expected to play a central role in meeting this additional demand while supporting the economic resilience of mining regions [3,4].
Nevertheless, achieving optimal performance and stability necessitates meticulous control over factors: pH, redox potential (Eh), oxidizing agent concentration (Fe3+, H2O2), irrigation rate, and temperature [5,6,7]. Consequently, industrial design and optimization face substantial challenges, especially due to mineralogical heterogeneity and the need for high-purity Pregnant Leach Solution (PLS).
1.1. Relevance and Knowledge Gap
Whilst studies cover copper dissolution kinetics using diverse models [8,9], significant gaps remain regarding the holistic integration of operational factors. This is particularly true when integrating green agents (e.g., glycine) and emerging techniques like bioleaching into standard heap leaching [10,11]. Furthermore, the need to minimize environmental impact and reagent costs drives the development of methodologies balancing metallurgical efficiency with process sustainability.
1.2. Problem Statement
Owing to mineral heterogeneity and complex redox thermodynamics, managing critical variables (e.g., pH, Eh, temperature, irrigation rate) presents a highly multivariable challenge [2,5]. In practice, for instance, incorrect Fe3+ use risks jarosite formation, whilst excessive irrigation can lead to saturation, fine migration, and heap clogging [12,13]. These operational uncertainties therefore demand a systematic scientific approach, integrating literature reviews, expert validation, and experimentation, to delineate optimal operating ranges.
1.3. Research Objectives
Based on these considerations, the following general and specific objectives were defined:
- General Objective: Develop and validate a methodological framework that integrates systematic literature review, kinetic modeling, and experimental assays for the optimization of copper heap leaching under dynamic conditions.
- Specific Objectives:
- (a)
- Obj O.1: Identify the most influential operational variables (pH, Eh, Fe3+, etc.) and their optimal ranges according to the scientific literature and the authors’ own experimentation.
- (b)
- Obj O.2: Develop a multivariable mathematical model that incorporates the effects of acidity, oxidant concentration, and reaction kinetics to predict copper recovery under different operating conditions.
- (c)
- Obj O.3: Assess the practical applicability of this model and compare it with pilot-scale data, validating its robustness in scenarios involving mineral heterogeneity and dynamic irrigation configurations.
- (d)
- Obj O.4: Propose criteria for the industrial implementation of advanced control and monitoring methodologies in heaps, focusing on maximizing metallurgical efficiency and minimizing operational costs.
1.4. Research Questions
Based on the problem statement and the objectives outlined, the following research questions were formulated to guide the analysis and discussion (Section 3, Section 4 and Section 5):
- P.1
- Which are the most decisive parameters in copper heap leaching (pH, Eh, temperature, irrigation rate, concentrations of H2SO4 and/or Fe3+), and how do they interact to influence copper recovery?
- P.2
- To what extent can a multivariable mathematical model describe the kinetics of copper dissolution under dynamic operating conditions and mineral heterogeneity?
- P.3
- What findings help delineate optimal operating and industrial upscaling routes, considering the possible inclusion of “green” agents (glycine, eco-friendly surfactants) and bioleaching techniques?
1.5. Manuscript Structure and Methodological Approach
To address the research questions, our robust methodology merges literature review (detailed in Section 2) with experimentation. This yields a classified corpus of 12 thematic clusters (Section 3), encompassing topics such as waste electronics leaching, bioleaching, and deep eutectic solvents [10,11,14]. Subsequently, Table 1 outlines key results alongside the formulated copper leaching mathematical model. These findings are then analyzed and contextualized against prior work and industrial practice within Section 4. Finally, Section 5 highlights significant contributions and future research pathways, emphasising the vital theory–practice nexus for advancing mining efficiency and sustainability.

Table 1.
Discussion Synthesis Table: Key Factors and Recommendations for Copper Heap Leaching.
1.6. Justification and Scope
Relevant to both academia and industry, this research tackles the strategic priority of optimizing heap leaching, particularly in copper-producing nations. It contributes an integral analytical method to the body of knowledge whilst providing practical guidelines for implementing novel operational and environmental practices. Our integrated approach—combining rigorous search, scientific validation, and empirical testing—yields a holistic perspective, covering physicochemical understanding of copper dissolution through to industrial planning and cost management. Accordingly, this Section 1 sets the stage by outlining the study’s context and motivations, leading into the detailed methodological and technical discussions presented hereafter.
2. Systematic Analysis Methodology
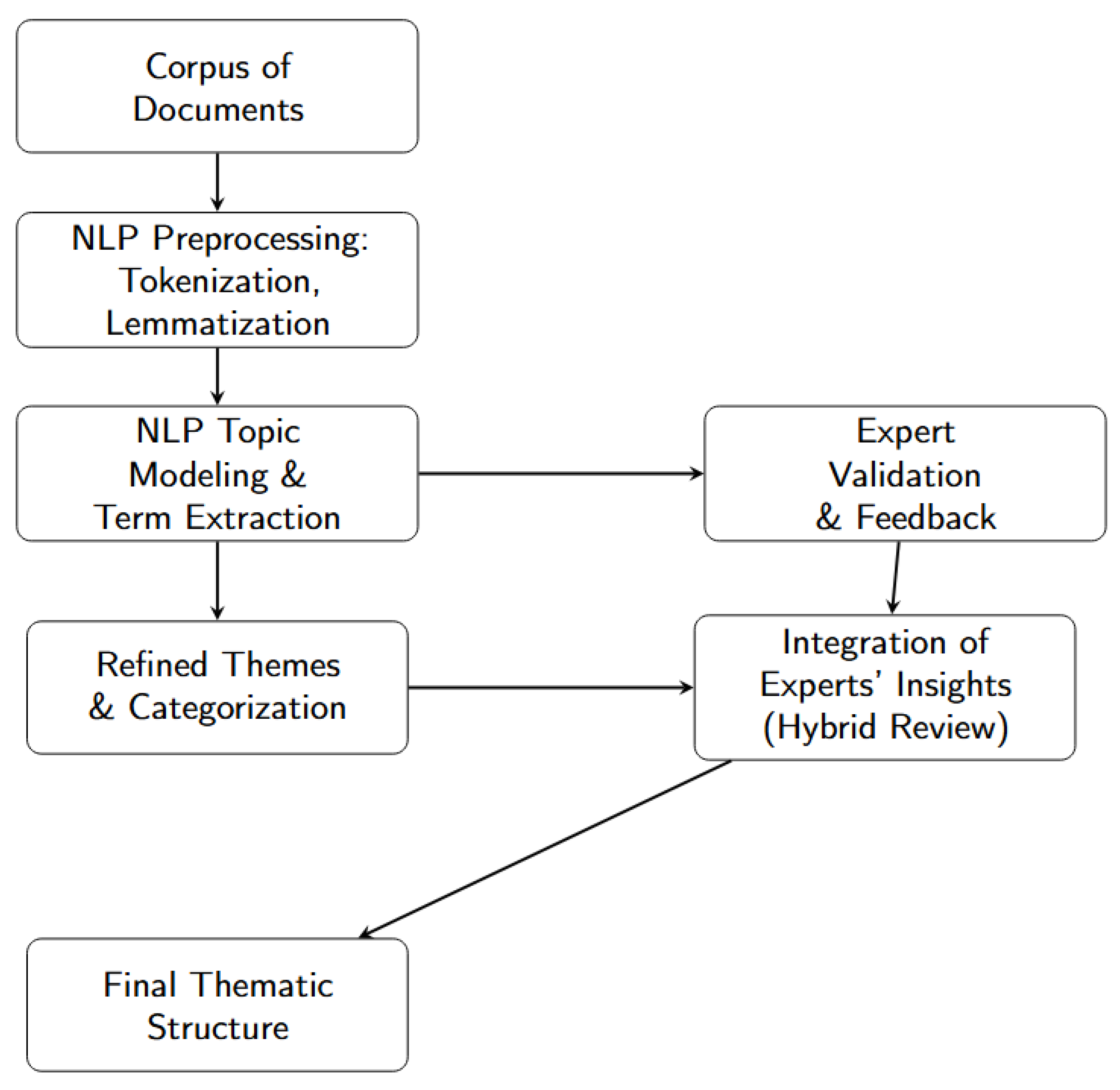
This section describes in detail the methodology adopted to conduct a systematic analysis, integrating both bibliographic search and filtering criteria as well as advanced Natural Language Processing (NLP) techniques and expert validation. The data were extracted and refined before subsequently being classified into thematic clusters, following a sequence of steps outlined below. Figure 1 provides an overview of the workflow.
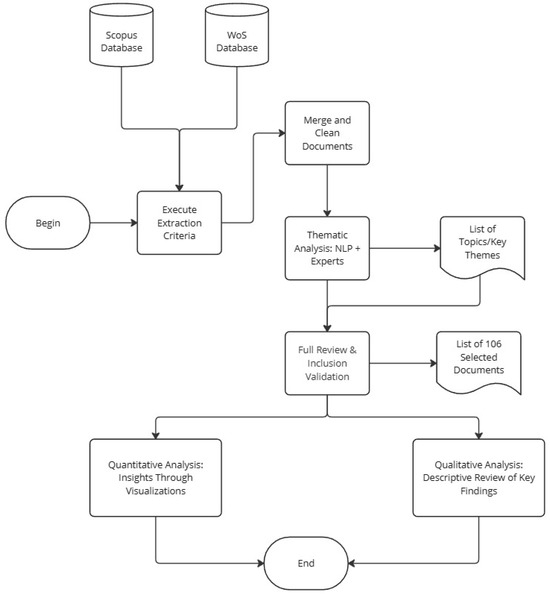
Figure 1.
General workflow for the systematic analysis, integrating procedures for data extraction, cleaning, thematic identification via NLP, and expert validation.
2.1. Document Extraction and Search Criteria
A systematic literature search was conducted using the Scopus database (Elsevier, Amsterdam, The Netherlands) and the Web of Science Core Collection (Clarivate Analytics, Philadelphia, PA, USA), selected for their quality and indexing breadth.The Scopus and Web of Science (WoS) databases were last searched on 15 April 2025. The search strategy employed combined key terms related to copper leaching, influencing factors, operational variables, and optimization approaches, guided by specific criteria:
| Key Queries: | |
| Document Types: | Research articles, reviews, conference proceedings, and indexed book chapters. |
| Time Range: | 2015–2025, focusing on recent and high-impact contributions. |
Following duplicate removal and initial screening (year/quality criteria; see Section 3), 1721 documents remained from an initial 2344. A semantic filter (semantic filter) was then applied, using a relevance threshold (e.g., 0.96) based on similarity to core topics (leaching kinetics, critical variables), yielding 116 documents (score ≥ 2.0). After final cleaning and meticulous cross-checking (DOI, title, author) to prevent redundancy, 106 documents were selected. Exclusion criteria included absent abstracts or low topical relevance, ensuring the study’s focus and consistency. Inclusion required alignment between automated language-based categorization and independent expert assessment. Data extraction was carried out without relying on author queries or software-based automation.
2.2. Merging and Cleaning Documents
The retrieved documents were consolidated into a single repository for normalization. In line with good practices for systematic review, column headers were harmonized (Merge and Clean Documents) between Scopus and WoS to ensure consistency in metadata such as Title, Authors, Year, Abstract, Keywords, and DOI. Next, inclusion and exclusion criteria were established.
2.2.1. Inclusion Criteria
- Articles and reviews with accessible and verified full text.
- Studies published in English or Spanish.
- Sources ranked at least Q2 in Scopus/WoS, indexed conferences, or journals with a high impact factor.
- Documents with five or more verified citations or, failing that, with a clear methodological contribution.
- Direct contribution to the optimization of copper leaching, key variables, and/or innovation in monitoring and control methodologies.
2.2.2. Exclusion Criteria
- Records lacking sufficient information (Title/Abstract).
- Publications in languages other than English or Spanish without an official translation.
- Duplicates between WoS and Scopus.
- Articles with restricted access that could not be verified.
- Conference abstracts without full peer-reviewed text.
Applying these criteria reduced the corpus to 108 high-quality relevant documents.
2.3. Refinements in NLP-Driven Analysis and Bias Mitigation
Greater Emphasis on NLP Methods and Tools: Our initial strategy combined conventional document filtering (Scopus/Web of Science) with machine-learning-based semantic analysis. However, we recognized the need for deeper scrutiny of potential biases and overlooked research. Consequently, the updated methodology (Section 2, final portion) compares our approach with recently proposed systematic-review frameworks [15,16], highlighting how transformer-based semantics (e.g., Sentence-BERT) can refine classification accuracy and expose thematic gaps [17].
Specifically, we evaluated whether restricting the corpus to Q2+ journals or imposing a high threshold for similarity () might systematically exclude emergent or cross-disciplinary studies. To mitigate this risk, we introduced a two-stage validation process:
(i) Contextual embedding and topic modelling. Abstracts were embedded with Sentence-BERT v0.4.0 (UKPLab, TU Darmstadt, Darmstadt, Germany) and processed with spaCy v3.7.2 (Explosion AI GmbH, Berlin, Germany). The embeddings fed an advanced topic-modelling workflow built on BERTopic v0.16.1 (open-source; https://github.com/MaartenGr/BERTopic, accessed on 9 April 2025). The resulting clusters were benchmarked against classical Latent Dirichlet Allocation (LDA) and TF–IDF pipelines implemented in Gensim v4.3.1 (RARE Technologies Ltd., Prague, Czech Republic) and keyword extraction with RAKE [18,19,20].
(ii) Expert review. A panel of three metallurgical specialists examined borderline documents, verifying their relevance to copper leaching and ensuring that niche but methodologically robust works were retained.
We also implemented an iterative feedback loop with domain experts to calibrate hyperparameters like the minimal cluster size and the embedding dimensionality [21]. This fine-tuning step notably improved the resolution in detecting subthemes (e.g., bio-assisted leaching, surfactant-enabled kinetics), reducing the risk of conflating diverse research fronts into a single cluster. Furthermore, by contrasting specialized sets of articles on, for instance, glycine-based approaches or deep eutectic solvents, the refined semantic thresholds unveiled additional synergies previously masked by simpler keyword-matching filters.
Overall, these enhancements represent a more holistic and adaptive NLP-driven strategy for large-scale bibliometric reviews. The integrated pipeline—merging advanced embeddings, domain-expert feedback, and multi-method cross-validation—enables us to capture both mainstream and emergent scholarship in the copper-leaching domain, thus reinforcing the methodological rigor and credibility of our systematic analysis.
2.4. Thematic Analysis: NLP + Expert Validation
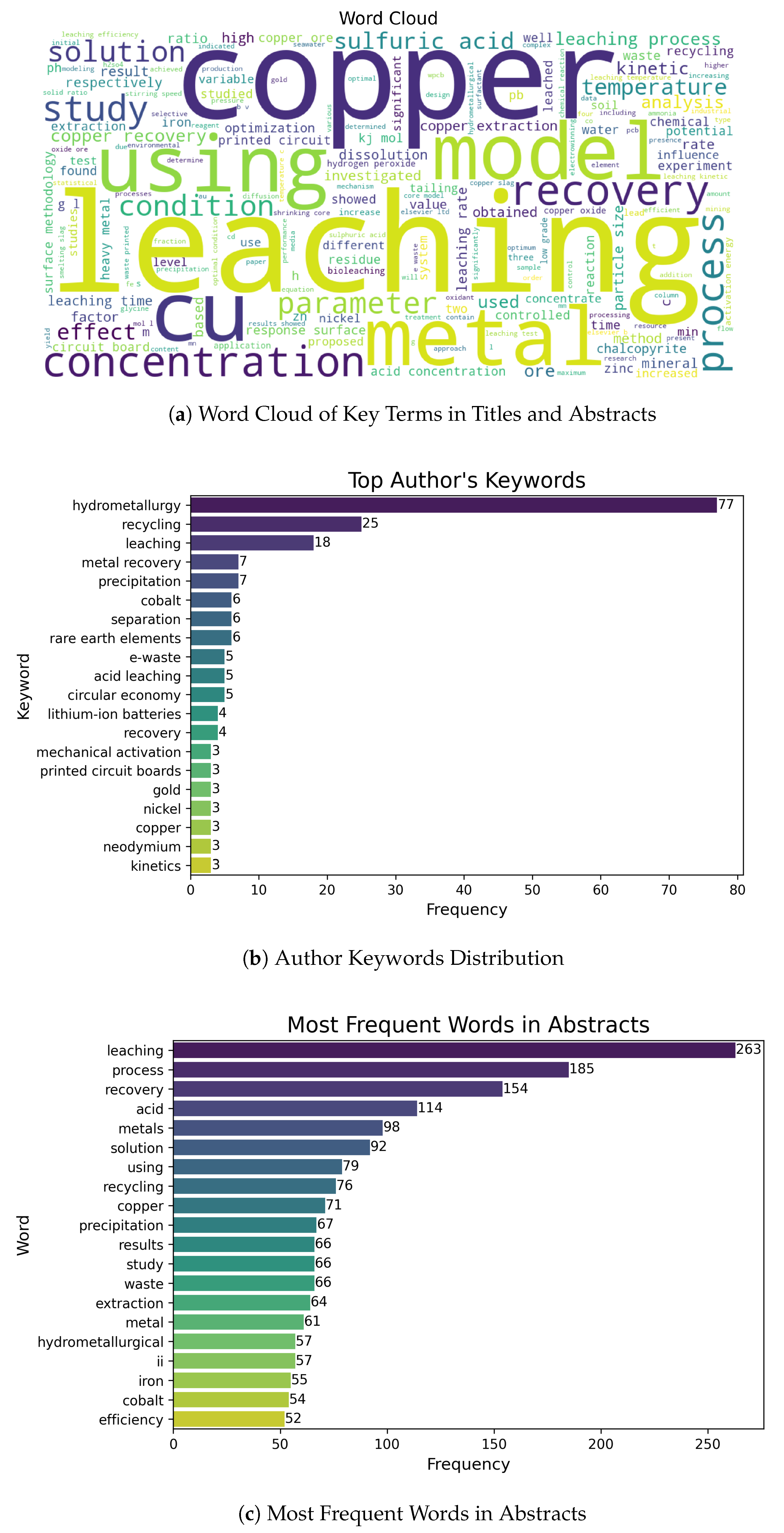
Once the pertinent documents were selected, we performed a thematic analysis that combined Natural Language Processing (NLP) with structured expert review. Following Garcia et al. [15], García et al. [16], we used standard algorithms for term extraction and unsupervised topic modeling to reveal semantic patterns; domain specialists then refined the output. Figure 2 summarizes the workflow.
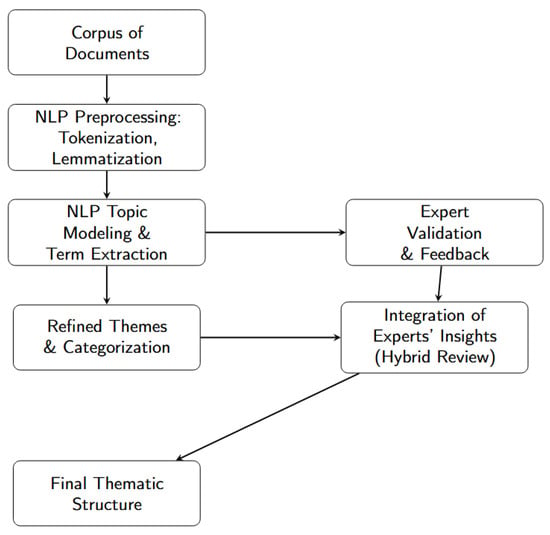
Figure 2.
Workflow for topic identification and refinement: (1) NLP pre-processing, (2) topic modeling and term extraction, (3) expert validation, (4) refinement of themes, (5) hybrid integration of experts’ insights, and (6) final thematic structure.
The process comprised six sequential phases:
- Phase 1
- NLP pre-processing: tokenization, lemmatization and stop-word removal were applied to the titles, abstracts, and author keywords, producing a clean text corpus.
- Phase 2
- Topic modeling and term extraction: unsupervised methods (latent Dirichlet allocation and k-means) grouped documents by semantic similarity, while key terms for each provisional topic were extracted automatically.
- Phase 3
- Expert validation and feedback: three metallurgical specialists examined the provisional clusters, marking documents as coherent or mis-assigned and providing qualitative comments.
- Phase 4
- Refined themes and categorization: algorithmic labels were adjusted, overly small clusters were merged, and ambiguous documents were reassigned on the basis of the experts’ feedback.
- Phase 5
- Hybrid integration of experts’ insights: a second review round reconciled any remaining discrepancies and approved the revised set of themes.
- Phase 6
- Final thematic structure: the agreed-upon clusters—twelve in total—were adopted for the bibliographic synthesis reported in Section 3.
This hybrid approach leverages the scalability of automated NLP while retaining the contextual depth provided by expert judgement, yielding a robust and transparent thematic classification of the literature.
2.5. Comprehensive Review and Inclusion Validation
The curated subset (166 documents) was then subjected to a detailed inspection (Full Review and Inclusion Validation). Each article was evaluated manually and supported by automated ranking, confirming:
- Methodological Relevance: leaching processes, critical parameters, and reproducible outcomes.
- Scientific Quality: statistical rigor, robust experimental design, and/or sound theoretical modeling.
- Industrial Applicability: focus on copper leaching (oxide/sulfide), tailings management, or innovations in condition monitoring.
A total of 113 articles were selected as most closely aligned with the research question, in accordance with the validation methodologies outlined in [15] for systematic reviews.
2.6. Quantitative Analysis
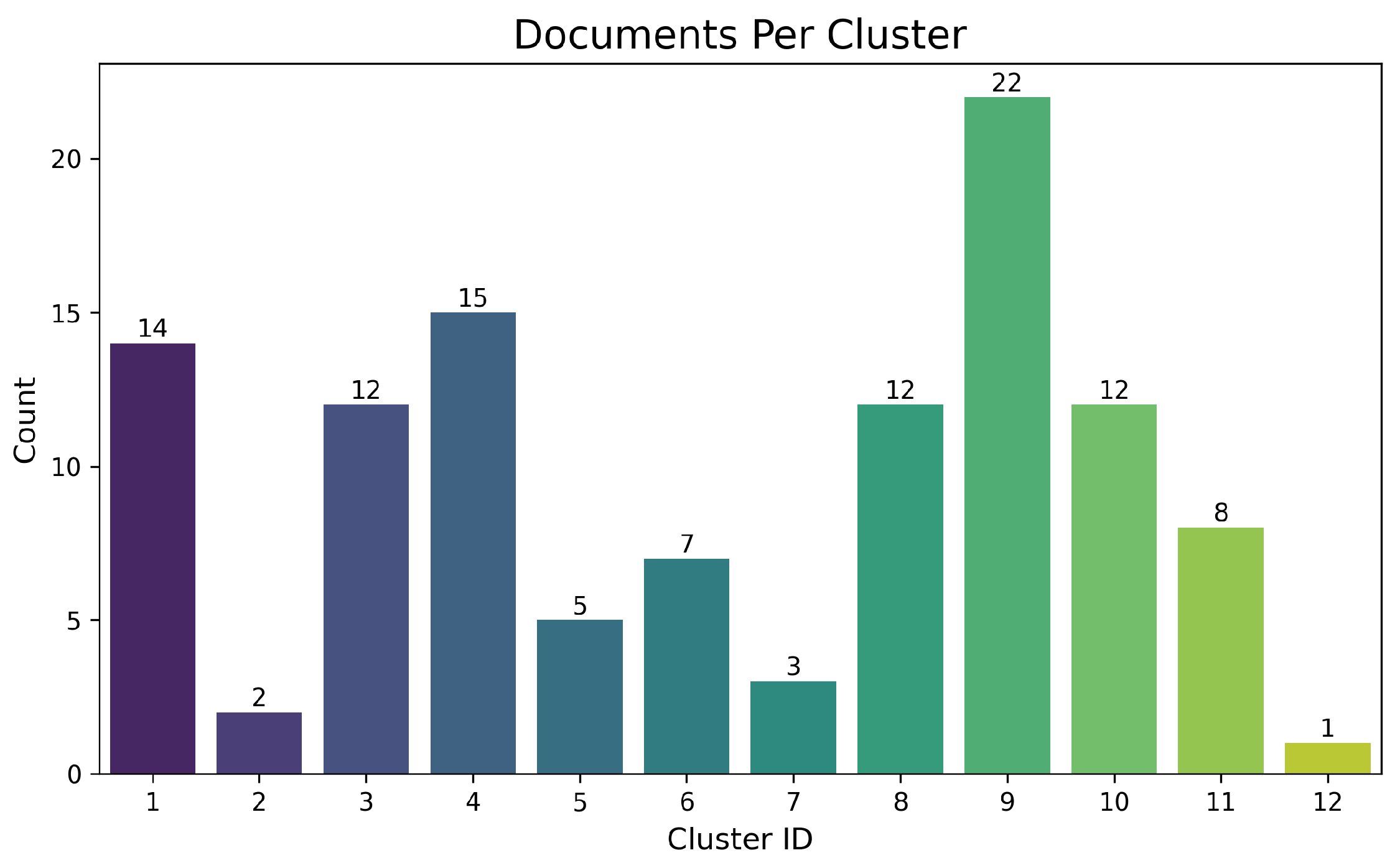
Using the final corpus, a Quantitative Analysis was performed to explore key bibliometric indicators (year of publication, document type, impact factor, countries of affiliation). Additionally, visualizations were generated to reveal trends and thematic relationships (e.g., co-occurrence networks, keyword histograms, and temporal evolution). As illustrated in Figure 3a, high frequency was detected in terms related to “copper leaching”, “kinetic models”, and “heap optimization”. These graphical representations provided a macroscopic view of dominant research lines and the multidisciplinary nature of the field, confirming the robustness of the selected body of literature.
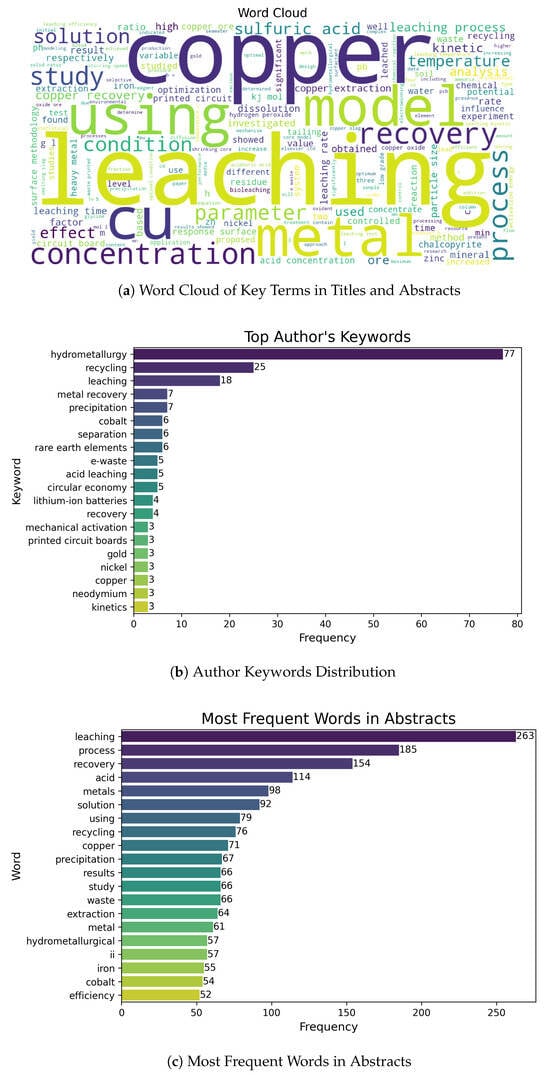
Figure 3.
Comparative Terminology Visualization. (a) Word cloud highlighting frequent terms related to hydrometallurgy and process optimization. (b) Author keywords showing the coexistence of traditional and emerging concepts. (c) Term frequency from abstracts emphasizing both conventional and sustainable copper leaching strategies.
2.7. Qualitative Analysis: Descriptive Review of Key Findings
The key data items sought from each included study for the qualitative synthesis included (but were not limited to) (1) reported optimal ranges and effects of operational variables (pH, Eh, temperature, irrigation rate, oxidant concentration, etc.); (2) types and efficacy of conventional and ’green’ leaching reagents (e.g., glycine, organic acids, surfactants); (3) details of kinetic models or statistical methodologies applied and their predictive success; (4) information on bioleaching approaches and their performance; (5) discussed environmental impacts, sustainability strategies, and scalability. When studies reported multiple results or conditions, those highlighted by the original authors as most significant or optimal or those directly addressing the research questions of this SLR were prioritized for discussion within the thematic synthesis.
Where available, details on the copper source (e.g., specific ores, tailings, or e-waste), study scale (e.g., laboratory, pilot, or industrial), and employed methodologies (e.g., reactor type, column dimensions, analytical techniques) were recorded to contextualize the findings. Funding sources of the included studies were not a specific data item for extraction. If information on these contextual variables was unclear or missing in a primary study, the synthesis relied on the available details, and no assumptions were made to fill gaps; the focus remained on the reported findings.
Finally, a Qualitative Analysis was carried out on the most relevant findings from the prioritized documents (110 articles). Under a meta-synthesis criterion, notable contributions were grouped in relation to the following:
- Leaching Kinetics Modeling (shrinking-core equations, diffusional models, chemical reactions).
- Operational Control and In Situ Monitoring Practices (pH, Eh, irrigation rate, particle size).
- Impurity Management and Oxidizing Agents (Fe3+, H2O2, complexing agents).
- Emerging Trends such as “green” surfactants, bioleaching, and eutectic solvents.
The combination of quantitative and qualitative findings, alongside methodological evaluation, resulted in a robust thematic map presented in Section 3. This map comprehensively addresses the challenges and advances in optimizing heap leaching for copper, as well as perspectives on sustainability and future efficiency.
2.8. Quantitative Analysis and Results Visualization
This subsection presents various charts and histograms illustrating the distribution of selected documents in the present SLR (Systematic Literature Review), as well as the publication sources and associated thematic trends. Each figure enables a quantitative and bibliometric perspective on dominant research lines in copper leaching and the optimization of its key variables.
Figure 4: Document count per 12 clusters (defined via NLP/expert validation; Section 2). High-volume clusters (9, 8, 10, 4) emphasise slag leaching, variable optimization (pH, redox, surfactants), and complex/residual ore processing. Lower-volume clusters (e.g., 2, 12) cover specialized, high-specificity topics (e.g., Cu precipitation [cyanide], F fixation [tailings]).
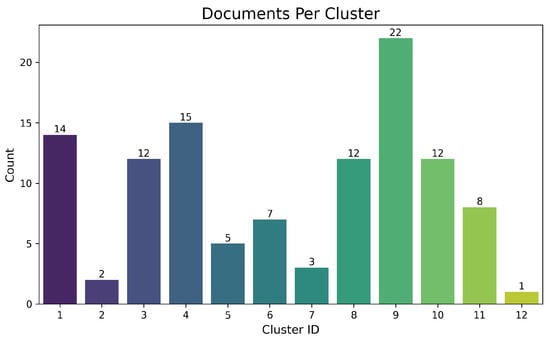
Figure 4.
Distribution of documents by cluster. The horizontal axis denotes the numbering of the 12 thematic clusters, whereas the vertical axis shows the document count in each.
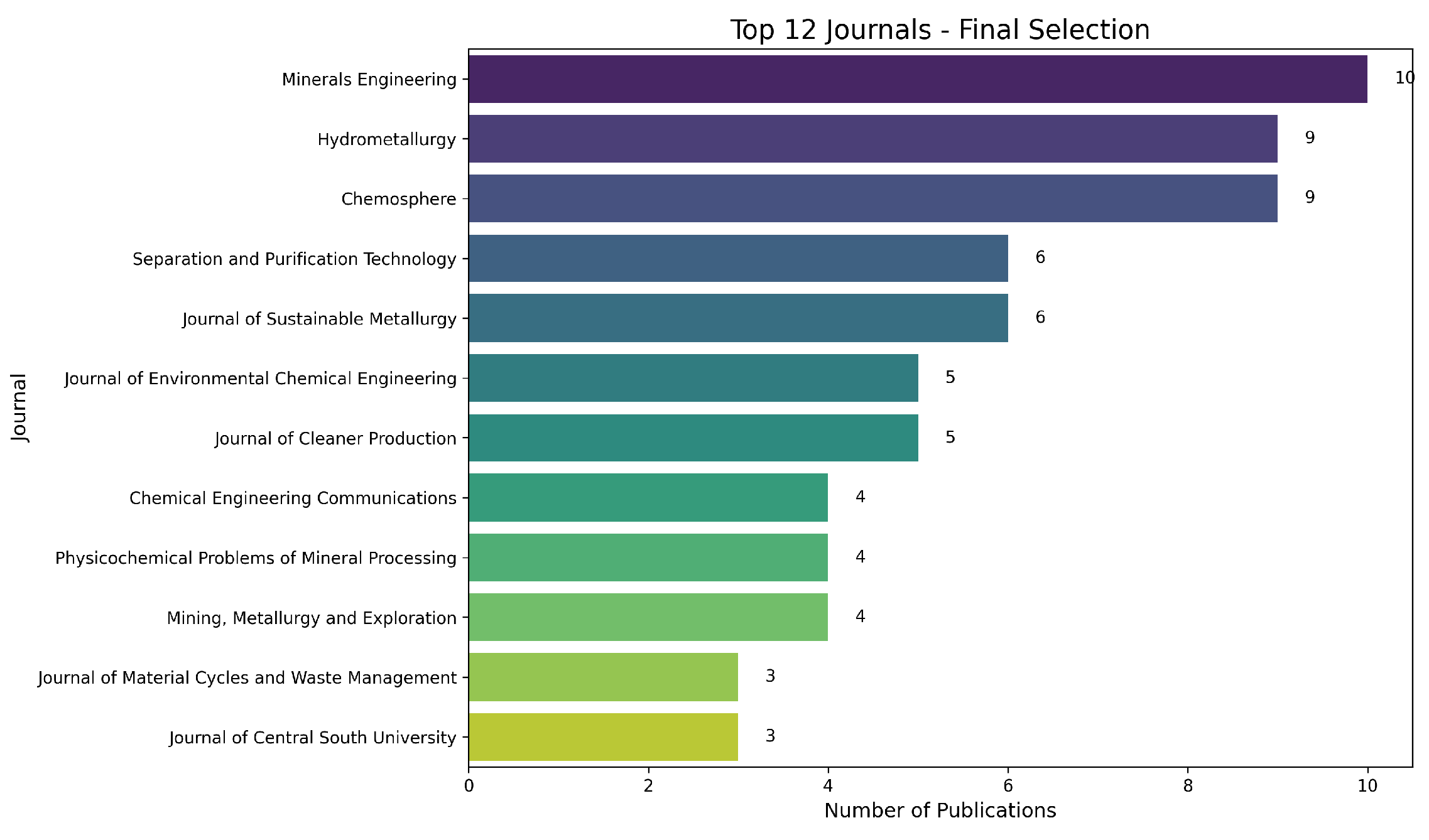
Figure 5 presents the 12 most representative publication sources in the final dataset. Minerals Engineering, Hydrometallurgy, and Chemosphere lead the list, confirming the multidisciplinary nature of the studies (extractive metallurgy, environmental chemistry, and process optimization). Collectively, these results indicate that the research on copper leaching and its critical variables is primarily found in high-impact (Q1/Q2) journals, with many articles specializing in process engineering, effluent treatment, and sustainability.
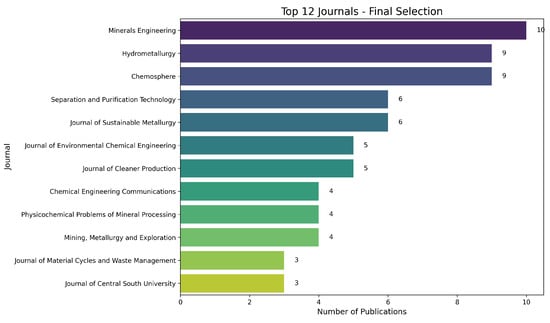
Figure 5.
Top 12 journals among the selected documents. The vertical axis lists the journals, and the horizontal axis shows the number of publications in each.
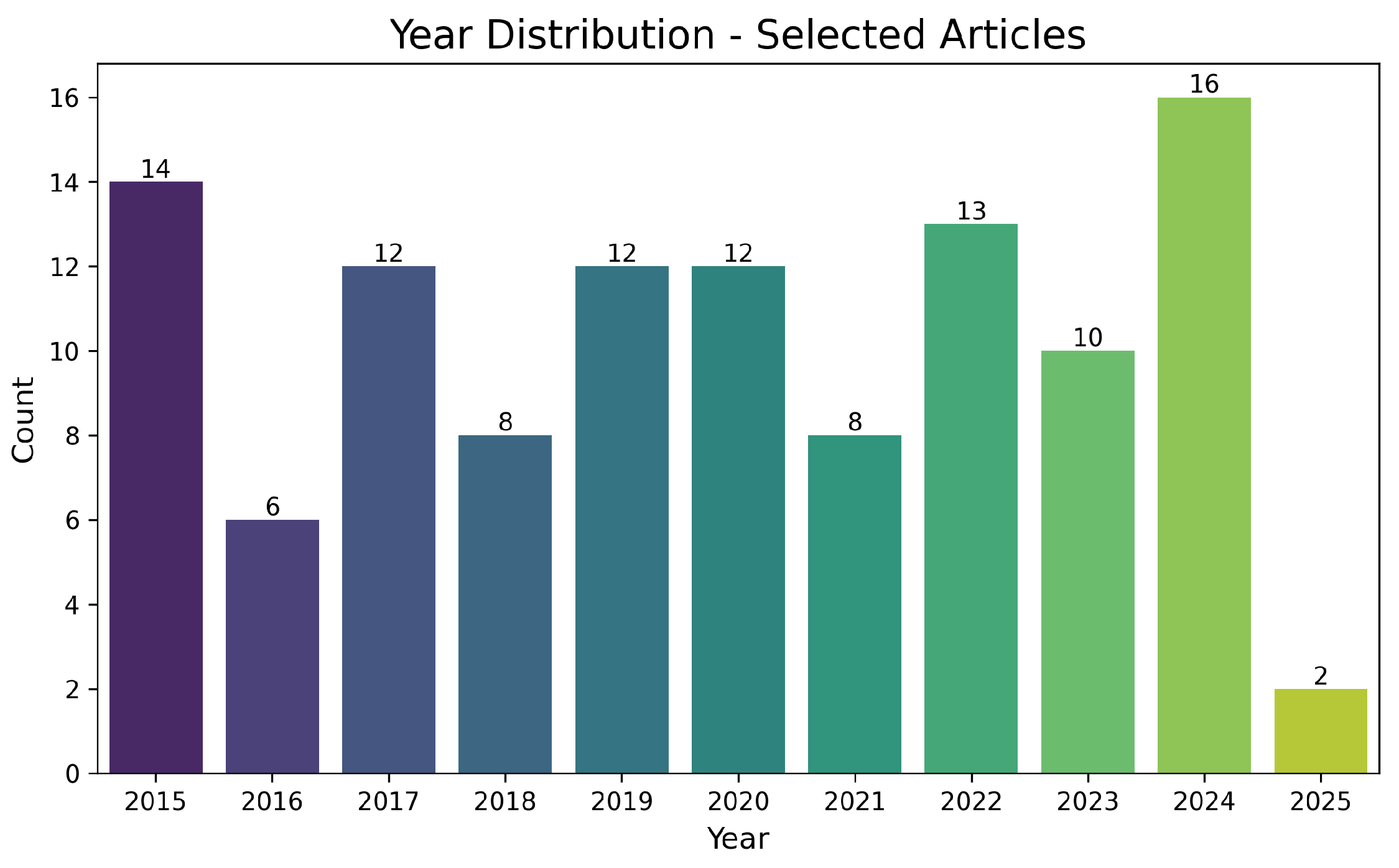
Figure 6 depicts the temporal evolution of selected publications, with a notable increase starting in 2020. This rise aligns with industrial interest in new intelligent mining technologies, process digitalization, and the quest for more sustainable methods of copper extraction. The expected peak in 2024 suggests that recent advances in leaching, complexing agents, and waste management continue to gain prominence, translating into a higher volume of publications.
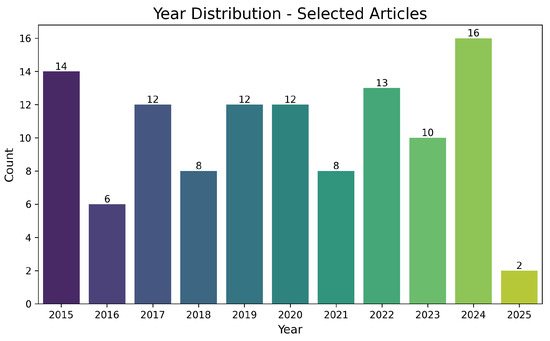
Figure 6.
Distribution of selected articles by publication year (2015–2025). The vertical axis represents the number of documents, and the horizontal axis the year.
Quantitative Finding Overview
Our analysis indicates research on copper leaching (kinetics, conditions, optimisation) spans leading journals (e.g., Minerals Engineering, Hydrometallurgy) but clusters heavily. Furthermore, NLP identifies key dimensions like environmental aspects, surfactants/bioreactors, and kinetic/thermodynamic influences. Recent trends also highlight the industry’s drive towards enhancing copper production efficiency whilst minimizing its environmental impact.
2.9. Assessment of Publication Bias and Transformer-Based Semantics
To ensure internal validity, potential biases from database (Scopus/WoS)/criteria (Q2+)/semantic threshold combination were scrutinized. Firstly, retained/discarded articles were compared checking exclusion reasons (relevance vs. methodological limitations) [15,16]. Secondly, filter impact on emerging areas (e.g., eco-surfactants, extreme temp. bio-leaching) was assessed.
Advanced NLP refined classification/gap ID. Specifically, Sentence-BERT [17] provided accurate semantic similarity (surpassing TF-IDF/LDA) by capturing nuances [21]. An iterative expert review of ”borderline” cases (assessed on soundness/alignment with review question) strengthened this stage, enhancing recall and sub-theme detection [15,16].
Consequently, combining transformer semantics with expert input yielded refined classification and mitigated publication bias. This methodological refinement enhances literature representativeness and allows clearer thematic gap delineation in recent copper leaching approaches.
A formal risk of bias assessment was not conducted for each individual studies included in this systematic review. The review relied on the peer-review process and the quality indicators (journal ranking, citations as per Section 2.2.1) as an indirect measure of the robustness of the included studies.
3. Findings and Topic Synthesis
This section presents the detailed Findings and Topic Synthesis concerning the most significant variables in dynamic copper heap leaching, aiming to enhance the quality of the Pregnant Leach Solution (PLS). To this end, a range of scientific works were grouped into several thematic clusters, numbered 1 through 12. Each cluster synthesizes a particular problem or approach related to copper leaching, and the most noteworthy results and methodological contributions are discussed. An in-depth analysis of each cluster is provided below in successive order, emphasizing aspects relevant to leaching optimization, the characterization of critical parameters, and the achievement of a high-purity PLS.
3.1. Cluster 1: Copper Recovery from PCB Residues and New Hydrometallurgical Strategies
3.1.1. Cluster Overview
This initial group of studies investigates copper (Cu) recovery from waste printed circuit boards (PCBs), focusing on optimizing diverse hydrometallurgical pathways such as acidic/alkaline leaching, solvent extraction, and organic or ”green” methods. Emphasis is placed on alternative approaches aiming to enhance selectivity, purity, environmental sustainability, and the efficient utilisation of secondary resources. Furthermore, these studies evaluate various reagents (e.g., H2SO4-H2O2, ammoniacal solutions, ethylene glycol complexes) and solvents (e.g., M5640, D2EHPA, ammonium solutions) for extraction performance. Collectively, this research outlines the challenges and opportunities in PCB copper recovery, underscoring the importance of industrial scaling, kinetic modeling, and cost analysis.
3.1.2. Key Findings
“Clean” Leaching Pathways and Micro-Nano Copper Powders
In Li et al. [22], the feasibility of a so-called “clean” leaching process for extracting copper present in waste printed circuit boards (WPCBs) is analyzed. Through subsequent chemical reduction processes, these authors achieve the synthesis of micro-nano-scale copper powders, emphasizing the viability of producing high-value-added products from electronic waste.
Use of H2SO4 and H2O2 for Copper Extraction
The study by Ganji et al. [23] delves into the efficiency of the sulfuric acid–hydrogen peroxide (H2SO4-H2O2) combination for recovering Cu from PCBs. High recoveries (exceeding 90%) are reported, emphasizing both the operational simplicity of the method and the importance of properly controlling the redox potential to avoid unwanted byproduct formation.
Selective Leaching with Ammoniacal Systems
An alternative pathway is presented by Sun et al. [24] who employ an ammoniacal medium for the selective recovery of copper from complex mixtures of electronic waste. By using ammonia and ammonium carbonate solutions, they preferentially dissolve copper over other metals, thereby simplifying the subsequent separation and purification of the metal.
Role of Organic Agents in Enhanced Leaching
In He et al. [25], the addition of organic agents—specifically ethylene glycol—is assessed for its capacity to boost copper dissolution in an acidic medium, stabilizing H2O2 and enhancing the electrochemical behavior on the PCB surface. Such changes in leaching kinetics yield copper recovery efficiencies near 98%.
Physical Preconcentration and Ammonium-Based Leaching
Aiming to propose a “green” route with low reagent consumption, Shi et al. [26] outline a physical preconcentration strategy followed by ammoniacal leaching. This method achieves over 90% efficiency, underscoring the importance of combining physical (e.g., density or magnetic separation) and selective chemical steps to maximize extraction.
Solvent Extraction with Acorga M5640 and Solvent Reuse
According to Wang et al. [27], copper extraction from a leach solution can be enhanced by using specific extractants such as Acorga M5640. This solvent can be recycled more than ten times without significant loss of efficiency, implying cost savings and lower environmental impact.
Application of D2EHPA in the Hydrometallurgical Process
Following the solvent-extraction route for copper recovery, Correa et al. [28] describe the use of D2EHPA in a combined oxidizing leach and liquid–liquid extraction protocol. The process achieves notable copper purification while minimizing the entrainment of other metals, thereby consolidating a flow sheet with high scale-up potential.
Comparison of Hydrometallurgical Methods
In a review, Yousefzadeh et al. [29] carry out an analysis of hydrometallurgical techniques for Cu recovery, comparing them through modeling and multi-criteria methodologies (AHP-TOPSIS). They conclude that, although cyanide-based and strong-acid approaches achieve high efficiencies, alternative methods (e.g., organic acids or ammoniacal systems) may be more environmentally sustainable, depending on the specific operational conditions.
Chemical Leaching Modeling
Mathematical modeling plays a pivotal role in predicting copper recovery from PCBs. Becci et al. [30] propose a second-order equation integrating Fe3+ concentration, temperature, and the copper grade in the waste, enabling accurate estimation of leaching kinetics and metallic ion release.
Optimal Parameters for Chemical Leaching
To optimize the recovery of base metals from telecommunications and desktop-computer boards, Mizero et al. [31] employ experimental design (DOE) methods. Their findings highlight the significance of factors such as agitation rate, particle size, and H2SO4 concentration, aligning with other investigations that underscore the need for precise control of process variables.
Joint Recovery of Cu, Zn, and Ni
Some studies broaden the focus to include other valuable metals. Pinho et al. [32] explore the simultaneous dissolution of copper, zinc, and nickel from PCBs in an ammonia–ammonium sulfate system, demonstrating the flexibility of the ammoniacal medium for sequential separation of multiple metals and for producing high-grade products.
Bio-Metallurgical Aspects and Operational Parameters
In the bioleaching domain, Janyasuthiwong et al. [33] demonstrate the feasibility of recovering copper and other metals through microbial processes, contingent on well-established pH and redox potential conditions. Although the study primarily focuses on Ni and Pb, it underscores the potential of biological approaches, especially when combined with chemical methods.
Alkaline Fusion and Precious-Metal Leaching
Guo et al. [34] investigate a prior fusion of the material followed by leaching and separation steps to recover both Cu and other precious metals (Au, Ag). While the central focus is phase conversion, the study illustrates the versatility of the method in managing precious metals contained within the PCB matrix.
Sustainability and Industrial Scale-Up
Lastly, Dutta et al. [35] present a comprehensive assessment of the economic and environmental feasibility of recovering metals from PCBs, highlighting the optimization of hydrometallurgical stages and stressing the need for multivariable control to minimize hazardous waste production and maximize process profitability.
3.1.3. Contributions to Research
In summary, the assembled works provide a multidimensional view of copper and other metal recovery from PCB waste, covering the following:
- Novel reagents and leaching routes (H2SO4-H2O2 systems, ammoniacal media, alkaline fusion).
- Mathematical models and experimental designs for predicting and optimizing copper dissolution.
- Purification and solvent-extraction methods (Acorga M5640, D2EHPA) with potential reuse, reducing both costs and environmental impact.
- “Green” perspectives and industrial scale-up pathways, including the addition of organic agents or bioleaching.
These studies are fundamental for consolidating methodologies that deliver integrated solutions in metal recovery, with direct implications for managing electronic waste and optimizing hydrometallurgical processes in the mining industry. The diversity of approaches underlines the need to continue exploring technological synergies (e.g., physical and/or thermal pretreatments, deep eutectic solvents, advanced kinetic modeling) to ultimately bolster the development of sustainable and competitive industrial schemes.
3.2. Cluster 2: Complementary Studies on Copper Recovery and Leaching Risk
3.2.1. Cluster Overview
The second group of investigations, while not exclusively focused on copper heap leaching, complements the broader perspective on challenges and opportunities pertaining to copper recovery and metal management under various scenarios. On the one hand, the optimized recovery of copper from cyanidic solutions in gold–copper ore processing is discussed; on the other, the risks of copper mobilization and other metals in an electric-field-assisted phytoremediation setup are analyzed. These studies offer valuable insights into the management of Pregnant Leach Solutions (PLS) in gold mining and the mitigation of environmental risks in contaminated soils.
3.2.2. Key Findings
Optimizing Copper Recovery from Cyanidic Solutions
According to Kassymova et al. [36], copper present in the cyanidic solutions used to process gold–copper ores can be precipitated and recovered through a probabilistic–deterministic design, underscoring the complexity of adjusting variables like sulfidizer stoichiometry or the addition of neutralizing reagents. The authors employ a two-level full-factorial design () followed by a central composite response-surface model to screen three factors—pH, S dosage and temperature—and then refine their optimum. The model (adj. ) predicts that pH 11.2, 0.85 g S and 45 °C would raise Cu recovery from 78% to 86% while cutting reagent cost by 11%. This work proposes an experimental model that combines statistical analysis and empirical validation, striking a balance between metallurgical efficiency and the chemical stability of the post-leach solution. Such a multivariable approach aligns with the necessity for strict operational control in copper heap leaching [6,7], given the interplay of different reagents and conditions (pH, Eh, etc.).
Copper Mobilization Risk in Phytoremediation
Meanwhile, Luo et al. [37] address the risks of copper and lead leaching during soil phytoremediation using Noccaea caerulescens, under both traditional conditions and the application of an electric field. The study highlights how electric voltage can increase the mobility of metals not hyperaccumulated by the plant, thereby raising contamination risks for groundwater. Such findings underscore the importance of avoiding excessive dosing of reagents or over-intensifying processes (e.g., irrigation or electric power) to prevent undesired metal carry-over, a phenomenon similarly noted in copper heap leaching when excessively high irrigation rates are applied [12,13].
3.2.3. Practical Application of Statistical Design to Heap Leaching
Although Cluster 2 focuses on cyanidic solutions and phytoremediation, the statistical tools employed are directly transferable to heap-leach optimization. Full-factorial and response-surface methodologies such as those used by Kassymova et al. [36] are the same techniques successfully applied by Hosseinzadeh and Hosseini [6] (Taguchi ) and Yavari et al. [7] (Box–Behnken) to maximize oxide-ore extraction in column heaps. In those works, the most sensitive variables are the following:
- pH. Hosseinzadeh and Hosseini [6] reported that lowering solution pH from 2.5 to 1.8 increased Cu recovery from 74% to 82% (+8 pp) but raised acid consumption by 14 kg ore.
- Eh. In Yavari et al. [7], boosting redox potential from 540 mV to 670 mV (Ag/AgCl) with 5 g shortened the time to reach 80% extraction by 18%.
- Reagent concentration. Kassymova et al. [36] observed that S dosages above 1.0 g caused colloidal CuS formation and cut dissolved-Cu grade by 12%; factorial design identified 0.8–0.9 g as the economic optimum.
Applying these optimized conditions to pilot-scale heap columns reduced acid usage by 10–15 kg ore and lowered raffinate recycle volumes by 8%, yielding operating cost savings of USD 0.7– Cu [6,7].
3.2.4. Contributions to Research
The works included in this Cluster 2 provide complementary perspectives on the global understanding of Sustainable Leachate Recovery (SLR) by demonstrating
- Probabilistic–Deterministic Experimental Design: It is proposed by Kassymova et al. [36] to optimize copper recovery in cyanidic environments, illustrating the versatility of full-factorial and response-surface methodologies and their potential application in copper heap leaching, especially when handling mixed-metal solutions.
- Environmental Risk Management: The findings of Luo et al. [37] underscore the need to assess unintentional metal mobilisation during assisted remediation or extraction processes, an aspect that can be extrapolated to controlling redox potential and irrigation rate in heap leaching.
Hence, the optimization of hydrometallurgical processes is shown to aim not only at maximum copper recovery but also at minimizing the environmental risks associated with the release and transport of metals. The statistical design examples discussed above confirm that systematic experimentation—rather than one-factor-at-a-time trials—can simultaneously improve copper yield and reduce operating costs in heap operations. Integrating these designs with real-time monitoring of pH, Eh, and reagent dosing therefore represents the best practice for efficient and sustainable heap leaching.
3.3. Cluster 3: Optimization Methodologies in Copper Leaching and Processing Dynamics
3.3.1. Cluster Overview
This third cluster assembles research focused on the development and optimization of various copper leaching processes, emphasizing experimental design methodologies, the use of alternative water resources, and analyses of washout dynamics in heaps. The studies encompass approaches such as countercurrent leaching, the use of desalinated seawater in heap leaching, and the combination of statistical techniques (e.g., Taguchi, ANOVA, Response Surface Methodology, neural networks) to refine process efficiency.
3.3.2. Key Findings
Countercurrent Leaching and Raffinate Reuse
As reported by Movahhedi et al. [38], implementing a countercurrent leaching process offers an opportunity to reuse hydrometallurgical raffinate in treating low-grade ores. Through Response Surface Methodology (RSM), optimal operating conditions are determined, resulting in lower fresh-water requirements and greater copper selectivity.
Advanced Optimization Algorithms in Ammoniacal Systems
Combining RSM with evolutionary algorithms, such as a backpropagation neural network coupled with a genetic algorithm (GA-BPNN), allows for more accurate performance prediction in the leaching of copper oxides under ammoniacal conditions [39]. This approach simultaneously optimizes the ammonium concentration, pH, and temperature—crucial variables for preventing the formation of passivating species and achieving high copper recovery.
Experimental Design Methods for Concentrates and Tailing Leaching
Other authors, including Mbuya et al. [40], have employed Taguchi and ANOVA to optimize the leaching of tailings containing copper and cobalt, demonstrating that integrating these statistical tools facilitates identifying robust conditions against parameter fluctuations (temperature, time, concentrations, etc.). Similarly, Zandevakili et al. [41] apply the Taguchi method to fine-tune the leaching of Sarcheshmeh concentrate, whereas Borsynbayev et al. [42] propose an electrohydropulse discharge-based method to improve copper extraction from tailings. These studies underscore the importance of experimental planning in the mining industry, where mineralogical and operational variability pose an ongoing challenge [2,5].
Optimization and Modeling in Alkaline and Ammoniacal Systems
Process optimization in ammoniacal and alkaline solutions is also demonstrated by Nadirov et al. [43] who use mathematical models to identify optimal conditions for copper and zinc leaching from smelting slags. Their work confirms the critical role of carefully managing medium chemistry and the liquid–solid ratio, aspects of equal relevance to heap leaching [12].
Environmental Assessment and Water Resource Utilization
Access to water resources constitutes another essential factor. Goodboy and Missimer [44], for instance, compare the use of desalinated versus filtered seawater in copper extraction at mines located at high altitudes and in arid regions. While the study is not primarily focused on leaching kinetics, its findings complement the debate on water management and sustainability—critical elements in copper heap leaching, where the water balance is paramount [1].
Innovations in Heap Leaching Techniques
The deep well rinsing methodology proposed by Rucker [13] demonstrates the feasibility of injecting leaching solutions into deep wells to enhance copper extraction rates in oxidized ores. This approach becomes especially relevant when the heap exhibits structural heterogeneity or when surface drainage is hindered.
Leaching Dynamics and Metallurgical Efficiency
Studies such as Yuan et al. [45] highlight the importance of kinetic characterization and dynamic testing for recovering copper and silver from tailings, emphasizing the synergy between leaching parameters (chemical agent, temperature) and the overall profitability of the process. Additionally, Estay and Díaz-Quezada [46] “deconstruct” the so-called leaching ratio, a widely used industrial-scale simplification, pointing out that adopting more detailed kinetic models could enhance predictions of copper extraction and enrich heap leaching planning.
Application of Advanced Materials for Metal Containment
Regarding impact mitigation, Mazarji et al. [47] work with metal–organic frameworks (MIL-101) and biocarbons as amendments in contaminated soils, demonstrating their ability to immobilize copper (among other contaminants). Although the primary focus is the retention of metals in soils, such encapsulation strategies can be extrapolated to stabilize tailings or reduce metal migration in discontinued heap leaching systems.
3.3.3. Contributions to Research
Overall, the studies in Cluster 3 reinforce several key aspects in Sustainable Leachate Recovery (SLR):
- Statistical and Computational Models: Using RSM, Taguchi, ANOVA, and GA-BPNN (among others) refines parameters and detects nonlinear relationships in copper leaching, bolstering the robustness and repeatability of the process.
- Closed-Loop Approaches and New Technologies: Countercurrent leaching, raffinate reuse, and the injection of leachants into deep wells exemplify operational innovation aimed at metallurgical efficiency and optimized water consumption.
- Water Management and Environmental Factors: The adoption of desalinated or filtered water and the application of advanced materials for contaminant containment underscore the significance of environmental considerations, aligning with the sustainability mandate prevalent in the industry.
- Industrial Scale-Up Considerations: Studies by Movahhedi et al. [38], Goodboy and Missimer [44], and Rucker [13] reveal the complexities of scaling up, highlighting the need for pilot-scale tests and the adaptation of operations to local conditions (altitude, arid climate, mineral characteristics).
From the viewpoint of copper heap leaching, these contributions help consolidate methodologies for controlling and optimizing irrigation rate, temperature, and chemical reagent concentrations, while opening the door to alternative water strategies and more precise, reliable statistical models. In line with the holistic perspective of this research, they underscore the importance of comprehensive, multivariable control to achieve higher recovery levels and minimize the environmental impacts inherent to mining activities.
3.4. Cluster 4: Advances in Leaching of Oxidized and Complex Copper Ores
3.4.1. Cluster Overview
This fourth cluster encompasses a range of studies on copper extraction from oxidized and complex ores, highlighting a variety of reagents and methodologies—organic acids, ammoniacal solutions, surfactants, etc.—as well as their integration with flotation processes and kinetic-control strategies. Overall, these works underscore the importance of selectively adjusting operational parameters (pH, leaching agent, temperature, additives) to increase the copper dissolution rate and reduce costs, aspects equally relevant in large-scale heap leaching [12,48,49].
3.4.2. Key Findings
Organic Acids in Leaching Oxidized Copper Ores
Yaras and Arslanoglu [50] demonstrate that formic acid is effective in dissolving low-grade copper (malachite). Their study highlights the possibility of extending this route to other similar species (e.g., cuprite), provided that physicochemical parameters such as proton activity and formate concentration are carefully managed. This “organic” leaching proposal aligns with the growing trend of exploring “green” and eco-friendly reagents [10,11] to mitigate the environmental risks associated with using strong mineral acids.
Ammoniacal Systems and Specific Chelating Agents
Xiao et al. [14] examine the behavior of an oxidized copper ore with high gangue alkalinity when EDTA · 2Na is employed, emphasizing the preferential complexation of copper and the mitigation of side reactions with alkaline components. The use of chelators or complexing agents emerges as an alternative in cases where the presence of carbonated or silicate gangues hinders copper extraction using conventional acids. Similarly, Han et al. [51] confirm that activation with NH4HF2 in an ammoniacal medium (NH3-NH4Cl) enhances the dissolution of low-grade complex ores.
Leaching of Refractory Oxides and Copper Loss in Tailings
From another viewpoint, Wang et al. [52] investigate the behavior of refractory oxidized ores from the Yulong region (Tibet), placing emphasis on agitation and the need to optimize costs and recovery efficiency. Likewise, Aghazadeh et al. [8] and Ambo et al. [53] demonstrate the feasibility of combining leaching processes with pre-flotation or physical preconcentration methods (e.g., near-infrared sensors) to increase copper grade and reduce inert gangue before the main dissolution stage.
Kinetic Contributions and Leaching Models
Robertson [12] delves into the significance of an integrated flow-and-mineral-leaching model, addressing the solution transport dynamics in large heaps. This approach aligns with the case studies of Hosseinzadeh et al. [54] and Shi et al. [55], who assess leaching kinetics in low-grade ores and smelting slags, respectively. Both adopt the shrinking-core model or other kinetic variants to describe copper extraction and distinguish the controlling step (surface chemical reaction vs. diffusion through the product layer).
Acidification and Reagent Support in Ore and Slag Dissolution
In line with these findings, Deng et al. [56] explore the action of lactic acid in leaching oxidized ores, observing that temperature and reagent concentration strongly influence dissolution kinetics. Additionally, Wang et al. [2] describe a complementary method combining sulfide ore flotation with acid leaching of tailings to optimize overall copper recovery. Meanwhile, Yingwei et al. [57] focus on recovering tellurium from a copper–tellurium material, underscoring the versatility of H2O2 as an oxidant in acidic systems.
Synergy with Electrometallurgical Techniques and Surfactants
El-Okazy et al. [58] demonstrate copper recovery by electrodeposition from an acidic leach liquor, underscoring the importance of integrating hydro- and electrometallurgical techniques to produce a high-purity product. In parallel, Ai et al. [48] and Ai et al. [49] highlight the accelerated copper leaching achieved with surfactants, reducing surface tension and improving particle wettability. This aspect can be critical in large-scale heaps, where permeability and uniform irrigation are vital [13].
Methods, Effective Reagents, and Influence of Key Variables
Studies using organic acids report high copper extractions when both acidity and temperature are controlled. For example, Yaras and Arslanoglu [50] and Deng et al. [56] leach malachite and other oxidized ores with 1.0 mol formic acid at 60 °C and 0.8 mol lactic acid at 65–70 °C, obtaining 85–95% Cu while keeping the pH < 2. In ammoniacal media, Xiao et al. [14] reach about 92% recovery by adding 0.15 mol EDTA·2Na (pH 9–10, 45 °C), showing that selective complexation can overcome gangue alkalinity. Activation with 0.10 mol in Cl, as tested by Han et al. [51], raised copper dissolution from 48% to 83% at 50 °C through removal of silicate coatings. Low-dose surfactants (0.05–0.10 g SDS) reduced surface tension and improved diffusion, giving an extra 10–12% Cu at ambient temperature [48,49]. Finally, kinetic tests on refractory Yulong ores show that increasing the temperature from 25 °C to 70 °C roughly doubles the rate constant, although energy costs and impurity precipitation rise markedly above 60 °C [52]. Overall, the evidence identifies practical operating windows—pH < 2 for organic acids, pH 9–10 for ammoniacal systems, and 40–70 °C for temperature—and confirms that maximising copper recovery depends on matching each reagent system with tight control of pH and temperature.
3.4.3. Contributions to Research
The works compiled in this Cluster 4 complement Sustainable Leachate Recovery (SLR) with proposals centered on
- New Reagents and Operating Conditions: Use of organic acids [50,56], complexing agents such as EDTA [14], and surfactants [48,49] to optimize dissolution kinetics and reduce passivating byproducts.
- Kinetic Control and Advanced Modeling: Assessing the controlling mechanisms in leaching (chemical interface vs. diffusion), developing integrated mineral flow models [12], and validating them experimentally in different mineral matrices [54,55].
- Utilizing Tailings and Slags: Combining flotation–leaching strategies [2], treating slags with H2SO4 [55], or addressing complex ores [51] to maximize copper recovery and make use of residues normally discarded.
- Integration with Electrometallurgical Stages: Cases where leaching is followed by electrodeposition to obtain higher-purity copper, thereby extending the value chain [58].
Overall, these studies support the premise that optimizing copper leaching—whether in heaps or agitated reactors—depends on a blend of approaches: rigorous control of operational variables, careful reagent selection, and the application of suitable kinetic models to predict extraction rates and the formation of detrimental species (jarosites, passivating compounds, etc.). From the SLR perspective, adopting organic reagents, chelating agents, and “eco-friendly” surfactants appears promising for improving both performance and environmental sustainability of the process, provided that the leaching process and potential environmental impacts are managed in a comprehensive manner.
3.5. Cluster 5: “Green” Applications and Innovative Oxidants for Copper and Other Metal Recovery
3.5.1. Cluster Overview
This fifth cluster compiles research on more ecological (green) methods for recovering copper and other metals, with special attention to alternative reagents such as glycine and to kinetic strategies involving novel oxidants. The unifying theme across these studies is the pursuit of a balance between metallurgical efficiency and environmental sustainability, in line with the notion of Sustainable Leachate Recovery (SLR).
3.5.2. Key Findings
Glycine as a Selective and Eco-Friendly Agent
Barragán-Mantilla et al. [10] investigate the use of glycine for recovering copper from mining residues and less conventional ores, emphasizing its low environmental impact and the possibility of implementing a closed circuit to reduce effluent generation. This approach aligns with trends favoring “green” agents and minimizing aggressive reagent addition in copper heap leaching [11,23].
Application of Glycine in Smelting Slags
Huang et al. [11] develop a method for copper leaching from copper smelting slags using an alkaline glycine solution. High selectivity in copper extraction is observed, facilitating subsequent separation of other metals. This underscores the potential of glycine-based complexing leaching in alkaline conditions, where pH control and metal–ligand coordination play a pivotal role.
Recovery in Sediments and E-Waste with Environmental Metrics
Li et al. [59] address copper recovery in acidified sediments containing cupric thiocyanate (CuSCN) from the gold industry, demonstrating the feasibility of a “green” method that reduces hazardous chemicals and toxic effluents. Separately, Rezaee et al. [60] examine a staged glycine–permanganate leaching process for the valorization of base and precious metals (Au, Ag) in printed circuit boards (PCBs). Their study includes a life-cycle assessment (LCA), illustrating the reduced environmental footprint compared to conventional hydrometallurgical routes.
Oxidant-Based Approaches and Synergy with Other Additives
Though primarily focused on molybdenite, Shoghian-Alanaghi et al. [61] underscore the importance of employing specialized oxidants (H2O2, MnO2, or even Cu2+) where an intensified dissolution rate is desired. Moreover, the effect of adding ethylene glycol and oxygen is evaluated, increasing surface reactivity. This type of “combined” strategy can be extrapolated to copper heap leaching when co-oxidants and surfactants are used to improve infiltration and leaching kinetics [5,48].
3.5.3. Contributions to Research
The works included in Cluster 5 highlight the importance of
- Promoting mild and eco-friendly reagents, such as glycine, to reduce process toxicity and meet stricter environmental regulations.
- Designing staged leaching sequences, employing permanganate or co-oxidants, which facilitate precious-metal extraction and e-waste management Rezaee et al. [60].
- Assessing the synergy between leaching agents and oxidants (as in the use of ethylene glycol combined with H2O2 and O2), a way to enhance dissolution rate and lower operating costs Shoghian-Alanaghi et al. [61].
- Incorporating environmental indicators and life-cycle modeling (LCA) to objectively compare “green” pathways against conventional methods.
In the context of copper heap leaching, these advances suggest adopting combined strategies—glycine, “mild” oxidants, multistage leaching sequences—that could boost overall recovery and reduce the load of contaminants in the Pregnant Leach Solution (PLS). This approach aligns with the Sustainable Leachate Recovery (SLR) framework, in which metallurgical efficiency and environmental sustainability constitute a dual objective that is crucial for twenty-first-century copper mining.
3.6. Cluster 6: Leaching and Metal Recovery from E-Waste, Anode Slimes, and Industrial Residues
3.6.1. Cluster Overview
The sixth cluster covers studies focusing on metal extraction and recovery—chiefly copper, gold, and other valuable elements—from complex secondary sources such as integrated circuits, waste electrical and electronic equipment (WEEE), anode slimes, and NMC-type battery residues. These investigations highlight diverse reagents (including bromotrihalide ionic liquids, Na2S2O8, and strong acids) and the implementation of pressure-leaching and electro-generation techniques to optimize leaching kinetics and selectivity. Additionally, statistical modeling strategies and the feasibility of catalytic electrodeposition technologies are discussed to promote more sustainable metal utilization.
3.6.2. Key Findings
Novel Ionic Liquids for Gold and Copper Recovery
With a view to exploiting precious and base metals from circuit boards, Yin et al. [62] propose bromotrihalide-based ionic liquids capable of selectively dissolving gold and copper via a redox mechanism. They emphasize low toxicity and high extraction efficiency, both factors aligning with the quest for “green” and cost-effective routes. Although this approach targets e-waste, it resonates with the need for similar strategies in copper heap leaching (SLR) when dealing with complex ores.
Statistical Assessment of WEEE Leaching with Na2S2O8
Popescu et al. [63] employ statistical methods to unravel the factors influencing the dissolution of copper, zinc, and brass from electronic devices in a sodium persulfate (Na2S2O8) solution. Their work underscores the roles of temperature, contact time, and oxidant concentration, findings consistent with studies on sulfide or oxidized ores where adding oxidants (e.g., H2O2, Fe3+) can accelerate leaching kinetics [5,7].
Anode Slime Treatment and Pressure Leaching
An emerging field involves the treatment of anode slimes generated during copper electrorefining. Li et al. [64] propose an alkaline fusion followed by leaching to recover both copper-bearing and precious metals, whereas Seisko et al. [65] emphasize pressurized leaching of decopperized anode slimes, attaining high recoveries under strongly acidic conditions. This underscores both the significance of pressure leaching as a complement to heap leaching and the necessity for stringent pH and Eh control to maximize yield [12].
Electrochemistry and E-Waste-Related Technologies
Electrochemical techniques figure prominently in Li et al. [66] and Fathima et al. [67], which describe recovering copper and gold from memory modules and other electronic components. While Li et al. [66] couple electrooxidation in an HCl-H2O2 medium to simultaneously dissolve and recover metals, Fathima et al. [67] provide a critical review of catalytic electrodeposition for optimizing copper extraction from e-waste. In both cases, electrochemical control—whether by voltage or current—enhances selectivity and avoids passivating by-product formation, a principle also transferable to heap-leaching operations with subsequent electrolytic refining.
Battery Residues and Statistical Modeling
Partinen et al. [68] broaden the scope to “black mass” derived from lithium-ion batteries, containing metals such as Ni, Mn, and Co. They use experimental tests combined with regression modeling to understand leaching kinetics. Although their research focuses on the battery industry, the methodological approach—combining experiments with statistical modeling—aligns with optimizing copper heap leaching, where predicting kinetic behavior under varying acidity and irrigation conditions is crucial [6,46].
3.6.3. Contributions to Research
The works in Cluster 6 advance Sustainable Leachate Recovery (SLR) by
- Broadening the boundaries of leaching operations to include complex industrial byproducts (anode slimes, e-waste, spent batteries) with high copper or gold content.
- Integrating chemical and electrochemical leaching methods, particularly where rapid dissolution and simultaneous electrolytic metal recovery are sought.
- Employing advanced statistical and modeling techniques (e.g., design of experiments, multivariable regression) to elucidate how different operational factors interact in the leaching kinetics.
- Proposing ionic liquids and oxidants with lower environmental impact, paralleling the exploration of glycine and “green” surfactants for primary minerals.
Overall, these contributions highlight the need for a holistic perspective in the mining and recycling industries, where heap leaching could be complemented by electrolytic refining and innovative reagents to unlock the value of post-consumer waste and metallurgical byproducts. This approach aligns with the circular-mining trend and with reducing the environmental footprint associated with copper and precious-metal production.
3.7. Cluster 7: Biohydrometallurgy and Hybrid Methods for Copper Recovery from Electronic Waste
3.7.1. Cluster Overview
This seventh cluster brings together studies focusing on copper recovery from electronic waste and electric cables, emphasizing biohydrometallurgical methods and hybrid approaches that combine chemical, biological, and/or physical separation stages. The primary objective is to exploit the capacity of microorganisms (e.g., Acidithiobacillus ferrooxidans) to catalyze leaching, reducing reliance on harsh reagents and fostering more environmentally benign processes. These techniques can in part be extrapolated to heap leaching when bioleaching is introduced as an alternative or complement to conventional chemistry [5].
3.7.2. Key Findings
Copper Recovery from Electrical Cables by Bioleaching
Lambert et al. [69] examine copper leaching from discarded electrical cables, comparing purely chemical leaching to Acidithiobacillus-catalyzed leaching in terms of kinetics and reagent needs. A higher efficiency in copper dissolution emerges under well-controlled redox conditions, aided by biologically regenerated ferric iron. Controlling Eh, pH, and temperature—comparable to requirements in heap leaching of copper ore [6,7]—proves crucial for optimizing recovery rates.
Bio-Assisted Leaching of Pyrolyzed Circuit Boards
Building on this trend, Arinanda et al. [5] investigate copper recovery from pyrolyzed printed circuit boards (PCBs), examining how different operational parameters (e.g., agitation, ferric/ferrous concentration, dissolved oxygen) affect the dissolution of metallic copper. The process merges bio-oxidation of iron with subsequent copper dissolution, underscoring the role of microorganisms in continuously regenerating ferric species and enhancing selectivity, in accordance with bioleaching approaches for sulfide or oxide ores [10].
Hybrid Approaches and Copper Reuse
Conversely, Sinha et al. [70] propose a hybrid methodology for recovering and reusing copper from e-waste, integrating chemical leaching stages and low-cost biomaterials. The study highlights the feasibility of reintroducing recovered copper into industrial processes, thus closing the value loop and curbing the need for primary extraction. Such a circular-economy model aligns with the Sustainable Leachate Recovery (SLR) paradigm by prioritizing both metallurgical efficiency and waste minimization.
3.7.3. Contributions to Research
From a sustainable leaching perspective, the articles in Cluster 7 offer
- Bio-Assistance and Ferric Regeneration: Whether in electronic waste (cables or PCBs), bacteria like Acidithiobacillus can facilitate Fe3+ generation and speed up extraction kinetics, reducing large-scale oxidant requirements.
- Synergy between Chemical and Biological Processes: Integrating thermal pretreatment (pyrolysis), chemical leaching, and biological catalysis helps to optimize copper recovery, an approach with potential adaptation to heap leaching of difficult ores or byproducts.
- Use of Biomaterials and Circular Economy: Direct reuse of extracted copper, as in Sinha et al. [70], reinforces the circular-economy principle, where recovered metal is reinserted as a raw material—potentially extending to heap leaching circuits directly supplying electrowinning processes.
Overall, these approaches reaffirm the versatility of biohydrometallurgy and combined methods for optimizing copper recovery in a sustainable context, broadening the scope beyond primary mineral extraction and effectively applying these strategies to e-waste valorization. Under SLR, incorporating microorganisms and hybrid methodologies represents a promising path toward greater efficiency and reduced environmental impact in copper leaching.
3.8. Cluster 8: Modeling, Statistical Analysis, and Process Design for Copper Leaching
3.8.1. Cluster Overview
This eighth cluster groups studies that highlight the significance of statistical, mathematical, and computational modeling in copper leaching, spanning various approaches—from chemometric methods, response surface methodology, and experimental design, to machine-learning tools and multiscale models for predicting metal recovery. The common denominator is the pursuit of optimal conditions—tuning parameters such as leaching-agent concentration, column height, temperature, or irrigation rate—and the validation of these models under conditions of mineral heterogeneity.
3.8.2. Key Findings
Chemometrics and Chlorides in Sulfide Leaching
In Sokić et al. [71], a chemometric approach is employed to study the dissolution kinetics of chalcopyrite concentrate using sodium nitrate (NaNO3) in a H2SO4 medium, confirming that controlling variables such as the NaNO3:H2SO4 ratio and temperature significantly affects copper extraction rate. Similarly, Saldaña et al. [72] develop an analytical model for secondary sulfide leaching in chloridic media, underscoring the importance of chloride chemistry in enhancing copper recovery and proposing industrial alternatives when traditional sulfate environments fail to provide the desired kinetics.
Optimization via Response Surface Methodology and Experimental Design Models
Response Surface Methodology (RSM) appears in multiple studies. For instance, Yu et al. [73] use RSM to extract copper from refractory oxidized ores, whereas Hayati et al. [74] integrate it with Monte Carlo simulations to estimate copper leaching from e-waste under uncertainty. Likewise, Harichandan and Mandre [75] (in two successive publications) and Hernández et al. [76] demonstrate how to design and operate leaching systems (in both reactors and heaps) through statistical optimization techniques. These works underscore the relevance of variable interactions (pH, Eh, acid concentration, particle size, column height, etc.) in achieving optimal copper recovery.
Multiscale Models and Flow Simulations
From a more computational perspective, Ortega-Tong et al. [77] apply a reactive-transport model to identify the mineralogical controls governing in situ copper recovery. Similarly, Paspureddi et al. [78] propose a multiscale reactive-flow model in porous microstructures, a highly pertinent approach for predicting large-scale heap behavior by accounting for textural variability and the geo-chemical complexity of the ore [2,5].
Machine Learning and Neural Networks for Recovery Prediction
Machine learning has also found application in leaching. Flores and Leiva [79] compare various supervised learning algorithms to forecast copper recovery based on operational parameters. With a similar perspective, Harichandan and Mandre [75] evaluate both statistical models and artificial neural networks, highlighting the latter’s ability to capture nonlinear interactions and improve metallurgical performance predictions.
Modeling and Validation in Columns and Pilot Operations
Studies such as Wang et al. [80] (ISR in Kapunda, Australia) and Hoseinian et al. [81] (column leaching model for oxides) emphasize the importance of empirically validating models at the pilot or semi-pilot scale. Their findings demonstrate how intermediate-scale experimentation can validate—or refine—theoretical frameworks, thereby enabling scalability to industrial applications.
3.8.3. Contributions to Research
The works compiled in Cluster 8 are fundamental to Sustainable Leachate Recovery (SLR) by
- Enhancing predictive capabilities in copper leaching, integrating statistical methods (RSM, DOE), machine learning, and multiscale reactive-flow models.
- Demonstrating the usefulness of simulations to anticipate heap behavior under different conditions (height, particle size distribution, acid vs. chloride media, etc.), reducing costly trial-and-error testing.
- Underscoring the importance of variable interactions (pH, Eh, oxidant dosage, temperature) in dissolution kinetics, validating their relevance for the efficient and robust design and operation of leaching heaps.
- Highlighting methodological versatility, such as leaching in chloride media Saldaña et al. [72] or modeling with AI Flores and Leiva [79], providing new pathways for more stringent process control.
In conclusion, Cluster 8 accentuates the demand for analytical and computational tools to tackle the complexity of copper heap leaching, especially where mineralogical heterogeneity and large-scale flow dynamics complicate operational planning. Through these contributions, global optimization (metallurgical, economic, and environmental) becomes more attainable, bolstering SLR principles in the mining industry.
3.9. Cluster 9: Novel Approaches and Leaching Alternatives for Copper Complex Ores and Byproducts
3.9.1. Cluster Overview
This ninth cluster encompasses studies focused on copper extraction (and other valuable metals) from diverse sources: smelting slags, secondary sulfides in chloride media, tailings, and byproducts such as plant residues (CPR), as well as hybrid (bio-chemical) and solvometallurgical methods. These works highlight both the variety of reagents and environments (organic acids, deep eutectic solvents, pressurized media, chloride solutions, etc.) and the need to optimize leaching kinetics while anticipating environmental impacts.
3.9.2. Key Findings
Copper Slag Treatment and Kinetic Models
Sun et al. [82] delve into the kinetics of copper leaching from smelting slag using H2SO4 under atmospheric-pressure oxidation. Their findings suggest that control is governed by diffusion through the product layer, enabling up to 90% copper extraction under optimal conditions. Meanwhile, Topçu et al. [83] propose a deep eutectic solvent (based on choline chloride) to recover copper from slag; this “green” route reduces dependence on strong mineral acids and includes a predictive extraction model. Consistently, Gargul et al. [84] investigate the application of citric acid for leaching lead and copper from flash slags, confirming the feasibility of organic acids as an environmentally friendly alternative.
Bio-, Reactor, and Column Testing in Low-Grade Resources
In a comparative study, Panda et al. [85] combine reactor and column tests for two low-grade resources, finding that the combination of flotation and leaching significantly enhances recovery. Likewise, Wang et al. [2] describe controlled bioextraction of low-grade sulfide ores in columns using microorganisms and well-defined conditions (pre-leaching, temperature, irrigation distribution). This “well-controlled” design suggests adaptability to industrial heap leaching, where irrigation homogeneity is crucial [5].
Pressure Technologies and Advanced Processes
Green et al. [86] present a decade of experience in pressure leaching of copper concentrates at Morenci (U.S.), demonstrating high extractions and less impact compared to traditional pyrometallurgical methods. In turn, Mohamed et al. [87] detail the simultaneous recovery of copper and nickel from Egyptian ores via a “pug leaching” technique at moderate temperatures, underscoring the importance of optimizing variables (H2SO4/ore ratio, contact time, etc.). Combined agitation or pressurized conditions can boost the leaching rate and reduce costs.
Chloride Solutions, Tailings, and Byproducts
Schueler et al. [88] investigate the recovery of Cu, Zn, and Pb from sulfide tailings in a H2SO4/Cl− medium, revealing kinetic advantages and a more selective dissolution compared to the sulfate-only approach. Meanwhile, Turan et al. [89] employ ammonium salts for the selective extraction of copper from tailings, demonstrating that ammoniacal complexation at certain concentrations can favor copper transfer without requiring large volumes of acid. The recovery of lead from plant residues (CPR) is discussed by Li et al. [66], integrating a brine leaching process with precipitation and calcination—a method potentially applicable to copper recovery from byproducts in saline mixtures.
Comparative Appraisal of Alternative Leaching Media and Their Sustainability Trade-Offs
Recent studies allow a quantitative comparison of the main “non-conventional” lixiviants applied to complex ores and by-products. Acidic oxidation with H2SO4 remains the kinetic benchmark, reaching ~90% Cu recovery from smelting slag at 80 °C (10 g acid, 120 min) but generates sulfate-rich effluents that demand costly [82]. Citric acid, a biodegradable organic reagent, delivered 55–72% dissolution of flash-smelter slag at pH 2–3 and ambient temperature while lowering acute-toxicity indicators, although its high COD must be managed [84]. A choline-chloride/ethylene-glycol deep-eutectic solvent (DES) extracted up to ~85% Cu after 2 h at 90 °C, and the spent DES was recycled for five successive cycles with <5% efficiency loss, evidencing good circularity; its higher viscosity, however, may limit percolation in large heaps [83]. Finally, column bioleaching of low-grade sulfide tailings achieved 60–68% Cu after 35 days with minimal reagent input, although longer residence times and strict aeration control are required [2]. These data confirm that reagent selection involves a performance–environment balance whereby DES and organic acids reduce hazardous discharge, whereas bio-routes offer the lowest chemical footprint at the expense of slower kinetics.
Innovative Applications and Pilot-Scale Testing
Smolinski et al. [90] report on the utility of nuclear techniques (radiotracers with 64Cu) to track leaching kinetics and clarify extraction pathways, whereas Atta Mends et al. [91] address nickel and copper recovery from sulfide flotation tailings, reiterating the relevance of controlled leaching methodologies. Ambo et al. [92] describe the “selective leaching” of copper in infrared sensor–preconcentrated ores, illustrating the synergy between preconcentration techniques and leaching to improve feed grade and reduce contaminants. Finally, Kamariah et al. [93] introduce a solvometallurgical process using monoethanolamine to extract copper from chrysocolla, achieving high recovery at elevated temperatures and demonstrating the possibility of recycling the leachant.
Bioleaching and Toxicity in Metallurgical Residues
Potysz et al. [94] examine the bioleaching of slags and the residual toxicity of byproducts, demonstrating the complexities of managing metallurgical wastes. Such environmental implications resonate with previous heap-leaching studies, where the Pregnant Leach Solution (PLS) must meet purity standards for subsequent processing [11,95]. Likewise, Praburaman et al. [96] assess copper bioleaching in contaminated soils using an organic formulation (panchakavya), confirming that certain natural amendments can positively influence metal solubilization.
3.9.3. Contributions to Research
The works in Cluster 9 advance Sustainable Leachate Recovery (SLR) by
- Expanding the range of resources and byproducts (slag, tailings, contaminated soils, metallurgical residues) that can serve as feedstock for copper leaching under environmentally acceptable conditions.
- Showcasing various extraction pathways (leaching with organic acids, pressure processes, deep eutectic solvents, ammoniacal complexation, etc.), each offering distinct advantages depending on mineralogy and gangue composition.
- Highlighting methodological integration (column tests, reagent reuse, preconcentration via infrared sensors, radiotracers) to enhance recovery rates and reduce environmental footprints.
- Addressing toxicity and byproduct management, an essential aspect of the SLR philosophy, given that copper recovery must be coupled with the proper handling of undesirable metals and final residue.
In summary, the reviewed literature in this cluster demonstrates that copper leaching extends beyond conventional ores and acidic technologies, instead exploring novel reactive pathways and sustainable approaches capable of maximizing recovered value while minimizing environmental impact. This diversification reinforces the relevance of SLR as a strategy for contemporary mining, wherein technological innovation and sustainability criteria jointly define competitiveness and social acceptance of the sector.
3.10. Cluster 10: Metal Extraction and Environmental Effects of Minerometallurgical Wastes in the Context of Copper Leaching
3.10.1. Cluster Overview
The tenth cluster concentrates on investigations involving recovery of copper (and other metals) from metallurgical wastes (e.g., from zinc, brass, or spent catalysts) and the characterization of heavy-metal release in soils and environments impacted by mining. It also includes several studies that explore the potential use of copper tailings in geotechnical applications and the effects of different leachants on the release of contaminant metals. All of this aligns with the quest for more sustainable leaching methodologies and impact mitigation under Sustainable Leachate Recovery (SLR).
3.10.2. Key Findings
Copper Integration in Valuable-Element Extraction
Zhang et al. [97] (first reference in the cluster) and Zhang et al. [97] (third reference, same authors in another study) propose adding or involving copper powder in metal extraction from zinc-leaching residues and zinc ferrite, respectively. These investigations underscore the possible reuse of copper as a reducing or dissolution-promoting agent for other metals, emphasizing copper’s versatility in hydrometallurgical circuits and reinforcing the circular economy. Moreover, Sharma et al. [98] apply the Taguchi design to optimize copper and zinc leaching from spent CuO/ZnO/Al2O3 catalysts, illustrating the potential to recover copper from refining or chemical-processing industries.
Recovery from Tailings and Industrial Wastes
Kilicarslan and Saridede [99] detail the treatment of brass residues to recover copper and zinc, demonstrating that hydrometallurgical routes can be competitive against simply disposing of the material. Separately, Turan et al. [89] (from a previous cluster) address the selective extraction of copper in mine tailings via ammonium salts. These approaches validate the adaptability of leaching to various byproducts, yielding both operational and environmental benefits.
Environmental Effects and Heavy-Metal Release
Regarding environmental impacts, Yin et al. [100] and Kim and Hyun [101] analyze the release of metals (e.g., Cu, Zn, Pb, As) from incineration ashes or abandoned mine soils, highlighting how pH and local geochemistry govern metal mobility. This is closely tied to controlling leaching parameters in heap leaching, given that the final Pregnant Leach Solution (PLS) composition and potential acid drainage depend on variables like pH, Eh, and the presence of complexing agents [2,7]. Additionally, Sumalatha et al. [102] and Fan et al. [103] present metal-removal techniques in industrial sludges or asphalt mixtures containing converter slag (BOF), confirming the importance of investigating metal release under different leaching conditions and its possible effect when reusing tailings materials in civil applications.
Risks and Metal Mobility in Tailings and Agricultural Soils
Fang et al. [104] examine metal release upon applying sewage-sludge compost to soils, whereas Sun et al. [105] and Yan et al. [106] study the transport and transformation of heavy metals (Cu, Pb, Zn, etc.) from mine tailings to agricultural soils (e.g., rice paddies), documenting potential human-health risks and the need to monitor metal bioaccumulation. Vinoth and Aswathy [107] review the environmental effects of using copper tailings as a geotechnical material, underscoring the relevance of characterizing long-term leachability and ensuring no groundwater contamination.
3.10.3. Contributions to Research
The literature presented in this Cluster 10 contributes to Sustainable Leachate Recovery (SLR) in several ways.
- Revalorization of Industrial Wastes and Catalysts: Multiple works demonstrate the feasibility of extracting copper from secondary streams and metallurgical tailings, supporting a circular-economy approach and reducing environmental liabilities Zhang et al. [97], Sharma et al. [98].
- Controlling Metal Mobility in Civil Engineering Applications: Studies on incorporating tailings into construction materials (e.g., asphalt mixes, fills) emphasize the need to evaluate the long-term leachability of metals Fan et al. [103], Vinoth and Aswathy [107].
- Mitigating Contamination and Health Risks: Several investigations, including Kim and Hyun [101], Sun et al. [105], Yan et al. [106], stress the urgency of monitoring heavy-metal release from soils or mine tailings, reinforcing the notion that success in copper heap leaching depends not only on metallurgical extraction but also on safeguarding the environment.
- New Approaches for Joint Extraction: Both copper-assisted leaching of zinc—Zhang et al. [97]—and the joint extraction of Cu and Zn from catalysts and brass—Sharma et al. [98], Kilicarslan and Saridede [99]—illustrate the versatility of hydrometallurgical methods when operational variables (pH, Eh, ammoniacal or organic ligands) are properly controlled.
Taken together, the contributions of this cluster underscore the importance of sustainable leachate recovery (SLR) as an integral strategy, wherein cost minimization and process optimization for extracting copper must go hand in hand with reducing environmental risks and exploring avenues for byproduct utilization in industry. All of this reaffirms the necessity for meticulous control over leaching parameters (temperature, acidity, Eh, among others) and for long-term evaluations encompassing final disposal or reuse of generated residues.
3.11. Cluster 11: Challenges and Advances in Chalcopyrite Leaching for Copper Recovery
3.11.1. Cluster Overview
This eleventh cluster centres on chalcopyrite leaching (main Cu mineral), covering process optimization, kinetics, and statistical designs. Findings are pivotal due to chalcopyrite’s known refractoriness and passivation tendency [1,9,108].
3.11.2. Key Findings
Condition Optimization via RSM and Factorial Designs
Statistical designs (Response Surface Methodology [RSM], factorial) refine chalcopyrite leaching variables. Examples include optimizing concentrate dissolution [108], using Ag-coated pyrite [109], controlling Eh [110], and leaching roasted concentrates [111], all identifying optimal conditions (time, H2SO4, temperature, etc.).
Use of Oxidising Agents and Additives
Reagent studies feature ammonium persulfate ((NH4)2S2O8) under pressure/temperature [1] and iodide (I−) assistance in ferric media [9], enhancing kinetics/oxidation pathways. This aligns with SLR principles: precise dosing promotes selective Cu dissolution and reduces passivation.
Bio-Intensification and Biochemical Optimization
Biohydrometallurgy is key. Yavari et al. [7] propose decoupling chemical/microbial stages for enhanced Fe3+/Eh control. This strategy could benefit heap leaching against passivation/jarosite challenges [5,6].
Novel Leaching Devices and Control Mechanisms
Yang et al. [112] introduce “ore-on-a-chip” microchannels for real-time dissolution observation. This enables rapid, resource-efficient lab-scale assessment of reagents/variables (pH, Eh, temp.) before heap scale-up.
3.11.3. Contributions to Research
From a Sustainable Leachate Recovery (SLR) standpoint, the compiled studies in Cluster 11 show the following:
- Experimental designs (RSM, Box–Wilson, composite factorials) are powerful tools for optimizing chalcopyrite-leaching conditions and concurrently managing multiple factors.
- Adding specific oxidants or additives (ammonium persulfate, iodide, silver on pyrite) can counteract surface passivation and accelerate the extraction kinetics, provided redox potential is well managed [9,109].
- Bioleaching intensification (decoupling chemical and biological stages) can enhance metallurgical efficiency while minimizing waste and reagent costs, aligning with sustainability goals [7].
- Micro-scale experimentation (ore-on-a-chip) or pressurized systems (autoclaves) fosters early identification of advantageous operating conditions, subsequently validated in column or heap tests.
Together, these works reaffirm the notion that although chalcopyrite is refractory, it can be optimized via a multivariable approach that integrates stringent control over pH, Eh, temperature, and the judicious selection of oxidant or catalytic additives. This perspective supports the imperative of comprehensive physical–chemical, biological, and statistical methodologies for sustainable copper extraction in heaps, reducing operational costs while curbing environmental impacts.
3.12. Cluster 12: Process Optimization for Sustainable Copper Recovery and Contaminant Fixation in Metallurgical Residues
3.12.1. Cluster Overview
This twelfth cluster focuses on copper recovery and contaminant (particularly fluoride) fixation from specialized metallurgical byproducts, such as slag derived from spent carbon cathode reduction. The central research relies on statistical optimization methodologies (Response Surface Methodology, RSM) to manage the variables of time, temperature, and CaO addition, aligning with the principles of Sustainable Leachate Recovery (SLR). The ultimate goal is to maximize copper extraction while concurrently immobilizing or transforming undesirable species (e.g., fluoride), thereby mitigating environmental risks.
3.12.2. Key Findings
Fluoride Control and Copper Recovery
Li et al. [113] investigate copper leaching/recovery alongside fluoride fixation in metallurgical industry byproducts linked to “reduction slag” and spent carbon cathodes. By applying RSM, the authors identify temperature, reaction time, and CaO proportion as critical factors to achieve both high copper recovery and fluoride retention in stable phases (e.g., CaF2). This approach confirms the duality targeted by SLR, balancing metallurgical efficiency with impact minimization by sequestering hazardous contaminants.
Multivariable Optimization Using RSM
The RSM approach described by Li et al. [113] underscores the robustness of experimental statistics for exploring interactions among multiple factors, thereby pinpointing optimal process conditions. Specifically, modeling allows simultaneous prediction of copper recovery rates and estimation of fluoride fixation—a frequent contaminant in the metallurgical sector. Such methodology resonates with prior contributions in other clusters where RSM and factorial designs have been successfully applied to ore and byproduct leaching optimization [73,108].
Implications for Copper Heap Leaching
Although Li et al. [113] focus on a specific residue (spent carbon cathode reduction copper slag) rather than primary ore leaching, the multivariable approach and interest in contaminant fixation bear direct relevance to copper heap leaching. In these systems, fixation or immobilization of potentially toxic species is a recurring challenge, especially where ore mineralogy contains fluoride, arsenic, or other undesirable elements [5,6]. Thorough control of pH, Eh, additives, and temperature could likewise curb the formation of contaminant precipitates and optimize the Pregnant Leach Solution (PLS) quality.
3.12.3. Contributions to Research
In the framework of Sustainable Leachate Recovery (SLR), the key insights from Cluster 12 can be summarized as follows:
- Integrating leaching kinetics with contaminant fixation: Validating the potential to maximize copper recovery while immobilizing an undesirable element (e.g., fluoride) by incorporating CaO and fine-tuning process conditions.
- Robust statistical approach: The adoption of RSM highlights the advantage of multivariable statistical methods for managing complex chemical and operational interactions, in line with industrial heap-leaching requirements.
- Scaling and broader applicability: Though the case study centres on reduction slag and spent carbon cathodes, the results indicate that strategic variable control (temperature, residence time, additives) is pivotal for converting impurities into stable forms and achieving high-purity PLS.
Overall, Li et al. [113] affirm the importance of holistic optimization approaches in the copper industry, whether for heap leaching of primary ores or for managing metallurgical slags and byproducts, simultaneously addressing efficiency and environmental protection.
Synthesis of the Thematic Analysis
A review of these clusters (1 through 12) reveals a convergence toward measuring and optimizing a set of key variables in copper heap leaching. To this end, we first summarize in a table the most noteworthy findings (Table 2).

Table 2.
Summary of relevant findings (Clusters 1–12) and their connection to key heap-leaching variables (pH, redox potential, oxidant concentration, irrigation rate, temperature, etc.) within the framework of Sustainable Leachate Recovery (SLR).
In summary, this robust and replicable methodological protocol enables the identification and quantification of the most influential variables in dynamic copper heap leaching, optimizing operational control and thereby the final quality of the PLS.
4. Discussion
Figure 7 presents a conceptual map from 106 studies. Five critical variables (pH, Eh, oxidant load, irrigation rate, temp.) converge on core heap system. Monitoring via IoT sensing/analytics is recommended. Key SLR interventions: green reagents, bio-oxidation, recirculation, RSM/ML optimization. Indicators (Cu recovery, reagent use, LCA) enable feedback loop for ’smart heaps’. Figure synthesizes collective evidence; provides visual framework for discussion.
Figure 7.
Conceptual synthesis derived from the reviewed literature. The five core variables most frequently cited (pH, Eh, oxidant concentration, flow rate, and temperature) act on the heap-leaching core, are monitored by IoT sensing, and are modulated through Sustainable Leachate Recovery (SLR) interventions.
Reviewing preceding clusters highlights heap leaching complexity and the need for rigorous multivariable control to maximize efficiency whilst ensuring sustainability. This section connects findings to the stated objectives (O.1–O.4) and research questions (P.1–P.3).
This revised section strengthens methodology via deeper statistical analysis, explicit validation routes, and bridging lab insights with industrial adoption. Aim: fortify evidence linking critical variables to robust kinetic control and minimised environmental impact [15,16].
4.1. Synthesizing the Findings in Relation to Critical Variables (pH, Eh, [Ox], Irrigation Rate, Temperature)
Studies concur: pH (1.5–2.0) and redox potential (Eh) (600–750 mV) are central for controlling Cu dissolution and preventing passivation (Clusters 1, 3, 5, 11). Oxidant concentration (Fe3+, H2O2, Na2S2O8, etc.) is decisive for inhibiting passivation in refractory minerals (e.g., chalcopyrite) [1,7,9].
New Insight: An integrated feedback system (sensors + analytics) is recommended for closed-loop pH/Eh/oxidant control via dynamic reagent delivery (supported by RSM/Taguchi). This mitigates risks (overdosage, jarosite, incomplete oxidation), aligning with industrial requirements [6,79].
Regarding irrigation rate, uniform percolation is crucial [2,12,13]; extremes are problematic (saturation/fines vs. dry zones/slow kinetics). Temperature accelerates kinetics (optimum 25–), but >50– increases cost/side-reaction risks. Balanced “operational windows” are necessary.
Refined Approach on Operating Windows: A multi-criteria framework (thermodynamics + pilot data + Bayesian updating) is proposed for defining/updating optimal windows (pH, Eh, irrigation, temp.), accounting for mineralogy/seasonal shifts for adaptive leaching [29].
4.2. Novel Reagents and Experimental Design Methodologies
Studies (Clusters 4, 5, 6) indicate a trend towards organic agents (e.g., acids, glycine) and deep eutectic solvents (DESs) as greener alternatives. Surfactants [48,49] and biohydrometallurgy (Cluster 7, requiring optimised conditions for, e.g., A. ferrooxidans) are also promising.
Enhanced Design Strategy: Investigating green reagent (e.g., glycine) synergy with partial metal recirculation using advanced DoE (factorial, Box-Behnken)—augmented with constraints (biodegradability, LCA, by-products)—is recommended. Cost–benefit modeling should be integrated [10,60].
Statistical optimization methods (Taguchi, ANOVA, RSM, etc.; Clusters 3, 8, 11, 12) effectively combine/rank variables and reveal interactions. Integrating multivariable modeling with in situ adjustment advances model development (O.2) and validation (O.3).
High-Level Methodological Perspective: Hybrid strategies coupling fuzzy/multi-criteria decision-making (AHP-TOPSIS) with RSM [29] are recommended for intangible factors (risk, toxicity). This balances predictions with real-world constraints (climate, ore variability), ensuring practical optimization.
4.3. Meeting the Objectives: Model and Methodology Validation
Objective O.1 (Identifying the most influential operational variables). Findings highlight five critical variables: pH, Eh, oxidant concentration, irrigation rate, temperature. These, alongside mineralogy, define Cu recovery and PLS quality. Secondary variables (surfactants, additives [glycine/NH4HF2], bacterial potential) also modulate kinetics (Clusters 4, 5, 7, 11).
Refinement for O.1: We expanded experimental factors (humidity control, partial pressure, dynamic infiltration) informed by Clusters 8, 11. It improves resolution for interactions, enabling precise detection of dominant variables under field conditions [75,76].
Objective O.2 (Development of a multivariable mathematical model). The literature (Clusters 3, 8, 11) supports advanced approaches (RSM, factorial designs, ML [NNs, GAs]). These flexibly describe/predict kinetics; integrating experiments with simulations enhances predictive capacity for various heap-leaching configurations.
Refinement for O.2: We propose coupling RSM with agent-based models (micro-scale percolation). Calibrating with column data allows capturing edge effects (channeling)/dynamic porosity shifts, revealing non-linear phenomena [77,78].
Objective O.3 (Assessing practical applicability at the pilot scale). Validation studies (Clusters 2, 3, 9) confirm model scalability in controlled column/semi-pilot tests. Whilst mineral heterogeneity is challenging, advances in reactive transport modeling (Clusters 8, 9) and countercurrent strategies (Cluster 3) suggest industrial feasibility.
Refinement for O.3: We incorporate geo-statistical data via block-models [89,97] simulating heterogeneity in pilot columns. Subdividing columns into mineralogical domains allows better mimicry of deposits, yielding realistic validation and smoother scale-up.
Objective O.4 (Proposing industrial implementation criteria). Industrial emphasis: integrated online monitoring (pH, Eh, flow), alternative reagents (glycine, ammoniacal), bioleaching. Studies (raffinate reuse, desalination [Cluster 3], contaminant fixation [F, Cluster 12]) highlight sustainable practices enhancing multivariable control, reducing risks/costs, meeting regulations.
Refinement for O.4: An integrated platform: sensor data + predictive analytics (5–15 min intervals) is recommended [16,36]. It enables real-time feedback for auto-calibrating inputs (acid, oxidants, surfactants). Anomaly detection alerts (Eh, fluid distribution) for rapid intervention are included.
4.4. Addressing the Research Questions
P.1 (Determinant parameters and their interactions). Core control variables: pH, Eh, oxidant conc. (Fe3+, H2O2, Na2S2O8), irrigation rate, temperature. Interactions nonlinear (e.g., excess Fe3+ → jarosite; poor irrigation → non-uniformity, lower yield).
Refinement for P.1: Interaction plots/PLS quantified Eh/oxidant effects, verifying optimal Fe3+ (approx. 3–8 g ) for oxide–sulfide blends. Significant temp.–irrigation synergy requires balancing against overheating/undersaturation [109,114].
P.2 (Multivariable mathematical model in heterogeneous environments). Methods (RSM, Taguchi, ANOVA, NN; Clusters 3, 8, 11) accurately describe kinetics with robust stats/validation. Continuous adjustment (pH, Eh, temp.) plus mineralogical characterization vital for predictive accuracy across geometries/sizes.
Refinement for P.2: Modeling enriched with microstructural images (SEM, X-ray tomography) for local porosity/morphology estimation. Data enhance NN predictions, linking micro/macro scales, boosting reliability in heterogeneous scenarios [30,38].
P.3 (Optimal operating and upscaling routes). Green agents (glycine, eco-surfactants) and bioleaching offer lower environmental impact with competitive recovery. Examples (fluoride fixation [Cluster 12], raffinate reuse [Cluster 3]) show reconfiguration yields high-purity PLS while reducing costs/risks. Industrial adoption requires refined heap engineering (drainage, irrigation, dust) and economic assessment.
Refinement for P.3: Suggested staged scale-up (building on green lixiviant synergy): (1) bench tests (glycine, varied oxidation); (2) pilot column validation (real raffinate); (3) heap segment demo [31,58]. Phased approach accounts for emergent complexities (scaling, weather).
4.5. Implications for Sustainable Leachate Recovery (SLR) and Future Directions
Integrating statistical models, RSM, and multiscale experimentation (lab, column, pilot) provides a robust framework for Cu leaching (ores, waste, tailings). This multivariable control underpins SLR, enabling the following:
- Optimizing copper dissolution kinetics and minimizing the formation of secondary compounds (jarosites, metallic precipitates).
- Enhancing the purity of the Pregnant Leach Solution (PLS), facilitating subsequent extraction or electrowinning of copper.
- Incorporating eco-friendly reagents (glycine, organic acids) and bioleaching, thereby reducing toxicity and the carbon footprint of the process.
- Adopting statistical design methodologies (RSM, Taguchi, Box–Behnken) to fine-tune operating ranges according to dynamic heap conditions.
- Converging with the circular economy, reusing solvents or byproducts (e.g., Cu0 as a reducing agent, raffinate, seawater) and fixing contaminants (F, Pb, As) into stable forms.
Extended Vision: Future SLR vision: merging advanced automation (digital twins, IoT) with robust metallurgy. Potential research includes dynamic irrigation (via redox mapping), ML reagent forecasting, and AI self-optimising heaps [46,86], enabling environmentally responsible and economically viable systems.
Key industrial challenges remain: mineral heterogeneity, method/reagent scalability, and real-time pH/Eh/irrigation control. Overcoming these necessitates detailed pilot tests, integrated online monitoring, and robust long-term simulation models.
Ultimately, copper heap leaching can achieve an optimal balance (profitability, efficiency, minimal environmental impact) if Sustainable Leachate Recovery (SLR) guides operational design and decisions.
4.6. Limitations and Avenues for Future Research
Although this SLR followed PRISMA-inspired guidelines and combined machine-learning filters with expert validation, several constraints should be borne in mind when interpreting the results:
- L1.
- Database and language scope. Only peer-reviewed records indexed in Web of Science and Scopus (2015–2025) and written in English or Spanish were retained. Relevant grey literature, patents and papers in other languages (e.g., Chinese, Russian) may therefore be under-represented.
- L2.
- Quality-screening bias. The Q2-or-better journal filter and minimum-citation threshold favor mature topics; emerging research with few citations could have been excluded despite methodological soundness.
- L3.
- Heterogeneous reporting. Reaction conditions are not reported uniformly across studies (e.g., some offer “acid concentration”, others “pH after 24 h”), limiting quantitative meta-analysis of kinetic constants.
- L4.
- Scale-up uncertainty. More than 70 % of the primary studies use batch or column tests ≤ 2 m height. Extrapolation to industrial heaps (>6 m) assumes that hydraulic and thermal gradients can be managed, an aspect still largely unverified in the field.
- L5.
- Economic and regional variability. Cost and LCA data come mainly from Chile, China, and Australia; reagent prices, energy mix and water availability differ elsewhere, so absolute OPEX figures should be adapted locally.
Research gaps. Addressing these limitations points to four priority lines:
- Long-duration (>6 month) heap trials that couple in situ redox mapping with IoT-driven control.
- Harmonized reporting templates for pH, Eh, oxidant dosage and mass-transfer data to enable kinetic meta-models.
- Techno-economic assessments of glycine and deep-eutectic solvents under different regional energy–water scenarios.
- Open datasets linking mineralogical heterogeneity to model parameters for machine-learning validation.
5. Conclusions
Synthesizing the literature via thematic cluster analysis yields robust conclusions and guidelines for optimizing copper heap leaching. This overview links key findings, challenges, contributions, and industrial implications.
Reviewed publications (12 clusters) converge on requiring tight control of key variables (pH 1.5–2.0, Eh 600–750 mV, oxidant concentration [Fe3+, H2O2, etc.]) for favorable kinetics and mitigating issues like jarosite formation or passivation. Statistical/computational methods (RSM, NN) proved central for integrating experiments and modeling. Scalability to column/pilot scale was confirmed, provided real-time monitoring is implemented. This validated methodological framework supports our findings’ internal consistency and guides future work.
Several contributions (Clusters 4, 5, 9) highlight integrating “green” reagents (glycine, organic acids, eco-surfactants) to reduce toxicity whilst enhancing metallurgical performance. Moderate doses (per Table 1) optimize heap wettability and complexation, reducing mineral acid reliance. Furthermore, findings on raffinate reuse, countercurrent leaching, and bioleaching (Clusters 3, 7, 11) support feasible closed-loop designs (including oxidant regeneration), improving economics and environmental acceptance.
Despite promising approaches, limitations persist, primarily ore heterogeneity and dynamic heap changes (permeability, thermal gradients). While multiscale modeling/DoE (Section 2, Cluster 8) address variability, long-term testing ( months) remains necessary for complex heaps/ores. Additionally, alternative reagent adoption (e.g., DES) faces infrastructure/PLS quality constraints, and ML implementation requires robust data acquisition and sensor maintenance.
We emphasized both findings and methodological limitations. Our critical review’s depth relies on qualitative analysis and cross-comparison (e.g., in situ oxidation roles, green reagent advantages, biohydrometallurgy relevance), integrating efficiency, economics, and environment [6,7]. Broader-scale studies (spatial/temporal, climate, mineralogy) are needed; gaps remain in multi-site validation and life-cycle integration despite advances [89,97]. Qualitative discussion ensures conclusion relevance and identifies urgent gaps. Finally, this review presents a rigorous subset illustrating diverse approaches—refined via automated screening plus expert validation—not an exhaustive literature survey [15,16].
Guidelines in Table 1 bridge science and practice. Key recommendations include:
- (a)
- Establish robust multivariable control: Integrate online sensors for continuous monitoring (pH, Eh, temp., flow); dynamically adjust dosages.
- (b)
- Adopt lower-toxicity reagents: Assess feasibility of glycine, surfactants, organic acids complementing/substituting H2SO4.
- (c)
- Scale up biohydrometallurgy: Utilize for refractory ores (e.g., chalcopyrite), ensuring microbial population maintenance for kinetic advantages.
- (d)
- Apply advanced design methodologies: Use RSM, factorial designs, metaheuristics for optimal points considering ore variability and heap dynamics.
We can conclude that the systematically gathered evidence confirms that copper heap leaching can achieve substantial improvements in both metallurgical efficiency and sustainability through rigorous multivariable control of pH, Eh, oxidant concentration, temperature, and irrigation rate. Furthermore, the adoption of alternative reagents (glycine, surfactants, deep eutectic solvents) and biohydrometallurgical techniques supports an industrial model oriented toward both operational success and environmental protection. The present study, together with the recommendations and proposed research lines, serves as a reference framework for advancing toward a more technologically mature, globally competitive, and environmentally aligned copper mining industry through the principles of Sustainable Leachate Recovery.
Author Contributions
Conceptualization, F.L. and L.R.; Methodology, J.G. and Y.M.; Software, J.G. and Y.M.; Validation, F.L., J.G. and L.R.; Formal Analysis, V.B. and F.L.; Investigation, F.L., L.R. and J.G.; Resources, J.G.; Data Curation, L.R. and Y.M.; Writing—Original Draft Preparation, F.L., L.R. and V.B.; Writing—Review and Editing, F.L., L.R. and V.B.; Visualization, J.G. and Y.M.; Supervision, J.G.; Project Administration, A.P.; Funding Acquisition, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the VINCI-DI initiative at Pontificia Universidad Católica de Valparaíso (PUCV), under project number 039.706/2025.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors express their gratitude to the institutions and funding bodies that supported this research. Special thanks to the Pontificia Universidad Católica de Valparaíso (PUCV) for its continuous academic support, particularly through the Doctorate in Intelligent Industry program.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| RSL | Systematic Literature Review (Revisión Sistemática de Literatura) |
| PLS | Pregnant Leach Solution |
| NLP | Natural Language Processing |
| Eh | Oxidation–Reduction Potential (Potencial de Óxido-Reducción) |
References
- Turan, M.; Arslanoğlu, H.; Altundoğan, H. Optimization of the leaching conditions of chalcopyrite concentrate using ammonium persulfate in an autoclave system. J. Taiwan Inst. Chem. Eng. 2015, 50, 49–55. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, S.; Feng, Q.; Xian, Y.; Liu, D. Leaching characteristics and mechanism of copper flotation tailings in sulfuric acid solution. Russ. J.-Non-Ferr. Met. 2015, 56, 127–133. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summary 2024: Copper. 2024. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-copper.pdf (accessed on 22 April 2025).
- International Energy Agency. World Energy Outlook 2024—Special Focus on Critical Minerals for Clean Energy. 2024. Available online: https://www.iea.org/reports/world-energy-outlook-2024 (accessed on 22 April 2025).
- Arinanda, M.; van Haute, Q.; Lambert, F.; Gaydardzhiev, S. Effects of operational parameters on the bio-assisted leaching of metals from pyrolized printed circuit boards. Miner. Eng. 2019, 134, 16–22. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Hosseini, M. Investigation and optimization of influencing parameters on the copper extraction from a low-grade oxide deposit by acid leaching. Metall. Res. Technol. 2019, 116, 305. [Google Scholar] [CrossRef]
- Yavari, M.; Ebrahimi, S.; Aghazadeh, V.; Ghashghaee, M. Intensified bioleaching of copper from chalcopyrite: Decoupling and optimization of the chemical stage. Iran. J. Chem. Chem. Eng. 2020, 39, 343–352. [Google Scholar] [CrossRef]
- Aghazadeh, S.; Olyaei, Y.; Noaparast, M. Leaching optimisation of oxide copper ore from Meskani mine. Int. J. Min. Miner. Eng. 2015, 6, 295–308. [Google Scholar] [CrossRef]
- Granata, G.; Miura, A.; Liu, W.; Pagnanelli, F.; Tokoro, C. Iodide-assisted leaching of chalcopyrite in acidic ferric sulfate media. Hydrometallurgy 2019, 186, 244–251. [Google Scholar] [CrossRef]
- Barragán-Mantilla, S.; Gascó, G.; Almendros, P.; Méndez, A. Insights into the use of green leaching systems based on glycine for the selective recovery of copper. Miner. Eng. 2024, 206, 108534. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Liu, H.; Fan, G.; Peng, W.; Cao, Y. Selective complexation leaching of copper from copper smelting slag with the alkaline glycine solution: An effective recovery method of copper from secondary resource. Sep. Purif. Technol. 2023, 326, 124619. [Google Scholar] [CrossRef]
- Robertson, S. Development of an integrated heap leach solution flow and mineral leaching model. Hydrometallurgy 2017, 169, 79–88. [Google Scholar] [CrossRef]
- Rucker, D. Deep well rinsing of a copper oxide heap. Hydrometallurgy 2015, 153, 145–153. [Google Scholar] [CrossRef]
- Xiao, F.; Luo, Z.; Peng, Y.; Luo, X.; Yan, Y.; Sun, S.; Tu, G. Leaching Kinetics of Cu from Low-Grade Oxidized Copper Ore with High Alkalinity Gangue Using Edta·2na Solution. JOM 2024, 76, 7023–7033. [Google Scholar] [CrossRef]
- Garcia, J.; Villavicencio, G.; Altimiras, F.; Crawford, B.; Soto, R.; Minatogawa, V.; Franco, M.; Martínez-Muñoz, D.; Yepes, V. Machine learning techniques applied to construction: A hybrid bibliometric analysis of advances and future directions. Autom. Constr. 2022, 142, 104532. [Google Scholar] [CrossRef]
- García, J.; Leiva-Araos, A.; Diaz-Saavedra, E.; Moraga, P.; Pinto, H.; Yepes, V. Relevance of Machine Learning Techniques in Water Infrastructure Integrity and Quality: A Review Powered by Natural Language Processing. Appl. Sci. 2023, 13, 12497. [Google Scholar] [CrossRef]
- Reimers, N.; Gurevych, I. Sentence-BERT: Sentence Embeddings using Siamese BERT-Networks. In Proceedings of the EMNLP 2019, Hong Kong, China, 3–7 November 2019; pp. 3982–3992. [Google Scholar] [CrossRef]
- Grootendorst, M. BERTopic: Neural Topic Modeling with a Class-Based TF–IDF Procedure. 2022. Available online: https://github.com/MaartenGr/BERTopic (accessed on 22 April 2025).
- Rose, S.; Engel, D.; Cramer, N.; Cowley, W. Automatic Keyword Extraction from Individual Documents. In Text Mining: Applications and Theory; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–20. [Google Scholar]
- Řehůřek, R.; Sojka, P. Software Framework for Topic Modelling with Large Corpora. In Proceedings of the LREC 2010 Workshop on New Challenges for NLP Frameworks, Valletta, Malta, 22 May 2010; pp. 45–50. [Google Scholar]
- Honnibal, M.; Montani, I.; Van Landeghem, S.; Boyd, A. spaCy 3.0: Advanced Natural Language Processing in Python. 2020. Available online: https://spacy.io (accessed on 22 April 2025).
- Li, X.G.; Shi, S.X.; Yan, S.; Li, L.; Qin, X.Z.; Zhu, X.N. Sustainable strategies for recovering metallic copper from waste printed circuit boards: Clean leaching and micro-nano copper powder preparation. J. Environ. Chem. Eng. 2024, 12, 113220. [Google Scholar] [CrossRef]
- Ganji, S.; Azizi, A.; Hayati, M. Copper leaching from waste printed circuit boards (PCBs) using sulphuric acid and hydrogen peroxide. Res. J. Chem. Environ. 2019, 23, 1–9. [Google Scholar] [CrossRef]
- Sun, Z.; Xiao, Y.; Sietsma, J.; Agterhuis, H.; Visser, G.; Yang, Y. Selective copper recovery from complex mixtures of end-of-life electronic products with ammonia-based solution. Hydrometallurgy 2015, 152, 91–99. [Google Scholar] [CrossRef]
- He, J.; Zhang, M.; Chen, H.; Guo, S.; Zhu, L.; Xu, J.; Zhou, K. Enhancement of leaching copper by organic agents from waste printed circuit boards in a sulfuric acid solution. Chemosphere 2022, 307, 135924. [Google Scholar] [CrossRef]
- Shi, S.X.; Nie, C.C.; Chang, H.H.; Wu, P.; Piao, Z.J.; Zhu, X.N. Physical pre-concentration and ammonium leaching of metal copper from waste printed circuit boards. J. Clean. Prod. 2021, 318, 128512. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Sun, X.; Wang, L. Separation and recovery of copper from waste printed circuit boards leach solution using solvent extraction with Acorga M5640 as extractant. Sep. Sci. Technol. 2019, 54, 1302–1311. [Google Scholar] [CrossRef]
- Correa, M.; Silvas, F.; Aliprandini, P.; de Moraes, V.; Dreisinger, D.; Espinosa, D. Separation of copper from a leaching solution of printed circuit boards by using solvent extraction with D2EHPA. Braz. J. Chem. Eng. 2018, 35, 919–930. [Google Scholar] [CrossRef]
- Yousefzadeh, S.; Yaghmaeian, K.; Mahvi, A.; Nasseri, S.; Alavi, N.; Nabizadeh, R. Comparative analysis of hydrometallurgical methods for the recovery of Cu from circuit boards: Optimization using response surface and selection of the best technique by two-step fuzzy AHP-TOPSIS method. J. Clean. Prod. 2020, 249, 119401. [Google Scholar] [CrossRef]
- Becci, A.; Amato, A.; Rodríguez Maroto, J.; Beolchini, F. Prediction Model for Cu Chemical Leaching from Printed Circuit Boards. Ind. Eng. Chem. Res. 2019, 58, 20585–20591. [Google Scholar] [CrossRef]
- Mizero, B.; Musongo, T.; Rene, E.; Battes, F.; Lens, P. Optimization of process parameters for the chemical leaching of base metals from telecom and desktop printed circuit boards. Process Saf. Environ. Prot. 2018, 120, 14–23. [Google Scholar] [CrossRef]
- Pinho, S.; Ribeiro, C.; Ferraz, C.; Almeida, M. Copper, zinc, and nickel recovery from printed circuit boards using an ammonia–ammonium sulphate system. J. Mater. Cycles Waste Manag. 2021, 23, 1456–1465. [Google Scholar] [CrossRef]
- Janyasuthiwong, S.; Ugas, R.; Rene, E.; Carucci, A.; Esposito, G.; Lens, P. Effect of operational parameters on the leaching efficiency and recovery of heavy metals from computer printed circuit boards. J. Chem. Technol. Biotechnol. 2016, 91, 2038–2046. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Qin, H.; Liu, Y.; Tian, Q.; Li, D. Recovery of metal values from waste printed circuit boards using an alkali fusion-leaching-separation process. Hydrometallurgy 2015, 156, 199–205. [Google Scholar] [CrossRef]
- Dutta, D.; Panda, R.; Kumari, A.; Goel, S.; Jha, M. Sustainable recycling process for metals recovery from used printed circuit boards (PCBs). Sustain. Mater. Technol. 2018, 17, e00066. [Google Scholar] [CrossRef]
- Kassymova, D.; Sapinov, R.; Kushakova, L.; Kulenova, N.; Shoshay, Z.; Adylkanova, M. Optimization of Copper Recovery from Cyanide Leaching Solutions Used in Gold–Copper Ore Processing Using Probabilistic–Deterministic Experimental Design. Processes 2025, 13, 61. [Google Scholar] [CrossRef]
- Luo, J.; Xing, X.; Qi, S.; Wu, J.; Gu, X. Comparing the risk of metal leaching in phytoremediation using Noccaea caerulescens with or without electric field. Chemosphere 2019, 216, 661–668. [Google Scholar] [CrossRef]
- Movahhedi, H.; Mohammad Beygian, A.; Keshavarz Alamdari, E.; Moradkhani, D. Developing of a Counter-Current Copper Leaching Process Using Response Surface Methodology. Miner. Process. Extr. Metall. Rev. 2024, 45, 824–834. [Google Scholar] [CrossRef]
- Zhao, M.; Fang, J.; Zhang, L.; Dai, Z.; Yao, Z. Improving the Estimation Accuracy of Copper Oxide Leaching in an Ammonia–Ammonium System Using RSM and GA-BPNN. Russ. J.-Non-Ferr. Met. 2017, 58, 591–599. [Google Scholar] [CrossRef]
- Mbuya, B.; Kime, M.B.; Tshimombo, A. Comparative Study of Approaches based on the Taguchi and ANOVA for Optimising the Leaching of Copper–Cobalt Flotation Tailings. Chem. Eng. Commun. 2017, 204, 512–521. [Google Scholar] [CrossRef]
- Zandevakili, S.; Akhondi, M.; Hosseini, R.; Mohammad, S. Leaching optimization of sarcheshmeh copper concentrate by application of taguchi experimental design method. Iran. J. Chem. Chem. Eng. 2020, 39, 229–236. [Google Scholar] [CrossRef]
- Borsynbayev, A.; Omarov, K.; Mustafin, Y.; Havlíček, D.; Absat, Z.; Muratbekova, A.; Kaikenov, D.; Pudov, A.; Shuyev, N. A study of copper leaching from the tailings of the Karagaily (Republic of Kazakhstan) concentrating factory using an electric hydropulse discharge. J. Serbian Chem. Soc. 2022, 87, 925–937. [Google Scholar] [CrossRef]
- Nadirov, R.; Syzdykova, L.; Zhussupova, A. Copper smelter slag treatment by ammonia solution: Leaching process optimization. J. Cent. South Univ. 2017, 24, 2799–2804. [Google Scholar] [CrossRef]
- Goodboy, K.; Missimer, T. Evaluation of desalinated seawater vs. Filtered raw seawater for healeach copper extraction on mountaintop mines in arid regions. Desalin. Water Treat. 2020, 194, 1–18. [Google Scholar] [CrossRef]
- Yuan, L.; Peng, X.; Li, S.; Chen, Y. Dynamic tests on recovering copper silver metal from tailings. J. Comput. Theor. Nanosci. 2016, 13, 2671–2676. [Google Scholar] [CrossRef]
- Estay, H.; Díaz-Quezada, S. Deconstructing the Leaching Ratio. Mining, Metall. Explor. 2020, 37, 1329–1337. [Google Scholar] [CrossRef]
- Mazarji, M.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Bayero, M.; Fedorenko, A.; Mahmoodi, N.; Sillanpää, M.; Bauer, T.; Soldatov, A. Metal-organic frameworks (MIL-101) decorated biochar as a highly efficient bio-based composite for immobilization of polycyclic aromatic hydrocarbons and copper in real contaminated soil. J. Environ. Chem. Eng. 2022, 10, 108821. [Google Scholar] [CrossRef]
- Ai, C.-M.; Sun, P.-P.; Wu, A.-X.; Chen, X.; Liu, C. Accelerating leaching of copper ore with surfactant and the analysis of reaction kinetics. Int. J. Miner. Metall. Mater. 2019, 26, 274–281. [Google Scholar] [CrossRef]
- Ai, C.-M.; Wu, A.-X.; Wang, Y.-M.; Hou, C.-L. Optimization and mechanism of surfactant accelerating leaching test. J. Cent. South Univ. 2016, 23, 1032–1039. [Google Scholar] [CrossRef]
- Yaras, A.; Arslanoglu, H. Leaching behaviour of low-grade copper ore in the presence of organic acid. Can. Metall. Q. 2018, 57, 319–327. [Google Scholar] [CrossRef]
- Han, J.; Liu, W.; Xue, K.; Qin, W.; Jiao, F.; Zhu, L. Influence of NH4HF2 activation on leaching of low-grade complex copper ore in NH3-NH4Cl solution. Sep. Purif. Technol. 2017, 181, 29–36. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Lu, Y.; Xu, W.; Yan, K.; Wang, R.; Pan, Y.; Li, J.; Xu, Z. Leaching Behaviors of Yulong Refractory Oxide Copper Ores from Tibet in Sulfuric Acid Solutions. J. Sustain. Metall. 2023, 9, 982–998. [Google Scholar] [CrossRef]
- Ambo, A.; Baba, A.; Amos, B.; Ogara, J.; Ayinla, K. Leaching kinetics of near infrared sensor-based pre-concentrated copper ores by sulphuric acid. Physicochem. Probl. Miner. Process. 2017, 53, 489–501. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Entezari Zarandi, A.; Pasquier, L.C.; Azizi, A. Kinetic Investigation on Leaching of Copper from a Low-Grade Copper Oxide Deposit in Sulfuric Acid Solution: A Case Study of the Crushing Circuit Reject of a Copper Heap Leaching Plant. J. Sustain. Metall. 2021, 7, 1154–1168. [Google Scholar] [CrossRef]
- Shi, G.; Liao, Y.; Su, B.; Zhang, Y.; Wang, W.; Xi, J. Kinetics of copper extraction from copper smelting slag by pressure oxidative leaching with sulfuric acid. Sep. Purif. Technol. 2020, 241, 116699. [Google Scholar] [CrossRef]
- Deng, J.; Wen, S.; Deng, J.; Wu, D.; Yang, J. Extracting copper by lactic acid from copper oxide ore and dissolution kinetics. J. Chem. Eng. Jpn. 2015, 48, 538–544. [Google Scholar] [CrossRef]
- Li, Y.; Fan, X.; Yang, K. Research on oxidizing leaching tellurium from copper telluride material in acid condition with hydrogen peroxide. Chem. Eng. Commun. 2021, 208, 1238–1244. [Google Scholar] [CrossRef]
- El-Okazy, M.; Zewail, T.; Farag, H.M. Recovery of copper from spent catalyst using acid leaching followed by electrodeposition on square rotating cylinder. Alex. Eng. J. 2018, 57, 3117–3126. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Chen, J.; Shen, X.; Cui, S.; Liu, K.; Han, Q. A novel green method for copper recovery from cuprous thiocyanate-containing acidified sediments in the gold industry. J. Clean. Prod. 2021, 329, 129729. [Google Scholar] [CrossRef]
- Rezaee, M.; Saneie, R.; Mohammadzadeh, A.; Abdollahi, H.; Kordloo, M.; Rezaee, A.; Vahidi, E. Eco-friendly recovery of base and precious metals from waste printed circuit boards by step-wise glycine leaching: Process optimization, kinetics modeling, and comparative life cycle assessment. J. Clean. Prod. 2023, 389, 136016. [Google Scholar] [CrossRef]
- Shoghian-Alanaghi, A.; Zamharir, A.; Aghajani, H.; Tabrizi, A. Improving the Leaching Rate of Molybdenite Concentrate Using Oxidants by Adding Ethylene Glycol and Oxygen: Kinetic Study. Min. Metall. Explor. 2022, 39, 1753–1761. [Google Scholar] [CrossRef]
- Yin, X.; Liu, R.; Cheng, M.; Sun, Q.; Yang, Y. Efficient leaching and recovery of metallic gold and copper from integrated circuits with the novel bromotrihalide ionic liquids based on the redox mechanism. Sep. Purif. Technol. 2023, 313, 123456. [Google Scholar] [CrossRef]
- Popescu, I.; Varga, T.; Fogarasi, S.; Imre-Lucaci, A.; Ilea, P. Statistical Evaluation of Factors Affecting the Leaching Process of Waste Electrical and Electronic Equipment using Sodium Persulfate. Chem. Eng. Commun. 2016, 203, 414–423. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Xu, Z.; Tian, Q.; Feng, Q. Leaching behavior of metals from copper anode slime using an alkali fusion-leaching process. Hydrometallurgy 2015, 157, 9–12. [Google Scholar] [CrossRef]
- Seisko, S.; Aromaa, J.; Latostenmaa, P.; Forsen, O.; Wilson, B.; Lundstrom, M. Pressure leaching of decopperized copper electrorefining anode slimes in strong acid solution. Physicochem. Probl. Miner. Process. 2017, 53, 465–474. [Google Scholar] [CrossRef]
- Li, F.; Cai, K.; Huang, Q.; Zhong, M.; Wang, L.; Song, Q.; Yuan, W. Recovery of Au and Cu from waste memory modules by electrolysis with hydrochloric acid-hydrogen peroxide system. Sep. Purif. Technol. 2023, 308, 122872. [Google Scholar] [CrossRef]
- Fathima, A.; Tang, J.; Giannis, A.; Ilankoon, I.; Chong, M. Catalysing electrowinning of copper from E-waste: A critical review. Chemosphere 2022, 298, 134340. [Google Scholar] [CrossRef]
- Partinen, J.; Halli, P.; Varonen, A.; Wilson, B.; Lundström, M. Investigating battery black mass leaching performance as a function of process parameters by combining leaching experiments and regression modeling. Miner. Eng. 2024, 215, 108828. [Google Scholar] [CrossRef]
- Lambert, F.; Gaydardzhiev, S.; Léonard, G.; Lewis, G.; Bareel, P.F.; Bastin, D. Copper leaching from waste electric cables by biohydrometallurgy. Miner. Eng. 2015, 76, 38–46. [Google Scholar] [CrossRef]
- Sinha, R.; Chauhan, G.; Singh, A.; Kumar, A.; Acharya, S. A novel eco-friendly hybrid approach for recovery and reuse of copper from electronic waste. J. Environ. Chem. Eng. 2018, 6, 1053–1061. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Pezo, L.; Stanković, S.; Patarić, A.; Janjušević, Z.; Lončar, B. Copper leaching from chalcopyrite concentrate by sodium nitrate in sulphuric acid solution—Chemometric approach. Bulg. Chem. Commun. 2019, 51, 457–463. [Google Scholar] [CrossRef]
- Saldaña, M.; Salinas-Rodríguez, E.; Castillo, J.; Peña-Graf, F.; Roldán, F. Development of an analytical model for copper heap leaching from secondary sulfides in chloride media in an industrial environment; [Razvoj analitičkog modela za iskorišćavanje bakra iz sekundarnih sulfida u hloridnim medijima u industrijskom okruženju]. Hem. Ind. 2022, 76, 183–195. [Google Scholar] [CrossRef]
- Yu, B.; Zhan, D.; Liu, J.; Wang, H.; Yang, S.; Hu, X. Response Surface Method Optimization to Improve Copper Extraction from Refractory Copper Oxide Ore. Min. Metall. Explor. 2022, 39, 2221–2228. [Google Scholar] [CrossRef]
- Hayati, M.; Ganji, S.; Shahcheraghi, S.; Khabir, R. Optimization of copper recovery from electronic waste using response surface methodology and Monte Carlo simulation under uncertainty. J. Mater. Cycles Waste Manag. 2023, 25, 211–220. [Google Scholar] [CrossRef]
- Harichandan, B.; Mandre, N. Studies on the potential recovery of copper from low-grade mixed sulfide-oxide ore and optimization of the process parameters. Sep. Sci. Technol. 2022, 57, 719–732. [Google Scholar] [CrossRef]
- Hernández, I.; Ordóñez, J.; Robles, P.; Gálvez, E.; Cisternas, L. A Methodology For Design And Operation Of Heap Leaching Systems. Miner. Process. Extr. Metall. Rev. 2017, 38, 180–192. [Google Scholar] [CrossRef]
- Ortega-Tong, P.; Jamieson, J.; Kuhar, L.; Faulkner, L.; Prommer, H. In Situ Recovery of Copper: Identifying Mineralogical Controls via Model-Based Analysis of Multistage Column Leach Experiments. ACS ES T Eng. 2023, 3, 773–786. [Google Scholar] [CrossRef]
- Paspureddi, A.; Salazar-Tio, R.; Balasubramanian, G.; Chatterjee, A.; Crouse, B. Multiscale modeling of reactive flow in heterogeneous porous microstructures. Hydrometallurgy 2024, 228, 106333. [Google Scholar] [CrossRef]
- Flores, V.; Leiva, C. A comparative study on supervised machine learning algorithms for copper recovery quality prediction in a leaching process. Sensors 2021, 21, 2119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, C.; Dowd, P.; Wang, Z.; Faulkner, L. Modelling in-situ recovery (ISR) of copper at the Kapunda mine, Australia. Miner. Eng. 2022, 186, 107752. [Google Scholar] [CrossRef]
- Hoseinian, F.; Bahadori, M.; Hashemzadeh, M.; Rezai, B.; Soltani-Mohammadi, S. Application of Mathematical Modeling on Copper Recovery Optimization of Oxide Ores. JOM 2017, 69, 1939–1944. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, T.; Shen, P.; Liu, D.; Dong, L. Kinetics analysis of copper extraction from copper smelting slag by sulfuric acid oxidation leaching. Miner. Eng. 2024, 216, 108886. [Google Scholar] [CrossRef]
- Topçu, M.; Çeltek, S.; Rüşen, A. Green leaching and predictive model for copper recovery from waste smelting slag with choline chloride-based deep eutectic solvent. Chin. J. Chem. Eng. 2024, 75, 14–24. [Google Scholar] [CrossRef]
- Gargul, K.; Boryczko, B.; Bukowska, A.; Jarosz, P.; Małecki, S. Leaching of lead and copper from flash smelting slag by citric acid. Arch. Civ. Mech. Eng. 2019, 19, 648–656. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, G.; Sarangi, C.; Sanjay, K.; Subbaiah, T.; Das, S.; Sarangi, K.; Ghosh, M.; Pradhan, N.; Mishra, B. Reactor and column leaching studies for extraction of copper from two low grade resources: A comparative study. Hydrometallurgy 2016, 165, 111–117. [Google Scholar] [CrossRef]
- Green, C.; Robertson, J.; Marsden, J. Pressure leaching of copper concentrates at Morenci, Arizona-10 years of experience. Miner. Metall. Process. 2018, 35, 109–116. [Google Scholar] [CrossRef]
- Mohamed, S.; Emam, A.; Fathy, W.; Salem, A.; Eldeeb, A. Enhanced extraction of copper and nickel based on the Egyptian Abu Swayeil copper ore. Anal. Sci. Technol. 2024, 37, 63–78. [Google Scholar] [CrossRef]
- Schueler, T.; de Aguiar, P.; Vera, Y.; Goldmann, D. Leaching of Cu, Zn, and Pb from Sulfidic Tailings Under the Use of Sulfuric Acid and Chloride Solutions. J. Sustain. Metall. 2021, 7, 1523–1536. [Google Scholar] [CrossRef]
- Turan, M.; Orhan, R.; Nizamoğlu, H. Use of Ammonia Salts in Selective Copper Extraction from Tailings. Min. Metall. Explor. 2020, 37, 1349–1356. [Google Scholar] [CrossRef]
- Smolinski, T.; Rogowski, M.; Brykala, M.; Pyszynska, M.; Chmielewski, A. Studies on hydrometallurgical processes using nuclear techniques to be applied in copper industry. I. Application of 64Cu radiotracer for investigation of copper ore leaching. Nukleonika 2018, 63, 123–129. [Google Scholar] [CrossRef]
- Atta Mends, E.; Manka Tita, A.; Hussaini, S.; Samuel Thella, J.; Pan, L.; Chu, P. Investigation of leaching of nickel sulfide flotation tailings to recover valuable metals. Miner. Eng. 2024, 212, 108716. [Google Scholar] [CrossRef]
- Ambo, A.; Iyakwari, S.; Glass, H. Selective leaching of copper from near infrared sensor-based preconcentrated copper ores. Physicochem. Probl. Miner. Process. 2020, 56, 204–218. [Google Scholar] [CrossRef]
- Kamariah, N.; Xanthopoulos, P.; Binnemans, K.; Spooren, J. Solvometallurgical Process for the Recovery of Copper from Chrysocolla in Monoethanolamine. Ind. Eng. Chem. Res. 2023, 62, 12880–12890. [Google Scholar] [CrossRef]
- Potysz, A.; Pedziwiatr, A.; Hedwig, S.; Lenz, M. Bioleaching and toxicity of metallurgical wastes. J. Environ. Chem. Eng. 2020, 8, 104450. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, X.; Liu, X.; Xu, W.; Pan, Y.; Li, J.; Wang, R.; Xiao, Y.; Xu, Z. A Novel and High-Efficiency Approach to Improve the Sustainability of Agitation Leaching Technology to Refractory Copper Oxide Ore Using the Self-Pressurizing Technology. J. Sustain. Metall. 2025, 1–17. [Google Scholar] [CrossRef]
- Praburaman, L.; Park, J.H.; Govarthanan, M.; Selvankumar, T.; Oh, S.G.; Jang, J.S.; Cho, M.; Kamala-Kannan, S.; Oh, B.T. Impact of an organic formulation (panchakavya) on the bioleaching of copper and lead in contaminated mine soil. Chemosphere 2015, 138, 127–132. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, Y.; Sun, Z.; Ke, Y.; Peng, C.; Zhu, M.; Luo, Y.; Min, X. Application of copper for sufficient metal extraction from zinc leaching residue: Process optimization and copper reuse. Miner. Eng. 2024, 214, 108763. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, G.; Dutta, N. Kinetic study on leaching of Zn and Cu from spent low-temperature shift catalyst (CuO/ZnO/Al2O3): Application of taguchi design. J. Mater. Cycles Waste Manag. 2020, 22, 1509–1520. [Google Scholar] [CrossRef]
- Kilicarslan, A.; Saridede, M. Treatment of Industrial Brass Wastes for the Recovery of Copper and Zinc. Sep. Sci. Technol. 2015, 50, 286–291. [Google Scholar] [CrossRef]
- Yin, K.; Chan, W.; Dou, X.; Ren, F.; Chang, V.C. Measurements, factor analysis and modeling of element leaching from incineration bottom ashes for quantitative component effects. J. Clean. Prod. 2017, 165, 477–490. [Google Scholar] [CrossRef]
- Kim, J.; Hyun, S. Nonequilibrium leaching behavior of metallic elements (Cu, Zn, As, Cd, and Pb) from soils collected from long-term abandoned mine sites. Chemosphere 2015, 134, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sumalatha, J.; Naveen, B.; Malik, R. Toxic Metals Removal from Industrial Sludge by Using Different Leaching Solutions. J. Inst. Eng. (India) Ser. A 2019, 100, 337–345. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, M.; Yang, T.; Li, N.; Yao, X. Leaching Characteristics of Heavy Metals from Asphalt Mixture with Basic Oxygen Furnace Slag as Aggregate. J. Test. Eval. 2024, 52, JTE20230279. [Google Scholar] [CrossRef]
- Fang, W.; Delapp, R.; Kosson, D.; van der Sloot, H.; Liu, J. Release of heavy metals during long-term land application of sewage sludge compost: Percolation leaching tests with repeated additions of compost. Chemosphere 2017, 169, 271–280. [Google Scholar] [CrossRef]
- Sun, R.; Gao, Y.; Yang, Y. Leaching of heavy metals from lead-zinc mine tailings and the subsequent migration and transformation characteristics in paddy soil. Chemosphere 2022, 291, 132792. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Xu, D.M.; Chen, T.; Yan, Z.A.; Li, L.L.; Wang, M.H. Leachability characteristic of heavy metals and associated health risk study in typical copper mining-impacted sediments. Chemosphere 2020, 239, 124748. [Google Scholar] [CrossRef]
- Vinoth, M.; Aswathy, M. Environmental effects of using copper mine tailings in various geotechnical applications. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2022, 175, 84–96. [Google Scholar] [CrossRef]
- Golaghaei, F.; Mohadesi, A.; Ataei, S.; Karimi, M.; Torabi, M. Optimizing the leaching conditions of chalcopyrite/pyrite concentrate in Sarcheshmeh Copper Complex using response surface methodology. Chem. Eng. Commun. 2024, 211, 221–228. [Google Scholar] [CrossRef]
- Salehi, S.; Noaparast, M.; Shafaei, S. Response surface methodology (RSM) for optimization of chalcopyrite concentrate leaching with silver-coated pyrite. Physicochem. Probl. Miner. Process. 2016, 52, 1023–1035. [Google Scholar] [CrossRef]
- Rissoni Toledo, A.; Tayar, S.; Arena, F.; Benedetti, A.; Bevilaqua, D. New insights into oxidative-reductive leaching of chalcopyrite concentrate using a central composite factorial design. Miner. Eng. 2022, 180, 107467. [Google Scholar] [CrossRef]
- Sonmez, I.; Sahbudak, K.; Kartal, L.; Alkan, B. Optimization of sulfuric acid leaching of roasted chalcopyrite concentrate with Box–Wilson experimental design. SN Appl. Sci. 2020, 2, 1557. [Google Scholar] [CrossRef]
- Yang, D.; Kirke, M.; Fan, R.; Priest, C. Investigation of Chalcopyrite Leaching Using an Ore-on-a-Chip. Anal. Chem. 2019, 91, 1557–1562. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Li, B.; Wei, Y.; Wang, H. Optimization of Copper Recovery and Fluorine Fixation from Spent Carbon Cathode Reduction Copper Slag by Response Surface Methodology. J. Sustain. Metall. 2024, 10, 2186–2204. [Google Scholar] [CrossRef]
- Mbuya, B.; Ntakamusthi, P.; Kime, M.B.; Zeka, L.; Nkulu, G.; Mwamba, A.; Mulaba-Bafubiandi, A. Metallurgical Evaluation of the Leaching Behavior of Copper–Cobalt-bearing Ores by the Principal Component Analysis Approach: Case Study of the DRC Copperbelt Ore Deposits. J. Sustain. Metall. 2021, 7, 985–994. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).