Abstract
Hydrogen is a key energy carrier, playing a vital role in sustainable energy systems. This review provides a comparative analysis of physical, chemical, and innovative hydrogen storage methods from technical, environmental, and economic perspectives. It has been identified that compressed and liquefied hydrogen are predominantly utilized in transportation applications, while chemical transport is mainly supported by liquid organic hydrogen carriers (LOHC) and ammonia-based systems. Although metal hydrides and nanomaterials offer high hydrogen storage capacities, they face limitations related to cost and thermal management. Furthermore, artificial intelligence (AI)- and machine learning (ML)-based optimization techniques are highlighted for their potential to enhance energy efficiency and improve system performance. In conclusion, for hydrogen storage systems to achieve broader applicability, it is recommended that integrated approaches be adopted—focusing on innovative material development, economic feasibility, and environmental sustainability.
1. Introduction
Hydrogen energy plays a central role in global energy transition strategies aimed at reducing the environmental impacts of fossil fuels. Due to its low environmental footprint, high energy density, and compatibility with renewable energy sources, hydrogen is increasingly considered a fundamental component of sustainable energy systems [1]. However, for hydrogen to be effectively utilized, the development of safe, cost-effective, and high-efficiency storage systems is essential [2].
In this context, significant technological advancements have been achieved in both physical (e.g., compressed gas, liquefaction, adsorption) and chemical (e.g., metal hydrides, liquid organic hydrogen carriers, compounds such as ammonia and methanol) hydrogen storage methods in recent years [3,4]. Nevertheless, the literature still lacks comprehensive comparative analyses that holistically examine these technologies from technical, economic, environmental, and safety perspectives [5]. Moreover, current studies that present up-to-date and extensive reviews on the optimization impacts of machine learning (ML)- and artificial intelligence (AI)-based modeling techniques on hydrogen storage systems remain limited [6,7].
The novelty of this study lies in its comprehensive comparative analysis of hydrogen storage technologies, with a particular focus on the performance evaluation of metal hydride systems and the growing contributions of AI/ML-based applications. Additionally, this study incorporates systematic evaluations aligned with the safety and performance criteria supported by the U.S. Department of Energy (DOE) [8].
The potential contributions of machine learning techniques—such as in material selection, thermodynamic equilibrium analysis, energy efficiency optimization, and cost reduction—are also thoroughly addressed. By integrating both conventional and ML-assisted approaches, this review provides a strategic perspective on the integration of hydrogen into sustainable energy systems.
2. Materials and Methods
This section outlines the methods applied for the technical, economic, and environmental analysis of different hydrogen storage technologies. In this study, both physical and chemical storage techniques were comprehensively examined, and their advantages, limitations, and applicability were evaluated. Various methods for storing hydrogen in different forms—such as compressed gas, liquefied hydrogen, adsorption, and chemical storage—were addressed, and these techniques were compared based on critical parameters including energy density, cost analysis, and system efficiency. Furthermore, the performance of chemical storage systems such as metal hydrides and liquid organic hydrogen carriers (LOHCs) was assessed through nanotechnological enhancements and thermodynamic modeling.
Machine learning (ML)- and artificial intelligence (AI)-based analytical methods have emerged as a significant area of research in the optimization and performance prediction of hydrogen storage systems [6]. In particular, data-driven modeling approaches are increasingly employed in the determination of thermodynamic stability, hydrogen absorption/desorption kinetics, and cost-effectiveness analysis of hydrogen storage materials [7,9].
The literature reports successful applications of ML-based regression and classification models in capacity estimation and material selection processes for hydrogen storage methods [7,8,9]. In this context, support vector machine (SVM), decision tree (DT), random forest (RF), and deep learning (DL) algorithms have been used in the efficiency analysis of metal hydride- and MOF-based hydrogen storage systems [10,11,12,13]. In our study, ML- and AI-based analyses related to hydrogen storage methods were reviewed, and the optimization processes these methods contribute to in the current literature were detailed. Specifically, data-driven approaches concerning energy density, environmental sustainability, and cost analysis of hydrogen storage systems were comparatively discussed. This comprehensive analysis provides a framework for understanding the role of hydrogen storage technologies within sustainable energy systems.
2.1. Hydrogen Storage Technologies
Hydrogen storage technologies are generally divided into two main categories: physical and chemical storage techniques [3]. Physical storage methods include high-pressure compression, liquefied hydrogen storage, and adsorption-based approaches [3,14], whereas chemical methods involve the storage of hydrogen through chemical compounds [15].
These two approaches differ significantly in terms of storage capacity, energy efficiency, and safety requirements, and the choice of method depends largely on the scale of application [4,16].
Machine learning (ML)- and artificial intelligence (AI)-assisted analytical techniques are increasingly employed for the optimization and performance evaluation of hydrogen storage systems. In particular, comparative analyses of different hydrogen storage methods can be conducted with greater accuracy using data-driven ML algorithms [10,17].
In the literature, regression-based models have been successfully applied for capacity prediction of hydrogen storage materials [10], while artificial neural networks (ANNs) are utilized for optimizing energy consumption [18], and ML algorithms such as decision tree (DT) and random forest (RF) have been implemented in the efficiency analysis of storage processes [19,20].
In addition to machine learning-based technical analyses, various studies in the literature have focused on improving the safety performance of hydrogen storage systems. Within this scope, risk assessment frameworks have been developed specifically for metal hydride-based systems, and the responses of compounds such as NaAlH4 under potential accident scenarios have been investigated [21]. Subsequent research has further evaluated fire and explosion risks associated with these materials through more comprehensive experimental tests, and science-based methodologies have been proposed to enhance safety performance [22]. Moreover, a report published by the U.S. Department of Energy (DOE) addressed the reactivity requirements of hydrogen storage materials and presented a systematic approach for their safe use, including new material testing protocols and risk mitigation strategies [8].
In addition to technical analyses based on machine learning, recent studies aimed at improving the efficiency and safety analysis of hydrogen storage technologies have led to the systematic classification of these methods. In this context, hydrogen storage technologies are generally divided into two main categories: physical and chemical storage techniques [3]. Figure 1 summarizes the current hydrogen storage technologies based on this classification. This figure has been developed by the authors based on the classification frameworks commonly found in the literature [3,23,24,25].
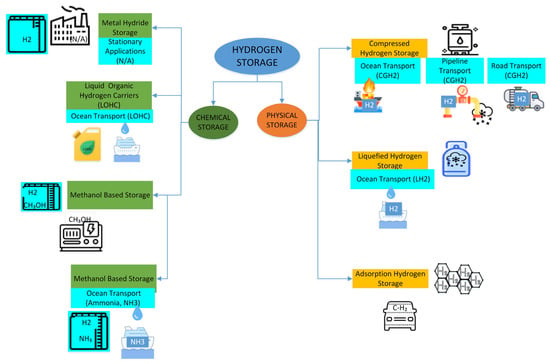
Figure 1.
Classification of hydrogen storage technologies and application areas.
Physical storage techniques encompass compressed hydrogen gas (CGH2), liquefied hydrogen (LH2), and adsorption-based storage methods [24]. These approaches allow for hydrogen to be stored in physical form—under high pressure, at cryogenic temperatures, or on adsorbent materials. In particular, compressed hydrogen storage is widely used in pipelines, land transportation, and refueling stations [24]. In contrast, liquefied hydrogen storage requires cryogenic conditions around −253 °C to enhance volumetric energy density and is typically used in large-scale applications such as marine transport [26]. Adsorption-based storage is an innovative method that enables the physical retention of hydrogen at low pressures on high surface area materials [4].
Chemical storage methods involve the storage of hydrogen through chemical bonds and are particularly realized through compounds such as methanol, liquid organic hydrogen carriers (LOHCs), and ammonia [25,27]. Methanol-based storage involves the reaction of CO2 with hydrogen to produce CH3OH. This process offers a sustainable solution for the carbon cycle, and the resulting methanol can either be used directly as fuel or converted back to hydrogen when needed [28,29]. Similarly, LOHC systems represent a promising technology for storing and transporting hydrogen using chemicals with high hydrogen-carrying capacity [25]. Ammonia-based storage is achieved by chemically binding hydrogen within ammonia (NH3) molecules, making it suitable for long-distance energy transport applications [30].
The choice among these technologies depends on several factors, including the scale of application, cost, energy density, environmental impact, and safety requirements [31]. Figure 1 presents the overall classification of hydrogen storage technologies, which are further detailed in the following sections under physical and chemical storage methods.
2.1.1. Physical Storage Techniques
Under the category of physical storage techniques, methods that store hydrogen based on its physical properties are detailed. This section discusses the basic principles, advantages, limitations, and technical requirements of various storage techniques. In the following subsections, the processes for storing hydrogen through compressed, liquefied, and adsorption-based methods are examined in detail.
Compressed hydrogen storage is based on the physical containment of hydrogen gas under high pressure in specially designed tanks [24,32]. The tanks used for this purpose are typically made of carbon fiber-reinforced composites and aluminum alloys and are capable of operating safely within a pressure range of 350–700 bar [33,34,35]. One major limitation of this method is the energy loss associated with compressing hydrogen, which results in a reduction in total system energy efficiency by approximately 20–30% [36]. This loss increases operational costs, particularly in industrial-scale applications. According to the literature, hydrogen is generally assumed to be stored at pressures ranging from 200 to 700 bar [33].
The energy density (MJ/L) and hydrogen storage capacity (wt%) values presented in Table 1 are derived from the literature based on the following equations [5,16,32,36,37,38,39,40,41]:
This equation expresses the amount of energy per unit volume (MJ/L) stored in the tank under specified temperature (T) and pressure (Ptank) conditions. Energy density is a critical engineering indicator in terms of system design and portability.
Here:
- Edensity: energy density (MJ/L);
- Psystem: system pressure ratio;
- Ptank: hydrogen pressure inside the tank (bar);
- Patmosphere: atmospheric pressure (1 bar);
- Vtank: tank volume (L);
- R: universal gas constant (8.314 J/mol·K);
- T: storage temperature (K).
The values calculated using Equation (1) form the basis of the technical comparisons presented under the “Energy Density (MJ/L)” column in Table 1. In this study, energy density is evaluated strictly in MJ/L as defined by Equation (1). Although the inverse metric—liters per megajoule (L/MJ), which indicates the required storage volume per unit of energy—is also found in the literature, it is not utilized in this study’s comparative analysis or tables.
The system pressure ratio, another critical design parameter, is calculated as follows [40]:
This ratio shows how much the hydrogen is compressed relative to atmospheric pressure, and it is particularly relevant for tank material selection and safety assessments, where Patmosphere is taken as 1 bar.
Liquefied hydrogen storage involves cooling hydrogen to approximately −253 °C to store it in liquid phase [4,42]. Compared to compressed gas storage, this method offers higher energy density but requires a significant amount of energy for the liquefaction process—approximately 8–12 kWh per kilogram of hydrogen. The energy consumption of the cryogenic cooling system is calculated using the following formula:
where
- Ecryo: cryogenic energy consumption (kWh/kg H2);
- c: specific heat capacity of hydrogen (J/kg·K);
- ∆T: temperature change (K);
- COP: coefficient of performance of the cooling system.
Equation (3) is widely used to analyze energy consumption in liquefied hydrogen systems and is consistent with thermodynamic models found in the current literature [42].
The adsorption-based hydrogen storage method is an innovative technique that stores hydrogen through weak physical forces on porous materials with high surface area, such as carbon nanotubes and metal–organic frameworks (MOFs) [43]. This method provides significant advantages, including high storage capacity and low energy loss under low-temperature and pressure conditions [44,45,46]. The adsorption capacity is calculated using the following formula:
where
- Cadsorpsiyon: adsorption capacity (g/L);
- Ssurface: surface area of the material (m2/g);
- Phydrogen: hydrogen pressure (bar).

Table 1.
Hydrogen Storage Methods—Technical Specifications.
Table 1.
Hydrogen Storage Methods—Technical Specifications.
| Storage Category | Storage Method | Operating Temperature (°C) | Operating Pressure (bar) | Hydrogen Storage Capacity (wt%) | Thermodynamic Properties (ΔH kJ/mol H2) | AI/ML Optimization Potential | References |
|---|---|---|---|---|---|---|---|
| Metal Hydride Storage | Magnesium Hydrides (MgH2) | 175–300 | 1–10 | 7–8 | ~75 | Prediction of hydrogen absorption kinetics via ML-based modeling | [47,48,49,50,51,52] |
| Thermochemical Storage (CaH2, SrH2) | 150–300 | 1–10 | 6–7 | ~60 | AI-assisted thermodynamic optimization studies | [51,53,54,55,56] | |
| Physical Storage | Underground Hydrogen Storage | 10–50 | 50–200 | - | - | ML-based modeling (MLP, RBF, LSSVM) for predicting surface wettability and contact angle | [57,58,59,60,61,62] |
| Metal–Organic Frameworks (MOFs) (MOF-5, -74) | 25–150 | 10–100 | 5–10 | ~25 | Prediction of adsorption capacity using artificial neural networks (ANNs) | [43,63,64,65,66] | |
| Carbon Nanotube and Graphene (CNT, Graphene) | 25–200 | 10–100 | 4–7 | ~20 | Prediction of storage capacity using support vector machines (SVMs) | [67,68,69,70,71,72] | |
| Physical Compression and Storage (H2) | 25–100 | 200–700 | 5–6 | ~10 | Deep learning-based risk analysis for gas leakage prediction | [14,32,73,74,75] | |
| Chemical Storage | Liquid Organic Hydrogen Carriers (C7H12, C6H14) | 25–150 | 1–10 | 6–7 | ~65 | Optimization of hydrogen conversion efficiency using decision trees (DTs) | [25,73,76,77,78,79,80,81,82] |
| Ammonia-Based Storage (NH3) | 20–300 | 1–10 | 7–8 | ~30 | AI-assisted prediction of energy efficiency in ammonia cracking reactors | [27,30,83,84,85,86,87] | |
| Boron-Based Hydrogen Storage (NaBH4, KBH4) | 20–200 | 10–100 | 7–8 | ~35 | Optimization of hydrogen recovery processes using multivariable regression (MLR) | [9,12,88,89,90,91] | |
| Chemical Hydrogen Storage (NaAlH4, LiAlH4) | 25–200 | 10–100 | 6–7 | ~30 | Use of machine learning (random forest, RF) in thermodynamic stability analysis | [1,2,9,12,92,93] | |
| Other Storage Methods | CO2 Combination Systems (H2 + CO2) | 150–300 | - | Not applicable | Not applicable | Modeling of hydrogen–CO2 reaction kinetics using AI | [94,95,96,97,98,99] |
| Storage with Ionic Liquids | 25–150 | 10–100 | 6–7 | ~25 | ML-assisted prediction of ionic liquid stability | [100,101,102,103,104] | |
| Electrochemical Storage (Metal-Air Batteries) | 25–50 | - | Not applicable | Not applicable | AI modeling of electrochemical reaction rates | [105,106,107,108,109] | |
| Layered Composite Materials (Polymer Composites) | 25–150 | 10–100 | Not applicable | Not applicable | ML-based analysis for structural optimization of polymer-based hydrogen storage materials | [110,111,112,113,114,115,116] | |
| Biological Hydrogen Storage (Biomass) | 25–50 | - | Not applicable | Not applicable | AI-assisted modeling of biochemical hydrogen production processes | [117,118] |
Equation (4) represents the theoretical adsorption capacity based on the surface area of the material and the hydrogen pressure, which is a critical performance metric for material selection and system design. The values obtained from Equation (4) correspond directly to the values presented in Table 1 under “Storage Capacity (g/L)” and “Operating Pressure (bar)”, and this equation serves as the basis for the technical comparisons of adsorption-based storage systems in this study.
In conclusion, each physical hydrogen storage method presents distinct advantages and limitations depending on factors such as application scale, energy density, system efficiency, and technical requirements. Table 1 enables a comparative analysis of these methods based on critical engineering parameters such as operating temperature, pressure, energy density, and adsorption capacity. This allows for the scientific determination of which storage method is more suitable for specific application scenarios. The technical parameters presented in the table are structured based on thermodynamic calculations and engineering equations widely cited in the literature.
For instance, Equation (1), which is used for calculating energy density in compressed hydrogen systems, and Equation (2), which defines the system pressure ratio, form the engineering basis for the operating pressure and capacity values presented in Table 1. Similarly, Equation (3), which evaluates energy consumption in liquefied hydrogen systems, and Equation (4), which defines capacity calculations based on surface area in adsorption-based systems, support the technical validity of the comparative data included in the table.
Additionally, the column titled “Optimization Potential with AI/ML” reflects applications commonly found in the literature that are based on algorithms such as artificial neural networks (ANNs), support vector machines (SVMs), and decision trees (DTs).
Table 1 presents a comparative overview of hydrogen storage methods in terms of key technical parameters (i.e., operating temperature, pressure, hydrogen storage capacity, thermodynamic properties) and their optimization potential through artificial intelligence (AI) and machine learning (ML) approaches. However, some methods are more related to hydrogen conversion, production, or indirect utilization, rather than direct physical or chemical storage. Therefore, the table reflects not only technical data but also the technological context of these systems.
Metal hydride storage methods—especially magnesium hydride (MgH2)-based systems—stand out with high hydrogen storage capacities (7–8 wt%) and relatively low pressure requirements. However, their high operating temperatures (~250 °C) can limit their practical applicability [52,93,119]. In such systems, absorption kinetics are successfully modeled using machine learning algorithms such as linear regression (LR), gradient descent (GD), and artificial neural networks (ANNs) [51].
Among chemical storage methods, liquid organic hydrogen carriers (LOHCs) offer the advantage of operating under low temperatures and pressures, but they pose challenges in terms of toxicity and recyclability. These disadvantages are being mitigated through process optimization using decision trees (DTs) and other AI-supported algorithms [2,37,79].
Nanomaterials such as metal–organic frameworks (MOFs) and carbon nanotubes (CNTs) are promising due to their high surface areas. However, their hydrogen storage capacities vary depending on the MOF structure and environmental conditions [43,120,121,122]. Optimization of storage performance in such systems is possible using techniques like artificial neural networks (ANNs) and multivariate linear regression (MLR) [9,12,13,18,123].
Physical compression methods are applicable for transportation due to their high working pressures (200–700 bar), but they also involve high energy losses [32,36,38]. Moreover, hydrogen leakage in these systems poses a significant safety risk. Deep learning-based risk assessment and sensor data processing techniques play a critical role in the development of gas leakage prediction and early warning systems [73,74].
Underground hydrogen storage offers large-volume and long-term solutions; however, risks such as geological safety and leakage are increasingly being analyzed through AI-based modeling methods [124,125]. Ammonia and boron compounds (e.g., NaBH4, NH3) provide high hydrogen storage capacities (~7–8 wt%), but they face limitations in energy recovery efficiency due to high enthalpy changes (~30–35 kJ/mol) [1,54,126,127,128].
Electrochemical systems (e.g., metal–air batteries), layered composite materials, CO2-coupled systems (H2 + CO2), and biologically based systems are not primarily designed for direct hydrogen storage. Therefore, technical metrics such as wt% or ΔH are not directly applicable, as these systems serve primarily in conversion processes or as supportive structures. In particular, CO2-coupled systems are intended for syngas production or methanation reactions rather than hydrogen storage. For this reason, these metrics are marked as “Not applicable” in the table.
Nonetheless, AI-based techniques have a significant impact on optimization and process efficiency in such systems [94,95,96,97,98,99,105,106,107,108,109,110,111,112,113,114,115,116,117,118].
2.1.2. Chemical Storage Techniques
Chemical storage techniques rely on the principle of storing hydrogen as an energy carrier within chemical bonds. These methods enable the storage of hydrogen in liquid or solid form by binding it with various chemical compounds. Owing to their high energy densities and low volumetric requirements, chemical hydrogen storage methods play a critical role in renewable energy systems and portable energy applications [12].
Materials such as metal hydrides [39], liquid organic hydrogen carriers (LOHCs) [78], boron-based compounds [127], and ammonia [129] are among the most widely studied and applied chemical storage solutions. The main advantage of these techniques is their ability to release hydrogen in a controlled manner while maintaining long-term stability throughout the storage process [130]. However, challenges such as energy efficiency, storage capacity limitations, reaction kinetics, and material recyclability remain significant [7].
Numerous studies in the literature aim to enhance the economic feasibility of these techniques and minimize their environmental impacts [131]. In this regard, machine learning (ML) and artificial intelligence (AI) techniques have broad applicability in the optimization of chemical hydrogen storage processes—from material selection and thermodynamic modeling to reactor design and recycling strategies [13].
In particular, models based on deep learning (DL) and support vector machines (SVMs) have been employed to improve the performance of hydrogen storage materials [132]. These techniques play a vital role in predicting material properties, optimizing synthesis processes, and enhancing storage capacity [18].
This section provides a detailed analysis of the general principles, advantages, limitations, and application areas of chemical storage techniques.
Metal hydrides represent an advanced storage method wherein hydrogen is chemically bonded with metals [119]. ML-based modeling techniques are widely used to optimize the thermodynamic stability and hydrogen absorption/desorption kinetics of metal hydrides [10,17,52]. Algorithms such as support vector machine (SVM) and random forest (RF) have been applied to model hydrogen diffusion within metal lattice structures, thereby enhancing storage capacity [10,133,134]. Furthermore, artificial neural networks (ANNs) have been used to develop models that minimize performance loss in recycling processes of metal hydrides [43,135].
Understanding how hydrogen atoms are incorporated into the metal lattice and how these structures influence storage capacity is critical for material design and development. Compounds like LaNi5H6 are prominent examples in this field, offering low-temperature operation and high hydrogen storage capacity (1.4–8.4 wt%). However, the need for high-pressure operation in such systems necessitates additional safety measures [39,93,136].
Figure 2 illustrates how metal atoms in the crystal structure of metal hydrides bind hydrogen atoms and the lattice positions they occupy [137,138]. These structural arrangements have a significant impact on the hydrogen absorption capacity and kinetic behavior of metal hydrides. The optimized lattice pathways enable rapid bonding and release of hydrogen atoms. Research on these crystal structures has greatly improved the practical applicability of metal hydrides in energy storage systems. In particular, these systems show great promise for expanding the hydrogen economy and integrating hydrogen with renewable energy sources.
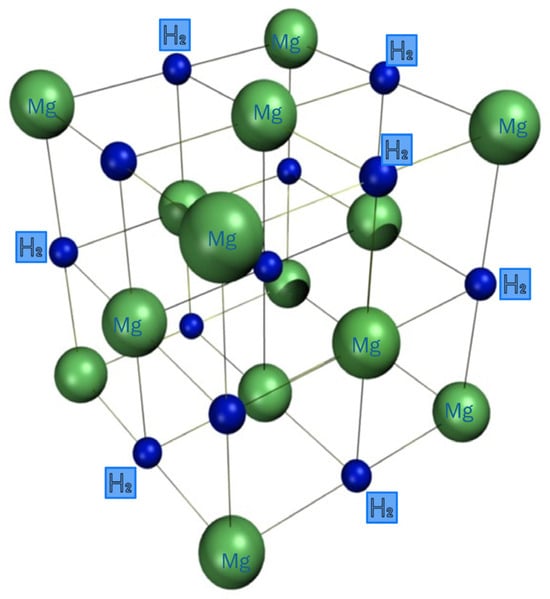
Figure 2.
Crystal structure of metal hydrides.
The gravimetric hydrogen storage capacity of metal hydrides—defined as the amount of hydrogen stored per unit mass of the host material—can be calculated using the following equation [137]:
where
- H/M: molar ratio of hydrogen to host atoms;
- MH: molar mass of hydrogen;
- MHost: molar mass of the host material.
This calculation is essential for evaluating the efficiency of metal hydrides in hydrogen storage technologies [137].
The cycling stability of metal hydrides is of critical importance for their applicability in long-term energy storage systems. In particular, maintaining performance over a high number of charge–discharge cycles is a key determinant of the effectiveness and economic sustainability of metal hydrides [119]. Cycling degradation is typically a direct result of physical and chemical changes occurring within the metal hydride structure. These changes may lead to a reduction in storage capacity, a decrease in hydrogen diffusion rates, and overall energy losses. Therefore, materials engineering studies aimed at improving the cycling durability of metal hydrides are of significant importance.
Nanotechnological enhancements have recently emerged as a prominent approach for improving the performance and cycling stability of metal hydrides. It has been scientifically demonstrated that nanoscale structures can enhance the hydrogen storage properties of metal hydrides and increase their storage capacity [52,119]. Nanoparticles, nanocomposites, and nanostructures offer substantial advantages in enhancing hydrogen diffusion mechanisms and accelerating reaction kinetics [119]. Specifically, the use of carbon-based nanomaterials such as graphene and carbon nanotubes provides an effective strategy for optimizing the storage performance of metal hydrides [52].
Figure 3 schematically illustrates the fundamental principles of metal hydride-based hydrogen storage and compression systems [139]. The diagram represents the process in which hydrogen is supplied from low-pressure storage tanks to metal hydride reactors, where it is stored through absorption, and then released via desorption by applying heat, subsequently transferred to high-pressure storage tanks. The figure includes both single-stage and two-stage system configurations, explaining the operational cycle of metal hydride-based storage systems and the role of thermal compressors used to increase pressure. Thermodynamic and physical effects encountered during the design and operation of such systems are particularly important in terms of cycling stability and must be considered in long-term performance assessments [139].
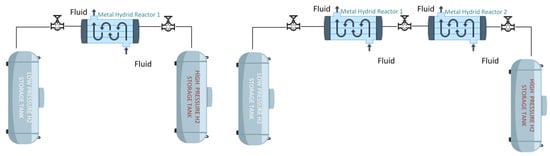
Figure 3.
Schematic representation of metal hydride-based hydrogen storage and compression system.
Liquid organic hydrogen carriers (LOHCs) represent an innovative technology that enables the chemical bonding and transport of hydrogen. This method allows for safe storage at ambient pressure and room temperature [140]. ML-based optimization approaches are employed to enhance the thermodynamic stability of LOHC systems. Algorithms such as decision tree (DT) and random forest (RF) are applied in thermodynamic modeling to maximize hydrogen conversion efficiency [77,140]. Additionally, reaction kinetics in LOHC systems have been optimized using AI-based reactor simulations, leading to minimized energy losses [141].
The energy efficiency of LOHC systems is calculated by considering the ratio between stored and lost energy during the storage process [140,142]:
Chemical compounds such as ammonia and methanol are commonly used to improve the portability of hydrogen. These compounds enable hydrogen storage at low-temperature and pressure conditions, contributing to the efficient operation of energy systems. For instance, methanol-based storage can achieve energy densities ranging from 8% to 12% [125,139,142,143].
Ammonia (NH3) contains three hydrogen (H2) molecules per NH3 molecule, offering high hydrogen content. The storage and release processes are defined based on the following thermochemical reaction [144,145]:
- Molar mass of NH3 = 17 g/mol;
- Molar mass of H2 = 2 g/mol;
- Total hydrogen content = (6/17) × 100 ≈ 17.65%.
This value represents the theoretical hydrogen storage capacity of ammonia. However, in practical applications, factors such as catalyst efficiency and energy losses may influence the system’s actual performance [30].
To enhance energy efficiency in ammonia-based storage systems, parameters such as adsorption capacity and surface area must be optimized. Their relationship with hydrogen pressure is particularly important for maximizing system efficiency [146].
Methanol (CH3OH) is an effective liquid organic hydrogen carrier commonly used in steam reforming reactions for hydrogen production. The reforming process of methanol is expressed as follows [147]:
- Molar mass of NH3 = 17 g/mol;
- Molar mass of H2 = 2 g/mol;
- Total hydrogen content = (6/17) × 100 ≈ 17.65%.
This value represents the amount of hydrogen per unit mass obtained from the methanol–water reaction. However, in real-world applications, catalytic efficiency and system losses must be taken into account [148].
2.2. Cost Analysis and Energy Efficiency
Studies on the economic feasibility of hydrogen storage systems generally consider capital expenditures (CAPEX), operational and maintenance costs (OPEX), and energy consumption expenses [5,24]. A comprehensive analysis of these cost elements, as well as a comparison of the cost structures and energy demands of different storage technologies, is critical for identifying sustainable solutions within the energy sector.
Different hydrogen storage methods show significant variation in terms of investment and operational costs. For instance, in compressed hydrogen storage systems, the CAPEX values vary depending on the materials used, while OPEX values are generally higher in liquefied hydrogen systems due to substantial energy consumption [32,34,149]. Although the initial investment cost of metal hydride-based systems is relatively high, their long-term low OPEX contributes to balancing the overall cost structure [39,119,150,151].
In the design and optimization of hydrogen storage systems, several parameters must be carefully evaluated, including storage category, storage method, operating pressure, installation cost, operating cost, and total cost [2,5,24]. Recently, ML- and AI-based models have been effectively applied to optimize hydrogen storage systems [11,12,51,132]. These approaches serve as the foundation for decision support systems developed to improve energy efficiency, minimize costs, and enhance safety levels in storage applications.
Table 2 provides a holistic comparison of cost analysis by storage method, presenting the installation, operating, and total costs for each system. The qualitative assessments—such as “Low”, “Medium”, and “High”—are based on the technical features, cost levels, and practical applicability of hydrogen storage methods. These classifications rely on investment and operational costs reported in the literature, and where quantitative data are unavailable, evaluations are based on qualitative assessments. Therefore, the categorizations presented in the table aim to scientifically reflect the relative economic feasibility of various hydrogen storage technologies [10,25,39,52,77,79,119,124,140,152,153,154].

Table 2.
Economic analysis of hydrogen storage methods: operating pressure, installation and operating costs, and AI/ML-based optimization applications.
The values of operating pressure, installation cost, operating cost, and total cost presented in Table 2 are derived from general trends reported in the current literature. In particular, it has been identified that the number of studies providing direct quantitative support for these parameters—especially in MgH2-based systems—remains limited [155]. Similarly, cost analyses for thermochemical storage systems are also notably scarce [11,39,54,152].
In terms of installation costs, metal hydride systems require high capital investment. Among them, magnesium hydride stands out due to its requirement for high purity and complex production processes. Likewise, MOFs, CNTs, and graphene-based systems are also considered costly due to sophisticated synthesis requirements [5,39,52,63,119,121]. In contrast, underground hydrogen storage, as a physical method, offers lower installation costs [124,154].
Operating costs vary based on the energy demands and technical complexity of the method. While underground storage exhibits low OPEX [47,49], systems based on MOFs and CNTs are more expensive due to higher energy consumption [95]. Chemical storage methods are considered moderately expensive; however, toxicity issues and recycling processes tend to increase overall costs [1,25,76].
Total cost must be considered as the sum of installation and operating expenditures. In this regard, underground storage is advantageous. However, MOF- and metal hydride-based systems are less favorable for large-scale applications due to their high total costs [124,125,139]. On the other hand, LOHC- and ammonia-based systems may offer more balanced total costs due to their relatively low operating expenses [76].
AI/ML-based methods play an effective role in optimizing all of these cost components. From the production of MOFs to the efficiency optimization of ammonia reactors, these approaches show great potential in reducing costs and improving system efficiency [133,160,161]. In addition, deep learning-based hydrogen leakage prediction systems contribute significantly to safety and sustainability evaluations [155,222].
A major gap in the literature is the lack of direct numerical cost analyses, particularly regarding the economic viability of MOF and metal hydride systems. There is a need for further experimental and theoretical research on this topic [20,220]. Moreover, comprehensive studies are required to address toxicity management, recycling processes, and the applicability of AI-supported decision support systems [19,39,45,76].
It should also be noted that some of the technologies presented in the table—such as biological systems, electrochemical cells, layered composites, and CO2-coupled systems—are not primarily intended for direct physical or chemical hydrogen storage. Instead, they should be considered in the context of temporary retention, conversion, or system-level integration where hydrogen acts as an energy vector [220].
The inclusion of these systems in the table is not limited to their classical storage capacities but rather aims to highlight their role in the entire hydrogen life cycle, their cost optimization potential, and their integration with AI/ML-based application scenarios from a holistic perspective [133,155,209]. Therefore, for some technical indicators, the term “Not applicable” is used to reflect the functional limitations of these technologies and the lack of data in the literature [52]. This approach is intended to provide an extended framework for assessing the multifaceted role of hydrogen in energy systems [155,222].
2.3. Evaluation of Energy Efficiency
The energy efficiency of hydrogen storage technologies varies according to the specific energy conversion processes involved in each system. Metal hydride-based systems, which store energy through chemical bonds, typically offer an efficiency range of approximately 60–75% [52,119]. In contrast, liquefied hydrogen systems operate at lower efficiencies, around 40–55%, due to the significant energy requirements for achieving cryogenic temperatures [38,125,149]. Compressed hydrogen storage systems, which operate under high pressure, can achieve relatively high efficiencies of 85–90% [8].
These differences highlight the importance of energy loss percentages as a key factor in technology selection, making energy efficiency a critical metric in the comparative evaluation of storage technologies [24].
The energy efficiency ratio (η) represents the relationship between the energy expended during storage and the recoverable energy output. This ratio is defined by the following equation:
where
- η: efficiency percentage;
- Usable Energy: energy recovered after storage;
- Input Energy: energy consumed during storage.
In chemical storage systems, particularly LOHC (liquid organic hydrogen carrier) technologies, energy losses during the recovery process can reach as high as 70–85% [25,77]. Therefore, the total system efficiency for such technologies is expressed as the product of both storage and recovery efficiencies:
where
- ηchemical: overall efficiency of chemical hydrogen storage systems;
- Storage Efficiency: effectiveness of hydrogen bonding within chemical compounds [223];
- Recovery Efficiency: efficiency of hydrogen release from the compounds [224].
In addition to energy efficiency, the environmental impact and sustainability characteristics of chemical hydrogen storage systems have become prominent evaluation criteria. Within this scope, various storage technologies have been analyzed in terms of carbon footprint, energy consumption, and recycling potential, based on the current literature findings [225,226].
Table 3 presents a comparative evaluation of the environmental impacts and sustainability performance of hydrogen storage methods. The assessments in the “Recyclability and Sustainability” column are qualitatively categorized based on criteria such as technological maturity, recyclability potential, formation of toxic by-products, and material circularity.

Table 3.
Environmental impacts and sustainability of hydrogen storage methods.
Accordingly:
- “High” refers to systems that are environmentally compatible or fully recyclable through natural processes.
- “Medium” refers to systems with recyclability or sustainability potential that are still under development.
- “Low” indicates systems with inadequate recycling technologies or where environmental risks remain insufficiently addressed.
Table 3, developed within the scope of this study, presents a comparative analysis of various hydrogen storage and utilization methods through different technological platforms. The analysis considers key environmental and technical indicators such as carbon footprint (kg CO2/kg H2), energy consumption (kWh/kg H2), and recyclability–sustainability potential. Although most of the methods listed in the table represent direct hydrogen storage technologies, some systems—such as electrochemical devices, ionic liquids, and layered composites—do not provide direct storage but are included due to their roles in hydrogen recovery, conversion, or as functional energy carriers. The rationale for including these systems is to visualize how indicators such as carbon footprint and energy consumption reflect not only the storage infrastructure itself, but also the broader systematic hydrogen cycle, enabling the relative environmental positioning of different technologies.
Key highlights include the following:
- Metal hydride systems (e.g., magnesium hydride) are known for their high energy consumption (~30 kWh/kg H2), due to the elevated temperatures required for hydrogen release. Depending on the production route of magnesium, carbon emissions may reach up to 8 kg CO2/kg H2, and even higher in some cases. Although specific carbon footprint values are not always directly reported, the high energy demand supports this approximation [227,228,229].
- Underground hydrogen storage, as a physical method, stands out for its large-scale and long-term storage capacity, offering relatively more sustainable environmental outcomes. While direct carbon emission data may be limited, the literature estimates suggest emissions in the range of ~5 kg CO2/kg H2, based on various LCA models [232,233].
In contrast:
- LOHC-based chemical storage methods exhibit moderate recyclability potential but face engineering challenges such as toxicity management [231,242]. On the other hand, biological hydrogen production and storage systems, due to their reliance on natural microbial processes, offer high sustainability potential and minimal carbon footprint [255,257].
Additionally:
- Nanostructured materials such as metal–organic frameworks (MOFs) are notable for their high surface areas yet lack comprehensive data regarding long-term recyclability and environmental compatibility [234,243]. Systems based on carbon nanotubes and graphene show high performance potential but still face underdeveloped recycling processes [238].
Strategic Insights and Forward-Looking Recommendations
The evaluation presented in Table 3 goes beyond energy efficiency, encompassing dimensions such as material sustainability, systematic recyclability, and long-term environmental integration. Based on this perspective, key findings and recommendations are as follows:
- There is a significant lack of literature regarding recyclability processes for advanced materials such as MOFs and carbon nanomaterials.
- For biological storage methods to achieve widespread commercialization, large-scale pilot demonstrations must be implemented.
- In chemical storage systems, it is essential to reduce efficiency losses by developing new catalyst designs and thermal management strategies.
In conclusion, Table 3 provides a multidimensional evaluation not only in terms of technological performance but also concerning carbon management, energy expenditure, and circular economy principles. In this respect, it serves as a strategic scientific reference for decision-makers and researchers aiming to compare the environmental impact of various hydrogen technologies within a unified framework [52,76,119,133,155,209,220,222,227,228,229,231,232,233,234,238,242,255,257].
2.4. Evaluation of Hydrogen Storage Systems Based on Artificial Intelligence and Machine Learning
The complex and multivariate nature of hydrogen storage systems—combined with nonlinear relationships among thermodynamic parameters and uncertainties across different storage environments—has led to the increasing use of machine learning (ML) and artificial intelligence (AI) techniques in this field. Although no software-based modeling was conducted within the scope of this study, a theoretical and analytical framework has been presented based on the literature findings, illustrating the integration of ML techniques into performance modeling [12], material selection [9], capacity prediction [10], and multi-criteria evaluation processes [134] for hydrogen storage systems.
ML algorithms are generally classified under four main types of learning: supervised learning, unsupervised learning, reinforcement learning, and deep learning. Each learning approach offers distinct advantages in solving specific problems associated with hydrogen storage systems. Table 4 provides an overview of these learning types, highlighting their areas of application and use cases in hydrogen storage technologies.

Table 4.
Analysis of TRL and commercial applicability of storage methods.
In the literature, numerous studies have demonstrated the implementation of these algorithms using open-source Python-based libraries such as scikit-learn, TensorFlow, and Keras, as well as platforms like MATLAB. These tools offer robust analytical capabilities for tasks such as multidimensional data modeling, system optimization, and material classification [7,12,132]. The ML algorithms summarized in Table 4 are integrated into hydrogen storage systems through these platforms, enabling performance analyses based on various learning types.
In this context, the literature review presented in this study emphasizes the significant potential of AI/ML-based approaches in the future design, efficiency enhancement, and decision support integration of hydrogen storage technologies.
3. Result and Discussion
This section provides a comprehensive analysis of the current state, performance criteria, and future potential of hydrogen storage technologies. First, the development stages of the field are assessed by examining the technology readiness levels (TRLs), current research status, and commercial applicability of various hydrogen storage methods.
Next, the contributions of machine learning techniques to optimization and efficiency enhancement in hydrogen storage systems are evaluated. The role of nanotechnology-based materials in improving hydrogen storage capacity is also thoroughly analyzed, highlighting innovative approaches in this domain.
Subsequently, the industrial integration and cost analysis of hydrogen storage methods are discussed, with a focus on their applicability across different industrial sectors.
Finally, a comparative performance evaluation of hydrogen storage systems is presented, outlining their advantages and limitations in terms of energy density, cost, safety, and environmental impact. In light of emerging technologies and future perspectives, potential development areas are discussed, and the critical role of hydrogen storage systems in enabling a sustainable energy transition is emphasized.
3.1. Performance Assessment of Hydrogen Storage Technologies
Hydrogen storage technologies hold significant potential to deliver sustainable and high-efficiency solutions for energy systems. In this context, evaluating the performance of these technologies provides critical insights into their technology readiness levels (TRLs), research status, and commercial applicability.
The technology readiness level (TRL) is a key metric that reflects a technology’s progress from laboratory-scale development to commercial deployment. It is widely used to compare the developmental maturity of technologies and to assess their commercialization potential. In hydrogen storage technologies, TRL helps determine the technical maturity, pilot-scale implementation status, and market readiness of each method [24,39,140].
Table 5 summarizes the TRLs of various hydrogen storage methods, outlines their current research status, and evaluates their suitability for commercial application.

Table 5.
TRL and commercial use analysis of hydrogen storage methods.
The classifications of “Commercial Applicability” and “Current Research Status” in Table 5 are based on the scale of application, testing phases, and the scope of technology development projects reported in the literature. For instance, the term “High” refers to technologies that have already entered commercial application and have been widely tested. “Medium” indicates pilot implementations or early commercialization phases, whereas “Low” refers to technologies that remain at laboratory or theoretical levels. The terms “R&D”, “laboratory”, and “pilot test”, used to define research status, do not reflect technical maturity directly but rather the scope of current experimental or field testing activities.
The data presented in this table classify hydrogen storage methods according to their technology readiness level (TRL), research status, and commercial applicability potential. TRLs are rated on a scale from 1 (basic research) to 9 (full-scale commercial deployment), following standards widely accepted by the European Commission, the U.S. Department of Energy (DOE), and the related academic literature. Notably, innovative and theoretically promising approaches (e.g., MOFs, ionic liquids) typically remain at the laboratory stage and are therefore generally categorized within TRLs 4–5 [275].
Research status is classified according to development stages such as laboratory-scale, pilot testing, or commercial demonstration. Commercial applicability, on the other hand, is defined based on the recent literature reporting on field implementations, techno-economic analyses, and market penetration levels, and is described using qualitative terms such as “Low”, “Medium”, and “High”.
The table includes not only direct hydrogen storage technologies but also systems that contribute indirectly to hydrogen storage, such as through energy conversion, transport, or component-level integration (e.g., electrochemical storage, layered composites). These technologies have been included due to their potential to support storage via temporary retention, hydrogen release, or transformation, enabling a broader and comparative analysis of both conventional and hybrid storage systems.
In this context, physical methods such as underground hydrogen storage have reached high TRLs and are already in use for commercial-scale applications [58,59]. In contrast, more innovative methods like MOFs and biological storage systems remain in the laboratory stage and are thus assigned lower TRLs [43,256]. The relationship between TRL and commercial viability provides valuable guidance for technology-oriented sectoral analyses, particularly in identifying priority areas for R&D investment.
Classifying hydrogen storage technologies according to their TRL, research status, and commercial applicability not only helps identify which technologies deserve further R&D funding but also offers insights into where AI- and ML-based optimization tools could be effectively integrated. In line with this, Table 6 summarizes the potential areas of ML application, suitable algorithms, and expected benefits for different hydrogen storage technologies.

Table 6.
ML-supported optimization applications in hydrogen storage methods.
Table 6 presents the application areas, optimization targets, and expected technical outcomes of machine learning (ML) algorithms in hydrogen storage technologies, structured based on the latest literature findings. This table not only provides a general classification but also offers a strategic framework to improve system performance by suggesting algorithmic solutions tailored to the technical challenges of each storage method.
In the context of physical compression and high-pressure storage systems, deep learning (DL) models have been effectively used in safety-oriented applications such as leak detection and risk analysis. For instance, the Temporal Convolutional Network (TCN) architecture has been successfully applied to predict leakage locations with high accuracy at hydrogen refueling stations [74]. Similarly, the LSTM-based autoencoder (LSTM-AE) model has demonstrated high performance in predicting both the location and severity of leaks in hydrogen-powered gas turbines [73].
For metal hydride storage systems, artificial neural networks (ANNs) and regression-based supervised learning methods are frequently employed to model hydrogen absorption kinetics. These models have shown high accuracy in capacity prediction, particularly for magnesium-based and complex hydrides, providing useful guidance for experimental design [10].
In chemical systems such as liquid organic hydrogen carriers (LOHCs), decision trees (DTs) and federated learning techniques have been highlighted in the literature for predicting conversion efficiency. Specifically, in dibenzyltoluene (DBT)-based systems, these algorithms have been reported to yield successful predictions of hydrogen storage performance and contribute significantly to system optimization [81].
For metal–organic frameworks (MOFs) and nanotechnology-based storage systems, support vector machines (SVMs) and regression algorithms have proven effective in modeling critical parameters such as surface area and adsorption capacity. Comparative analyses performed on real MOF structures have demonstrated that several ML algorithms can predict both gravimetric and volumetric hydrogen capacities with over 95% accuracy [166].
Accordingly, the classification presented in Table 6 enables performance evaluation not only in terms of algorithm–method pairing but also across multidimensional outputs such as safety, efficiency, and cost-effectiveness. These pairings are directly linked to the learning types and algorithm classifications discussed in Section 2.4 and should be interpreted in conjunction with the system performance and sustainability evaluations elaborated in Section 3.2.
3.2. Nanotechnology and Metal Hydrides
Nanotechnology-based materials offer significant innovations for hydrogen storage systems and play a key role in enhancing energy storage capacity, reducing system costs, and improving overall efficiency. In particular, the high surface area, rapid hydrogen absorption, and low-temperature operation capabilities of nanostructures have positioned these technologies at the forefront of sustainable energy transformation systems.
Metal hydride-based systems are widely preferred in hydrogen storage applications due to their safety, reversibility, and recyclability. In addition, nanotechnology-driven advancements—such as carbon-based materials, metal–organic frameworks (MOFs), and lightweight hydrides—offer advanced solutions for increasing storage capacity.
Table 7 provides an in-depth analysis of nanotechnology-enhanced hydrogen storage materials by evaluating their technology readiness levels (TRLs) and the innovations they bring to the field [275].

Table 7.
Nanotechnology-enhanced hydrogen storage materials.
Table 7 presents a comparative analysis of nanotechnology-enhanced hydrogen storage materials, focusing on their technical capabilities, technology readiness levels (TRLs), and the structural or functional innovations they provide. In particular, nanostructures with high surface areas—such as graphene and carbon nanotubes—have been shown to improve hydrogen adsorption capacity while reducing overall system costs and energy losses, as emphasized in the literature [67,192]. Similarly, metal–organic frameworks (MOFs) have shown great promise for the future of the hydrogen economy due to their ability to deliver high storage capacities at low pressures [170,280]. Lightweight and metal hydrides are also notable for their high storage capacity and low operating temperatures; however, they pose certain limitations in terms of recycling cost and environmental sustainability [119]. On the other hand, advanced systems such as solid oxide fuel cells (SOFCs) have significant potential to improve overall system efficiency by integrating hydrogen production and storage processes [279].
3.3. Industrial Applications and Cost Considerations
Nanotechnology has revolutionized hydrogen storage and energy technologies, significantly enhancing cross-industry integration. In this context, studies focusing on nanotechnology-based materials and their areas of application have been assessed in terms of technological compatibility and production cost.
The following table presents a comparative overview of different nanotechnology-driven materials, highlighting their application domains, industrial compatibility, and associated production costs.
Table 8 offers a classification that evaluates the industrial integration potential of nanotechnology-enhanced hydrogen storage materials, based on cost trends and industrial adaptability. This table has been structured using updated data from the literature and sector-specific reports to reflect current developments.

Table 8.
Application areas, cost trends, and industrial compatibility of nanotechnology.
Table 8 provides a comparative evaluation of nanotechnology-based materials used in hydrogen storage and conversion technologies, focusing on their application domains, cost trends, and industrial compatibility. This assessment is structured based on findings from recent scientific studies and data derived from industrial implementations. In particular, cost-effectiveness and scalability are identified as key determinants in material selection for energy conversion technologies.
The classifications of “Industrial Compatibility” and “Cost Trend” are based on several criteria: the material’s technology readiness level (TRL), scalability of production processes, current industrial usage, and reported cost ranges per kilogram of hydrogen. A “High” compatibility rating indicates that the material is already in commercial use, while “Medium” suggests usage at the pilot scale. Cost classification is generally based on USD/kg H2 production cost estimates.
High surface area nanostructures, such as carbon nanotubes (CNTs) and graphene, are prominent in stationary energy storage and vehicular applications due to their medium production cost and high to medium industrial compatibility. These materials are preferred for mobile energy systems thanks to their rapid response times and excellent thermal and electrical conductivity [22,24,52,173,175,176,281].
Metal hydrides, such as MgH2 nanostructures, offer strong potential for stationary storage systems. However, despite their promising properties, their integration into industry remains moderate, mainly due to medium-to-high production costs [8,19,22,119,139,282].
Metal–organic frameworks (MOFs) like MOF-5 and HKUST-1 show high hydrogen storage capacity at large surface areas and are applicable in industrial and stationary settings. Nevertheless, their limited production scalability and high cost keep their industrial compatibility at a medium level [22,43,283,284,285].
Lightweight hydrides, such as LiBH4 and NaBH4, are highly promising for portable hydrogen storage but face limited industrial uptake due to high production costs and poor regeneration efficiency [21,22,48,194,286].
Solid oxide fuel cells (SOFCs) are applicable in both stationary and mobile energy systems. They are classified as having medium cost and moderate industrial compatibility. Given their advanced design and reversible operation capabilities (r-SOC), they are expected to play a greater role in future applications [288,289].
From a broader perspective, carbon-based nanomaterials (graphene and CNTs) stand out with high industrial compatibility despite their medium cost, making them attractive for automotive and portable systems. Conversely, materials like MOFs and lightweight hydrides, although possessing high theoretical capacity, show moderate industrial compatibility due to high production costs and limited scalability.
Similarly, metal hydrides such as MgH2 nanostructures offer substantial potential for stationary storage, but their widespread deployment is hindered by technical complexity and cost-intensive manufacturing. Likewise, while SOFC technology presents advanced energy conversion potential, it still faces technical and economic barriers related to long-term durability and system integration.
3.4. Future Perspectives of Hydrogen Storage Technologies
Hydrogen stands out as a key enabler in clean energy transition, and in this context, storage technologies play a crucial role. The limitations of conventional storage methods necessitate the development of alternative approaches. This section discusses the future perspectives of hydrogen storage technologies and highlights emerging and innovative storage solutions.
For hydrogen to achieve broader adoption in the coming decades, advancements in research and development, along with improvements in technology readiness levels (TRLs) and application potential, must be prioritized. A thorough analysis of these factors is essential to identify scalable, cost-effective, and sustainable storage methods.
Table 9 provides a summary of alternative hydrogen storage approaches, including their innovative potential and current feasibility. The table aims to classify novel methods not only by their technical characteristics but also by their prospective contributions to next-generation hydrogen systems.

Table 9.
Future perspectives and alternative approaches.
Table 9 classifies alternative hydrogen storage methods based on innovative research trends and technology readiness levels (TRLs) reported in the literature. TRL values reflect the transition of technologies from laboratory testing to field implementation, while future applicability potential is assessed based on current technical maturity, scalability, and economic feasibility. This table offers a visual and systematic summary of all the technological perspectives discussed in this section.
Key Findings from the Literature
- Crystalline Hydrogen Storage (Clathrate Hydrates—H2O): This approach shows significant potential for hydrogen storage and release under low-temperature conditions. However, its low TRL (3) and early-stage development currently limit its large-scale application. Further theoretical and experimental research is required [145,289,290].
- CO2-Coupled Systems (H2 + CO2 Reactions): This method aims to produce synthetic fuels while reducing carbon emissions by reacting hydrogen with carbon dioxide. Demonstrated in industrial pilot-scale setups, the technology has reached TRL 5, though broader commercial deployment still requires in-depth economic assessments [31,135,267].
- Biological Hydrogen Storage (Enzyme Catalysis): This method presents a bio-inspired innovation for hydrogen generation and storage. However, with a TRL of 3, it remains in the experimental phase and needs significant development to become a scalable solution [16,291].
- Ionic Liquid-Based Storage: Known for reversible hydrogen storage characteristics, this approach has shown promising results at the laboratory scale. With a TRL of 4, it may become a feasible option for higher-capacity applications in the medium term [43,292,295].
- Layered Composite Materials: These materials offer lightweight construction and high storage capacity, showing promising outcomes at the pilot test level. With a TRL of 5, they hold significant potential for use in mobile hydrogen applications [93,140,143].
- Electrochemical Storage (Metal–Air Batteries): This technology, which plays a key role in integrating renewable energy systems, is currently undergoing commercial testing. At TRL 5, it shows strong potential to support the sustainability of hydrogen energy storage systems [131,293,294].
Uncovered and Overlooked Aspects in the Literature
- Lack of Economic Assessments: Many of these technologies lack detailed analyses of their economic feasibility. This is particularly true for TRL 3 systems, where cost models and deployment studies are scarce [24,25,145].
- Cross-Technology Integration: Research is limited on combining innovative approaches—such as ionic liquids, composite materials, and biological pathways—to create more effective hybrid storage systems [31,119].
- Long-Term Stability and Environmental Impact: Few studies address the long-term performance, safety, and environmental implications of hydrogen storage methods, creating a critical knowledge gap before commercial implementation [21,22,124,135].
Table 9 reveals a significant gap between the technical maturity (TRL) and practical applicability of innovative hydrogen storage technologies. In particular, for approaches at lower TRLs, economic feasibility, scalability, and environmental impact assessments must be further developed to support their future deployment.
3.5. Comparative Performance Analysis of Hydrogen Storage Technologies
Hydrogen is widely recognized as a key component of sustainable energy systems, serving as a versatile and transportable energy carrier through various storage technologies. In this context, the performance of hydrogen storage technologies must be assessed based on multiple criteria, including storage capacity, energy consumption, cost, and carbon footprint.
Table 10 provides a comparative summary of hydrogen storage technologies implemented through physical, chemical, and emerging methods. The objective of this analysis is to evaluate the advantages and limitations of each storage method in light of operational requirements and technology readiness levels (TRLs).

Table 10.
Comparative summary of hydrogen storage technologies.
By mapping these parameters across storage types, the table offers an integrated performance-based perspective that can support strategic decision-making in research, development, and industrial deployment.
The comparative data presented in Table 10 offers a comprehensive assessment of hydrogen storage technologies by integrating technical, environmental, and economic dimensions. Physical storage methods, particularly those with high technology readiness levels (TRL 6–8), offer short-term feasibility advantages. However, their reliance on high-pressure or extremely low-temperature conditions imposes significant energy consumption and infrastructure cost burdens.
Chemical storage systems, especially those using LOHCs or ammonia-based carriers, enable storage under lower pressures and more flexible conditions. Nevertheless, these systems face limitations due to the high energy demands of recovery processes and the complexity of reactor systems. Although metal hydride-based systems offer high storage capacities, their requirement for elevated operating temperatures restricts their broader application potential.
Alternative technologies, such as ionic liquids and biological systems, are still in the early stages of R&D (TRL 3–4). Despite their lower carbon footprints and environmental advantages, these solutions face ongoing challenges in terms of scalability, cost, and process continuity.
According to the current literature, quantitative comparisons of installation costs and carbon footprint values are not consistently available across all technologies [8,25,203,217]. Therefore, the terms used in Table 10 such as “low”, “medium”, and “high” reflect qualitative assessments based on processing conditions, energy input, material type, and infrastructure requirements [25,93,158].
In most cases, cost evaluations of hydrogen storage technologies are drawn from technology-specific studies, and a lack of standardized, comparative datasets remains a critical gap. Accordingly, the cost classifications used in the table are qualitative in nature, as the absence of detailed life cycle assessments (LCAs) and techno-economic analyses (TEAs) precludes robust numerical comparisons.
In conclusion, this assessment highlights the relative suitability of each hydrogen storage technology under specific conditions, offering strategic guidance for researchers, policy-makers, and industry stakeholders seeking to promote the widespread and efficient integration of hydrogen into future energy systems.
3.6. Future Prospects of Hydrogen Storage Technologies
Hydrogen plays a critical role in energy transition, and the advancement of storage technologies significantly enhances its potential for industrial, transportation, and mobile applications in alignment with sustainable energy goals. Evaluating different hydrogen storage methods based on their application domains is essential to determine which technologies are most suitable for specific sectors.
In this context, hydrogen storage methods are assessed in terms of their feasibility for transportation (e.g., automotive, aviation, and marine), stationary energy storage (e.g., grid or industrial use), and mobile applications (e.g., portable devices, drones, and electric tools).
Table 11 compares the application potential of hydrogen storage technologies across these three categories. The table indicates each method’s suitability for a given sector using “✅” for applicable and “❌” for not suitable, and the evaluations are supported by relevant references from the literature.

Table 11.
Comparison of hydrogen storage methods by application area.
The classifications presented in Table 11 are based on documented use cases and technological feasibility assessments reported in the literature. Evaluating each hydrogen storage method by its application domain enables the identification of sector-specific priorities based on both technical performance and economic feasibility.
Insights Identified in the Literature
- Physical Storage: Compressed and liquefied hydrogen exhibit high applicability for transport and stationary energy storage. However, their use in mobile applications remains limited. Compressed hydrogen has been extensively studied in the transport sector [24,32], while liquefied hydrogen stands out in marine and aerospace sectors due to its high energy density and transportability. Underground hydrogen storage holds great potential for large-scale stationary applications but lacks direct feasibility for transport and mobile uses [24,125,300].
- Chemical Storage: Technologies such as LOHCs and ammonia-based systems offer significant benefits for transport and stationary storage, given their high energy density and long-distance transport capability [25,77,140]. Some lightweight metal hydrides have demonstrated broad applicability across transport, stationary, and mobile sectors [21,22,39,88,119], while specific solutions like magnesium hydride are primarily designed for stationary systems [93,273].
- Other Methods: Ionic liquids and biological hydrogen storage represent emerging technologies offering promising features for mobile and stationary applications. In particular, biological storage shows strong potential for environmental sustainability [16,40]. However, these technologies are not yet mature for large-scale commercial deployment.
Unobserved and Overlooked Aspects in the Literature: The comparison in Table 11 reveals significant differences in technology readiness levels (TRLs) among hydrogen storage methods. While compressed and liquefied hydrogen are widely deployed in industry, many chemical and biological storage solutions remain in development phases [8,77,124,135]. Moreover, technologies aimed at mobile applications require further studies addressing efficiency, energy density, and safety parameters [39,119,204].
Conclusion: Each hydrogen storage method provides distinct advantages in specific sectors, but due to technological and economic constraints, no single technology is universally applicable. To ensure a sustainable and sector-specific deployment of hydrogen across the energy economy, storage methods must be tailored to the unique requirements of each application domain.
3.7. Technological Developments and Innovative Solutions
Hydrogen storage technologies hold strategic importance in achieving the energy sector’s low-carbon goals. In this context, ongoing innovation efforts aim not only to increase existing storage capacities but also to provide more sustainable, safer, and cost-effective solutions. Beyond conventional methods, emerging technologies such as nanotechnology-enhanced structures, metal–organic frameworks (MOFs), metal hydrides, carbon-based materials, and fuel cells offer next-generation alternatives for high-density, low-pressure, and secure hydrogen storage.
Table 12 presents a systematic overview of current research areas in hydrogen storage technologies, along with their potential innovations, technology readiness levels (TRLs), and future applicability prospects. The TRL rating system, as used here, reflects the developmental pathway of a technology, from basic research (TRL 1) to full market readiness (TRL 9). The terms “High” and “Medium” used to describe future applicability potential are based on assessments of both technical feasibility and economic sustainability. Below, a detailed analysis of the technologies presented in Table 12 is provided.

Table 12.
Future perspectives and technological developments.
Nanostructures with high surface area have received significant attention in the literature due to their potential to enhance energy density and hydrogen uptake capacity. Reported hydrogen storage capacities range between 7 and 9 wt%, making these structures particularly advantageous for portable applications and fuel cell systems [119,122,302]. However, complex production processes and high manufacturing costs remain barriers to widespread commercialization. Metal hydride systems, especially those operating at low temperatures, can achieve storage capacities up to 8 wt% [8,22,48,273]. Nevertheless, kinetic limitations and thermal management requirements present technical challenges for industrial-scale deployment.
Carbon-based nanostructures such as graphene and carbon nanotubes offer improved storage potential due to their high surface area and gas diffusion characteristics [67,175,303]. These features are ideal for high energy density applications. Yet, their elevated production costs currently hinder economic viability. Metal–organic frameworks (MOFs) present a promising option due to their high porosity and ability to adsorb hydrogen at low pressures [43,63,64]. Still, structural stability concerns and limitations in large-scale manufacturing restrict their industrial implementation.
Lightweight borohydrides such as LiBH4 and NaBH4 stand out in mobile applications for their ability to reach hydrogen storage capacities of up to 10 wt% [8,88,89,119]. Nonetheless, low recyclability efficiency significantly reduces their overall energy efficiency and sustainability.
Solid oxide fuel cells (SOFCs) are particularly relevant for integrated systems that combine hydrogen extraction from carriers like ammonia with direct energy generation [83,124,304]. While this technology is advantageous for carbon emissions reduction and energy efficiency enhancement, it faces limitations due to its high-temperature operating conditions and material stability concerns.
In conclusion, the technologies summarized in Table 12 offer promising solutions for the safe, efficient, and sustainable storage of hydrogen. Their current technology readiness levels and applicability prospects, as supported by the literature, serve as strategic indicators for the global advancement of the hydrogen economy. This assessment also contributes to shaping future research directions.
3.8. General Assessment of Hydrogen Storage Technologies
Hydrogen is widely recognized as a key energy carrier in sustainable energy transitions and is considered a central component of low-carbon energy systems [2,135]. This study offers a comprehensive analysis of hydrogen storage technologies under physical, chemical, and innovative nanotechnology-assisted approaches. Based on a detailed literature review and technical evaluation, the potential of these methods in providing sustainable solutions for the energy sector is thoroughly discussed across technical, economic, and environmental dimensions.
- Physical Storage
- Compressed and liquefied hydrogen storage technologies are already commercially implemented for transportation and stationary energy applications [145]. Despite their advantage of high energy density, both methods face significant drawbacks such as high energy consumption—particularly for liquefaction, which requires approximately 8–12 kWh/kg H2—along with cost-related and operational challenges [31].
- Underground hydrogen storage offers a cost-effective and large-scale solution for stationary energy storage, but its broader applicability is constrained by geological limitations and potential leakage risks [124].
- Chemical Storage
- Liquid organic hydrogen carriers (LOHCs) and ammonia-based systems are emerging as promising technologies for long-distance hydrogen transport and stationary storage [140]. LOHCs provide reversibility and operational flexibility, while ammonia-based storage exhibits a higher energy density [77]. However, both methods require further investigation regarding carbon footprint and toxicity management [241,304].
- Metal hydrides and lightweight hydrides deliver high hydrogen storage capacities ranging from 7 to 10 wt%, along with improved safety profiles [119]. Yet, their widespread commercial deployment is limited due to high costs, thermal management complexities, and kinetic limitations [52].
- Innovative and Nanotechnology-Based Methods
- Nanotechnology-enhanced materials, such as graphene, carbon nanotubes, and MOFs, significantly improve hydrogen storage performance through increased surface area and energy density [18]. MOFs offer high storage at low pressure, while nanostructures support rapid hydrogen uptake [43]. Nevertheless, high production costs and stability issues hinder their scalability [4].
- Biological hydrogen storage and ionic liquids represent environmentally friendly and innovative approaches [20]. In particular, biological storage offers low energy consumption and sustainability benefits yet suffers from low TRL and limited capacity, which currently restricts its broader development [2,39,218].
4. Conclusions and Recommendations
In this study, hydrogen storage technologies were comprehensively evaluated from a multidimensional perspective, encompassing technology readiness level (TRL), cost analysis, environmental impact, energy density, and machine learning (ML) integration. Based on the findings from Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11 and Table 12, the technical, economic, and environmental performance of physical, chemical, and nanotechnology-based storage systems were comparatively analyzed, and their application potentials were assessed.
Physical storage technologies, including compressed hydrogen, liquefied hydrogen, and underground storage, have emerged as the most mature solutions in terms of TRL, having reached levels TRL 7–8, as indicated in Table 5. While these technologies are suitable for large-scale deployment in transportation and stationary energy systems (Table 2 and Table 11), they also pose sustainability challenges due to high energy consumption and carbon emissions, as shown in Table 3.
Chemical storage systems, such as metal hydrides, LOHCs, and ammonia-based carriers, offer high energy densities (Table 1 and Table 6), but limitations in recyclability and economic feasibility continue to hinder their widespread adoption. As highlighted in Table 11, LOHCs and ammonia-based technologies show promise for long-distance hydrogen transportation, but their environmental impacts, particularly carbon emissions, require careful management (Table 3).
Nanotechnology-based solutions, including MOFs, carbon nanotubes, and lightweight hydrides, offer high hydrogen storage capacities and low environmental impact potentials (Table 4 and Table 6). However, due to their TRLs generally ranging between 3 and 5 (Table 5), these technologies remain at pre-commercial stages.
Regarding the integration of ML and AI, detailed in Table 6 and Table 12, these methods have shown great potential in enhancing system efficiency and optimizing costs. Notably, support vector machines (SVMs), artificial neural networks (ANNs), and deep learning (DL) algorithms have demonstrated high accuracy in predicting hydrogen storage capacities.
From an economic perspective, Table 2 indicates that compressed and underground hydrogen storage systems involve relatively lower capital costs, whereas liquefied hydrogen is associated with higher energy requirements and therefore elevated costs. Chemical systems such as boron-based compounds and LOHCs still require optimization in their production processes to improve economic feasibility.
In terms of environmental impact, data from Table 3 reveal that chemical storage systems generally produce higher carbon footprints, whereas biological systems and MOF-based solutions are more aligned with sustainable goals. However, their TRLs remain insufficient for commercial implementation.
Application-specific evaluations in Table 11 suggest that compressed hydrogen, liquefied hydrogen, and LOHCs are most viable for transportation, while underground and metal hydride systems are favorable for stationary storage. For mobile applications, light metal hydrides and ionic liquids present promising potential.
Strategic Recommendations
In conclusion, hydrogen storage technologies hold strategic value in the energy transition due to their diverse capabilities. However, to facilitate their widespread deployment, holistic advancements in energy efficiency, economic feasibility, environmental sustainability, and AI integration are essential.
Funding
This research received no external funding. The author personally covered the article processing charge (APC) without any institutional or grant support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, W.; Tupe, J.A.; Aguey-Zinsou, K. Metal Hydride Storage Systems: Approaches to Improve Their Performances. Part. Part. Syst. Charact. 2024, 41, e202400163. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gupta, R.; Rohit, M.V.; Lee, J.-K. Recent Developments in Hydrogen Production, Storage, and Transportation: Challenges, Opportunities, and Perspectives. Fire 2024, 7, 233. [Google Scholar] [CrossRef]
- Panigrahi, P.K.; Chandu, B.; Motapothula, M.; Puvvada, N. Potential Benefits, Challenges and Perspectives of Various Methods and Materials Used for Hydrogen Storage. Energy Fuels 2024, 38, 2630–2649. [Google Scholar] [CrossRef]
- Bosu, S.; Rajamohan, N. Recent Advancements in Hydrogen Storage—Comparative Review on Methods, Operating Conditions and Challenges. Int. J. Hydrogen Energy 2023, 52, 352–371. [Google Scholar] [CrossRef]
- Mehr, A.S.; Phillips, A.D.; Brandon, M.P.; Pryce, M.T.; Carton, J.G. Recent Challenges and Development of Technical and Technoeconomic Aspects for Hydrogen Storage, Insights at Different Scales; A State of Art Review. Int. J. Hydrogen Energy 2024, 70, 786–812. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, Y.; Li, Q.; Yang, T.; Jia, H. Crystal Structure Prediction and Performance Assessment of Hydrogen Storage Materials: Insights from Computational Materials Science. Energies 2024, 17, 3591. [Google Scholar] [CrossRef]
- Mortazavi, B. Recent Advances in Machine Learning-Assisted Multiscale Design of Energy Materials. Adv. Energy Mater. 2024, 14, 2403876. [Google Scholar] [CrossRef]
- Khalil, Y.F. Quantifying and Addressing the DOE Material Reactivity Requirements with Analysis and Testing of Hydrogen Storage Materials & Systems; U.S. Department of Energy Technical Report; United Technologies Research Center (UTRC): East Hartford, CT, USA, 2012. [Google Scholar] [CrossRef]
- Jia, Z.; Xie, J.; Zhang, L.; Lin, H.; Wang, X.; Wu, G.; Liu, W. Machine Learning Accelerates Design of Bilayer-Modified Graphene Hydrogen Storage Materials. Sep. Purif. Technol. 2024, 352, 128229. [Google Scholar] [CrossRef]
- Bhaskar, A.; Muduli, R.C.; Kale, P. Prediction of Hydrogen Storage in Metal Hydrides and Complex Hydrides: A Supervised Machine Learning Approach. Int. J. Hydrogen Energy 2024, 98, 1212–1225. [Google Scholar] [CrossRef]
- Tasneem, S.; Subramani, T.; Hussain, S.M.; Mohamed, A.R.; Jusoh, N.; Nadarajah, M. Machine Learning Modeling of Reversible Thermochemical Reactions Applicable in Energy Storage Systems. J. Taiwan Inst. Chem. Eng. 2023, 148, 104926. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Al-Hinai, A.M.; Rooney, D.W.; Al-Muhtaseb, A.H. Advances in Hydrogen Storage Materials: Harnessing Innovative Technology, from Machine Learning to Computational Chemistry, for Energy Storage Solutions. Int. J. Hydrogen Energy 2024, 67, 1270–1293. [Google Scholar] [CrossRef]
- Vishnyakov, A. Machine Learning in Computational Design and Optimization of Disordered Nanoporous Materials. Materials 2025, 18, 534. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhao, W.; Wang, W.; Li, X.; Zhou, H. Large Scale of Green Hydrogen Storage: Opportunities and Challenges. Int. J. Hydrogen Energy 2023, 50, 379–399. [Google Scholar] [CrossRef]
- Ali, M.; Rehman, A.; Khan, S.U.; Ahmed, S.; Iqbal, M.T.; Al-Hinai, A.M. First-Principles Evaluation of LiCaF3-αHα as an Effective Material for Solid-State Hydrogen Storage. J. Energy Storage 2024, 83, 110731. [Google Scholar] [CrossRef]
- Faye, O.; Szpunar, J.A.; Eduok, U. A Critical Review on the Current Technologies for the Generation, Storage, and Transportation of Hydrogen. Int. J. Hydrogen Energy 2022, 47, 13771–13795. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y.; Li, J.Y.; Li, Y.; Wang, L.; Zhang, J. Exploration and Design of Mg Alloys for Hydrogen Storage with Supervised Machine Learning. Int. J. Hydrogen Energy 2023, 48, 38412–38425. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Ma, X.; Li, Q.; Zhang, X.; Liu, J.; Zhou, C. Predictive Modeling for Hydrogen Storage in Functionalized Carbonaceous Nanomaterials Using Machine Learning. J. Energy Storage 2024, 97, 112914. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Zhang, Y.; Zhao, M.; Liu, Q. A Techno-Economic Study of Photovoltaic-Solid Oxide Electrolysis Cells Coupled Magnesium Hydrides-Based Hydrogen Storage and Transportation toward Large-Scale Applications of Green Hydrogen. Energy Environ. Sci. 2024, 17, 8429–8456. [Google Scholar] [CrossRef]
- Alagumalai, A.; Kumar, M.; Kale, P.; Patel, M.; Ali, M.; Kumar, R. Machine Learning in Biohydrogen Production: A Review. Biofuel Res. J. 2023, 10, 1844–1856. [Google Scholar] [CrossRef]
- Khalil, Y.F. Risk Quantification Framework of Hydride-Based Hydrogen Storage Systems for Light-Duty Vehicles. J. Loss Prev. Process Ind. 2015, 38, 187–195. [Google Scholar] [CrossRef]
- Khalil, Y.F. Science-Based Framework for Ensuring Safe Use of Hydrogen as an Energy Carrier and an Emission-Free Transportation Fuel. Process Saf. Environ. Prot. 2018, 117, 326–336. [Google Scholar] [CrossRef]
- Alanazi, A.; Alarifi, A.; Al-Sulaiman, F.A.; Habib, M.A. Hydrogen Adsorption Kinetics in Organic-Rich Shale Reservoir Rocks for Seasonal Geological Storage. Fuel 2024, 379, 132964. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, Q.; Li, H.; Wang, Y.; Zhao, J. Techno-Economic Analysis of Hydrogen Storage and Transportation from Hydrogen Plant to Terminal Refueling Station. Int. J. Hydrogen Energy 2023, 52, 547–560. [Google Scholar] [CrossRef]
- Tsogt, N.; Gbadago, D.Q.; Hwang, S. Exploring the Potential of Liquid Organic Hydrogen Carrier (LOHC) System for Efficient Hydrogen Storage and Transport: A Techno-Economic and Energy Analysis Perspective. Energy Convers. Manag. 2023, 299, 117856. [Google Scholar] [CrossRef]
- Ghafri, S.Z.S.A.; Al-Attili, A.; Al-Kayiem, H.H.; Abed, A.M.; Basrawi, F. Modelling of Liquid Hydrogen Boil-Off. Energies 2022, 15, 1149. [Google Scholar] [CrossRef]
- Negro, V.; Noussan, M.; Chiaramonti, D. The Potential Role of Ammonia for Hydrogen Storage and Transport: A Critical Review of Challenges and Opportunities. Energies 2023, 16, 6192. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, H.; Du, J.; Liao, J. Research Progress of Methanol Production via CO2 Hydrogenation: Mechanism and Catalysts. Process Saf. Environ. Prot. 2024, 189, 1071–1088. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Liu, C.; Huang, Y.; Li, J.; Yang, G. Carbon Dioxide Enabled Hydrogen Storage by Methanol: Highly Selective and Efficient Catalysis with Well-Defined Heterogeneous Catalysts. Coord. Chem. Rev. 2024, 508, 215775. [Google Scholar] [CrossRef]
- Ishaq, H.; Crawford, C. Review of Ammonia Production and Utilization: Enabling Clean Energy Transition and Net-Zero Climate Targets. Energy Convers. Manag. 2024, 300, 117869. [Google Scholar] [CrossRef]
- Alabbadi, A.A.; Obaid, O.A.; AlZahrani, A.A. A Comparative Economic Study of Nuclear Hydrogen Production, Storage, and Transportation. Int. J. Hydrogen Energy 2023, 54, 849–867. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Liu, X.; Wang, Y.; Zhao, M.; Chen, L.; Wu, C. Small-Scale High-Pressure Hydrogen Storage Vessels: A Review. Materials 2024, 17, 721. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Peng, W.; Hua, Z.; Zheng, J. A Comparative Analysis of the Regulations, Codes and Standards for On-Board High-Pressure Hydrogen Storage Cylinders. Int. J. Hydrogen Energy 2023, 54, 894–910. [Google Scholar] [CrossRef]
- Magliano, A.; Carrera, C.P.; Pappalardo, C.M.; Guida, D.; Berardi, V.P. A Comprehensive Literature Review on Hydrogen Tanks: Storage, Safety, and Structural Integrity. Appl. Sci. 2024, 14, 9348. [Google Scholar] [CrossRef]
- Nachtane, M.; Boukhriss, H.; Saifaoui, D.; Benyahia, M.; Benyahia, A.; Assarar, M. An Overview of the Recent Advances in Composite Materials and Artificial Intelligence for Hydrogen Storage Vessels Design. J. Compos. Sci. 2023, 7, 119. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q.; Cao, J.; Wang, Y.; Peng, X. Performance Assessment and Optimisation of a Concentric Two-Piston Compressor System for Hydrogen Storage. Energy Convers. Manag. 2024, 310, 118470. [Google Scholar] [CrossRef]
- Yan, X.; Zheng, W.; Wei, Y.; Yan, Z. Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain. Processes 2024, 12, 315. [Google Scholar] [CrossRef]
- Wang, J.; Webley, P.A.; Hughes, T.J. Thermodynamic Modelling of Pressurised Storage and Transportation of Liquid Hydrogen for Maritime Export. Int. J. Hydrogen Energy 2024, 62, 1273–1291. [Google Scholar] [CrossRef]
- Nivedhitha, K.S.; Sathya, S.; Arunkumar, R.; Nagarajan, V.; Ganesan, R. Advances in Hydrogen Storage with Metal Hydrides: Mechanisms, Materials, and Challenges. Int. J. Hydrogen Energy 2024, 61, 1259–1275. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Jaszczur, M.; Salman, H.M. Hydrogen as an Energy Carrier: Properties, Storage Methods, Challenges, and Future Implications. Environ. Syst. Decis. 2023, 44, 327–344. [Google Scholar] [CrossRef]
- Esposito, L.; van der Wiel, M.; Acar, C. Hydrogen Storage Solutions for Residential Heating: A Thermodynamic and Economic Analysis with Scale-Up Potential. Int. J. Hydrogen Energy 2024, 79, 579–592. [Google Scholar] [CrossRef]
- Song, Z.; Xu, J.; Chen, X. Design and Optimization of a High-Density Cryogenic Supercritical Hydrogen Storage System Based on Helium Expansion Cycle. Int. J. Hydrogen Energy 2023, 49, 1401–1412. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Q.; Li, M.; Wang, Y.; Liu, F.; Wang, X. Pd-Doped HKUST-1 MOFs for Enhanced Hydrogen Storage: Effect of Hydrogen Spillover. RSC Adv. 2023, 13, 14980–14988. [Google Scholar] [CrossRef]
- Anuchitsakol, S.; Dilokekunakul, W.; Khongtor, N.; Chaemchuen, S.; Klomkliang, N. Combined Experimental and Simulation Study on H2 Storage in Oxygen and Nitrogen Co-Doped Activated Carbon Derived from Biomass Waste: Superior Pore Size and Surface Chemistry Development. RSC Adv. 2023, 13, 36009–36019. [Google Scholar] [CrossRef] [PubMed]
- Hirscher, M.; Zhang, L.; Oh, H. Nanoporous Adsorbents for Hydrogen Storage. Appl. Phys. A 2023, 129, 132. [Google Scholar] [CrossRef]
- de Oliveira, J.C.A.; Gonçalves, D.V.; Montenegro, D.L.; Lucena, S.M.P. Predicting Hydrogen Storage at 298 K in Activated Carbons. Adsorption 2023, 30, 403–415. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Li, Y.; Zhang, Z.; Wu, C.; Wang, J. Structural Inhomogeneity: A Potential Strategy to Improve the Hydrogen Storage Performance of Metal Hydrides. J. Mater. Chem. A 2023, 11, 13255–13267. [Google Scholar] [CrossRef]
- Ren, L.; Li, Q.; Wang, H.; Yang, Y.; Zhang, W. Nanostructuring of Mg-Based Hydrogen Storage Materials: Recent Advances for Promoting Key Applications. Nano-Micro Lett. 2023, 15, 91. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Zhang, T.; Zhou, Q.; Zhao, M.; He, X. Core–Shell Nanostructured Magnesium-Based Hydrogen Storage Materials: A Critical Review. Ind. Chem. Mater. 2023, 1, 282–296. [Google Scholar] [CrossRef]
- Kanti, P.K.; Shrivastav, A.P.; Sharma, P.; Maiya, M.P. Thermal Performance Enhancement of Metal Hydride Reactor for Hydrogen Storage with Graphene Oxide Nanofluid: Model Prediction with Machine Learning. Int. J. Hydrogen Energy 2023, 52, 470–485. [Google Scholar] [CrossRef]
- Gao, J.; Guo, X.; Wu, Y.; Xiao, W.; Hao, L. The Hydrogen Absorption Process Prediction of AB2 Hydrogen Storage Device Based on Data-Driven Approach. Int. J. Hydrogen Energy 2024, 58, 657–670. [Google Scholar] [CrossRef]
- Desai, F.; Uddin, M.N.; Rahman, M.M.; Asmatulu, R. A Critical Review on Improving Hydrogen Storage Properties of Metal Hydride via Nanostructuring and Integrating Carbonaceous Materials. Int. J. Hydrogen Energy 2023, 48, 29256–29277. [Google Scholar] [CrossRef]
- Çolak, A.B.; Aydın, D.; Al-Ghosini, A.; Dalkılıç, A.S. Discharging Performance Prediction of Experimentally Tested Sorption Heat Storage Materials with Machine Learning Method. J. Energy Storage 2022, 56, 106159. [Google Scholar] [CrossRef]
- Humphries, T.D.; Paskevicius, M.; Alamri, A.; Buckley, C.E. Thermodynamic Destabilization of SrH2 Using Al for the Next Generation of High Temperature Thermal Batteries. J. Alloys Compd. 2021, 894, 162404. [Google Scholar] [CrossRef]
- Errahoui, H.; Hafidi, A.; Bouachrine, M.; Belmouden, M.; Ez-Zahraouy, H. Impact of Calcium Doping on the Electronic and Optical Characteristics of Strontium Hydride (SrH2): A DFT Study. Atoms 2024, 12, 55. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Wu, H.; Li, J.; Liu, B.; Zhou, Q. Transparent AI-Assisted Chemical Engineering Process: Machine Learning Modeling and Multi-Objective Optimization for Integrating Process Data and Molecular-Level Reaction Mechanisms. J. Clean. Prod. 2024, 448, 141412. [Google Scholar] [CrossRef]
- Sarı, E.; Çiftçi, E. Underground Hydrogen Storage in a Depleted Gas Field for Seasonal Storage: A Numerical Case Study of the Tekirdağ Gas Field. Fuel 2023, 358, 130310. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Z.; Cheng, J.; Sun, Q.; Liu, K.; Zhang, Q. Are Palygorskite and Sepiolite the Potential Materials for Underground Hydrogen Storage? J. Energy Storage 2024, 81, 110397. [Google Scholar] [CrossRef]
- Malki, M.L.; Chellal, H.A.K.; Mao, S.; Rasouli, V.; Mehana, M. A Critical Review of Underground Hydrogen Storage: From Fundamentals to Applications, Unveiling Future Frontiers in Energy Storage. Int. J. Hydrogen Energy 2024, 79, 1365–1385. [Google Scholar] [CrossRef]
- Gao, K.; Creasy, N.; Huang, L.; Gross, M.R. Underground Hydrogen Storage Leakage Detection and Characterization Based on Machine Learning of Sparse Seismic Data. Int. J. Hydrogen Energy 2024, 61, 137–150. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, Y.; Wang, X.; Chen, Y.; Sun, J. Efficient Prediction of Hydrogen Storage Performance in Depleted Gas Reservoirs Using Machine Learning. Appl. Energy 2024, 361, 122914. [Google Scholar] [CrossRef]
- Kohzadvand, K.; Kouhi, M.M.; Barati, A.A.; Omrani, S.; Ghasemi, M. Prediction of Interfacial Wetting Behavior of H2/Mineral/Brine; Implications for H2 Geo-Storage. J. Energy Storage 2023, 72, 108567. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, X.; Lu, Z.; Xü, Q. Metal-Organic Framework-Based Catalysts for Hydrogen Production from Liquid-Phase Chemical Hydrides. Coord. Chem. Rev. 2023, 493, 215302. [Google Scholar] [CrossRef]
- Wright, A.M.; Kapelewski, M.T.; Marx, S.; Farha, O.K.; Morris, W. Transitioning Metal–Organic Frameworks from the Laboratory to Market through Applied Research. Nat. Mater. 2024, 24, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Yulia, F.; Zulys, A.; Saha, B.B.; Mabuchi, T.; Gonçalves, W.; Nasruddin, N. Bio-Metal-Organic Framework-Based Cobalt Glutamate for CO2/N2 Separation: Experimental and Multi-Objective Optimization with a Neural Network. Process Saf. Environ. Prot. 2022, 162, 998–1012. [Google Scholar] [CrossRef]
- Formalik, F.; Shi, K.; Joodaki, F.; Wang, X.; Snurr, R.Q. Exploring the Structural, Dynamic, and Functional Properties of Metal-Organic Frameworks through Molecular Modeling. Adv. Funct. Mater. 2023, 34, 2308130. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Züttel, A. Continuous Production Prototype for Scaling up of Graphene Oxide/Carbon Nanotube Composite Synthesis towards Efficient Hydrogen Storage. Green Chem. 2024, 27, 756–769. [Google Scholar] [CrossRef]
- Baloochiyan, A.; Öztürk, H.; Eruçar, İ. Atomistic Investigation of Porous Amorphous Materials for CH4/H2 Separation. Chem. Eng. Sci. 2024, 287, 120741. [Google Scholar] [CrossRef]
- Beniwal, P.; Chakraborty, B.; Kumar, T.J.D. Zirconium Decorated 2D Holey Graphyne for High Capacity Hydrogen Storage: Insights from First Principles Simulations. Int. J. Hydrogen Energy 2023, 53, 29–41. [Google Scholar] [CrossRef]
- Bajgirani, M.A.; Biglari, Z.; Sahihi, M. Boosting Hydrogen Storage Capacity in Modified-Graphdiyne Structures: A Comprehensive Density Functional Study. Mater. Today Commun. 2024, 39, 108787. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, S. Support Vector Machine-Based Prediction of Unconfined Compressive Strength of Multi-Walled Carbon Nanotube Doped Soil-Fly Ash Mixes. Multiscale Multidiscip. Model. Exp. Des. 2024, 7, 5365–5381. [Google Scholar] [CrossRef]
- Vivanco-Benavides, L.E.; Martínez-González, C.L.; Mercado-Zúñiga, C.; Torres-Torres, C. Machine Learning and Materials Informatics Approaches in the Analysis of Physical Properties of Carbon Nanotubes: A Review. Comput. Mater. Sci. 2021, 201, 110939. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Zhang, R.; Chen, X.; Zhao, Y. Deep Learning-Based Source Term Estimation of Hydrogen Leakages from a Hydrogen Fueled Gas Turbine. Int. J. Hydrogen Energy 2024, 86, 875–890. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Gao, Y.; Xu, K.; Zhang, Q. Deep Learning-Based Hydrogen Leakage Localization Prediction Considering Sensor Layout Optimization in Hydrogen Refueling Stations. Process Saf. Environ. Prot. 2024, 189, 549–560. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Yu, M.; Zhang, X.; Jiang, L. Techno-Economic Analysis on Low-Temperature and High-Pressure Cryo-Adsorption Hydrogen Storage. Fuel 2024, 381, 133532. [Google Scholar] [CrossRef]
- Tang, J.; Xie, R.; Pishva, P.; Shen, X.; Zhu, Y.; Peng, Z. Recent Progress and Perspectives of Liquid Organic Hydrogen Carrier Electrochemistry for Energy Applications. J. Mater. Chem. A 2024, 12, 15580–15597. [Google Scholar] [CrossRef]
- Sage, V.; Patel, A.; Chen, H.; Nguyen, T. Recent Progress and Techno-Economic Analysis of Liquid Organic Hydrogen Carriers for Australian Renewable Energy Export—A Critical Review. Int. J. Hydrogen Energy 2024, 56, 1419–1438. [Google Scholar] [CrossRef]
- Lin, A.; Bagnato, G. Revolutionising Energy Storage: The Latest Breakthrough in Liquid Organic Hydrogen Carriers. Int. J. Hydrogen Energy 2024, 63, 315–328. [Google Scholar] [CrossRef]
- Sultanova, M.U.; Samoilov, V.O.; Borisov, R.S.; Ramazanov, D.N.; Maximov, A.L. Hydrogen Storage with a Naphthenic Liquid Organic Hydrogen Carrier (LOHC) Obtained from Coal Tar. Int. J. Hydrogen Energy 2024, 68, 1251–1264. [Google Scholar] [CrossRef]
- Bollmann, J.; Schneider, A.; Krause, M.; Hupfer, M.; Meier, G.; Schäfer, C. Temperature Imaging during the Hydrogen Release Reaction from a Liquid Organic Hydrogen Carrier (LOHC) System Using Phosphor Thermometry. Exp. Fluids 2024, 65, 174. [Google Scholar] [CrossRef]
- Ali, A.; Khan, M.A.; Choi, H. Hydrogen Storage Prediction in Dibenzyltoluene as Liquid Organic Hydrogen Carrier Empowered with Weighted Federated Machine Learning. Mathematics 2022, 10, 3846. [Google Scholar] [CrossRef]
- Men, J.; Chen, G.; Reniers, G.; Rao, X.; Zeng, T. A Hybrid Deep Belief Network-Based Label Distribution Learning System for Seismic Damage Estimation of Liquid Storage Tanks. Process Saf. Environ. Prot. 2023, 172, 908–919. [Google Scholar] [CrossRef]
- El-Adawy, M.; Nemitallah, M.A.; Abdelhafez, A. Towards Sustainable Hydrogen and Ammonia Internal Combustion Engines: Challenges and Opportunities. Fuel 2024, 364, 131090. [Google Scholar] [CrossRef]
- Yan, T.; Xie, T.; Pan, W.; Wang, L. Experimental Study on Ammonia-Based Thermochemical Resorption Thermal Energy Storage System. Renew. Energy 2024, 229, 120696. [Google Scholar] [CrossRef]
- Mucci, S.; Stumm, M.; Rix, M.J.; Mitsos, A. Model-Based Evaluation of Ammonia Energy Storage Concepts at High Technological Readiness Level. Appl. Energy 2024, 377, 124495. [Google Scholar] [CrossRef]
- Yue, W.; Zhang, B.; Zhang, S.; Wang, B.; Xia, Y.; Liang, Z. A Deep Learning Model for Predicting the Laminar Burning Velocity of NH3/H2/Air. Appl. Sci. 2024, 14, 9603. [Google Scholar] [CrossRef]
- Lan, P.; Chen, S.; Li, Q.; Li, K.; Wang, F.; Zhao, Y. Intelligent Hydrogen-Ammonia Combined Energy Storage System with Deep Reinforcement Learning. Renew. Energy 2024, 233, 121725. [Google Scholar] [CrossRef]
- Park, K.; Kim, M.; Kwon, S.; Kang, S.; Kim, T. Performance Evaluation of Solid NaBH4-Based Hydrogen Generator for Fuel-Cell-Powered Unmanned Autonomous Systems. Appl. Energy 2023, 337, 120882. [Google Scholar] [CrossRef]
- Dansirima, P.; Grinderslev, J.B.; Kristensen, L.G.; Utke, R.; Jensen, T.R. Tailoring Dehydrogenation in Lithium Borohydride–Magnesium Nickel Hydride Hydrogen Storage Systems with Metal Halide Additives. Int. J. Hydrogen Energy 2024, 98, 908–921. [Google Scholar] [CrossRef]
- Salem, H.M.; El-Aty, D.M.A.; Zaki, E.G.; Aman, D. Photocatalytic Hydrogen Production from Sodium Borohydride Using Low Carbon Footprint Substrate Azadirachta indica Oil. Environ. Technol. 2024, 45, 1–12. [Google Scholar] [CrossRef]
- Filiz, B.C.; Yörüklü, H.C.; Açıkalın, K.; Demirci, U.B.; Fıgen, A.K. Boron-Based Hydrogen Storage Materials towards Power-to-X Technology on the Path to Carbon Neutrality. Int. J. Hydrogen Energy 2023, 48, 39389–39406. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, L.; Zhao, Y.; Li, H.; Wang, Y. Hydrolysis Kinetics of LiAlH4 at Subzero Temperatures. ACS Appl. Energy Mater. 2023, 6, 2550–2559. [Google Scholar] [CrossRef]
- Bishnoi, A.; Pati, S.; Sharma, P. Architectural Design of Metal Hydrides to Improve the Hydrogen Storage Characteristics. J. Power Sources 2024, 608, 234609. [Google Scholar] [CrossRef]
- Al-Qahtani, R.A.; Abdelnaby, M.M.; Abdulazeez, I.; Hamouz, O.C.S.A. Synthesis and Optimization of 3D Porous Polymers for Efficient CO2 Capture and H2 Storage. Carbon Capture Sci. Technol. 2024, 5, 100330. [Google Scholar] [CrossRef]
- Shen, Z.; Liang, S.; Chen, K.; Zhu, Y.; Wang, B. Analysis of Cyclic Thermodynamic System Combining Ammonia Gas Turbine and Transcritical Carbon Dioxide. Appl. Energy 2024, 376, 124238. [Google Scholar] [CrossRef]
- Liang, Y.; Ju, Y.; Lin, Q. An Integrated Solution of Energy Storage and CO2 Reduction: Trans-Critical CO2 Energy Storage System Combining Carbon Capture with LNG Cold Energy. J. Clean. Prod. 2024, 482, 144228. [Google Scholar] [CrossRef]
- Hou, L.; Elsworth, D.; Wang, J.; Zhou, J.; Zhang, F. Feasibility and Prospects of Symbiotic Storage of CO2 and H2 in Shale Reservoirs. Renew. Sustain. Energy Rev. 2023, 189, 113878. [Google Scholar] [CrossRef]
- Uddin, Z.; Yu, B.; Lee, H. Evaluation of Alternative Processes of CO2 Methanation: Design, Optimization, Control, Techno-Economic and Environmental Analysis. J. CO2 Util. 2022, 60, 101974. [Google Scholar] [CrossRef]
- Sayani, J.K.S.; Sivabalan, V.; Foo, K.S.; Pedapati, S.R.; Lal, B. Development of a Prediction Model for Gas Hydrate Formation in Multiphase Pipelines by Artificial Intelligence. Chem. Eng. Technol. 2022, 45, 1482–1490. [Google Scholar] [CrossRef]
- Otero-Lema, M.; González, P.; Sánchez, B.; Pereiro, A.B.; Rodríguez, H. On the Molecular Mechanisms of CO2 Uptake in Confined Ionic Liquids: A Computational Study. J. Mol. Liq. 2024, 405, 124909. [Google Scholar] [CrossRef]
- Mirzaei-Saatlo, M.; Asghari, E.; Shekaari, H.; Pollet, B.G.; Vinodh, R. Performance of Ethanolamine-Based Ionic Liquids as Novel Green Electrolytes for the Electrochemical Energy Storage Applications. Electrochim. Acta 2023, 474, 143499. [Google Scholar] [CrossRef]
- Gu, R.; Song, Z. Machine Learning-Aided Rational Screening of Task-Specific Ionic Liquids. In Artificial Intelligence in Process Systems Engineering; Wiley-VCH: Weinheim, Germany, 2024; Chapter 3; pp. 79–104. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, Y.; Wang, L.; Diao, A.; Peng, X. Optimal Design of the Piston Trajectory for the Ionic Liquid Compressor Applied in Hydrogen Storage. Int. J. Hydrogen Energy 2023, 56, 709–721. [Google Scholar] [CrossRef]
- Sun, J.; Sato, Y.; Sakai, Y.; Kansha, Y. A Review of Ionic Liquids and Deep Eutectic Solvents Design for CO2 Capture with Machine Learning. J. Clean. Prod. 2023, 414, 137695. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Liu, W.; Huang, R.; Ma, Y. Effects of Sn on the Electrochemistry and Discharge Performances of As-Extruded Mg-1.2Ga as an Anode for Mg-Air Batteries. Next Mater. 2024, 7, 100342. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Okoye, C.O.; Li, X.; Wang, Z.; Sun, Z. Advances on Lithium, Magnesium, Zinc, and Iron-Air Batteries as Energy Delivery Devices—A Critical Review. J. Solid State Electrochem. 2024, 28, 2999–3025. [Google Scholar] [CrossRef]
- Wang, T.; Yang, T.; Luo, D.; Fowler, M.; Yu, A.; Chen, Z. High-Energy-Density Solid-State Metal–Air Batteries: Progress, Challenges, and Perspectives. Small 2023, 20, 2309306. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Li, Z.; Gu, C.; Sun, X.; Liu, Y. Data-Driven Systematic Parameter Identification of an Electrochemical Model for Lithium-Ion Batteries with Artificial Intelligence. Energy Storage Mater. 2021, 44, 557–568. [Google Scholar] [CrossRef]
- Xu, L.; Lin, X.; Xie, Y.; Hu, X. Enabling High-Fidelity Electrochemical P2D Modeling of Lithium-Ion Batteries via Fast and Non-Destructive Parameter Identification. Energy Storage Mater. 2021, 45, 952–962. [Google Scholar] [CrossRef]
- Ćwik, J.; Baran, J.; Krawczyk, P.; Milewski, J.; Komorowski, P. Layered Composite Magnetic Refrigerants for Hydrogen Liquefaction. Int. J. Hydrogen Energy 2024, 87, 485–499. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Y.; Xia, X. Performance Investigations of the Easily Manufactured Composite All-Day Radiative Cooling Materials Based on PDMS. Mater. Lett. 2024, 357, 137743. [Google Scholar] [CrossRef]
- Bąk, A.; Mikuła, J.; Oliinyk, I.; Łach, M. Basic Research on Layered Geopolymer Composites with Insulating Materials of Natural Origin. Sci. Rep. 2024, 14, 9610. [Google Scholar] [CrossRef]
- Champa-Bujaico, E.; García-Díaz, P.; Díez-Pascual, A.M. Machine Learning for Property Prediction and Optimization of Polymeric Nanocomposites: A State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 10712. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-B.; Fan, K.; Huang, X.; Ge, J.; Liu, Y.; Kang, H. Recent Advances in Artificial Intelligence Boosting Materials Design for Electrochemical Energy Storage. Chem. Eng. J. 2024, 490, 151625. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Y.; Zhao, H.; Liu, M.; Chen, Z.; Li, J. Machine Learning Approaches in Polymer Science: Progress and Fundamental for a New Paradigm. SmartMat 2025, 6, 1320. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Zhou, Q.; Fang, Y.; Li, Y.; Zhang, S. Transcend the Boundaries: Machine Learning for Designing Polymeric Membrane Materials for Gas Separation. Chem. Phys. Rev. 2024, 5, 041301. [Google Scholar] [CrossRef]
- Orejuela-Escobar, L.M.; Venegas-Vásconez, D.; Méndez, M. Opportunities of Artificial Intelligence in Valorisation of Biodiversity, Biomass and Bioresidues—Towards Advanced Bio-Economy, Circular Engineering, and Sustainability. Int. J. Sustain. Energy Environ. Res. 2024, 13, 105–118. [Google Scholar] [CrossRef]
- Workie, E.; Tesfaye, T.; Zhou, Y.; Liu, G.; Wang, H. Advancing the Bioconversion Process of Food Waste into Methane: A Systematic Review. Waste Manag. 2022, 156, 187–199. [Google Scholar] [CrossRef]
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A Review on Metal Hydride Materials for Hydrogen Storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Dun, C.; Wang, X.; Chen, L.; Li, S.; Breunig, H.; Urban, J.J. Nano-Enhanced Solid-State Hydrogen Storage: Balancing Discovery and Pragmatism for Future Energy Solutions. Nano Res. 2024, 17, 8729–8742. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S.; Yang, Z. Hydrogen Adsorption on Metal-Organic Framework (MOF-5) Synthesized by DMF Approach. J. Porous Mater. 2008, 16, 141–148. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Gao, L.; Liu, Y.; Ding, Z. Advances and Prospects of Nanomaterials for Solid-State Hydrogen Storage. Nanomaterials 2024, 14, 1036. [Google Scholar] [CrossRef]
- Witman, M.; Ek, G.; Ling, S.; Chames, J.; Agarwal, S.; Wong, J.; Allendorf, M.D.; Sahlberg, M.; Stavila, V. Data-Driven Discovery and Synthesis of High Entropy Alloy Hydrides with Targeted Thermodynamic Stability. Chem. Mater. 2021, 33, 4067–4077. [Google Scholar] [CrossRef]
- Du, Z.; Liu, Y.; Chen, Y.; Sun, Y.; Li, H.; Xu, Q. Exploring Hydrogen Geologic Storage in China for Future Energy: Opportunities and Challenges. Renew. Sustain. Energy Rev. 2024, 196, 114366. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Y.; Wang, B.; Pan, Q.; Gan, Z. Perspective for the Safe and High-Efficiency Storage of Liquid Hydrogen: Thermal Behaviors and Insulation. Hydrogen 2024, 5, 559–573. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Yu, L.; Chen, J.; Lin, D.; Wu, Q. Modification Strategies of Magnesium-Based Materials Originating from Other Materials for Hydrogen Storage: A Review. Next Mater. 2024, 6, 100311. [Google Scholar] [CrossRef]
- Zabelin, D.; Wei, W.; Sun, C.; Zhang, H.; Zhang, D. Enhancing Hydrogen Storage Efficiency: Surface-Modified Boron Nanosheets Combined with IRMOF-20 for Safe and Selective Hydrogen Storage. Int. J. Hydrogen Energy 2024, 57, 1025–1038. [Google Scholar] [CrossRef]
- Šagud, M.; Vuksan-Ćusa, B.; Alfirević, A.; Vuksan, D.; Jakovljević, M. An Expert Review of Clozapine in Eastern European Countries: Use, Regulations and Pharmacovigilance. Schizophr. Res. 2023, 268, 53–61. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Li, Y.; Xu, C.; Gao, Y. Porous Framework Materials for Energy & Environment Relevant Applications: A Systematic Review. Green Energy Environ. 2023, 9, 217–233. [Google Scholar] [CrossRef]
- Qureshi, T.; Khan, M.M.; Pali, H.S. The Future of Hydrogen Economy: Role of High Entropy Alloys in Hydrogen Storage. J. Alloys Compd. 2024, 1004, 175668. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Chen, M.; Liu, X.; Wang, F. Evaluating the Techno-Economic Feasibility of Hydrogen-Fuelled Reciprocating Engines for Renewable Base-Load Power Generation. Energy Convers. Manag. 2024, 311, 118515. [Google Scholar] [CrossRef]
- Verma, A.; Wilson, N.; Joshi, K. Solid State Hydrogen Storage: Decoding the Path through Machine Learning. Int. J. Hydrogen Energy 2023, 50, 1518–1532. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, R.; Wu, Y.; Sun, C.; Lin, D. Machine Learning in Solid-State Hydrogen Storage Materials: Challenges and Perspectives. Adv. Mater. 2024, 37, 2413430. [Google Scholar] [CrossRef] [PubMed]
- Seyyedattar, M.; Zendehboudi, S.; Ghamartale, A.; Afshar, M. Advancing Hydrogen Storage Predictions in Metal-Organic Frameworks: A Comparative Study of LightGBM and Random Forest Models with Data Enhancement. Int. J. Hydrogen Energy 2024, 69, 158–172. [Google Scholar] [CrossRef]
- Evro, S.; Oni, B.A.; Tomomewo, O.S. Carbon Neutrality and Hydrogen Energy Systems. Int. J. Hydrogen Energy 2024, 78, 1449–1461. [Google Scholar] [CrossRef]
- Ugaddan, E.; Navarro, A.; Reyes, M.; del Rosario, J.; Santiago, A. AB5-Based Metal Hydride Embedded in Polyethylene and Polymethylmethacrylate for Hydrogen Storage. Int. J. Hydrogen Energy 2024, 78, 952–962. [Google Scholar] [CrossRef]
- Du, Y.; Yang, M.; Tang, L.; Huang, Y.; Zhang, Q. First-Principles Study of the Hydrogen Storage Properties of Hydride Perovskites XCuH3 (X = K, Rb) for Hydrogen Storage Applications. Int. J. Hydrogen Energy 2024, 78, 713–725. [Google Scholar] [CrossRef]
- Ahmed, B.; Tahir, M.B.; Sagir, M.; Parveen, A.; Abbas, Z.; Nassani, A.A. First-Principles Study of Ti-Based X2TiH5 (X = Mg, Ca, Sr) Hydrides for Advanced Hydrogen Storage Applications. Chem. Phys. 2024, 589, 112499. [Google Scholar] [CrossRef]
- Cortina, M.D.; Aramburu, M.R.d.T.; Neves, A.M.; Hurtado, L.; Jepsen, J.; Ulmer, U. The Integration of Thermal Energy Storage Within Metal Hydride Systems: A Comprehensive Review. Inorganics 2024, 12, 313. [Google Scholar] [CrossRef]
- Bárkányi, Á.; Tarcsay, B.L.; Lovas, L.; Mérő, T.; Chován, T.; Egedy, A. Future of Hydrogen Economy: Simulation-Based Comparison of LOHC Systems. Clean Technol. Environ. Policy 2023, 26, 1521–1536. [Google Scholar] [CrossRef]
- Rao, N.; Lele, A.K.; Patwardhan, A.W. Optimization of Liquid Organic Hydrogen Carrier (LOHC) Dehydrogenation System. Int. J. Hydrogen Energy 2022, 47, 28530–28545. [Google Scholar] [CrossRef]
- Wang, C. Research Progress of Hydrogen Storage Materials. Highl. Sci. Eng. Technol. 2024, 83, 121–128. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, Q.; Zhang, T.; Liu, H.; Wei, Q. Hydrogen Storage in MXenes: Controlled Adjustment of Sorption by Interlayer Distance and Transition Metal Elements. Int. J. Hydrogen Energy 2023, 50, 1555–1570. [Google Scholar] [CrossRef]
- Sun, S.; Wang, L.; Li, M.; Zhou, X.; Zhang, Y. Ammonia as Hydrogen Carrier: Advances in Ammonia Decomposition Catalysts for Promising Hydrogen Production. Renew. Sustain. Energy Rev. 2022, 169, 112918. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Shen, C.; Zhao, Y.; Huang, W. Hydrogen Used for Renewable Energy Storage: Techno-Economic Analysis of Different Technology Routes. In Springer Proceedings in Physics; Springer Nature: Singapore, 2024; p. 269. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, F.; Liu, X.; Tang, Z.; Lin, Q. High Capacity Ammonia Adsorption in a Robust Metal–Organic Framework Mediated by Reversible Host–Guest Interactions. Chem. Commun. 2022, 58, 5753–5756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, L.; Chen, R.; Huang, J.; Wang, T. Recent Advances in Methanol Steam Reforming Catalysts for Hydrogen Production. Catalysts 2025, 15, 36. [Google Scholar] [CrossRef]
- Huang, M.; Li, Z.; Du, W.; Zhou, Y.; Wang, C. Hydrogen Production via Steam Reforming of Methanol on Cu/ZnO/Al2O3 Catalysts: The Effect of TiO2 Addition Mode. New J. Chem. 2024, 48, 3276–3285. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Stoppato, A.; Prattico, L. Techno-Economic Analysis of Hydrogen Separation and Purification Technologies for Fuel Cell Application. Available online: https://thesis.unipd.it/retrieve/4d222abc-d171-420a-9211-161c361f0151/Rizzo_Michael.pdf (accessed on 10 April 2025).
- Abdin, Z.; Khalilpour, K.; Catchpole, K. Projecting the Levelized Cost of Large Scale Hydrogen Storage for Stationary Applications. Energy Convers. Manag. 2022, 270, 116241. [Google Scholar] [CrossRef]
- Tomme, L.; Bernard, L.; Camesasca, M. Machine Learning Applications for Thermochemical and Kinetic Property Prediction. Rev. Chem. Eng. 2024, 41, 419–449. [Google Scholar] [CrossRef]
- Ibrahim, A.; Veremieiev, S.; Gaskell, P.H. An Advanced, Comprehensive Thermochemical Equilibrium Model of a Downdraft Biomass Gasifier. Renew. Energy 2022, 194, 912–926. [Google Scholar] [CrossRef]
- Wang, H.; Hu, K.; Fan, W.; Zhang, M.; Xia, X.; Cai, J. Prediction and Mechanism of Underground Hydrogen Storage in Nanoporous Media: Coupling Molecular Simulation, Pore-Scale Simulation and Machine Learning. Int. J. Hydrogen Energy 2024, 101, 303–318. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Wulf, C.; Schonhoff, A.; Kuckshinrichs, W. Life Cycle Costing Approaches of Fuel Cell and Hydrogen Systems: A Literature Review. Int. J. Hydrogen Energy 2023, 54, 361–377. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Ivanov, A.V.; Petrov, N.I.; Frolov, A.I. Metal Hydride Hydrogen Storage (Compression) Units Operating at Near-Atmospheric Pressure of the Feed H2. Inorganics 2023, 11, 290. [Google Scholar] [CrossRef]
- Drawer, C.; Lange, J.; Kaltschmitt, M. Metal Hydrides for Hydrogen Storage—Identification and Evaluation of Stationary and Transportation Applications. J. Energy Storage 2023, 77, 109988. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Wu, F.; Jiang, Y.; Yu, X. Efficient Catalysis of FeNiCu-Based Multi-Site Alloys on Magnesium-Hydride for Solid-State Hydrogen Storage. Chin. Chem. Lett. 2024, 36, 109566. [Google Scholar] [CrossRef]
- Sharma, S.; Deshpande, R.; Khanna, N.; Gupta, S. Prediction of Thermochemical Properties of Long-Chain Alkanes Using Linear Regression: Application to Hydroisomerization. J. Phys. Chem. B 2024, 128, 9619–9628. [Google Scholar] [CrossRef]
- Papadias, D.D.; Ahluwalia, R. Bulk Storage of Hydrogen. Int. J. Hydrogen Energy 2021, 46, 34527–34541. [Google Scholar] [CrossRef]
- Maleki, M.; Dehghani, M.R.; Akbari, A.; Kazemzadeh, Y.; Ranjbar, A.M. Investigation of Wettability and IFT Alteration During Hydrogen Storage Using Machine Learning. Heliyon 2024, 10, e38679. [Google Scholar] [CrossRef]
- Dehghani, M.R.; Nikravesh, H.; Aghel, M.; Kafi, M.; Kazemzadeh, Y.; Ranjbar, A.A. Estimation of Hydrogen Solubility in Aqueous Solutions Using Machine Learning Techniques for Hydrogen Storage in Deep Saline Aquifers. Sci. Rep. 2024, 14, 17672. [Google Scholar] [CrossRef]
- Yang, G.; Kong, D.; He, X.; Yu, X.; Jiang, K. Prediction of Hydrogen Leakage Location and Intensity in Hydrogen Refueling Stations Based on Deep Learning. Int. J. Hydrogen Energy 2024, 68, 209–221. [Google Scholar] [CrossRef]
- Raza, A.; Mahmoud, M.; Arif, M.; Alafnan, S. Underground Hydrogen Storage Prospects in the Kingdom of Saudi Arabia. Fuel 2023, 357, 129665. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Furukawa, H.; Barnett, B.R.; Jiang, H.Z.H.; Long, J.R.; Breunig, H. Technoeconomic Analysis of Metal–Organic Frameworks for Bulk Hydrogen Transportation. Energy Environ. Sci. 2021, 14, 1083–1097. [Google Scholar] [CrossRef]
- Borja, N.K.; Fabros, C.J.E.; Doma, B.T. Prediction of Hydrogen Adsorption and Moduli of Metal–Organic Frameworks (MOFs) Using Machine Learning Strategies. Energies 2024, 17, 927. [Google Scholar] [CrossRef]
- Abdimomyn, S.; Malik, S.; Skakov, M.; Koyanbayev, Y.; Miniyazov, A.Z.; Malchik, F. Hydrogen Storage Materials: Promising Materials for Kazakhstan’s Hydrogen Storage Industry. Eurasian Chem.-Technol. J. 2024, 26, 113–123. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, M.; Shin, D.Y.; Kim, N.; Park, J.; Lee, J.H.; Oh, H.; Hong, C.S. Fine-Tuned MOF-74 Type Variants with Open Metal Sites for High Volumetric Hydrogen Storage at Near-Ambient Temperature. Chem. Eng. J. 2024, 489, 151500. [Google Scholar] [CrossRef]
- Peng, P.; Wang, Y.; Li, X.; Liu, L.; Xu, Y. Cost and Potential of Metal–Organic Frameworks for Hydrogen Back-Up Power Supply. Nat. Energy 2022, 7, 448–460. [Google Scholar] [CrossRef]
- Xiao, C.; Tian, J.; Chen, Q.; Hong, M. Water-Stable Metal–Organic Frameworks (MOFs): Rational Construction and Carbon Dioxide Capture. Chem. Sci. 2024, 15, 1570–1584. [Google Scholar] [CrossRef]
- Fathalian, F.; Aarabi, S.; Ghaemi, A.; Hemmati, A. Intelligent Prediction Models Based on Machine Learning for CO2 Capture Performance by Graphene Oxide-Based Adsorbents. Sci. Rep. 2022, 12, 9261–9272. [Google Scholar] [CrossRef]
- Nikzad, M.H.; Heidari-Rarani, M.; Navarchian, A.H. Multi-Objective Optimization and Machine Learning-Based Prediction of Tensile Properties of an Armchair Graphene Sheet. Diam. Relat. Mater. 2024, 144, 111014. [Google Scholar] [CrossRef]
- Verma, R.; Jaggi, N. A DFT Investigation of Osmium Decorated Single Walled Carbon Nanotubes for Hydrogen Storage. Int. J. Hydrogen Energy 2023, 54, 1507–1519. [Google Scholar] [CrossRef]
- Atakoohi, S.E.; Saidi, M.; Hossain, G.; Sharma, S.; Malhotra, P. Graphene-Based Material Supports for Ni- and Ru-Catalysts in CO2 Hydrogenation: Ruling Out Performances and Impurity Role. ChemSusChem 2024, 17, e202400993. [Google Scholar] [CrossRef]
- Bharadwaj, G.; Sharma, K.; Pandey, A.K.; Gupta, A. Carbon Nanotube–Graphene-Based Nanofluids: A Comprehensive Review on the Role of Thermal Conductivity and Its Solar Energy Applications. J. Therm. Anal. Calorim. 2024, 149, 1859–1872. [Google Scholar] [CrossRef]
- Fan, C.; Gong, H.; Zhang, Y.; Ma, W.; Yu, Q. Fast Dynamic Prediction of Consequences of Heavy Gas Leakage Accidents Based on Machine Learning. Front. Environ. Sci. 2024, 12, 1409072. [Google Scholar] [CrossRef]
- Lyu, S.; Qi, Q.; Huang, X.; Peng, S.; Yang, D.; Chen, L. Machine Learning-Based Method for Gas Leakage Source Term Estimation in Highway Tunnels. Tunneling Undergr. Space Technol. 2024, 154, 106114. [Google Scholar] [CrossRef]
- Attallah, O. Multitask Deep Learning-Based Pipeline for Gas Leakage Detection via E-Nose and Thermal Imaging Multimodal Fusion. Chemosensors 2023, 11, 364. [Google Scholar] [CrossRef]
- Barkani, M.E.; Benamar, N.; Talei, H.; Bagaa, M. Gas Leakage Detection Using Tiny Machine Learning. Electronics 2024, 13, 4768. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Liu, Y.; Zhao, C.; Zhang, Z. A Leakage Detection Method for Hydrogen-Blended Natural Gas Pipelines in Utility Tunnels Based on Multi-Task LSTM and CFD Simulation. Int. J. Hydrogen Energy 2024, 97, 1335–1345. [Google Scholar] [CrossRef]
- Halder, P.; Mishra, S.; Ghosh, R. Advancements in Hydrogen Production, Storage, Distribution and Refuelling for a Sustainable Transport Sector: Hydrogen Fuel Cell Vehicles. Int. J. Hydrogen Energy 2023, 52, 973–983. [Google Scholar] [CrossRef]
- Tatar, A.; Esmaeili-Jaghdan, Z.; Shokrollahi, A.; Zeinijahromi, A. Hydrogen Solubility in n-Alkanes: Data Mining and Modelling with Machine Learning Approach. Int. J. Hydrogen Energy 2022, 47, 35999–36009. [Google Scholar] [CrossRef]
- Gorji, A.E.; Alopaeus, V. Prediction of Solubility of Hydrogen (H2) in Hydrocarbons Using QSPR Method: MLR Data-Driven as a Simple Machine Learning (ML) Algorithm. Int. J. Hydrogen Energy 2024, 90, 803–812. [Google Scholar] [CrossRef]
- Ali, A.; Khan, M.A.; Choi, H. Supervised Machine Learning-Based Prediction of Hydrogen Storage Classes Utilizing Dibenzyltoluene as an Organic Carrier. Molecules 2024, 29, 1280. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Y.; Gao, B.; Wu, X.; Zhao, L.; Li, X. High-Efficiency Ammonia-Fueled Hybrid Power Generation System Combining Ammonia Decomposition, Proton Exchange Membrane Fuel Cell and Micro Gas Turbine: A Thermodynamic Model and Performance Optimization. Energy Convers. Manag. 2025, 325, 119358. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Xie, Z.; Li, Z.; Shen, L.; Zhang, X. Energy-Efficient and Cost-Effective Ammonia Electrolysis for Converting Ammonia to Green Hydrogen. Cell Rep. Phys. Sci. 2024, 5, 102171. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, X.; Xie, J.; Li, F.; Yao, J.; Chen, Z. A Perspective to Ammonia-Hydrogen Energy Industry in China. Sci. China Chem. 2024, 67, 1765–1775. [Google Scholar] [CrossRef]
- Wessels, P.; Müller, J.; Strobl, A.; Appel, J.; Lönneke, P.; Scherf, U. Beyond Hydrolysis: Scalable, On-Demand Dihydrogen Release from NaBH4 Enables Circular and Sustainable Process Design. Sustain. Circ. NOW 2024, 2, 2332. [Google Scholar] [CrossRef]
- Thakur, V.; Kumar, P.; Sharma, S.; Ahir, P.; Thakur, A.; Kumar, S. Advanced Techno-Economic Assessment Methods of Green Hydrogen Storage Processes. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2024; p. 249. [Google Scholar] [CrossRef]
- Dragan, M. Hydrogen Storage in Complex Metal Hydrides NaBH4: Hydrolysis Reaction and Experimental Strategies. Catalysts 2022, 12, 356. [Google Scholar] [CrossRef]
- Peng, X.; Wang, L.; Li, C.; Zheng, M.; Chen, Y.; Shi, X. Machine Learning-Based Screening of Organic Frameworks for Separation of CF4/N2, C2F6/N2, and SF6/N2. Chem. Eng. Sci. 2024, 296, 120280. [Google Scholar] [CrossRef]
- Wahab, M.A.; Khan, M.; Laskar, M.; Ghosh, S.; Dhakate, S.; Chaudhuri, M. Advancing Catalysts by Nanoconfinement and Catalysis for Enhanced Hydrogen Production from Magnesium Borohydride: A Review. Chem. Asian J. 2024, 19, 1127–1143. [Google Scholar] [CrossRef]
- Olalde-López, D.; Rodríguez-Kessler, P.L.; Rodríguez-Carrera, S.; Muñoz-Castro, A. Hydrogen Storage Properties for Bimetallic Doped Boron Clusters M2B7 (M = Fe, Co, Ni). Int. J. Hydrogen Energy 2024, 49, 1678–1691. [Google Scholar] [CrossRef]
- Jason, J.I.; Wang, Z.; Tian, Y.; Zhang, C.; Huang, X.; Zhang, S. Defects Induced Metallized Boron Hydride Monolayers as High-Performance Hydrogen Storage Architecture. Int. J. Hydrogen Energy 2023, 50, 455–467. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, N.; Hillmansen, S.; Roberts, C.; Yan, Y. Techno-Economic Analysis of Hydrogen Storage Technologies for Railway Engineering: A Review. Energies 2022, 15, 6467. [Google Scholar] [CrossRef]
- Pratthana, C.; Aguey-Zinsou, K. Surfactant Induced Synthesis of LiAlH4 and NaAlH4 Nanoparticles for Hydrogen Storage. Appl. Sci. 2022, 12, 4742. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, T.; Qian, Q.; He, C.; Ren, J. Protocol for the Design and Accelerated Optimization of a Waste-to-Energy System Using AI Tools. STAR Protoc. 2023, 4, 102685. [Google Scholar] [CrossRef] [PubMed]
- Forootan, M.M.; Ahmadi, A. Machine Learning-Based Optimization and 4E Analysis of Renewable-Based Polygeneration System by Integration of GT-SRC-ORC-SOFC-PEME-MED-RO Using Multi-Objective Grey Wolf Optimization Algorithm and Neural Networks. Renew. Sustain. Energy Rev. 2024, 200, 114616. [Google Scholar] [CrossRef]
- Rakić, E.; Grilc, M.; Likozar, B. Liquid Organic Hydrogen Carrier Hydrogenation–Dehydrogenation: From Ab Initio Catalysis to Reaction Micro-Kinetics Modelling. Chem. Eng. J. 2023, 472, 144836. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Liu, M.; Liu, J.; Liu, Q.; Zhang, W. Ionic Liquid Binary Mixtures: Machine Learning-Assisted Modeling, Solvent Tailoring, Process Design, and Optimization. AIChE J. 2024, 70, 18392. [Google Scholar] [CrossRef]
- Dhakal, P.; Shah, J.K. A Generalized Machine Learning Model for Predicting Ionic Conductivity of Ionic Liquids. Molecular Syst. Des. Eng. 2022, 7, 1344–1358. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Li, W.; Zhao, X.; Yang, X. Machine Learning-Assisted Screening of Efficient Ionic Liquids for Catalyzing CO2 Cycloaddition Reaction. Mol. Catal. 2024, 569, 114630. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Wang, H.; Yang, J.; Liu, C.; Zhang, Y. An Ionic Liquid Supramolecular Gel Electrolyte with Unique Wide Operating Temperature Range Properties for Zinc-Ion Batteries. Polymers 2024, 16, 1680. [Google Scholar] [CrossRef]
- Pereira, J.; de Souza, R.R.; Moita, A. A Review of Ionic Liquids and Their Composites with Nanoparticles for Electrochemical Applications. Inorganics 2024, 12, 186. [Google Scholar] [CrossRef]
- Mokhtarpour, M.; Rostami, A.; Shekaari, H.; Zarghami, A.; Faraji, S. Protic Ionic Liquids Mono, Di, Triethanolamine Laurate as Green Phase Change Materials: Thermal Energy Storage Capacity and Conversion to Electricity. J. Therm. Anal. Calorim. 2024, 149, 7169–7180. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Farooq, A.; Martis, R.; Al-Othman, A. Optimization Techniques for Electrochemical Devices for Hydrogen Production and Energy Storage Applications. Int. J. Hydrogen Energy 2023, 52, 1058–1070. [Google Scholar] [CrossRef]
- Khan, F.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. A Computational Analysis of Effects of Electrode Thickness on the Energy Density of Lithium-Ion Batteries. Energy 2023, 288, 129774. [Google Scholar] [CrossRef]
- Park, J.; Lee, J. Electrochemical Energy Conversion and Storage Processes with Machine Learning. Trends Chem. 2024, 6, 302–314. [Google Scholar] [CrossRef]
- Adamu, H.; Abba, S.I.; Anyin, P.B.; Sani, Y.; Qamar, M. Artificial Intelligence-Navigated Development of High-Performance Electrochemical Energy Storage Systems through Feature Engineering of Multiple Descriptor Families of Materials. Energy Adv. 2023, 2, 615–627. [Google Scholar] [CrossRef]
- Li, S.; Gao, H.O.; You, F. AI for Science in Electrochemical Energy Storage: A Multi-Scale Systems Perspective on Transportation Electrification. Deleted J. 2024, 1, 100026. [Google Scholar] [CrossRef]
- Goussian, A.; Assaud, L.; Baghdadi, I.; Nouillant, C.; Franger, S. A Novel Reaction Rate Parametrization Method for Lithium-Ion Battery Electrochemical Modelling. Batteries 2024, 10, 205. [Google Scholar] [CrossRef]
- Akhmetkhanov, R.S. Features of Distributions of Local Elastic Strains in Single-Layer Composite Materials. J. Mach. Manuf. Reliab. 2024, 53, 1048–1060. [Google Scholar] [CrossRef]
- Yang, W.; Ye, Y.; Cheng, H.; Liu, J.; Yan, K.; Miao, H. Performance Optimization of a U-Tube Heat Exchanger Type Hydrogen Storage Reactor with a Novel Fin Structure. Int. J. Hydrogen Energy 2024, 82, 272–284. [Google Scholar] [CrossRef]
- Yin, L.; Yang, H.; Ju, Y. Review on the Key Technologies and Future Development of Insulation Structure for Liquid Hydrogen Storage Tanks. Int. J. Hydrogen Energy 2024, 57, 1302–1313. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, S.; Wei, J.; Gao, Y.; Zhu, Y.; Wang, H. Materials Selection, Design, and Regulation of Polymer-Based Hydrogen Barrier Composite Coatings, Membranes and Films for Effective Hydrogen Storage and Transportation: A Comprehensive Review. Int. J. Hydrogen Energy 2024, 91, 555–568. [Google Scholar] [CrossRef]
- Vieira, A.F.C.; Filho, M.R.T.; Eguea, J.P.; Ribeiro, M.L. Optimization of Structures and Composite Materials: A Brief Review. Eng 2024, 5, 3192–3211. [Google Scholar] [CrossRef]
- Jana, S.; Parthiban, A.; Rusli, W. Polymer Materials Innovations for Green Hydrogen Economy. Chem. Commun. 2025, 61, 3233–3249. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, L.; Yang, W.; Hou, J.; Liao, Z. Optimization of H2O2 Production in Biological Systems for Design of Bio-Fenton Reactors. Microorganisms 2024, 12, 1488. [Google Scholar] [CrossRef] [PubMed]
- Joshua, S.R.; Yeon, A.N.; Park, S.; Kwon, K.-H. Solar–Hydrogen Storage System: Architecture and Integration Design of University Energy Management Systems. Appl. Sci. 2024, 14, 4376. [Google Scholar] [CrossRef]
- Haq, Z.; Ullah, H.; Khan, M.N.A.; Naqvi, S.R.; Ahsan, M. Hydrogen Production Optimization from Sewage Sludge Supercritical Gasification Process Using Machine Learning Methods Integrated with Genetic Algorithm. Process Saf. Environ. Prot. 2022, 184, 614–624. [Google Scholar] [CrossRef]
- Su, S.; Yan, X.; Agbossou, K.; Chahine, R.; Zong, Y. Artificial Intelligence for Hydrogen-Based Hybrid Renewable Energy Systems: A Review with Case Study. J. Phys. Conf. Ser. 2022, 2208, 12013. [Google Scholar] [CrossRef]
- Patil, R.R.; Calay, R.K.; Mustafa, M.Y.; Thakur, S. Artificial Intelligence-Driven Innovations in Hydrogen Safety. Hydrogen 2024, 5, 312–326. [Google Scholar] [CrossRef]
- Chin, S.X.; Zhang, Y.; Zhou, Y.; Jiang, J.; Li, X.; Yang, S. Advancements and Challenges in Sodium Borohydride Hydrogen Storage: A Comprehensive Review of Hydrolysis, Regeneration, and Recycling Technologies. J. Renew. Sustain. Energy 2025, 17, 169–181. [Google Scholar] [CrossRef]
- Cava, C.; Gagliardi, G.G.; Piscolla, E.; Borello, D. Techno-Economic Analysis of Hydrogen Transport via Truck Using Liquid Organic Hydrogen Carriers. Processes 2025, 13, 1081. [Google Scholar] [CrossRef]
- Anand, C.; Singh, G.; Kaur, P.; Tripathi, M.; Sharma, S. Green Hydrogen for a Sustainable Future: A Review of Production Methods, Innovations, and Applications. Int. J. Hydrogen Energy 2025, 111, 319–332. [Google Scholar] [CrossRef]
- Maleki, F.; Ledari, M.B.; Fani, M. Integrated Analysis of a Hydrogen-Based Port: Energy, Exergy, Environmental, and Economic Sustainability. Int. J. Hydrogen Energy 2024, 100, 1402–1414. [Google Scholar] [CrossRef]
- Hong, H.; Guo, H.; Cui, Z.; Ball, A.; Nie, B. Structure Modification of Magnesium Hydride for Solid Hydrogen Storage. Int. J. Hydrogen Energy 2024, 78, 793–802. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Hao, Y.; Wu, P.; Ding, Z. Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications. Molecules 2024, 29, 2525. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yang, W.; Chen, X.; Zhang, Y.; Peng, Q. Rapid Hydrogen Energy Storage of Self-Supporting VS2/NC Modified MgH2. Energy 2024, 314, 134284. [Google Scholar] [CrossRef]
- Padamurthy, A.; Gandla, P.K.; Sheelwant, A.; Vemanaboina, H.; Paramasivam, P.; Ayanie, A.G. Emerging Trends and Future Prospects of Thermochemical Energy Storage Systems for Building Space and Water Heating Applications. Int. J. Energy Res. 2025, 1, 6685290. [Google Scholar] [CrossRef]
- Chilunda, M.D.; Talipov, S.A.; Farooq, H.; Biddinger, E.J. Electrochemical Cycling of Liquid Organic Hydrogen Carriers as a Sustainable Approach for Hydrogen Storage and Transportation. ACS Sustainable Chem. Eng. 2025, 1, 102685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Mao, S.; Mehana, M. Techno-Economic Analysis and Site Screening for Underground Hydrogen Storage in Intermountain-West Region, United States. Int. J. Hydrogen Energy 2025, 109, 275–289. [Google Scholar] [CrossRef]
- Thiyagarajan, S.R.; Emadi, H.; Hussain, A.; Patange, P.; Watson, M. A Comprehensive Review of the Mechanisms and Efficiency of Underground Hydrogen Storage. J. Energy Storage 2022, 51, 104490. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mandal, R.; Shankar, A.; Tyagi, A.; Arora, M.; Kumar, R. Make Metal–Organic Frameworks Safe and Sustainable by Design for Industrial Translation. Nat. Rev. Mater. 2025, 10, 167–169. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, L.; Xu, H.; He, X. Metal–Organic Framework-Assisted Atmospheric Water Harvesting Enables Cheap Clean Water Available in an Arid Climate: A Perspective. Materials 2025, 18, 379. [Google Scholar] [CrossRef]
- Cheng, Y.; Datta, S.J.; Zhou, S.; Jia, J.; Shekhah, O.; Eddaoudi, M. Advances in Metal–Organic Framework-Based Membranes. Chem. Soc. Rev. 2022, 51, 8300–8312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, C.; Yan, S.; Xu, X.; Yang, J.; Zhang, W. Synergistic Enhancement of Mechanical and Thermal Properties in Basalt Fiber Reinforced Composites through Nanotube and Graphene Bridging Structure: A Multi-Scale Simulation. Compos. Part B Eng. 2025, 112289. [Google Scholar] [CrossRef]
- Baidak, V.A.; Zavidovskiy, I.A.; Tatarintsev, A.A.; Bychkov, V.L.; Streletskiy, O.A. Energy-Effective Synthesis of Multiwalled Carbon Nanotubes via Ambient-Air Atmospheric-Pressure Plasma Jet Treatment of Graphite. Surfaces 2025, 8, 16. [Google Scholar] [CrossRef]
- Filho, V.; de Azevedo, A.C.S.; de Oliveira, R.; Bonfim, R.L.P.; da Silva Amaral, A.K.; da Cunha, R.B.; Agrawal, P.; Cunha, C.T.C.; Brito, G.; Jeferson, T. Properties of Recycled ABS and HIPS Polymers From WEEE and Their Blends With Virgin ABS Prepared by 3D Printing and Compression Molding. J. Appl. Polym. Sci. 2025, 142, e56797. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Z.; Wang, K.; Hou, Y.; Nie, B.; He, Q. Performance Analysis of a Novel Adsorption Type Carbon Dioxide Energy Storage System with High Energy Density and High Efficiency. J. Energy Storage 2025, 107, 115004. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, Y.; Xu, W.; Wang, Y.; Liu, Y.; Wang, Y.; Cui, P.; Yang, S.; Wang, B.; Zhao, X. Comprehensive Assessment of Carbon Footprint and Water Footprint of Ammonia Synthetic Process Based on Liquefied Natural Gas Cold Energy Utilization. Fuel 2025, 390, 134678. [Google Scholar] [CrossRef]
- Beber, V.C.; Abels, G.; Hesebeck, O. Material Selection of Tanks for Storage and Transport of Liquid Organic Hydrogen Carriers: A Lightweight and Lifecycle Assessment Comparative Study of Metal, Polymer, and Composite Alternatives. Energy Technol. 2024, 13, 1521–1536. [Google Scholar] [CrossRef]
- Corte, D.L.; Neves, M.F.; Ribeiro, C.J.; Torres, R.A.; Vicente, M.R. Recovered Ammonia as a Sustainable Energy Carrier: Innovations in Recovery, Combustion, and Fuel Cells. Energies 2025, 18, 508. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. Life cycle assessment of renewable hydrogen transport by ammonia. Int. J. Hydrogen Energy 2024, 94, 1018–1035. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Wang, X.; Tan, L.; Li, Q. Recent Advances on Non-Noble Metal Catalysts toward N-Ethylcarbazole Hydrogen Storage. J. Mater. Chem. A 2024, 13, 20–35. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Yang, J.; Li, Y.; Han, Y.; Yin, M. Performance Analysis and Multi-Objective Optimization of CO2 Transcritical Rankine Cycle Systems Driven by Solar Energy. Sol. Energy 2024, 286, 113179. [Google Scholar] [CrossRef]
- Salas, R.; Sefat, A.; Tuck, K.; Zhang, M.; Scharff, P. Ionic Liquids in Polymer Technology. Green Chem. 2024, 27, 1620–1651. [Google Scholar] [CrossRef]
- Kaur, H.; Thakur, A.; Thakur, R.C.; Kumar, A. A Review on Multifaceted Role of Ionic Liquids in Modern Energy Storage Systems: From Electrochemical Performance to Environmental Sustainability. Energy Fuels 2025, 39, 1123–1138. [Google Scholar] [CrossRef]
- Dash, M.; Dubey, A.K.; Choudhary, T.; Liu, Y.; Nanda, H.S.; Pati, S. Critical Metal Extraction from Spent Battery Cathodes and Anticipated Developments Using Next Generation Green Solvents for Achieving a Net-Zero Future. Chem. Eng. J. 2025, 507, 160324. [Google Scholar] [CrossRef]
- Fang, Z.; Zhu, P.; Zhang, X.; Feng, Y.; Wang, H. Self-Looped Electrochemical Recycling of Lithium-Ion Battery Cathode Materials to Manufacturing Feedstocks. Nat. Chem. Eng. 2025, 2, 142–151. [Google Scholar] [CrossRef]
- Pi, Y.; Zhang, L.; Hu, X.; Liu, S.; Hu, Q. Sustainable Recycling of Na3V2(PO4)3 Cathodes: A Pathway to High-Safety Na-Ni Dual-Ion Batteries for Scalable Energy Storage. J. Energy Storage 2025, 113, 115593. [Google Scholar] [CrossRef]
- Vogiantzi, C.; Tserpes, K. A Comparative Environmental and Economic Analysis of Carbon Fiber-Reinforced Polymer Recycling Processes Using Life Cycle Assessment and Life Cycle Costing. J. Compos. Sci. 2025, 9, 39. [Google Scholar] [CrossRef]
- Tong, J.; Liu, Z.; Liu, Y.; Huang, X.; Wang, G. ‘Back-to-Back’ Radial Layered Skeleton Converging Heat Flow to Assist in Thermal Conduction of Aramid Nanofibers/Graphene Phase Change Composite Materials. Adv. Funct. Mater. 2024, 35, 2411744. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, G.; Lai, J.; Yu, T.; Zhang, X. Interlaminar Toughening and Self-Healing Mechanism for Hard-and-Soft Layered Composite Laminates. Compos. Part A Appl. Sci. Manuf. 2024, 189, 108623. [Google Scholar] [CrossRef]
- Vilcáez, J.; Chowdhury, E. Biogenic Hydrogen Production from Oil Hydrocarbons at Geological Carbon Storage Conditions. Energy Convers. Manag. 2024, 325, 119438. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, Y.; Xu, H.; He, X. Hydrogen storage mechanism of metal–organic framework materials based on metal centers and organic ligands. Carbon Neutrality 2023, 2, 91. [Google Scholar] [CrossRef]
- Osman, A.I.; Nasr, M.; Mohamed, A.R.; Abdelhaleem, A.; Ayati, A.; Farghali, M.; Al-Muhtaseb, A.H.; Al-Fatesh, A.S.; Rooney, D.W. Life cycle assessment of hydrogen production, storage, and utilization toward sustainability. WIREs. Energy Environ. 2024, 13, e526. [Google Scholar] [CrossRef]
- Maghsoudy, S.; Zakerabbasi, P.; Baghban, A.; Esmaeili, A.; Habibzadeh, S. Connectionist Technique Estimates of Hydrogen Storage Capacity on Metal Hydrides Using Hybrid GAPSO-LSSVM Approach. Sci. Rep. 2024, 14, 52086–52098. [Google Scholar] [CrossRef]
- Meduri, S.; Nandanavanam, J. Prediction of Hydrogen Uptake of Metal Organic Frameworks Using Explainable Machine Learning. Energy AI 2023, 12, 100230. [Google Scholar] [CrossRef]
- Salehi, K.; Rahmani, M.; Atashrouz, S. Machine Learning Assisted Predictions for Hydrogen Storage in Metal-Organic Frameworks. Int. J. Hydrogen Energy 2023, 48, 33260–33275. [Google Scholar] [CrossRef]
- Sunshine, E.M.; Gupta, R.; Gao, M.; Smith, C.; Fu, J. Multiscale Optimization of Formic Acid Dehydrogenation Process via Linear Model Decision Tree Surrogates. Comput. Chem. Eng. 2024, 194, 108921. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Z.; Sundmacher, K. A New Machine Learning Framework for Efficient MOF Discovery: Application to Hydrogen Storage. Comput. Aided Chem. Eng. 2022, 49, 1807–1812. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Das, P.; Ram, S.; Lee, S. A Semi-Supervised Machine Learning Framework for Predicting Hydrogen Storage Capacities in Metal Hydrides. arXiv 2024, arXiv:2401.17587. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Z.-C. Deep Reinforcement Learning for Optimizing the Thermoacoustic Core in a Supercritical CO2 Thermoacoustic Engine. Energy 2025, 325, 135950. [Google Scholar] [CrossRef]
- Nguyen, K.H.N.; Rivas, A.; Delipei, G.; Hou, J. Reinforcement Learning-Based Control Sequence Optimization for Advanced Reactors. J. Nucl. Eng. 2024, 5, 209–225. [Google Scholar] [CrossRef]
- Ortiz, C.; García, S.; Carro, A.; Carvajal, E.; Chacartegui, R. Techno-Economic Analysis of a Modular Thermochemical Battery for Electricity Storage Based on Calcium-Looping. Appl. Energy 2024, 367, 123366. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, Y.; Yang, F.; Wu, L.; Xie, S.; Jiang, H. New Framework of Low-Carbon City Development of China: Underground Space Based Integrated Energy Systems. Undergr. Space 2023, 14, 300–311. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Liu, J.; Yang, W.; Fan, M.; Zhao, X. Transient Flow Characteristics for Fluid-Structure Interaction on Hydrogen Decompression Valve in High-Pressure Hydrogen Storage Systems. Int. J. Hydrogen Energy 2024, 79, 1250–1262. [Google Scholar] [CrossRef]
- Kong, H.; Tan, L.; Shen, G.; Zeng, Z.; Xu, J. Vanadium-Based Alloy for Hydrogen Storage: A Review. Rare Met. 2024, 43, 6201–6211. [Google Scholar] [CrossRef]
- Park, H.; Oh, J.; Nguyen, T.-B.; Lee, J. Hydrogen Storage and Release Characteristics of Polycyclic Aromatic By-Products for LOHC Systems. Appl. Catal. A Gen. 2022, 636, 118583. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, T.; Qian, Q.; He, C.; Ren, J. Comparative Thermodynamic Study of CO2 Transcritical Supermarket Booster Refrigeration System Combined with Vapor Injection and Parallel Compression. Int. J. Refrig. 2024, 170, 70–85. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Liu, S.; Sun, Y.; Huang, J.; Xu, Y. A Unique Amorphous Porous BiSbOx Nanotube with Abundant Unsaturated Sb-Stabilized BiO₈−ₓ Sites for Efficient CO2 Electroreduction in a Wide Potential Window. Adv. Funct. Mater. 2024, 34, 202402220. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, W.; Liu, X.; Zhang, J.; Yang, S.; He, L. Magnesium Nickel Hydride Monocrystalline Nanoparticles for Reversible Hydrogen Storage. Mater. Rep. Energy 2023, 4, 100246. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sadiq, M.; Arora, R.; Ramachandran, A.; Shankar, A. A Review on Biohydrogen Sources, Production Routes, and Its Application as a Fuel Cell. Sustainability 2023, 15, 12641. [Google Scholar] [CrossRef]
- McQueen, S.; Walters, L.; Allen, H.; Berriman, S. Department of Energy Hydrogen Program Plan; US Department of Energy (USDOE): Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- Ratoi, A.; Munteanu, C.; Eliezer, D. The Potential of Polymers and Glass to Enhance Hydrogen Storage Capacity: A Mathematical Approach. Materials 2024, 17, 6065. [Google Scholar] [CrossRef]
- Manzoor, S.; Thomas, M.; Fazio, A.; Mohiuddin, A.; Lufkin, T. Exploring Nanomaterials for Hydrogen Storage: Advances, Challenges, and Perspectives. Chem.-Asian J. 2024, 19, 202400365. [Google Scholar] [CrossRef]
- Othman, W.; Falasi, W.A.; Hussain, T.; Tit, N. Light-Metal Functionalized Boron Monoxide Monolayers as Efficient Hydrogen Storage Material: Insights from DFT Simulations. J. Energy Storage 2024, 98, 113014. [Google Scholar] [CrossRef]
- Hauch, A.; Jensen, S.H.; Ramousse, S.; Mogensen, M.B. Performance and Durability of Solid Oxide Electrolysis Cells. J. Electrochem. Soc. 2006, 153, A221–A227. [Google Scholar] [CrossRef]
- Cong, S.; Lee, J.; Xu, K.; Zhang, Y.; Wong, S.; Li, M. Designing Metal–Organic Framework (MOF) Membranes for Isomer Separation. Angew. Chem. 2024, 136, 202319894. [Google Scholar] [CrossRef]
- Simanullang, M.; Prost, L. Nanomaterials for On-Board Solid-State Hydrogen Storage Applications. Int. J. Hydrogen Energy 2022, 47, 29808–29817. [Google Scholar] [CrossRef]
- Wang, X.; Peng, P.; Witman, M.; Stavila, V.; Allendorf, M.D.; Breunig, H. Technoeconomic Insights into Metal Hydrides for Stationary Hydrogen Storage. Adv. Sci. 2025, 10, 22415736. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Jiang, H.Z.H.; Collins, S.; Furukawa, H.; Long, J.R.; Breunig, H. Long Duration Energy Storage Using Hydrogen in Metal–Organic Frameworks: Opportunities and Challenges. ACS Energy Lett. 2024, 9, 2727–2736. [Google Scholar] [CrossRef]
- Park, J.; Ha, J.; Moon, H.R. Synthesis of Single-Crystalline Core-Shell Metal-Organic Frameworks. J. Vis. Exp. 2023, 192, 64978. [Google Scholar] [CrossRef]
- Zelenka, T.; Dvořák, P.; Zou, Y.; Zhang, P.; Castro, M. The Influence of HKUST-1 and MOF-76 Hand Grinding/Mechanical Activation on Stability, Particle Size, Textural Properties and Carbon Dioxide Sorption. Sci. Rep. 2024, 14, 66432. [Google Scholar] [CrossRef]
- Openshaw, D.; Lang, W.T.; Goldstone, L.; Wildsmith, J.; Freeman, B.; Bagnato, G. Hydrogen Storage Methods by Lithium Borohydride. Int. J. Hydrogen Energy 2024, 69, 1188–1200. [Google Scholar] [CrossRef]
- Noring, A.; Buchheit, K.; Iyengar, A.; Hackett, G. Techno-Economic Analysis of Reversible and Paired Solid Oxide Cell Systems for Hydrogen Production. ECS Trans. 2023, 111, 2445–2458. [Google Scholar] [CrossRef]
- Kiang, Y. Solid Oxide Fuel Cell (SOFC) Module Pricing. Unpublished Work. 2025. [Google Scholar] [CrossRef]
- Boretti, A. Technology Readiness Level of Hydrogen Storage Technologies for Transport. Energy Storage 2024, 6, e546. [Google Scholar] [CrossRef]
- Ciocarlan, R.G.; Farrando-Perez, J.; Arenas-Esteban, D.; Houlleberghs, M.; Daemen, L.L.; Cheng, Y.; Ramirez-Cuesta, A.J.; Breynaert, E.; Martens, J.; Bals, S.; et al. Tuneable Mesoporous Silica Material for Hydrogen Storage Application via Nano-Confined Clathrate Hydrate Construction. Nat. Commun. 2024, 15, 8697. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, M.K.; Singh, T.; Kalita, P.; Dewan, A. Sustainable Hydrogen Generation and Storage—A Review. RSC Adv. 2023, 13, 25253–25267. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, H.F.; Wang, Q.; Zheng, Y.; Li, Q. Anti-Hydrogen Embrittlement Aluminum Coating Electrodeposited from Ionic Liquids. Mater. Today Commun. 2025, 44, 111877. [Google Scholar] [CrossRef]
- Mellouli, S.; Askri, F.; Alqahtani, T.; Algarni, S.; Zribi, S. Numerical Assessment of a Thermal Energy Storage System Based on a Metal Hydride Reactor and a Mechanical Hydrogen Compressor. Appl. Therm. Eng. 2024, 243, 122670. [Google Scholar] [CrossRef]
- Sgaramella, A.; Pastore, L.M.; Basso, G.L.; Mojtahed, A.; de Santoli, L. HCNG Refuelling Station to Accelerate the Transition Towards a Real Hydrogen Economy: A Techno-Economic Analysis. Int. J. Hydrogen Energy 2024, 69, 1403–1414. [Google Scholar] [CrossRef]
- Patel, S.; Wilson, I.H.; Sreenivasan, H.; Krishna, S. Numerical Simulations of Proppant Transportation in Cryogenic Fluids: Implications on Liquid Helium and Liquid Nitrogen Fracturing for Subsurface Hydrogen Storage. Int. J. Hydrogen Energy 2024, 56, 924–930. [Google Scholar] [CrossRef]
- Lee, K.; Ray, J.; Safta, C. Predictive Skill of Deep Learning Models Trained on Limited Sequence Data; Sandia National Lab. (SNL-CA): Livermore, CA, USA, 2020. [Google Scholar] [CrossRef]
- Emrani, A.; Berrada, A. A Comprehensive Review on Techno-Economic Assessment of Hybrid Energy Storage Systems Integrated with Renewable Energy. J. Energy Storage 2024, 84, 111010. [Google Scholar] [CrossRef]
- Li, K.; Wen, J.; Wang, S. Insulation and Cost Optimization of Vapor-Cooled Shield Coupled with Para-to-Ortho Hydrogen Conversion Based on Self-Pressurization Model of Liquid Hydrogen Tank and NSGA-II. J. Energy Storage 2024, 99, 113229. [Google Scholar] [CrossRef]
- Feng, X.; Liu, J.; Shi, J.; Hu, P.; Zhang, T.; Sun, S. Phase Equilibrium, Thermodynamics, Hydrogen-Induced Effects and the Interplay Mechanisms in Underground Hydrogen Storage. Comput. Energy Sci. 2024, 1, 46–56. [Google Scholar] [CrossRef]
- Kwon, H.; Park, J.; Koo, B. 4E Analysis for a Novel Cryogenic Hydrogen Liquefaction Process Using Various Refrigerants: Energy, Exergy, Economic, and Environment. J. Clean. Prod. 2024, 469, 143146. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Züttel, A. Nanoscale Engineering of Solid-State Materials for Boosting Hydrogen Storage. Chem. Soc. Rev. 2023, 53, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, F.; Zhou, R.; Guo, C.; Liu, X.; Zhu, Y. Phosphorous-Doped Carbon Nanotube/Reduced Graphene Oxide Aerogel Cathode Enabled by Pseudocapacitance for High Energy and Power Zinc-Ion Hybrid Capacitors. Chinese Chem. Lett. 2023, 35, 108354. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, X.; Zhang, Y.; Xie, Z.; Zhang, T.; Zhao, G. Enhanced Stability and Electrochemical Investigations of Ni/ZSM-5 Catalyst Layer on Nickel-Based Anodes for Ammonia-Fed Solid Oxide Fuel Cells. J. Power Sources 2023, 592, 233939. [Google Scholar] [CrossRef]
- Noh, H.; Kang, K.; Seo, Y. Environmental and Energy Efficiency Assessments of Offshore Hydrogen Supply Chains Utilizing Compressed Gaseous Hydrogen, Liquefied Hydrogen, Liquid Organic Hydrogen Carriers and Ammonia. Int. J. Hydrogen Energy 2022, 48, 7515–7532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).