Abstract
Transition metal oxide aerogels (AGLs) have attracted considerable attention in recent years due to their exceptional properties, including high surface area, significant porosity, and ultralow density. In this study, we report the first-time synthesis of zinc oxide nano-wafers and zinc aerogels for application as supercapacitor electrodes. The aerogels were synthesized via a novel one-pot hydrolysis method using NaBH4 as a reducing agent and subsequently annealed at 200 °C (ZnAGL(200)) and 450 °C (ZnAGL(450)) to investigate the influence of temperature on their electrochemical properties. Structural and morphological characterizations were conducted using XRD, FTIR, BET, XPS, SEM, and TEM analyses. Among the fabricated electrodes, the aerogel annealed at 200 °C (ZnAGL(200)) exhibited superior energy storage performance, attributed to its amorphous, continuous network structure, which enhanced its surface area and reduced its density compared to both the as-synthesized (ZnAGL(RT)) and 450 °C-annealed (ZnAGL(450)) counterparts. A two-electrode device demonstrated excellent cycling stability over 10,000 cycles, achieving an energy density of 7.97 Wh/kg and a power density of 15 kW/kg. These findings highlight the potential of zinc aerogels as materials for next-generation lightweight energy storage systems, with promising applications in industrial, mechanical, and aerospace technologies.
1. Introduction
The global demand for efficient energy storage has highlighted the limitations of present technologies, such as capacitors, batteries, and fuel cells, in meeting the needs of a growing population [1,2]. Supercapacitors (SCs) have emerged as a viable option due to their high power density, short charge–discharge times, and excellent cycle stability [3]. However, there are certain shortcomings, the most noticeable of which is their higher rates of self-discharge when compared to lithium-ion batteries, which can result in significant energy loss over time [4,5]. Studies suggest that a high surface area plays a crucial role in enhancing the electrochemical performance of pseudocapacitive supercapacitors by offering more Faradaic active sites [6,7]. Additionally, larger pore volume facilitates ion transport within the electrode, thereby improving the rate capability [8].
Aerogels (AGLs) have recently sparked widespread interest because of their unusual structure-dependent optical, mechanical, electrical, and magnetic capabilities [9,10]. Their high porosity, huge surface area, and ultralightweight properties make them suitable scaffolds for high-performance electrode materials in energy storage applications [11,12]. Notably, their exceptionally low density offers a significant advantage over traditional nanoscale materials. Initially, aerogel research concentrated on carbon-based matrices like graphene, with metals utilized as conductive and capacitive components [13]. However, recent advances have moved beyond carbon frameworks to investigate the potential of metal oxide aerogels as freestanding electrode materials, widening their applicability in next-generation supercapacitors and energy storage devices [14,15].
Among many transition metals, zinc oxide (ZnO) is a low-cost metallic oxide with sufficient electrochemical activity (an energy density of 650 A g−1) [16]. ZnO’s high theoretical capacitance, eco-friendliness, and stability make it a promising contender for supercapacitor electrodes [17,18,19]. Recent research has investigated the combination of ZnO with graphene-based materials to improve electrochemical performance. Das et al. used waste biomass-derived 3D graphene aerogels (GAs) as cathode materials for zinc-ion hybrid supercapacitors (ZHSCs), reaching a specific capacitance of 353.1 F g−1 at 0.1 A g−1 and ~84.2% capacity retention over 10,000 cycles at 10 A g−1 [19]. Their porous structure enabled efficient charge storage and ion transport, making them promising for energy storage applications. Ghazitabar et al. successfully synthesized an N-doped graphene aerogel/Co3O4/ZnO ternary nanocomposite, which demonstrated improved electrical conductivity and enhanced electrochemical properties with a high specific capacitance of 543 F g−1 [16]. Hassan et al. reported a specific capacitance of 108.95 mV/s for graphene aerogel integrated with ZnO materials [20]. Despite these promising advances, the synthesis and application of ZnO-based aerogel nanocomposites in electrochemical energy storage remain underexplored. While numerous studies have concentrated on producing ZnO aerogels for a variety of applications, their potential as high-performance supercapacitor electrodes has yet to be fulfilled.
In this study, we devised a one-pot synthesis technique for zinc aerogels and investigated their structural and electrochemical properties by annealing at 200 °C and 450 °C, respectively. These aerogels were used as electrode materials in supercapacitor applications, and the 200 °C-annealed sample (ZnAGL(200)) demonstrated improved electrochemical performance, with a maximum energy density of 7.97 Wh/kg and a power density of 15 kW/kg. This improved performance is due to its amorphous nanocrystalline, continuous network structure, which provides a large surface area and ultralow density when compared to the as-synthesized (ZnAGL(RT)) and 450 °C-annealed (ZnAGL(450)) counterparts. These findings emphasize zinc aerogels’ potential for enhanced energy storage as well as other industrial and therapeutic uses.
2. Materials and Methods
The detailed characterizations of aerogels, electrochemical analysis, and device fabrications are provided in the Electronic Supplementary Materials.
2.1. Materials
Zinc nitrate (Zn(NO3)2, 99%), sodium borohydride (NaBH4, 99%), sodium sulfate (Na2SO4, 99%), and potassium hydroxide (KOH, 99%) were purchased from Sigma-Aldrich. Ethanol, iso propyl alcohol, and acetone were purchased from Alfa Aesar. Carbon black was purchased from Merck Chemicals. The nitrogen gas cylinder was purchased from a local dealer (Republic of Korea). All experiments were performed with deionized water, and the temperature was maintained at 25 °C, unless otherwise specified.
2.2. Preparation of ZnAGL(RT), ZnAGL(200), and ZnAGL(450) Aerogels
Zinc aerogels were synthesized by dissolving Zn(NO3)2 in a mixture of ethanol and deionized water, followed by nitrogen bubbling for 20 min. A 0.5 M NaBH4 solution in ethanol was then added, leading to an instant color change to pale white, indicating aerogel formation. The resulting hydrogels were washed multiple times with DI water and ethanol and then freeze-dried for two days. The obtained Zn aerogels were stored in a desiccator. To prepare ZnO aerogels, the Zn aerogels were annealed at 200 °C and 450 °C for 2 h at a heating rate of 10 °C/min. Amorphous aerogels exhibited higher solubility in ethanol than their crystalline counterparts.
3. Results and Discussion
The SEM images of the as-prepared zinc aerogel samples (ZnAGL(RT)) and annealed samples (ZnAGL(200) and ZnAGL(450)), shown in Figure 1a–c, reveal thin wafer-like flaky nanoporous structures. The ZnAGL(RT) samples show significantly large flakes with sharp edges and a highly porous nature, as observed from the SEM studies, and when annealed at 200 °C, the resultant ZnAGL(200) samples show more open flaky structures with nanoparticle formation resulting from annealing. The ZnAGL(450) samples resulting from annealing the aerogel at 450 °C show more nanoparticle nature and less aggregation of nanoflakes. The EDS (Figure 1d) of the zinc aerogels annealed at 200 °C shows the presence of zinc and no impurities (the carbon and oxygen obtained from the background). Although the SEM images appear similar, the TEM analysis reveals significant differences. The HR-TEM analysis of the as-synthesized ZnAGL(RT), ZnAGL(200), and ZnAGL(450) is presented in Figure 2a,c,e and Figure 2b,d,f, respectively. The HR-TEM images of the as-synthesized ZnAGL(RT) (Figure 2c,d) confirm the presence of nanoflake structures with edge widths ranging from 5 to 10 nm, incorporating aggregated zinc nanoparticles within the nanostructured framework. Upon annealing at 200 °C, the nanoflakes exhibit a more interconnected and continuous morphology, suggesting the fusion of nanoparticles into a well-developed network. This transformation is likely driven by water evaporation and the subsequent filling of void spaces by zinc moieties, facilitated by thermal energy. In contrast, the 450 °C-annealed samples display significant aggregation of nanoflakes, leading to the formation of distorted aerogel structures. The SAED analysis further supports these structural transitions, with diffuse rings observed in the as-synthesized aerogel (ZnAGL(RT)) indicating an amorphous nature, while the ZnAGL(200) exhibits distinct diffraction spots in a ring-like pattern, suggesting the presence of nanocrystalline domains with some degree of structural order. The ZnAGL(450) aerogel presents well-defined diffraction rings with bright spots, confirming its highly crystalline nature. The measured d-spacing corresponding to the (103) plane of ZnO further substantiates the crystalline phase transformation occurring at elevated temperatures.
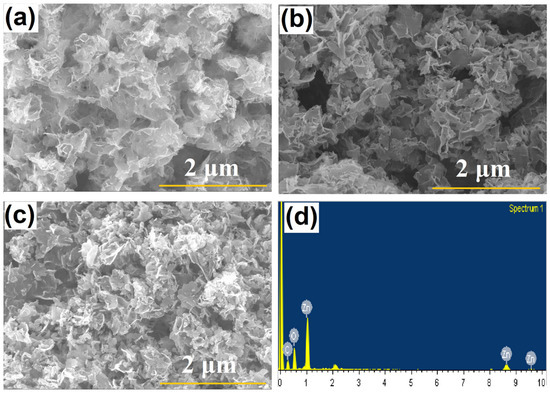
Figure 1.
FESEM images of (a) ZnAGL(RT), (b) ZnAGL(200), (c) ZnAGL(450), and (d) EDS spectrum for sample ZnAGL(RT).

Figure 2.
TEM and HRTEM images of (a,b) ZnAGL(RT), (c,d) ZnAGL(200), and (e,f) ZnAGL(450). The inset shows the corresponding SAED patterns of (b,d,e).
The specific surface area, pore volume, and average pore diameter of the ZnAGL(RT) and the annealed aerogel samples ZnAGL(200) and ZnAGL(RT) were analyzed using nitrogen adsorption studies, as illustrated in Figure 3a,b. All samples exhibited type II isotherms, indicating monolayer formation and suggesting a predominantly macroporous structure. The marked point in Figure 3 signifies the completion of monolayer formation. The absence of hysteresis further confirmed that adsorption and desorption occurred on a non-porous or macroporous surface. The as-synthesized zinc aerogels (ZnAGL(RT)) demonstrated the highest BET surface area of 90.5 m2/g with a pore volume of 0.45 cm3/g. Upon annealing, the surface area decreased to 63.8 m2/g for the ZnAGL(200) sample and 14.3 m2/g for the ZnAGL(450) sample, following the typical trend where increasing annealing temperatures lead to a reduction in BET surface area.
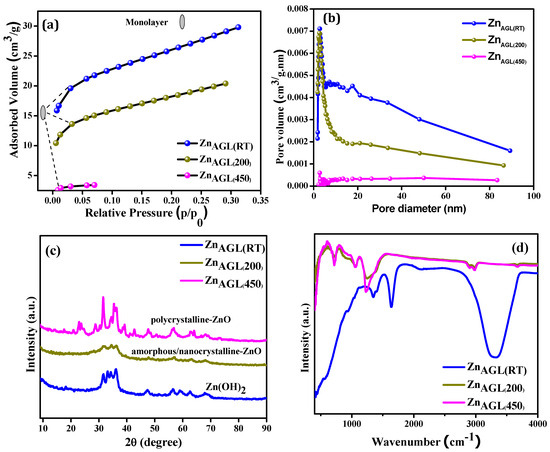
Figure 3.
(a) BET absorption graph and (b) pore volume of as-synthesized zinc aerogels (ZnAGL(RT)), ZnAGL(200) and ZnAGL(450), (c) XRD, and (d) FTIR of (ZnAGL(RT)), ZnAGL(200), and ZnAGL(450).
The XRD and FTIR of the zinc aerogels are given in Figure 3c,d, respectively. The XRD of the as-synthesized samples revealed an amorphous nature for the as-synthesized zinc aerogels (ZnAGL(RT)) and the annealed sample ZnAGL(200). The annealed sample at 450 °C showed more crystalline peaks, as also confirmed by HR-TEM analysis. The ZnAGL(RT) showed peaks corresponding to zinc metal, and the annealed samples ZnAGL(200) and ZnAGL(450) showed that for zinc hydroxide and oxides, respectively. The peak at 33.3° corresponding to the (002) face of zinc was the preferred orientation for the as-synthesized sample, whereas the strong crystalline peak at 62.4° for the annealed sample at 450 °C corresponded to the (103) plane of the zinc oxide aerogel. The annealing of the as-synthesized sample at 200 °C produced largely amorphous nature aerogels, as also confirmed by the HR-TEM analysis in Figure 2c,d.
Materials’ FTIR spectra are useful in determining the functional groups present in them. The FTIR of the as-synthesized zinc aerogels showed peaks at 1397 cm−1, 1639 cm−1, and 3250–3350 cm−1. The three peaks present in the as-synthesized samples are characteristic of zinc aerogels. The bandwidth from 1500 to 600 denotes the fingerprint region for zinc oxide materials. The peaks present for the annealed samples at 720, 1051, 1230, 2900, and 2985 cm−1 indicated the C-H, C=O, C-H, CH2, and CH3 vibrations, respectively, occurring due to the presence of carbon groups during the annealing process (200 and 450 °C). The Zn-O peaks appeared below 500 cm−1 in ZnAGL(200) and ZnAGL(450) samples and were more pronounced in both annealed samples.

Table 1.
Comparison of various zinc ion and aerogel-based material electrodes and their performances for supercapacitor applications.
Table 1.
Comparison of various zinc ion and aerogel-based material electrodes and their performances for supercapacitor applications.
| System | Electrolyte | Sp. Capacitance(F/g) | ED//PD (Wh/kg//kW/kg) | Ref. |
|---|---|---|---|---|
| ZHSC | Zn(CF3SO3)2 | 540(0.1 A/g) | 188/49 | [18] |
| MXene/ZHSC | ZnSO4 | - | 55/3 | [21] |
| TI2CTx-MXene/ZISC | ||||
| - | 233 (1 A/g) | 130/1 | [22] | |
| GA/ZHSC | ZnSO4 | 353 (1 A/g) | 77/15 | [19] |
| Zn/chitosan aerogels | KOH | 230 (0.5 A/g) | 4.67/5 | [17] |
| ZnO/CA | KOH | 375 (75 mA/cm2) | - | [23] |
| Zn-Co-S aerogel | KOH | 820 (1 A/g) | 47/0.7 | [24] |
| ZnAGL(200) | KOH | 288 (1 A/g)/ | 8/15 | This work |
The XPS studies were carried out for the as-synthesized (ZnAGL(RT)) and the annealed aerogels ZnAGL(200) and ZnAGL(450) in order to deduce the elemental composition and purity of the samples, and the results are shown in Figure 4a–f. The Zn 2p spectra showed two distinct peaks related to the Zn 2p1/2 and Zn 2P3/2, revealing Zn+2 in the prepared AGL compounds [25]. For the ZnAGL(RT), Zn 2p1/2 and Zn 2P3/2 were observed at 1044.7 to 1021.5 eV, respectively, with a spin–orbit splitting ratio (ΔBE) of 23.04 eV. In the ZnAGL(200) aerogel, the peaks slightly shifted to 1044.9 and 1021.8 eV, with a ΔBE of 23 eV, suggesting an enhanced defect density and hydroxylation due to increased oxygen vacancies [26]. The 450 °C-annealed AGL (ZnAGL(450)) showed peaks at 1021.5 and 1044.6 eV, with a ΔBE of 23.1 eV, revealing a reduced defect density and improved crystallinity. The O1s spectra of AGL samples were deconvoluted into three distinct peaks, representing the various chemical states of oxygen elements on the AGL surface. The deconvolution of the O 1s XPS spectra revealed three unique peaks, each representing a different chemical state of oxygen on the Zn aerogel surface. The signal at 530.0–530.5 eV is due to lattice oxygen (OL), suggesting that oxygen elements are linked within the Zn-O crystal lattice. The second peak at 531.0–531.5 eV is linked to defect-related oxygen (OD) or oxygen vacancies, indicating non-stoichiometry and structural defects in the ZnO lattice [27]. The third peak, which ranges from 532.0 to 533.5 eV, represents adsorbed oxygen species or hydroxyl groups (OH) formed by surface hydroxylation or physiosorbed water. The ZnAGL(RT) had an OH/OL ratio of 0.53, indicating considerable surface defects and hydroxylation. This ratio increased to 0.89 for the ZnAGL(200) sample, indicating greater enrichment of oxygen vacancies and hydroxylation [28]. The ZnAGL(450) sample showed a lower OH/OL ratio of 0.36 after higher temperature annealing, indicating enhanced crystallinity and decreased defect density and surface hydroxylation.
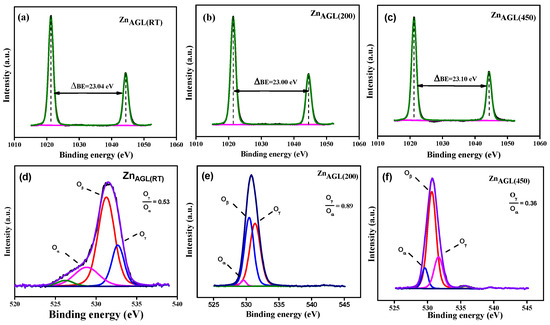
Figure 4.
(a–c) Zinc 2p spectra of ZnAGL(RT), ZnAGL(200), and ZnAGL(450); (d–f) O 1s spectra of ZnAGL(RT)), ZnAGL(200)), and ZnAGL(450).
Initially, to estimate the comparative energy storage capacity of the ZnAGL(RT), ZnAGL(200), and ZnAGL(450) aerogels, cyclic voltammetry (CV) was implemented using a three-electrode arrangement. The setup consisted of Ni-foam working electrodes loaded with the prepared samples, a Ag/AgCl reference electrode, and a Pt counter electrode in an aqueous 1 M KOH solution. The three zinc aerogel samples were subjected to electrochemical analysis, starting with cyclic voltammetry to measure the current generated at various scan rates after 200 stabilization cycles. The stabilization CV was carried out at 50 mV/s from 0 to 0.5 V. Figure 5a–c illustrate the change in the redox potentials of ZnAGL(RT), ZnAGL(200), and ZnAGL(450) at various scan rates from 5 mV/S to 100 mV/S at a potential window of 0 to 0.5 V. Enhanced current values were observed for ZnAGL(200) compared to ZnAGL(RT) and ZnAGL(450) (Figure 5j). The shape of the CV curves indicates the pseudocapacitive behavior of ZnAGL(RT), ZnAGL(200), and ZnAGL(450) [26], which is well established in most aerogel-based supercapacitors exhibiting capacitive behavior. Moreover, to understand the optimized materials loading and the effect of excess loading, we performed CV analysis for 2, 2.5, and 3 mg of ZnAGL(200) materials loading, and the obtained results are presented in Figure S1. Further, the specific capacity was calculated from the CV, and the obtained plots are displayed in Figure S2. After undergoing the CV tests, all the zinc aerogel samples were subjected to galvanostatic charge–discharge studies at various current densities, ranging from 1 to 20 A/g within a potential range of 0 to 0.4 V (Figure 5d,e). The curves also denoted pseudocapacitive behavior, which is consistent with the behavior typically observed in transition metal aerogel-based supercapacitor electrodes. Figure 5g–i present the specific capacitance of ZnAGL(RT), ZnAGL(200), and ZnAGL(450) across varying current densities (1–20 A/g), calculated using Equation (S1). Notably, ZnAGL(200) exhibited the highest specific capacity of 115 C/g (specific capacitance of 288 F/g), surpassing both ZnAGL(RT) and ZnAGL(450). This enhanced electrochemical performance can be attributed to their nanocrystalline network structures exhibiting unique defect-rich surfaces and the presence of oxygen vacancies, as confirmed by XPS analysis. These structural features facilitate improved charge storage, enhance ion diffusion kinetics, and contribute to superior electrochemical activity compared to the as-synthesized ZnAGL(RT) and highly crystalline ZnAGL(450) counterparts.
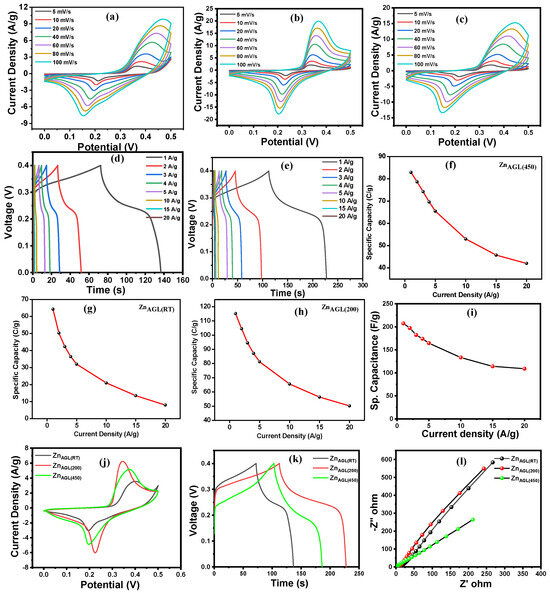
Figure 5.
(a–c) CV curves, (d–f) GCD curves, and (g–i) the relationship between the specific capacitance and current density of ZnAGL(RT), ZnAGL(200), and ZnAGL(450) electrodes, respectively; (j) comparative CV curves, (k) comparative GCD, and (l) the EIS plots of ZnAGL(RT), ZnAGL(200), and ZnAGL(450) electrodes.
Impedance spectroscopy is an effective tool for analyzing various electrochemical parameters, including solution resistance, charge-transfer resistance, Warburg impedance, and double-layer capacitance, among others. Hence, in order to evaluate the effect of Zn aerogels in the presence of KOH electrolyte, Nyquist plots were generated at OCV for all samples, and the results were analyzed. The solution resistance was found to vary slightly, with 4, 2.4, and 0.8 ohms for the ZnAGL(RT), ZnAGL(200), and ZnAGL(450) electrodes. Also, the charge-transfer resistance values were 18 and 20 ohms for the ZnAGL(RT) and ZnAGL(200) aerogels. By contrast, in the ZnAGL(450) samples, the Rct resistance could not be determined due to the absence of a semicircle in the high-frequency region. Moreover, the performance of the ZnAGL(200) nanostructure electrode compared favorably against previously reported electrodes as shown in Table 1.
In order to evaluate the performance of the zinc aerogels in real-time applications, a two-electrode arrangement was constructed using Zn aerogels as positive electrodes and rGO as the negative counterparts. The mass loading of the electrodes was adjusted as per the following equation:
where m+ and m− are the mass of the positive and negative electrodes; C+ and C− the specific capacitance; and V+ and V− the potential of the positive and negative electrodes, respectively. The mass loading for the positive electrode was 1.6 mg, and that for the negative electrode was 3 mg, respectively.
The CV for the device was carried out from 0 to 1.5 V within scan rates of 5–100 mV/s (Figure 6a). The device exhibited a pseudocapacitive behavior, and the current increased with increasing scan rates. The peak current increase at fast scan rates indicates the capacitance of the redox reactions. The galvanostatic charge–discharge curves were then generated in order to determine the real-time capacitance properties of the zinc aerogel electrode materials (Figure 6b). The specific capacitance was calculated to be from ~50 F/g to 26 F/g when the current density varied from 1 to 20 A/g (Figure 6c). Figure 6d displays the relationships between ED, PD, and discharge time, along with Ragone plots for the fabricated device. Notably, the ZnAGL(200)//AC device achieved an energy density of 8 Wh/kg at a power density of 15,000 W/kg and maintained an energy density of 2.1 Wh/kg at a power density of 1760 W/kg while operating at a potential of 1.5 V. In addition, to understand the stable potential window of the devices, they were employed using various voltages ranging from 1.2 to 1.6 V (Figure 6e), revealing the broad operational potential of the device.
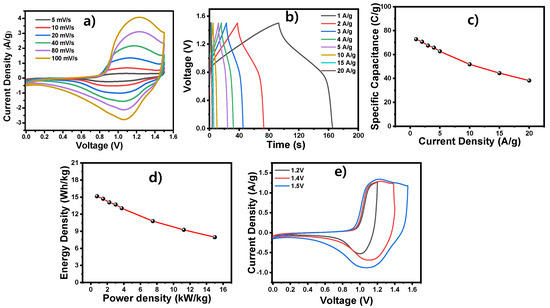
Figure 6.
(a–c) ZnAGL(200)//Ac device: (a) CV profile with various scan rates; (b) GCD profile at various current densities; (c) specific capacitance; (d) Ragone plot; (e) CV profile at various potentials.
The impedance spectra were also carried out for the device at an amplitude of 5 mV/s and a frequency range of 0.1 to 105 Hz. The EIS was run for the device before cycling, wherein the solution resistance and charge-transfer resistance values were found to be 1.1 and 91.9 ohms. The EIS resistance values for the device after cycling were found to be 73.6 and 292.5 ohms, respectively (Figure 7a). The increase in resistance after cycling may be due to the decreased activity of the material. Furthermore, to understand the stability of the constructed device, 10,000 cycles of consecutive GCD tests at 10 A/g current density were performed, and the obtained cyclic stability and columbic efficiency results are presented in Figure 7b. The device also showed excellent cyclability, up to 10,000 cycles with a Coulombic efficiency of 97%.
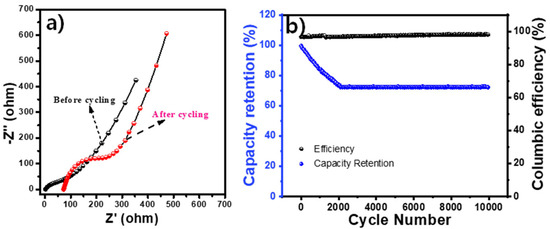
Figure 7.
ZnAGL(200)//Ac device: (a) EIS curves of the device before and after the cycling study; (b) cycling retention study.
The unique structural and chemical properties of ZnAGL(200) as a supercapacitor electrode are responsible for its superior electrochemical performance, which synergistically improves ion transport and charge storage. Initially, the amorphous nanocrystalline structure, which was verified by XRD (Figure 3c) and HR-TEM (Figure 2c,d), generated a continuous, interconnected nanoflake network with edge widths of 5–10 nm. This morphology provided a high density of active sites for Faradaic redox reactions, as demonstrated by the pseudocapacitive behavior observed in CV curves (Figure 5a–c). The amorphous nature of ZnAGL(200) minimized lattice constraints, enabling greater structural flexibility and ion accessibility during charge–discharge cycles, in contrast to the highly crystalline ZnAGL(450). Secondly, the XPS analysis (Figure 4d–f) revealed a high Oh/Ol ratio of 0.89 for ZnAGL(200), which suggested significant oxygen vacancies and surface hydroxylation. These oxygen vacancies functioned as defect sites, which facilitated the transfer of electrons between the Zn2+ and Zn0 states, thereby enhancing electronic conductivity and promoting redox activity. The pseudocapacitive reactions in the KOH electrolyte were further facilitated by the presence of hydroxyl groups, as illustrated by the subsequent reaction:
Zn(OH)2 + OH− ↔ ZnOOH + H2O + e−
Thirdly, the BET surface area of 63.8 m2/g and pore volume of 0.35 cm3/g (Figure 3a,b) in ZnAGL(200) offered a substantial surface area for electrolyte ion adsorption and diffusion, thereby enhancing the rate capability and specific capacitance (288 F/g at 1 A/g). The efficient ion transport was ensured by the macroporous structure, as evidenced by type II isotherms, which mitigated the diffusion limitations observed in the more aggregated ZnAGL(450). Lastly, the enhanced electrochemical kinetics of ZnAGL(200) were confirmed by the low solution resistance (2.4 Ω) and charge-transfer resistance (20 Ω) in EIS measurements (Figure 5i), which were influenced by its interconnected morphology and defect-rich surface. Collectively, these factors enabled ZnAGL(200) to attain a high energy density of 7.97 Wh/kg and a power density of 15 kW/kg in the asymmetric supercapacitor configuration, rendering it a promising material for next-generation energy storage applications.
4. Conclusions
In summary, Zn aerogel materials enriched with oxygen vacancies and exhibiting ZnO defect chemistry were successfully synthesized in a sol–gel one-pot hydrolysis method using a borohydride. The resulting aerogels featured nano-wafer architectures composed of a Zn particle network with moderate porosity. XRD analysis confirmed that the annealed Zn aerogel samples maintained a predominantly amorphous nanocrystalline structure with embedded Zn polycrystalline domains. Electrochemical characterization, including cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS), demonstrated an exceptional highest specific capacity of 115 C/g (specific capacitance (Csp) of 288 F/g) in a three-electrode configuration. Due to its superior electrochemical properties, ZnAGL(200) was selected for asymmetric supercapacitor (ASC) fabrication, employing reduced graphene oxide (rGO) as the negative electrode, yielding a specific capacitance (Csp) of 49 F/g. The Ragone plot indicated an energy density (ED) of 7.97 Wh/kg and a power density (PD) of 15 kW/kg, underscoring its high-performance characteristics. Additionally, the unique morphology and lightweight properties of the aerogel, facilitated by the borohydride-assisted synthesis, make it an outstanding candidate for next-generation ultralight supercapacitors, addressing the growing energy storage demands of advanced technological applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13051461/s1, General Procedures, Materials, Characterizations.
Author Contributions
Conceptualization, methodology, investigation, writing—original draft preparation, R.R.; software, validation, data curation, writing—review and editing, G.K.; formal analysis, M.R.A.R.A.; visualization, T.R.G.; supervision, funding acquisition, resources, W.K.K.; supervision, funding acquisition, J.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Csp | Specific capacitance |
| ED | Energy density |
| PD | Power density |
| ASC | Asymmetric supercapacitor |
| AGLs | Aerogels |
| EIS | Electrochemical impedance spectroscopy |
| CV | Cyclic voltammetry |
| GCD | Galvanostatic charge–discharge |
References
- Lim, J.M.; Jang, Y.S.; Van, T.; Nguyen, H.; Kim, J.S.; Yoon, Y.; Park, B.J.; Seo, D.H.; Lee, K.-K.; Han, Z.; et al. Advances in high-voltage supercapacitors for energy storage systems: Materials and electrolyte tailoring to implementation. Nanoscale Adv. 2023, 5, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Lv, T.; Liu, Y.; Wang, X.; Yang, Y.; Chen, Z.; Qi, Y.; Cao, S.; Chen, T. Flexible Asymmetric Supercapacitors with Extremely Slow Self-Discharge Rate Enabled by a Bilayer Heterostructure Polymer Electrolyte. Adv. Funct. Mater. 2022, 32, 2108794. [Google Scholar] [CrossRef]
- Shuja, A.; Khan, H.R.; Murtaza, I.; Ashraf, S.; Abid, Y.; Farid, F.; Sajid, F. Supercapacitors for energy storage applications: Materials, devices and future directions: A comprehensive review. J. Alloys Compd. 2024, 1009, 176924. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, X.; Li, W.; Shi, M.; Ren, K.; Lu, X. Reduced Self-Discharge of Supercapacitors Using Piezoelectric Separators. ACS Appl. Energy Mater. 2021, 4, 8070–8075. [Google Scholar] [CrossRef]
- Šedajová, V.; Nandi, D.; Langer, P.; Lo, R.; Hobza, P.; Plachá, D.; Bakandritsos, A.; Zbořil, R. Direct upcycling of highly efficient sorbents for emerging organic contaminants into high energy content supercapacitors. J. Colloid Interface Sci. 2025, 692, 137481. [Google Scholar] [CrossRef]
- Hou, L.; Yang, W.; Li, Y.; Wang, P.; Jiang, B.; Xu, C.; Zhang, C.; Huang, G.; Yang, F.; Li, Y. Dual-Template endowing N, O co-doped hierarchically porous carbon from potassium citrate with high capacitance and rate capability for supercapacitors. Chem. Eng. J. 2021, 417, 129289. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Nandi, D.; Yeetsorn, R.; Wanchan, W.; Devi, C.; Singh, R.P.; Vasistha, A.; Kumar, M.; Koinkar, P.; Yadav, K. A machine learning approach for estimating supercapacitor performance of graphene oxide nano-ring based electrode materials. Energy Adv. 2025, 4, 119–139. [Google Scholar] [CrossRef]
- Pan, G.; Zhu, J.; Ma, S.; Sun, G.; Yang, X. Enhancing the electromagnetic performance of Co through the phase-controlled synthesis of hexagonal and cubic Co nanocrystals grown on graphene. ACS Appl. Mater. Interfaces 2013, 5, 12716–12724. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Ramkumar, R.; Suganthi, S.; Milton, A.; Park, J.; Shim, J.-J.; Oh, T.H.; Kim, W.K. MnO/Mn2O3 aerogels as effective materials for supercapacitor applications. Energies 2024, 17, 2258. [Google Scholar] [CrossRef]
- Ramkumar, R.; Dhakal, G.; Shim, J.-J.; Kim, W.K. NiO/Ni nanowafer aerogel electrodes for high performance supercapacitors. Nanomaterials 2022, 12, 3813. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, R.; Rajkumar, C.; Do, H.; Kim, H.; Kim, W.K. A remarkable oxygen vacancy-rich rare earth aerogel with high activity towards electro and catalytic reduction of 5-nitroquinoline. J. Clean. Prod. 2023, 423, 138683. [Google Scholar] [CrossRef]
- Li, G.-R.; Feng, Z.-P.; Ou, Y.-N.; Wu, D.; Fu, R.; Tong, Y.-X. Mesoporous MnO2/carbon aerogel composites as promising electrode materials for high-performance supercapacitors. Langmuir 2010, 26, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, X.; Tervoort, E.; Zeng, G.; Liu, T.; Chen, X.; Sologubenko, A.; Niederberger, M. Nano-Sized Structurally Disordered Metal Oxide Composite Aerogels as High-Power Anodes in Hybrid Supercapacitors. ACS Nano 2018, 12, 2753–2763. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Q.; Gao, H.; Shi, Z.; Wu, J.; Zhi, M.; Hong, Z. Nickel oxide aerogel for high performance supercapacitor electrodes. RSC Adv. 2016, 6, 112620–112624. [Google Scholar] [CrossRef]
- Ghazitabar, A.; Naderi, M.; Fatmehsari Haghshenas, D.; Rezaei, M. Synthesis of N-doped graphene aerogel/Co3O4/ZnO ternary nanocomposite via mild reduction method with an emphasis on its electrochemical characteristics. J. Alloys Compd. 2019, 794, 625–633. [Google Scholar] [CrossRef]
- Liu, L.; Du, B.; Liu, R.; Chen, X.; Song, H. N-Doped Hierarchically Porous Carbon Aerogels by Controlling the Zn–Chitosan Complex Ratio for High-Performance Supercapacitors. Energy Fuels 2022, 36, 5920–5927. [Google Scholar] [CrossRef]
- Valentini, C.; Montes-García, V.; Ciesielski, A.; Samorì, P. Boosting Zinc Hybrid Supercapacitor Performance via Thiol Functionalization of Graphene-Based Cathodes. Adv. Sci. 2024, 11, 2309041. [Google Scholar] [CrossRef]
- Das, G.S.; Jangir, L.K.; Raju, K.S.; Sharma, Y.C.; Tripathi, K.M. Waste biomass-based 3D graphene aerogels for high performance zinc-ion hybrid supercapacitors. Chem. Commun. 2024, 60, 10568–10571. [Google Scholar]
- Hassan, K.; Hossain, R.; Sahajwalla, V. Recycled ZnO-fused macroporous 3D graphene oxide aerogel composites for high-performance asymmetric supercapacitors. J. Am. Ceram. Soc. 2022, 105, 7467–7478. [Google Scholar] [CrossRef]
- Cui, H.; Mi, H.; Ji, C.; Guo, F.; Chen, Y.; Wu, D.; Qiu, J.; Xie, H. A durable MXene-based zinc ion hybrid supercapacitor with sulfated polysaccharide reinforced hydrogel/electrolyte. J. Mater. Chem. A 2021, 9, 23941–23954. [Google Scholar] [CrossRef]
- Mateen, A.; Ansari, M.Z.; Hussain, I.; Eldin, S.M.; Albaqami, M.D.; Bahajjaj, A.A.A.; Javed, M.S.; Peng, K.-Q. Ti2CTx–MXene aerogel based ultra–stable Zn–ion supercapacitor. Compos. Commun. 2023, 38, 101493. [Google Scholar] [CrossRef]
- Kalpana, D.; Omkumar, K.; Kumar, S.S.; Renganathan, N. A novel high power symmetric ZnO/carbon aerogel composite electrode for electrochemical supercapacitor. Electrochim. Acta 2006, 52, 1309–1315. [Google Scholar] [CrossRef]
- Kim, Y.; Parale, V.G.; Kim, T.; Kim, S.-H.; Choi, H.; Patil, U.M.; Kanamori, K.; Park, H.-H. Synthesis of Zn-Co-S nanowire bundle type aerogel electrodes for asymmetric supercapacitors. Chem. Eng. J. 2023, 474, 145875. [Google Scholar] [CrossRef]
- Iaiche, S.; Djelloul, A. ZnO/ZnAl2O4 Nanocomposite Films Studied by X-Ray Diffraction, FTIR, and X-Ray Photoelectron Spectroscopy. J. Spectrosc. 2015, 2015, 836859. [Google Scholar] [CrossRef]
- Guan, S.; Hao, L.; Murayama, M.; Xie, X.; Komuro, S.; Zhao, X. Influence of anneal temperature in air on surface morphology and photoluminescence of ZnO thin films. IOP Conf. Ser. Mater. Sci. Eng. 2019, 522, 012004. [Google Scholar] [CrossRef]
- Chang, F.-M.; Brahma, S.; Huang, J.-H.; Wu, Z.-Z.; Lo, K.-Y. Strong correlation between optical properties and mechanism in deficiency of normalized self-assembly ZnO nanorods. Sci. Rep. 2019, 9, 905. [Google Scholar] [CrossRef]
- Li, F.; Bu, Y.; Han, G.-F.; Noh, H.-J.; Kim, S.-J.; Ahmad, I.; Lu, Y.; Zhang, P.; Jeong, H.Y.; Fu, Z. Identifying the structure of Zn-N2 active sites and structural activation. Nat. Commun. 2019, 10, 2623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).