Abstract
In this study, a microwave-assisted sulfuric acid recovery method is proposed for the efficient recovery of high-value carbon fibers at 100–140 °C. The recycled carbon fibers (RCF) were characterized, and recycled carbon fiber-reinforced plastics (RCFRP) were fabricated using their fibers. The recycling process preserved the surface morphology of the carbon fibers, with the RCF maintaining the axial groove structure on the surface of the virgin carbon fiber (VCF). X-ray diffraction (XRD) and Raman spectroscopy analyses confirmed that the degree of graphitization and crystalline structure of the RCF remained largely unchanged compared to the original carbon fibers. Surface oxidation occurred during the recycling process, leading to an increase in O–C=O content on the surface of the RCF compared to that of the VCF, which facilitated interfacial chemical bonding with the resin and enhanced the wettability. Compared to virgin carbon fiber-reinforced plastics (VCFRP), RCFRP retained up to 95.25% of the tensile strength, 97.47% of the shear strength, and 96.76% of the bending stress, demonstrating excellent mechanical properties. This study provides a simple and effective approach for the low-temperature and high-efficiency recycling of carbon fiber composites.
1. Introduction
Carbon fiber-reinforced plastics (CFRP) are lightweight, high-strength, fatigue-resistant, and thermally stable materials widely used in industries such as construction, transportation, sports, and wind energy [1]. With the expanding range of applications, global demand for CFRP has grown significantly. Industry forecasts predict that global CFRP consumption will reach 285,000 metric tons by 2025 [2]. At the end of their designated service life, carbon fibers within CFRP retain high mechanical properties, resulting in significant waste generation. Therefore, the efficient recycling of these discarded carbon fibers is imperative.
Currently, three primary methods exist for CFRP recycling: mechanical, thermal, and chemical. Mechanical recycling is not suitable for recovering long, high-modulus fibers, as it involves shearing or crushing the CFRP waste into 50 µm–100 mm particles, followed by separation to obtain short carbon fibers [3]. While this method is simple and environmentally friendly, it significantly degrades the fibers’ mechanical properties, limiting their reuse in high-performance applications [4]. More specifically, the recycled carbon fibers (RCF) obtained from recycled carbon fiber-reinforced plastics (RCFRP) typically exhibit poor interfacial performance [5]. Therefore, they are often relegated to be used as fillers in construction [6]. The thermal recovery method uses high temperatures to break down the resin into small molecules, thereby separating the carbon fibers from the resin matrix. Meyer et al. [7] found that RCF recovered at 700 °C were bound together by pyrolytic carbon, which hindered their reuse. Wei et al. [8] developed a two-step process for carbon fiber recovery, involving pyrolysis of CFRP at 425 °C followed by oxidation of the pyrolysis products at 550 °C. The RCF obtained exhibited a 19.7% reduction in tensile strength. Pickering et al. [9] recovered glass fibers using a fluidized gas process at 450 °C, yielding RCF with an average length of 5 mm and approximately half the tensile strength of virgin fibers. Jeong et al. [10] decomposed CFRP resin in steam at 600–800 °C, resulting in RCF with a 35% reduction in tensile strength. Pyrolysis-based methods face challenges such as high recycling temperatures, complex processes, reduced fiber strength, and the production of harmful gases. Chemical recycling has drawn significant interest due to its ability to recover long carbon fibers with minimal damage. This method uses various solvents to break down resin matrices into small molecules, thereby releasing carbon fibers. For instance, Pinero et al. [11] used an alkali catalyst to treat CFRP waste in supercritical water at pressures ranging from 4 MPa to 27 MPa. The resulting RCF retained 90% to 98% of the tensile strength of virgin carbon fiber (VCF), with a resin removal efficiency of approximately 79.3%. Jiang et al. [12] recovered carbon fibers using supercritical n-butanol, and the RCF maintained mechanical properties comparable to those of VCF. Although treating CFRP in supercritical or subcritical solutions can yield high-modulus carbon fibers, this approach requires specialized experimental equipment and poses challenges for industrial application. In recent years, acidic solutions have gained considerable attention for treating CFRP waste. Hanaoka et al. [13] successfully recovered carbon fibers by processing prepregs in nitric acid at 80 °C for 30 min. The interfacial shear strength of the RCF was nearly equivalent to that of the VCF, and the RCFRP exhibited tensile properties comparable to those of virgin carbon fiber-reinforced plastics (VCFRP). Jiang et al. [14] pretreated carbon fiber/epoxy resin composites (CF/EP) with nitric acid to initiate delamination, followed by treatment in polyethylene glycol at 160 °C for 200 min. This method achieved over 95 wt% resin removal, with RCF retaining 95% of the tensile strength of the VCF and exhibiting improved surface wettability, which enhanced bonding with the resin. Rijo et al. [15] demonstrated that the RCF recovered by treating CFRP with nitric acid had clean surfaces and retained the tensile strength of the VCF. Compared to other recycling methods, chemical recycling enables the recovery of long fibers, with RCF maintaining high mechanical properties. These advantages highlight its effectiveness and potential for producing high-quality RCF. Amine-cured epoxy resins are less resistant to nitric acid corrosion [16]. Current efforts to recover carbon fibers with acid are focused on treating amine-cured resins with nitric acid [14,17,18,19,20]. Less research has been done on the recycling of epoxy composites where the resin matrix type is anhydride cured. Microwave heating has the advantages of selective heating, rapid warming and uniform heating. Compared with the traditional heating method, the use of microwave heating to recover carbon fiber can shorten the recovery time and improve the recovery efficiency [21,22].
This study proposes a method for recovering carbon fibers by processing CFRP waste with microwave-assisted concentrated sulfuric acid. The concentrated sulfuric acid degrades the CFRP resin matrix into smaller molecules, enabling the recovery of carbon fibers. The effects of reaction temperature and time on the resin degradation rate were investigated. Additionally, the impact of the degradation process on the surface morphology, crystal structure, graphitization degree, and chemical structure of the surface layer of RCF was studied. RCFRP were fabricated using RCF, and their mechanical properties were evaluated.
2. Materials and Methods
2.1. Materials
The CFRP constituents used in the recycling experiments were as follows: Toray Carbon Fiber Cloth (T300, Toray Industries, Inc., Tokyo, Japan), E51 Epoxy Resin, methyltetrahydrophthalic anhydride (MeTHPA, industrial grade), and DMP-30 (industrial grade). The carbon fiber mass fraction in the CFRP is 65%. The sulfuric acid used has a concentration of 98%.
2.2. Experimental Process
2.2.1. Recovery of CFRP in Concentrated Sulfuric Acid
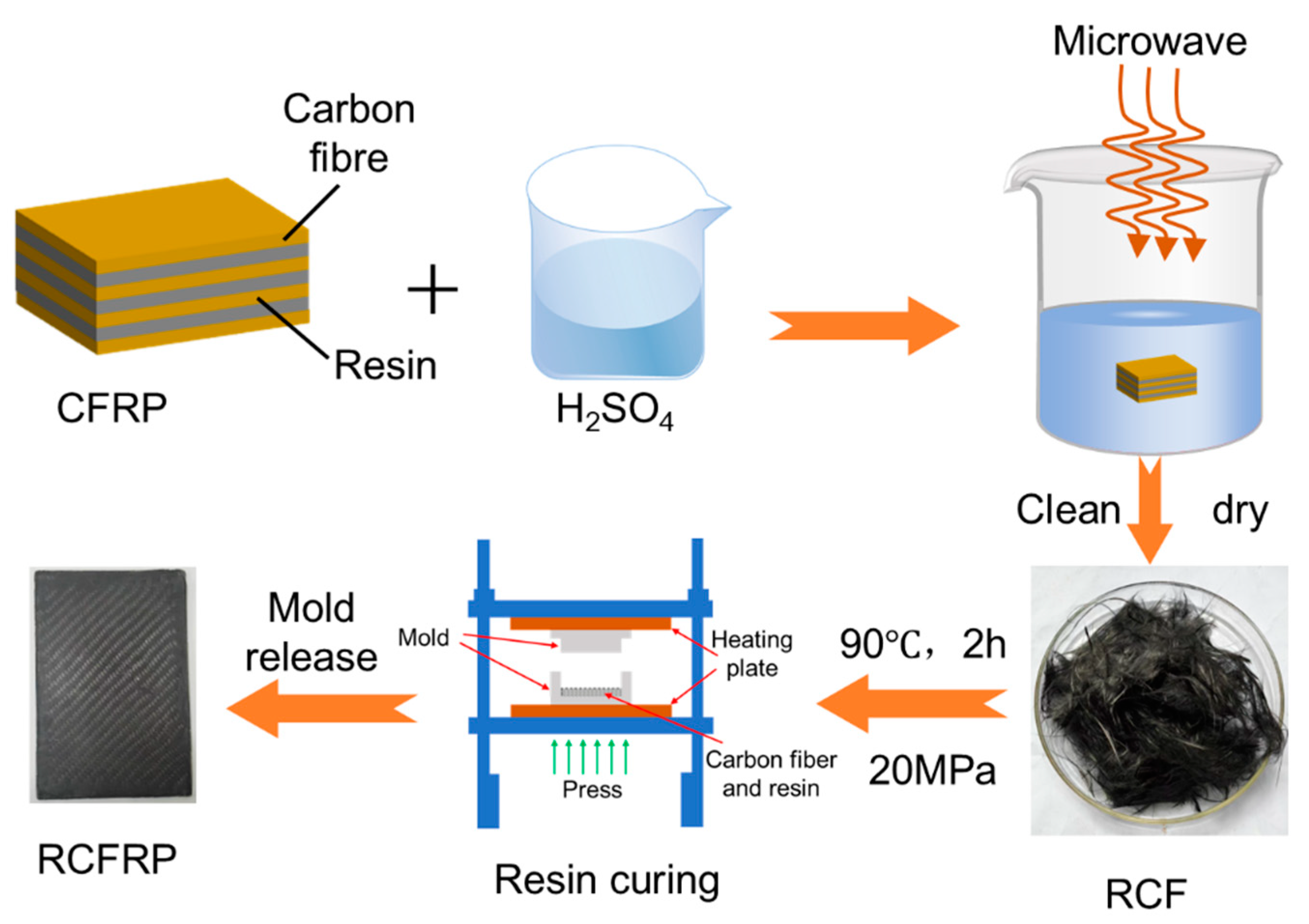
The recovery experiments were conducted using a microwave digestion instrument with a heating power of 1200 W and a microwave frequency of 2450 MHz. Concentrated sulfuric acid and CFRP samples were placed in a reactor, where the resin matrix was rapidly dissolved and degraded under the combined action of microwave irradiation and concentrated sulfuric acid, releasing the carbon fibers. The reaction temperature ranged from 80 °C to 140 °C, and the holding time varied from 20–60 min. After the reaction, the reactor was cooled to room temperature. The liquid in the reactor was diluted to a safe pH, and filtration separated the solid and liquid phases. The solid phase consisted of RCF, while the liquid phase was dilute sulfuric acid. Unsizing-treated Toray T300 carbon fibers, designated as the VCF, were used as a reference to evaluate RCF performance. The carbon fibers recovered at 100 °C for 60 min were designated RCF1, those recovered at 120 °C for 60 min were designated RCF2, and those recovered at 140 °C for 60 min were designated RCF3 (Figure 1).
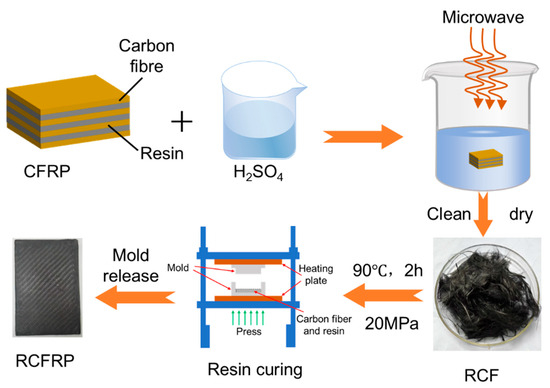
Figure 1.
Experimental Process Flow Chart.
The degradation rate (DR) was calculated using the following equation:
where M1 is the mass of the CFRP sample, M2 is the mass of the solid recovered at the end of the reaction, and ω is the mass fraction of the resin matrix in the composite.
2.2.2. Manufacturing of Carbon Fiber-Reinforced Plastics
The RCFRP were fabricated using RCF. In order to evaluate the performance of RCFRP, the VCFRP were prepared using Toray Carbon Fiber (T300, Toray Industries, Inc., Tokyo, Japan) fibers under identical processing conditions. The composite material was produced using YT-CC302S two-component epoxy resin (YT-CC302S, Kunshan ETO Composites Co., Kunshan, China), with curing performed via hot compression molding. The fabrication process was as follows: the two-component epoxy resin was mixed at a mass ratio of 3:1 (A:B), uniformly applied to the surface of the carbon fiber cloth, placed in a mold, and cured under a pressure of 20 MPa for two hours at a temperature of 90 °C.
2.3. Characterization
The surface morphology of the RCF and VCF was examined using scanning electron microscopy (SEM, GeminiSEM 300, Zeiss, Oberkochen, Germany). The crystal structure of the VCF and RCF was analyzed with an X-ray diffractometer (XRD, Rigaku SmartLab SE, Rigaku, Tokyo, Japan). The graphitization degree of the carbon fibers was characterized using Raman spectroscopy (Roman, Horiba LabRAM HR Evolution, Horiba, Kyoto, Japan). The surface structures of the VCF and RCF were analyzed using an X-ray photoelectron spectrometer (XPS, Thermo Scientific K-Alpha, Thermo Fisher Scientific Inc., Waltham, MA, USA) and a Fourier-transform infrared spectrometer (FTIR, Nicolet iS20, Thermo Fisher Scientific Inc., Waltham, MA, USA). The tensile, shear, and flexural properties of RCFRP and the VCFRP were examined using a universal testing machine. Gas and liquid products from the recycling process were analyzed by gas chromatography–mass spectrometry (GC–MS, Agilent 7890A-5975C, Agilent Technologies Inc., Santa Clara, CA, USA).
3. Results and Discussion
3.1. Effect of Reaction Conditions on Resin Degradation
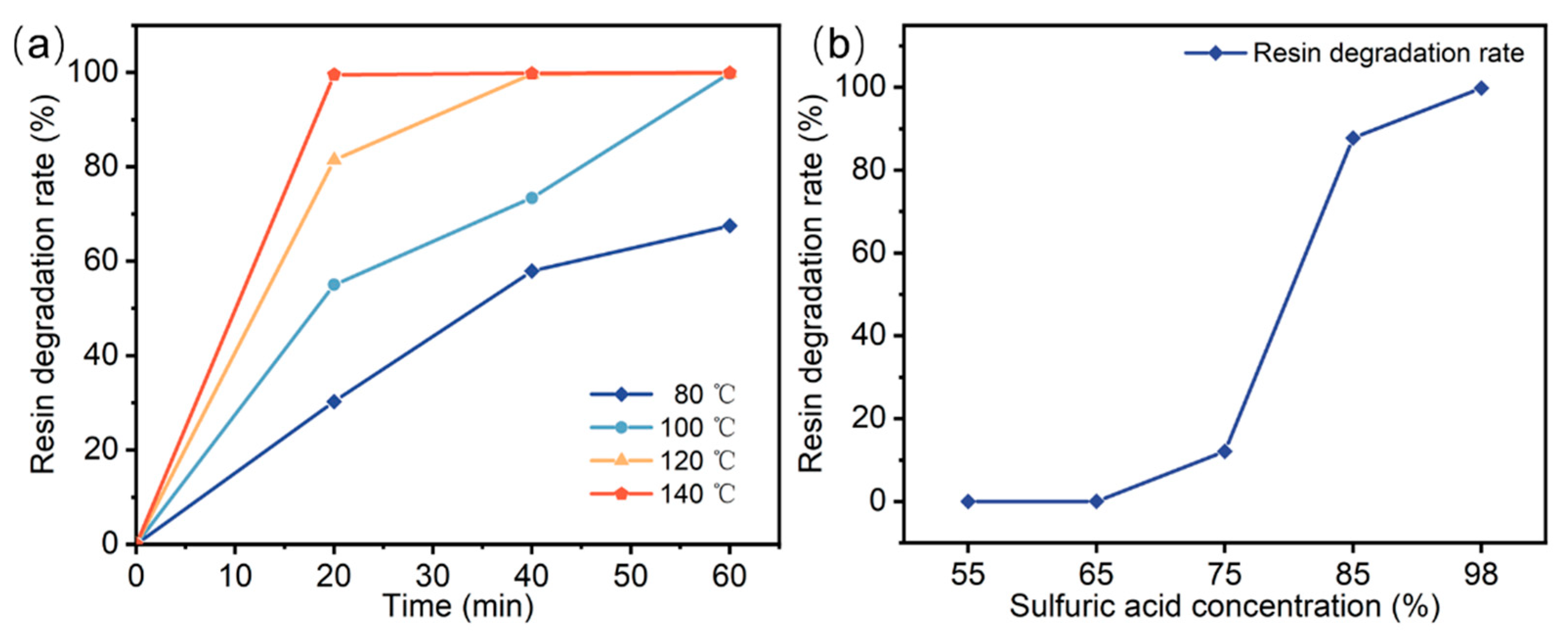
The resin degradation experiments utilized concentrated sulfuric acid with a mass fraction of 98% and a boiling point of approximately 337 °C. The composites were degraded at temperatures ranging from 80–140 °C. Figure 2a shows the effects of sulfuric acid temperature and holding time on the resin degradation rate. At a reaction temperature of 80 °C, extending the reaction time from 20 to 60 min increased the resin degradation rate from 30.26% to 67.5%. For a fixed temperature, longer reaction times promoted the degradation of the resin. When the reaction time was held constant at 20 min, increasing the degradation temperature from 80 °C to 140 °C raised the resin degradation rate from 30.26% to 99.5%. Therefore, the resin degradation rate increases with higher reaction temperatures for a given reaction time. Figure 2b shows the effect of sulfuric acid concentration on the resin degradation rate at a reaction temperature of 140 °C and a holding time of 60 min. When the sulfuric acid concentration is less than 65%, the resin cannot be degraded. The resin degradation rate increased with increasing sulfuric acid concentration. When the sulfuric acid concentration was 98%, the degradation rate of the resin was the highest. Table 1 compares the recycling method proposed in this study with existing methods. Compared to conventional recycling technologies, this approach offers significant technical advantages, including lower reaction temperatures, shorter reaction times, and a simplified process.
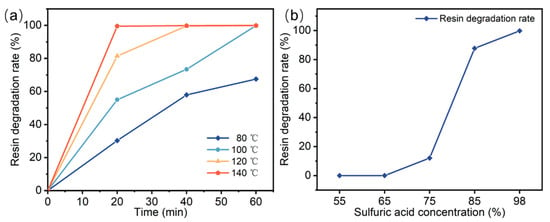
Figure 2.
Effect of experimental conditions on resin degradation rate: (a) temperature and time, (b) sulfuric acid concentration.

Table 1.
Comparison of different recycling methods.
3.2. Carbon Fiber Morphology
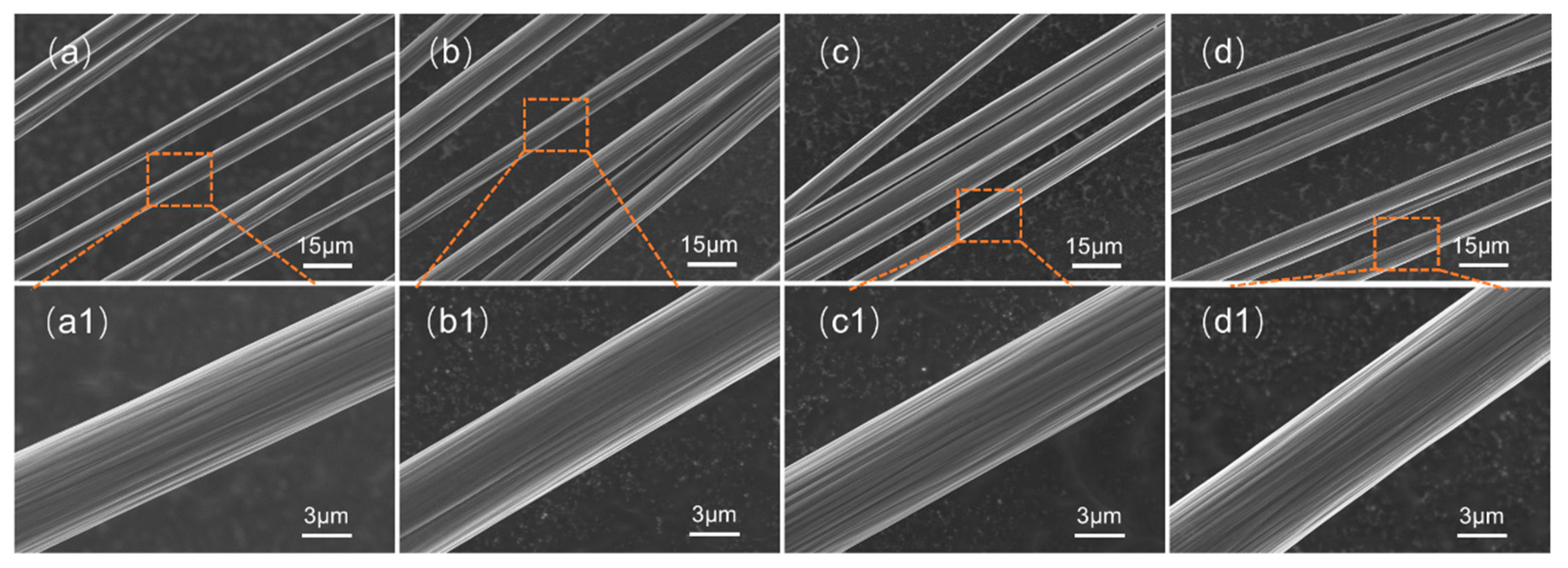
The SEM image of carbon fiber is shown in Figure 3. Carbon fiber recovered under various conditions exhibited smooth surfaces with no resin residue, indicating complete separation of the resin and carbon fibers. No cracks or defects were observed on the RCF. The morphology of the RCF was comparable to that of the VCF, both displaying prominent axial grooves. These grooves facilitate interfacial mechanical interlocking between the resin matrix and carbon fibers [25]. The preservation of VCF surface morphology in the RCF indicates that the sulfuric acid degradation process does not alter the morphology of the carbon fibers.
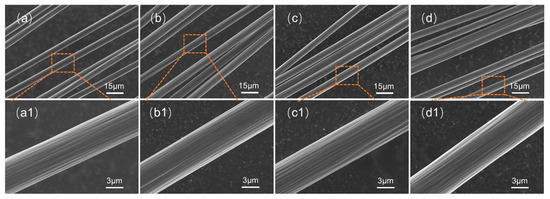
Figure 3.
SEM image of carbon fiber: (a) SEM-VCF; (a1) SEM-VCF; (b) SEM-RCF1; (b1) SEM-RCF1; (c) SEM-RCF2; (c1) SEM-RCF2; (d) SEM-RCR3; (d1) SEM-RCR3.
3.3. Raman and XRD Analysis
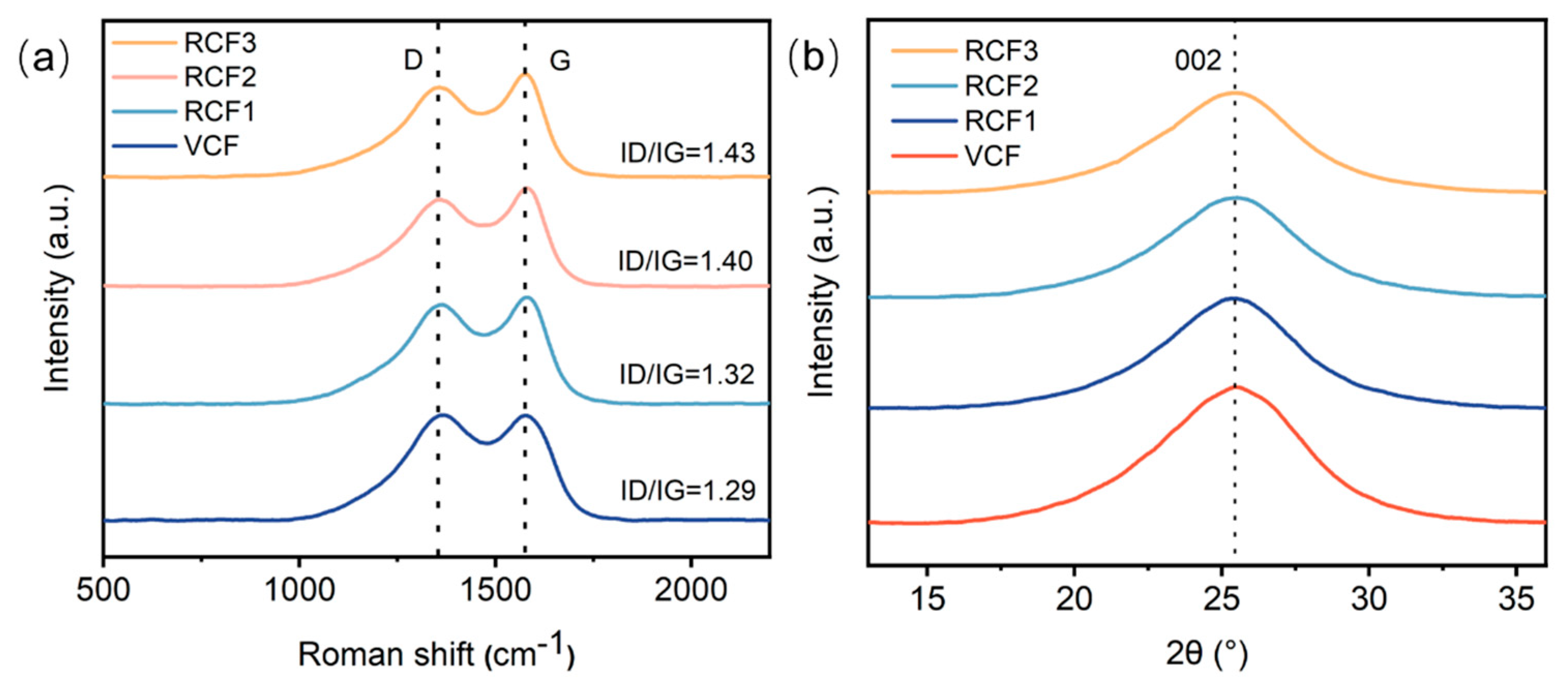
Figure 4a presents the Raman spectroscopy results for the carbon fibers. All Raman spectra exhibit two prominent bands: the D (disorder-induced) band at 1350 cm−1, and the G (graphitic) band at 1580 cm−1. The G band originates from the in-plane stretching motion of sp² carbon pairs, while the D band is attributed to disordered structures within the carbon fibers [26]. The degree of carbon atom order is evaluated using R = ID/IG, where lower R values indicate fewer defects and a higher degree of order [15,22]. The Raman spectra revealed that the R values of RCF were slightly higher than that of the VCF, indicating that the recycling process introduced defects in RCF, resulting in a reduction in graphitization. However, the smaller change increase in R values for RCF compared to the VCF suggests that the graphitization degree of RCF remained largely preserved, maintaining a high degree of carbon atom order.
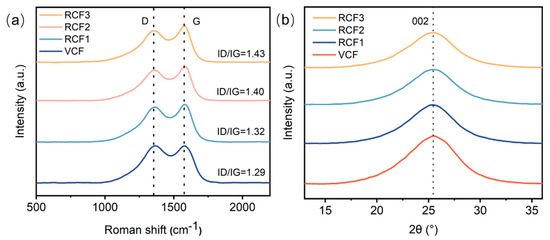
Figure 4.
(a) Raman spectroscopy test results; (b) XRD test results.
Figure 4b displays the XRD results for the VCF and RCF. The XRD patterns show a prominent (002) peak at approximately 2θ = 25.2°, attributed to the presence of imperfect graphite in the carbon fibers [27]. The layer spacing d002 in the (002) plane and the stacking height Lc of the carbon layers along the C-axis were calculated using the Scherrer Equation and Bragg Equation [28]. The results are summarized in Table 2. Compared to the VCF, RCF exhibited an increased half-peak width β and a reduced carbon layer stacking thickness Lc, indicating a decrease in the orderliness of RCF, consistent with the Raman spectroscopy findings. However, the half-peak width values for the VCF and RCF were approximately 0.09, with a crystal layer spacing d002 of around 0.35 nm and a carbon layer thickness of approximately 1.5 nm. These lattice parameters for RCF remained nearly identical compared to that of the VCF, suggesting that the sulfuric acid recycling process did not significantly alter the structure of carbon fibers [29].

Table 2.
Lattice parameters of carbon fiber 002 surface.
3.4. XPS and FTIR Analysis
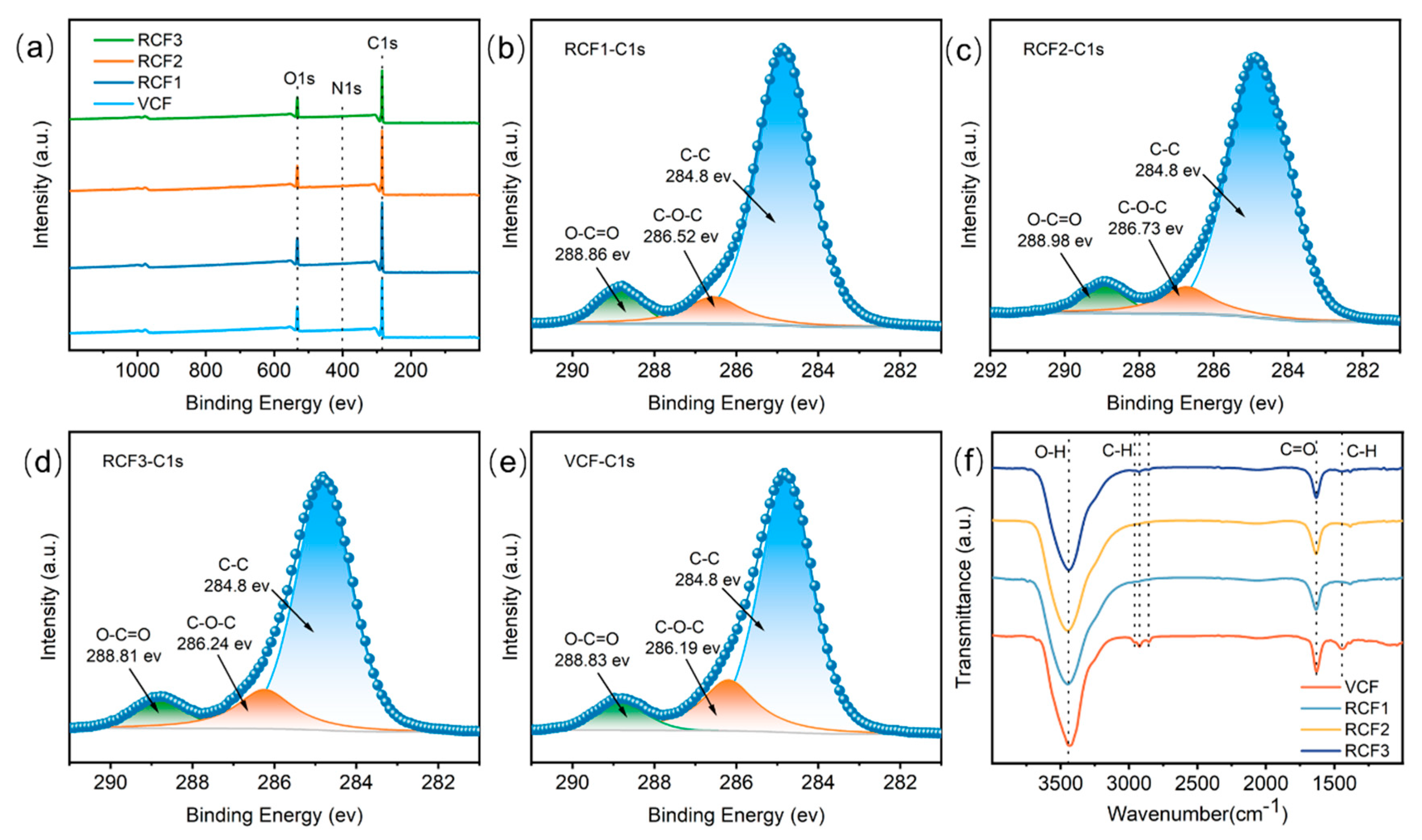
Figure 5a–e presents XPS results for the carbon fibers. The full-spectrum XPS scan shows three characteristic peaks at 284.9 eV, 532.4 eV, and 399.8 eV, corresponding to C1s, O1s, and N1s, respectively, with O/C values around 0.19 for RCF and the VCF. Further analysis of the carbon fiber surface using C1s spectroscopy identified three distinct peaks through curve fitting: 284.8 eV (-C–C-), 286.2 eV (-C–OH), and 288.8 eV (-COOH) [30]. The C1s spectral fitting results, summarized in Table 3, indicate that the C–C content in RCF treated with concentrated sulfuric acid decreased, while the -COOH content increased. Compared to the VCF, RCF exhibited a higher abundance of oxygen-containing functional groups, which improves adhesion between fibers and the resin matrix [31], thereby enhancing the potential for fiber reuse. The C–C bond content was most significantly reduced in RCF recovered at 140 °C, probably due to enhanced oxidation of the carbon fibers under high temperature and strongly acidic conditions.
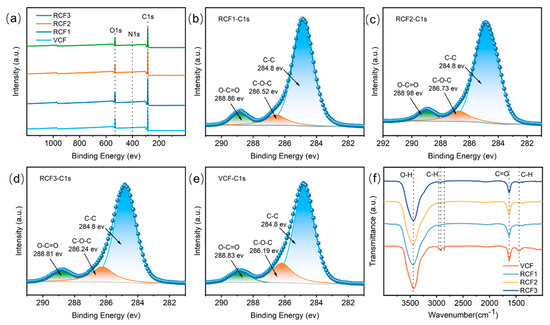
Figure 5.
Surface structure of carbon fibers: (a) XPS Survey; (b) RCF1-C1s; (c) RCF2-C1s; (d) RCF3-C1s; (e) VCF-C1s; (f) FTIR test results.

Table 3.
Surface structure of carbon fiber.
Figure 5f displays the FTIR spectroscopy results for the VCF and RCF. Characteristic peaks were observed at 3440 cm−1 (O–H stretching vibration), 2850 cm−1 and 2930 cm−1 (-CH2), 1450 cm−1 and 2960 cm−1 (-CH3), and 1630 cm−1 (C=O stretching vibration) [32]. Compared to the VCF, RCF showed a reduction or complete disappearance of C–H peaks, likely due to the strong oxidizing properties of concentrated sulfuric acid, which converted surface C–H bonds into other functional groups.
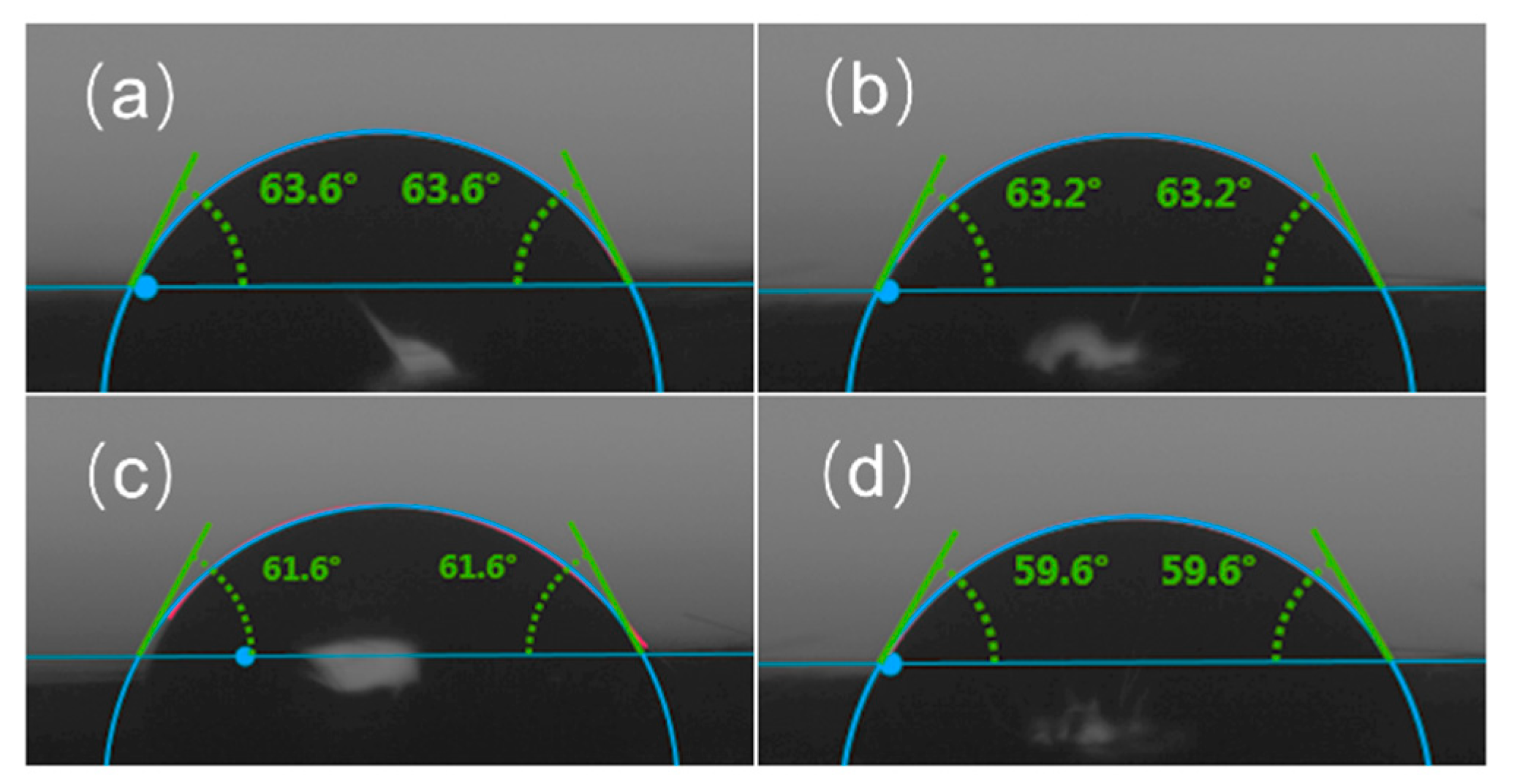
3.5. Contact Angle Analysis
Figure 6 presents the contact angle test results for the carbon fibers. The contact angle reflects the affinity between carbon fibers and resin, with a small contact angle indicating better wettability of the resin on the fiber surface [33]. The wettability of the VCF and RCF was evaluated using E51 epoxy resin as the wetting solution. The contact angles of sulfuric acid-treated carbon fibers were slightly lower than that of the VCF, indicating improved affinity between RCF and the resin. Among the RCF, RCF3 exhibited the smallest contact angle with E51 epoxy resin, likely because RCF3 has the highest -COOH content, which enhances resin affinity. Tian et al. [34] treated carbon fibers in concentrated nitric acid at 90 °C and reported improved wettability compared to the untreated VCF.
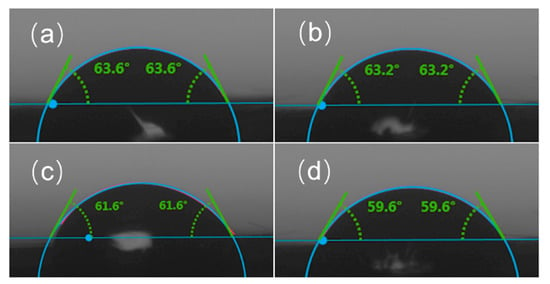
Figure 6.
Wetting angle of carbon fiber and resin: (a) VCF; (b) RCF1; (c) RCF2, (d) RCF3.
3.6. Properties of VCFRP and RCFRP
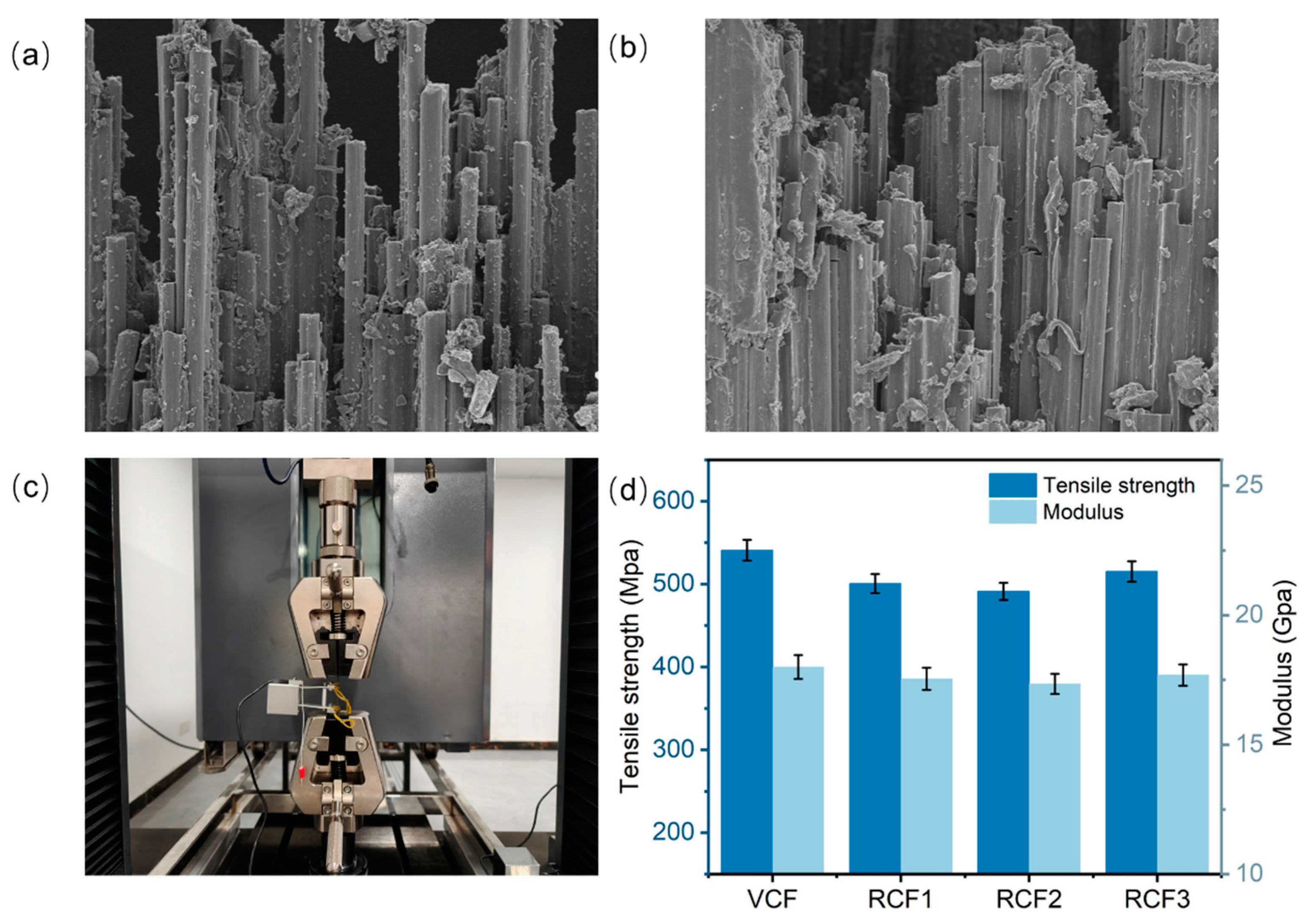
The mechanical properties of CFRP are influenced by two primary factors: the inherent mechanical properties of the fibers and the interfacial properties between the fibers and the resin matrix [35]. Figure 7a,b show the tensile section morphology of CFRP and RCFRP, respectively. The morphology reveals tight adhesion between the carbon fibers and the resin matrix, with no visible gaps at the fiber–resin interface. This strong interfacial bonding enables effective stress transfer from the resin matrix to the stress to the carbon fibers. Figure 7d shows the tensile property test results of CFRP. The tensile strength of the VCFRP was 540.93 MPa, while that of RCFRP exceeded 491.22 MPa. Compared to the VCFRP, RCFRP exhibited some attenuation in tensile properties but maintained over 91% of the tensile strength. Notably, RCFRP prepared with RCF3 achieved 95.25% of the tensile strength. Despite minor attenuation, RCFRP substantially preserved the tensile strength of the VCFRP.
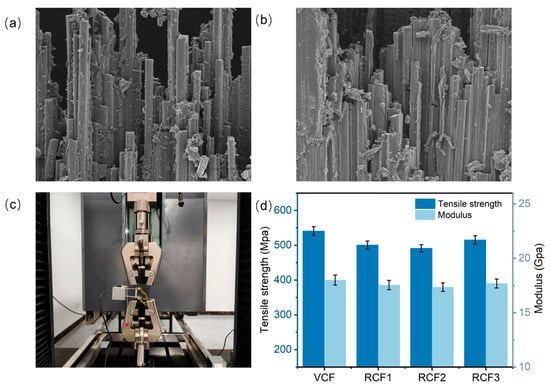
Figure 7.
(a) Tensile section morphology of CFRP; (b) tensile section morphology of RCFRP; (c) tensile test pictures; (d) tensile test results.
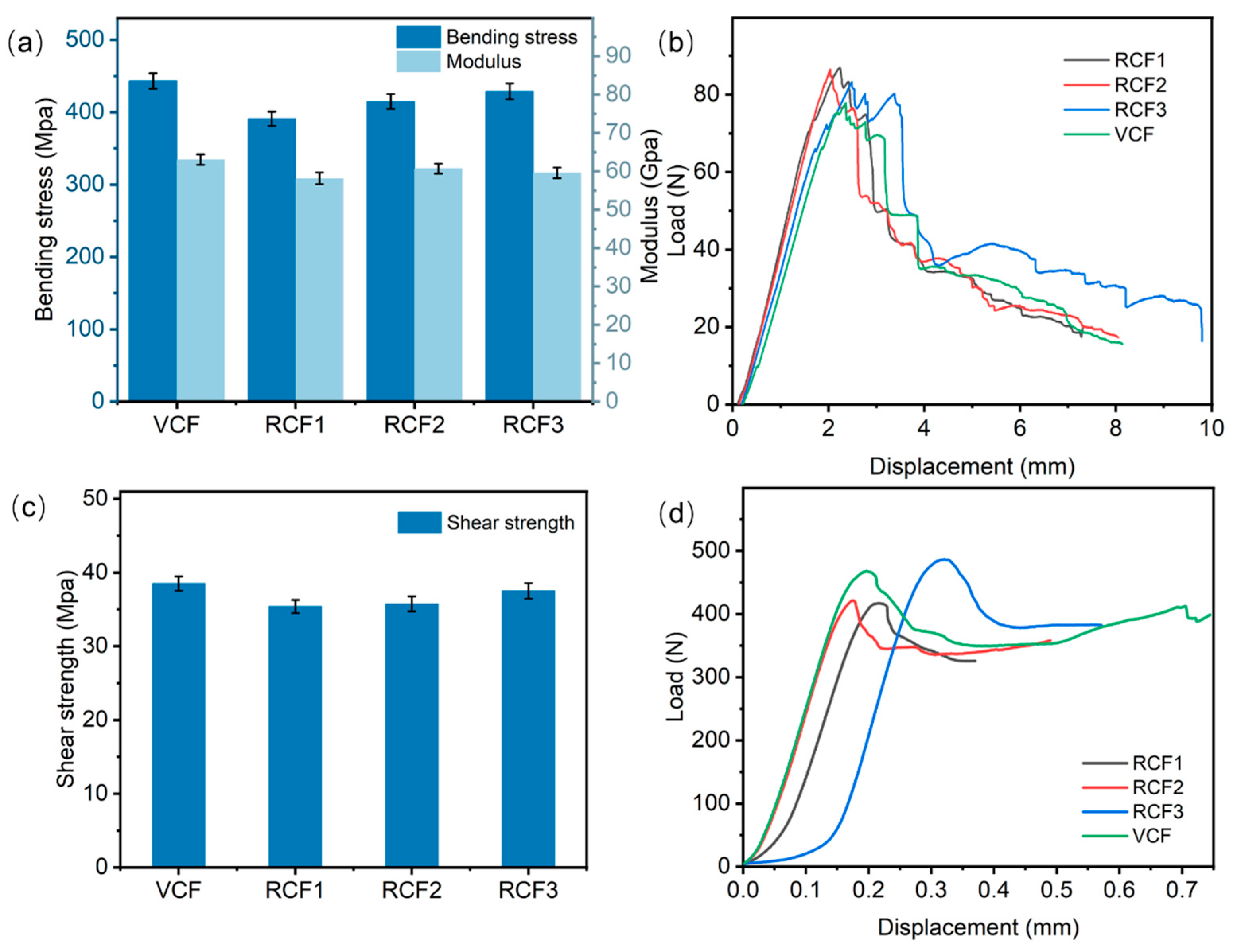
Figure 8a presents the test results of composite bending properties. The maximum bending stress of the VCFRP was 443.21 MPa, while the bending stresses of RCFRP were 390.97 MPa, 414.94 MPa, and 428.85 MPa for RCF1, RCF2, and RCF3, respectively. Carbon fiber-reinforced composites fabricated with RCF3 retained 97% of the bending stress of the VCFRP. In short-beam shear tests, specimens primarily experience interlayer shear stresses, which are largely determined by the interfacial bonding between the resin matrix and the fibers. Figure 8c shows the shear strength test results for the composites. The shear strength of the VRCRP and VCFRP was reduced by 0.97–3.1 MPa compared to the VCFRP, indicating that RCFRP substantially preserved the shear properties of the VCFRP.
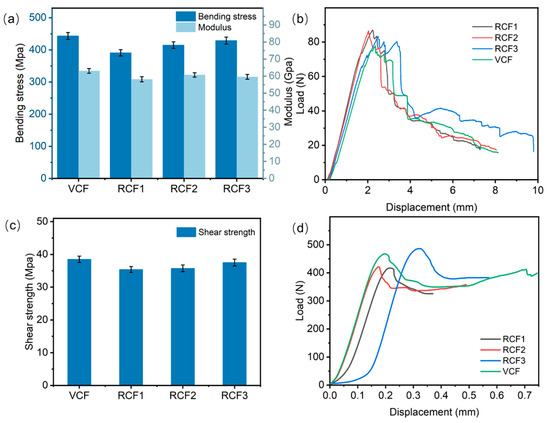
Figure 8.
(a) Flexural properties of CFRP; (b) displacement-load curve for flexural testing; (c) shear properties of CFRP; (d) displacement-load curve for shear test.
3.7. Resin Decomposition Products
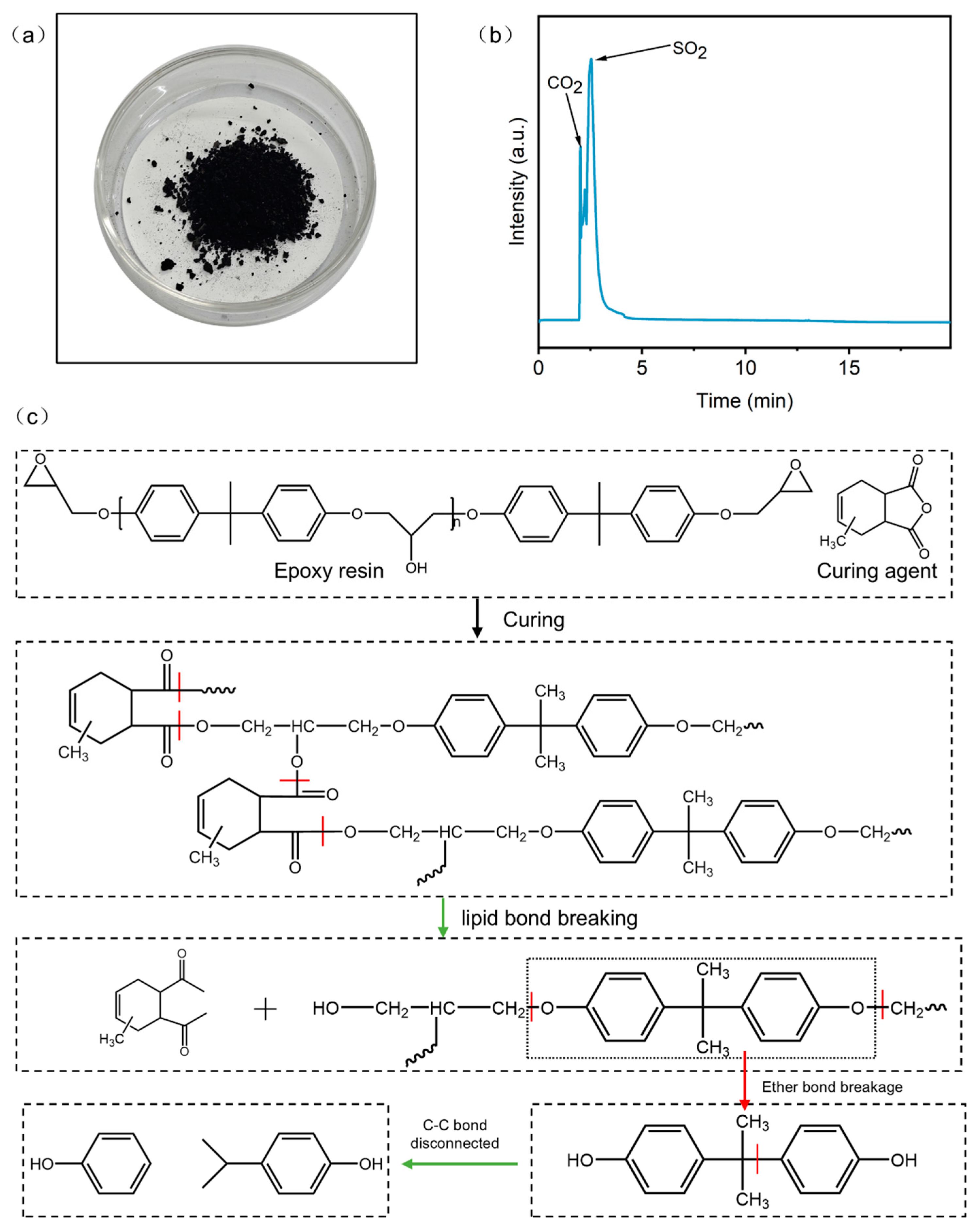
The degradation of CFRP in concentrated sulfuric acid yielded carbon slag, gaseous products, and liquid-phase products. Carbon slag formation resulted from the dehydrating properties of concentrated sulfuric acid, which dehydrated and carbonized the resin, ultimately producing carbon slag [36]. The gaseous products, primarily sulfur dioxide and carbon dioxide, were likely generated through reactions between concentrated sulfuric acid and organic components of the resin. The liquid-phase products were characterized using GC–MS, revealing phenol and 4-isopropylphenol as primary components. These compounds were likely formed through the following pathway: concentrated sulfuric acid cleaves the lipid and ether bonds in the cross-linked resin structure, degrading it into bisphenol A and other small molecule fragments [37,38]. As the reaction progresses, the C–C bonds in bisphenol A break, yielding phenol and 4-isopropylphenol (Figure 9 and Table 4).
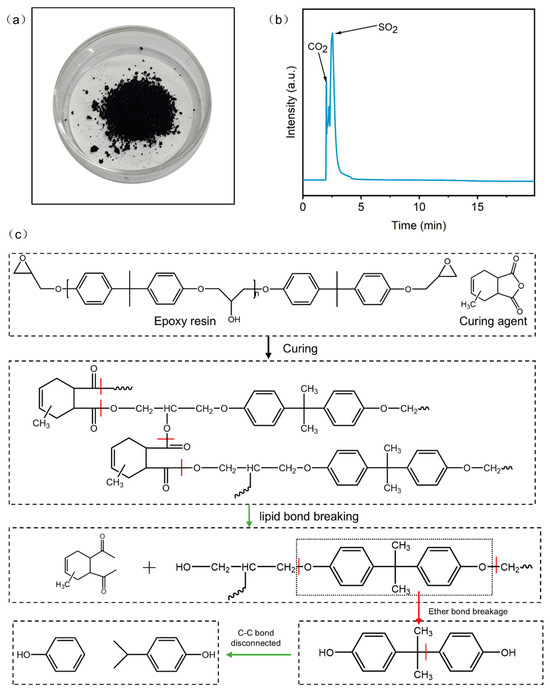
Figure 9.
(a) Carbon slag; (b) gas product; (c) possible bond breaking paths.

Table 4.
Liquid phase products.
4. Conclusions
This study introduces a microwave-assisted sulfuric acid recovery method for the efficient recycling of carbon fibers at low temperatures (80–140 °C). The results demonstrate that the resin degradation rate increases with higher reaction temperatures and longer reaction times. Complete resin degradation was not achieved at 80 °C within 60 min; however, at 100 °C, 120 °C, and 140 °C, complete degradation was achieved in 60, 40, and 20 min, respectively. During degradation, the resin undergoes dehydration and carbonization in concentrated sulfuric acid, forming carbon slag. The gaseous products include carbon dioxide and sulfur dioxide, while GC–MS identified phenol and 4-isopropylphenol as the primary liquid-phase products. RCF exhibited clean surfaces with no resin residue, cracks, or defects, retaining the axial groove structure of the VCF, which promotes interfacial mechanical interlocking with the resin matrix. XRD and Raman spectroscopy analyses confirmed that the graphitization degree and crystal structure of RCF remained largely unchanged compared with the VCF. XPS and FTIR analyses revealed an increased abundance of oxygen-containing functional groups on RCF surfaces, enhancing chemical bonding with the resin. This led to reduced contact angles and improved wettability of RCF compared to the VCF. RCFRP retained over 91% of the tensile strength, 88% of the bending stress, and 92% of the shear strength of the VCFRP. These results indicate that RCFRP maintain high mechanical consistency and reliability, enabling their use in recycled carbon fiber composites and facilitating waste recycling. This study provides a simple, effective approach for the low-temperature, efficient recycling of carbon fiber composites, with significant potential for industrial applications.
Author Contributions
Conceptualization, Z.N.; Data curation, Z.N., Y.R. and Y.S.; Formal analysis, Z.N.; Investigation, L.X., J.L., Y.S. and D.Z.; Methodology, Z.N.; Project administration, L.X.; Supervision, L.X.; Validation, Z.N., L.X., Y.R., J.L., Y.S., D.Z. and J.H.; Visualization, Y.R. and J.H.; Writing—original draft, Z.N.; Writing—review & editing, L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 52374305 and 51864030), China Petrochemical Corporation (Nos. 224113 and 223114), National Key R&D Program of China (Nos. 2023YFA1507703), Yunnan Fundamental Research Projects (Nos. 202301AV070009 and 202101AS070023), Yunling Scholars Program for Xingdian Talent Support Plan, and Yunnan Provincial youth top-notch talent support program.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Authors would like to acknowledge the National Natural Science Foundation of China, China Petrochemical Corporation, National Key R&D Program of China, Yunnan Fundamental Research Projects, Yunling Scholars Program for Xingdian Talent Support Plan, and Yunnan Provincial youth top-notch talent support program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, H.-H.; Kim, B.-J. Recovery of Carbon Fibers from Carbon Fiber-Reinforced Epoxy-Isophorone Diamine Composites via Step Thermolysis. Compos. Part B Eng. 2023, 260, 110757. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, G.; Vaidya, U.; Wang, H. Past, Present and Future Prospective of Global Carbon Fibre Composite Developments and Applications. Compos. Part B Eng. 2023, 250, 110463. [Google Scholar] [CrossRef]
- Pickering, S.J. Recycling Technologies for Thermoset Composite Materials—Current Status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Aldosari, S.M.; AlOtaibi, B.M.; Alblalaihid, K.S.; Aldoihi, S.A.; AlOgab, K.A.; Alsaleh, S.S.; Alshamary, D.O.; Alanazi, T.H.; Aldrees, S.D.; Alshammari, B.A. Mechanical Recycling of Carbon Fiber-Reinforced Polymer in a Circular Economy. Polymers 2024, 16, 1363. [Google Scholar] [CrossRef]
- Butenegro, J.A.; Bahrami, M.; Abenojar, J.; Martínez, M.Á. Recent Progress in Carbon Fiber Reinforced Polymers Recycling: A Review of Recycling Methods and Reuse of Carbon Fibers. Materials 2021, 14, 6401. [Google Scholar] [CrossRef]
- García, D.; Vegas, I.; Cacho, I. Mechanical Recycling of GFRP Waste as Short-Fiber Reinforcements in Microconcrete. Constr. Build. Mater. 2014, 64, 293–300. [Google Scholar] [CrossRef]
- Meyer, L.O.; Schulte, K.; Grove-Nielsen, E. CFRP-Recycling Following a Pyrolysis Route: Process Optimization and Potentials. J. Compos. Mater. 2009, 43, 1121–1132. [Google Scholar] [CrossRef]
- Wei, Y.; Hadigheh, S.A. Enhancing Carbon Fibre Recovery through Optimised Thermal Recycling: Kinetic Analysis and Operational Parameter Investigation. Mater. Today Sustain. 2024, 25, 100661. [Google Scholar] [CrossRef]
- Pickering, S.J.; Kelly, R.M.; Kennerley, J.R.; Rudd, C.D.; Fenwick, N.J. A Fluidised-Bed Process for the Recovery of Glass Fibres from Scrap Thermoset Composites. Compos. Sci. Technol. 2000, 60, 509–523. [Google Scholar] [CrossRef]
- Jeong, J.-S.; Kim, K.-W.; An, K.-H.; Kim, B.-J. Fast Recovery Process of Carbon Fibers from Waste Carbon Fibers-Reinforced Thermoset Plastics. J. Environ. Manag. 2019, 247, 816–821. [Google Scholar] [CrossRef]
- Piñero-Hernanz, R.; Dodds, C.; Hyde, J.; García-Serna, J.; Poliakoff, M.; Lester, E.; Cocero, M.J.; Kingman, S.; Pickering, S.; Wong, K.H. Chemical Recycling of Carbon Fibre Reinforced Composites in Nearcritical and Supercritical Water. Compos. Part A Appl. Sci. Manuf. 2008, 39, 454–461. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.J.; Lester, E.H.; Turner, T.A.; Wong, K.H.; Warrior, N.A. Characterisation of Carbon Fibres Recycled from Carbon Fibre/Epoxy Resin Composites Using Supercritical n-Propanol. Compos. Sci. Technol. 2009, 69, 192–198. [Google Scholar] [CrossRef]
- Hanaoka, T.; Ikematsu, H.; Takahashi, S.; Ito, N.; Ijuin, N.; Kawada, H.; Arao, Y.; Kubouchi, M. Recovery of Carbon Fiber from Prepreg Using Nitric Acid and Evaluation of Recycled CFRP. Compos. Part B Eng. 2022, 231, 109560. [Google Scholar] [CrossRef]
- Jiang, J.; Deng, G.; Chen, X.; Gao, X.; Guo, Q.; Xu, C.; Zhou, L. On the Successful Chemical Recycling of Carbon Fiber/Epoxy Resin Composites under the Mild Condition. Compos. Sci. Technol. 2017, 151, 243–251. [Google Scholar] [CrossRef]
- Rijo, B.; Dias, A.P.S.; Carvalho, J.P.S. Recovery of Carbon Fibers from Aviation Epoxy Composites by Acid Solvolysis. Sustain. Mater. Technol. 2023, 35, e00545. [Google Scholar] [CrossRef]
- Kubouchi, M.; Tsuda, K.; Nishiyama, T.; Hojo, H. A Study on an Application of Corrosion Behavior to Glass Fiber Composite Disposal and Recycling. Adv. Compos. Lett. 1995, 4, 13–15. [Google Scholar] [CrossRef]
- Hanaoka, T.; Arao, Y.; Kayaki, Y.; Kuwata, S.; Kubouchi, M. Analysis of Nitric Acid Decomposition of Epoxy Resin Network Structures for Chemical Recycling. Polym. Degrad. Stab. 2021, 186, 109537. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Sembokuya, H.; Tsuda, K. Chemical Recycling of Glass Fiber Reinforced Epoxy Resin Cured with Amine Using Nitric Acid. Polymer 2005, 46, 1905–1912. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Yamamoto, S.; Sembokuya, H.; Tsuda, K. An Approach to Chemical Recycling of Epoxy Resin Cured with Amine Using Nitric Acid. Polymer 2002, 43, 2953–2958. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, H.-O.; Kim, J.-S.; Lee, C.-K.; Kim, Y.-K.; Ju, C.-S. Circulating Flow Reactor for Recycling of Carbon Fiber from Carbon Fiber Reinforced Epoxy Composite. Korean J. Chem. Eng. 2011, 28, 449–454. [Google Scholar] [CrossRef]
- Deng, J.; Xu, L.; Zhang, L.; Peng, J.; Guo, S.; Liu, J.; Koppala, S. Recycling of Carbon Fibers from CFRP Waste by Microwave Thermolysis. Processes 2019, 7, 207. [Google Scholar] [CrossRef]
- Salas, A.; Berrio, M.E.; Martel, S.; Díaz-Gómez, A.; Palacio, D.A.; Tuninetti, V.; Medina, C.; Meléndrez, M.F. Towards Recycling of Waste Carbon Fiber: Strength, Morphology and Structural Features of Recovered Carbon Fibers. Waste Manag. 2023, 165, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jiang, J.; Zhang, L.; Wang, S.; Chen, J.; Yao, X.; Li, Y. Simple and Mild Method for the Recycling of Carbon-Fiber-Reinforced Bismaleimide Resin Composite Waste. ACS Sustain. Chem. Eng. 2023, 11, 2830–2839. [Google Scholar] [CrossRef]
- Wu, T.; Zhan, W.; Jia, X.; Li, H.; Sui, G.; Yang, X. Solvent-Free Rapid Degradation of Epoxy Composites and Recycling Application of High Performance Carbon Fibers through the Synergic Catalysis Effect of Molten Salts and Titanium Dioxide. Polym. Degrad. Stab. 2022, 196, 109849. [Google Scholar] [CrossRef]
- Pan, Y.; Mao, J.; Ding, J. Effect of Carbon Fiber Surface Modification on the Mechanical Properties of Carbon-Fiber-Reinforced Ultrahigh-Molecular-Weight Polyethylene Composite. J. Mater. Eng. Perform. 2019, 28, 1995–2005. [Google Scholar] [CrossRef]
- Huson, M.G.; Church, J.S.; Kafi, A.A.; Woodhead, A.L.; Khoo, J.; Kiran, M.S.R.N.; Bradby, J.E.; Fox, B.L. Heterogeneity of Carbon Fibre. Carbon 2014, 68, 240–249. [Google Scholar] [CrossRef]
- Wang, X.; Qian, X.; Zhang, Y.; Wang, X.; Song, S.; Zhang, C. Surface Oxidation of PAN-Based Ultrahigh Modulus Carbon Fibers (UHMCFs) and Its Effect on the Properties of UHMCF/EP Composites. Carbon Lett. 2021, 31, 449–461. [Google Scholar] [CrossRef]
- Liu, F.; Wang, H.; Xue, L.; Fan, L.; Zhu, Z. Effect of Microstructure on the Mechanical Properties of PAN-Based Carbon Fibers during High-Temperature Graphitization. J. Mater. Sci. 2008, 43, 4316–4322. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Ding, J. Chemical Recycling of Carbon Fibre/Epoxy Composites in a Mixed Solution of Peroxide Hydrogen and N,N-Dimethylformamide. Compos. Sci. Technol. 2013, 82, 54–59. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, W.; Jin, X.; Liang, X.; Sui, G.; Yang, X. Efficient Reclamation of Carbon Fibers from Epoxy Composite Waste through Catalytic Pyrolysis in Molten ZnCl2. RSC Adv. 2018, 9, 377–388. [Google Scholar] [CrossRef]
- Guo, H.; Huang, Y.D.; Meng, L.H.; Liu, L.; Fan, D.P.; Liu, D.X. Interface Property of Carbon Fibers/Epoxy Resin Composite Improved by Hydrogen Peroxide in Supercritical Water. Mater. Lett. 2009, 63, 1531–1534. [Google Scholar] [CrossRef]
- Huang, H.; Yin, Y.; Cheng, H.; Zhao, Z.; Zhang, B. Degradation Mechanism of CF/EP Composites in Supercritical n-Butanol with Alkali Additives. J Polym. Environ. 2017, 25, 115–125. [Google Scholar] [CrossRef]
- Ma, L.; Li, N.; Wu, G.; Song, G.; Li, X.; Han, P.; Wang, G.; Huang, Y. Interfacial Enhancement of Carbon Fiber Composites by Growing TiO2 Nanowires onto Amine-Based Functionalized Carbon Fiber Surface in Supercritical Water. Appl. Surf. Sci. 2018, 433, 560–567. [Google Scholar] [CrossRef]
- Tian, H.; Yao, Y.; Liu, D.; Li, Y.; Jv, R.; Xiang, G.; Xiang, A. Enhanced Interfacial Adhesion and Properties of Polypropylene/Carbon Fiber Composites by Fiber Surface Oxidation in Presence of a Compatibilizer. Polym. Compos. 2019, 40, E654–E662. [Google Scholar] [CrossRef]
- Ansari, M.S.; Zafar, S.; Pathak, H. A Comprehensive Review of Surface Modification Techniques for Carbon Fibers for Enhanced Performance of Resulting Composites. Results Surf. Interfaces 2023, 12, 100141. [Google Scholar] [CrossRef]
- Mozes, M.S. Volume Reduction of Spent Ion-Exchange Resin by Acid Digestion. Nucl. Technol. 1982, 59, 270–278. [Google Scholar] [CrossRef]
- Shen, M.; Robertson, M.L. Degradation Behavior of Biobased Epoxy Resins in Mild Acidic Media. ACS Sustain. Chem. Eng. 2021, 9, 438–447. [Google Scholar] [CrossRef]
- Savou, V.; Grause, G.; Kumagai, S.; Saito, Y.; Kameda, T.; Yoshioka, T. Pyrolysis of Sugarcane Bagasse Pretreated with Sulfuric Acid. J. Energy Inst. 2019, 92, 1149–1157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).