Critical Success Factors for Supplier Selection and Performance Enhancement in the Medical Device Industry: An Industry 4.0 Approach
Abstract
1. Introduction
2. Literature Review
2.1. Critical Success Factors in Supplier Selection for MDM Companies
2.2. The Relationship Between Quality as a Critical Factor in Supplier Selection and Organizational Performance in MDM Companies
2.3. The Medical Device Manufacturing (MDM) Industry in Mexico
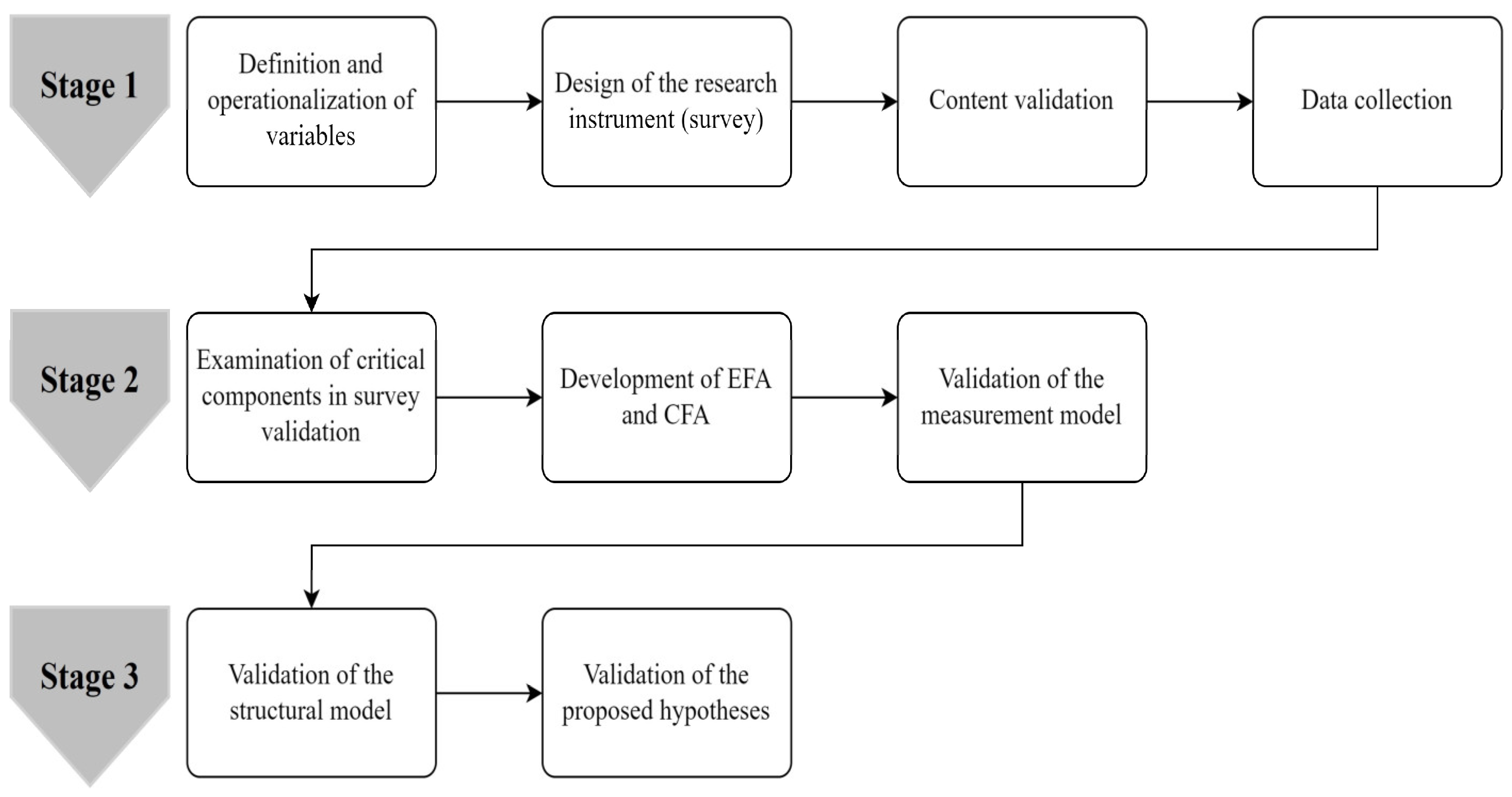
3. Materials and Methods
3.1. Survey Development and Sampling
3.2. Statistical Validation of the Survey
3.3. Structural Equation Modeling (SEM)
4. Results
4.1. Application of the Survey
4.2. Data Analysis and Results
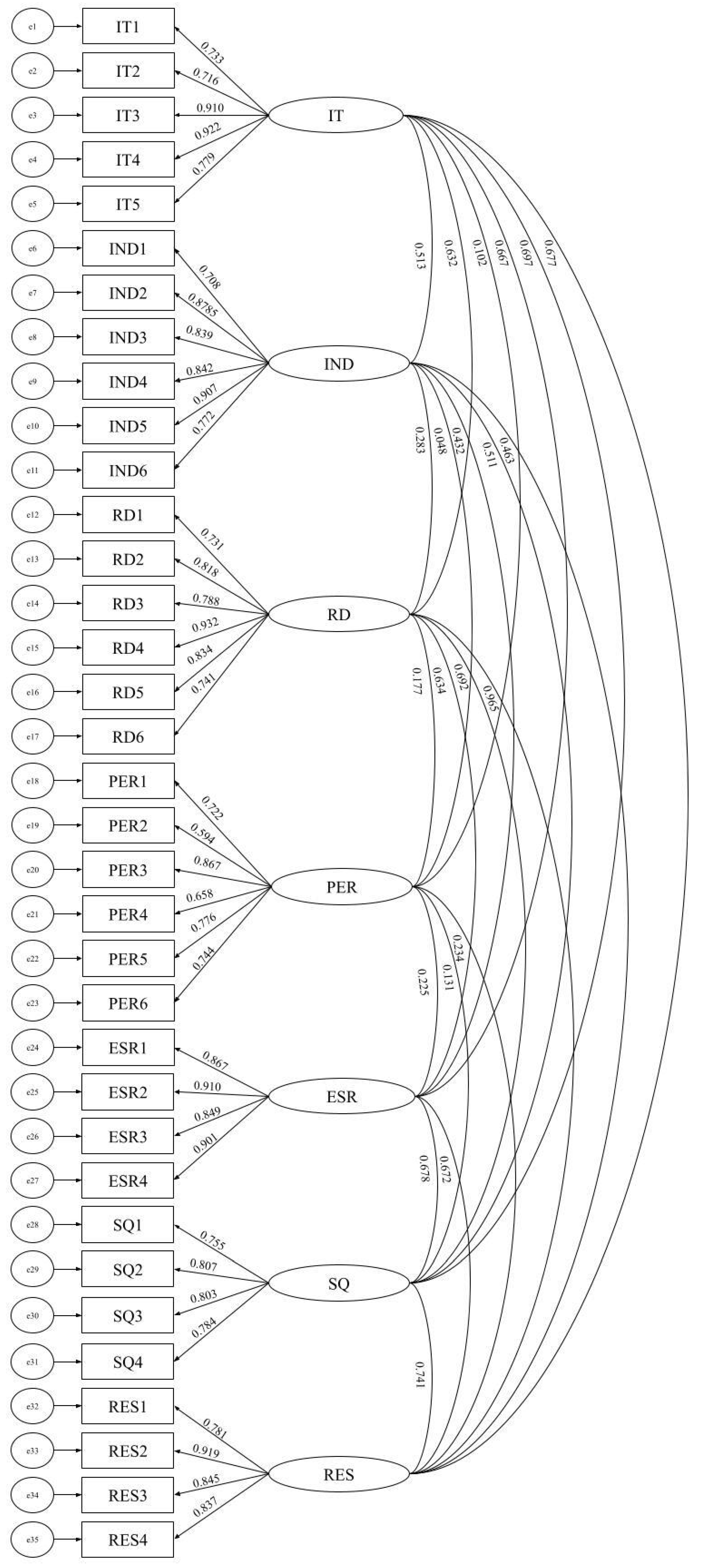
4.2.1. Results of the Exploratory Factor Analysis (EFA)
4.2.2. Results of the Confirmatory Factor Analysis (CFA)
4.2.3. Results of the Construct Validity
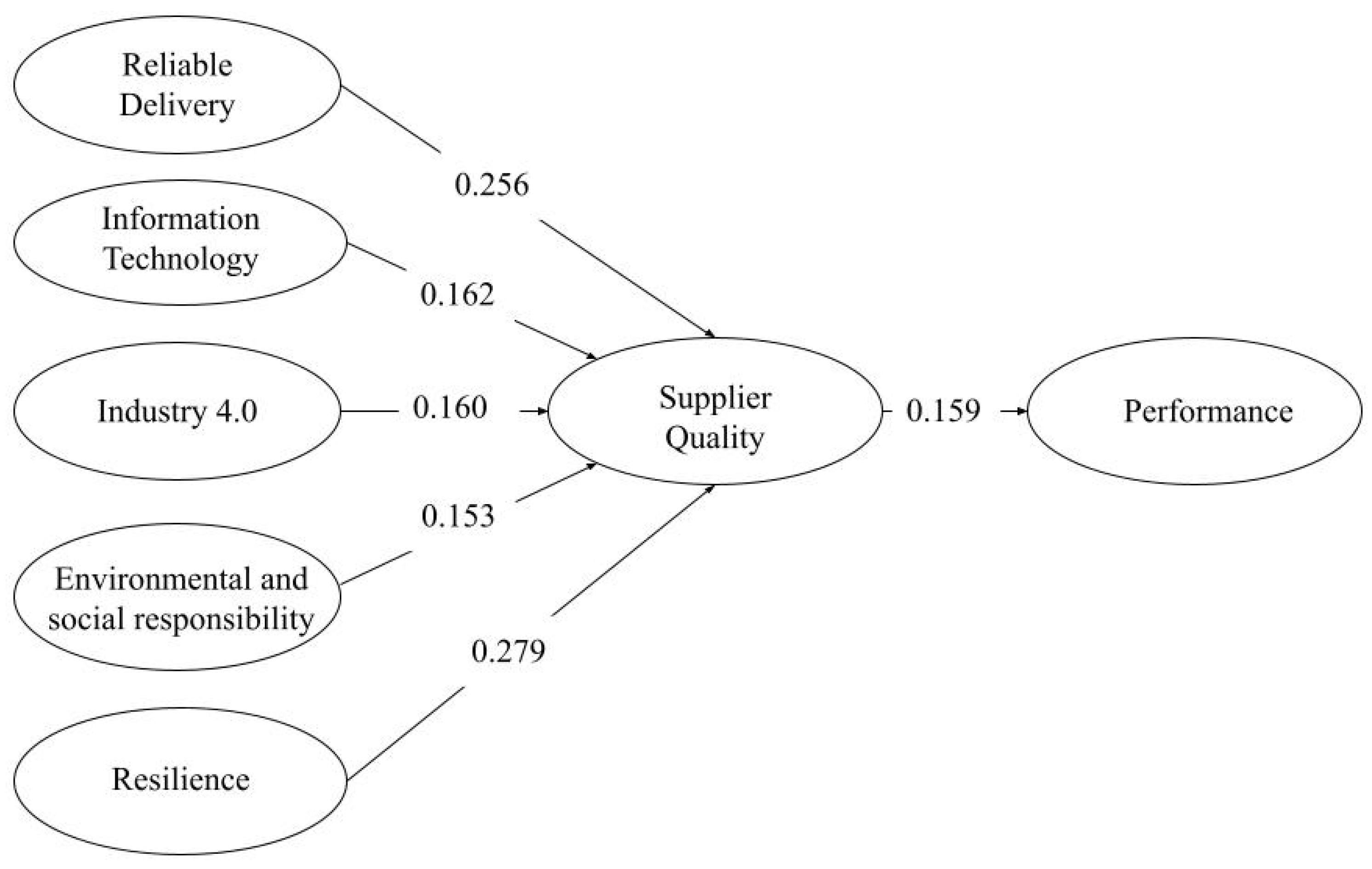
4.3. Evaluating Hypothesized Relationships Using SEM
5. Discussion
5.1. Theoretical Implications
5.2. Practical Implications
5.3. Challenges and Limitations
6. Conclusions
Research Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDM | Medical Device Manufacturing |
| CSF | Critical Success Factors |
| SEM | Structural Equation Modeling |
| SCM | Supply Chain Management |
| SC | Supply Chains |
| HSC | Healthcare Supply Chain |
| RE | Resilience Engineering |
| HSC4.0 | Healthcare Supply Chain 4.0 |
| GMRF | Global Model Regulatory Framework |
| RD | Reliable Delivery |
| IT | Information Technology |
| PER | Performance |
| ERP | Enterprise Resource Planning |
| RFID | Radio Frequency Identification |
| IND | Industry 4.0 Technologies |
| ESR | Environmental and Social Responsibility |
| CSR | Corporate Social Responsibility |
| RES | Resilience |
| EFA | Exploratory Factor Analysis |
| CFA | Confirmatory Factor Analysis |
| SQ | Supplier Quality |
| AMOS® | Analysis of Moment Structures |
| VIF | Variance Inflation Factors |
| KMO | Kaiser–Meyer–Olkin |
| CMIN | Minimum Discrepancy Coefficient |
| DF | Degrees of Freedom |
| RMSEA | Root Mean Square Error of Approximation |
| SRMR | Standardized Root Mean Residual |
| TLI | Tucker–Lewis Index |
| CFI | Comparative Fit Index |
| AVE | Average Variance Extracted |
| PNFI | Parsimony Normed Fit Index |
| IOT | Internet of Things |
| SRW | Standardized Regression Weights |
| CR | Critical Ratio |
| P | p-Value |
| SE | Standardized Error |
| SECIHTI | Secretaría de Ciencia, Humanidades, Tecnología e Innovación |
| UABC | Universidad Autónoma de Baja California |
Appendix A
| Construct | Item |
|---|---|
| Supplier Quality | |
| SQ1 | To what extent do suppliers demonstrate a robust quality system? |
| SQ2 | To what extent do suppliers ensure that their processes are of quality? |
| SQ3 | To what extent do suppliers have a quality philosophy aligned with my company’s quality philosophy? |
| SQ4 | To what extent do suppliers have a system for evaluating their suppliers’ performance that allows them to select them better? |
| Reliable Delivery | |
| RD1 | To what extent do suppliers meet delivery schedules on time? |
| RD2 | To what extent do suppliers deliver in full according to what is established in the order? |
| RD3 | To what extent are suppliers performing to an established compliance rate? |
| RD4 | To what extent do suppliers demonstrate adequate handling and conservation processes for the products/services required? |
| RD5 | To what extent do suppliers demonstrate a product identification and traceability system? |
| RD6 | To what extent do providers offer greater benefits that can be reflected in costs, prices, and care? |
| Information technology | |
| IT1 | To what extent do suppliers have cutting-edge and updated technology in their production processes? |
| IT2 | To what extent do suppliers have the technological capacity to meet the needs and/or requirements of my company? |
| IT3 | To what extent do suppliers use Information Technology (IT)-based support for exchanging shipping and delivery information? |
| IT4 | To what extent do suppliers use IT for inventory management and/or reporting their warehouse stocks? |
| IT5 | To what extent do suppliers share information in real-time to work on common demand forecasts? |
| Environmental and social responsibility | |
| ESR1 | To what extent do suppliers show commitment to the environment in the design of their products? |
| ESR2 | To what extent do suppliers have environmental policies? |
| ESR3 | To what extent do suppliers implement recycling programs (for relevant materials and/or resources)? |
| ESR4 | To what extent do suppliers have activities that have a social impact inside and outside their facilities? |
| Resilience | |
| RES1 | To what extent can suppliers keep us alert of any situation at all times? |
| RES2 | To what extent can suppliers cope with the changes brought about by SC disruption? |
| RES3 | To what extent can suppliers recover normal operations quickly after SC disruption? |
| RES4 | To what extent do suppliers offer flexibility to changes or modifications to product and/or process requirements? |
| Industry 4.0 | |
| IND1 | To what extent do suppliers use artificial intelligence? |
| IND2 | To what extent do providers use automation? |
| IND3 | To what extent do providers use simulation? |
| IND4 | To what extent are providers using remote sensing? |
| IND5 | To what extent are suppliers using collaborative robot systems? |
| IND6 | To what extent are suppliers using 3D printing/additive manufacturing? |
| Performance | |
| PER1 | Considering the operational efficiency of the last year, to what extent does my company comply with production plans? |
| PER2 | Considering operational efficiency over the past year, to what extent does my company have a program for developing new products to meet customer needs? |
| PER3 | Considering cost competitiveness over the past year, to what extent can my company compete on price within the market? |
| PER4 | Considering cost competitiveness during the past year, to what extent has my company managed to reduce production costs due to innovation in production processes? |
| PER5 | Considering cost competitiveness over the past year, to what extent does my company offer competitive prices as a result of product innovation? |
| PER6 | Considering the responsiveness over the past year, to what extent is my company able to satisfy customers in terms of volume and delivery time? |
References
- Zhu, Q.; Liu, A.; Li, Z.; Yang, Y.; Miao, J. Sustainable Supplier Selection and Evaluation for the Effective Supply Chain Management System. Systems 2022, 10, 166. [Google Scholar] [CrossRef]
- Mojtaba, H.H. Risk Assessment in the Global Supplier Selection Considering Supply Disruption: A Simulation Optimization Approach. Int. J. Supply Oper. Manag. 2023, 10, 501–522. [Google Scholar] [CrossRef]
- Aljuneidi, T.; Bhat, S.A.; Boulaksil, Y. A Comprehensive Systematic Review of the Literature on the Impact of the COVID-19 Pandemic on Supply Chains. Suppl. Chain. Anal. 2023, 3, 100025. [Google Scholar] [CrossRef]
- Hrishikesh, S.M.; Peddireddy, S.; Prashant, K.; Mansi. Importance of Supply Chain & Logistics Post Pandemic. EPRA Int. J. Econ. Bus. Manag. Stud. 2022, 9, 10–14. [Google Scholar] [CrossRef]
- Evcıoğlu, H.E.; Kabak, M. Supplier Selection in Supply Chain Network Using MCDM Methods. Sigma J. Eng. Nat. Sci. 2023, 41, 1–16. [Google Scholar] [CrossRef]
- Milovanović, G.; Milenović, J. Selection of Suppliers in the Supply Chain. Facta Univ. Ser. Econ. Organ. 2022, 19, 69–81. [Google Scholar] [CrossRef]
- Koufteros, X.; Vickery, S.K.; Dröge, C. The Effects of Strategic Supplier Selection on Buyer Competitive Performance in Matched Domains: Does Supplier Integration Mediate the Relationships? J. Supply Chain. Manag. 2012, 48, 93–115. [Google Scholar] [CrossRef]
- Ionel, E.-S. Supplier Selection Strategy—NPV Comparison. Rom. Econ. J. 2023, 26, 78–85. [Google Scholar] [CrossRef]
- Ngam, M.C.; Thiruchelvam, S.; Mustapha, K.N.; Rusli, M.E.; Mohd Hashim, A.; Ghazali, A.; Hakimie, H. Critical Success Factors for Supplier Selection in the Construction Industry: The Case of Public Works Department. Indian J. Sci. Technol. 2016, 9, 48. [Google Scholar] [CrossRef]
- Voeng, S.; Kritchanchai, D. Factors Influencing Supplier Selection for Vendor Managed Inventory Adoption in Hospitals. In Proceedings of the TIMES-iCON 2019—2019 4th Technology Innovation Management and Engineering Science International Conference, Bangkok, Thailand, 11–13 December 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Gündüz, Ç.; Gündüz, G.Ş. Supplier Selection under Fuzzy Environment. Tekst. Ve Konfeksiyon 2019, 29, 344–352. [Google Scholar] [CrossRef]
- Haris, J.; Abdul Rahim, S.; Haris, M.; Zahari, M.S. Critical Success Factors of Supplier Selection: Findings from Social Commerce Micro-Businesses in Malaysia. Int. J. Acad. Res. Bus. Soc. Sci. 2021, 11, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Niaz, M.; Nwagwu, U. Managing Healthcare Product Demand Effectively in the Post-Covid-19 Environment: Navigating Demand Variability and Forecasting Complexities. Am. J. Econ. Manag. Bus. 2023, 2, 316–330. [Google Scholar] [CrossRef]
- Harer, J. Management for Critical Medical Device and IVD Suppliers. In Medical Devices and In Vitro Diagnostics: Requirements in Europe; Baumgartner, C., Harer, J., Schröttner, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–26. ISBN 978-3-030-98743-5. [Google Scholar]
- Mcbride, M.L. Predicting Vulnerabilities in the Delivery of Secure Healthcare Supply Chain Services. Cybersecur. Innov. Technol. J. 2024, 2, 26–40. [Google Scholar] [CrossRef]
- Sastri, V.R. 11—Purchasing Controls and Supplier Quality for Medical Device Manufacturers and Their Suppliers. In Plastics in Medical Devices, 3rd ed.; Sastri, V.R., Ed.; William Andrew Publishing: Norwich, NY, USA, 2022; pp. 423–439. ISBN 978-0-323-85126-8. [Google Scholar]
- Ebrahimi, A. Identifying and Ranking Hospital Suppliers and Choosing the Right Supplier in Supply Chain Management. J. Rescue Relief 2023, 15, 153–161. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Eltoukhy, M.M.; Al Ruqeishi, K.; Salah, A. An Adapted Multi-Objective Genetic Algorithm for Healthcare Supplier Selection Decision. Mathematics 2023, 11, 1537. [Google Scholar] [CrossRef]
- Rockart, J.F. Chief Executives Define Their Own Data Needs.Pdf. Harv. Bus. Rev. 1979, 57, 81–93. [Google Scholar]
- Boynton, A.C.; Zmud, R.W. Assessment of Critical Success Factors. Sloan Manag. Rev. 1984, 25, 17–27. [Google Scholar]
- Belassi, W.; Tukel, O.I. A New Framework for Determining Critical Success/Failure Factors in Projects. Int. J. Proj. Manag. 1996, 14, 141–151. [Google Scholar] [CrossRef]
- Babandi, I.G.; Bardai, B.B. A Review of Critical Success Factors Influencing the Success of SMEs. SEISENSE Bus. Rev. 2023, 3, 37–61. [Google Scholar] [CrossRef]
- Taherdoost, H.; Brard, A. Analyzing the Process of Supplier Selection Criteria and Methods. Procedia Manuf. 2019, 32, 1024–1034. [Google Scholar] [CrossRef]
- Aguezzoul, A. Overview on Supplier Selection of Goods versus 3PL Selection. In Proceedings of the 2011 4th International Conference on Logistics, LOGISTIQUA’2011, Hammamet, Tunisia, 4 July 2011; pp. 248–253. [Google Scholar]
- Al Hazza, M.; Dapit, A.; Bourini, I.F.; Muataz, Z.; Ali, M.Y. Multicriteria Decision Making on Supplier Selection Using Soccer Model Integrated With Analytical Hierarchy Process. IIUM Eng. J. 2023, 24, 239–257. [Google Scholar] [CrossRef]
- Zu, X.; Cui, Y. An Empirical Model of Supplier Relation and Management for Better Quality. Int. J. Appl. Manag. Sci. 2013, 5, 217–233. [Google Scholar] [CrossRef]
- Kurniawan, R.B. Causal Relations on Beneficial and Non-Beneficial Factors for A Supplier Selection Problem. Ind. Eng. J. Univ. Sarjanawiyata Tamansiswa 2021, 5, 35–40. [Google Scholar]
- Frej, E.A.; Roselli, L.R.P.; Araújo De Almeida, J.; De Almeida, A.T. A Multicriteria Decision Model for Supplier Selection in a Food Industry Based on FITradeoff Method. Math. Probl. Eng. 2017, 2017, 41914. [Google Scholar] [CrossRef]
- Mohammad Sabbaghi, M.; Allahyari, A. A Supplier Selection Model Emphasizing the Project Risk Management in Drug Production in Pharmaceutical Industry. Teh. Glas. 2020, 14, 111–120. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Model Regulatory Framework for Medical Devices Including In Vitro Diagnostic Medical Devices (Who Medical Device Technical); World Health Organization: Geneva, Switzerland, 2017; pp. 1–68. ISBN 9789241512350.
- Patel, M.; Bhojak, K.; Patel, P. Analysis of Supplier Selection Process on Product Quality. Int. J. Sci. Res. 2013, 2, 182–185. [Google Scholar]
- Jayatilleka, S. Effective Supplier Reliability Introduction to Supply Chain & Supplier Quality Programs. In Proceedings of the 2024 Annual Reliability and Maintainability Symposium, Albuquerque, NM, USA, 22–25 January 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Hussein, M.; Elmelhy, M.T.; Salia, A.M. Operations Management, and Quality Control in the U.S. Medical Device Industry. Glob. Acad. J. Econ. Buss 2023, 5, 7–15. [Google Scholar] [CrossRef]
- Fragapane, G.I.; Bertnum, A.B.; Strandhagen, J.O. Possibilities and Benefits of Using Material Flow Information to Improve the Internal Hospital Supply Chain. In IFIP Advances in Information and Communication Technology; Springer: New York, NY, USA, 2019; Volume 567, pp. 240–247. [Google Scholar]
- Irfan, M.; Wang, M.; Akhtar, N. Impact of IT Capabilities on Supply Chain Capabilities and Organizational Agility: A Dynamic Capability View. Oper. Manag. Res. 2019, 12, 113–128. [Google Scholar] [CrossRef]
- Hwang, W.; Min, H. Assessing the Impact of ERP on Supplier Performance. Ind. Manag. Data Syst. 2013, 113, 1025–1047. [Google Scholar] [CrossRef]
- Furstenau, L.B.; Zani, C.; Terra, S.X.; Sott, M.K.; Choo, K.K.R.; Saurin, T.A. Resilience Capabilities of Healthcare Supply Chain and Supportive Digital Technologies. Technol. Soc. 2022, 71, 102095. [Google Scholar] [CrossRef]
- Grant, O. A Qualitative Study on the Impact of Advanced Technologies on Supplier Collaboration. Bus. Manag. 2025. preprint. [Google Scholar] [CrossRef]
- Pratiwi, T.W. Firms’ Technological Capabilities toward the Introduction of Industry 4.0: The Case of Supplier Firms in the Indonesia Automotive Industries. J. Perenc. Pembang. Indones. J. Dev. Plan. 2021, 5, 94–105. [Google Scholar] [CrossRef]
- Tortorella, G.L.; Prashar, A.; Antony, J.; Fogliatto, F.S.; Gonzalez, V.; Godinho Filho, M. Industry 4.0 Adoption for Healthcare Supply Chain Performance during COVID-19 Pandemic in Brazil and India: The Mediating Role of Resilience Abilities Development. Oper. Manag. Res. 2023, 17, 389–405. [Google Scholar] [CrossRef]
- Fatorachian, H.; Kazemi, H. Impact of Industry 4.0 on Supply Chain Performance. Prod. Plan. Control 2021, 32, 63–81. [Google Scholar] [CrossRef]
- Xu, L.D.; Xu, E.L.; Li, L. Industry 4.0: State of the Art and Future Trends. Int. J. Prod. Res. 2018, 56, 2941–2962. [Google Scholar] [CrossRef]
- Arji, G.; Ahmadi, H.; Avazpoor, P.; Hemmat, M. Identifying Resilience Strategies for Disruption Management in the Healthcare Supply Chain during COVID-19 by Digital Innovations: A Systematic Literature Review. Inform. Med. Unlocked 2023, 38, 101199. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Chu, C.H.; Hayya, J.; Mullen, T. An Exploratory Study of RFID Adoption in the Retail Sector. Oper. Manag. Res. 2010, 3, 80–89. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Petrick, I.; Mullen, T.; Kvasny, L. A Delphi Study of RFID Applicable Business Processes and Value Chain Activities in Retail. J. Technol. Manag. Innov. 2011, 6, 63–81. [Google Scholar] [CrossRef]
- Abbas, K.; Afaq, M.; Khan, T.A.; Song, W.C. A Blockchain and Machine Learning-Based Drug Supply Chain Management and Recommendation System for Smart Pharmaceutical Industry. Electronics 2020, 9, 852. [Google Scholar] [CrossRef]
- Omar, I.A.; Jayaraman, R.; Debe, M.S.; Salah, K.; Yaqoob, I.; Omar, M. Automating Procurement Contracts in the Healthcare Supply Chain Using Blockchain Smart Contracts. IEEE Access 2021, 9, 37397–37409. [Google Scholar] [CrossRef]
- Sharma, M.; Joshi, S. Digital Supplier Selection Reinforcing Supply Chain Quality Management Systems to Enhance Firm’s Performance. TQM J. 2020, 35, 102–130. [Google Scholar] [CrossRef]
- Zaid, A.A.; Jaaron, A.A.M.; Talib Bon, A. The Impact of Green Human Resource Management and Green Supply Chain Management Practices on Sustainable Performance: An Empirical Study. J. Clean. Prod. 2018, 204, 965–979. [Google Scholar] [CrossRef]
- Al-Sheyadi, A.; Muyldermans, L.; Kauppi, K. The Complementarity of Green Supply Chain Management Practices and the Impact on Environmental Performance. J. Environ. Manag. 2019, 242, 186–198. [Google Scholar] [CrossRef]
- García Alcaraz, J.L.; Díaz Reza, J.R.; Arredondo Soto, K.C.; Hernández Escobedo, G.; Happonen, A.; Puig I Vidal, R.; Jiménez Macías, E. Effect of Green Supply Chain Management Practices on Environmental Performance: Case of Mexican Manufacturing Companies. Mathematics 2022, 10, 1877. [Google Scholar] [CrossRef]
- Xu, J.; Yu, Y.; Wu, Y.; Zhang, J.Z.; Liu, Y.; Cao, Y.; Eachempati, P. Green Supply Chain Management for Operational Performance: Anteceding Impact of Corporate Social Responsibility and Moderating Effects of Relational Capital. J. Enterp. Inf. Manag. 2022, 35, 1613–1638. [Google Scholar] [CrossRef]
- Althaqafi, T. Environmental and Social Factors in Supplier Assessment: Fuzzy-Based Green Supplier Selection. Sustainability 2023, 15, 15643. [Google Scholar] [CrossRef]
- Bruckler, M.; Wietschel, L.; Messmann, L.; Thorenz, A.; Tuma, A. Review of Metrics to Assess Resilience Capacities and Actions for Supply Chain Resilience. Comput. Ind. Eng. 2024, 192, 110176. [Google Scholar] [CrossRef]
- Chowdhury, M.M.H.; Quaddus, M. Supply Chain Resilience: Conceptualization and Scale Development Using Dynamic Capability Theory. Int. J. Prod. Econ. 2017, 188, 185–204. [Google Scholar] [CrossRef]
- Dolgui, A.; Ivanov, D.; Sokolov, B. Ripple Effect in the Supply Chain: An Analysis and Recent Literature. Int. J. Prod. Res. 2018, 56, 414–430. [Google Scholar] [CrossRef]
- Villanueva, A. Mexico Nearshoring: Potential for Economic Boost. Available online: https://tecscience.tec.mx/en/business-innovation/mexico-nearshoring/ (accessed on 30 May 2024).
- Mohamed Khalifa, N. Global Supply Chain Resilience: Offshoring, Nearshoring or Reshoring Post COVID Pandemic. Rev. Cient. Estud. Empres. Ambient. 2022, 13, 99–121. [Google Scholar] [CrossRef]
- Burkhart, D.; Bode, C. On Supplier Resilience: How Supplier Performance, Disruption Frequency, and Disruption Duration Are Interrelated. J. Purch. Supply Manag. 2024, 30, 100921. [Google Scholar] [CrossRef]
- Albalushi, J.; Mishra, R.; Abebe, M. Supply Chain Resilience Meets Quality Management. Int. J. Prof. Bus. Rev. 2023, 8, e04165. [Google Scholar] [CrossRef]
- Nwachukwu, C.; Hieu, V. Assessing Supplier-Customer Relationship Management Practice and Business Performance; Bharath University: Chennai, India, 2021. [Google Scholar]
- Salam, M.A.; Khan, S.A. Achieving Supply Chain Excellence through Supplier Management: A Case Study of Fast Moving Consumer Goods. Benchmarking 2018, 25, 4084–4102. [Google Scholar] [CrossRef]
- Patil, D. Enhancing Supply Chain Performance: A Comprehensive Review of Supplier Quality Management Strategies. Res. J. Pharmacol. Pharmacodyn. 2024, 16, 239–242. [Google Scholar] [CrossRef]
- ISO 13485:2016; Medical Devices–Quality Management Systems–Requirements for Regulatory Purposes. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- Fernandes, A.C.; Vilhena, E.; Oliveira, R.; Sampaio, P.; Carvalho, M.S. Supply Chain Quality Management Impact on Organization Performance: Results from an International Survey. Int. J. Qual. Reliab. Manag. 2022, 39, 630–646. [Google Scholar] [CrossRef]
- Alomari, K.M.; Salah, A.A.; Mansour, A.D.; Alshaketheep, K.M.K.I.; Altarawneh, M.I.; Jray, A.A.A. Supply Chain Quality and Organizational Performance: Moderating Role of Competitive Advantages. WSEAS Trans. Bus. Econ. 2020, 17, 806–817. [Google Scholar] [CrossRef]
- Tarigan, Z.J.H.; Mochtar, J.; Basana, S.R.; Siagian, H. The Effect of Competency Management on Organizational Performance through Supply Chain Integration and Quality. Uncertain Supply Chain Manag. 2021, 9, 283–294. [Google Scholar] [CrossRef]
- Famiyeh, S.; Kwarteng, A. Supplier Selection and Firm Performance: Empirical Evidence from a Developing Country’s Environment. Int. J. Qual. Reliab. Manag. 2018, 35, 690–710. [Google Scholar] [CrossRef]
- Al-Qahtani, N.D.; Alshehri, S.S.A.; Aziz, A.A. The Impact of Total Quality Management on Organizational Performance in SMEs. Eur. J. Bus. Manag. 2015, 7, 119–127. [Google Scholar] [CrossRef]
- Liu, H.C.; Liu, R.; Gu, X.; Yang, M. From Total Quality Management to Quality 4.0: A Systematic Literature Review and Future Research Agenda. Front. Eng. Manag. 2023, 10, 191–205. [Google Scholar] [CrossRef]
- Cahyo, D.; Amaruddin, H. The Effect of Total Quality Management Implementation on Operational Performance through 5S and Corporate Culture at Food and Beverage Producer. In Proceedings of the Third International Conference on Government Education Management and Tourism, Bandung, Indonesia, 24 February 2024; Volume 3. [Google Scholar]
- Statista Medical Devices: Market Data & Analysis. Available online: https://www.statista.com/outlook/hmo/medical-technology/medical-devices/worldwide (accessed on 1 June 2024).
- Arredondo-Soto, K.C.; Carrillo-Gutiérrez, T.; Solís-Quinteros, M.; Ávila-López, L.A. Creation of Technology-Based Companies: Challenges to Innovate in the Manufacturing Sector of Medical Devices, the Case of Baja California, México. In Managing Innovation in Highly Restrictive Environments: Lessons from Latin America and Emerging; Springer: Cham, Switzerland, 2018; pp. 59–80. ISBN 9783319937151. [Google Scholar]
- Valdivia-Márquez, F.G.; Hernandez-Grageda, P.; Durán-Aguilar, G.; Rossa-Sierra, A. The Importance of Industrial Design in Medical Devices in the 21 St Century. In Human Systems Engineering and Design. IHSED 2018. Advances in Intelligent Systems and Computing; Ahram, T., Karwowski, W., Taiar, R., Eds.; Springer: Cham, Switzerland, 2019; Volume 876, pp. 469–474. ISBN 978-3-030-02052-1. [Google Scholar]
- Rubio, R.; Zurita-Barrón, M.A.; Contreras, H.H. A Medical Devices Cluster in Baja California-Mexico: A Strategic Proposal. In Proceedings of the IIE Annual Conference and Expo 2014, Montréal, QC, Canada, 31 May–3 June 2014; pp. 1770–1779. [Google Scholar]
- Fitch Solutions BMI. Mexico Medical Devices Report | Q3 2023; BMI: London, UK, 2023. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 8th ed.; Cengage Learning EMEA: Hampshire, UK, 2019; ISBN 978-1-4737-5654-0.
- Price, L.R. Psychometric Methods: Theory into Practice, 1st ed.; Guilford Publications: New York, NY, USA, 2016; ISBN 9781462524778. [Google Scholar]
- Padua, J. Técnicas de Investigación Aplicadas a Las Ciencias Sociales, 1st ed.; Fondo de Cultura Economica de España: México, México, 1979; ISBN 9786071650160. [Google Scholar]
- Badri, M.A.; Selim, H.; Alshare, K.; Grandon, E.E.; Younis, H.; Abdulla, M. The Baldrige Education Criteria for Performance Excellence Framework: Empirical Test and Validation. Int. J. Qual. Reliab. Manag. 2006, 23, 1118–1157. [Google Scholar] [CrossRef]
- Carpita, M.; Manisera, M. Constructing Indicators of Unobservable Variables from Parallel Measurements. Electron. J. Appl. Stat. Anal. 2012, 5, 320–326. [Google Scholar] [CrossRef]
- Durmić, E. Evaluation of Criteria for Sustainable Supplier Selection Using FUCOM Method. Oper. Res. Eng. Sci. Theory Appl. 2019, 2, 91–107. [Google Scholar] [CrossRef]
- Thanaraksakul, W.; Phruksaphanrat, B. Supplier Evaluation Framework Based on Balanced Scorecard with Integrated Corporate Social Responsibility Perspective. In Proceedings of the IMECS 2009, Hong Kong, 18 March 2009; Volume II, pp. 5–10. [Google Scholar]
- Erboz, G. How To Define Industry 4.0: Main Pillars Of Industry 4.0. In Proceedings of the 7th International Scientific Conference on Managerial Trends in the Development of Enter Prises in Globalization Era (ICoM)—Managerial Trends in the Development of Enterprises in Globalization Era, Nitra, Slovakia, 1–2 June 2017; pp. 761–767. [Google Scholar]
- Culot, G.; Nassimbeni, G.; Orzes, G.; Sartor, M. Behind the Definition of Industry 4.0: Analysis and Open Questions. Int. J. Prod. Econ. 2020, 226, 107617. [Google Scholar] [CrossRef]
- Raykov, T.; Marcoulides, G.A. An Introduction to Applied Multivariate Analysis, 1st ed.; Taylor & Francis Group: New York, NY, USA, 2008; ISBN 978-0-8058-6375-8. [Google Scholar]
- Byrne, B.M. Structural Equation Modeling with Amos: Basic Concepts, Applications and Programming, 3rd ed.; Routledge: New York, NY, USA, 2016; ISBN 9781138797031. [Google Scholar]
- DeCarlo, L.T. On the Meaning and Use of Kurtosis. Psychol. Methods 1997, 2, 292–307. [Google Scholar] [CrossRef]
- Kline, R. Principles and Practice of Structural Equation Modeling, 4th ed.; The Guilford Press: New York, NY, USA, 2016; ISBN 978-1-4625-2335-1.
- Brown, T.A. Confirmatory Factor Analysis for Applied Research, 2nd ed.; The Guilford Press: New York, NY, USA, 2015; ISBN 9781462515363. [Google Scholar]
- Vandenbosch, M.B. Confirmatory Compositional Approaches to the Development of Product Spaces. Eur. J. Mark. 1996, 30, 23–46. [Google Scholar] [CrossRef]
- Hatcher, L.; Stepanski, E.J. A Step-by-Step Approach to Using the SAS System for Univariate and Multivariate Statistics, 1st ed.; SAS Institute: Cary, NC, USA, 1994; ISBN 978-1555446345. [Google Scholar]
- Domínguez, L.C.; Sanabria, A.E. Construct Validity and Reliability of ROTA-Q for the Evaluation of Academic Quality of Clinical Clerkships in Medical Undergraduates. Educ. Médica 2019, 20, 71–78. [Google Scholar] [CrossRef]
- Nunnally, J.C. Psychometric Theory, 2nd ed.; McGraw-Hill: New York, NY, USA, 1978. [Google Scholar]
- Fornell, C.; Larcker, D.F. Evaluating Structural Equation Models with Unobservable Variables and Measurement Error. J. Mark. Res. 1981, 18, 39–50. [Google Scholar] [CrossRef]
- Dash, G.; Paul, J. CB-SEM vs PLS-SEM Methods for Research in Social Sciences and Technology Forecasting. Technol. Forecast. Soc. Change 2021, 173, 121092. [Google Scholar] [CrossRef]
- De los Santos, S.; Carrillo, J. La Industria de Dispositivos Médicos en México: Dualidad de Modelos Productivos; Comercio Exterior Bancomext: Ciudad de Mexico, Mexico, 2021; pp. 75–80. Available online: https://revistacomercioexterior.com/la-industria-de-dispositivos-medicos-en-mexico-dualidad-de-modelos-productivos (accessed on 16 June 2024).
- La Asociación Médica Mundial (AMM). Declaración de Helsinki de La AMM—Principios Éticos para Las Investigaciones Médicas en Seres Humanos. Available online: https://www.wma.net/es/policies-post/declaracion-de-helsinki-de-la-amm-principios-eticos-para-las-investigaciones-medicas-en-seres-humanos/ (accessed on 16 June 2024).
- Curran, P.J.; West, S.G.; Finch, J.F. The Robustness of Test Statistics to Nonnormality and Specification Error in Confirmatory Factor Analysis. Psychol. Methods 1996, 1, 16–29. [Google Scholar] [CrossRef]
- Khine, M.S. Application of Structural Equation Modeling in Educational Research and Practice, 1st ed.; Khine, M.S., Ed.; Sense Publishers: Rotterdam, The Netherlands, 2013; ISBN 978-94-6209-332-4. [Google Scholar]
- Kaiser, H.F.; Rice, J. Little Jiffy, Mark Iv. Educ. Psychol. Meas. 1974, 34, 111–117. [Google Scholar] [CrossRef]
- Thakkar, J.J. Applications of Structural Equation Modelling with R. In Structural Equation Modelling: Application for Research and Practice (with AMOS and R); Thakkar, J.J., Ed.; Springer: Singapore, 2020; pp. 91–99. ISBN 978-981-15-3793-6. [Google Scholar]
- Shin, H.; Collier, D.A.; Wilson, D.D. Supply Management Orientation and Supplier/Buyer Performance. J. Oper. Manag. 2000, 18, 317–333. [Google Scholar] [CrossRef]
- Boomsma, A.; Hoogland, J.J. The Robustness of LISREL Modeling Revisited. Struct. Equ. Models Present. Future A Festschr. Honor. Karl Jöreskog 2001, 2, 139–168. [Google Scholar]
- Bollen, K.A. A New Incremental Fit Index for General Structural Equation Models. Sociol. Methods Res. 1989, 17, 303–316. [Google Scholar] [CrossRef]
- Khan, B.A.; Naeem, H. Measuring the Impact of Soft and Hard Quality Practices on Service Innovation and Organisational Performance. Total Qual. Manag. Bus. Excell. 2018, 29, 1402–1426. [Google Scholar] [CrossRef]
- Kharub, M.; Sharma, R. An Integrated Structural Model of QMPs, QMS and Firm’s Performance for Competitive Positioning in MSMEs. Total Qual. Manag. Bus. Excell. 2020, 31, 312–341. [Google Scholar] [CrossRef]
- Görçün, Ö.F.; Aytekin, A.; Korucuk, S.; Tirkolaee, E.B. Evaluating and Selecting Sustainable Logistics Service Providers for Medical Waste Disposal Treatment in the Healthcare Industry. J. Clean. Prod. 2023, 408, 137194. [Google Scholar] [CrossRef]
- Sinha, K.K.; Kohnke, E.J. Health Care Supply Chain Design: Toward Linking the Development and Delivery of Care Globally. Decis. Sci. 2009, 40, 197–212. [Google Scholar] [CrossRef]
- Daú, G.; Scavarda, A.; Scavarda, L.F.; Portugal, V.J.T. The Healthcare Sustainable Supply Chain 4.0: The Circular Economy Transition Conceptual Framework with the Corporate Social Responsibility Mirror. Sustainability 2019, 11, 3259. [Google Scholar] [CrossRef]
- Pucci, J.U.; Christophe, B.R.; Sisti, J.A.; Connolly, E.S. Three-Dimensional Printing: Technologies, Applications, and Limitations in Neurosurgery. Biotechnol. Adv. 2017, 35, 521–529. [Google Scholar] [CrossRef]
- Ben-Daya, M.; Hassini, E.; Bahroun, Z. Internet of Things and Supply Chain Management: A Literature Review. Int. J. Prod. Res. 2019, 57, 4719–4742. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, S. Building Supply Chain Risk Resilience: Role of Big Data Analytics in Supply Chain Disruption Mitigation. Benchmarking 2019, 26, 2318–2342. [Google Scholar] [CrossRef]
- Panch, T.; Mattie, H.; Celi, L.A. The “Inconvenient Truth” about AI in Healthcare. NPJ Digit. Med. 2019, 2, 77. [Google Scholar] [CrossRef]
- Carroll, A.B. Corporate Social Responsibility Evolution of a Definitional Construct. Bus. Soc. 1999, 4, 268–295. [Google Scholar] [CrossRef]
- Le, T.T.; Vo, X.V.; Venkatesh, V.G. Role of Green Innovation and Supply Chain Management in Driving Sustainable Corporate Performance. J. Clean. Prod. 2022, 374, 133875. [Google Scholar] [CrossRef]
- Geng, R.; Mansouri, S.A.; Aktas, E. The Relationship between Green Supply Chain Management and Performance: A Meta-Analysis of Empirical Evidences in Asian Emerging Economies. Int. J. Prod. Econ. 2017, 183, 245–258. [Google Scholar] [CrossRef]
- Hengboriboon, L.; Sayut, T.; Srisathan, W.A.; Naruetharadhol, P. Strengthening a Company– Customer Relationship from Sustainable Practices: A Case Study of Petrotrade in Laos. Cogent Soc. Sci. 2022, 8, 2038355. [Google Scholar] [CrossRef]
- Katsaliaki, K.; Galetsi, P.; Kumar, S. Supply Chain Disruptions and Resilience: A Major Review and Future Research Agenda. Ann. Oper. Res. 2022, 319, 965–1002. [Google Scholar] [CrossRef]
- Ivanov, D.; Dolgui, A.; Sokolov, B.; Ivanova, M. Literature Review on Disruption Recovery in the Supply Chain. Int. J. Prod. Res. 2017, 55, 6158–6174. [Google Scholar] [CrossRef]
- Rubio-Romero, J.C.; del Carmen Pardo-Ferreira, M.; Torrecilla-García, J.A.; Calero-Castro, S. Disposable Masks: Disinfection and Sterilization for Reuse, and Non-Certified Manufacturing, in the Face of Shortages during the COVID-19 Pandemic. Saf. Sci. 2020, 129, 104830. [Google Scholar] [CrossRef]
- Lücker, F.; Seifert, R.W. Building up Resilience in a Pharmaceutical Supply Chain through Inventory, Dual Sourcing and Agility Capacity. Omega 2017, 73, 114–124. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Shivdas, A.; Ananthu, S. Redefining Supplier Selection in Healthcare Sector: A Novel Framework for Supplier Classification. J. Health Organ. Manag. 2025. ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Delcea, C.; Cotfas, L.-A. Supplier Selection Using Grey Systems Theory. In Advancements of Grey Systems Theory in Economics and Social Sciences; Delcea, C., Cotfas, L.-A., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 85–138. ISBN 978-981-19-9932-1. [Google Scholar]
- Rocha, L.A.; Rego, N. Reorganisation of the Internal Storage and Distribution Logistics in a Hospital. Procedia Comput. Sci. 2023, 219, 1357–1364. [Google Scholar] [CrossRef]
- Božic, D.; Šego, D.; Stankovic, R.; Šafran, M. Logistics in Healthcare: A Selected Review of Literature from 2010 to 2022. Transp. Res. Procedia 2022, 64, 288–298. [Google Scholar] [CrossRef]
- Gardeva, A. 4 Challenges Impacting the Healthcare Supply Chain. Available online: https://www.ibm.com/blog/4-challenges-impacting-the-healthcare-supply-chain/ (accessed on 15 August 2023).
- LaPointe, J. Exploring the Role of Supply Chain Management in Healthcare. Healthcare Supply Chain Management Leaders Are Looking to Become More Efficient and Resilient as They Continue to Face Significant Supply Chain Disruptions. Available online: https://www.techtarget.com/revcyclemanagement/feature/Exploring-the-Role-of-Supply-Chain-Management-in-Healthcare (accessed on 25 September 2023).
- Kumar, A.; Mani, V.; Jain, V.; Gupta, H.; Venkatesh, V.G. Managing Healthcare Supply Chain through Artificial Intelligence (AI): A Study of Critical Success Factors. Comput. Ind. Eng. 2023, 175, 108815. [Google Scholar] [CrossRef]

| Supplier quality is the degree to which a set of product features meets customer requirements [23,82]. It is the ability to provide products and services that meet necessary standards and requirements, thereby ensuring the safety and proper functioning of medical devices [63]. |
| Reliable delivery is the ability to meet specific delivery schedules, including lead times, punctuality, fulfillment rate, returns management, and location and transportation [23], while minimizing costs and maintaining quality [33]. |
| Information technology refers to systems compatibility, ease of communication, information exchange, and information technologies [83]; technological capacity and the ability to acquire new technologies and technical resources for research and development practices and processes [23]; and the sum of all the knowledge of a company in support of technological innovation [82]. |
| Environmental social responsibility is the responsible use of natural resources, minimizing damage and ensuring that these resources are available for future generations [23]. |
| Resilience is the capacity to absorb, adapt to, and restore after disruptions [54]. |
| Industry 4.0 refers to the use and/or integration of advanced technologies in processes to improve efficiency, quality, and innovation [84,85]. |
| Issues | Results | Recommended Values |
|---|---|---|
| Outliers | 162 significant responses. | Mahalanobis distance, with a statistical significance level of p < 0.001 [89]. |
| Univariate Normality | Kurtosis (−0.999, 0.936), skewness (−0.747, 0.206). | Kurtosis: range of ±3 [88]. Skewness: range of ±2 [99]. |
| Multivariate Normality | Multivariate kurtosis 159.078, obtained through SPSS® AMOS ® version 23. | A value lower than that derived from the formula p(p + 2), where p represents the number of measured variables in the model [100], resulting in a value of 1295. |
| Multicollinearity | Correlation coefficients below the recommended maximum value. | The correlation coefficient between pairs of measured variables >0.85 [100]. |
| VIF’s maximum calculated value: 4.913. | Variance inflation factor (VIF) with values >10 [89]. |
| EFA | CFA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Eigenvalues | Cronbach’s Alpha | Standardized Loading | AVE | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||
| RD4 | 0.817 | 13.989 | 0.923 | 0.932 | 0.656 | ||||||
| RD5 | 0.789 | 0.834 | |||||||||
| RD2 | 0.789 | 0.818 | |||||||||
| RD3 | 0.755 | 0.788 | |||||||||
| RD6 | 0.697 | 0.741 | |||||||||
| RD1 | 0.592 | 0.731 | |||||||||
| IND5 | 0.900 | 3.724 | 0.913 | 0.907 | 0.658 | ||||||
| IND6 | 0.807 | 0.772 | |||||||||
| IND4 | 0.797 | 0.842 | |||||||||
| IND1 | 0.689 | 0.708 | |||||||||
| IND3 | 0.684 | 0.839 | |||||||||
| IND2 | 0.671 | 0.785 | |||||||||
| PER3 | 0.853 | 3.170 | 0.899 | 0.867 | 0.536 | ||||||
| PER5 | 0.835 | 0.776 | |||||||||
| PER4 | 0.722 | 0.658 | |||||||||
| PER6 | 0.717 | 0.744 | |||||||||
| PER1 | 0.691 | 0.722 | |||||||||
| PER2 | 0.568 | 0.594 | |||||||||
| ESR2 | 0.791 | 1.598 | 0.932 | 0.910 | 0.778 | ||||||
| ESR1 | 0.748 | 0.867 | |||||||||
| ESR4 | 0.722 | 0.901 | |||||||||
| ESR3 | 0.697 | 0.849 | |||||||||
| IT3 | 0.792 | 1.327 | 0.910 | 0.910 | 0.667 | ||||||
| IT4 | 0.769 | 0.922 | |||||||||
| IT5 | 0.615 | 0.779 | |||||||||
| IT1 | 0.547 | 0.733 | |||||||||
| IT2 | 0.518 | 0.716 | |||||||||
| RES2 | 0.694 | 1.179 | 0.905 | 0.919 | 0.717 | ||||||
| RES3 | 0.658 | 0.845 | |||||||||
| RES4 | 0.644 | 0.837 | |||||||||
| RES1 | 0.537 | 0.781 | |||||||||
| SQ2 | 0.725 | 1.107 | 0.866 | 0.807 | 0.620 | ||||||
| SQ1 | 0.581 | 0.755 | |||||||||
| SQ3 | 0.555 | 0.803 | |||||||||
| SQ4 | 0.478 | 0.784 | |||||||||
| Goodness-of-Fit Statistics | Measurement Model | Structural Model Results | Recommended Values |
|---|---|---|---|
| CMIN/DF | 1.644 | 1.664 | <3 [105] |
| CFI | 0.924 | 0.923 | >0.9 [77] |
| TLI | 0.915 | 0.915 | >0.9 [77] |
| RMSEA | 0.063 | 0.063 | <0.08 [77] |
| SRMR | 0.075 | 0.079 | <0.08 [93] |
| PNFI | 0.743 | 0.749 | ≥0.5 [87] |
| Construct | RD | IND | PER | ESR | IT | RES | SQ |
|---|---|---|---|---|---|---|---|
| RD | 0.810 a | ||||||
| IND | 0.283 | 0.811 a | |||||
| PER | 0.177 | 0.048 | 0.732 a | ||||
| ESR | 0.634 | 0.432 | 0.225 | 0.882 a | |||
| IT | 0.632 | 0.513 | 0.102 | 0.667 | 0.817 a | ||
| RES | 0.695 | 0.463 | 0.234 | 0.672 | 0.677 | 0.847 a | |
| SQ | 0.692 | 0.511 | 0.131 | 0.678 | 0.697 | 0.741 | 0.788 a |
| Hypotheses | Path | SRW | SE | CR | p | Results | ||
|---|---|---|---|---|---|---|---|---|
| H1 | RD | → | SQ | 0.265 | 0.100 | 2.662 | 0.008 * | Supported |
| H2 | IT | → | SQ | 0.131 | 0.078 | 1.667 | 0.095 *** | Supported |
| H3 | IND | → | SQ | 0.106 | 0.047 | 2.266 | 0.023 ** | Supported |
| H4 | ESR | → | SQ | 0.106 | 0.063 | 1.672 | 0.094 *** | Supported |
| H5 | RES | → | SQ | 0.254 | 0.094 | 2.696 | 0.007 * | Supported |
| H6 | SQ | → | PER | 0.144 | 0.081 | 1.786 | 0.074 *** | Supported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltran-Salomon, E.; Saavedra-Leyva, R.E.; Tortorella, G.; Limon-Romero, J.; Tlapa, D.; Baez-Lopez, Y. Critical Success Factors for Supplier Selection and Performance Enhancement in the Medical Device Industry: An Industry 4.0 Approach. Processes 2025, 13, 1438. https://doi.org/10.3390/pr13051438
Beltran-Salomon E, Saavedra-Leyva RE, Tortorella G, Limon-Romero J, Tlapa D, Baez-Lopez Y. Critical Success Factors for Supplier Selection and Performance Enhancement in the Medical Device Industry: An Industry 4.0 Approach. Processes. 2025; 13(5):1438. https://doi.org/10.3390/pr13051438
Chicago/Turabian StyleBeltran-Salomon, Erika, Rafael Eduardo Saavedra-Leyva, Guilherme Tortorella, Jorge Limon-Romero, Diego Tlapa, and Yolanda Baez-Lopez. 2025. "Critical Success Factors for Supplier Selection and Performance Enhancement in the Medical Device Industry: An Industry 4.0 Approach" Processes 13, no. 5: 1438. https://doi.org/10.3390/pr13051438
APA StyleBeltran-Salomon, E., Saavedra-Leyva, R. E., Tortorella, G., Limon-Romero, J., Tlapa, D., & Baez-Lopez, Y. (2025). Critical Success Factors for Supplier Selection and Performance Enhancement in the Medical Device Industry: An Industry 4.0 Approach. Processes, 13(5), 1438. https://doi.org/10.3390/pr13051438










