Versatile Emulsion-Based Encapsulation System Production Processes: A Review
Abstract
1. Introduction
2. Emulsions
2.1. Definitions and Classification
2.2. Components
- Oil phase: lipids, in particular oils, which are components that are soluble in organic solvents but immiscible or poorly miscible with water. The specific type of oil is selected based on the intended application. For instance, edible oils derived from plants or seeds are employed in food applications.
- Internal water phase: aqueous phase containing active agents;
- Oil phase: oil or polymer solution, depending on the desired product;
- External water phase: water.
Stabilizers
- Emulsifier: surface-active molecules that adsorb to the surface of freshly formed droplets during homogenization, forming a protective layer that prevents the droplets from aggregation.
- Texture modifiers are divided into thickening agents and gelling agents, depending on their mode of operation and the rheological characteristics of their solutions. Both thickening agents and gelling agents act on the continuous phase of the emulsion, either by increasing its viscosity or by forming a gel, respectively. As a result, texture modifiers enhance emulsion stability by slowing the movement of the droplets.
- Weighting agent: a substance added to the dispersed phase to reduce the density difference between the droplets and the surrounding liquid, thereby slowing down gravitational separation.
- Ripening inhibitors are highly non-polar substances, with very low water solubility, that are added to the oil phase to inhibit Ostwald ripening through an entropy of mixing effect.
- Ionic surfactants: there are two types of ionic surfactants, that is, anionic, in which the head part is negatively charged, such as fatty acid salts, stearoyl lactylate salts, diacetyl tartatric acid esters of monoglycerides, and citric acid esters of monoglycerides, and cationic, in which the head part is positively charged, such as lauric arginate.
- Non-ionic surfactants: the head part contains no charge. The most common are monoglycerides, acetic acid esters of monoglycerides, lactic acid esters of monoglycerides, Tweens, and Spans. Some polymers are also used as non-ionic surfactants, such as Pluronic F-127, also known as Poloxamer 407, and Polyvinyl alcohol (PVA).
- Zwitterionic surfactants: the head part contains both charges, such as lecithin.
- A surfactant with a low HLB number (3–6) is predominantly hydrophobic, meaning it dissolves preferentially in oil and is typically used to stabilize water-in-oil emulsions.
- A surfactant with a high HLB number (10–18) is predominantly hydrophilic, so it dissolves preferentially in water and stabilizes oil-in-water emulsions.
- A surfactant with an intermediate HLB number (7–9) shows no strong affinity for either phase and is classified as a “wetting agent”.
- Molecules with HLB numbers below 3 (very hydrophobic) and above 18 (very hydrophilic) tend to accumulate in the bulk oil or water phases rather than at the oil–water interface, without having surface activity.
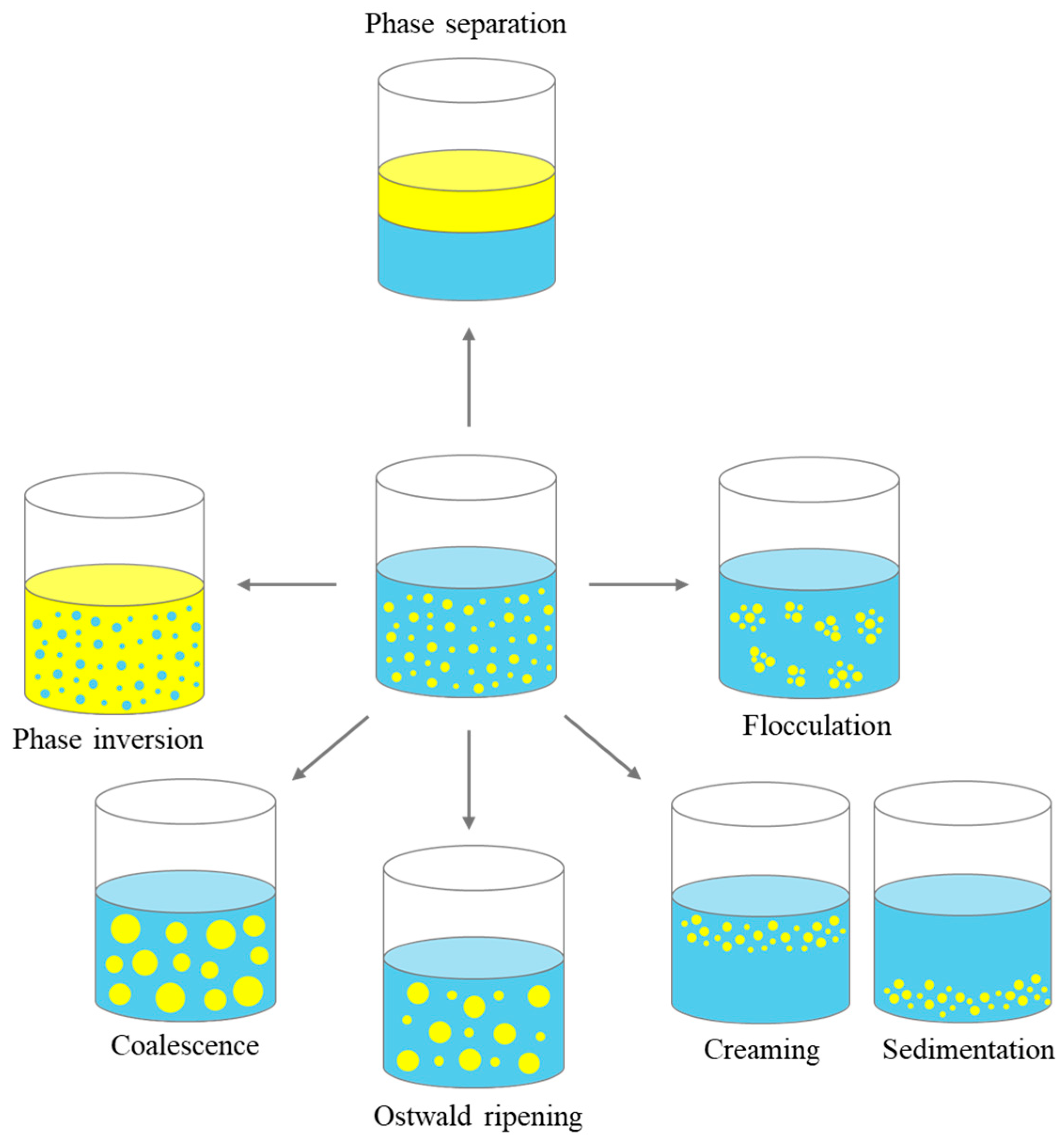
2.3. Mechanisms of Emulsion Instability
2.3.1. Gravitational Separation
2.3.2. Flocculation
2.3.3. Coalescence
2.3.4. Ostwald Ripening and Phase Inversion
2.4. Properties and Characterization of Emulsions
2.4.1. Droplet Morphology
2.4.2. Droplet Size Distribution
2.4.3. Optical Properties
2.4.4. Rheological Properties
2.4.5. Droplet Charge
3. Emulsion Production Processes
- High energy, which uses conventional emulsification devices such as rotor–stators, high-pressure homogenizers, and ultrasound homogenizers, and provides high shear rates and energy input per unit of volume. They are widely used in industrial applications and for large-scale production.
- Intermediate energy, which uses membrane homogenizers and microfluidic systems and provides intermediate shearing rates and energy inputs per unit volume. They are used in both industrial and laboratory-scale applications.
3.1. Rotor–Stator Emulsifier
3.2. High-Pressure Homogenizer
3.3. Ultrasound Homogenizer
- Direct: a probe sonicator is used, in which an ultrasonic horn or sonotrode is immersed in the liquid, transmitting high-intensity ultrasonic waves directly through the sonotrode;
- Indirect: ultrasonic waves are transmitted through the vessel and into the liquid via a water bath.
3.4. Membrane Homogenizer
3.5. Microfluidic Emulsification
4. Polymeric Particles Production Processes Based on Emulsions
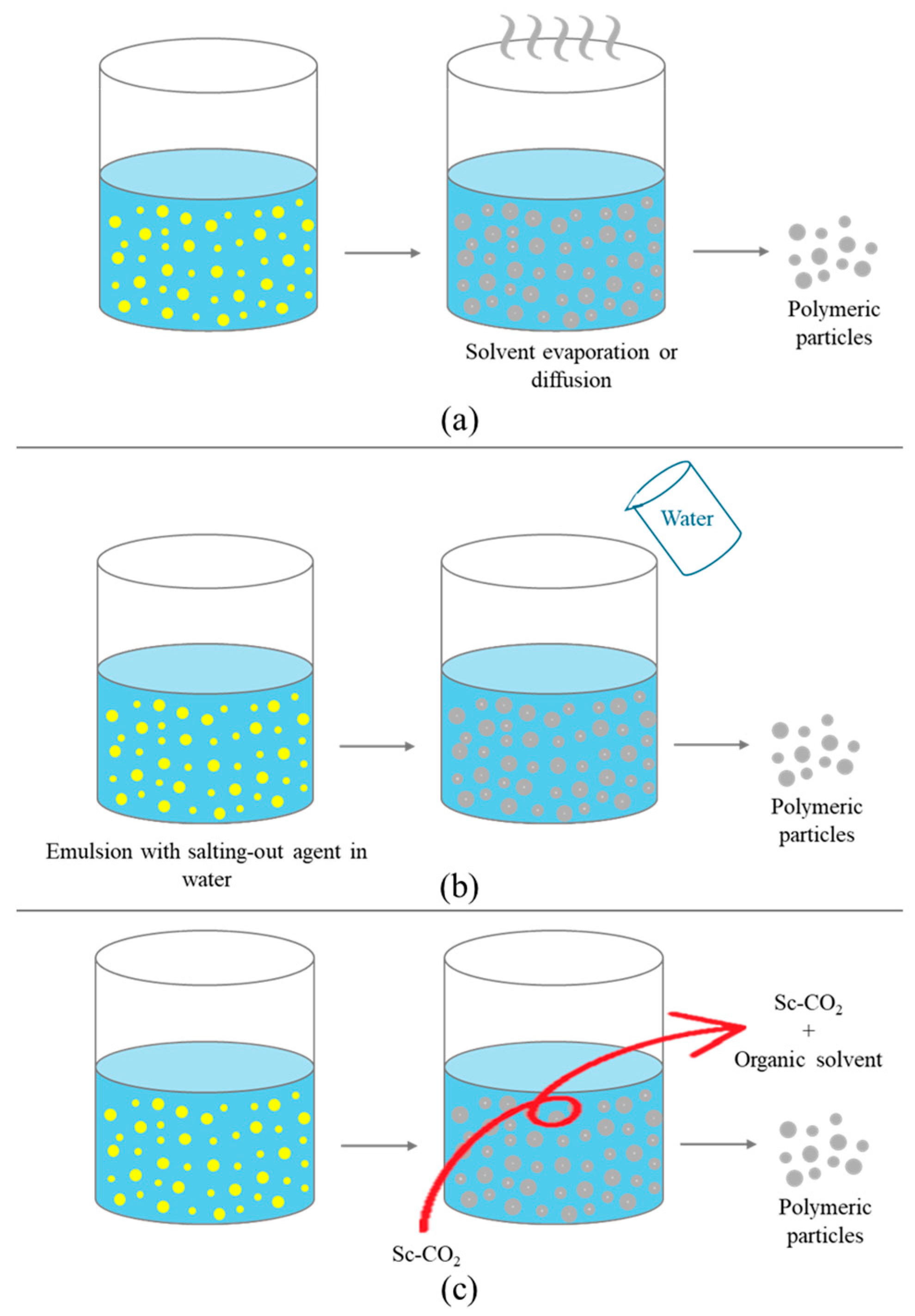
4.1. Emulsion Solvent Evaporation
4.2. Emulsion Solvent Diffusion
4.3. Salting-Out
4.4. Supercritical Emulsion Extraction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| o/w | Oil-in-water emulsion |
| w/o | Water-in-oil emulsion |
| w/o/w | Water-in-oil-in-water emulsion |
| HIPE | High internal phase emulsion |
| HLB | Hydrophile–Lipophile Balance |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| DLS | Dynamic light scattering |
| SLS | Static light scattering |
| DME | Direct membrane emulsification |
| PME | Premix membrane emulsification |
References
- Alu’datt, M.H.; Alrosan, M.; Gammoh, S.; Tranchant, C.C.; Alhamad, M.N.; Rababah, T.; Zghoul, R.; Alzoubi, H.; Ghatasheh, S.; Ghozlan, K.; et al. Encapsulation-Based Technologies for Bioactive Compounds and Their Application in the Food Industry: A Roadmap for Food-Derived Functional and Health-Promoting Ingredients. Food Biosci. 2022, 50, 101971. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef] [PubMed]
- Klojdová, I.; Milota, T.; Smetanová, J.; Stathopoulos, C. Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals 2023, 16, 362. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, X.; Zheng, H.; Li, J.; Wu, X.; Xu, J.; Zhen, Z.; Du, C. The Application of Encapsulation Technology in the Food Industry: Classifications, Recent Advances, and Perspectives. Food Chem. X 2024, 21, 101240. [Google Scholar] [CrossRef]
- de Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for Preservation of Functionality and Targeted Delivery of Bioactive Food Components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Amiri, S.; Mousavi Khaneghah, A. The Application of the Coacervation Technique for Microencapsulation Bioactive Ingredients: A Critical Review. J. Agric. Food Res. 2024, 18, 101431. [Google Scholar] [CrossRef]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of Active Ingredients in Polysaccharide–Protein Complex Coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- de Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro- and Nano Bio-Based Delivery Systems for Food Applications: In Vitro Behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and Nanoencapsulation of Bioactive Compounds for Agri-Food Applications: A Review. Ind. Crops Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Dalbhagat, C.G.; Mishra, H.N. Emerging Technologies and Coating Materials for Improved Probiotication in Food Products: A Review. Food Bioproc. Technol. 2022, 15, 998–1039. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion Micro Emulsion and Nano Emulsion: A Review. Syst. Rev. Pharm. 2016, 8, 39–47. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-Based Encapsulation and Delivery Systems for Polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Mamusa, M.; Resta, C.; Sofroniou, C.; Baglioni, P. Encapsulation of Volatile Compounds in Liquid Media: Fragrances, Flavors, and Essential Oils in Commercial Formulations. Adv. Colloid Interface Sci. 2021, 298, 102544. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food. Sci. Technol 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Lovell, P.A.; Schork, F.J. Fundamentals of Emulsion Polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef]
- Oberoi, K.; Tolun, A.; Sharma, K.; Sharma, S. Microencapsulation: An overview for the survival of probiotic bacteria. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 280–287. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Preparation of Curcumin-Loaded Emulsion Using High Pressure Homogenization: Impact of Oil Phase and Concentration on Physicochemical Stability. LWT 2017, 84, 34–46. [Google Scholar] [CrossRef]
- Marefati, A.; Pitsiladis, A.; Oscarsson, E.; Ilestam, N.; Bergenståhl, B. Encapsulation of Lactobacillus reuteri in W1/O/W2 Double Emulsions: Formulation, Storage and in Vitro Gastro-Intestinal Digestion Stability. LWT 2021, 146, 111423. [Google Scholar] [CrossRef]
- Bufalini, C.; Ferrari, P.F.; Li, J.; Campardelli, R. Double Emulsion with Improved Stability for Lactobacillus acidophilus Encapsulation in a Liquid Formulation. J. Food Eng. 2025, 391, 112463. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H.; Zhang, R.; McClements, D.J. Vitamin E Encapsulation within Oil-in-Water Emulsions: Impact of Emulsifier Type on Physicochemical Stability and Bioaccessibility. J. Agric. Food Chem. 2019, 67, 1521–1529. [Google Scholar] [CrossRef]
- Inapurapu, S.P.; Ibrahim, A.; Kona, S.R.; Pawar, S.C.; Bodiga, S.; Bodiga, V.L. Development and Characterization of ω-3 Fatty Acid Nanoemulsions with Improved Physicochemical Stability and Bioaccessibility. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125515. [Google Scholar] [CrossRef]
- Bufalini, C.; Palazzo, I.; Casazza, A.A.; Ferrari, P.F.; Campardelli, R.; Firpo, G.; Perego, P.; Reverchon, E. Encapsulation of Arthrospira Platensis Polyphenolic Extract Using Supercritical Emulsion-Based Process. J. Supercrit. Fluids 2024, 212, 106335. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. Supercritical Fluid Extraction of Emulsions to Nanoencapsulate Vitamin E in Polycaprolactone. J. Supercrit. Fluids 2017, 119, 274–282. [Google Scholar] [CrossRef]
- Paulo, F.; Paula, V.; Estevinho, L.M.; Santos, L. Propolis Microencapsulation by Double Emulsion Solvent Evaporation Approach: Comparison of Different Polymeric Matrices and Extract to Polymer Ratio. Food Bioprod. Process. 2021, 127, 408–425. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, J.F. The Release Kinetics of β-Carotene Nanocapsules/Xanthan Gum Coating and Quality Changes in Fresh-Cut Melon (Cantaloupe). Carbohydr. Polym. 2017, 157, 1874–1882. [Google Scholar] [CrossRef]
- Lv, X.; Cong, Z.; Liu, Z.; Ma, X.; Xu, M.; Tian, Y.; Zhang, X.; Xu, B.; Zhang, J.; Tang, Z. Improvement of the Solubility, Photostability, Antioxidant Activity and UVB Photoprotection of Trans-Resveratrol by Essential Oil Based Microemulsions for Topical Application. J. Drug Deliv. Sci. Technol. 2018, 48, 346–354. [Google Scholar] [CrossRef]
- Dario, M.F.; Oliveira, C.A.; Cordeiro, L.R.G.; Rosado, C.; Mariz, I.d.F.A.; Maçôas, E.; Santos, M.S.C.S.; Minas da Piedade, M.E.; Baby, A.R.; Velasco, M.V.R. Stability and Safety of Quercetin-Loaded Cationic Nanoemulsion: In Vitro and in Vivo Assessments. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 591–599. [Google Scholar] [CrossRef]
- Wei, Y.; Niu, Z.; Wang, F.; Feng, K.; Zong, M.; Wu, H. A Novel Pickering Emulsion System as the Carrier of Tocopheryl Acetate for Its Application in Cosmetics. Mater. Sci. Eng. C 2020, 109, 110503. [Google Scholar] [CrossRef]
- Goudon, F.; Clément, Y.; Ripoll, L. Controlled Release of Retinol in Cationic Co-Polymeric Nanoparticles for Topical Application. Cosmetics 2020, 7, 29. [Google Scholar] [CrossRef]
- Sharipova, A.A.; Aidarova, S.B.; Grigoriev, D.; Mutalieva, B.; Madibekova, G.; Tleuova, A.; Miller, R. Polymer–Surfactant Complexes for Microencapsulation of Vitamin E and Its Release. Colloids Surf. B Biointerfaces 2016, 137, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Lacour, B.; Grossiord, J.-L.; Seiller, M.; Cournarie, F.; Rosilio, V.; Chéron, M. Improved Formulation of W/O/W Multiple Emulsion for Insulin Encapsulation. Influence of the Chemical Structure of Insulin. Colloid Polym. Sci. 2004, 282, 562–568. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, N.; Gao, Y.; Wang, X.; Wu, J.; Ma, G. Alum Pickering Emulsion as Effective Adjuvant to Improve Malaria Vaccine Efficacy. Vaccines 2021, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Falco, N.; Reverchon, E.; Della Porta, G. Continuous Supercritical Emulsions Extraction: Packed Tower Characterization and Application to Poly(Lactic- Co -Glycolic Acid) + Insulin Microspheres Production. Ind. Eng. Chem. Res. 2012, 51, 8616–8623. [Google Scholar] [CrossRef]
- Kluge, J.; Fusaro, F.; Mazzotti, M.; Muhrer, G. Production of PLGA Micro- and Nanocomposites by Supercritical Fluid Extraction of Emulsions: II. Encapsulation of Ketoprofen. J. Supercrit. Fluids 2009, 50, 336–343. [Google Scholar] [CrossRef]
- Murakami, Y.; Shimoyama, Y. Production of Nanosuspension Functionalized by Chitosan Using Supercritical Fluid Extraction of Emulsion. J. Supercrit. Fluids 2017, 128, 121–127. [Google Scholar] [CrossRef]
- Umerska, A.; Gaucher, C.; Oyarzun-Ampuero, F.; Fries-Raeth, I.; Colin, F.; Villamizar-Sarmiento, M.; Maincent, P.; Sapin-Minet, A. Polymeric Nanoparticles for Increasing Oral Bioavailability of Curcumin. Antioxidants 2018, 7, 46. [Google Scholar] [CrossRef]
- Kim, M.R.; Feng, T.; Zhang, Q.; Chan, H.Y.E.; Chau, Y. Co-Encapsulation and Co-Delivery of Peptide Drugs via Polymeric Nanoparticles. Polymers 2019, 11, 288. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Prévost, D.; Surampalli, R.Y. Effect of Emulsion Formulation of Sinorhizobium Meliloti and Pre-Inoculated Seeds on Alfalfa Nodulation and Growth: A Pouch Study. J. Plant. Nutr. 2013, 36, 231–242. [Google Scholar] [CrossRef]
- Sohail, M.; Cheadle, J.; Khan, R.; Mane, H.; Samaher Salem, K.; Ernst, K.; San Miguel, A.; Opperman, C.H.; Pirzada, T.; Crook, N.; et al. Pickering Emulsion for Enhanced Viability of Plant Growth Promoting Bacteria and Combined Delivery of Agrochemicals and Biologics. Adv. Funct. Mater. 2025, 2418272. [Google Scholar] [CrossRef]
- Yaakov, N.; Kottakota, C.; Mani, K.A.; Naftali, S.M.; Zelinger, E.; Davidovitz, M.; Ment, D.; Mechrez, G. Encapsulation of Bacillus Thuringiensis in an Inverse Pickering Emulsion for Pest Control Applications. Colloids Surf. B Biointerfaces 2022, 213, 112427. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.F.; Bufalini, C.; Campardelli, R.; Brondolo, A.; Ercole, E.; Palombo, D.; Perego, P. Stabilization of Microbial Strains in Long-Lasting Double Emulsions as a New Strategy for Liquid Biofertilizer Formulation. Food Bioprod. Process. 2024, 148, 611–618. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; He, Y.; Ye, B.-C.; Wang, J. Preparation and Characterization of Monodisperse Microcapsules with Alginate and Bentonite via External Gelation Technique Encapsulating Pseudomonas Putida Rs-198. J. Biomater. Sci. Polym. Ed. 2017, 28, 1556–1571. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Grillo, R.; Mello, N.F.S.; Rosa, A.H.; Fraceto, L.F. Application of Poly(Epsilon-Caprolactone) Nanoparticles Containing Atrazine Herbicide as an Alternative Technique to Control Weeds and Reduce Damage to the Environment. J. Hazard Mater. 2014, 268, 207–215. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Oliveira, J.L.d.; da Silva, C.M.G.; Pascoli, M.; Pasquoto, T.; Lima, R.; Abhilash, P.C.; Fernandes Fraceto, L. Polymeric and Solid Lipid Nanoparticles for Sustained Release of Carbendazim and Tebuconazole in Agricultural Applications. Sci. Rep. 2015, 5, 13809. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar]
- McClements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Calvo, F.; Gómez, J.M.; Ricardez-Sandoval, L.; Alvarez, O. Integrated Design of Emulsified Cosmetic Products: A Review. Chem. Eng. Res. Des. 2020, 161, 279–303. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review. Processes 2022, 10, 738. [Google Scholar] [CrossRef]
- Hema, S.K.; Karmakar, A.; Das, R.K.; Srivastava, P. Simple Formulation and Characterization of Double Emulsion Variant Designed to Carry Three Bioactive Agents. Heliyon 2022, 8, e10397. [Google Scholar] [CrossRef]
- Leister, N.; Karbstein, H.P. Evaluating the Stability of Double Emulsions—A Review of the Measurement Techniques for the Systematic Investigation of Instability Mechanisms. Colloids Interfaces 2020, 4, 8. [Google Scholar] [CrossRef]
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems: Preparation, Stability, and Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Rojas, O.J.; McClements, D.J. Recent Innovations in Emulsion Science and Technology for Food Applications. J. Agric. Food Chem. 2021, 69, 8944–8963. [Google Scholar] [CrossRef]
- Lamba, H.; Sathish, K.; Sabikhi, L. Double Emulsions: Emerging Delivery System for Plant Bioactives. Food Bioproc. Technol. 2015, 8, 709–728. [Google Scholar] [CrossRef]
- Mohd Isa, N.S.; El Kadri, H.; Vigolo, D.; Gkatzionis, K. Optimisation of Bacterial Release from a Stable Microfluidic-Generated Water-in-Oil-in-Water Emulsion. RSC Adv. 2021, 11, 7738–7749. [Google Scholar] [CrossRef]
- Gao, H.; Ma, L.; Cheng, C.; Liu, J.; Liang, R.; Zou, L.; Liu, W.; McClements, D.J. Review of Recent Advances in the Preparation, Properties, and Applications of High Internal Phase Emulsions. Trends Food Sci. Technol. 2021, 112, 36–49. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef]
- de Carvalho-Guimarães, F.B.; Correa, K.L.; de Souza, T.P.; Rodríguez Amado, J.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. A Review of Pickering Emulsions: Perspectives and Applications. Pharmaceuticals 2022, 15, 1413. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-Stabilized Pickering Emulsions: Formation, Stability, Properties, and Applications in Foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Niu, H.; Wang, W.; Dou, Z.; Chen, X.; Chen, X.; Chen, H.; Fu, X. Multiscale Combined Techniques for Evaluating Emulsion Stability: A Critical Review. Adv. Colloid Interface Sci. 2023, 311, 102813. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Lopes, L. Overcoming the Cutaneous Barrier with Microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef]
- Komaiko, J.S.; McClements, D.J. Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Whey Protein Peptides as Components of Nanoemulsions: A Review of Emulsifying and Biological Functionalities. J. Food Eng. 2014, 122, 15–27. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin Polymers as Wall Materials for the Nano-Encapsulation of Bioactive Compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Thakore, S.D.; Patel, B.G. Nanoemulsion as a Valuable Nanostructure Platform for Pharmaceutical Drug Delivery. In Nano- and Microscale Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 321–341. [Google Scholar]
- Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Nanotechnology Applications in Drug Controlled Release. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 81–116. [Google Scholar]
- Banasaz, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions. Molecules 2020, 25, 3966. [Google Scholar] [CrossRef]
- Tamilvanan, S. Oil-in-Water Lipid Emulsions: Implications for Parenteral and Ocular Delivering Systems. Prog. Lipid Res. 2004, 43, 489–533. [Google Scholar] [CrossRef]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Recent Advances in Polysaccharides Stabilized Emulsions for Encapsulation and Delivery of Bioactive Food Ingredients: A Review. Carbohydr. Polym. 2020, 242, 116388. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Double Emulsion Solvent Evaporation Approach as a Novel Eugenol Delivery System—Optimization by Response Surface Methodology. Ind. Crops Prod. 2018, 126, 287–301. [Google Scholar] [CrossRef]
- Ding, S.; Serra, C.A.; Vandamme, T.F.; Yu, W.; Anton, N. Double Emulsions Prepared by Two–Step Emulsification: History, State-of-the-Art and Perspective. J. Control. Release 2019, 295, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Muñoz, N.; Alcalá-Alcalá, S.; Quintanar-Guerrero, D. Preparation of Polymer Nanoparticles by the Emulsification-Solvent Evaporation Method: From Vanderhoff’s Pioneer Approach to Recent Adaptations. In Polymer Nanoparticles for Nanomedicines; Springer International Publishing: Cham, Switzerland, 2016; pp. 87–121. [Google Scholar]
- Panigrahi, D.; Sahu, P.K.; Swain, S.; Verma, R.K. Quality by Design Prospects of Pharmaceuticals Application of Double Emulsion Method for PLGA Loaded Nanoparticles. Appl. Sci. 2021, 3, 638. [Google Scholar] [CrossRef]
- Piñón-Segundo, E.; Llera-Rojas, V.G.; Leyva-Gómez, G.; Urbán-Morlán, Z.; Mendoza-Muñoz, N.; Quintanar-Guerrero, D. The Emulsification-Diffusion Method to Obtain Polymeric Nanoparticles. In Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–83. [Google Scholar]
- Deshmukh, R.; Wagh, P.; Naik, J. Solvent Evaporation and Spray Drying Technique for Micro- and Nanospheres/Particles Preparation: A Review. Dry. Technol. 2016, 34, 1758–1772. [Google Scholar] [CrossRef]
- Nava-Arzaluz, M.G.; Pinon-Segundo, E.; Ganem-Rondero, A.; Lechuga-Ballesteros, D. Single Emulsion-Solvent Evaporation Technique and Modifications for the Preparation of Pharmaceutical Polymeric Nanoparticles. Recent Pat. Drug Deliv. Formul. 2012, 6, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Han, B.; Zhao, Y.; Yang, G. Formation of Multiple Water-in-Ionic Liquid-in-Water Emulsions. J. Colloid Interface Sci. 2012, 368, 395–399. [Google Scholar] [CrossRef]
- Hejazifar, M.; Lanaridi, O.; Bica-Schröder, K. Ionic Liquid Based Microemulsions: A Review. J. Mol. Liq. 2020, 303, 112264. [Google Scholar] [CrossRef]
- Kuchlyan, J.; Kundu, N.; Sarkar, N. Ionic Liquids in Microemulsions: Formulation and Characterization. Curr. Opin. Colloid Interface Sci. 2016, 25, 27–38. [Google Scholar] [CrossRef]
- Xie, R.; Tan, Z.; Fan, W.; Qin, J.; Guo, S.; Xiao, H.; Tang, Z. Deep-Eutectic-Solvent-in-Water Pickering Emulsions Stabilized by Starch Nanoparticles. Foods 2024, 13, 2293. [Google Scholar] [CrossRef]
- Miao, J.; Zuo, X.; McClements, D.J.; Zou, L.; Liang, R.; Zhang, L.; Liu, W. Fabrication of Natural Deep Eutectic Solvent Based Water-in-Oil High Internal Phase Emulsion: Improving Loading Capacity and Stability of Curcumin. J. Food Eng. 2024, 366, 111862. [Google Scholar] [CrossRef]
- Sekharan, T.R.; Chandira, R.M.; Tamilvanan, S.; Rajesh, S.C.; Venkateswarlu, B.S. Deep Eutectic Solvents as an Alternate to Other Harmful Solvents. Biointerface Res. Appl. Chem. 2022, 12, 847–860. [Google Scholar] [CrossRef]
- Syed, U.T.; Calzada, J.; Mendoza, G.; Arruebo, M.; Piacentini, E.; Giorno, L.; Crespo, J.G.; Brazinha, C.; Sebastian, V. Drug Delivery Applications of Hydrophobic Deep Eutectic Solvent-in-Water Nanoemulsions: A Comparative Analysis of Ultrasound Emulsification and Membrane-Assisted Nanoemulsification. ACS Appl. Mater. Interfaces 2025, 17, 4075–4086. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Liu, K.; Zhang, M.; Yuan, P.; Cai, J. Microparticles and Microcapsules from the Solvent Extraction of Deep Eutectic Solvent-Based Emulsion. Ind. Eng. Chem. Res. 2020, 59, 2892–2898. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double Emulsion Solvent Evaporation Techniques Used for Drug Encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef]
- Halmos, E.P.; Mack, A.; Gibson, P.R. Review Article: Emulsifiers in the Food Supply and Implications for Gastrointestinal Disease. Aliment. Pharmacol. Ther. 2019, 49, 41–50. [Google Scholar] [CrossRef]
- Ozogul, Y.; Karsli, G.T.; Durmuş, M.; Yazgan, H.; Oztop, H.M.; McClements, D.J.; Ozogul, F. Recent Developments in Industrial Applications of Nanoemulsions. Adv. Colloid Interface Sci. 2022, 304, 102685. [Google Scholar] [CrossRef]
- Piorkowski, D.T.; McClements, D.J. Beverage Emulsions: Recent Developments in Formulation, Production, and Applications. Food Hydrocoll. 2014, 42, 5–41. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef]
- Luo, T.; Wei, Z. Recent Progress in Food-grade Double Emulsions: Fabrication, Stability, Applications, and Future Trends. Food Front. 2023, 4, 1622–1642. [Google Scholar] [CrossRef]
- McClements, D.J.; Bai, L.; Chung, C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving Emulsion Formation, Stability and Performance Using Mixed Emulsifiers: A Review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, C.E.; Ambigaipalan, P.; Shahidi, F. Emulsifiers for the Food Industry. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2020; pp. 1–36. [Google Scholar]
- Zembyla, M.; Murray, B.S.; Sarkar, A. Water-in-Oil Emulsions Stabilized by Surfactants, Biopolymers and/or Particles: A Review. Trends Food Sci. Technol. 2020, 104, 49–59. [Google Scholar] [CrossRef]
- Khalfallah, A. Structure and Applications of Surfactants. In Surfactants—Fundamental Concepts and Emerging Perspectives; IntechOpen: London, UK, 2023. [Google Scholar]
- Marhamati, M.; Ranjbar, G.; Rezaie, M. Effects of Emulsifiers on the Physicochemical Stability of Oil-in-Water Nanoemulsions: A Critical Review. J. Mol. Liq. 2021, 340, 117218. [Google Scholar] [CrossRef]
- Sarkar, R.; Pal, A.; Rakshit, A.; Saha, B. Properties and Applications of Amphoteric Surfactant: A Concise Review. J. Surfactants Deterg. 2021, 24, 709–730. [Google Scholar] [CrossRef]
- Che Marzuki, N.H.; Wahab, R.A.; Abdul Hamid, M. An Overview of Nanoemulsion: Concepts of Development and Cosmeceutical Applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.I.A.; Hussein, I.A.; Sultan, A.S.; Al-Muntasheri, G.A. Use of Organoclay as a Stabilizer for Water-in-Oil Emulsions under High-Temperature High-Salinity Conditions. J. Pet. Sci. Eng. 2018, 160, 302–312. [Google Scholar] [CrossRef]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and Emulsion Stability: The Role of the Interfacial Properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef]
- Yamashita, Y.; Miyahara, R.; Sakamoto, K. Emulsion and Emulsification Technology. In Cosmetic Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 489–506. [Google Scholar]
- Capek, I. Degradation of Kinetically-Stable o/w Emulsions. Adv. Colloid Interface Sci. 2004, 107, 125–155. [Google Scholar] [CrossRef]
- Hu, Y.-T.; Ting, Y.; Hu, J.-Y.; Hsieh, S.-C. Techniques and Methods to Study Functional Characteristics of Emulsion Systems. J. Food Drug Anal. 2017, 25, 16–26. [Google Scholar] [CrossRef]
- Kupikowska-Stobba, B.; Domagała, J.; Kasprzak, M.M. Critical Review of Techniques for Food Emulsion Characterization. Appl. Sci. 2024, 14, 1069. [Google Scholar] [CrossRef]
- Lobo, L.; Svereika, A.; Nair, M. Coalescence during Emulsification. J. Colloid Interface Sci. 2002, 253, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Strategies to Control and Inhibit the Flocculation of Protein-Stabilized Oil-in-Water Emulsions. Food Hydrocoll. 2019, 96, 209–223. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. Factors Affecting the Stability of Emulsions Stabilised by Biopolymers. In Science and Technology Behind Nanoemulsions; InTech: London, UK, 2018. [Google Scholar]
- Cai, Z.; Wei, Y.; Shi, A.; Zhong, J.; Rao, P.; Wang, Q.; Zhang, H. Correlation between Interfacial Layer Properties and Physical Stability of Food Emulsions: Current Trends, Challenges, Strategies, and Further Perspectives. Adv. Colloid Interface Sci. 2023, 313, 102863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Y.; Jia, X.; Li, J.; Zhang, M.; Yin, L. Review on the Stability Mechanism and Application of Water-in-Oil Emulsions Encapsulating Various Additives. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1660–1675. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qi, D.; Lin, C.; Li, J. A Technical Review on Characterization Methods for Structures and Properties of Emulsion. APL Mater. 2024, 12, 110602. [Google Scholar] [CrossRef]
- Wang, X.; Anton, H.; Vandamme, T.; Anton, N. Updated Insight into the Characterization of Nano-Emulsions. Expert Opin. Drug Deliv. 2023, 20, 93–114. [Google Scholar] [CrossRef]
- Medina, S.C.; Anjum, D.H.; Behzad, A.R.; Vilagines, R.D.; Tabatabai, A.; Leiknes, T. Microscopy Techniques Applied to Submicron Characterization of Oilfield Produced Water. J. Pet. Sci. Eng. 2021, 206, 108930. [Google Scholar] [CrossRef]
- Dudkiewicz, A.; Tiede, K.; Loeschner, K.; Jensen, L.H.S.; Jensen, E.; Wierzbicki, R.; Boxall, A.B.A.; Molhave, K. Characterization of Nanomaterials in Food by Electron Microscopy. TrAC Trends Anal. Chem. 2011, 30, 28–43. [Google Scholar] [CrossRef]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron Microscopy of Nanoemulsions: An Essential Tool for Characterisation and Stability Assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Yeoh, T.; Shah, J.C.; Walsh, T. Assessing and Predicting Physical Stability of Emulsion-Based Topical Semisolid Products: A Review. J. Pharm. Sci. 2023, 112, 1772–1793. [Google Scholar] [CrossRef]
- Liao, W.; Gharsallaoui, A.; Dumas, E.; Elaissari, A. Understanding of the Key Factors Influencing the Properties of Emulsions Stabilized by Sodium Caseinate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5291–5317. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Orsi, D.; Salerni, F.; Liggieri, L.; Ravera, F.; McMillin, R.; Ferri, J.; Cristofolini, L. Recent Developments in Emulsion Characterization: Diffusing Wave Spectroscopy beyond Average Values. Adv. Colloid Interface Sci. 2021, 288, 102341. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Shang, J.; Mao, Z.; Yang, C. Measurement Methods of Particle Size Distribution in Emulsion Polymerization. Chin. J. Chem. Eng. 2021, 39, 1–15. [Google Scholar] [CrossRef]
- Roig, A.R.; Alessandrini, J.L. Particle Size Distributions from Static Light Scattering with Regularized Non-Negative Least Squares Constraints. Part. Part. Syst. Charact. 2006, 23, 431–437. [Google Scholar] [CrossRef]
- McClements, D.J. Theoretical Prediction of Emulsion Color. Adv. Colloid Interface Sci. 2002, 97, 63–89. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Colloidal Basis of Emulsion Color. Curr. Opin. Colloid Interface Sci. 2002, 7, 451–455. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioproc. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Goñi, S.M.; Salvadori, V.O. Color Measurement: Comparison of Colorimeter vs. Computer Vision System. J. Food Meas. Charact. 2017, 11, 538–547. [Google Scholar] [CrossRef]
- Lee, C.C.; Tomas, M.; Jafari, S.M. Optical Analysis of Nanoencapsulated Food Ingredients by Color Measurement. In Characterization of Nanoencapsulated Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–528. [Google Scholar]
- Zhou, Y.; Yin, D.; Chen, W.; Liu, B.; Zhang, X. A Comprehensive Review of Emulsion and Its Field Application for Enhanced Oil Recovery. Energy Sci. Eng. 2019, 7, 1046–1058. [Google Scholar] [CrossRef]
- Kulkarni, V.S.; Shaw, C. Rheological Studies. In Essential Chemistry for Formulators of Semisolid and Liquid Dosages; Elsevier: Amsterdam, The Netherlands, 2016; pp. 145–182. [Google Scholar]
- Tavares, L.; Zapata Noreña, C.P.; Barros, H.L.; Smaoui, S.; Lima, P.S.; Marques de Oliveira, M. Rheological and Structural Trends on Encapsulation of Bioactive Compounds of Essential Oils: A Global Systematic Review of Recent Research. Food Hydrocoll. 2022, 129, 107628. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.; Genot, C. Lipid Oxidation in Oil-in-Water Emulsions: Involvement of the Interfacial Layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef]
- Pinto, I.; Buss, A. ζ Potential as a Measure of Asphalt Emulsion Stability. Energy Fuels 2020, 34, 2143–2151. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting Zeta Potential, the Key Feature of Interfacial Phenomena, with Applications and Recent Advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental Methods in Chemical Engineering: Zeta Potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as Pharmaceutical Carrier for Dermal and Transdermal Drug Delivery: Formulation Development, Stability Issues, Basic Considerations and Applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Håkansson, A. Emulsion Formation by Homogenization: Current Understanding and Future Perspectives. Annu. Rev. Food Sci. Technol. 2019, 10, 239–258. [Google Scholar] [CrossRef]
- Santana, R.C.; Perrechil, F.A.; Cunha, R.L. High- and Low-Energy Emulsifications for Food Applications: A Focus on Process Parameters. Food Eng. Rev. 2013, 5, 107–122. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic Emulsification: An Overview on the Preparation of Different Emulsifiers-Stabilized Emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Håkansson, A. Rotor-Stator Mixers: From Batch to Continuous Mode of Operation—A Review. Processes 2018, 6, 32. [Google Scholar] [CrossRef]
- Rodgers, T.L.; Cooke, M. Rotor–Stator Devices: The Role of Shear and the Stator. Chem. Eng. Res. Des. 2012, 90, 323–327. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, S.; Li, W. High Shear Mixers: A Review of Typical Applications and Studies on Power Draw, Flow Pattern, Energy Dissipation and Transfer Properties. Chem. Eng. Process. 2012, 57–58, 25–41. [Google Scholar] [CrossRef]
- Gazolu-Rusanova, D.; Lesov, I.; Tcholakova, S.; Denkov, N.; Ahtchi, B. Food Grade Nanoemulsions Preparation by Rotor-Stator Homogenization. Food Hydrocoll. 2020, 102, 105579. [Google Scholar] [CrossRef]
- Kumar, A.; Kanwar, R.; Mehta, S.K. Nanoemulsion as an Effective Delivery Vehicle for Essential Oils: Properties, Formulation Methods, Destabilizing Mechanisms and Applications in Agri-Food Sector. Next Nanotechnol. 2025, 7, 100096. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent Insights into Nanoemulsions: Their Preparation, Properties and Applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef]
- Yadav, K.S.; Kale, K. High Pressure Homogenizer in Pharmaceuticals: Understanding Its Critical Processing Parameters and Applications. J. Pharm. Innov. 2020, 15, 690–701. [Google Scholar] [CrossRef]
- Ocampo-Salinas, I.O.; Ellez-Medina, D.I.; Jimenez-Martinez, C.; Davila-Ortiz, G. Application of High Pressure Homogenization to Improve Stability and Decrease Droplet Size in Emulsion-Flavor Systems. Int. J. Environ. Agric. Biotechnol. 2016, 1, 646–662. [Google Scholar] [CrossRef]
- Leong, T.S.H.; Wooster, T.J.; Kentish, S.E.; Ashokkumar, M. Minimising Oil Droplet Size Using Ultrasonic Emulsification. Ultrason. Sonochem. 2009, 16, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Bates, D. Ultrasonic Innovations in the Food Industry: From the Laboratory to Commercial Production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Silva, E.K.; Rosa, M.T.M.; Meireles, M.A.A. Ultrasound-Assisted Formation of Emulsions Stabilized by Biopolymers. Curr. Opin. Food Sci. 2015, 5, 50–59. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Xing, L.; Zhang, W. Applications and Effects of Ultrasound Assisted Emulsification in the Production of Food Emulsions: A Review. Trends Food Sci. Technol. 2021, 110, 493–512. [Google Scholar] [CrossRef]
- Hwangbo, S.-A.; Lee, S.-Y.; Kim, B.-A.; Moon, C.-K. Preparation of Surfactant-Free Nano Oil Particles in Water Using Ultrasonic System and the Mechanism of Emulsion Stability. Nanomaterials 2022, 12, 1547. [Google Scholar] [CrossRef]
- Yao, C.; Zhao, S.; Liu, L.; Liu, Z.; Chen, G. Ultrasonic Emulsification: Basic Characteristics, Cavitation, Mechanism, Devices and Application. Front. Chem. Sci. Eng. 2022, 16, 1560–1583. [Google Scholar] [CrossRef]
- Alliod, O.; Charcosset, C. Membrane Emulsification for Encapsulation of Bioactives: Application to the Encapsulation of Antioxidants. In Emulsion-Based Encapsulation of Antioxidants; Springer: Cham, Switzerland, 2020; pp. 225–247. [Google Scholar]
- Charcosset, C. Preparation of Emulsions and Particles by Membrane Emulsification for the Food Processing Industry. J. Food Eng. 2009, 92, 241–249. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent Advances in Encapsulation of Curcumin in Nanoemulsions: A Review of Encapsulation Technologies, Bioaccessibility and Applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Nazir, A.; Vladisavljević, G.T. Droplet Breakup Mechanisms in Premix Membrane Emulsification and Related Microfluidic Channels. Adv. Colloid Interface Sci. 2021, 290, 102393. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Integrated Membrane Processes for the Preparation of Emulsions, Particles and Bubbles. In Integrated Membrane Systems and Processes; Wiley: Hoboken, NJ, USA, 2016; pp. 79–140. [Google Scholar]
- Piacentini, E.; Drioli, E.; Giorno, L. Membrane Emulsification Technology: Twenty-Five Years of Inventions and Research through Patent Survey. J. Memb. Sci. 2014, 468, 410–422. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Preparation of Microparticles and Nanoparticles Using Membrane-Assisted Dispersion, Micromixing, and Evaporation Processes. Particuology 2024, 84, 30–44. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic Methods for Generating Continuous Droplet Streams. J. Phys. D Appl. Phys. 2007, 40, R319–R336. [Google Scholar] [CrossRef]
- Engl, W.; Backov, R.; Panizza, P. Controlled Production of Emulsions and Particles by Milli- and Microfluidic Techniques. Curr. Opin. Colloid Interface Sci. 2008, 13, 206–216. [Google Scholar] [CrossRef]
- Fan, R.; Wu, J.; Duan, S.; Jin, L.; Zhang, H.; Zhang, C.; Zheng, A. Droplet-Based Microfluidics for Drug Delivery Applications. Int. J. Pharm. 2024, 663, 124551. [Google Scholar] [CrossRef]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of Droplets and Bubbles in a Microfluidic T-Junction—Scaling and Mechanism of Break-Up. Lab A Chip 2006, 6, 437. [Google Scholar] [CrossRef] [PubMed]
- Kole, S.; Bikkina, P. A Parametric Study on the Application of Microfluidics for Emulsion Characterization. J. Pet. Sci. Eng. 2017, 158, 152–159. [Google Scholar] [CrossRef]
- Vladisavljević, G.; Al Nuumani, R.; Nabavi, S. Microfluidic Production of Multiple Emulsions. Micromachines 2017, 8, 75. [Google Scholar] [CrossRef]
- Wei, X.; Yao, X.; Yue, J.; Li, G.; Liu, N.; Li, D.; Yang, D.; Fang, Y.; Nishinari, K.; Zhao, M. Recent Advances on the Generation, Stabilization, and Potential Applications of Double Emulsions Based on the Microfluidic Strategy. Food Eng. Rev. 2024, 16, 129–145. [Google Scholar] [CrossRef]
- Moon, S.-K.; Cheong, I.W.; Choi, S.-W. Effect of Flow Rates of the Continuous Phase on Droplet Size in Dripping and Jetting Regimes in a Simple Fluidic Device for Coaxial Flow. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 84–88. [Google Scholar] [CrossRef]
- Wu, L.; Qian, J.; Liu, X.; Wu, S.; Yu, C.; Liu, X. Numerical Modelling for the Droplets Formation in Microfluidics—A Review. Microgravity Sci. Technol. 2023, 35, 26. [Google Scholar] [CrossRef]
- Han, W.; Chen, X. A Review on Microdroplet Generation in Microfluidics. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 247. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, X.; Hou, J.; Huang, L.; Yao, Y.; Ding, Z.; Wei, J.; Hao, N. Droplet Microfluidic Devices: Working Principles, Fabrication Methods, and Scale-Up Applications. Small Methods 2024, 8, e2301406. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.K.; Tsai, S.S.H.; Wan, J.; Stone, H.A. Dripping and Jetting in Microfluidic Multiphase Flows Applied to Particle and Fibre Synthesis. J. Phys. D Appl. Phys. 2013, 46, 114002. [Google Scholar] [CrossRef]
- Elvira, K.S.; Gielen, F.; Tsai, S.S.H.; Nightingale, A.M. Materials and Methods for Droplet Microfluidic Device Fabrication. Lab A Chip 2022, 22, 859–875. [Google Scholar] [CrossRef]
- Fu, T.; Wu, Y.; Ma, Y.; Li, H.Z. Droplet Formation and Breakup Dynamics in Microfluidic Flow-Focusing Devices: From Dripping to Jetting. Chem. Eng. Sci. 2012, 84, 207–217. [Google Scholar] [CrossRef]
- Ho, T.M.; Razzaghi, A.; Ramachandran, A.; Mikkonen, K.S. Emulsion Characterization via Microfluidic Devices: A Review on Interfacial Tension and Stability to Coalescence. Adv. Colloid Interface Sci. 2022, 299, 102541. [Google Scholar] [CrossRef]
- Kamnerdsook, A.; Juntasaro, E.; Khemthongcharoen, N.; Chanasakulniyom, M.; Sripumkhai, W.; Pattamang, P.; Promptmas, C.; Atthi, N.; Jeamsaksiri, W. On Classification of Water-in-Oil and Oil-in-Water Droplet Generation Regimes in Flow-Focusing Microfluidic Devices. Colloids Interfaces 2023, 7, 17. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef] [PubMed]
- Schroen, K.; Berton-Carabin, C.; Renard, D.; Marquis, M.; Boire, A.; Cochereau, R.; Amine, C.; Marze, S. Droplet Microfluidics for Food and Nutrition Applications. Micromachines 2021, 12, 863. [Google Scholar] [CrossRef]
- Kakran, M.; Antipina, M.N. Emulsion-Based Techniques for Encapsulation in Biomedicine, Food and Personal Care. Curr. Opin. Pharmacol. 2014, 18, 47–55. [Google Scholar] [CrossRef]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.-A.; Sudesh, K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.R.; Ambrosi, A.; Luccio, M. Di Encapsulated Essential Oils: A Perspective in Food Preservation. Future Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- Jenjob, R.; Phakkeeree, T.; Seidi, F.; Theerasilp, M.; Crespy, D. Emulsion Techniques for the Production of Pharmacological Nanoparticles. Macromol. Biosci. 2019, 19, e1900063. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.A.; Patel, D.M.; Patel, J.K.; Patel, D.H. Solvent Emulsification Evaporation and Solvent Emulsification Diffusion Techniques for Nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Springer International Publishing: Cham, Switzerland, 2021; pp. 287–300. [Google Scholar]
- Lim, K.; Hamid, Z.A.A. Polymer Nanoparticle Carriers in Drug Delivery Systems. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 217–237. [Google Scholar]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N. Production of Micro and Nano Particles of Pharmaceutical by Supercritical Carbon Dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.-S.; Kim, S.; Ha, E.-S.; Kim, M.-S.; Hwang, S.-J. Pharmaceutical Applications of Supercritical Fluid Extraction of Emulsions for Micro-/Nanoparticle Formation. Pharmaceutics 2021, 13, 1928. [Google Scholar] [CrossRef]
- Tabernero, A.; Martín del Valle, E.M.; Galán, M.A. Supercritical Fluids for Pharmaceutical Particle Engineering: Methods, Basic Fundamentals and Modelling. Chem. Eng. Process. 2012, 60, 9–25. [Google Scholar] [CrossRef]
- Santos, D.T.; Santana, Á.L.; Meireles, M.A.A.; Petenate, A.J.; Silva, E.K.; Albarelli, J.Q.; Johner, J.C.F.M.S.; Gomes, M.T.; Torres, R.A.D.C.; Hatami, T. Recent Developments in Particle Formation with Supercritical Fluid Extraction of Emulsions Process for Encapsulation. In Supercritical Antisolvent Precipitation Process; Springer: Cham, Switzerland, 2019; pp. 51–64. [Google Scholar]
- Della Porta, G.; Falco, N.; Reverchon, E. Continuous Supercritical Emulsions Extraction: A New Technology for Biopolymer Microparticles Production. Biotechnol. Bioeng. 2011, 108, 676–686. [Google Scholar] [CrossRef] [PubMed]

| Sector of Application | Active Agents | Carrier Agent | Encapsulating System | Size | Ref. |
|---|---|---|---|---|---|
| Food | Curcumin | Medium chain triglyceride, canola oil or linseed oil | Emulsion | 400–800 nm | [19] |
| Lactobacillus reuteri | Medium chain triglyceride oil | Emulsion | 13–15 μm | [20] | |
| Lactobacillus acidophilus | Sunflower oil | Emulsion | 5 μm | [21] | |
| Vitamin E | Corn oil | Emulsion | 0.1–0.5 μm | [22] | |
| Omega-3 fatty acid | Sunflower oil | Emulsion | 150 nm | [23] | |
| Arthrospira platensis polyphenolic extracts | Polycaprolactone | Polymeric particles | 140–800 nm | [24] | |
| Vitamin E | Polycaprolactone | Polymeric particles | 60–150 nm | [25] | |
| Propolis | Poly(lactide co-glycolides) (PLGA), ethyl cellulose or polycaprolactone | Polymeric particles | 2–5 μm | [26] | |
| β-carotene | Polycaprolactone and xanthan gum | Polymeric particles | 122–220 nm | [27] | |
| Cosmetic | Resveratrol | Rose oil, jasmine oil or lemon oil, | Emulsion | 10–70 nm | [28] |
| Quercitin | Capric triglyceride | Emulsion | – | [29] | |
| Tocopheryl acetate | Medium chain triglyceride | Emulsion | 120 nm | [30] | |
| Retinol | Eudragit RS 100 | Polymeric particles | 180–300 nm | [31] | |
| Vitamine E | Sodium polystyrene sulfonate | Polymeric particles | 1–1.3 μm | [32] | |
| Pharmaceutical | Insulin | Medium chain triglyceride oil | Emulsion | 10–30 μm | [33] |
| Malaria vaccine adjuvant | Squalene | Emulsion | 2.8–18 μm | [34] | |
| Insulin | Poly(lactide co-glycolides) (PLGA) | Polymeric particles | 1.8–16.9 μm | [35] | |
| Ketoprofen | Poly(lactide co-glycolides) (PLGA) | Polymeric particles | 100–200 nm | [36] | |
| Ibuprofen | Chitosan | Polymeric particles | 200–280 nm | [37] | |
| Curcumin | Poly(lactide co-glycolides) (PLGA) | Polymeric particles | 250–500 nm | [38] | |
| Peptide | poly(ethylene glycol)-block-polycaprolactone | Polymeric particles | 100–200 nm | [39] | |
| Agriculture | Sinorhizobium meliloti | Canola oil | Emulsion | – | [40] |
| Pseudomonas simiae and Azospirillum brasilense | Isopropyl palmitate | Emulsion | 80–150 μm | [41] | |
| Bacillus thuringiensis | Mineral oil | Emulsion | 5–8 μm | [42] | |
| Two bacteria, a yeast, and two saprophytic fungi | Soybean oil | Emulsion | 5 μm | [43] | |
| Pseudomonas putida | Sodium alginate and bentonite | Polymeric particles | 25–100 μm | [44] | |
| Atrazine | Poly(epsilon-caprolactone) | Polymeric particles | 408–483 nm | [45] | |
| Carbendazim and tebuconazole | Polycaprolactone | Polymeric particles | 520–540 nm | [46] |
| Process | Droplet Size | Advantages | Disadvantages | Scale Up |
|---|---|---|---|---|
| Rotor–stator emulsifier | >1 µm | Ease to install, user friendly, low investment cost, high throughput, compatible with viscous systems, large volume production | Wide droplet size distribution, nanoemulsions cannot be produced | Laboratory and industrial scale |
| High-pressure homogenizer | >0.1 µm | Possibility to produce nanoemulsion | Wide droplet size distribution, not suitable for sensitive compounds | Laboratory and industrial scale |
| Ultrasound homogenizer | >0.1 µm | Ease of operation, low cost, ease of maintenance, excellent cleanliness, reduced energy consumption | High increase in temperature, not suitable for sensitive compounds, small product volume | Laboratory scale |
| Membrane homogenizer | >0.3 µm | Highly energy efficient, narrow particle size distribution, reduced shear stress, low energy requirements, high versatility | Fouling phenomenon, low dispersed phase flux | Laboratory scale |
| Microfluidic emulsification | >0.1 µm | Precise emulsification, high homogeneity of droplet size, highly versatility, ability of customize microfluidic chip | Facility of clogging, complexity of scale up | Laboratory scale |
| Process | Advantages | Disadvantages |
|---|---|---|
| Emulsion solvent evaporation | Versatile and simple | Difficulty in controlling the particle size distribution, residual solvent in the product |
| Emulsion solvent diffusion | Simple and highly reproducible | High amount of required water, residual solvent in the product, long process time |
| Salting-out | Less hazardous solvents, no increase in temperature | Extensive washing step, ability to encapsulate only lipophilic compounds |
| Supercritical emulsion extraction | High control of particle size and morphology, reduction of solvent residues, versatile, possibility to work in continuous mode | High investment costs, safety issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bufalini, C.; Campardelli, R. Versatile Emulsion-Based Encapsulation System Production Processes: A Review. Processes 2025, 13, 1409. https://doi.org/10.3390/pr13051409
Bufalini C, Campardelli R. Versatile Emulsion-Based Encapsulation System Production Processes: A Review. Processes. 2025; 13(5):1409. https://doi.org/10.3390/pr13051409
Chicago/Turabian StyleBufalini, Chiara, and Roberta Campardelli. 2025. "Versatile Emulsion-Based Encapsulation System Production Processes: A Review" Processes 13, no. 5: 1409. https://doi.org/10.3390/pr13051409
APA StyleBufalini, C., & Campardelli, R. (2025). Versatile Emulsion-Based Encapsulation System Production Processes: A Review. Processes, 13(5), 1409. https://doi.org/10.3390/pr13051409








