Abstract
Cuboid packed-bed devices developed for chromatographic separation typically have shorter bed heights and larger cross-sectional areas than their equivalent cylindrical columns. These devices can be operated at low back pressures and give comparable or even better resolution than their equivalent columns. However, the bed height of a cuboid packed-bed device could potentially affect its separation performance. To examine this, three devices having 5, 10 and 19.5 mm bed heights were fabricated and packed with the same resin media. A mathematical model was first developed to predict the effect of bed height on the performance of these cuboid devices. This prediction was performed based on the residence time heterogeneity (RTH) in these devices, which increased slightly as the bed height was decreased. However, this was not likely to affect the separation efficiency very significantly. The relative performances of these three cuboid devices were then compared based on the resolution obtained during ion-exchange chromatography of multi-protein mixtures. As predicted by the mathematical model, the loss in resolution due to the decrease in bed height was relatively small (0.83 to 0.73 in binary protein separation). Also, this loss could easily be compensated for by slightly lowering the flow rate or by extending the elution gradient. The results discussed in this paper demonstrate that with cuboid packed-bed devices, the dimensions could be altered in a reasonably flexible manner without adversely affecting separation performance. Such flexibility is advantageous from the point of view of process design and optimization, which is critically important for developing large-scale processes for the purification of biologics.
1. Introduction
A cylindrical column is widely considered the default device for conducting chromatographic separation and purification. The high resolution in separation obtained with chromatography is vital in different areas of application, e.g., chemicals, pharmaceuticals, biopharmaceuticals, food, the environment, renewable resources, water and waste-water treatment. Chromatography is considered the gold-standard technique for the purification of biologics such as vaccines and monoclonal antibodies. The dimensions of columns (i.e., bed height and diameter) could have a significant impact on their performance metrics (such as resolution and productivity), and such an effect is usually scale-dependent [1,2,3,4,5,6,7,8]. In analytical chromatography, long columns with a small diameter are preferred, as they give higher resolution in separation. However, in many preparative applications, a smaller bed height and a relatively larger lateral dimension may be advantageous. For example, protein A affinity chromatography devices with a small bed height may be preferable to tall columns for optimal production during antibody manufacture [5]. The effect of column dimension, resin particle size and operating conditions such as linear velocity on column performance is extensively discussed in the scientific literature [6,7,8]. The pressure drop across a column will increase with an increase in column length, as described by Darcy’s Law. While a tall column might be better from a resolution perspective in analytical chromatography, the resulting high pressure typically seen with an ultrahigh-pressure liquid chromatography (UPLC) system may result in frictional heating [9] and ultimately on-column aggregation and degradation of biological samples [10]. In preparative chromatography columns, which typically use softer and compressible resin particles, high pressure may result in severe degradation of column performance due to resin compaction [11,12,13,14,15,16,17].
An ideal preparative chromatography column is one that gives high productivity (i.e., can be operated at a high flow rate) while maintaining sufficient separation efficiency (i.e., resolution, yield and purity). A high flow rate results in a high pressure drop and problems linked to it. Different strategies have been employed for maintaining a high flow rate in chromatographic separation at a reasonably low flow pressure: (1) simultaneously increasing the area of the cross-section of the column and reducing its bed height, (2) developing new resin media with improved hydraulic properties [18], (3) using monoliths [19], and (4) using membrane-based separation media [20]. When the choice of separation media is restricted, option (1) is usually used [7,8,21,22,23]. However, as discussed in the next paragraph, a chromatography column with a short bed height and large cross-sectional area (i.e., a squat column) is particularly prone to flow maldistribution.
Flow maldistribution that exists in a typical squat column could affect the separation performance very significantly [24]. Figure 1A shows the idealized flow paths within a squat column. The shortest flow path from the inlet to the outlet is the one along the axis of the column, while the longest paths are those that travel radially from the inlet through the header to the periphery, then travel down along the side of the column and then travel radially inward in the bottom header to the column outlet. In addition to being the longest path, these are also the slowest, as the radial velocity in the header decreases exponentially from the center to the periphery [25]. The outcome of such variability is a wide residence time distribution and consequently lower separation efficiency. As the column bed height is reduced, this flow unevenness is further exacerbated. This problem is typically addressed by improving the design of the column headers [26,27,28] and by introducing alternative flow arrangements [29].
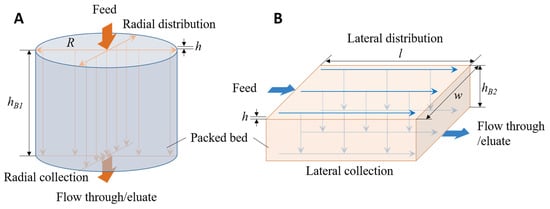
Figure 1.
Diagrams showing idealized fluid flow paths in (A) a squat column and (B) a cuboid packed-bed device with the same bed volume but different axial and radial (or lateral) dimensions.
Box-shaped cuboid packed-bed devices have been proposed as a solution to non-uniform flow distribution in short bed height chromatography devices [25]. As shown in Figure 1B, the cuboid packed bed is fed and drained in a complimentary fashion using a pair of flow channels. By this approach, the residence time distribution is narrowed down, and higher efficiency compared to squat columns can therefore be obtained using these cuboid devices [25,30,31,32]. In these studies, squat columns and cuboid devices of same bed volume and bed height packed with the same resin were compared. In each case, the cuboid device outperformed its equivalent column in terms of all separation metrics compared. More recently, a 1 mL cuboid device having a bed height of 10 mm was shown to have comparable performance (in terms of the number of theoretical plates and resolution in binary separation) as a 1 mL column having a 25.8 mm bed height [31]. The implication of this study was that operational advantages such as low pressure drop and a high flow rate could be achieved with a short bed height cuboid device without compromising resolution. The option of being able to use short bed height cuboid devices is attractive in the manufacture of biologics where a fast separation cycle is attractive not only from the point of view of productivity but also from the angle of product stability. However, how cuboid devices having different bed heights perform relative to each other has not yet been systematically examined. Knowing this will make it easier to select the appropriate dimensions for cuboid devices for specific applications. However, such determination will need to be based on an objective comparison of separation metrics.
In this paper, we first discuss a mathematical model for comparing residence time heterogeneity (RTH) in cuboid devices having different bed heights. Three cuboid devices with the same cross-sectional dimensions but different bed heights (i.e., 5, 10 and 19.5 mm) were fabricated to verify the validity of the proposed model and to compare actual separation performance by conducting protein separation experiments at different conditions such as flow rate and linear gradient length during elution. The results obtained are discussed.
2. Material and Methods
Sodium chloride (SOD002.205) was purchased from Bioshop (Burlington, ON, Canada). Sodium phosphate monobasic (S0751), sodium phosphate dibasic (S0876) and proteins, i.e., ribonuclease A (R6513, pI = 9.6), cytochrome C (C7752, pI = 10–10.5) and lysozyme (L6876, pI = 11.35) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Capto S cation exchange resin media (17-5441-03) was purchased from GE Healthcare Biosciences, QC, Canada. Water used for buffer/solution making was obtained from a Diamond NANOpure water purification unit (Barnstead, IA, USA). Buffers were filtered and degassed before being used in chromatography experiments.
Three custom-designed and in-house fabricated cuboid packed-bed devices with the same cross-sectional dimensions (i.e., length: 20 mm, width: 10 mm) and different bed heights (i.e., 5 mm, 10 mm and 19.5 mm) were used in this study. These cuboid devices had bed volumes of 1 mL, 2 mL and 3.9 mL, respectively. The height of the lateral channels in these devices was 0.25 mm. Exploded-view drawings of these three devices are shown in Figure 2. The function of each component (i.e., two plates with lateral channels for flow distribution and collection and the central frame for housing the packed bed) and the resin packing procedure are discussed in detail elsewhere [25,30]. Briefly, the resin was packed within the cuboid devices using a flow packing technique. The flow rate used in the packing step was higher than that used during actual separation. This ensured that the resin was properly packed and that gaps did not appear in the resin bed during separation. The main difference between the three cuboid devices was the thickness of the central frame, which determined the bed height and thereby the bed volume.
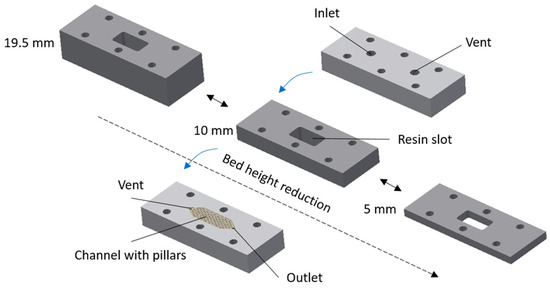
Figure 2.
Exploded-view drawings of cuboid packed-bed devices with different bed heights.
Bubbles were purged from the lateral channels of the cuboid devices using the vent ports prior to connecting these to an AKTA prime liquid chromatography system (GE Healthcare Biosciences, QC, Canada). Protein separation experiments were carried out first using a binary protein mixture and then a ternary protein mixture to assess the impact of bed height on resolution. The protein mixture containing the feed sample was injected in the devices after equilibration with buffer A (20 mM phosphate buffer, pH 7). A 100 μL loop was used for sample injection. The bound proteins were eluted using buffer B (20 mM phosphate buffer, 1 M NaCl, pH 7) using different elution strategies. The resolution (R) in the binary and ternary protein separation experiments was calculated using the following equation:
where VR is the retention volume, w0.5 is the peak width at 50% peak height, and a and b, shown as superscripts in Equation (1), represent the two sequentially eluted species.
3. Results and Discussion
It has been shown that the residence time varies with radial or lateral locations in columns and the cuboid devices [25]. Briefly, the residence time in a column increases significantly in flow paths located further away for the axis. The residence time in a cuboid device also increases slightly along paths located further away from the middle of the device [25,30]. The residence time heterogeneity (RTH) could be defined as ((τ − τmin)/τmin), where τ is the residence time along a given path, while τmin is the minimum residence time within the device. The residence time along a flow path in the cuboid device could be determined as shown below [30]:
where hB is the height of the packed bed within the cuboid device, vS is the superficial velocity, z is the lateral position, v0 is the lateral velocity at the inlet, vU is the lateral velocity at position z, and l is the length of the channel.
The minimum residence time (τmin) is at z = l/2 [30]:
Therefore, the difference between τ and τmin is
Therefore, RTH is obtained by
The rate of change in velocity in the lateral channel could be obtained by the equation shown below [25]:
where h is the channel height.
The lateral velocity at the inlet (v0) is given by [32]
where V is the volumetric flow rate and w is the width of the channel.
The superficial velocity (vS) is given by [32]
From Equations (5)–(8)
Therefore, RTH depends on the position (z), bed height (hB) and channel height (h).
The maximum residence time (τmax) could be expected along a flow path travelling through the regions of the packed bed adjacent to the inlet and the outlet [30], i.e., at z = 0 and z = l. Putting these conditions in in Equation (9), the maximum residence time heterogeneity in the cuboid device (RTHmax) is given by
Figure 3 shows the simulated RTH along the lateral lengths of the cuboid packed-bed devices with different bed heights as determined using Equation (9). The RTH is clearly a function of the lateral position, with a minimum value of zero at the center of the device (z/l = 0.5) and maximum values at the two ends (z/l = 0 or 1). The RTH value increased with the decrease in bed height, the maximum RTH values being 0.031, 0.016 and 0.008 for bed heights of 5 mm, 10 mm and 19.5 mm, respectively. However, the maximum percentage deviations from the minimum were relatively small in all cases, these being 3.1, 1.6 and 0.8%, respectively. Based on this, it could be anticipated that the separation performance of the cuboid chromatography devices would decrease with the decrease in bed height, though not as significantly as observed with cylindrical columns.
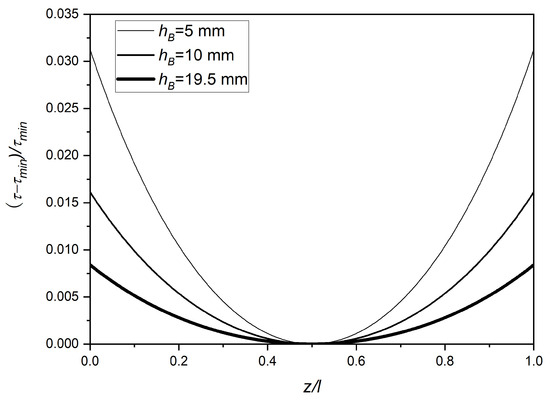
Figure 3.
Simulated RTH along the lateral lengths of cuboid packed-bed devices having different bed heights (cuboid length = 20 mm, cuboid width = 10 mm).
Binary protein separation experiments were carried out using a mixture of ribonuclease A and lysozyme as the feed solution with cuboid devices of different bed heights packed with Capto S cation exchange resin media. These proteins were selected as these bound to the Capto S media at the operating pH used in these binary protein separation experiments, i.e., pH 7.0, and could be eluted using the appropriate salt gradient. These experiments were carried out at very high flow rates, i.e., at 5 mL/min and 10 mL/min, using 100 μL of feed sample (2.5 mg/mL ribonuclease A+ 0.75 mg/mL lysozyme). For the cuboid device having a 5 mm bed height (i.e., 1 mL bed volume), the 10 mL/min flow rate corresponded to an equivalent of 10 column volumes per minute (or 10 CVM), which is very high for resin-based chromatography. Both proteins bound to the resin in the presence of the binding buffer, and these were eluted using 25 mL linear gradient elution, which was initiated immediately after sample injection. Ribonuclease A (pI = 9.6) was eluted first, followed by lysozyme (pI = 11.35). Figure 4 shows the chromatograms obtained from experiments carried out at 10 mL/min flow. Table 1 shows resolution obtained during the separation of ribonuclease A and lysozyme using the cuboid packed-bed devices at both flow rates examined. At a flow rate of 10 mL/min, the resolutions in ribonuclease A and lysozyme separation (R) obtained with 5 mm and 10 mm bed heights were similar (i.e., 0.73 and 0.71, respectively). The resolution increased slightly to 0.83 when the bed height was increased to 19.5 mm. A similar trend was also observed for the three devices at a 5 mL/min flow rate. This difference in resolution could be explained based on the mathematical-model-based RTH analysis described in the previous paragraphs.
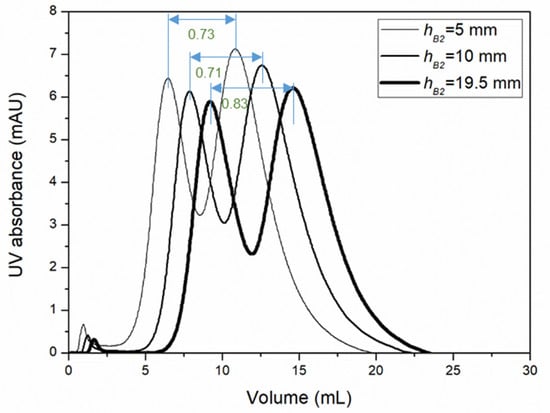
Figure 4.
Chromatograms for ribonuclease A and lysozyme separation by the Capto S media packed cuboid devices with different bed heights at a 10 mL/min flow rate (loop: 100 μL, sample: 2.5 mg/mL ribonuclease A+ 0.75 mg/mL lysozyme, buffer A: 20 mM phosphate buffer, pH 7, buffer B: 20 mM phosphate buffer, 1 M NaCl, pH 7, elution: 25 mL linear gradient started immediately after injection, first peak: ribonuclease A, second peak: lysozyme, resolution values obtained are indicated in the figure using double sided arrows).

Table 1.
Resolution in protein separation obtained with cuboid packed-bed devices with different bed heights at different flow rates (loop: 100 μL, sample: 2.5 mg/mL ribonuclease A+ 0.75 mg/mL lysozyme, buffer A: 20 mM phosphate buffer, pH 7, buffer B: 20 mM phosphate buffer, 1 M NaCl, pH 7, elution: 25 mL linear gradient).
Ternary protein separation was carried out using a feed solution consisting of a mixture of ribonuclease A, cytochrome C and lysozyme with the cuboid devices having different bed heights. All of these proteins being positively charged bound to the Capto S media in the presence of the binding buffer and were eluted using a salt (sodium chloride) gradient. The order of elution was ribonuclease A (pI = 9.6) followed by cytochrome C (pI = 10–10.5) and finally followed by lysozyme (pI = 11.35). These experiments were carried out at a flow rate of 0.5 mL/min in order to obtain high resolution in the separation of these proteins. Three different linear gradient lengths were examined in these studies, i.e., 25, 37.5 and 50 mL. Feed sample (100 μL volume; 1.5 mg/mL ribonuclease A+ 0.5 mg/mL cytochrome C+ 0.5 mg/mL lysozyme) was injected in these studies. The resolution of ribonuclease between the ribonuclease A and cytochrome peaks (the first and second eluted peaks) was designated as R1, while the resolution between the cytochrome C and lysozyme peaks (the second and third eluted peaks) was designated as R2. The resolution obtained in these ternary protein separation experiments is summarized in Table 2. Quite clearly, the resolution increased when the bed height increased. However, even with the 5 mm bed height device, good ternary protein separation was obtained. The results summarized in Table 2 also suggest that when using the 5 mm bed height cuboid device, comparable separation (in terms of resolution) to that obtained with the 19.5 mm bed height device could be obtained by extending the length of the linear gradient. For instance, the resolution combinations obtained with the 19.5 mm bed height cuboid device using a 25 mL linear gradient were R1 = 1.38 and R2 = 1.05. Using a 37.5 mL linear gradient, the resolution combinations obtained with the 5 mm bed height device were 1.48 (R1) and 0.95 (R2). When the gradient length was further increased to 50 mL, the resolution combinations obtained with the 5 mm bed height device were 1.64 (R1) and 1.05 (R2).

Table 2.
Resolution in ternary protein separation obtained with cuboid packed-bed devices having different bed heights at a 0.5 mL/min flow rate using different elution conditions (loop: 100 μL, sample: 1.5 mg/mL ribonuclease A+ 0.5 mg/mL cytochrome C+ 0.5 mg/mL lysozyme, buffer A: 20 mM phosphate buffer, pH 7, buffer B: 20 mM phosphate buffer, 1 M NaCl, pH 7).
Figure 5 shows chromatograms for ribonuclease A, cytochrome C and lysozyme separation obtained with the 5 mm bed height device using a 37.5 and 50 mL linear gradient elution and with the 19.5 mm bed height device using a 25 mL linear gradient elution. These results show that comparable resolution in ternary separation could be obtained with the 5 mm bed height device in about the same time scale as that with the 19.5 mm bed height device. Also, the buffer consumptions in these separations were comparable. Overall, these results demonstrate the suitability of very short bed height cuboid devices for conducting the high-resolution separation of proteins. These results are consistent with those reported in an earlier study [31], where it was demonstrated that a short bed height cuboid device could actually give superior separation compared to an equivalent tall column. While the resolution increased with the increase in bed height, comparable resolution in separation with a comparable time scale could be obtained with the 5 mm bed height device by using a longer gradient. This was possible due to the smaller bed volume of the 5 mm bed height device compared to the 19.5 mm bed height device. The lessons learned from this study would also be applicable to other chromatography devices with short bed heights, such as those based on membranes and monoliths [33].
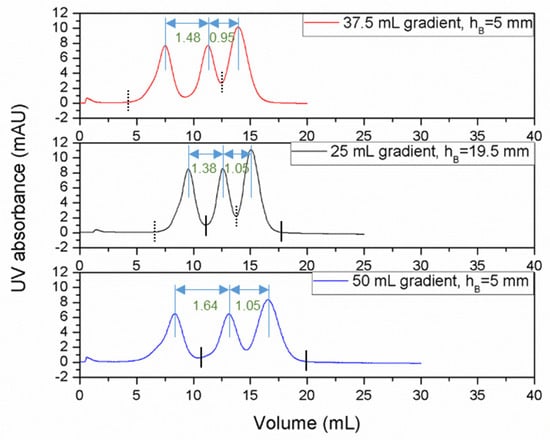
Figure 5.
Chromatograms showing comparable resolution during the separation of the ternary protein mixture of ribonuclease A, cytochrome C and lysozyme using cuboid devices of 5 mm and 19.5 mm bed heights at a 0.5 mL/min flow rate (first peak: ribonuclease A, second peak: cytochrome C, third peak: lysozyme, loop: 100 μL, sample: 1.5 mg/mL ribonuclease A+ 0.5 mg/mL cytochrome C+ 0.5 mg/mL lysozyme, buffer A: 20 mM phosphate buffer, pH 7, buffer B: 20 mM phosphate buffer, 1 M NaCl, pH 7, elution: 25, 37.5 and 50 mL linear gradient started immediately after injection; the bed height and gradients used in these experiments are indicated in the respective figures; resolution obtained are indicated in the figures using double sided arrows).
4. Conclusions
Cuboid chromatography devices have been shown to be suitable for conducting fast, high-resolution chromatography of proteins. One of the main advantages of the cuboid format is that it is possible to carry out preparative separations at very high flow rates with short bed height devices without problems such as resin compaction. This study demonstrates and explains why low bed height cuboid devices can be used for conducting such chromatographic separations. A model was developed to examine the effect of bed height on the efficiency of separation obtained using cuboid chromatography devices. The degree of dispersion within cuboid chromatography devices having different bed heights could be objectively compared using the residence time heterogeneity (RTH) parameter. The mathematical model developed for calculating RTH predicted that while the efficiency of separation was likely to decrease with a decrease in bed height, such an effect would not be as significant as that observed with conventional cylindrical columns. Binary and ternary protein separation experiments showed that good separation could be obtained even using a cuboid chromatography device having a very low bed height, i.e., 5 mm. The resolution obtained in such separations decreased to some extent with the decrease in bed height, but this could easily be offset by using a longer elution gradient. Due to its smaller bed volume, comparable separation was obtained with the shorter bed height device in the same time scale as that with the taller device. Overall, the short bed height cuboid device could be used for fast protein separation without compromising the resolution.
Author Contributions
G.C. carried out the experiments and wrote the manuscript. R.G. supervised the research work and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Natural Science and Engineering Research Council (NSERC) of Canada for funding this study. The authors also thank Paul Gatt for fabricating the cuboid packed-bed devices used in this study.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Wu, N.; Bradley, A.C. Effect of column dimension on observed column efficiency in very high pressure liquid chromatography. J. Chromatogr. A 2012, 1261, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Finch, J.W.; Lavallee, M.J.; Collamati, R.A.; Benevides, C.C.; Gebler, J.C. Effects of column length, particle size, gradient length and flow rate on peak capacity of nano-scale liquid chromatography for peptide separations. J. Chromatogr. A 2007, 1147, 30–36. [Google Scholar] [CrossRef]
- Lestremau, F.; Wu, D.; Szücs, R. Evaluation of 1.0mm i.D. Column performances on ultra high pressure liquid chromatography instrumentation. J. Chromatogr. A 2010, 1217, 4925–4933. [Google Scholar] [CrossRef]
- Schweiger, S.; Jungbauer, A. Scalability of pre-packed preparative chromatography columns with different diameters and lengths taking into account extra column effects. J. Chromatogr. A 2018, 1537, 66–74. [Google Scholar] [CrossRef]
- Fahrner, R.L.; Iyer, H.V.; Blank, G.S. The optimal flow rate and column length for maximum production rate of protein a affinity chromatography. Bioprocess Eng. 1999, 21, 287–292. [Google Scholar] [CrossRef]
- Katz, E.; Ogan, K.L.; Scott, R.P.W. Liquid chromatography column design. J. Chromatogr. A 1984, 289, 65–83. [Google Scholar] [CrossRef]
- Golshan-Shirazi, S.; Guiochon, G. Theory of optimization of the experimental conditions of preparative elution chromatography: Optimization of the column efficiency. Anal. Chem. 1989, 61, 1368–1382. [Google Scholar] [CrossRef]
- Carr, P.W.; Wang, X.; Stoll, D.R. Effect of pressure, particle size, and time on optimizing performance in liquid chromatography. Anal. Chem. 2009, 81, 5342–5353. [Google Scholar] [CrossRef]
- Gritti, F.; Martin, M.; Guiochon, G. Influence of viscous friction heating on the efficiency of columns operated under very high pressures. Anal. Chem. 2009, 81, 3365–3384. [Google Scholar] [CrossRef]
- Fekete, S.; Ganzler, K.; Guillarme, D. Critical evaluation of fast size exclusion chromatographic separations of protein aggregates, applying sub-2μm particles. J. Pharm. Biomed. Anal. 2013, 78–79, 141–149. [Google Scholar] [CrossRef]
- Dorn, M.; Hekmat, D. Simulation of the dynamic packing behavior of preparative chromatography columns via discrete particle modeling. Biotechnol. Prog. 2016, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Norling, L.; Lute, S.; Emery, R.; Khuu, W.; Voisard, M.; Xu, Y.; Chen, Q.; Blank, G.; Brorson, K. Impact of multiple re-use of anion-exchange chromatography media on virus removal. J. Chromatogr. A 2005, 106, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Dorn, M.; Eschbach, F.; Hekmat, D.; Weuster-Botz, D. Influence of different packing methods on the hydrodynamic stability of chromatography columns. J. Chromatogr. A 2017, 1516, 89–101. [Google Scholar] [CrossRef]
- Rathore, A.S.; Mittal, S.; Lute, S.; Brorson, K. Chemometrics applications in biotechnology processes: Predicting column integrity and impurity clearance during reuse of chromatography resin. Biotechnol. Prog. 2012, 28, 1308–1314. [Google Scholar] [CrossRef]
- Keener, R.N.; Maneval, J.E.; Fernandez, E.J. Toward a robust model of packing and scale-up for chromatographic beds. 1. Mechanical compression. Biotechnol. Prog. 2004, 20, 1146–1158. [Google Scholar] [CrossRef]
- Kirkland, J.J.; DeStefano, J.J. The art and science of forming packed analytical high-performance liquid chromatography columns. J. Chromatogr. A 2006, 1126, 50–57. [Google Scholar] [CrossRef]
- Keener, R.N.; Maneval, J.E.; Östergren, K.C.E.; Fernandez, E.J. Mechanical deformation of compressible chromatographic columns. Biotechnol. Prog. 2002, 18, 587–596. [Google Scholar] [CrossRef]
- Hayes, R.; Ahmed, A.; Edge, T.; Zhang, H. Core–shell particles: Preparation, fundamentals and applications in high performance liquid chromatography. J. Chromatogr. A 2014, 1357, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, K. Applications of silica-based monolithic hplc columns. J. Sep. Sci. 2004, 27, 843–852. [Google Scholar] [CrossRef]
- Boi, C.; Malavasi, A.; Carbonell, R.G.; Gilleskie, G. A direct comparison between membrane adsorber and packed column chromatography performance. J. Chromatogr. A 2020, 1612, 460629. [Google Scholar] [CrossRef]
- Vanderheyden, Y.; Cabooter, D.; Desmet, G.; Broeckhoven, K. Isocratic and gradient impedance plot analysis and comparison of some recently introduced large size core–shell and fully porous particles. J. Chromatogr. A 2013, 1312, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, A.; Yamamoto, S.; Peterka, M.; Krajnc, N.L. Fast separation of large biomolecules using short monolithic columns. J. Chromatogr. B 2013, 927, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Teeters, M.A.; Root, T.W.; Lightfoot, E.N. Performance and scale-up of adsorptive membrane chromatography. J. Chromatogr. A 2002, 944, 129–139. [Google Scholar] [CrossRef]
- Yuan, Q.S.; Rosenfeld, A.; Root, T.W.; Klingenberg, D.J.; Lightfoot, E.N. Flow distribution in chromatographic columns1. J. Chromatogr. A 1999, 831, 149–165. [Google Scholar] [CrossRef]
- Ghosh, R. Using a box instead of a column for process chromatography. J. Chromatogr. A 2016, 1468, 164–172. [Google Scholar] [CrossRef]
- Johnson, C.; Natarajan, V.; Antoniou, C. Evaluating two process scale chromatography column header designs using cfd. Biotechnol. Progr. 2014, 30, 837–844. [Google Scholar] [CrossRef]
- Gebauer, K.H.; Luo, X.L.; Barton, N.G.; Stokes, A.N. Efficiency of preparative and process column distribution systems. J. Chromatogr. A 2003, 1006, 45–60. [Google Scholar] [CrossRef]
- Broyles, B.S.; Shalliker, R.A.; Guiochon, G. Visualization of solute migration in chromatographic columns influence of the frit porosity. J. Chromatogr. A 2001, 917, 1–22. [Google Scholar] [CrossRef]
- Shalliker, R.A.; Ritchie, H. Segmented flow and curtain flow chromatography: Overcoming the wall effect and heterogeneous bed structures. J. Chromatogr. A 2014, 1335, 122–135. [Google Scholar] [CrossRef]
- Ghosh, R.; Chen, G. Mathematical modelling and evaluation of performance of cuboid packed-bed devices for chromatographic separations. J. Chromatogr. A 2017, 1515, 138–145. [Google Scholar] [CrossRef]
- Chen, G.; Roshankhah, R.; Ghosh, R. A cuboid chromatography device having short bed-height gives better protein separation at a significantly lower pressure drop than a taller column having the same bed-volume. J. Chromatogr. A 2021, 1647, 462167. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ghosh, R. Effect of the length-to-width aspect ratio of a cuboid packed-bed device on efficiency of chromatographic separation. Processes 2018, 6, 160. [Google Scholar] [CrossRef]
- Hagemann, F.; Wypysek, D.; Baitalow, K.; Adametz, P.; Thom, V.; Wessling, M. Why device design is crucial for membrane adsorbers. J. Chromatogr. Open 2022, 2, 100029. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).