Abstract
SO2 and NOx emissions from iron and steel production pollute the atmosphere. With the implementation of ultra-low emission standards, the requirements for flue gas purification have become more stringent. Activated carbon, due to its rich surface chemistry, stable physical structure, and excellent adsorption and renewability, has a significant effect on the synergistic removal of multiple pollutants from industrial flue gas, and its industrial application has achieved a SO2 removal rate of ≥98% and a NOx removal rate of ≥83%. Firstly, we analyze the structure of activated carbon and the adsorption principle, discuss the mechanism of desulfurization and denitrification, and explore the shortcomings of the technology; then, we summarize the modification methods of activated carbon, determine the impregnation method of loading non-precious metal oxides as the optimal solution, and elucidate the loading conditions, process, and reaction mechanism; finally, we discuss the current status of the research, analyze the process deficiencies and the direction of optimization, and look forward to the prospect of development.
1. Introduction
The flue gas produced by the iron and steel production process mainly contains acidic gaseous pollutants such as SO2 and NOx that destroy the ozone layer; cause acid rain and greenhouse effects; damage the human respiration, digestion, cardiovascular, and immune systems; and contain carcinogenic SO2 and NOx (Figure 1) [1,2,3,4,5,6]. The mass concentration of SO2 emission in the flue gas of domestic iron and steel enterprises is 300–800 mg/Nm3, and the maximum can reach 2000–4000 mg/Nm3. The NOx mass concentration is 0.15–0.30g/Nm3 and can reach up to the 0.5–0.6 g/Nm3 range [7]. Therefore, improving and optimizing the treatment capacity of pollutants such as sulfur and nitrate in flue gas plays a vital role in the green and sustainable development of the steel industry. Although the existing flue gas desulfurization and denitrification technology can meet the requirements of production and environmental protection, there are still many problems, such as the complex process, low efficiency, poor water resistance, high input and operating costs, difficult recovery and reuse of by-products, and secondary pollution [8,9,10].
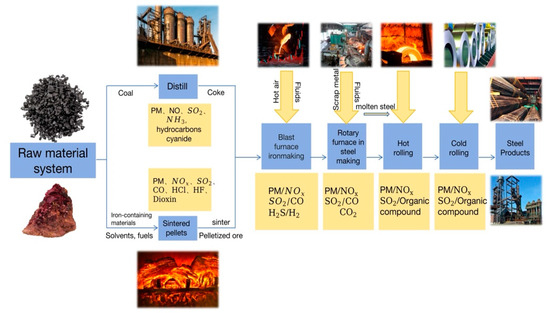
Figure 1.
Pollutants produced by various processes in iron and steel production.
Among many flue gas treatment methods, activated carbon desulfurization and denitrification technology is the only way to simultaneously remove various pollutants such as sulfides, nitrogen oxides, dioxins, and heavy metals in flue gas [11]. Activated carbon flue gas desulfurization and denitrification mainly includes three technologies: ‘downstream’, ‘cross-flow’, and ‘countercurrent’. Compared with the ‘downstream’ and ‘cross-flow’ technology, the ‘countercurrent’ technology has more advantages in adsorption kinetics. In the countercurrent technology, the reverse flow mode of activated carbon and flue gas increases the mass transfer driving force by 30% through concentration gradient optimization to ensure that the SO2 adsorption efficiency is stable at ≥98.5% [12]. The axial saturation gradient of the bed reaches 0.8–1.2 kg SO2/kg AC·m, the horizontal saturation deviation is less than 5%, and the bottom sulfur capacity can reach 400–500 mg/g (15–20% higher than that of the downstream process). This design increases the treatment capacity per unit volume to 1200–1500 m3/(m2·h), maximizing the utilization of the adsorption capacity and optimizing energy consumption [13]. Therefore, the ‘countercurrent’ technology is more widely used, such as by Handan Steel [14], Anyang Steel [15], and other large iron and steel enterprises. Activated carbon desulfurization and denitrification technology has the advantages of a high desulfurization rate (close to 100%), low energy consumption, recyclable by-products, and simple process. This has been widely recognized by the academic community. However, its denitrification effect is unsatisfactory. In the actual production process, the denitrification effect can only be achieved by spraying ammonia at multiple places [16]. In addition, when activated carbon is used for simultaneous desulfurization and denitrification, SO2 and NOx will form a competitive adsorption at the active sites on the surface of activated carbon, affecting the desulfurization efficiency. Therefore, in order to improve the desulfurization and denitrification efficiency of activated carbon and avoid the waste of resources, research on the modification of activated carbon in the desulfurization and denitrification process of activated carbon came into being.
This review covers the process technology and the reaction mechanism of activated carbon desulfurization and denitrification. At the same time, it reviews the research results of scholars in recent years in improving the efficiency of flue gas desulfurization and denitrification by modified activated carbon. On this basis, the advantages and shortcomings of the flue gas desulfurization and denitrification process with activated carbon are highlighted.
2. Reaction Mechanism of Activated Carbon and Flue Gas Sulfur Nitrate
2.1. Activated Carbon and Its Characteristics
Activated carbon is a black porous solid carbon made of carbon-containing materials such as high-quality coal, wood chips, and fruit shells. Activated carbon is refined through a series of processes such as carbonization, cooling, activation, and washing [17]. The density is 1.8–2.1 g/cm3, the apparent density is 0.08–0.45 g/cm3, and the carbon content is 10–98%. Activated carbon is odorless and porous and has a strong adsorption capacity for gas or colloidal solids. The total surface area per gram of activated carbon can reach 500–1700 m2, and the ignition point is above 300 °C. The large specific surface area and microporous structure of activated carbon provide it with unique multi-functional adsorption [18,19,20,21,22].
According to the different classification criteria depicted in Figure 2 and cited in References [23,24,25,26,27], they can be categorized according to the raw material used to make them, the state of appearance presentation, and the size of the stomata.
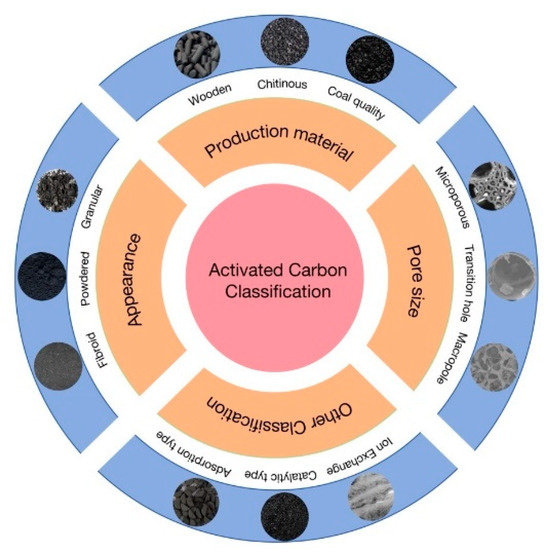
Figure 2.
Different kinds of activated carbon.
Different raw materials can be divided into wooden activated carbon, shell activated carbon (coconut shell activated carbon), coal activated carbon [23], and other raw activated carbon (such as animal bone activated carbon, mineral raw activated carbon, synthetic resin activated carbon, rubber or plastic activated carbon, regenerated activated carbon, etc.). Among them, wood activated carbon, shell activated carbon, and coal activated carbon are most widely used in production and life. According to the appearance of the present form, it can be divided into powder activated carbon, granular activated carbon, and fiber activated carbon [24]. Activated carbon can also be classified according to the pore size. Dubinin, a scholar of the former Soviet Union, proposed that a pore diameter between 100 and 2000 nm is a large pore activated carbon; a pore diameter between 2 and 100 nm is mesoporous (also known as transition pore) activated carbon; and microporous activated carbon has a pore diameter of less than 2 nm [25]. The International Union of Theoretical and Applied Chemistry (IUPAC) lists macroporous activated carbon with a pore diameter above 50 nm, mesoporous activated carbon with a pore diameter between 2 and 50 nm, and microporous activated carbon with pore diameter below 2 nm [26]. In addition to the above three classifications, activated carbon can also be classified differently according to the specific use and manufacturing process [27].
2.2. Adsorption Function and Principle of Activated Carbon
The adsorption function of activated carbon is mainly affected by the pore structure, specific surface area, and functional groups, and the pore size plays a decisive role in the adsorption performance [28,29]. The pores are divided into macropores, micropores, and transition pores according to the diameter. The proportion of macropores and transition pores in activated carbon is relatively small, which plays a role in the adsorption pathway process and dominates the adsorption rate. Most of the actual adsorption is carried out in micropores, and the number of micropores plays a dominant role in the adsorption capacity (Figure 3).
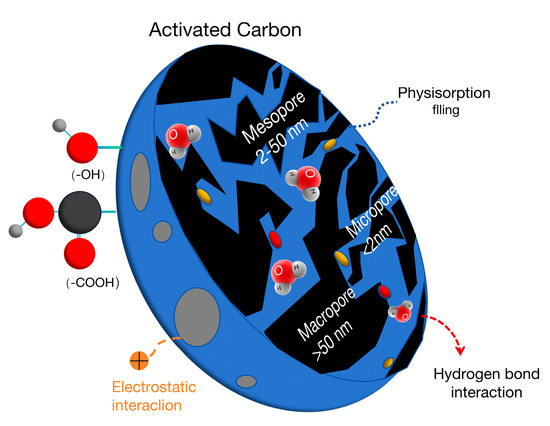
Figure 3.
Pore structure and adsorption principle of activated carbon [30].
The porous structure of activated carbon provides a huge specific surface area for it. The more developed the pore structure is, the larger the specific surface area will be, and the stronger the adsorption function.
The adsorption function of activated carbon is divided into physical adsorption and chemical adsorption. The physical adsorption of activated carbon is generated by intermolecular forces, and van der Waals force is the main force [31]. Intermolecular forces are ubiquitous among all molecules. Any substance has a certain adsorption capacity, and the adsorbed molecules can also adsorb other molecules. Therefore, physical adsorption is multi-molecular layer adsorption. As adsorbents, substances can often adsorb various other substances, so physical adsorption has no selectivity. Due to the relatively weak intermolecular forces, the adsorbed substances are often easier to desorb, so the physical adsorption is reversible [32]. For chemical adsorption, the adsorbate molecules and the surface atoms of the adsorbent will undergo electron transfer, exchange, or sharing, thereby forming chemical bonds, such as covalent bonds and ionic bonds. Chemisorption has strong selectivity, that is, an adsorbent only chemically adsorbs a specific adsorbate. Chemical adsorption requires sufficient energy to overcome the activation energy of the reaction, so it is usually significant at high temperatures. In the flue gas desulfurization and denitrification of activated carbon, some functional groups on the surface of activated carbon will produce weak chemical adsorption with some adsorbates, but physical adsorption is dominant. Some scholars have studied the adsorption of SO2 molecules on the surface of perfect graphene and vacancy defect graphene and calculated the corresponding properties of SO2 adsorbed on perfect graphene (PG) by means of density functional theory (DFT). The results showed that the adsorption process was achieved by physical adsorption, and the adsorption energy (Eads) of different adsorption configurations ranged from −0.125 eV to −0.314 eV. In addition, it is found that the presence of vacancies can improve the sensitivity of graphene to detect SO2 molecules and promote the strong adsorption of surface SO2 molecules on the surface of vacancy defect graphene. Further calculation of the electronic properties shows that there is a hybridization between the S-2p orbital of the SO2 molecule and the C-2p orbital of the vacancy defect graphene (VG), which is the reason for the increase in the SO2/VG adsorption energy [33]. At the same time, other scholars have studied the diffusion behavior of NO molecules in the pores of activated carbon through dynamic simulation. The diffusion coefficient of NO molecules in the pores of activated carbon is calculated to be 1.2 × 10−4 cm2/s, which indicates that the diffusion rate of NO molecules in the pores of activated carbon is relatively slow. It further explains why a certain residence time is required in the actual desulfurization and denitrification process [34,35].
Activated carbon has two functional groups, oxygen-containing and nitrogen-containing [36]. The oxygen-containing functional groups contain carboxyl, lactone, carbonyl, phenolic hydroxyl, and so on. The nitrogen-containing functional groups contain pyrimidine, amide group, milk amine group, phthalamido group, etc. (Figure 4).
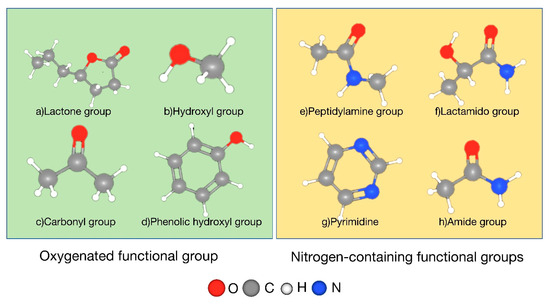
Figure 4.
Partial oxygen and nitrogen functional groups on the surface of activated carbon.
Different functional groups on the surface of activated carbon have different effects on desulfurization and denitrification: acidic functional groups such as carboxyl and hydroxyl groups can enhance the physical adsorption and chemical reaction ability of sulfur dioxide [37,38]. The presence of acidic functional groups can increase the wettability of the surface of activated carbon, thereby promoting the dissolution and absorption of sulfur dioxide in the aqueous phase and improving the desulfurization efficiency. However, acidic functional groups usually inhibit the adsorption of nitrogen oxides; basic functional groups such as pyrrole nitrogen and pyridine nitrogen are conducive to the adsorption and conversion of nitrogen oxides [39,40]. Basic functional groups can be used as Lewis base centers to react with acidic sites in nitrogen oxides to promote the adsorption and reduction of nitrogen oxides. However, the contribution of basic functional groups to the adsorption of sulfur dioxide is relatively small. Carbonyl and quinone groups promote the redox reaction of sulfur and nitrogen oxides by redox activity [39], and lactone groups indirectly affect the surface properties and pore structure. In practice, functional groups can be regulated by surface modification to improve the desulfurization and denitrification performance. Activated carbon is mainly composed of carbon elements. Its chemical properties are relatively stable, and it is not easy to react with adsorbates, which makes it able to maintain good adsorption performance in various environments. At the same time, the above oxygen-containing, nitrogen-containing, and other functional groups on the surface of activated carbon can be chemically adsorbed with some adsorbates to further enhance their adsorption capacity.
2.3. Activated Carbon Flue Gas Desulfurization and Denitrification Technology
In addition to the strong adsorption performance, the catalytic, reduction, and loading capacity of activated carbon enable it to have a good performance in flue gas treatment, and it is an excellent desulfurization and denitrification agent. Since the early 1990s, activated carbon flue gas desulfurization and denitrification technology has become one of the hot spots in domestic academic research. Since 2019, with the deepening of research, activated carbon desulfurization and denitrification technology has been increasingly improved, which has achieved the combined removal of multiple pollutants in flue gas. The removal rate of SO2 and SO3 reached 98%, the removal rate of NOx reached more than 80%, and the removal effect of dust reached less than 10 mg/Nm3. Because of gas–solid adsorption, there is no liquid water involved in the whole process, so there is no need to treat wastewater. The by-products are mainly H2SO4 and S, so they can be recycled [41,42] (Table 1).

Table 1.
Activated carbon desulfurization and denitrification technology [43,44,45,46,47,48].
Due to its developed pore structure and huge specific surface area, activated carbon can physically adsorb SO2, O2, H2O, and NOx in flue gas. The active sites such as oxygen-containing functional groups on the surface can also catalyze the oxidation reaction of sulfur dioxide to produce sulfuric acid and store it in the pores. When heated to 400 °C in the regeneration tower, the thermal desorption and chemical reaction are coordinated, and the carbon reacts with sulfuric acid to release SO2, so as to realize the regeneration of activated carbon. However, excessive regeneration will destroy its pore structure. When ammonia is injected for denitrification, activated carbon provides an interface for the reaction. However, due to the poor catalytic selectivity of active sites, frequent side reactions, and the influence of gas–solid mass transfer resistance and the operating conditions, the denitrification efficiency is limited. In the process of sulfur recovery, the catalytic oxidation method converts SO2 into SO3 with the help of the catalyst to prepare sulfuric acid, and the oxidation absorption method uses a strong oxidant to achieve this conversion. Ammonium sulfate can be prepared by introducing oxygen and ammonia. In view of the existing problems, it is expected to improve the adsorption and catalytic activity of nitrogen oxides, improve the gas–solid mass transfer efficiency, and promote the further development of this technology by loading transition metal or non-noble metal elements to modify activated carbon and regulate the pore structure of activated carbon [49].
3. Different Modification Methods of Activated Carbon and Its Removal Effect on Sulfur and Nitrate in Flue Gas
In order to seek high denitrification efficiency of activated carbon, scholars have conducted in-depth research on its pore structure, pore size, specific surface area, and surface functional group type. Studies have shown that the non-polarity, hydrophobicity, chemical stability, and thermal stability of activated carbon itself have created good conditions for its activation and modification [50,51,52]. Through physical and chemical modification methods, the adsorption and catalysis of activated carbon are improved to meet the high-efficiency denitrification requirements of activated carbon for flue gas.
3.1. Common Modification Methods of Activated Carbon
The modification methods of activated carbon can be divided into physical modification and chemical modification [53]. Among them, physical modification includes high-temperature heat treatment modification and microwave modification. Chemical modification includes oxidation modification, acid–base modification, metal modification, and plasma modification, as shown in Table 2:

Table 2.
Modification of activated carbon [54,55,56,57,58,59,60].
Physical modification is performed by heating the activated carbon by providing an inert gas atmosphere (high-temperature heat treatment) or microwaves. The purpose is to increase the micropore volume and specific surface area of the activated carbon, thereby increasing its adsorption capacity. However, in the case of local sintering, the pore size will shrink to a certain extent, which will affect the adsorption capacity of activated carbon. In chemical modification, oxidant, reducing agent, and non-redox means are used to change the structure and quantity of functional groups and enhance the adsorption capacity of activated carbon. For example, in reduction modification (taking H2 as an example), R-OH (hydroxyl group on the surface of activated carbon) + H2→R-H + H2O, the non-polarity of the activated carbon surface is enhanced, but the adsorption of polar substances is weakened. On the contrary, with oxidation modification (taking ozone as an example), C + O3→C-O-O + O2 forms peroxide functional groups, the surface polarity of activated carbon is enhanced, and the adsorption of non-polar substances is weakened. Acid–base modification will change the pore structure of activated carbon while strengthening the adsorption. As the concentration of the modified solution increases, the degree of collapse and crack deepens, resulting in a decrease in the specific surface area and affecting the adsorption capacity.
For metal loading modification, metal ions or metal compounds are introduced into the surface or pores of activated carbon. Additionally, the metal is combined with the functional groups on the surface of activated carbon by chemical reaction. After metal loading, first of all, the chemical properties of the activated carbon surface can be changed, new active sites can be generated, and the adsorption and catalytic ability of the material can be improved. Secondly, through the interaction between the metal and activated carbon, the synergistic effect is exerted, the selectivity is improved, the target is accurately adsorbed, and the overall performance of the material is enhanced. Thirdly, the stability of activated carbon after metal loading modification is good, which prolongs the service life of activated carbon. The reusability of the metal can also be improved by the reducibility of the activated carbon itself and the characteristics of the loaded metal.
3.2. Modification of Metal-Loaded Activated Carbon
According to the characteristics of flue gas and the needs of desulfurization and denitrification, combined with the advantages and disadvantages of various activated carbon modifications, metal loading modification is an ideal activated carbon modification method in activated carbon desulfurization and denitrification technology. Metal loading modification can not only repair the pore structure of activated carbon but also enhance the chemical properties of activated carbon [53]. In the calcination stage, the pore structure evolution of activated carbon showed a significant temperature–time–concentration synergistic effect. When the treatment temperature is in the range of 400–500 °C, the constant temperature time is 1–1.5 h and the solution concentration is in the range of 10–15%. The pores of activated carbon show the characteristics of multi-level pore development: the specific surface area is significantly increased from the original value, the highest increase can reach about 50%, the average pore volume is increased to 0.5–0.7 cm3/g, the proportion of micropores is increased to about 80%, while the average pore size shows a shrinking trend, down to 1.7–2.0 nm. This structural optimization is due to the fact that the high temperature promotes the pyrolysis and recombination of the precursor, and the increase in the solution concentration strengthens the chemical etching effect. This further refines the micropore structure and promotes the enhancement of the meso-micropore connectivity [61]. When activated carbon is used as a catalytic carrier, it can produce a catalytic adsorption effect on different gases by loading different metal elements or metal compounds. For SO2 and NOx in sintering flue gas, metal or metal compounds can be selectively incorporated into activated carbon for modification to improve the adsorption of activated carbon to two gases. There are three main methods for loading metal-modified activated carbon, as shown in Table 3:

Table 3.
Modification method of supported metal activated carbon [61,62,63,64].
In the field of activated carbon supported metal modification, the impregnation method, sol-gel method, and solid-phase synthesis method show different technical characteristics. The impregnation method realizes the load through the capillary penetration and thermal decomposition of the metal salt solution. For example, after the CuO-loaded coal-based activated carbon is calcined at 500 °C, the SO2 adsorption capacity is increased to 350 mg/g [65], but there are problems of uneven metal dispersion and pore blockage [66]. The sol-gel method generates nano-metal oxides in situ by the hydrolysis–condensation reaction. For example, after calcination at 500 °C, the photocatalytic degradation efficiency of phenol reached 90% [67], but the process is complicated and the cost is high. The solid-phase synthesis method forms an alloy system by mechanical mixing and high-temperature calcination. For example, after calcination at 800 °C, the methane conversion rate of Ni-MoO2 supported petroleum coke-based activated carbon reaches 95%, and the carbon deposition resistance is excellent, but the energy consumption is significantly higher than other methods [68,69]. The three methods are suitable for gas adsorption, photocatalysis, and high-temperature catalysis, respectively. The choice depends on the type of supported metal, dispersion requirements, and process economy.
The impregnation method achieves loading by soaking activated carbon in metal salt solution and calcining at high temperature. The process has simple steps, requiring no complex sol preparation or mechanical grinding, and the raw material and operation cost are lower. The process only involves soaking and calcination; thus, the technical threshold is low, the equipment requirements are not high, and it is easy to implement. From the perspective of application scenarios, the impregnation method is suitable for the field of gas adsorption (such as CuO loading coal-based activated carbon to increase the SO2 adsorption capacity). Although there is the problem of uneven metal dispersion, its basic loading mechanism still has practical value in specific fields (such as adsorption scenarios with relatively non-extreme dispersion requirements). With the characteristics of a low cost and easy operation, it has become the preferred solution for basic modification requirements. Using the characteristics of the strong adsorption of activated carbon, metal ions, metal elements, or metal compounds are first adsorbed to the surface of activated carbon. Next, the metal ions are reduced to low-valent ions or elements by the reduction of carbon atoms under certain conditions. During the reaction of metal elements with activated carbon, the pore structure of activated carbon changes, and the surface chemical functional groups are abundant. Some metal elements also have redox ability. For example, the multivalent characteristics of transition metal oxides (such as MnO2) (Mn+/Mn3+/Mn2+) can undergo reversible redox reactions during the adsorption process, which can provide more adsorption sites for activated carbon and increase the adsorption capacity of activated carbon. Therefore, the activated carbon can be changed from a single physical adsorption to a physical and chemical double adsorption [70]. The loading process is shown in Figure 5.
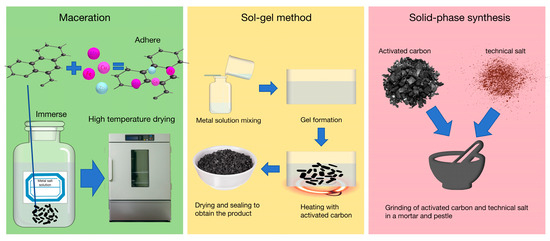
Figure 5.
Modification method of metal-loaded activated carbon.
Smathi et al. [71] prepared carbon-based adsorbents by impregnating four metal oxides of Ni, V, Fe, and Ce with palm shell activated carbon. The removal of SO2 and NOx in simulated flue gas was studied in a fixed bed reactor. The results showed that the activated carbon impregnated with CeO2 had the strongest adsorption capacity for SO2 and NOx. Quan [72] prepared Mn/AC, Fe/AC, and Cu/AC carbon-based metal catalysts by the equal volume impregnation method and studied the removal of SO2 and NOx in simulated flue gas in a fixed bed reactor. The results showed that the Mn/AC catalyst had a good denitrification function at a certain temperature (150–200 °C), but there was also a rapid decrease in catalytic activity. In view of the poor stability of metal elements, a series of Fe-Mn/AC catalysts were prepared by the equal volume impregnation method. The results showed that Fe and Mn had a strong synergistic effect, and a new active component, Fe3Mn3O8, was formed, which significantly improved the denitration performance and catalytic stability of the catalyst. Li Yuantao [73] et al. prepared single-component Cu and two-component Cu-Ni metal oxide supported activated carbon by the impregnation method. The results showed that 6% CuO/AC had the best desulfurization and denitrification performance at a certain temperature (120 °C). Under this condition, loading 5% NiO could further improve the desulfurization and denitrification performance, and 5% NiO-2% CuO/AC had the best effect. Sun [74] used Fe2O3 load to modify activated carbon to prepare an Fe2O3/AC adsorbent. The preparation conditions of the Fe2O3-modified activated carbon adsorbent and the effect of the adsorption process conditions on the removal of hydrogen sulfide by activated carbon were investigated in a fixed bed reactor. The results showed that the desulfurization rate of the Fe2O3/AC adsorbent was as high as 99.4% when the mass of Fe2O3 was 1:1, the activated carbon was dried at 80 °C for 36 h, and the adsorption condition was 60 °C. The supported metal oxides provided the reaction driving force through the redox cycle and enhanced the chemical adsorption specificity through the surface acidic sites, thereby improving the adsorption capacity and stability of activated carbon.
In experiments on loading metal-modified activated carbon, it was found that the cost of loading noble metal-modified activated carbon was high, and the stability of loading metal-modified activated carbon was poor. Meanwhile, the adsorption effect of loading non-precious metal oxide-modified activated carbon was good, the production cost was low, the preparation process was simple, and it was easy to store, which was the best metal type for loading metal-modified activated carbon.
3.3. Loading Non-Noble Metal Oxide Activated Carbon Desulfurization and Denitrification
Among the many non-precious metal oxides, activated carbon modified with Fe2O3, CuO, ZnO, and Mn2O3 has the best desulfurization and denitrification effect. Taking Fe2O3-modified activated carbon as an example, the characterization of modified activated carbon was explored, and the optimum preparation conditions for the desulfurization and denitrification of activated carbon modified by non-precious metal oxides were summarized.
Xue Limei et al. [75] used coconut shell activated carbon as the raw material, with 3% Fe2O3 loading, heated at 500 °C for 180 min to modify activated carbon. The pore size of the modified activated carbon was significantly reduced, and the Fe2O3 crystal was relatively evenly distributed on the surface of the activated carbon. This was embedded in the inner wall of the activated carbon macropores, so that the original macropores became numerous small micropores, thereby increasing the specific surface area of the modified activated carbon. Yin Shoulai [76] et al. obtained Fe2O3/AC catalyst by the impregnation method. To obtain this, they added activated carbon ground powder to deionized water and Fe(NO3)3·9H2O solution, stirred it magnetically for 120 min, followed by drying and roasting. The modified Fe2O3/AC catalyst had strong stability, and the main pore size was less than 4 nm. The active component, iron oxide, mainly existed in the form of γ-Fe2O3 crystals. The increase in the Fe3+ and surface adsorbed oxygen Oβ contents improved the low temperature selectivity and reactivity of Fe2O3/AC. Hao Ke [61] prepared Fe2O3/AC by the impregnation method. The activated carbon was soaked in Fe2O3 nitrate solution with different concentrations (5%, 10%, 15%, 20%) for 12 h, then placed in a constant temperature water bath at 60 °C for 6 h, and then ammonia was added to neutralize the pH value of the activated carbon. Four parts of quantitative activated carbon were taken and heated under N2 protection at a certain temperature (300 °C, 400 °C, 500 °C, 600 °C) and a certain time (1 h, 1.5 h, 2 h, 2.5 h). The characterization of the modified activated carbon was detected by XRD, SEM and BET, and the optimal characterization preparation conditions for loading Fe2O3/AC were obtained, as shown in Figure 6:
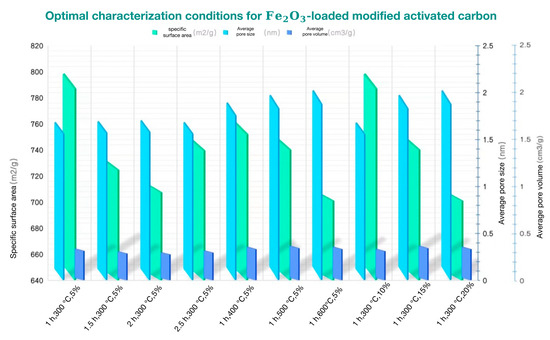
Figure 6.
The best characterization conditions of Fe2O3-modified activated carbon.
From Figure 6, it can be seen that the characterization change of the loaded Fe2O3/AC prepared by the impregnation solution concentration of 10%, the heating temperature of 400 °C, and the heating time of 1 h is the most conducive to flue gas desulfurization and denitrification. The enhancement of the desulfurization and denitrification ability of activated carbon loaded with non-noble metal oxides is mainly due to the change in pore structure of activated carbon after modification from the perspective of physical adsorption. The pore structure and intermolecular force of activated carbon are enhanced, and the total amount of gas molecules captured by the activated carbon pores due to van der Waals force is increased. From the perspective of electrostatic forces, if the surface of the activated carbon involved in the load is positively charged, it will attract ions with negative charge. On the contrary, if the surface is negatively charged, it will attract positively charged ions.
In terms of chemical adsorption, the introduction of hydroxyl groups on the surface of Fe2O3 by chemical modification can significantly improve the removal performance of activated carbon for sulfur dioxide. The mechanism of action is reflected in two aspects: firstly, as an active site, hydroxyl groups can react with sulfur dioxide molecules by the acid–base neutralization reaction or coordination reaction and strengthen the fixation of sulfur dioxide on the surface of the material by chemical adsorption (1); secondly, the activated carbon loaded with Fe2O3 reacts in the presence of oxygen and water (2) and can react by catalytic oxidation (3). Sulfur dioxide is converted into sulfur trioxide, and sulfur trioxide is desorbed by Reaction (4). Sulfur trioxide reacts with water to form sulfuric acid to achieve sulfur fixation and conversion, that is, Reaction (5). This synergistic effect not only enhances the adsorption capacity through surface hydroxyl groups but also enhances the conversion efficiency of sulfur through the catalytic oxidation process, thereby significantly improving the comprehensive performance of activated carbon-based materials in flue gas desulfurization. The reaction process is as follows (* represents the adsorption state in the equation):
The absorption of NOx by Fe2O3/AC is absorbed by the redox reaction (taking NO as an example). Fe2O3 has a certain oxidizability. In the presence of oxygen, nitric oxide can be oxidized to nitrogen dioxide (10). The generated nitrogen dioxide can react with water (the activated carbon adsorption system may contain a small amount of water) to generate nitric acid. The adsorption of acidic gases in nitrogen oxides (NO2 as an example) occurs. When nitrogen oxides exist in this acidic gas, the basic sites on the activated carbon or the hydroxyl groups on the surface of Fe2O3 react (11) (the hydroxyl groups on the surface of Fe2O3 are involved in the reaction) to increase the adsorption capacity of activated carbon for nitrogen oxides.
The above reaction process is as follows:
Although the adsorption of metal-loaded activated carbon has been improved, there are still some problems. For example, the catalytic efficiency of non-noble metal active sites is limited. In some reactions that require high activity, the ideal reaction rate may not be achieved. After microwave treatment of activated carbon, this problem can be well improved. Microwave irradiation loading non-noble metal oxide activated carbon can improve the dispersion of metals on activated carbon, so that the metals are more evenly distributed on the surface of activated carbon, so as to improve the exposure of metal active sites and accelerate the reaction rate. Microwave heating is an internal heating method, which can heat the activated carbon and the loaded metal at the same time. Therefore, the redox reaction on the surface can be carried out efficiently, so that the flue gas desulfurization and denitrification is more efficient.
4. Loaded Non-Noble Metal Oxide Activated Carbon Microwave Flue Gas Desulfurization and Denitrification
4.1. Activated Carbon Microwave Desulfurization and Denitrification
Microwaves are high-frequency electromagnetic waves with a wavelength of 1–1000 mm and a wave frequency range of 300–3000 GHz, which is between general radio waves and light waves [77]. It mainly has three characteristics: penetration, reflection, and absorption [78]. Microwave heating is a process involving a microwave directly coupled with the polar molecules in the reaction substance to achieve the effect of targeted heating [79]. In addition, the containers used for microwave heating are mostly transparent materials such as polypropylene and glass. Based on the loss mechanism of electromagnetic waves interacting with substances, and combining the advantages of microwave heating with the characteristics of the container materials, this approach realizes synchronous heating of the entire material being heated [80] (Figure 7). Compared with traditional heating methods, microwave heating has the advantages of high energy utilization, high heating efficiency, strong heating selectivity, and safe and controllable heating process [81].
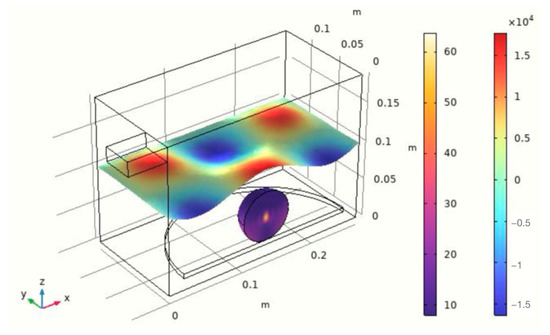
Figure 7.
Heat distribution map of microwave heating.
Activated carbon microwave flue gas desulfurization and denitrification technology involves using activated carbon to adsorb harmful substances in flue gas. When the flue gas passes through the activated carbon bed, harmful gases (SO2 and NOx) are adsorbed by activated carbon in its own internal pores. Under the action of microwave, SO2 is reduced to sulfur, and NOx is reduced to nitrogen, so as to achieve the purpose of the simultaneous removal of sulfur and nitrate from the flue gas [82,83]. The thermal effect and non-thermal effect under microwave irradiation play an important role in flue gas desulfurization and denitrification [84,85,86,87]. The phenomenon that matter absorbs microwave energy to produce heat during microwave irradiation is called the thermal effect of microwave [88]. The reason why microwave irradiation of activated carbon can produce the thermal effect and high efficiency desulfurization and denitrification is that microwave heating belongs to intramolecular heating. Moreover, the activated carbon material has delocalized π electrons due to the sp2 hybrid carbon network. On the one hand, it can interact with the microwaves, prompting activated carbon to effectively absorb microwave energy and provide an energy source for desulfurization and denitrification reactions; on the other hand, under the influence of high-speed rotating electromagnetic waves, the delocalized π electrons change the internal electromagnetic field of the activated carbon. Therefore, the internal molecules exhibit dipole polarization motion characteristics, triggering the rotation, collision, and friction of the reaction molecules. The internal molecules also convert microwave energy into traditional heat energy, realizing the overall temperature rise of activated carbon. This can also create a suitable reaction environment and promote the reduction of sulfur dioxide to elemental sulfur and nitrogen oxides to nitrogen and achieve the goal of efficient desulfurization and denitrification [89].
The non-thermal effect of microwave refers to the effect of the microwaves acting on the reactants other than the thermal effect [90]. The heating rate of microwave irradiation is related to the polarity of the material. The complete tiled structure of activated carbon is a non-polar state. The uneven distribution or fracture of charge will cause local resonance coupling energy transfer with microwaves, and many regions with different temperatures will appear to form local active sites. The temperature of these active sites is significantly higher than that of the other regions, so they are called ‘hot spots’. In the process of microwave irradiation of activated carbon, the heat or temperature distribution of activated carbon will be uneven, resulting in a local temperature that is too high, the formation of an obvious temperature gradient, and the promotion of the desulfurization and denitrification reaction, the so-called hot spot effect [91]. During the desulfurization and denitrification of activated carbon by microwave irradiation, SO2 and NOx can be reduced without a higher temperature, and a higher temperature gradient is formed on the activated carbon. From the center of the activated carbon to the surface, the temperature gradually decreases, so that the product gas of the flue gas reduction rapidly diffuses out [92], as shown in Figure 8.
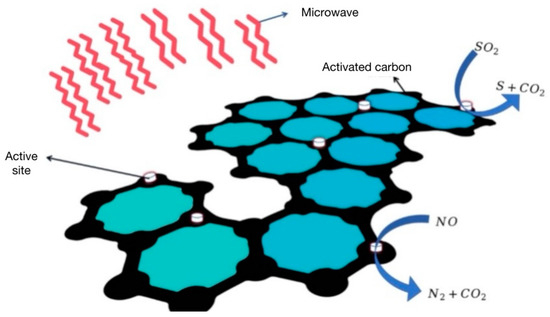
Figure 8.
Mechanism of desulfurization and denitrification of activated carbon by microwave irradiation.
In the microwave desulfurization and denitrification reaction of activated carbon, activated carbon is not only the adsorbent of SO2 and NOx but also the reducing agent. The main redox reactions are as follows [93]:
xC + 2NOx → N2 + xCO2
xC + NOx → 0.5N2 + xCO
xC + SO2 → S + CO2
2C + SO2 → S + 2CO
In the whole process of desulfurization and denitrification, Reactions (13) and (15) are exothermic reactions, which can be carried out at a lower temperature. Furthermore, Reactions (14) and (16) are endothermic reactions, which can be carried out at a higher temperature under the conventional heating mode. The microwave irradiation activated carbon thermal reduction of sulfur nitrogen oxide reaction does not need to heat the activated carbon to the conventional reaction temperature. Additionally, it can lead to SO2 and NOx reduction, to achieve a higher efficiency of sulfur and nitrate removal.
In the process of microwave desulfurization and denitrification of activated carbon, the pore volume and pore size of activated carbon increase under the combined action of the thermal effect and the non-thermal effect, and the rapid increase in temperature caused by the thermal effect reduces the specific surface area of the activated carbon skeleton [94]. Under the same conditions, the microwave power affects the removal efficiency of sulfur and nitrate in the flue gas. The oxygen content on the surface of activated carbon decreases with the increase in microwave power. This is due to the fact that heating the activated carbon under microwave irradiation in the flue gas atmosphere will make its surface part of the oxygen-containing functional groups in the form of CO or CO2 removal [95]. The greater the microwave power, the more oxygen-containing functional groups are removed. With the continuous increase in microwave power, the alkaline characteristics of the activated carbon surface are continuously enhanced. After microwave irradiation, the acidic groups of the activated carbon are decomposed, and its chemical characteristics tend to be alkaline, which is more conducive to the efficient removal of pollutants such as SO2 and NOx [96].
4.2. Loaded Non-Noble Metal Oxide Activated Carbon Microwave Desulfurization and Denitrification
The synergistic technology of microwave and activated carbon loaded with non-precious metal oxides for flue gas desulfurization and denitrification can make full use of the propagation and heating characteristics of microwaves in flue gas. It can also combine the advantages of adsorption, catalysis, and regeneration of modified activated carbon in the process of flue gas desulfurization and denitrification. This provides a new path for flue gas desulfurization and denitrification.
Microwave desulfurization and denitrification of activated carbon loaded with non-noble metal oxides has been widely studied in academia. Jinxin [97] used 420 W microwave to irradiate activated carbon loaded with ZnO, Mn2O3, and CuO to carry out desulfurization and denitrification comparative experiments on flue gas with a flow rate of 0.15 m3/h. The results showed that under the same conditions, the denitrification efficiency of unsupported non-noble metal oxide activated carbon could reach 80.5% under microwave irradiation. After loading the three non-noble metal oxides, the denitrification efficiency reached 87.4%, 89.1%, and 92.5%, respectively. The desulfurization efficiency did not change much, or even decreased, because the non-noble metal oxides hindered the reduction of SO2 by activated carbon. Firstly, non-precious metal oxides will occupy the active sites on the surface of activated carbon for the adsorption and reduction of sulfur dioxide, reducing its contact area. Secondly, the loading of non-noble metal oxides changes the surface chemical properties and electronic structure of activated carbon, affecting the adsorption and reduction of sulfur dioxide. Thirdly, the side reactions catalyzed by non-precious metal oxides consume the substances required for the reduction of sulfur dioxide or generate intermediate products that hinder the reduction reaction, thus inhibiting the reaction. Li [98] used microwave irradiation to load Mn2O3 activated carbon for flue gas denitrification experiments and explored the effects of the temperature, non-metallic oxide loading, microwave power, space velocity, and oxygen content on flue gas denitrification. The results showed that the optimum loading of Mn2O3 is 3–5%. Under the same conditions, the higher the microwave power is, the higher the denitrification efficiency, the higher the oxygen content, and the higher the denitrification rate, but the higher the space velocity is, the lower the denitrification rate. When the temperature reaches 400 °C, the denitrification rate is close to 100%. Liu [99] obtained Fe2O3-loaded coal-based carbon by the precipitation method and carried out flue gas denitrification experiments under microwave irradiation. The results showed that the denitrification rate could reach 95% when the microwave power was 250 W. Hao Ke [62] modified activated carbon by loading four non-precious metal oxides of Fe2O3, CuO, Mn2O3, and ZnO by the impregnation method and explored the effect of different conditions on the efficiency of flue gas desulfurization and denitrification under microwave irradiation. The results showed that under the same conditions, the desulfurization and denitrification effect of activated carbon loaded with the four non-metallic oxides was better than that of ordinary activated carbon. When the temperature is 420 °C, the desulfurization effect of four non-metallic oxides is close to 100%, while the denitrification effect is different. According to the amount of denitrification, the order is Fe2O3/AC > CuO/AC > Mn2O3/AC > ZnO/AC. The experimental process of the above conclusions is shown in Figure 9:
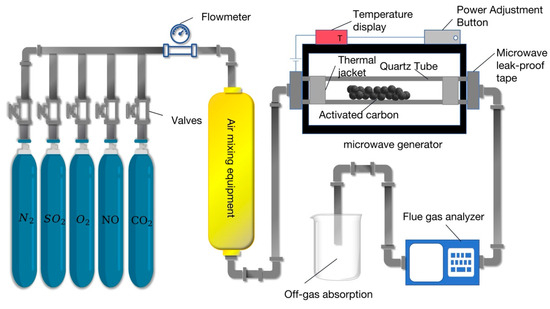
Figure 9.
Microwave desulfurization and denitrification experimental device loaded with non-noble metal oxide activated carbon.
Compared with the traditional activated carbon microwave flue gas desulfurization and denitrification, the non-noble metal oxide loaded activated carbon microwave flue gas desulfurization and denitrification mainly reflects three advantages. The advantages are improving the efficiency of flue gas sulfur and nitrate removal, reducing energy consumption and operating costs, and reducing secondary pollution.
- (1)
- The removal efficiency of sulfur and nitrate in the flue gas is improved. First of all, the traditional activated carbon microwave flue gas desulfurization and denitrification technology mainly depends on the adsorption of activated carbon. After the activated carbon is loaded with non-noble metal oxides, the original activated carbon becomes a catalyst or catalyst carrier under the action of microwaves. While exerting the adsorption effect, it can also effectively promote the reaction of desulfurization and denitrification. Secondly, after the non-metallic oxide is loaded on the surface of the activated carbon, it will provide more active sites for the activated carbon under the action of microwaves. The abundant active sites provide more adsorption space for SO2 and NOx, which effectively alleviates the competitive adsorption of SO2 and NOx. Thirdly, the loading of non-noble metal oxides enhances the microwave absorption performance of activated carbon, which is beneficial to the rapid heating of activated carbon, forming a high temperature gradient and increasing the reaction rate.
- (2)
- Reduced energy consumption and operating costs. Firstly, activated carbon loaded with non-precious metal oxides is not easily saturated by sulfur oxides and nitrogen oxides in the process of flue gas desulfurization and denitrification, which prolongs the service life of activated carbon. Secondly, there is no need for tedious and accurate technical control in the whole technical link, which reduces the cost of human resources and other material costs. Thirdly, due to the lack of some cumbersome technology to control the environment, the cost of equipment in construction is relatively reduced.
- (3)
- It reduces the by-products such as CO, sulfate, and nitrate and reduces the secondary pollution to the environment. Firstly, microwave radiation loading of non-noble metal oxides will cause electrons inside the activated carbon to transition, resulting in a thermal effect. This thermal effect can promote non-noble metal oxides becoming better dispersed on the surface of activated carbon and prevent their agglomeration. Secondly, the microwave field can also induce the formation of a local electromagnetic field between the metal and the activated carbon. This affects the conductivity of the activated carbon, and the electron transfer becomes easier between the activated carbon and the non-noble metal. The change changes the electron cloud density around the non-noble metal, which affects the adsorption and activation ability of the reactant molecules. Thirdly, microwave loading of non-noble metal activated carbon will also cause changes in the functional groups on the surface of activated carbon. These changed functional groups interact with non-precious metals to form a special catalytic environment conducive to complete oxidation, making the reaction more inclined to generate carbon dioxide and reducing the generation of carbon monoxide.
4.3. Dilemma Analysis of Microwave Desulfurization and Denitrification of Loaded Non-Noble Metal Oxide Activated Carbon
The microwave desulfurization and denitrification technology of loaded non-noble metal oxide activated carbon has achieved certain results in the experimental research stage. Its desulfurization and denitrification efficiency is better than that of ordinary activated carbon microwave desulfurization and denitrification technology. However, the relevant parameters of the experimental study are set under artificial conditions. There are some differences between the experimental flue gas composition and the real industrial flue gas composition. Therefore, the actual desulfurization and denitrification effect of microwave desulfurization and denitrification technology loaded with non-noble metal oxide activated carbon needs to be further studied. In view of the excellent performance of the microwave desulfurization and denitrification technology of loaded non-noble metal oxide activated carbon in the experimental process, we strive to make the technology more mature and stable and apply it to the actual production field as soon as possible. In the future, we should continue to carry out in-depth research from the following aspects:
- (1)
- Strive to make the flue gas composition and flue gas environment, through a significant amount of research, more similar to actual industrial emissions. In the current research process, scholars do not fully consider the flue gas environment. In most cases, only the flue gas components that have a direct impact on the experimental results are retained. Although there are studies on other factors such as heavy metal harmful substances, oxygen content, humidity, temperature, acidity, and alkalinity in flue gas, they remain at the basic level of separate research on single index parameters. In the existing literature, there is a lack of research on the microwave desulfurization and denitrification efficiency of non-noble metal oxide activated carbon in real flue gas environments. The amount of flue gas is far from the actual industrial production unit time or standard capacity of flue gas emission standards. Therefore, this technology not only has huge research space but also great research potential.
- (2)
- Strive to explore the microwave irradiation time and the actual ratio and dosage of non-noble metal oxide loaded activated carbon more specifically in the study. In the existing research, in order to reduce the carbon loss of activated carbon, the use of microwave irradiation to load non-noble metal oxides in the research process is relatively short. In the actual production process, as long as the production line does not stop, flue gas emissions will not stop. How to rationally use microwave irradiation in the production practice to maximize the adsorption and catalytic effects of activated carbon loaded with non-noble metal oxides in order to achieve the ideal desulfurization and denitrification efficiency is another subject. The subject must be overcome in the application field of this technology. According to the different application fields and flue gas compositions, exploring the ratio of non-precious metal oxides and activated carbon and finally scientifically controlling the amount of activated carbon are effective ways to control costs.
- (3)
- Strive to design and manufacture the equipment from the perspective of specific practical applications in the research and calculate and control the operating costs. In the current research process, scholars use microwave tube furnaces as microwave generating devices and corundum furnace tubes as activated carbon loading devices. In the production practice, how to design and install the microwave-generating device and how to arrange the activated carbon to achieve the ideal desulfurization and denitrification effect of flue gas are key to the application of this technology. Some scholars have estimated the construction and operation costs of this technology. However, with the change of the times and the upgrading of production capacity, the economics of microwave desulfurization and denitrification technology of non-precious metal oxide activated carbon under the existing production environment have yet to be further verified by scholars.
Conclusions and Prospects
- (1)
- The advantages and disadvantages of the existing activated carbon flue gas desulfurization and denitrification process coexist. The advantage is that it can remove a variety of harmful substances in flue gas at the same time, and the activated carbon itself is cheap; the disadvantage is that the process is old and that the process is too long, resulting in high loss of activated carbon. Improper operation may also produce by-products such as CO and CO2, resulting in secondary pollution. Therefore, the process does not occupy a dominant position in flue gas desulfurization and denitrification in the field of iron and steel production.
- (2)
- The modification of activated carbon can not only improve the removal efficiency of flue gas sulfur and nitrate by activated carbon but also improve the regeneration ability of activated carbon. The special physical and chemical properties of activated carbon provide it with good adsorption, catalysis, and reduction properties, and it also has good wave absorption properties. Under the action of microwave, activated carbon can improve its activity and accelerate the reaction rate with sulfur and nitrate in flue gas. The modified activated carbon loaded with non-noble metal oxides (mainly loaded with Fe2O3, CuO, ZnO, Mn2O3) can also reduce the production of CO at the reaction terminal under the synergistic effect of microwave and improve the microwave absorption performance of activated carbon. It can also increase the active sites on activated carbon and improve the removal efficiency of sulfur and nitrate as a whole, so as to achieve the requirements of low cost, high efficiency, strong stability, and no pollution.
- (3)
- Activated carbon flue gas desulfurization and denitrification technology has great development potential and broad research space. Future research should focus on the optimization of the activated carbon modified load and the development of composite carbon-based materials. The regeneration of activated carbon, the batch loading process, and the multiple influencing factors of activated carbon desulfurization and denitrification under the synergistic effect of microwave irradiation should also be paid attention to. In addition, the advantages and disadvantages of the existing process technology should be summarized in close connection with the actual needs of production. In view of the good removal effect of load non-metallic oxides and microwave irradiation, the equipment should be continuously optimized and upgraded, so as to make greater contributions to flue gas desulfurization and denitrification in the iron and steel industry.
Author Contributions
Writing—original draft preparation, L.M.; Diagramming, W.L.; Translation, Proofreading, J.W.; Data collection, Organizational science, C.H.; Project management, Funding acquisition, Supervision, Writing—review and editing, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “National Natural Science Foundation of China funded project, grant number 52174312” and “Hebei Provincial Natural Science Foundation-High-end Iron and Steel Metallurgy Joint Fund Sponsored Projects, grant number E2021209151”.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garin, F.O. Mechanism of NOx decomposition. Appl. Catal. A Gen. 2001, 222, 183–219. [Google Scholar] [CrossRef]
- Fritz, A.; Pitchon, V. The current state of research on automotive lean NOx catalysis. Appl. Catal. B Environ. 1997, 13, 1–25. [Google Scholar] [CrossRef]
- Mauzerall, D.; Sultan, B.; Kim, N.; Bradford, D. NOx emissions from large point sources: Variability in ozone production, resulting health damages and economic costs. Atmos. Environ. 2005, 39, 2851–2866. [Google Scholar] [CrossRef]
- Rigby, D.; Batts, B. The isotopic composition of nitrogen in Australian coals and oil shales. Chem. Geol. Isot. Geosci. Sect. 1986, 58, 273–282. [Google Scholar] [CrossRef]
- Burchill, P.; Welch, L. Variation of nitrogen content and functionality with rank for some UK bituminous coals. Fuel 1989, 68, 100–104. [Google Scholar] [CrossRef]
- Snyder, L. Petroleum nitrogen compounds and oxygen compounds. Acc. Chem. Res. 1970, 3, 290–299. [Google Scholar] [CrossRef]
- Zhuang, J. Discussion on Desulfurization and Denitrification Technology of Sintering Flue Gas. Steel Manag. 2021, 7, 47–48. (In Chinese) [Google Scholar]
- Ma, S.; Ma, J.; Jin, X.; Yao, J. Experimental study on simultaneous removal of SO2 and NOx by microwave irradiation activated carbon bed. J. Eng. Thermophys. 2013, 34, 26–29. (In Chinese) [Google Scholar]
- Kwon, D.; Park, K.; Hong, S. Enhancement of SCR activity and SO2 resistance on VOx/TiO2 catalyst by addition of molybdenum. Chem. Eng. J. 2016, 284, 315–324. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, R.; Yuan, B.; Jiang, J. Simultaneous removal of SO2, NO and H2O through an integrative process utilizing a cost-effective complex oxidant. J. Hazard. Mater. 2016, 301, 74–83. [Google Scholar] [CrossRef]
- Debarr, J.; Lizzio, A. Adsorption of SO2 on Bituminous Coal and Activated Carbon Fiber. Energy Fuel 1997, 11, 267–271. [Google Scholar] [CrossRef]
- Gao, J.; Wang, T.; Shu, Q.; Nawaz, Z.; Wen, Q.; Wang, D.; Wang, J. An Adsorption Kinetic Model for Sulfur Dioxide Adsorption by ZL50 Activated Carbon. Chin. J. Chem. Eng. 2010, 18, 223–230. [Google Scholar] [CrossRef]
- Papangelakis, P.; Miao, R.; Lu, R.; Liu, H.; Wang, X.; Ozden, A.; Liu, S.; Sun, N.; O’Brien, C.; Hu, Y.; et al. Improving the SO2 tolerance of CO2 reduction electrocatalysts using a polymer/catalyst/ionomer heterojunction Design. Nat. Energy 2024, 9, 1011–1020. [Google Scholar] [CrossRef]
- Han, J.; Yan, Z.; Shao, J. Characteristics and Application of Countercurrent Activated Carbon Flue Gas Desulfurization and Denitrification Technology. Sinter. Pelletizing 2018, 43, 13–18. (In Chinese) [Google Scholar]
- Wang, J. Application of activated carbon flue gas countercurrent integrated purification (CCMB) technology in coke oven of Anyang Steel. Wide Heavy Plate 2023, 29, 43–45+48. (In Chinese) [Google Scholar]
- Zhang, P.; Helmut, W.; Joachim, U. Adsorption of SO2 on activated carbon for low gas concentrations. Chem. Eng. Technol. 2007, 30, 635. [Google Scholar] [CrossRef]
- Montagnaro, F.; Silvestre-Albero, A.; Silvestre-Albero, J.; Rodríguez-Reinoso, F.; Erto, A.; Lancia, A.; Balsamo, M. Post-combustion CO`adsorption on activated carbons with different textural properties. Microporous Mesoporous Mater. 2015, 209, 157–164. [Google Scholar] [CrossRef]
- Hao, L. (Ed.) Food Additives; Agricultural University Press: Beijing, China, 2016; Volume 7, p. 260. (In Chinese) [Google Scholar]
- The ‘Concise Chemical Reagent Manual’ was compiled. In Concise Handbook of Chemical Reagents; Shanghai Science and Technology Press: Shanghai, China, 1991; Volume 1, p. 338. (In Chinese)
- Chen, K. Treatment and Disposal of Agricultural Solid Waste; Guo, C., Xue, Z., Eds.; Henan Science and Technology Press: Zhengzhou, China, 2016; Volume 11, pp. 144–145. (In Chinese) [Google Scholar]
- Gu, J. (Ed.) Antibiotics; Shanghai Science and Technology Press: Shanghai, China, 2001; Volume 9, p. 144. (In Chinese) [Google Scholar]
- Ma, W. Solid-Water Interface Chemistry and Adsorption Technology; Metallurgical Industry Press: Beijing, China, 2011; Volume 10, pp. 65–66. (In Chinese) [Google Scholar]
- Wang, H.; Xu, Z.; Kohandehghan, A.; Li, Z.; Cui, K.; Tan, X.; Stephenson, T.; King’ondu, C.; Holt, C.; Olsen, B.; et al. Interconnected Carbon Nanosheets Derivedfrom Hemp for Ultrafast Supercapacitors with High Energy. ACS Nano 2013, 6, 5131–5141. [Google Scholar] [CrossRef]
- Nguyen, T.; Hur, J.; Kim, I.; Bui, V.; Lee, Y.-C. Development of Antimicrobial CuO/(3-aminopropyl)Triethoxysilane Activated Carbon Fiber. J. Nanosci. Nanotechnol. 2021, 21, 4519–4523. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Serpinsky, V.V. Isotherm Equationfor Water Vapor Adsorption by Microporous Carbonaceous Adsorbents. Carbon 1981, 19, 402–403. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Wang, Y. Coal Chemical Technology; Zhou, G., Wang, L., Liu, S., Xu, D., Eds.; China University of Mining and Technology Press: Xuzhou, China, 2014; Volume 9, p. 384. (In Chinese) [Google Scholar]
- Shirra, G.; Ilan, K.; Carlos, G.D. Removal of dissolved organic matter by granular-activated carbonadsorption as a pretreatment to reverse osmosis of membrane bioreactor effluents. Water Res. 2008, 42, 6–7. [Google Scholar]
- Chiang, Y.; Chiang, P.; Huang, C. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Li, H. Preparation of high-efficiency radon-absorbing material by water immersion-freeze-thaw cycle and multi-process coupling modified activated carbon. J. Radioanal. Nucl. Chem. 2022, 331, 3125–3133. (In Chinese) [Google Scholar] [CrossRef]
- Durigon, A.M.M.; da Silveira, G.D.; Sokal, F.R.; Pires, R.A.C.V.; Dias, D. Food dyes screeningusing electrochemistry approach in solid state: The case of sunset yellow dyeelectrochemical behavior. J. Solid State Electrochem. 2020, 24, 2907–2921. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.Z. Study of rejuvenators dynamic diffusion behavior into aged asphalt and its effects. Constr. Build. Mater. 2020, 261, 120673. [Google Scholar] [CrossRef]
- Zhou, Q.; Ju, W.; Su, X.; Yong, Y.; Li, X. Adsorption behavior of SO2 on Vacancy-defected graphene: A DFT Study. J. Phys. Chem. Solids 2017, 109, 40–45. [Google Scholar] [CrossRef]
- Greenwood, T.; Koehler, S.P.K. Molecular Dynamics Simulations of Nitric Oxide Scattering Off Graphene. ChemPhysChem 2022, 23, e202200216. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; She, X.; Wang, J.; Xue, Q. Numerical study on the distribution of flue gas residence time in the desulfurization and denitrification system by the optimization of the Model. Int. J. Chem. React. Eng. 2022, 20, 1095–1105. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Xu, Z.; Xiong, J.; Zhu, T. Transformation of functional groups in the reduction of NO with NH3 over nitrogen-enriched activated carbons. Fuel 2018, 223, 312–323. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y. Effect of acidic surface functional groups on Cr(VI) removal by activated carbon from aqueous Solution. Rare Met. 2010, 29, 333–338. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, G.; Wang, Z.; Wang, Q.; Yang, Y.; Luo, J. Influences of surface functional groups on catalytic activity over activated carbon catalysts for sulfur dioxide removal from flue Gases. Appl. Catal. B Environ. 1994, 3, 229–238. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Li, G.; Zhang, J.; Guo, Y. NO removal activity and surface characterization of activated carbon with oxidation Modification. J. Energy Inst. 2017, 90, 813–823. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Xu, S.; Cao, F.; Ren, X.; Sun, Q.; Yang, L.; Wennersten, R.; Mei, N. Simulation of the VOC Adsorption Mechanism on Activated Carbon Surface by Nitrogen-Containing Functional Groups. Appl. Sci. 2024, 14, 1793. [Google Scholar] [CrossRef]
- Jin, C.; Zhao, T. Engineering application of activated carbon desulfurization and denitrification integrated technology. CFHI Technol. 2017, 4, 45–47. (In Chinese) [Google Scholar]
- Li, F.; Zhang, J.; Qi, Y.; Gu, Q. Activated carbon combined desulfurization and denitrification process. Chem. Enterp. Manag. 2020, 4, 189–190. (In Chinese) [Google Scholar]
- Rubio, B.; Izquierdo, M.T.; Mastral, A.M. Influence of low-rank coal char properties on their SO2 removal capactity from flue gases.2. Activated chars. Carbon 1998, 36, 263–268. [Google Scholar] [CrossRef]
- Lisovskii, A.; Semiat, R.; Aharoni, C. Adsorption of sulfur dioxide by active carbon treated by nitric acid: I. Effect of the treatment on adsorption of SO2 and extractability of the acid formed. Carbon 1997, 35, 1639–1643. [Google Scholar] [CrossRef]
- Schröder, E.; Thomauske, K.; Weber, C.; Hornung, A.; Tumiatti, V. Experiments on the generation of activated carbon from biomass. J. Anal. Appl. Pyrolysis 2007, 79, 106–111. [Google Scholar] [CrossRef]
- Lin, W.; Johnsson, J.E.; Dam-Johansen, K.; van den Bleek, C.M. Interaction between emissions of sulfur dioxide and nitrogen oxides in fluidizedbed combustion. Fuel 1994, 73, 1202–1208. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduc-tion of NO and NO2 at low temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, L.; Zhang, Y.; Liang, J.; Wang, J.; Zhang, Z.; Wang, X. Activated semi-coke in SO2 removal from flue gas:selection of activation methodology and desulfurization mechanism study. Energy Fuels 2013, 27, 3080–3089. [Google Scholar] [CrossRef]
- Tang, Q. Experimental Study on Competitive Adsorption of SO2 and NOx Mixed Gas; Xi’an Jiaotong University: Xi’an, China, 2003. (In Chinese) [Google Scholar]
- Caglar, A.; Kaya, S.; Saka, C.; Yildiz, D.; Kivrak, H. Investigation of electrooxidation and methanolysis of sodium borohydride on activated carbon supported Co catalysts from poplar sawdust. Int. J. Hydron-Gen Energy 2024, 75, 171–178. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, H.; Yang, F.; Zhang, N.; Cao, X. Inorganic composite sorbents for water vapor sorption: A research progress. Renew. Sustain. Energy Rev. 2016, 54, 761–776. [Google Scholar] [CrossRef]
- Kumar, N.; Pandey, A.; Sharma, Y.C. A review on sustainable mesoporous activated carbon as adsorbent for efficientremoval of hazardous dyes from industrial wastewater. J. Water Process Eng. 2023, 54, 104054. [Google Scholar] [CrossRef]
- Liu, W.; Xie, H.; Zhang, J.; Zhang, C. Sorption removal of cephalexin by HNO3 and H2O2 oxidized activated carbons. Sci. China Chem. 2012, 55, 1959–1967. [Google Scholar] [CrossRef]
- Blazewicz, S.; Swiatkouski, A.; Trznadel, B.J. The influence of heat treatment on activated carbon structure anporosity. Carbon 1999, 37, 693–700. [Google Scholar] [CrossRef]
- Qiu, W.; Dou, K.; Zhou, Y.; Huang, H.; Chen, Y.; Lu, H. Hierarchical pore structure of activated carbon fabricated by CO2/microwave for volatile organic compounds adsorption. Chin. J. Chem. Eng. 2018, 26, 81–88. [Google Scholar] [CrossRef]
- Haydar, S.; Ferro-Garcıa, M.A.; Rivera-Utrilla, J.; Joly, J.P. Adsorption of p-nitrophenol on an activated carbon with different oxidation. Carbon 2003, 41, 387–395. [Google Scholar] [CrossRef]
- Boudou, J.P.; Chehimi, M.; Broniek, E.; Siemieniewska, T.; Bimer, J. Adsorption of H2S or SO2 on an activated carbon cloth modified by ammonia treatment. Carbon 2003, 41, 1999–2007. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.; Wu, H.; Li, J.; Yang, L. Adsorption of volatile organic compounds at medium-high temperature conditions by activated carbons. Energy Fuels 2019, 34, 3679–3690. [Google Scholar] [CrossRef]
- Teresa, B.; Camille, P. On the reactive adsorption of ammonia on activated carbons modified by impregnation with inorganic compounds. J. Colloid Interface Sci. 2009, 338, 329–345. [Google Scholar]
- Zhang, J.; Duan, Y.; Zhou, Q.; Zhu, C.; She, M.; Ding, W. Adsorptive removal of gas-phase mercury by oxygen non-thermalplasma modified activated carbon. Chem. Eng. J. 2016, 294, 281–289. [Google Scholar] [CrossRef]
- Hawke. Effect of Activated Carbon Loading on Microwave Desulfurization and Denitrification of Sintering Flue Gas; North China University of Science and Technology: Tangshan, China, 2023. (In Chinese) [Google Scholar]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Ning, P.; Yu, L.; Yi, H.; Tang, X.; Li, H.; Wang, H.; Yang, L. Effect of Fe/Cu/Ce loading on the coal-based activated carbons for hydrolysis of carbonyl sulfide. J. Rare Earths 2010, 28, 205–210. [Google Scholar] [CrossRef]
- Chukov, D.I.; Stepashkin, A.A.; Maksimkin, A.V.; Tcherdyntsev, V.V.; Kaloshkin, S.D.; Kuskov, K.V.; Bugakov, V.I. Investigation of structure, mechanical and tribological properties of short carbon fiber reinforced UHMWPE-matrix composites. Compos. Part B Eng. 2015, 76, 79–88. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Z.; Gong, H.; Wang, X. Copper oxide modified activated carbon for enhanced adsorption performance of siloxane: An experimental and DFT Study. Appl. Surf. Sci. 2022, 601, 154200. [Google Scholar] [CrossRef]
- Natrayan, L.; Arul Kumar, P.V.; Dhanraj, J.A.; Kaliappan, S.; Sivakumar, N.S.; Patil, P.P.; Sekar, S.; Paramasivam, P. Synthesis and Analysis of Impregnation on Activated Carbon in Multiwalled Carbon Nanotube for Cu Adsorption from Wastewater. Bioinorg. Chem. Appl. 2022, 2022, 7470263. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Karagianni, P.; Giannakas, A.; Makrigianni, V.; Mouzourakis, E.; Deligiannakis, Y.; Konstantinou, I.; Giannakas, A. Photocatalytic degradation of phenol by char/N-TiO2 and char/N-F-TiO2 composite Photocatalysts. Catal. Today 2017, 280, 114–121. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Xie, W.; Hao, Q.; Chen, H.; Ma, X. Handy synthesis of robust Ni/carbon catalysts for methane decomposition by selective gasification of pine Sawdust. Int. J. Hydrogen Energy 2018, 43, 19414–19419. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F. Design Strategy, Synthesis, and Mechanism of Ni Catalysts for Methane Dry Reforming Reaction: Recent Advances and Future Perspectives. Energy Fuels 2022, 36, 5594–5621. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Sumathi, S.; Bhatia, S.; Lee, K.T.; Mohamed, A.R. Selection of best impregnatedpalm shell activated carbon(PSAC) for simultaneous removal of SO2 and NOx. J. Hazard. Mater. 2010, 176, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Quan, W. Study on the Performance of Activated Carbon Supported Metal Oxide HC-SCR Denitration Catalyst; China University of Mining and Technology: Xuzhou, China, 2019. (In Chinese) [Google Scholar]
- Li, Y.; Yi, H.; Tang, X.; Liu, X.; Wang, Y.; Cui, B. Study on simultaneous desulfurization and denitrification performance of Ni-Cu metal oxide modified activated carbon. Ind. Saf. Environ. Prot. 2017, 43, 4–8. (In Chinese) [Google Scholar]
- Sun, D. Preparation of Modified Activated Carbon Adsorbent and Its Desulfurization Performance for Natural Gas; Northeast Petroleum University: Daqing, China, 2018. (In Chinese) [Google Scholar]
- Xue, L.; Jin, Y.; Liu, L.; Wei, L.; Liu, Z.; Zhao, K. Preparation and characterization of iron oxide modified activated carbon. Carbon 2017, 1, 16–19. (In Chinese) [Google Scholar]
- Yin, S.; Zhu, B.; Sun, Y.; Cao, Z.; Chen, G.; Jiang, R.; Xu, T.; Liu, F. Low temperature selective catalytic reduction denitrification performance of Fe2O3/AC catalysts. Chin. J. Process Eng. 2018, 18, 330–336. (In Chinese) [Google Scholar]
- Adachi, M.; Nakagawa, T.; Fujioka, T.; Mori, M.; Kubota, K.; Oda, G.; Kikkawa, T. Feasibility of portable microwave imaging device for breastcancer detection. Diagnostics 2021, 12, 27. [Google Scholar] [CrossRef]
- Koppel, T.; Shishkin, A.; Haldre, H.; Toropovs, N.; Vilcane, I.; Tint, P. Reflection and transmission properties of common construction materials at 2.4 GHz frequency. Energy Procedia 2017, 113, 158–165. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Eskicioglu, C. Recent developments on thermal municipal sludge pretreatment technologies for enhanced anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 110, 423–443. [Google Scholar] [CrossRef]
- Xue, L.; Gao, R.; Shen, L.; Zheng, X.; Gao, M. Dependence of degradation of anthocyanins on non-uniformity of microwave heating in blueberry puree. Food Bioprod. Process. 2023, 139, 129–143. [Google Scholar] [CrossRef]
- Zhang, X.; Rajagopalan, K.; Lei, H.; Ruan, R.; Sharma, B.K. An overview of a novel concept in biomass pyrolysis: Microwave irradiation. Sustain. Energy Fuels 2017, 1, 1664–1699. [Google Scholar] [CrossRef]
- Wei, Z.; Zeng, G.; Xie, Z.; Sun, J. Simultaneous Desulfurization and Denitrification by Microwave Catalytic over FeCoCu/Zeolite 5A Catalyst. J. Environ. Eng. 2010, 136, 1403–1408. [Google Scholar] [CrossRef]
- DeGroot, W.F.; Osterheld, T.H.; Richards, G.N. Chemisorption of oxygen and of nitricoxide on cellulosic chars. Carbon 1991, 29, 185–189. [Google Scholar] [CrossRef]
- Kong, Y.; Chang, Y. NOx Abatement with Carbon Adsorbents and Microwave Energy. Fuel Energy Abstr. 1995, 9, 971–975. [Google Scholar] [CrossRef]
- Cha, C. Microwave-induced reactions of SO2 and NOx decom-position in the char-bed. Res. Chem. Inter-Mediat. 1994, 20, 13–28. [Google Scholar] [CrossRef]
- Ma, S.; Li, Z.; Ma, J.; Chai, F.; Zhu, S. Influence study of oxygen in the flue gas on physical and chemical properties of activated carbon under microwave irradiation. J. Environ. Chem. Eng. 2015, 3, 1312–1319. [Google Scholar] [CrossRef]
- Ma, S.; Gao, L.; Ma, J.; Jin, X.; Yao, J.; Zhao, Y. Advances on simultaneous desulfurization and denitrification using activated carbon irradiated by microwaves. Environ. Technol. 2012, 33, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.; Stadler, A.; Dallinger, D. Microwaves in Organic and Medicinal Chemistry; Wiley VCH: Weinheim, Germany, 2005. [Google Scholar]
- Kim, T.; Lee, J.; Lee, K.-H. Microwave heating of carbon-based solid materials. Carbon Lett. 2014, 15, 15–24. [Google Scholar] [CrossRef]
- Wang, N.; Zou, W.; Li, X.; Liang, Y.; Wang, P. Study and application status of the nonthermal effects of microwaves in chemistry and materials science-a brief review. RSC Adv. 2022, 12, 17158–17181. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Experimental and Simulation Study of Hot Spot Effect in Microwave Heating Process; Shandong University: Jinan, China, 2017. (In Chinese) [Google Scholar]
- Ma, S.; Gao, L.; Jin, X.; Yao, J.; Zhao, Y. Research progress of microwave irradiation activated carbon desulfurization and denitrification technology. Environ. Sci. Technol. 2011, 34, 70–74. (In Chinese) [Google Scholar]
- Wang, X.; Wang, A.; Wang, X.; Zhang, T. Microwave plasma enhanced reduction of SO2 to sulfur with carbon. Energy Fuels 2007, 21, 867–869. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, W.J.; Jin, Y.; Zhu, X.F.; Zhong, B.H.; Jiang, X. Activation and modification of activated carbon materials. Environ. Pollut. Control. Technol. Equip. 2002, 3, 25. (In Chinese) [Google Scholar]
- Ye, P. Effect of Microwave Heating on the Surface Properties of Activated Carbon. Carbon Tech. 2004, 23, 5–9. (In Chinese) [Google Scholar]
- Wang, X.; Wang, P.; Liu, Y.; Ren, J. Study on the desulfurization and denitrification performance of activated carbon based on microwave. Resour. Econ. Environ. Prot. 2015, 5, 15–16+20. (In Chinese) [Google Scholar]
- Jin, X. Experimental Study on Simultaneous Desulfurization and Denitrification of Activated Carbon Bed by Microwave Irradiation; North China Electric Power University: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Li, H. Mn2O3/AC Microwave Catalysts for Microwave Selective Catalytic Reduction of Nitrogen Oxides; Xiangtan University: Xiangtan, China, 2013. (In Chinese) [Google Scholar]
- Liu, B. Research on Coal-Based Carbon Microwave Flue Gas Denitrification; Wuhan University of Science and Technology: Wuhan, Chian, 2016. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).