Abstract
Olive leaves (Olea europaea L.) are a source of natural bioactive compounds with various health benefits and are often considered agricultural waste. This study aimed to evaluate the antioxidant, antibacterial, and antidiabetic activities of an infused aqueous extract and ultrasonic-assisted extracts (aqueous, methanol, ethyl acetate, and hexane) from wild olive leaves grown in the United Arab Emirates (UAE). The aqueous-infused extract exhibited the highest total phenolic content (TPC; 91.9 mg GAE/g extract), the highest reducing power activity, the lowest IC50 value for diphenyl-1-picrylhydrazyl (DPPH) inhibition (97.3% and 81.1 µg/mL, respectively), and the highest α-amylase and α-glucosidase inhibition activities (77.1% and 83.3%, respectively). Meanwhile, the ultrasonic-assisted methanolic extract exhibited the highest total flavonoid content (31.2 mg RE/g extract) and significant α-amylase and α-glucosidase inhibition activities (61.8% and 77.2%, respectively). The antimicrobial activity of wild olive leaf extracts (WOLEs) at concentrations of 1, 2.5, and 5 mg, tested using the disc diffusion method against Gram-positive and Gram-negative bacterial strains, was weak or ineffective at the studied concentrations. The study concluded that WOLEs are high in total phenolic and flavonoid content and antioxidant and antidiabetic activities, suggesting the potential use of WOLE in folk medicine.
1. Introduction
The olive tree (OT), being a source of olive oil, is largely cultivated in the Mediterranean region. As an adapted crop, OT can grow in sub-humid, semi-arid, and arid areas with modern and efficient agricultural practices, and its cultivation is expanding to new growing countries, such as Australia, California, Saudi Arabia, Java, Argentina, Chile, Bermuda, and Uruguay [1]. OTs have been grown in different climate conditions with a wide range of cultivars. Currently, around 900 million OTs span over 10.5 million hectares at the global level [2]. According to the Food and Agriculture Organization of the United Nations Statistics (2022), Spain, Turkey, and Italy are the leading olive-producing countries, while 98% of production around the world comes from the Mediterranean region [https://www.fao.org/statistics/en/ (accessed on 20 February 2025)].
Olive leaves (OLs) are primarily considered by-products of horticultural practices used for the traditional management of OT orchards. Unfortunately, this conventional disposal method results in the loss of valuable bioactive components, particularly phenolic elements, present in these leaves and serious environmental problems. OLs are regarded as a potential source of bioactive elements, such as polyphenols, primarily oleuropein, and phenolic acids, including caffeic, gallic, and coumaric acids. Additionally, they contain other compounds, like tyrosol, hydroxytyrosol, ligstroside, and lignans [3,4]. However, the phenolic constituents of OLs differ by cultivar and are thus dependent on different factors, like sampling time, environmental and geographical factors, tree biological cycle, agriculture production, and extraction technique practices, along with the isolation, processing, and storage techniques [5,6].
Different extracts of olive leaf cultivars have been reported to exhibit various properties, including antioxidant activity [7], anticancer effects [4], antidiabetic and anti-inflammation effects [8,9], anti-inflammatory activity [10], and antimicrobial activity [11,12]. Herbs and nutraceuticals containing food-derived bioactive components are beneficial and used by multiple drug and supplement manufacturers. Also, intake of herbal extracts is very common in certain population groups due to their potential synergistic effects [9].
OTs are not common in the United Arab Emirates (UAE) due to its subtropical–arid desert climate. They grow on an industrial scale in some regions, such as Sir Bani Yas Island near Abu Dhabi, and the number of cultivated OTs has reached 16,000, yielding 20,000 tons of olive fruits per year. However, wild OTs grow as nonbearing trees with oblong and silvery-green leaves in various parts of the country. Despite existing within one of the driest regions in the world, several crops, including OTs, have spread successfully due to scientific efforts supported by modern agricultural practices, such as efficient irrigation systems, fertilization, and effective soil management [13,14].
In various countries, including the UAE, boiled aqueous extracts of OLs are frequently consumed in traditional medicine to treat numerous diseases, such as hyperglycemia, hypertension, inflammation, and cancer [10,15]. While previous studies have investigated the bioactive properties of OL extracts from different cultivars, no research has yet been conducted on the antioxidant, antidiabetic, and antimicrobial properties of wild olive leaf extracts (WOLEs) grown in the UAE. Therefore, this study aims to evaluate the in vitro antioxidant, antibacterial, and antidiabetic activities of WOLEs from the UAE. Additionally, we assess the total phenolic content (TPC) and flavonoid content of aqueous, methanolic, ethyl acetate, and hexane extracts of wild olive leaves grown under severe drought conditions in the UAE.
2. General Experimental
2.1. Chemicals
All the chemicals used in this study were of analytical grade: Aluminum trichloride (AlCl3 ≥ 99.9%), gallic acid (anhydrous), rutin hydrate, DPPH (1,1-diphenyl-2- picrylhydrazyl), 2-chloro-4-nitrophenyl-α-maltrotrioside (CNP-G3), P-nitrophenyl-α-D-glucopyranoside (PNP-G), α-glucosidase and α-amylase of porcine pancreas, potassium thiocyanate (KSCN), sodium carbonate (Na2CO3), potassium ferricyanide (K3[Fe(CN)6]6), and ethyl acetate were purchased from Glentham Life Sciences (Corsham, Wiltshire, UK). Folin–Ciocalteu reagent and trichloro acetic acid were obtained from Sisco Research Laboratories (Mumbai, India), while ferric chloride (FeCl3), methanol, hexane, BHT, and L-ascorbic acid were purchased from Sigma-Aldrich (Buchs, Switzerland).
2.2. Collection, Preparation, and Sample Extraction
Wild OLs were collected from the Al Gharbia region in the western part of Abu Dhabi, United Arab Emirates (UAE; latitude: 23.3722° N; longitude: 53.4249° E), in January 2024. A voucher specimen for the wild OLs (OEW-1-2024) has been deposited at the College of Pharmacy, Al-Ain University, Abu Dhabi, UAE. The juvenile and early grown wild OLs of an irrigated olive tree were washed with water and dried at 45 °C for 4 days in an air-circulated conventional oven (Labtron, Model LDO-A10, Camberley, UK). Samples were pulverized using a stainless-steel grinder to obtain a fine powder. The ultrasonic-assisted extraction at low frequency (20 kHz) was performed for leaf powders. The ultrasound technique has advantages in terms of efficiency, yield, and preservation of bioactive compounds during extraction. Briefly, 10 g of the pulverized leaf sample was mixed with 50 mL of de-ionized water, methanol, ethyl acetate, and hexane for 15 min using a Nordberg NU20 ultrasonic bath (Moscow, Russia) at 40 °C, separately. All extracts were passed through Whatman filter paper No. 1 to eliminate residues. For efficient extraction of bioactive constituents, the residues were re-extracted twice using 25 mL portions of each solvent, filtered, and the combined extracts were adjusted to 100 mL with their respective solvents. WOLEs were kept refrigerated at 4 °C until use. Aqueous infusion is a traditional and widely used method for extracting bioactive compounds from herbs by soaking them in hot or boiling water. The technique uses only water as the solvent, making it safe, non-toxic, and environmentally friendly compared to organic solvents. For the preparation of the aqueous infusion extract (AIE), 10 g of the collected green leaves was washed with water, sliced into approximately 1 cm × 1 cm pieces, and gently boiled for 15 min in 100 mL of de-ionized water. The mixture was filtered to remove residues, adjusted to a final volume of 100 mL, and stored in a refrigerator at 4 °C until use.
2.3. Extract Yield Determination
The yield of WOLEs (%) was measured in triplicate for each extract using an air-circulated conventional oven (Labtron, Model LDO-A10, Camberley, UK) at 105 °C until a constant weight was attained.
2.4. Total Phenolic Content Determination
The total phenolic content of each extract was determined using the Folin–Ciocalteu reagent [4]. An aliquot of 0.1 or 0.5 mL from each extract (100 mg/mL) was combined with 2.5 mL of de-ionized water in a 10 mL volumetric flask and thoroughly mixed with 250 μL of the Folin–Ciocalteu reagent. After 3 min, 0.5 mL of 10% sodium carbonate solution was added, and the final volume was adjusted to 10 mL with de-ionized water. The absorbance was recorded at 760 nm using a spectrophotometer (VWR, Model UV-3100PC, Beijing, China). Gallic acid was used as the standard for the calibration curve (0–10 ppm). The total phenolic content (mg/g of dry extract) was expressed as gallic acid equivalent (GAE) and determined using the established calibration curve, derived from the following regression equation:
where Y represents the absorbance, and X denotes the gallic acid concentration in µg/mL. All measurements were performed in triplicate.
2.5. Determination of Total Flavonoids Content
For total flavonoids content, 1 mL from each extract (100 mg/mL) was combined with 1 mL of 2% aluminum trichloride in absolute ethanol. The mixture was then diluted with absolute ethanol to a final volume of 25 mL and allowed to stand for 40 min at 20 °C. The absorbance was recorded at 415 nm using a spectrophotometer (VWR, Model UV-3100PC, Beijing, China). For the blank, 1 mL of each extract and 1 drop of acetic acid were diluted with absolute ethanol to 25 mL, and the absorbance was recorded as described earlier. The total flavonoid content (mg/g of dry extract) was expressed as rutin equivalent (RE). Rutin was used as a standard for the calibration curve (0–40 ppm), and the content was calculated using the following regression equation:
where Y represents the absorbance, and X denotes the rutin concentration in µg/mL. All measurements were performed in triplicate.
Y = 0.0301X − 0.0089, R2 = 0.9997,
2.6. Antioxidant Activity Determination
2.6.1. DPPH Radical Scavenging Activity
The potential antioxidant activities of WOLEs were determined using DPPH free- radical scavenging activity at different concentrations to determine the IC50 value for each extract. The reaction mixture consisted of 0–1000 µL (1000 µg/mL) of aqueous, methanol, ethyl acetate, and hexane extract. An aliquot from each extract was adjusted to 1 mL with its respective solvent, vortexed, and then 3 mL of a methanolic DPPH solution (6 × 10−5 M) was added. The solutions were mixed by vortexing, and the absorbance of each extract and control was recorded at 517 nm after 30 min using a spectrophotometer (VWR, Model UV-3100PC, Beijing, China) against a blank. The percentage of DPPH radical inhibition was calculated using the following equation:
where C is the control absorbance, E is the extract absorbance, and B is the blank absorbance. All measurements were performed in triplicate
DPPH free radical inhibition activity (%) = [C − (E − B)/C] × 100,
2.6.2. Percentage of Reducing Power
The reducing power activity of the WOLE, an aliquot of 1.0 mL of each extract, previously dissolved in its solvent (1 g/100 mL), was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide (1 g/100 mL). The mixture was then incubated at 50 °C for 30 min, followed by the addition of 2.5 mL of trichloroacetic acid (10 g/100 mL) and centrifugation at 1650× g for 10 min. After centrifugation, 2.5 mL of the upper layer was collected and mixed with 2.5 mL of ferric chloride solution (0.1 g/100 mL). The absorbance was recorded at 700 nm for each extract, with a blank prepared using a similar concentration for each treatment. Ascorbic acid (30 µg) was used as the standard, and all measurements were performed in triplicate:
Reducing power activity (%) = (Sample absorbance − sample blank)/(Ascorbic acid absorbance) × 100.
2.7. Antidiabetic Activity
2.7.1. α-Glucosidase Inhibitory Activity Determination
The α-glucosidase inhibitory activity (%) of the WOLEs was assessed by measuring the ability of the α-glucosidase enzyme to release p-nitrophenol (PNP) and glucose from p-nitrophenyl-α-D-glucopyranoside (PNP-G) [9]. The PNP level was determined by recording the increase in absorbance at 415 nm during the reaction against each extract blank as a reference.
The reaction mixture (400 µL) consisted of 250 µL of 20 mM PNP-G in 0.05 M phosphate buffer (pH 7.0) and 50 µL of aqueous, methanol, ethyl acetate, or hexane extract (100 µg/mL). The mixture was then incubated at 37 °C for 5 min, after which 100 µL of freshly prepared α-glucosidase enzyme solution (1 mg/mL) in phosphate buffer (pH 7.0) was added. The mixture was left to stand for 15 min at room temperature, and then 1000 µL of 2.1% Na2CO3 was added, and the absorbance was recorded. The α-glucosidase enzyme inhibition percentage was calculated using the following formula:
where X represents the reaction in the absence of the extract, and Y represents the increase in absorbance in its presence. All measurements were performed in triplicate.
The inhibitory activity of extract (%) = [(X − Y)/X] × 100,
2.7.2. Determination of α-Amylase Inhibitory Activity
The α-amylase inhibitory activity was evaluated by measuring the ability of porcine pancreas α-amylase to release 2-chloro-4-nitrophenol (CNP) from CNP-G3 [4]. The level of CNP was determined by measuring the increase in absorbance at 405 nm during the reaction against each extract blank as a reference. The reaction mixture consisted of 450 µL of 0.15 mM CNP-G 3, 0.2 M potassium thiocyanate solution in 0.05 M phosphate buffer (pH 7.0), and 50 µL of aqueous, methanol, ethyl acetate, or hexane (100 µg/mL) extract from WOLE. Then, 100 µL of freshly prepared α-amylase solution (1 mg/mL) in phosphate buffer (pH 7.0) was added:
where X represents the reaction in the absence of the extract, and Y represents the increase in absorbance in its presence. All measurements were performed in triplicate.
The inhibitory activity of extract (%) = [(X − Y)/X] × 100,
2.8. Determination of Antibacterial Activity
2.8.1. Microorganism Strains
The following bacterial strains were sourced from the laboratory of microbiology of the college of pharmacy (Al Ain University, Abu Dhabi, United Arab Emirates): Gram-positive bacteria (Staphylococcus aureus (S. aureus; WDCM00032 vitroids–Sigma) and Bacillus subtilis (B. subtilis; WDCM00003 vitroids–Sigma)), and Gram-negative bacteria (Escherichia coli (E. coli; ATCC-25922) and Pseudomonas aeruginosa (P. aeruginosa; WDCM00026 vitroids–Sigma)) sourced from (Sigma-Aldrich Production GmbH, Buchs, Switzerland).
2.8.2. Antibacterial Assay
A measured volume from each WOLE was concentrated by nitrogen gas flushing to a final concentration of 250 mg/mL. The antimicrobial testing was performed using the paper disc diffusion method [16]. In brief, sterilized 6 mm paper discs (Himedia Laboratories, Mumbai, India) were impregnated with 4, 10, or 20 μL (1.0, 2.5, and 5 mg/disc) from each extract and left to dry under aseptic conditions. The antibiotic chloramphenicol (30 µg/disc) was used as a positive control. A sterile cotton swab (BROMED, Mesquite, TX, USA) was dipped into the previously prepared inoculum suspension of each bacterium, adjusted to 0.5 McFarland standard turbidity. The discs were carefully placed on hard nutrient agar plates (Sigma-Aldrich, Mumbai, India) that had been previously streaked with the microorganisms. The plates were incubated at 37 °C for 18–24 h. Bacterial growth inhibition was assessed by measuring the net diameter of the clear inhibition zone around each disc. The average of three measurements was recorded.
2.9. Statistical Analysis
Statistical analyses were conducted using the Statistical Analysis System (SAS) software, version 2000 (SAS Institute Inc., Cary, NC, USA). Significant differences between the means of the measurements were determined using an LSD test, with p ≤ 0.05 considered statically significant. Regression equations and correlation coefficients (R) were determined using MS Excel software. All measurements were performed in triplicate.
3. Results and Discussion
3.1. Extraction Yields
The results of extraction yields of WOLEs are shown in Table 1. The aqueous ultrasonic-assisted extract (AU) of wild OLs showed the highest extraction yield (18.4%), followed by methanol extract (12.9%), while the AIE showed an extraction yield of 8.5%, and 4.1% for ethyl acetate and 0.7% for hexane extracts. Results showed that the extraction yields of WOLEs were highly affected by solvent polarity and the extraction technique. The yields of extracts were related to the extraction solvent polarity and were in the following decreasing order: AU > methanol > ethyl acetate > hexane extracts. Along with that, the factors, including temperature, time, pulverization content, leaf quality, cultivars, and the solvent-to-sample ratio, appeared to affect yield extraction [17,18]. The WOLE yield increased solvent polarization because of its phenolic and non-phenolic extraction. Likewise, a higher extraction yield using polar solvents was reported in recent studies [4,12]. The extraction yield for the aqueous ultrasonic-assisted extract (18.4%) was higher as compared to the aqueous-infused extract (8.5%). Some other studies reported that other factors, like extraction techniques, temperature, and duration, were included among the most important factors affecting yields.

Table 1.
Percentage yields, phenolic and flavonoid contents, DPPH activity (IC50), and reducing power activities of various extracts of WOLEs grown in UAE *.
3.2. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
The TPC in WOLEs was reported as23.3–91.9 mg GAE/g dry extract, while TFC was observed as9.0–31.2 mg RE/g dry extract (Table 1). The AIE exhibited the maximum TPC of 91.9 and methanolic extract at 44.4, and later a decreasing trend was seen for the aqueous ultrasonic-assisted extract (AU), ethyl acetate extract, and then hexane extract. Meanwhile, TFC was highest in methanolic extract with 31.2, followed by a decrease in value for AIE, AU, and hexane extract. A previous study reported more variable results for TPC of aqueous OL extracts, from 7.5 to 250 mg/g [19]. Similarly, another recent study observed a varied TPC of 29.2 to 45.4 mg GAE/g in five different olive aqueous extracts [20].
In addition to that, another study concluded that TPC and stability of OLs were higher at high temperatures [21], which might be due to the higher value of TPC extracted from AIE than AU. Moreover, an elevated TPC in OLs was documented while using more polar solvents [4,12,18]. Similarly, another study found that the method of extraction is also very important, even using a similar solvent with regards to TPC and TFC [16,22].
A few other studies have found that mild–moderate drought conditions for OLs are most often related to increases in TPC, TFC, and antioxidant activities, and a decrease in oxidative damage [23,24,25,26], whereas severe drought can reduce the phenol production, leading to oxidative damage and a decrease in TPC, TFC, and antioxidant properties [27,28]. Adding to that, many other factors, including solvent properties and polarity, pH, along with geographical and environmental conditions, affect extraction [13]. A previous study found high TPC, TFC, and antioxidant activities of WOLEs grown in Jordan as compared to those grown in the UAE due to different climate conditions [4].
Although the aqueous extract (AU) showed the highest yield among those obtained using ultrasonic-assisted extraction, its TPC and TFC was lower than the methanol extract. This is likely because the aqueous extract facilitates the extraction of more non-phenolic compounds, whereas methanol helps to enhance the phenolic compounds’ solubility and extraction [29,30]. The variations were primarily due to the solvent’s polarity, which affected the solubility of specific bioactive compounds. For example, polar solvents are more effective at extracting hydrophilic compounds, such as phenolics and flavonoids, whereas non-polar solvents like hexane may extract less polar compounds, like lipids.
The results clearly showed that WOLE contained a considerable amount of phenolics and flavonoids, which suggests a direct relationship with antioxidant activities. Considering that, the associations among the different values from different models were determined to observe TPC, TFC, antioxidant, antidiabetic, and antimicrobial activities.
3.3. Antioxidant Properties
3.3.1. DPPH Free-Radical Scavenging Activity
Figure 1 presents the DPPH activity of various WOLEs and the observed results in a dose-dependent manner. Also, the IC50 values of WOLE are reported in Table 1. The BHT standard exhibited the lowest IC50 value (44.8 µg/mL), indicating the highest DPPH scavenging activity, while AIE reported an IC50 value of 81.1 (µg/mL). This indicates the highest scavenging activity among other WOLEs. In addition, methanol extract showed an IC50 of 212.7 µg/mL. The results showed a non-significant relationship between the aqueous extract (AU) and the ethyl acetate extract. However, the hexane extract exhibited the highest IC50 value, which was not detected at the concentrations used, and reported low DPPH activity. Although, the ability to quench the DPPH radical, indicating greater antioxidant activity, increased with low IC50 values.
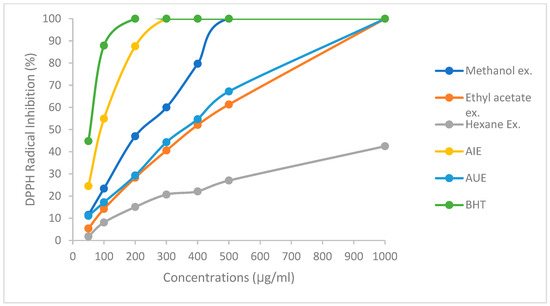
Figure 1.
Percentage of DPPH activity of WOLE at different concentrations.
In addition, a strong association was seen among the TPC and DPPH activity (R = 0.71), while total flavonoids exhibited a correlation of 0.83 (Table 2). In agreement with our result, Martin-Garcia et al. found a significant correlation between DPPH inhibition activity and TPC (R = 0.82), as well as the sum of oleuropein (R = 0.77), in different olive leaf cultivars [3]. Furthermore, a strong correlation was observed between TPC and TFC of olive leaf extracts and DPPH activity [4,18], suggesting the beneficial role of WOLE in disease prevention through reducing the oxidative stress.

Table 2.
Correlation between phenolic and flavonoid contents and antioxidant and antidiabetic activities of WOLE.
3.3.2. Percentage of Reducing Power Activity
The reducing power of WOLE was quantitatively calculated by assessing its reducing power from Fe3⁺ to Fe2⁺ ions. Table 1 presents the reducing power activity (%) of WOLE at 100 µg/mL by comparing to vitamin C (30 µg). Among different extracts, AIE exhibited the highest reducing power activity (97.3%). Also, AIE values were significantly higher as compared to other extracts and comparable to standard vitamin C (p ≤ 0.05). Additionally, the methanol, AU, ethyl acetate, and hexane extracts demonstrated reducing power activities of 82.9%, 65.6%, 58.4%, and 21.7%, respectively. We found no difference in reducing power activity among the AU and ethyl acetate extracts, however, we found a strong association with TPC and TFC of WOLE (R = 0.81 and 0.87, respectively; Table 2). Moreover, phenolic compounds, particularly oleuropein and hydroxytyrosol, have been reported to exhibit strong reducing power by donating electrons to stabilize free radicals [4,31,32]. It has been reported that OL ethanolic and methanolic extracts have increased in reducing power compared to aqueous extracts due to their different bioactive properties [33,34].
3.3.3. Antidiabetic Activity
Table 3 shows the percentages of α-amylase and α-glucosidase inhibitory properties of different WOLEs. The results showed that the aqueous-infused extract exhibited elevated α-amylase (77.1%) and α-glucosidase inhibitory activities (83.3%), while methanol extract showed α-amylase and α-glucosidase inhibitory activities at 61.8% and 77.2%, respectively. Additionally, the ultrasonic-assisted aqueous (AU), ethyl acetate, and hexane extracts demonstrated α-amylase inhibitory activities of 52.3%, 33.2%, and 22.4%, respectively, while α-glucosidase inhibitory properties were reported with 60.5%, 52.7%, and 35.6%, respectively. Significant correlations were found between α-amylase and α-glucosidase properties (%), and the TPC contents of the extracts were 0.88 and 0.82, while the relationship with TFC was 0.82 and 0.92, respectively (Table 2). However, WOLEs exhibited stronger α-glucosidase inhibition activities as compared to α-amylase inhibition activities.

Table 3.
Percentage of α-amylase and α-glucosidase inhibition activities from WOLEs grown in UAE *.
In agreement with our study, it has been found that α-glucosidase inhibitory activity is linked to flavonoids and secoiridoids, whereas phenolic compounds of the extracts were used to inhibit α-amylase enzyme [8,35]. A recent study reported the percentage of the WOLE α-amylase inhibition activity grown in Jordan, showing the inhibitory activities of 65.1% and 56.7% of ethanolic and ethyl acetate extracts, respectively [4], while the aqueous extract reported a lower inhibition of 51.8% at a 100 µg/mL concentration. Also, a strong association between α-amylase inhibitory activity and TPC (0.80), TFC (0.74), and total flavonol content of the extracts (0.72) was found. OL extracts from three other olive cultivars showed dose-dependent α-glucosidase inhibition activity and an IC50 value of 14 µg/mL [9]. Furthermore, another study found α-glucosidase inhibitory enzyme activity of 81.34% at 3.85 mg/mL for OL extracts [36].
Several studies reported earlier that the ability of phenolic compounds to inhibit carbohydrate-hydrolyzing enzymes is primarily attributed to their interaction with the enzymes, reducing their activity. Therefore, WOLEs may offer potential health benefits in managing diabetic complications and reducing postprandial blood glucose levels [37,38].
3.3.4. Antibacterial Activity of WOLEs
The antibacterial activity of WOLEs compared to the control antibiotic chloramphenicol (30 µg/disc) is shown in Table 4. No inhibition zones were detected against any of the tested bacteria at concentrations of 1 and 2.5 mg/disc. However, at the 5 mg/disc concentration, the maximum inhibition zone observed was for the methanolic extract against Staphylococcus aureus (4.5 mm). The results also showed that all tested strains, except Pseudomonas aeruginosa, were sensitive to the standard antibiotic chloramphenicol (30 µg/disc), with varying inhibition diameters. These findings indicate that the WOLEs studied, grown in the UAE, exhibited little to no antibacterial properties as compared to the tested bacteria at the concentrations used.

Table 4.
Inhibition zone net diameter (mm) of the WOLEs at a concentration of 5.0 mg/disc against tested bacteria strains *.
Our results, aligning with previously reported studies, suggested that olive leaf extracts do not exhibit significant antibacterial activity against certain bacterial strains, indicating that their inhibitory effects may be limited or strain dependent. Studies have shown that the extent of inhibition varies depending on factors such as bacterial strain, olive leaf cultivar, extraction solvent, concentration, assay type, and specific experimental conditions [39,40]. Olive leaf extracts have been widely studied for their antimicrobial properties, with numerous studies demonstrating their effectiveness against various bacteria. This activity is primarily attributed to their phenolic content, including hydroxytyrosol, the dialdehydic form of decarboxymethyl elenolic acid, oleacein, verbascoside, oleuropein, and other compounds that exert synergistic effects [41,42,43].
Although the high phenolic compounds enhance their antimicrobial activity, antimicrobial effects are not solely related to phenolic content. Pseudomonas aeruginosa is inherently resistant to chloramphenicol due to multiple resistance mechanisms, such as enzymatic inactivation, low outer membrane permeability, and intrinsic and acquired resistance [44]. However, comparing results from different studies is challenging because of the different test methods. Generally, Gram-positive bacteria are more vulnerable to phenolic compounds due to their membrane’s interaction with the hydrophobic properties of polyphenols. In contrast, Gram-negative bacteria are further resistant to polyphenols because of their hydrophilic cell walls [45].
4. Conclusions
Our study concluded that WOLE is high in total phenolic and flavonoid content and antioxidant and antidiabetic activities. Due to its high correlation between the contents of its phenolics and flavonoids and these biological activities, it is a promising lead as a potential natural therapeutic agent. The antibacterial activity of WOLE grown in the UAE was limited and appeared to be strain dependent. Despite this, the combination of WOLE with conventional drugs has the potential to enhance disease management through the exploitation of its bioactive properties. Further research is needed to explore its full pharmacological potential, optimize extraction methods, and establish its safety and efficacy for applications.
Author Contributions
M.M.A.-D. and R.A.-J. conceptualized the study; M.M.A.-D. and H.J.H. conducted data collection and analysis; M.M.A.-D., R.A.-J., M.A., A.A. and S.I. wrote the initial manuscript; B.A.A.-N. proofread the paper, while S.I. and D.A.-R. performed the final editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Al Ain University of Science and Technology, UAE (Grant No. Ph2023-4-100).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Maher M. Al-Dabbas conducted this research during a period of sabbatical leave from the University of Jordan and worked at Al Ain University (UAE).
Conflicts of Interest
Author Bha’a Aldin Al-Nawasrah was employed by Nabeel Ghunaim and Partners company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Sala, G.; Caruso, T.; Marra, F.P.; Zafonte, F.; Amico Roxas, A.; Schiavo, B.; Galia, A.; Brunori, A.; Dini, F.; Regni, L.; et al. Study of Energetic Properties of Different Tree Organs in Six Olea europaea L. Cultivars. Sci. Rep. 2021, 11, 17047. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, B.A.; Alotaibi, M.D.; Alothman, O.Y.; Sanjay, M.R.; Kian, L.K.; Almutairi, Z.; Jawaid, M. A New Study on Characterization and Properties of Natural Fibers Obtained from Olive Tree (Olea europaea L.) Residues. J. Polym. Environ. 2019, 27, 2334–2340. [Google Scholar] [CrossRef]
- Martín-García, B.; De Montijo-Prieto, S.; Jiménez-Valera, M.; Carrasco-Pancorbo, A.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Comparative Extraction of Phenolic Compounds from Olive Leaves Using a Sonotrode and an Ultrasonic Bath and the Evaluation of Both Antioxidant and Antimicrobial Activity. Antioxidants 2022, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbas, M.M.; Ramadan, A.; Hamad, H.J.; Al-Bashabsheh, Z.; Abughoush, M.; Kayed, K.; Al-Nawasra, B.A.; Aldabbas, A.; Iqbal, S. Cytotoxic, Antioxidant and Alpha-Amylase Inhibitory Activities of Wild and Nabali Olive Leaf Extracts from Jordan. Pak. J. Pharm. Sci. 2024, 37, 571–581. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Durazzo, A.; Akli, H.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P.; Calokerinos, A.; Mati, A.; Lydakis-Simantiris, N. Extraction of Polyphenols from Olive Leaves Employing Deep Eutectic Solvents: The Application of Chemometrics to a Quantitative Study on Antioxidant Compounds. Appl. Sci. 2022, 12, 831. [Google Scholar] [CrossRef]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-Glucosidase and α-Amylase by Spanish Extra Virgin Olive Oils: The Involvement of Bioactive Compounds Other than Oleuropein and Hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef]
- Mansour, H.M.M.; Zeitoun, A.A.; Abd-Rabou, H.S.; El Enshasy, H.A.; Dailin, D.J.; Zeitoun, M.A.A.; El-Sohaimy, S.A. Antioxidant and Anti-Diabetic Properties of Olive (Olea europaea) Leaf Extracts: In Vitro and In Vivo Evaluation. Antioxidants 2023, 12, 1275. [Google Scholar] [CrossRef]
- Fayez, N.; Khalil, W.; Abdel-Sattar, E.; Abdel-Fattah, A.F.M. In Vitro and in Vivo Assessment of the Anti-Inflammatory Activity of Olive Leaf Extract in Rats. Inflammopharmacology 2023, 31, 1529–1538. [Google Scholar] [CrossRef]
- Ferreira, D.M.; de Oliveira, N.M.; Lopes, L.; Machado, J.; Oliveira, M.B. Potential Therapeutic Properties of the Leaf of Cydonia oblonga Mill. Based on Mineral and Organic Profiles. Plants 2022, 11, 2638. [Google Scholar] [CrossRef] [PubMed]
- Debib, A.; Boukhatem, M.N. Phenolic Content, Antioxidant and Antimicrobial Activities of “Chemlali” Olive Leaf (Olea europaea L.) Extracts. Int. J. Pharmacol. Phytochem. Ethnomed. 2017, 6, 38–46. [Google Scholar] [CrossRef]
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the Usage of Olive Leaves, Bioactive Compounds, Biological Activities, and Food Applications: A Comprehensive Review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef] [PubMed]
- Barazani, O.; Dag, A.; Dunseth, Z. The History of Olive Cultivation in the Southern Levant. Front. Plant Sci. 2023, 14, 1131557. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Sbeih, M.; Amayreh, M.; Rahhal, B.; Mudalal, S. Evaluation of the Antibacterial and Antifungal Properties of Oleuropein, Olea europea Leaf Extract, and Thymus Vulgaris Oil. BMC Complement. Med. Ther. 2024, 24, 297. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Moumneh, M.; Hamad, H.J.; Abughoush, M.; Abuawad, B.; Al-Nawasrah, B.A.; Al-Jaloudi, R.; Iqbal, S. Impact of Processing and Preservation Methods and Storage on Total Phenolics, Flavonoids, and Antioxidant Activities of Okra (Abelmoschus esculentus L.). Foods 2023, 12, 3711. [Google Scholar] [CrossRef]
- Cifá, D.; Skrt, M.; Pittia, P.; Di Mattia, C.; Poklar Ulrih, N. Enhanced Yield of Oleuropein from Olive Leaves Using Ultrasound-Assisted Extraction. Food Sci. Nutr. 2018, 6, 1128–1137. [Google Scholar] [CrossRef]
- Cho, W.Y.; Kim, D.H.; Lee, H.J.; Yeon, S.J.; Lee, C.H.; Khan, M.K. Evaluation of Effect of Extraction Solvent on Selected Properties of Olive Leaf Extract. J. Food Qual. 2020, 2020, 3013649. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; García, P.; Brenes, M. Characterization of Bioactive Compounds in Commercial Olive Leaf Extracts, and Olive Leaves and Their Infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef]
- Filgueira-Garro, I.; González-Ferrero, C.; Mendiola, D.; Marín-Arroyo, M.R. Effect of Cultivar and Drying Methods on Phenolic Compounds and Antioxidant Capacity in Olive (Olea europaea L.) Leaves. AIMS Agric. Food 2022, 18, 250–264. [Google Scholar] [CrossRef]
- Elboughdiri, N. Effect of Time, Solvent-Solid Ratio, Ethanol Concentration and Temperature on Extraction Yield of Phenolic Compounds From Olive Leaves; Engineering, Technology & Applied Science Research: Gastouni, Greece, 2018; Volume 8. [Google Scholar]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic Profile (HPLC-UV) of Olive Leaves According to Extraction Procedure and Assessment of Antibacterial Activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef] [PubMed]
- Gholami, R.; Fahadi Hoveizeh, N.; Zahedi, S.M.; Padervand, M.; Dawi, E.A.; Carillo, P. Nanostructure-Assisted Drought Tolerance in Olive Trees (Olea europaea L.): The Role of Fe2O3-Graphitic Carbon. Front. Plant Sci. 2024, 15, 1454619. [Google Scholar] [CrossRef]
- Cerri, L.; Parri, S.; Dias, M.C.; Fabiano, A.; Romi, M.; Cai, G.; Cantini, C.; Zambito, Y. Olive Leaf Extracts from Three Italian Olive Cultivars Exposed to Drought Stress Differentially Protect Cells against Oxidative Stress. Antioxidants 2024, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of Drought Stress on Phenolic Accumulation in Greenhouse-Grown Olive Trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Jiménez-Herrera, R.; Pacheco-López, B.; Peragón, J. Water Stress, Irrigation and Concentrations of Pentacyclic Triterpenes and Phenols in Olea europaea L. cv. Picual Olive Trees. Antioxidants 2019, 8, 294. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C.M. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Haleem Abdul-Wajid, H.; Leonardo Battaglia, M. Plants Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 251. [Google Scholar] [CrossRef]
- Moudache, M.; Colon, M.; Nerín, C.; Zaidi, F. Phenolic Content and Antioxidant Activity of Olive By-Products and Antioxidant Film Containing Olive Leaf Extract. Food Chem. 2016, 212, 521–527. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila Aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Ivanov, M.; Vajic, U.J.; Mihailovic-Stanojevic, N.; Miloradovic, Z.; Jovovic, D.; Grujic-Milanovic, J.; Karanovic, D.; Dekanski, D. Highly Potent Antioxidant Olea europaea l. Leaf Extract Affects Carotid and Renal Haemodynamics in Experimental Hypertension: The Role of Oleuropein. EXCLI J. 2018, 17, 29–44. [Google Scholar] [CrossRef]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant Activity of Phenolics Extracted from Olea europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and Removal of Phenolic Compounds from Olive Mill Wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Khelouf, I.; Karoui, I.J.; Lakoud, A.; Hammami, M.; Abderrabba, M. Comparative Chemical Composition and Antioxidant Activity of Olive Leaves Olea europaea L. of Tunisian and Algerian Varieties. Heliyon 2023, 9, e22217. [Google Scholar] [CrossRef] [PubMed]
- Adera, K.T.; Inami, Y.M.; Akamatsu, K.T.; Atsuoka, T.M. Inhibition of-Glucosidase and-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar]
- Alshaal, S. Determination of the antioxidant properties of the Syrian olive leaves extracts and isolation oleuropein by HPLC techniques. Anal. Bioanal. Chem. Res. 2019, 6, 97–110. [Google Scholar]
- Hammoudi, R.; Dehak, K.; Tlili, M.L.; Khenfer, S.; Medjouel, M.; Hadj-Mahammed, M. Biological Activities of Phenolic Extracts of a Medicinal Plant, Endemic to the Algerian Sahara: Salvia Chudaei Batt. & Trab. Int. J. Biosci. 2017, 11, 108–115. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.B.; Adibi, H.; Khodarahmi, R. Differential α-Amylase/α-Glucosidase Inhibitory Activities of Plant-Derived Phenolic Compounds: A Virtual Screening Perspective for the Treatment of Obesity and Diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Šimat, V.; Skroza, D.; Tabanelli, G.; Čagalj, M.; Pasini, F.; Gómez-Caravaca, A.M.; Fernández-Fernández, C.; Sterniša, M.; Smole Možina, S.; Ozogul, Y.; et al. Antioxidant and Antimicrobial Activity of Hydroethanolic Leaf Extracts from Six Mediterranean Olive Cultivars. Antioxidants 2022, 11, 1656. [Google Scholar] [CrossRef]
- Silvan, J.M.; Casado, R.; Villalva, M.; Diaz, S.; Gutierrez-Docio, A.; Guerrero-Hurtado, E.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive Leaf Extracts as a Source of Antibacterial Compounds against Campylobacter spp. Strains Isolated from the Chicken Food Chain . In Proceedings of the 2nd International Electronic Conference on Foods—“Future Foods and Food Technologies for a Sustainable World” (Foods 2021), Online, 15–30 October 2021; p. 84. [Google Scholar]
- Wei, J.; Wang, S.; Pei, D.; Qu, L.; Li, Y.; Chen, J.; Di, D.; Gao, K. Antibacterial Activity of Hydroxytyrosol Acetate from Olive Leaves (Olea europaea L.). Nat. Prod. Res. 2018, 32, 1967–1970. [Google Scholar] [CrossRef]
- Yakhlef, W.; Arhab, R.; Romero, C.; Brenes, M.; de Castro, A.; Medina, E. Phenolic Composition and Antimicrobial Activity of Algerian Olive Products and By-Products. LWT 2018, 93, 323–328. [Google Scholar] [CrossRef]
- Breidenstein, E.B.M.; de la Fuente-Núñez, C.; Hancock, R.E.W. Pseudomonas aeruginosa: All Roads Lead to Resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; McKeever, L.C.; Malik, N.S.A. Assessment of the Antimicrobial Activity of Olive Leaf Extract against Foodborne Bacterial Pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).