Potential Natural Antioxidant and Anti-Inflammatory Properties of Carthamus caeruleus L. Root Aqueous Extract: An In Vitro Evaluation
Abstract
1. Introduction
2. Materiel and Methods
2.1. Chemicals and Instruments
2.2. Plant Collection
2.3. Aqueous Extraction
2.4. Phytochemical Studies
2.4.1. Total Phenolic Content
2.4.2. Total Flavonoid Content
2.4.3. Condensed Tannins
2.4.4. RP-HPLC Analysis
2.5. Antioxidant Activity
2.5.1. Total Antioxidant Capacity (TAC)
2.5.2. Ferric Reducing Antioxidant Power (FRAP)
2.5.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Test
2.5.4. Inhibition of Lipid Peroxidation: Thiobarbituric Acid (TBA) Method
2.5.5. Ferrous Ion-Chelating Activity
2.5.6. Hydroxyl Radical Scavenging Assay
2.5.7. Hydrogen Peroxide (H2O2) Decomposition
2.6. Erythrocyte Suspension Preparation
2.7. Analysis of the Extract’s Toxicity
2.8. Anti-Inflammatory Activity
2.8.1. Protection Against Hypotonic Stress-Induced Hemolysis
2.8.2. Protection Against Heat Stress-Induced Hemolysis
2.8.3. Protection Against Oxidative Stress-Induced Hemolysis
2.8.4. Inhibition of Ovalbumin Denaturation
2.8.5. Assessment of Lipid Peroxidation in Animal Tissues Using the TBARS Method (Thiobarbituric Acid Reactive Substance)
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. RP-HPLC Profile
3.3. Antioxidant Activity
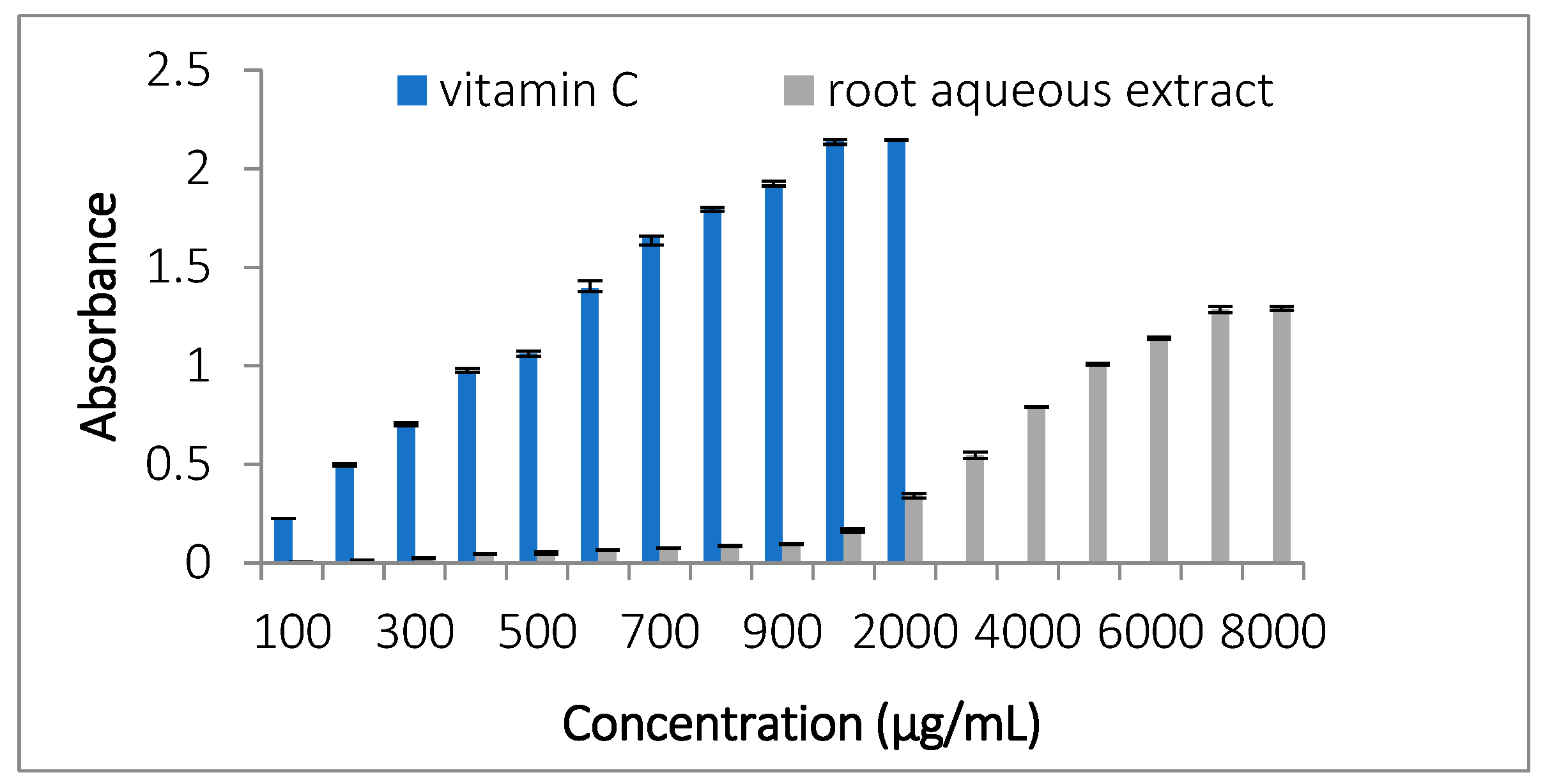
3.3.1. Total Antioxidant Capacity (TAC)
3.3.2. Ferric Reducing Antioxidant Power (FRAP)
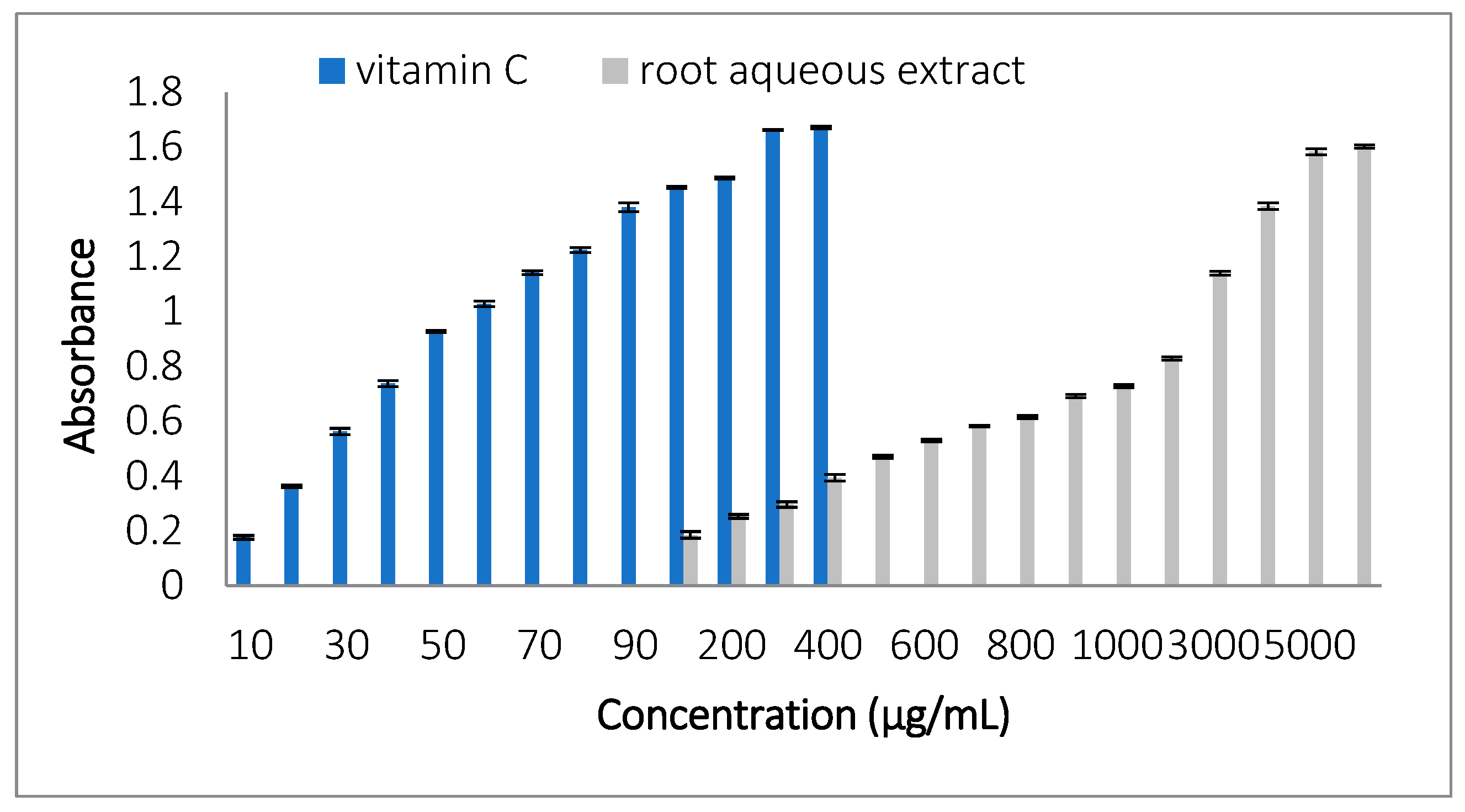
3.3.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Test
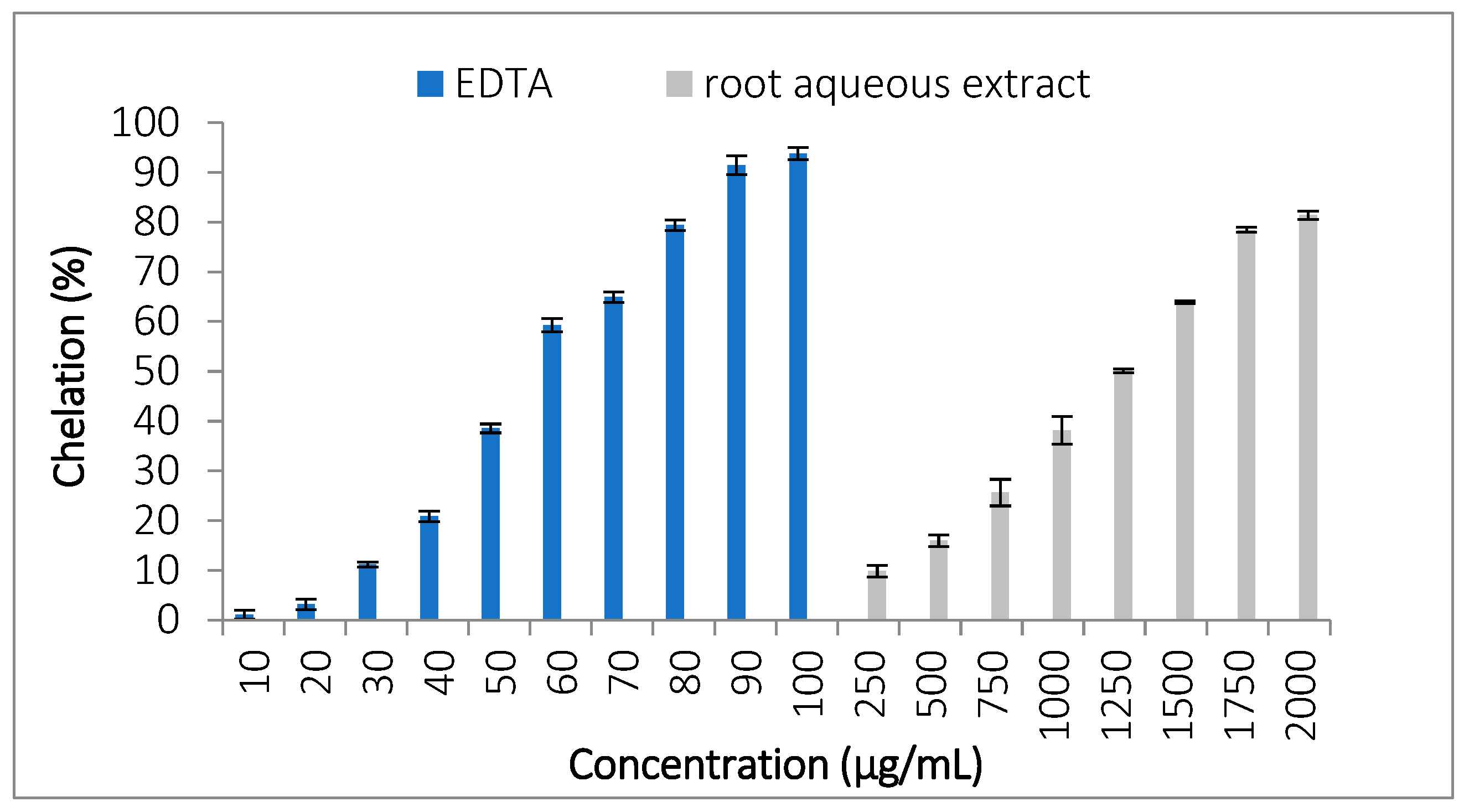
3.3.4. Ferrous Ion Chelating Activity
3.3.5. Antioxidant Activity in the Linoleic Acid System: Thiobarbituric Acid (TBA) Method
3.3.6. Hydroxyl Radical Scavenging Assay
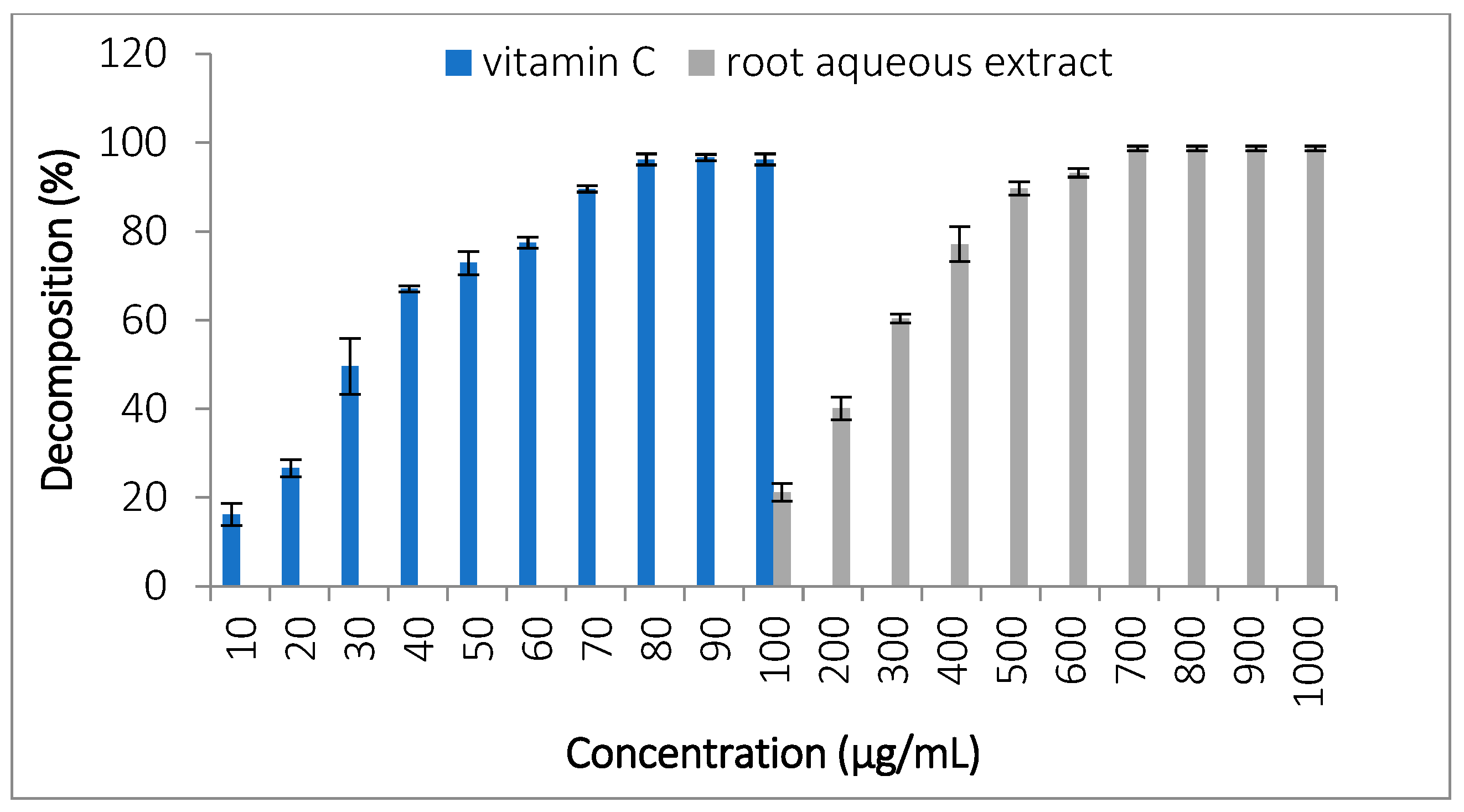
3.3.7. Hydrogen Peroxide (H2O2) Decomposition
3.4. Analysis of the Extract’s Toxicity
3.5. Anti-Inflammatory Activity
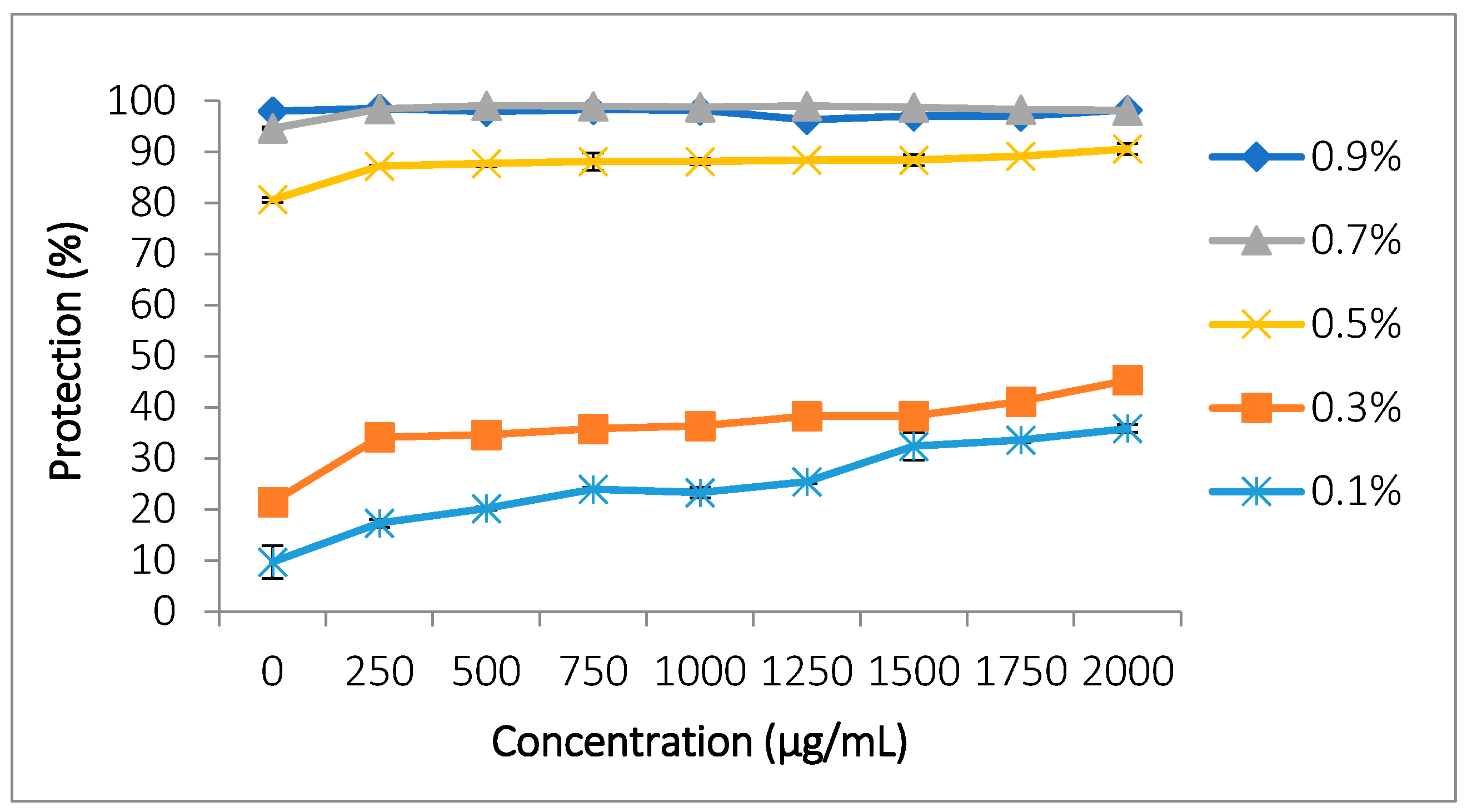
3.5.1. Protection Against Hemolysis Induced by Hypotonic Stress
3.5.2. Protection Against Heat-Induced Hemolysis
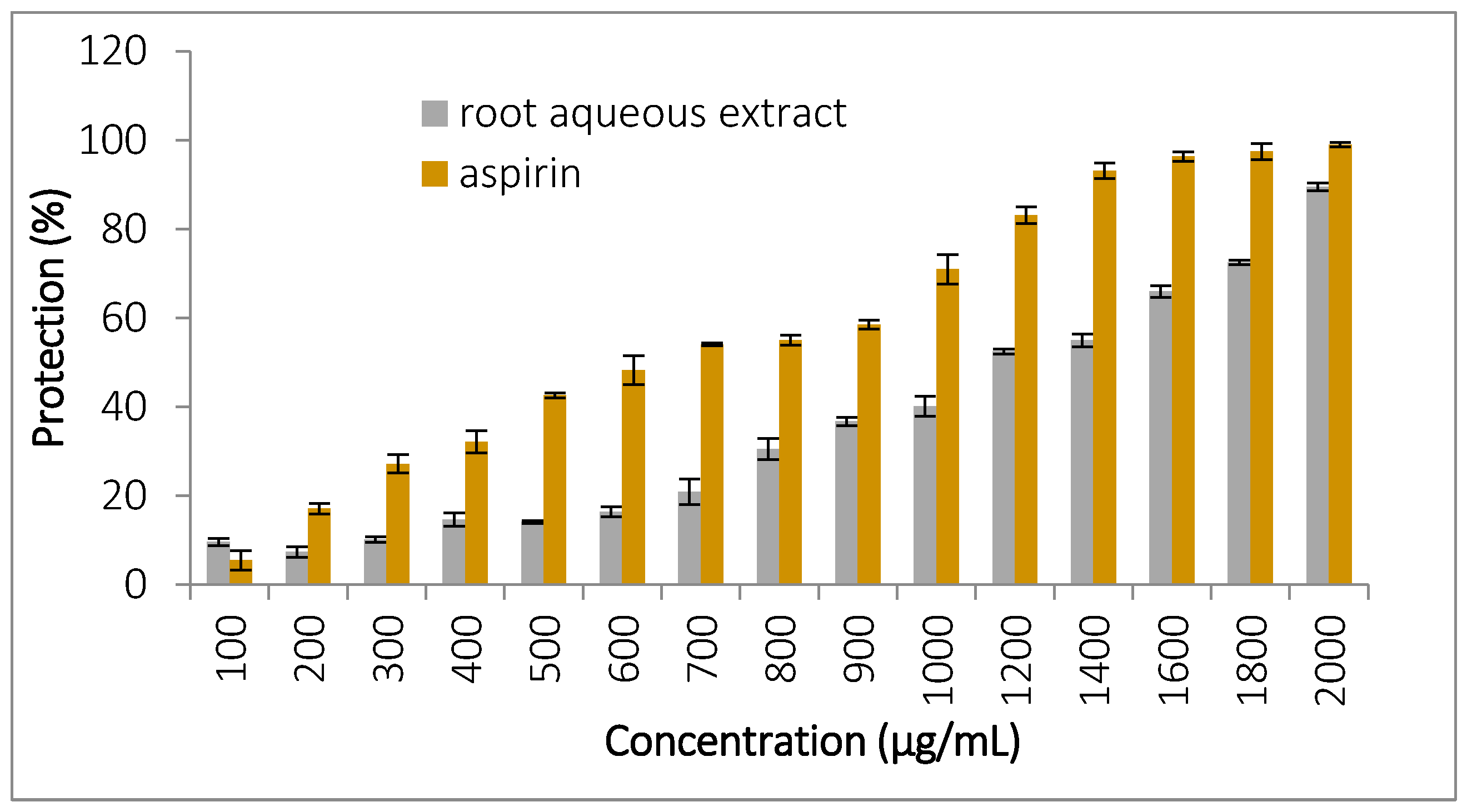
3.5.3. Protection Against Oxidative Stress-Induced Hemolysis
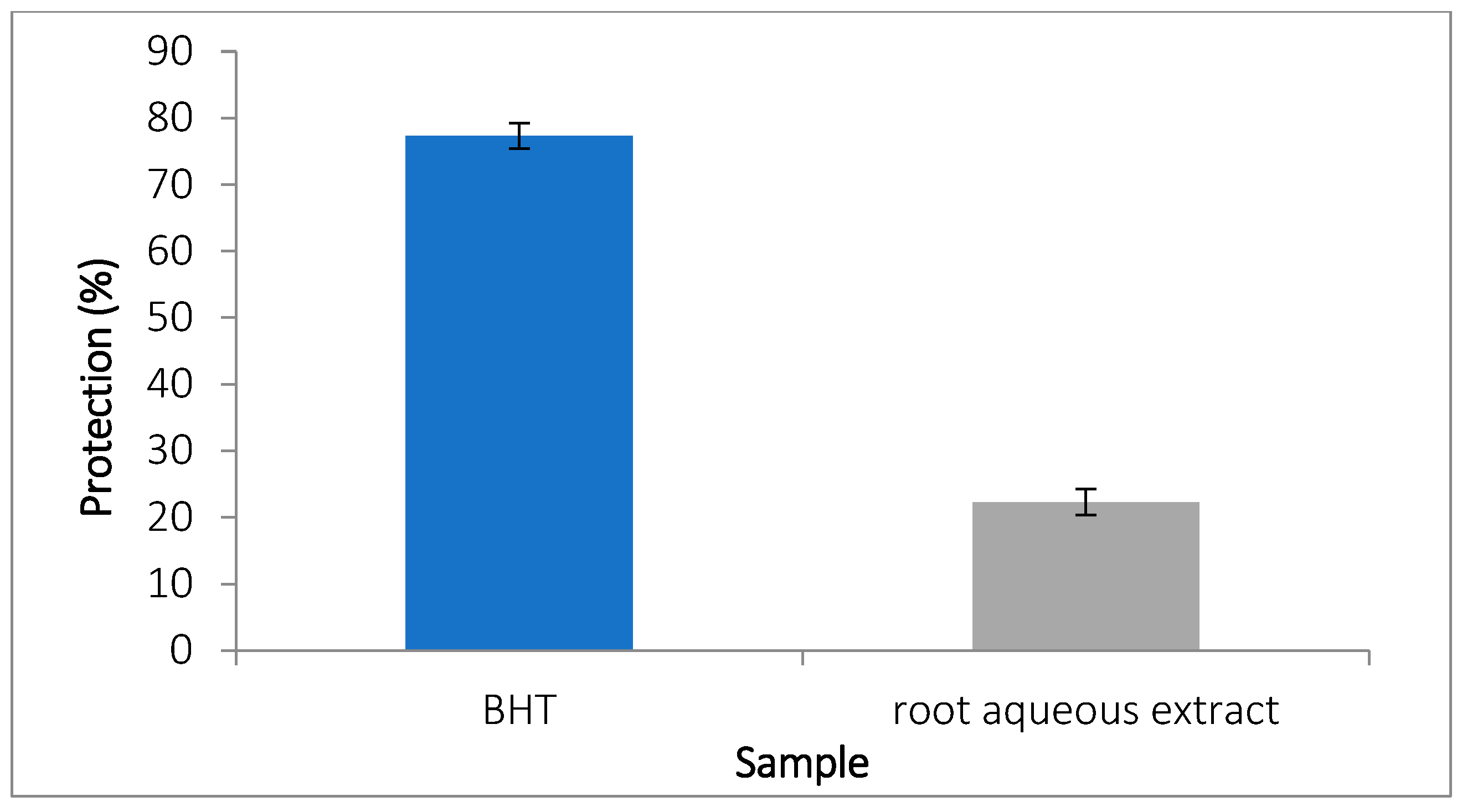
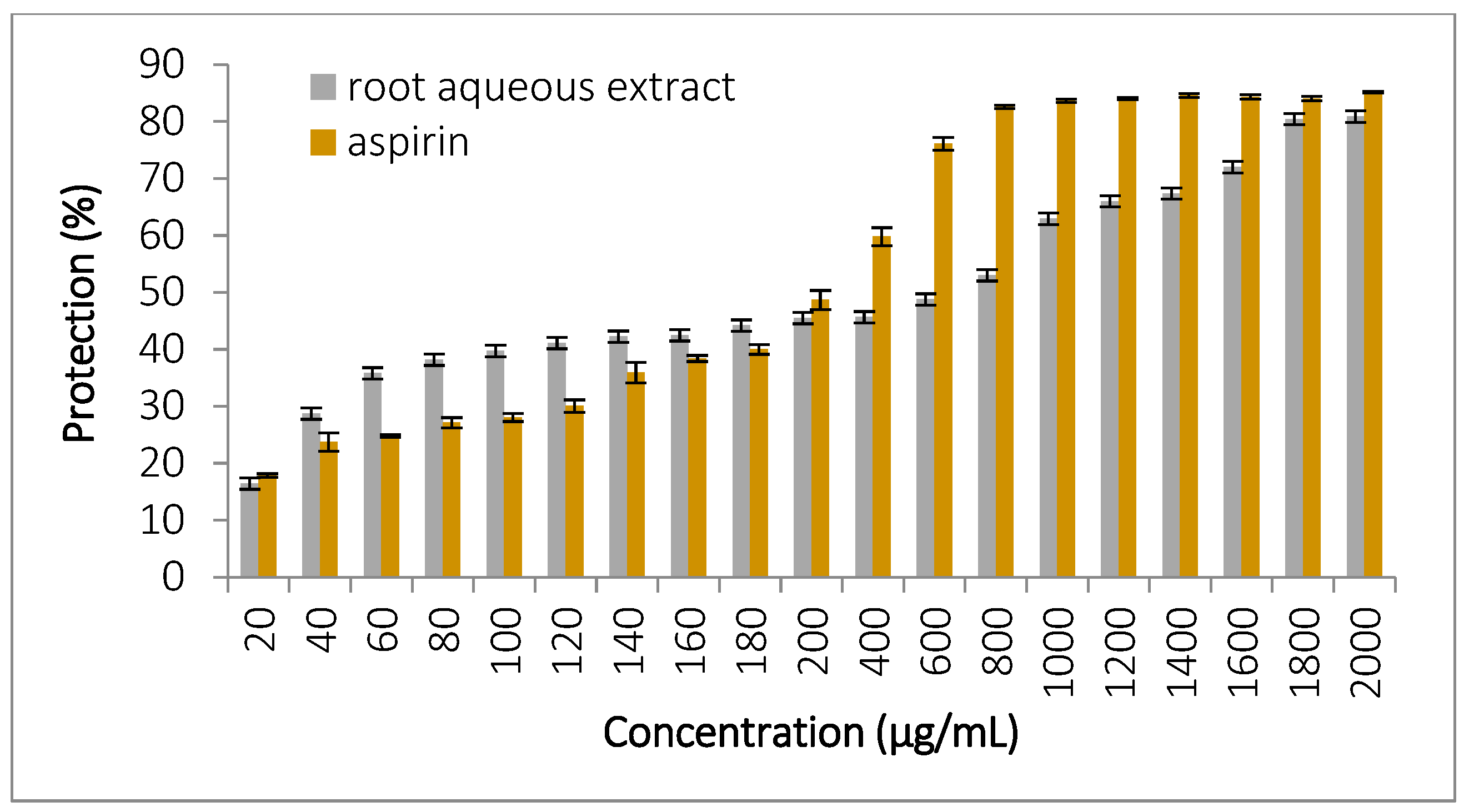
3.5.4. Inhibition of Ovalbumin Denaturation
3.5.5. Assessment of Lipid Peroxidation in Animal Tissues Using the TBARS Method
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- GBD. Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017; University of Leicester: Leicester, UK, 2017. [Google Scholar]
- Abegunde, D.O.; Mathers, C.D.; Adam, T.; Ortegon, M.; Strong, K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 2007, 370, 1929–1938. [Google Scholar] [CrossRef]
- Lucero-Prisno, D.E., III; Shomuyiwa, D.O.; Kouwenhoven, M.B.N.; Dorji, T.; Odey, G.O.; Miranda, A.V.; Ogunkola, I.O.; Adebisi, Y.A.; Huang, J.; Xu, L.; et al. Top 10 public health challenges to track in 2023: Shifting focus beyond a global pandemic. Public Health Chall. 2023, 2, e86. [Google Scholar] [CrossRef]
- Alkhateeb, H.H. Evaluation of antidiabetic, antioxidant, and antilipidemic potential of natural dietary product prepared from Cyphostemma digitatum in rats’ model of diabetes. J. Herbmed Pharmacol. 2022, 11, 197–203. [Google Scholar] [CrossRef]
- Jasemi, S.V.; Khazaei, H.; Fakhri, S.; Mohammadi-Noori, E.; Farzaei, M.H. Naringenin improves ovalbumin-induced allergic asthma in rats through antioxidant and anti-inflammatory effects. Based Complement. Altern. Med. 2022, 1, 9110798. [Google Scholar] [CrossRef] [PubMed]
- Migdal, C.; Serres, M. Espèces réactives de l’oxygène et stress oxydant. Médecine/Sci. 2011, 27, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Mobashar, A.; Shabbir, A.; Shahzad, M.; Gobe, G. Preclinical Rodent Models of Arthritis and Acute Inflammation Indicate Immunomodulatory and Anti-Inflammatory Properties of Juglans Regia Extracts. Evid. Based Complement. Altern. Med. 2022, 2022, 1695701. [Google Scholar] [CrossRef] [PubMed]
- Kizil, G.; Kizil, M.; Ceken, B.; Yavuz, M.; Demir, H. Protective ability of ethanol extracts of Hypericum scabrum L. and Hypericum Retusum Aucher Against Protein Oxidation DNA Damage. Int. J. Food Prop. 2011, 14, 926–940. [Google Scholar] [CrossRef]
- Asadi, M. Antioxidant and antimicrobial activities in the different extracts of Caspian saffron, Crocus caspius Fisch & CA Mey. Ex Hohen. Casp. J. Environ. Sci. 2016, 14, 331–338. [Google Scholar]
- Toubane, A.; Rezzoug, S.A.; Besombes, C.; Daoud, K. Optimization of Accelerated Solvent Extraction of Cart. Caeruleus L. Eval. Antioxid. Anti-Inflamm. Act. Extr. Ind. Crops Prod. 2017, 97, 620–631. [Google Scholar]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecule 2022, 27, 2494. [Google Scholar] [CrossRef]
- Gorniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A. The pharmacological potential of hesperidin. Indian J. Biochem. Biophys. (IJBB) 2019, 56, 287–300. [Google Scholar]
- Strudwick, X.L.; Cowin, A.J. The Role of the Inflammatory Response in Burn Injury. In Hot Topics in Burn Injuries; InTech: London, UK, 2018. [Google Scholar]
- Sahib, A.S.; Al-Jawad, F.H.; Al-Kaisy, A.A. Burns, endothelial dysfunction, and oxidative stress: The role of antioxidants. Ann. Burn. Fire Disasters 2009, 22, 6. [Google Scholar]
- Khémiri, I.; Essghaier, B.; Sadfi-Zouaoui, N.; Bitri, L. Antioxidant and antimicrobial potentials of seed oil from Carthamus tinctorius L. in the management of skin injuries. Oxidative Med. Cell. Longev. 2020, 2020, 4103418. [Google Scholar] [CrossRef]
- Khalil, H.E.; Al Ahmed, A. Phytochemical Analysis and Free Radical Scavenging Activity of Carthamus oxyacantha growing in Saudi Arabia: A Comparative Study. Int. J. Pharm. Sci. Rev. Res. 2017, 45, 51–55. [Google Scholar]
- Saffidine, K.; Sahli, F.; Mihoub, Z.M. Antimicrobial activity of an Algerian medicinal plant: Cart. Caeruleus L. Pharmacogn. Commun. 2013, 3, 71–76. [Google Scholar]
- Haddouchi, F.; Chaouche, T.M.; Halla, N. Phytochemical screening, antioxidant activities and hemolytic power of four Saharan plants from Algeria. Phytothérapie 2016, 16, 1–9. [Google Scholar]
- Akrout, A.; Gonzalez, L.A.; El Jani, H.; Madrid, P.C. Antioxidant and antitumor activities of Artemisia campestris and Thymelaea hirsuta from southern Tunisia. Food Chem. Toxicol. 2011, 49, 342–347. [Google Scholar] [CrossRef]
- Hmid, I.; Hanine, H.; Elothmani, D.; Oukabli, A. The physicochemical characteristics of Morrocan pomegranate and evaluation of the antioxidant activity for their juices. J. Saudi Soc. Agric. Sci. 2018, 17, 302–309. [Google Scholar]
- Jurišić Grubešić, R.; Srečnik, G.; Kremer, D.; Vuković Rodríguez, J.; Nikolić, T.; Vladimir-Knežević, S. Simultaneous RP-HPLC-DAD separation, and determination of flavonoids and phenolic acids in Plantago L. species. Chem. Biodivers. 2013, 10, 1305–1316. [Google Scholar] [CrossRef]
- Trinel, M.; Dubois, C.; Burger, P.; Plainfossé, H.; Azoulay, S.; Verger-Dubois, G.; Fernandez, X. Phytochemical Investigation of an Ostrya carpinifolia L. Extract: An Effective Anti-Pollution Cosmetic Active Ingredient. Chem. Biodivers. 2024, 22, e202402139. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.; Reddy, S.G.; Babu, P.P.; Reddy, A.R. The antioxidant and antiproliferative activities of methanolic extracts from Njavara rice bran. BMC Complement. Altern. Med. 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Moualek, I.; Benarab, K.; Belounis, Y.; Houali, K. Anti-Inflammatory and Antioxidant Activities of Cow’s Milk Supplemented with Aqueous Extract of Malva Sylvestris. Eurasia Proc. Health Environ. Life Sci. 2023, 11, 1–11. [Google Scholar] [CrossRef]
- Kızıl, G.; Kızıl, M.; Yavuz, M.; Emen, S.; Hakimoğlu, F. Antioxidant Activities of Ethanol Extracts of Hypericum triquetrifolium. and Hypericum Scabroides. Pharm. Biol. 2008, 46, 231–242. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Nabavi, S.M.; Hellio, C.; Alinezhad, H.; Zare, M.; Azimi, R.; Baharfar, R. Antioxidant and antihemolytic activities of methanol extract of Hyssopus angustifolius. J. Appl. Bot. Food Qual. 2012, 85, 198–201. [Google Scholar]
- Rajamanikandan, S.; Sindhu, T.; Durgapriya, D.; Sophia, D.; Ragavendran, P.; Gopalakrishnan, V.K. Radical scavenging and antioxidant activity of ethanolic extract of Mollugo nudicaulis by in vitro assays. Indian J. Pharm. Educ. Res. 2011, 45, 310–316. [Google Scholar]
- Serteser, A.; Kargıoğlu, M.; Gök, V.; Bağci, Y.; Özcan, M.M.; Arslan, D. Antioxidant properties of some plants growing wild in Turkey. Grasas Y Aceites 2009, 60, 147–154. [Google Scholar] [CrossRef]
- Henneh, I.T.; Ameyaw, E.O.; Biney, R.P.; Armah, F.A.; Obese, E.; Konjah, D.; Eric, T.O. Ziziphus abyssinica hydro-ethanolic root bark extract attenuates acute inflammation, possibly through membrane stabilization and inhibition of protein denaturation and neutrophil degranulation. Front. Pharmacol. 2018, 29, 81–94. [Google Scholar]
- Suwalsky, M.; Villena, F.; Gallardo, M.J. In vitro protective effects of resveratrol against oxidative damage in human erythrocytes. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.A.; Zaman, S.; Juhara, F.; Akter, L.; Tareq, S.M.; Masum, E.H.; Bhattacharjee, R. Evaluation of antinociceptive, In-vivo & In-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complement. Altern. Med. 2014, 14, 1–12. [Google Scholar]
- Sakat, S.; Juvekar, A.R.; Gambhire, M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int. J. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar]
- Anoopkumar-Dukie, S.; Walker, R.B.; Daya, S. A sensitive and reliable method for the detection of lipid peroxidation in biological tissues. J. Pharm. Pharmacol. 2001, 53, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure-activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef]
- Heng, Y.Z.; Zhou, Y.; Guo, R.; Fu, Z.M.; Chen, D.F. Structure-antioxidant activity relationship of ferulic acid derivatives: Effect of ester groups at the end of the carbon side chain. Lwt 2020, 120, 108932. [Google Scholar]
- Sompong, W.; Cheng, H.; Adisakwattana, S. Protective effects of ferulic acid on high glucose-induced protein glycation, lipid peroxidation, and membrane ion pump activity in human erythrocytes. PLoS ONE 2015, 10, e0129495. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy-and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Martinka Maksymiak, M.; Zięba, M.; Orchel, A.; Musiał-Kulik, M.; Kowalczuk, M.; Adamus, G. Bioactive (co) oligoesters as potential delivery systems of p-anisic acid for cosmetic purposes. Materials 2020, 13, 41–53. [Google Scholar] [CrossRef]
- Boz, H. p-Coumaric acid in cereals: Presence, antioxidant, and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Kesari, K.K. Scavenging properties of plant-derived natural biomolecule para-coumaric acid in the prevention of oxidative stress-induced diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials-A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M.; Wianowska, D. Antioxidant Properties of Selected Flavonoids in Binary Mixtures-Considerations on Myricetin, Kaempferol and Quercetin. Int. J. Mol. Sci. 2023, 24, 10070. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; Islam, S. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef] [PubMed]
- Borges-Bubols, G.; da Rocha Vianna, D.; Medina-Remon, A.; Von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Morabito, R. Antioxidant activity of quercetin in an H2O2-induced oxidative stress model in red blood cells: Functional role of Band 3 protein. Int. J. Mol. Sci. 2022, 23, 10991. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, H.R.; Lee, K.A. comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential anti-inflammatory effects of hesperidin from the genus citrus. Curr. Med. Chem. 2018, 25, 4929–4945. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Lee, S.H.; Lee, K.A. A comparative study of hesperetin, hesperidin, and hesperidin glucoside: Antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Wang, S.; Yuan, D.; Gong, Y.; Wang, S. Attenuation of oxidative stress of erythrocytes by plant-derived flavonoids, orientin, and luteolin. Evid. Based Complement. Altern. Med. 2016, 2016, 3401269. [Google Scholar] [CrossRef] [PubMed]
- Praveena, R.; Sadasivam, K.; Deepha, V.; Sivakumar, R. Antioxidant potential of orientin: A combined experimental and DFT approach. J. Mol. Struct. 2014, 1061, 114–123. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef]
- Milekn, M.; Młodecki, Ł.; Dżugan, M. Caffeine content and antioxidant activity of various brews of specialty grade coffee. Acta Sci. Pol. Technol. Aliment. 2021, 20, 179–188. [Google Scholar]
- Vieira, A.J.; Gaspar, E.M.; Santos, P.M. Mechanisms of potential antioxidant activity of caffeine. Radiat. Phys. Chem. 2020, 174, 108968. [Google Scholar] [CrossRef]
- Hennebelle, T.; Sahpaz, S.; Bailleul, F. Polyphénols végétaux, sources, utilisations et potentiel dans la lutte contre le stress oxydatif. Phytothérapie 2004, 2, 3–6. [Google Scholar] [CrossRef]
- Bountagkidou, O.G.; Ordoudi, S.A.; Tsimidou, M.Z. Structure–antioxidant activity relationship study of natural hydroxybenzaldehydes using in vitro assays. Food Res. Int. 2010, 43, 2014–2019. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Resorcinols, flavonoids, and stilbene phenols: Redox, radicals, and physiological effects. In Systems Biology of Free Radicals and Antioxidants; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Rahen, M.; De Tran, Q.; Negar, S.S.; Khadija, A.P.; Pranoy, S.; Sukumar, B.; Shamima, A. In vitro anti-inflammatory resorcinol derivatives and their in silico analysis. Can Tho Univ. J. Sci. 2020, 12, 80–84. [Google Scholar]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta (BBA) Gen. Subj. 2011, 1810, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Costantini, E.; Sinjari, B.; Falasca, K.; Reale, M.; Caputi, S.; Jagarlapodii, S.; Murmura, G. Assessment of the vanillin anti-inflammatory and regenerative potentials in inflamed primary human gingival fibroblast. Mediat. Inflamm. 2021, 2021, 5562340. [Google Scholar] [CrossRef]
- Baghiani, A.; Boumerfeg, S.; Belkhiri, F.; Khennouf, S.; Charef, N.; Harzallah, D.; Arrar, L.; Mosaad Attia, A.W. Antioxidant and radical scavenging properties of Carthamus caeruleus L extracts grow wild in Algeria flora. Comun. Sci. 2009, 1, 128–136. [Google Scholar]
- Ouda, A.N.; Fatiha, M.; Sadia, M.; Zohra, S.F.; Noureddine, D. In vivo Anti-inflammatory Activity of Aqueous Extract of Carthamus caeruleus L Rhizome Against Carrageenan-Induced Inflammation in Mice. Jordan J. Biol. Sci. 2021, 14, 529–535. [Google Scholar]
- Ali-Rachedi, F.; Meraghni, S.; Touaibia, N.; Mesbah, S. Analyse quantitative des composés phénoliques d’une endémique algérienne Scabiosa atropurpurea sub. Maritima L. Bull. La Société R. Des Sci. Liège 2018, 87, 13–21. [Google Scholar] [CrossRef]
- Fuad, F.M.; Nadzir, M.M.; Harun, A. Hydrophilic natural deep eutectic solvent: A review on physicochemical properties and extractability of bioactive compounds. J. Mol. Liq. 2021, 339, 116923. [Google Scholar] [CrossRef]
- Moussa, H.; Dahmoune, F.; Hentabli, M.; Remini, H.; Mouni, L. Optimization of ultrasound-assisted extraction of phenolic-saponin content from Carthamus caeruleus L. rhizome and predictive model based on support vector regression optimized by dragonfly algorithm. Chemom. Intell. Lab. Syst. 2022, 222, 104493. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar]
- Karagoz, A.; Artun, F.T.; Özcan, G.; Melikoğlu, G.; Anıl, S.; Kültür, Ş.; Sütlüpınar, N. In vitro evaluation of antioxidant activity of some plant methanol extracts. Biotechnol. Biotechnol. Equip. 2015, 29, 1184–1189. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Bidie, A.P.; N’guessan, B.B.; Yapo, A.F.; N’Guessan, J.D.; Djaman, A.J. Activités antioxydantes de dix plantes medicinales de la pharmacopée ivoirienne. Sci. Nat. 2011, 8, 1–12. [Google Scholar]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Habibou, H.H.; Idrissa, M.; Ikhiri Khalid, P.; Benjamin, O. Activité Antioxydante des Extraits Méthanoliques de Differents Organes de Detarium microcarpum Guill. & Perr. Eur. Sci. J. 2019, 15, 1857–7881. [Google Scholar]
- Hidalgo, M.; Sánchez-Moreno, C.; De Pascual-Teresa, S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Bouzid, W.; Yahia, M.; Abdeddaim, M.; Aberkane, M.C.; Ayachi, A. Évaluation de l’activité antioxydante et antimicrobienne des extraits de l’aubépine monogyne. Leban. Sci. J. 2011, 12, 59–69. [Google Scholar]
- Akar, Z.; Küçük, M.; Doğan, H. A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzym. Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M. Determination of antioxidant activity of caffeic acid and p-coumaric acid by using electrochemical and spectrophotometric assays. Int. J. Electrochem. Sci. 2016, 11, 10644–10658. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Sharma, K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease, and xanthine oxidase inhibitors. Food Chem. 2017, 235, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ghedadba, N.; Hambaba, L.; Ayachi, A.; Aberkane, M.C.; Bousselsela, H.; Oueld-Mokhtar, S.M. Polyphenols content, antioxidant and antimicrobial activities of leaf extracts of Marrubium deserti de Noé ex Coss. Phytothérapie 2015, 13, 118–129. [Google Scholar] [CrossRef]
- Ozyurek, M.; Bektaşoğlu, B.; Güçlü, K.; Güngör, N.; Apak, R. A novel hydrogen peroxide scavenging assay of phenolics and flavonoids using cupric reducing antioxidant capacity (CUPRAC) methodology. J. Food Compos. Anal. 2010, 23, 689–698. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M. Antioxidant compounds, assays of determination, and mode of action. Afr. J. Pharm. Pharmacol. 2013, 7, 528–539. [Google Scholar] [CrossRef]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulski, M. Synthesis and antioxidant activity of caffeic acid derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef]
- Kumari, C.S.; Yasmin, N.; Hussain, M.R.; Babuselvam, M. Invitro anti-inflammatory and anti-arthritic property of Rhizopora mucronata leaves. Int. J. Pharma Sci. Res. 2015, 6, 482–485. [Google Scholar]
- Freitas, M.V.; Rita de Cássia, M.N.; Da Costa Huss, J.C.; De Souza, T.M.T.; Costa, J.O.; Firmino, C.B.; Penha-Silva, N. Influence of aqueous crude extracts of medicinal plants on the osmotic stability of human erythrocytes. Toxicol. Vitr. 2008, 22, 219–224. [Google Scholar] [CrossRef]
- Ansari, P.; Shofiul, A.J.; Sumonto, S.; Kallol, K.M.; Tasnim, T.; Sanjeeda, S.B. Potential investigation of anti-inflammatory and anti-oxidative properties of ethanolic extract of Ixora nigricans leaves. IJPR 2015, 5, 104–111. [Google Scholar]
- Louerrad, Y.; Haddi, R.; Kaid, H.M. Etude de la peroxydation lipidique chez une plante médicinale Haloxylon scoparium Pomel. J. Biores. Val. 2016, 1, 28–33. [Google Scholar]
- Padmanabhan, P.; Jangle, S.N. Evaluation of in-vitro anti-inflammatory activity of herbal preparation, a combination of four medicinal plants. Int. J. Basic Appl. Med. Sci. 2012, 2, 109–116. [Google Scholar]
- AlSaffar, R.M.; Rashid, S.; Ahmad, S.B.; Rehman, M.U.; Hussain, I.; Parvaiz Ahmad, S.; Ganaie, M.A. D-limonene (5 (one-methyl-four-[1-methylethenyl]) cyclohexane) diminishes CCl4-induced cardiac toxicity by alleviating oxidative stress, inflammatory and cardiac markers. Redox Rep. 2022, 27, 92–99. [Google Scholar] [CrossRef]
- Orlov, S.N.; Aksentsev, S.L.; Kotelevtsev, S.V. Extracellular calcium is required for the maintenance of plasma membrane integrity in nucleated cells. Cell Calcium 2005, 38, 53–57. [Google Scholar] [CrossRef]
- Iba, T.; Sawada, T.; Kondo, Y.; Kondo, K.; Levy, J.H. Morphological changes in blood cells in a rat model of heatstroke: A pilot study. J. Clin. Med. 2022, 11, 4821. [Google Scholar] [CrossRef]
- Kampinga, H.H. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int. J. Hyperth. 2006, 22, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.F.; Campos, M.L.; Irioda, A.C.; Stremel, D.P.; Trindade, A.C.L.B.; Pontarolo, R. Anti-inflammatory effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens is related to inhibition of prostanoid production. Molecules 2017, 22, 1883. [Google Scholar] [CrossRef] [PubMed]
- Meddour, R.; Meddour-Sahar, O. Medicinal plants and their traditional uses in Kabylia (Tizi Ouzou, Algeria). Arab. J. Med. Aromat. Plants 2015, 1, 137–151. [Google Scholar]
- Meddour, R.; Sahar, O.; Abdoune, N.; Dermouche, M. Quantitative ethnobotanical investigation of medicinal plants used by local population in the rural municipalities of Haizer and El Asnam, province of Bouira, Northern Algeria. Mediterr. Bot. 2022, 43, e71190. [Google Scholar] [CrossRef]
- Suwalsky, M.; Orellana, P.; Avello, M.; Villena, F. Protective effect of Ugni molinae Turcz against oxidative damage of human erythrocytes. Food Chem. Toxicol. 2007, 45, 130–135. [Google Scholar] [CrossRef]
- Balcao, V.M.; Vila, M.M. Structural and functional stabilization of protein entities: State-of-the-art. Adv. Drug Deliv. Rev. 2015, 93, 25–41. [Google Scholar] [CrossRef]
- Saso, L.; Valentini, G.; Casini, M.L.; Grippa, E.; Gatto, M.T.; Leone, M.G.; Silvestrini, B. Inhibition of heat-induced denaturation of albumin by nonsteroidal antiinflammatory drugs (NSAIDs): Pharmacological implications. Arch. Pharmacal Res. 2001, 24, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Fetni, S.; Bertella, N. Composés bioactifs: Etude in vitro des propriétés anti-inflammatoires de l’extrait méthanolique des fruits de Rosa canina L. (rosacees). Nutr. Santé 2020, 9, 117–125. [Google Scholar] [CrossRef]
- Lavialle, M.; Layé, S. Acides gras poly-insaturés (omega 3, omega 6) et fonctionnement du système nerveux central. Innov. Agron. 2010, 10, 25–42. [Google Scholar]
- Pazzini, C.E.F.; Colpo, A.C.; Poetini, M.R.; Pires, C.F.; de Camargo, V.B.; Mendez, A.S.L.; Folmer, V. Effects of red wine tannat on oxidative stress induced by glucose and fructose in erythrocytes in vitro. Int. J. Med. Sci. 2015, 12, 478–486. [Google Scholar] [CrossRef]
- Dahmani, M.M.; Laoufi, R.; Selama, O.; Arab, K. Gas chromatography coupled to mass spectrometry characterization, anti-inflammatory effect, wound-healing potential, and hair growth-promoting activity of Algerian Carthamus caeruleus L. (Asteraceae). Indian J. Pharmacol. 2018, 50, 123–129. [Google Scholar] [CrossRef]





| Class | Components | References for Classification | Retention Time (min) | Area (%) | Biological Activity/ References | |
|---|---|---|---|---|---|---|
| Phenolic acids | 2,3-dimethyl cinnamic acid | [37] | 14.760 | 1.5895 | ND | |
| 3,4,5-Trimethoxy benzoic acid | [37] | 10.908 | 2.1882 | ND | ||
| Dihydroxycinnamic acid | [37] | 7.256 | 2.1875 | ND | ||
| Ferulic acid | [37] | 9.250 | 2.8991 | Antioxidant [38] | ||
| Anti-inflammatory [39] | ||||||
| Isovanillic acid | [40] | 7.545 | 4.1639 | Antioxidant [40] | ||
| m-Anisic acid | [41] | 12.169 | 0.9167 | ND | ||
| p-Coumaric acid | [42] | 9.616 | 2.0014 | Antioxidant [42] | ||
| Anti-inflammatory [43] | ||||||
| Rosmarinic acid | [44] | 10.196 | 3.0362 | Antioxidant [44] | ||
| Anti-inflammatory [45] | ||||||
| Tannic acid | [46] | 3.538 | 2.4771 | Antioxidant [47] | ||
| Anti-inflammatory [47] | ||||||
| Flavonoids | Flavonols | Myricetin | [48] | 11.255 | 1.2076 | Antioxidant [48] |
| Anti-inflammatory [49] | ||||||
| Quercetin | [48] | 12.932 | 1.6805 | Antioxidant [50] | ||
| Anti-inflammatory [51] | ||||||
| Rutin | [52] | 8.832 | 2.5702 | Antioxidant [52] | ||
| Anti-inflammatory [52] | ||||||
| Flavanones | Hesperidin | [53] | 15.579 | 0.4340 | Antioxidant [54] | |
| Anti-inflammatory [54] | ||||||
| Flavones | Luteolin | [55] | 12.658 | 1.2398 | Antioxidant [55] | |
| Anti-inflammatory [56] | ||||||
| Orientin | [57] | 8.260 | 4.9447 | Antioxidant [57] | ||
| Anti-inflammatory [56] | ||||||
| Vitexin | [58] | 7.024 | 2.9931 | Antioxidant [58] | ||
| Anti-inflammatory [58] | ||||||
| Other | Caffeine (alkaloids) | [59] | 6.370 | 1.5039 | Antioxidant [60] | |
| Coumarin | [61] | 11.784 | 1.4114 | Antioxidant [50] | ||
| Anti-inflammatory [50] | ||||||
| Hydroxy-quinone (quinone) | [61] | 3.895 | 3.3714 | ND | ||
| P-hydroxybenzaldehyde | [62] | 7.892 | 2.7577 | ND | ||
| Resorcinol | [63] | 5.173 | 1.4332 | Antioxidant [63] | ||
| Anti-inflammatory [64] | ||||||
| Vanillin | ND | 8.415 | 4.5950 | Antioxidant [65] | ||
| Anti-inflammatory [66] | ||||||
| Tests | Concentration (µg/mL) | Maximal Protection (%) | ||
|---|---|---|---|---|
| Extract | Standard | |||
| Hypotonicity-induced hemolysis at different concentrations of NaCl | 0.7% | 2000 | 98.13 ± 0.15 a | 98.2 ± 0.13 a |
| 0.5% | 2000 | 90.59 ± 1.07 a | 98.2 ± 0.13 b | |
| 0.3% | 2000 | 45.3 ± 0.89 a | 98.2 ± 0.13 b | |
| 0.1% | 2000 | 35.8 ± 0.75 a | 98.2 ± 0.13 b | |
| Heat-induced hemolysis | 3000 | 70 ± 1.27 a | 78.53 ± 0.51 b | |
| HClO-induced hemolysis | 2000 | 89 ± 0.87 a | 99 ± 0.5 b | |
| Albumin denaturation | 2000 | 81.05 ± 2.2 a | 85.28 ± 0.11 a | |
| TBARS | 4000 | 69.25 ± 0.89 a | 77.2 ± 1.02 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belounis, Y.; Moualek, I.; Sebbane, H.; Ait Issad, H.; Saci, S.; Saoudi, B.; Nabti, E.-h.; Trabelsi, L.; Houali, K.; Cruz, C. Potential Natural Antioxidant and Anti-Inflammatory Properties of Carthamus caeruleus L. Root Aqueous Extract: An In Vitro Evaluation. Processes 2025, 13, 878. https://doi.org/10.3390/pr13030878
Belounis Y, Moualek I, Sebbane H, Ait Issad H, Saci S, Saoudi B, Nabti E-h, Trabelsi L, Houali K, Cruz C. Potential Natural Antioxidant and Anti-Inflammatory Properties of Carthamus caeruleus L. Root Aqueous Extract: An In Vitro Evaluation. Processes. 2025; 13(3):878. https://doi.org/10.3390/pr13030878
Chicago/Turabian StyleBelounis, Yousra, Idir Moualek, Hillal Sebbane, Hakima Ait Issad, Sarah Saci, Bilal Saoudi, El-hafid Nabti, Lamia Trabelsi, Karim Houali, and Cristina Cruz. 2025. "Potential Natural Antioxidant and Anti-Inflammatory Properties of Carthamus caeruleus L. Root Aqueous Extract: An In Vitro Evaluation" Processes 13, no. 3: 878. https://doi.org/10.3390/pr13030878
APA StyleBelounis, Y., Moualek, I., Sebbane, H., Ait Issad, H., Saci, S., Saoudi, B., Nabti, E.-h., Trabelsi, L., Houali, K., & Cruz, C. (2025). Potential Natural Antioxidant and Anti-Inflammatory Properties of Carthamus caeruleus L. Root Aqueous Extract: An In Vitro Evaluation. Processes, 13(3), 878. https://doi.org/10.3390/pr13030878







