Antioxidant and Antibacterial Activities and Phytochemical Analysis of Olea europaea subsp. laperrinei Leaves Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Procedure
2.3. Phytochemical Studies
2.3.1. Determination of Total Phenol Content
2.3.2. Determination of Total Flavonoid Content
2.3.3. Determination of Total Tannin Content
2.3.4. HPLC-UV Analysis
2.4. In Vitro Antioxidant Activities
2.4.1. Total Antioxidant Capacity
2.4.2. Ferric-Reducing Antioxidant Power Assay
2.4.3. DPPH Radical Scavenging Assay
- -
- Effective concentration (EC50), representing the antioxidant quantity required to reduce the initial DPPH radical concentration by half. A lower EC50 indicates stronger radical scavenging activity.
- -
- The antioxidant activity index (AAI) corresponds to the final concentration of DPPH in the control (μg/mL)/EC50 (μg/mL). The extracts have low antioxidant activity when AAI is <0.5, moderate antioxidant activity when AAI is between 0.5 and 1.0, high antioxidant activity when AAI is between 1.0 and 2.0, and very strong antioxidant activity when AAI is >2.0 [37].
2.4.4. β-Carotene Bleaching Assay
2.4.5. Hydrogen Peroxide Radical Scavenging Activity
2.5. Antibacterial Activity
2.5.1. Bacterial Strains
2.5.2. Disk Diffusion Method
2.6. Statistical Analysis
3. Results
3.1. Total Phenolic, Flavonoid, and Tannin Content
3.2. HPLC-UV Analysis
3.3. In Vitro Antioxidant Activities
3.3.1. Total Antioxidant Activity
3.3.2. Ferric-Reducing Antioxidant Power Assay
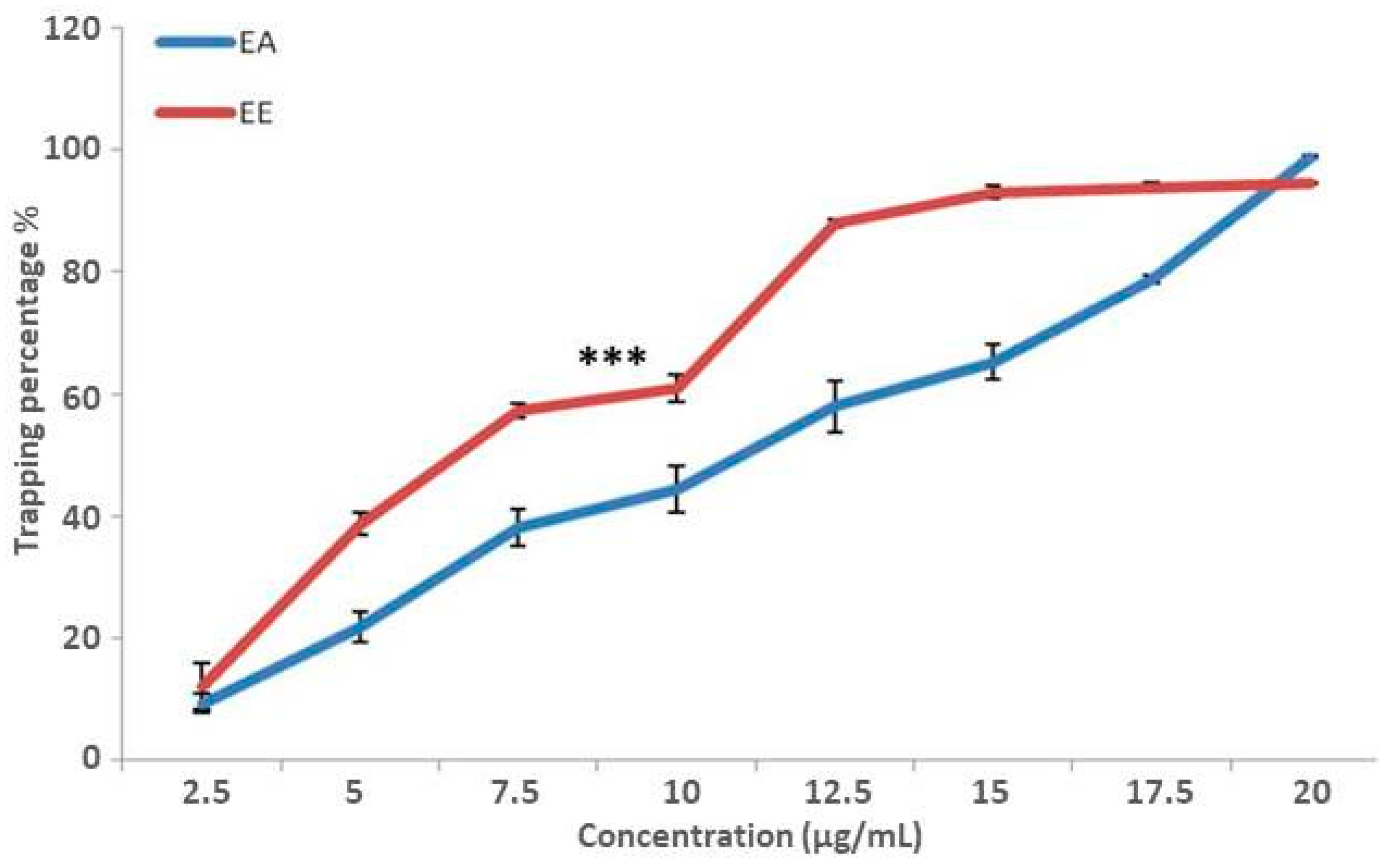
3.3.3. DPPH• Free Radical Scavenging Assay
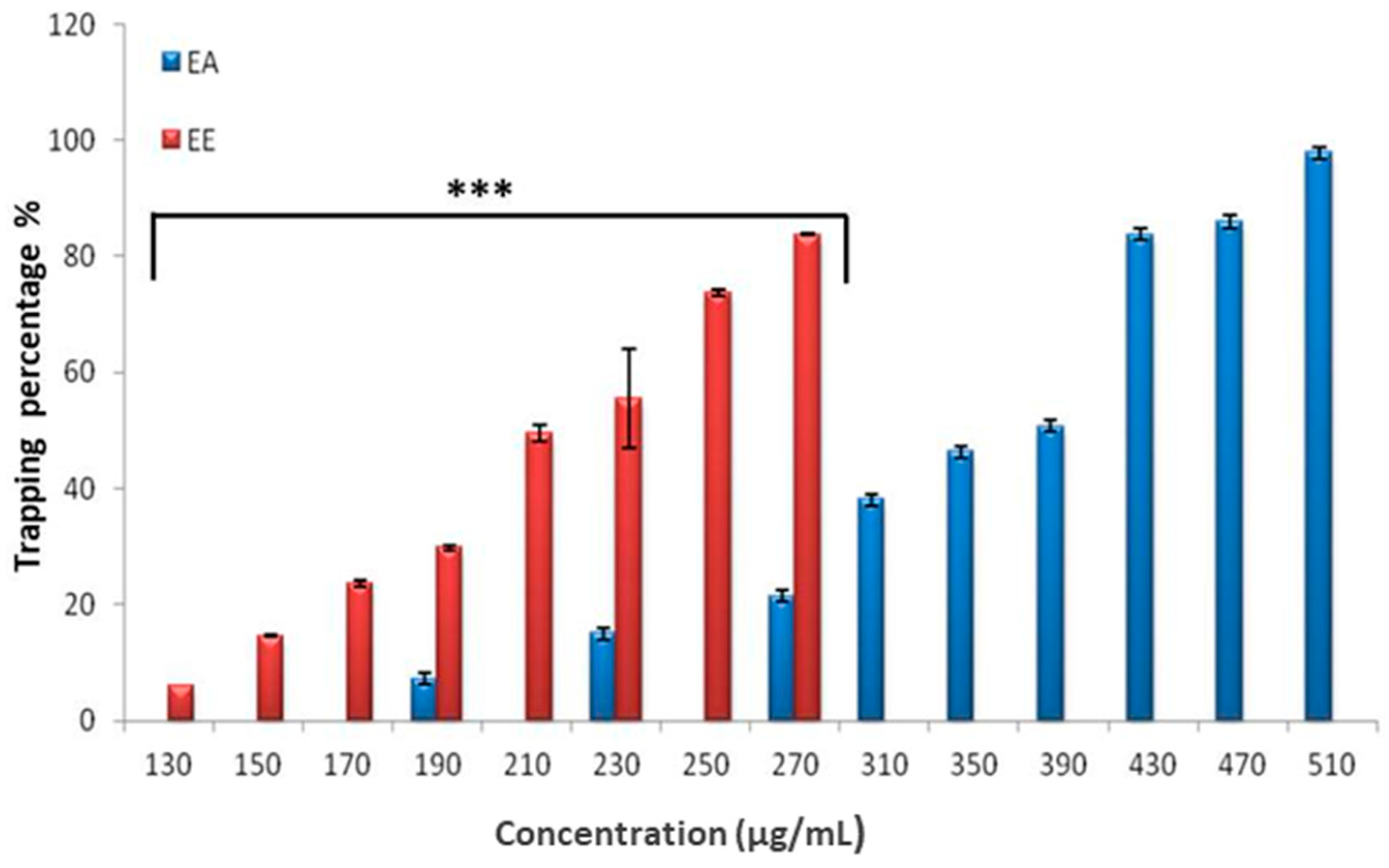
3.3.4. β-Carotene Bleaching Assay
3.3.5. Hydrogen Peroxide Radical Scavenging Activity
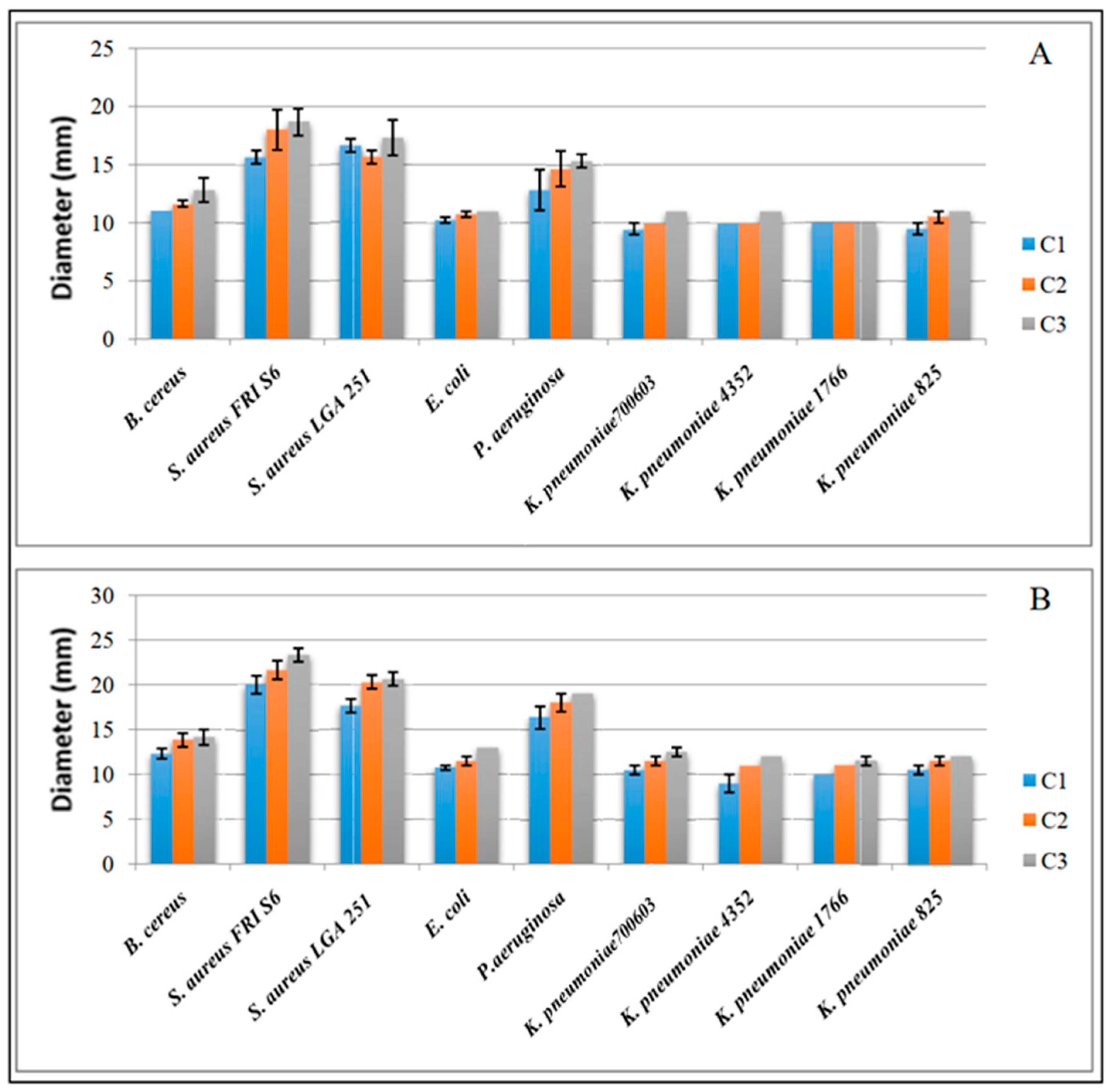
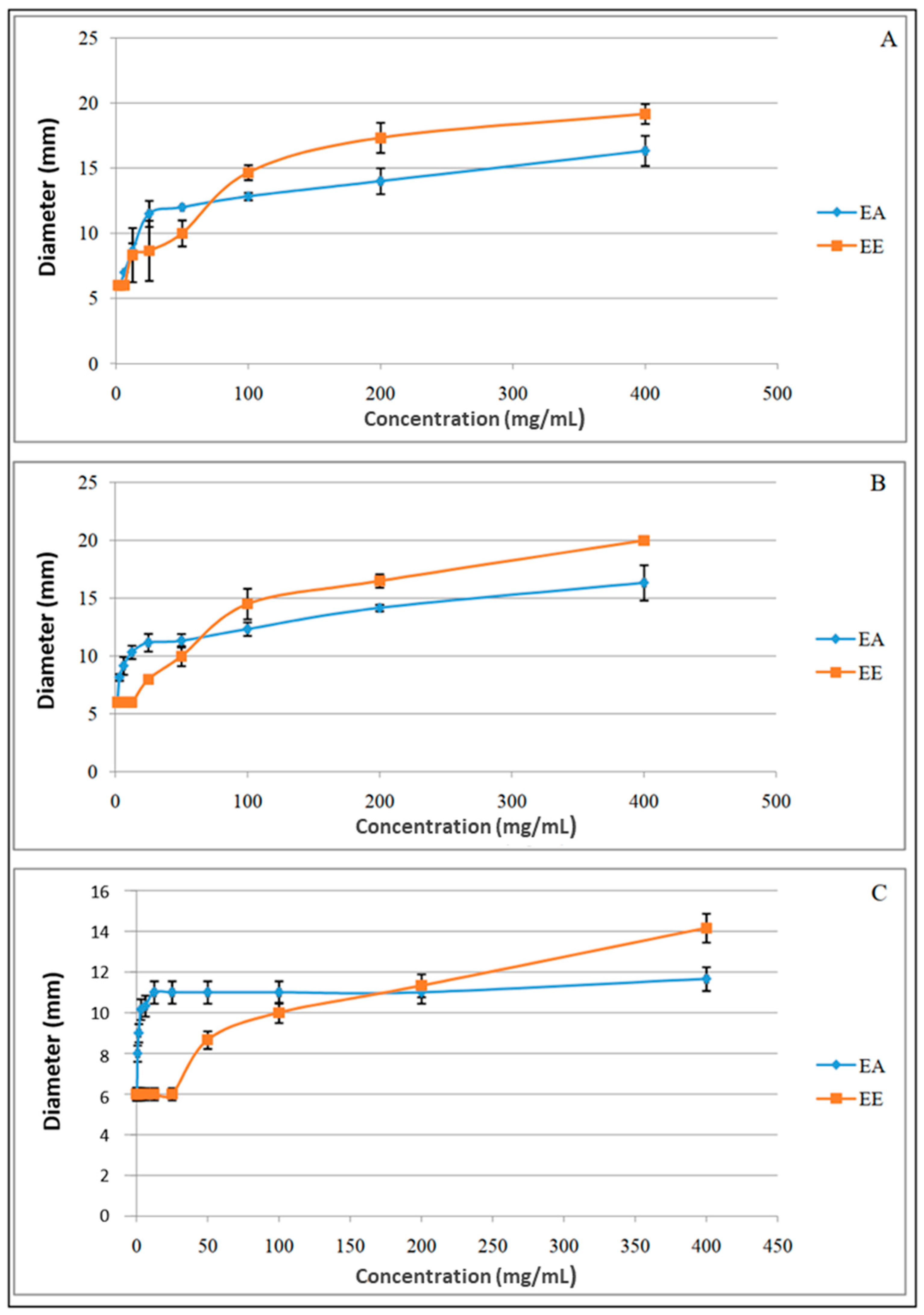
3.4. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, I.C.F.; Barros, L.; Soares, M.E.; Bastos, M.L.; Pereira, J.A. Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. Food Chem. 2006, 103, 188–195. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Dekanski, D.; Janićijević-Hudomal, S.; Tadić, V.; Marković, G.; Arsić, I.; Mitrović, D.M. Phytochemical analysis and gastroprotective activity of an olive leaf extract. Serbian J. Chem. Soc. 2009, 74, 367–377. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Lahcene, S.; Trabelsi, L.; Salem-Bekhit, M.M.; Rabea, S.; Refat, H.M.; Selim, M.; Alehaidib, S.M.; Sebbane, H.; Msela, A.; Benguerba, Y.; et al. Anti-leishmanial activities of Olea europaea subsp. laperrinei extracts. Cell. Mol. Biol. 2023, 69, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Maazoun, A.M.; Ben Hlel, T.; Haouel-Hamdi, S.; Belhadj, F.; Mediouni-Ben Jemâa, J.; Marzouki, M.N. Screening for insecticidal potential and acetylcholinesterase activity inhibition of Urginea maritima bulbs extract for the control of Sitophilus oryzae (L.). J. Asia Pac. Entomol. 2017, 20, 752–760. [Google Scholar] [CrossRef]
- Stefani, T.; Morales-San Claudio, P.D.C.; Rios, M.Y.; Aguilar-Guadarrama, A.B.; Gonzãlez-Maya, L.; Sãnchez-Carranza, J.N.; Gonzãlez-Ferrara, M.; Camacho-Corona, M.D.R. UPLC-QTOF-MS analysis of cytotoxic and antibacterial extracts of Hechtia glomerata Zucc. Nat. Prod. Res. 2020, 34, 644–648. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; El Kadri, H.; Gutierrez-Merino, J.; Wantock, T.; Harle, T.; Seggiani, M.; Danti, S.; Di Stefano, R.; Velliou, E. Combined antimicrobial effect of bio-waste olive leaf extract and remote cold atmospheric plasma effluent. Molecules 2021, 26, 1890. [Google Scholar] [CrossRef]
- Lahcene, S.; Taibi, F.; Mestar, N.; Ali Ahmed, S.; Boumendjel, M.; Ouafi, S.; Houali, K. Insecticidal effects of the Olea europaea subsp. laperrinei extracts on the flour pyralid Ephestia kuehniella. Cell. Mol. Biol. 2018, 64, 6–12. [Google Scholar]
- Mestar, N.G.; Boudiaf, M.N.; Lahcene, S.; Abbaci, H.; Aiche, G.I.; Metna, B.; Saadoun, N.S.; Taibi, F.; Houali, K. Bio-insecticidal effects of oleaster leaves aqueous extracts against psylla larvae (Euphyllura olivina (Costa)), a primary pest of Olea europaea L. Cell. Mol. Biol. 2018, 64, 35–40. [Google Scholar] [CrossRef]
- Kovalíková, Z.; Kubeš, J.; Skalický, M.; Kuchtickova, N.; Mašková, L.; Tuma, J.; Vachová, P.; Hejnak, V. Changes in content of polyphenols and ascorbic acid in leaves of white cabbage after pestinfestation. Molecules 2019, 24, 2622. [Google Scholar] [CrossRef] [PubMed]
- Karami, S.; Basaki, T.; Khaneghah, A.M. The inhibitory effects of polyphenolic compounds on the damage caused by safflower fly (Acanthiophilus helianthi) in Carthamus spp. Qual. Assur. Saf. Crop Foods 2021, 13, 87–93. [Google Scholar]
- Goldsmith, C.D.; Vuong, Q.V.; Sadeqzadeh, E.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Phytochemical properties and anti-proliferative activity of Olea europaea L. leaf extracts against pancreatic cancer cells. Molecules 2015, 20, 12992–13004. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Torras-Ceballos, A.; Gómez-Mora, J.; Fernández-Rojas, B. Luteolin, quercetin, genistein, and quercetagetin inhibit the effects of lipopolysaccharide obtained from Porphyromonas gingivalis in H9c2 cardiomyoblasts. Cell. Mol. Biol. Lett. 2017, 22, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a bioactive compound from Olea europaea L., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The olive biophenols oleuropein and hydroxytyrosol selectively reduce proliferation, influence the cell cycle, and induce apoptosis in pancreatic cancer cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the main polyphenol of Olea europaea leaf extract, has an anticancer effect on human BRAF melanoma cells and potentiates the cytotoxicity of current chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; Abd El-Hack, M.E.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Peyrol, J.; Riva, C.; Amiot, M. Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef]
- Monteiro, M.; Silva, A.F.R.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Strategies to broaden the applications of olive biophenols oleuropein and hydroxytyrosol in food products. Antioxidants 2021, 10, 444. [Google Scholar] [CrossRef]
- Cheurfa, M.; Abdallah, H.H.; Allem, R.; Noui, A.; Picot-Allain, C.M.N.; Mahomoodally, F. Hypocholesterolaemic and antioxidant properties of Olea europaea L. leaves from Chlef province, Algeria using in vitro, in vivo and in silico approaches. Food Chem. Toxicol. 2019, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: A review. J. Food Biochem. 2021, 46, e13950. [Google Scholar] [CrossRef]
- Djenane, D.; Yangüela, J.; Derriche, F.; Bouarab, L.; Roncales, P. Utilisation des composés de feuilles d’olivier comme agents antimicrobiens; application pour la conservation de la viande fraîche de dinde. Rev. Nat. Technol. 2012, 7, 53–61. [Google Scholar]
- Djenane, D.; Aboudaou, M.; Djenane, F.; García-Gonzalo, D.; Pagán, R. Improvement of the shelf-life status of modified atmosphere packaged camel meat using nisin and Olea europaea subsp. laperrinei leaf extract. Foods 2020, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Baali-Cherif, D.; Besnard, G. High genetic diversity and clonal growth in relict populations of Olea europaea subsp. laperrinei (Oleaceae) from Hoggar, Algeria. Ann. Bot. 2005, 96, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Besnard, G.; Baali-Cherif, D. Coexistence of diploids and triploids in a Saharan relict olive: Evidence from nuclear microsatellite and flow cytometry analyses. C. R. Biol. 2009, 332, 1115–1120. [Google Scholar] [CrossRef]
- Bernal, M.; Llorens, L.; Julkunen-Tiitto, R.; Badosa, J.; Verdaguer, D. Altitudinal and seasonal changes of phenolic compounds in Buxus sempervirens leaves and cuticles. Plant Phys. Biochem. 2013, 70, 471–482. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar]
- Bouchoucha, S.; Boukhebti, H.; Belhadj, H.; Oulmi, A.; Chaker, A.N. Identification of bioactive compounds and determination of total phenolic and flavonoid contents in leaf extracts originated from Algerian desert Olea europaea subsp. laperinei and Olea europaea subsp. europaea var. sylvestris and evaluation their potential as antioxidants. Prog. Nutr. 2023, 25, 1–15. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Hagerman, A.E.; Butler, L.G. Protein precipitation method for the quantitative Determination of tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the Determination of vitamin E. Methods Enzymol. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006, 95, 200–204. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Ponce, A.G.; Fritz, R.; Del Valle, C.; Roura, S.I. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT-Food Sci. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Luis, A.; Gil, N.; Amaral, M.E.; Duarte, A.P. Antioxidant activities of extracts from Acacia melanoxylon, Acacia dealbata, and Olea europaea and alkaloids estimation. Int. J. Pharm. Pharm. Sci. 2012, 4, 225–231. [Google Scholar]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef]
- Addab, N.; Fetni, S.; Hamlaoui, F.; Zerguine, A.; MahlouL, K. Comparative evaluation of antioxidant activity of ethanolic extracts from leaves of Olea europaea L. from Eastern Algeria. J. Fac. Med. 2020, 4, 579–586. [Google Scholar]
- Katalinic, V.; Milo, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; Zhang, Y.L. Phenolic compositions and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure-activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Brahmi, F.; Mechri, B.; Dhibi, M.; Hammami, M. Variations in phenolic compounds and antiradical scavenging activity of Olea europaea leaves and fruits extracts collected in two different seasons. Ind. Crops Prod. 2013, 49, 256–264. [Google Scholar] [CrossRef]
- Abaza, L.; Ben Youcef, N.; Manai, H.; Mahjoub Haddada, F.; Methenni, K.; Zarrouk, M. Chetoui olive leaf extracts: Influence of the solvent type on phenolics and antioxidant activities. Grsas Y Aceites 2011, 62, 96–104. [Google Scholar] [CrossRef]
- Mebirouk-Boudechiche, L.; Cherif, M.; Boudechiche, L.; Sammar, F. Teneurs en composés primaires et secondaires des feuilles d’arbustes fourragers de la région humide d’Algérie. Rev. Méd. Vét. 2014, 165, 344–352. [Google Scholar]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef]
- Mailoa, M.N.; Mahendradatta, M.; Laga, A.; Djide, N. Tannin extract of guava leaves (Psidium guajava L) variation with concentration organic solvents. Int. J. Sci. Technol. Res. 2013, 2, 106–110. [Google Scholar]
- Fernando, C.D.; Soysa, P. Total phenolic, flavonoid contents, in-vitro antioxidant activities, and hepatoprotective effect of aqueous leaf extract of Atalantia ceylanica. BMC Complement. Altern. Med. 2014, 14, 395. [Google Scholar] [CrossRef] [PubMed]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Mohammedi, Z.; Atik, F. Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) karst. Int. J. Pharma Bio Sci. 2011, 2, 609–615. [Google Scholar]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic profile (HPLC-UV) of olive leaves according to extraction procedure and assessment of antibacterial activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef] [PubMed]
- Rouibah, Z.; Ben Mensour, A.; Rekik, O.; Boumendjel, M.; Taibi, F.; Bouaziz, M.; El Feki, A.; Messarah, M.; Boumendjel, A. Chemical composition, antioxidant activities, in an allergic asthma model, of Olea europaea L. leaf extracts from Collo (Skikda, Algeria). Drug Chem. Toxicol. 2019, 45, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Khlif, I.; Jellali, K.; Michel, T.; Halabalaki, M.; Skaltsounis, A.L.; Allouche, N. Characteristics, phytochemical analysis, and biological activities of extracts from Tunisian Chetoui Olea europaea Variety. J. Chem. 2015, 2418731. [Google Scholar] [CrossRef]
- Jan, S.; Khan, M.R.; Rashid, U.; Bokhari, J. Assessment of antioxidant potential, total phenolics, and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public Health Res. Perspect. 2013, 4, 246–254. [Google Scholar] [CrossRef]
- Hayes, J.E.; Allen, P.; Brunton, N.; O’Grady, M.N.; Kerry, J.P. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol, and ellagic acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F.; Eslami, B. Antioxidant activity of the bulb and aerial parts of Ornithogalum sintenisii L. (Liliaceae) at flowering stage. Trop. J. Pharma Res. 2010, 9, 141–148. [Google Scholar] [CrossRef][Green Version]
- Arab, K.; Bouchenak, O.; Yahiaoui, K. Evaluation de l’activité biologique des feuilles de l’olivier sauvage et cultivé. Afr. Sci. 2013, 9, 159–166. [Google Scholar]
- Mkaouar, S.; Krichen, F.; Bahloul, N.; Allaf, K.; Kechaou, N. Enhancement of Bioactive Compounds and Antioxidant Activities of Olive (Olea europaea L.) Leaf Extract by Instant Controlled Pressure Drop. Food Bioprocess Technol. 2018, 11, 1222–1229. [Google Scholar] [CrossRef]
- Al-Saeghi, S.S.; Hossain, M.; Al-Touby, S.S.J. Characterization of antioxidant and antibacterial compounds from aerial parts of Haplophyllum tuberculatum. J. Bioresour. Bioprod. 2022, 7, 52–62. [Google Scholar] [CrossRef]
- Saiah, H.; Allem, R.; El Kebir, F.Z. Antioxidant and antibacterial activities of six Algerian medicinal plants. Int. J. Pharm. Pharm. Sci. 2016, 8, 367–374. [Google Scholar]
- Kubula, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem, and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Orak, H.H.; Isbilir, S.S.; Yagar, H. Determination of antioxidant properties of lyophilized olive leaf water extracts obtained from 21 different cultivars. Food Sci. Biotechnol. 2012, 21, 1065–1074. [Google Scholar] [CrossRef]
- Alsulaymani, F.A.; Elmhdwi, M.F.; Gaber, S.; El Aali, N.M.; Mohammed, M.I.; Abduulsalam, A.A. In vitro antioxidant and antibacterial activity of olive leave extract. J. Pharm. Appl. Chem. 2021, 7, 41–47. [Google Scholar]
- Šimat, V.; Skroza, D.; Tabanelli, G.; Čagalj, M.; Pasini, F.; Gómez-Caravaca, A.M.; Fernández-Fernández, C.; Sterniša, M.; Smole Možina, S.; Ozogul, Y.; et al. Antioxidant and antimicrobial activity of hydroethanolic leaf extracts from six Mediterranean olive cultivars. Antioxidants 2022, 11, 1656. [Google Scholar] [CrossRef]
- Martín-García, B.; De Montijo-Prieto, S.; Jiménez-Valera, M.; Carrasco-Pancorbo, A.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Comparative extraction of phenolic compounds from olive leaves using a sonotrode and an ultrasonic bath and the evaluation of both antioxidant and antimicrobial activity. Antioxidants 2022, 11, 558. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Sbeih, M.; Amayreh, M.; Rahhal, B.; Mudalal, S. Evaluation of the antibacterial and antifungal properties of oleuropein, Olea europaea leaf extract, and Thymus vulgaris oil. BMC Complement. Med. Ther. 2024, 24, 297. [Google Scholar] [CrossRef] [PubMed]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Silvan, J.M.; Guerrero-Hurtado, E.; Gutierrez-Docio, A.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive leaf as a source of antibacterial compounds active against antibiotic-resistant strains of Campylobacter jejuni and Campylobacter coli. Antibiotics 2023, 12, 26. [Google Scholar] [CrossRef]
- Ben Hassena, A.; Abidi, J.; Miled, N.; Kulinowski, Ł.; Skalicka-Woźniak, K.; Bouaziz, M. New insights into the antibacterial activity of hydroxytyrosol extracted from olive leaves: Molecular docking simulations of its antibacterial mechanisms. Chem. Biodivers. 2024, 22, e202401714. [Google Scholar] [CrossRef]
- Mumcu, A. Olive leaf: Antimicrobial and antioxidant properties, food applications, and current studies. Eurasian J. Food Sci. Technol. 2023, 7, 23–43. [Google Scholar]
- Khan, H.; Ahmad, W.; Hussain, I.; Imran, M.; Afridi, M.S.; Ullah, S. Phytochemical composition, antioxidant and antimicrobial activities of leaves of Olea europaea wild variety. J. Food Meas. Charact. 2020, 14, 640–648. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as inhibitors of antibiotic-resistant bacteria—Mechanisms underlying rutin interference with bacterial virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Magyari-Pavel, I.Z.; Moacă, E.-A.; Avram, Ș.; Diaconeasa, Z.; Haidu, D.; Ștefănuț, M.N.; Rostas, A.M.; Muntean, D.; Bora, L.; Badescu, B.; et al. Antioxidant extracts from Greek and Spanish olive leaves: Antimicrobial, anticancer and antiangiogenic effects. Antioxidants 2024, 13, 774. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. olive leaves through the evaluation of their extracts: Antioxidant and antimicrobial activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef]



| Aqueous Extract (EA) | Ethanolic Extract (EE) | |||
|---|---|---|---|---|
| Compound | Retention Time (min) | Area (%) | Retention Time (min) | Area (%) |
| Gallic acid | 3.054 | 0.94 | 3.054 | 0.97 |
| Hydroxy quinone | 3.748 | 1.79 | - | - |
| Hydroxytyrosol | 4.885 | 1.33 | 4.850 | 1.39 |
| Resorcinol | 5.972 | 1.09 | 5.972 | 0.96 |
| POH benzaldehyde | - | - | 6.170 | 2.32 |
| Catechol | 6.371 | 0.87 | - | - |
| Catechin | - | - | 6.358 | 1.12 |
| Caffeine | - | - | 6.789 | 1.02 |
| Vanillic acid | 7.056 | 1.14 | 7.044 | 1.09 |
| Aesculetin | 7.031 | 1.21 | 7.374 | 1.26 |
| Cafeic acid | 7.188 | 1. 39 | 7.197 | 1.60 |
| Orientin | 7.984 | 2.20 | 7.982 | 2.35 |
| Vitexine 2 O rhamnoside | - | - | 8.648 | 1.61 |
| Rutin | 8.947 | 1.61 | 8.925 | 1.54 |
| Quercetin | 9.222 | 1.16 | - | - |
| O Anisic acid | 9.528 | 1.33 | 9.494 | 1.23 |
| Luteolin 7 glucoside | - | - | 9.499 | 2.14 |
| Salicylic acid | 9.960 | 2.89 | 9.961 | 2.66 |
| Rosmarinic acid | 10.232 | 2.34 | 10.225 | 2.21 |
| Naringenin7 glucoside | 10.520 | 4.80 | 10.520 | 4.44 |
| 3-4-5-TrimitoxyBenzoic acid | - | - | 10.834 | 1.09 |
| Myricetin | - | - | 11.013 | 1.11 |
| Oleuropein | 11.239 | 7.47 | 11.244 | 5.23 |
| m Anisic acid | 11.789 | 2.44 | 11.783 | 7.29 |
| Luteolin | - | - | 12.783 | 0.92 |
| Cinnamic acid | 13.631 | 2.30 | 13.639 | 3.69 |
| Hesperidin | 14.984 | 1.41 | 14.853 | 0.82 |
| Bacterial Strains | MICs(mg/mL) | |
|---|---|---|
| EE | EA | |
| S. aureus FRIS6 | 12.5 | 6.25 |
| S. aureus LGA251mecC | 25 | 1.56 |
| P. aeruginosa ATCC 27853 | 50 | 0.78 |
| Type of Extract | Total Polyphenol Contents | References |
|---|---|---|
| Methanolic extract | 216.5 ± 2.9 mg GAE/100 g dry Weight (Olea europaea subsp. laperrenei Tassili N’ajjer | [24] |
| Ethanolic extract | 245.2 ± 0.65 mg GAE/g dry residue | [45] |
| Methanolic extract | 464.27 mg H*E/100 g dry weight (Chemlal) 270.53 mg H*E/100 g dry weight (Neb Jmel) | [46] |
| Aqueous extract | 16.52 ± 0.62 mg GAE/g dry weight (Chetoui) | [47] |
| Ethanolic extract | 24.36 ± 0.85 mg GAE/g dry weight (Chetoui) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahcene, S.; Moualek, I.; Bariz, K.; Benramdane, E.; Alenazy, R.; Alqasmi, M.; Almufarriji, F.M.; Thabet, M.; Fallata, G.; Alqurainy, N.; et al. Antioxidant and Antibacterial Activities and Phytochemical Analysis of Olea europaea subsp. laperrinei Leaves Extracts. Processes 2025, 13, 1113. https://doi.org/10.3390/pr13041113
Lahcene S, Moualek I, Bariz K, Benramdane E, Alenazy R, Alqasmi M, Almufarriji FM, Thabet M, Fallata G, Alqurainy N, et al. Antioxidant and Antibacterial Activities and Phytochemical Analysis of Olea europaea subsp. laperrinei Leaves Extracts. Processes. 2025; 13(4):1113. https://doi.org/10.3390/pr13041113
Chicago/Turabian StyleLahcene, Souad, Idir Moualek, Karim Bariz, Elias Benramdane, Rawaf Alenazy, Mohammed Alqasmi, Fawaz M. Almufarriji, Mohammed Thabet, Ghaith Fallata, Nasser Alqurainy, and et al. 2025. "Antioxidant and Antibacterial Activities and Phytochemical Analysis of Olea europaea subsp. laperrinei Leaves Extracts" Processes 13, no. 4: 1113. https://doi.org/10.3390/pr13041113
APA StyleLahcene, S., Moualek, I., Bariz, K., Benramdane, E., Alenazy, R., Alqasmi, M., Almufarriji, F. M., Thabet, M., Fallata, G., Alqurainy, N., Saoudi, B., Sadoun, N., Trabelsi, L., & Houali, K. (2025). Antioxidant and Antibacterial Activities and Phytochemical Analysis of Olea europaea subsp. laperrinei Leaves Extracts. Processes, 13(4), 1113. https://doi.org/10.3390/pr13041113








