Injection-Molded Poly(butylene succinate)/Wheat Flour By-Product Biocomposites: Mechanical, Thermal, and Structural Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
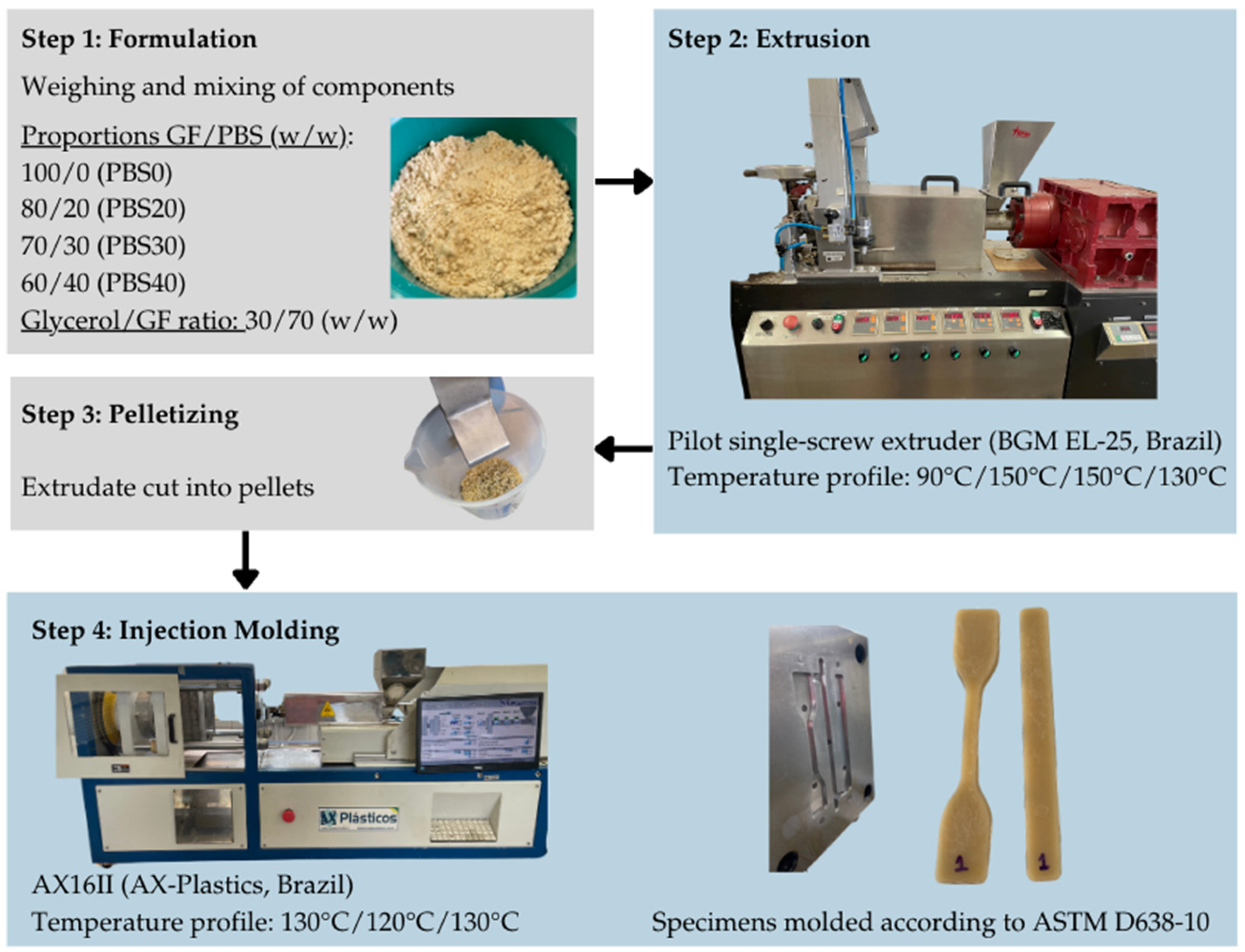
2.2. Biocomposites Production
2.3. Biocomposite Characterization
2.3.1. Instrumental Color Measurement
2.3.2. Linear Contraction Index (LCI)
2.3.3. Solubility in Water
2.3.4. Mechanical Properties
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.7. Differential Scanning Calorimetry (DSC)
2.4. Statistical Analysis
3. Results and Discussion
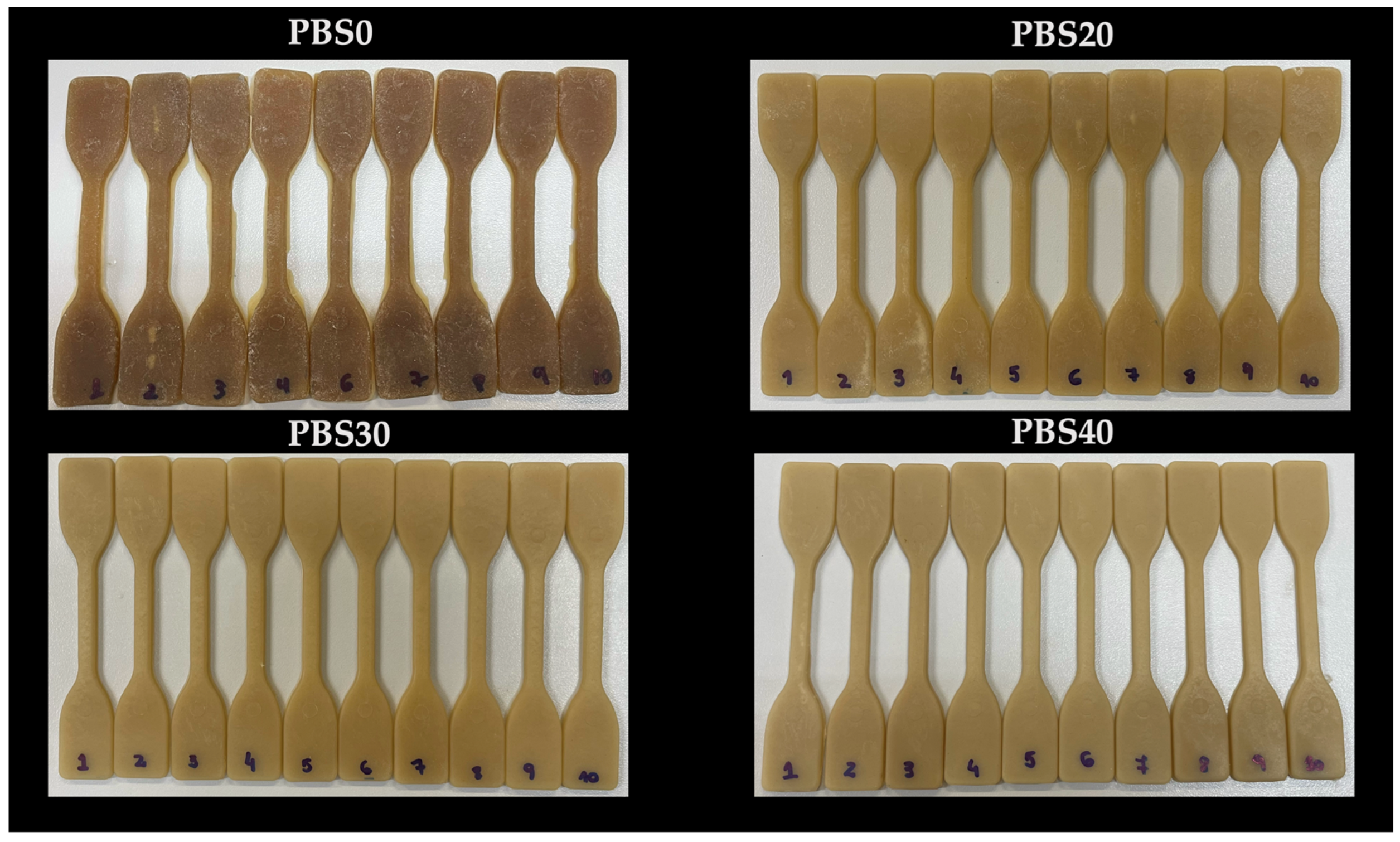
3.1. Visual Appearance and Color Parameters of Biocomposites
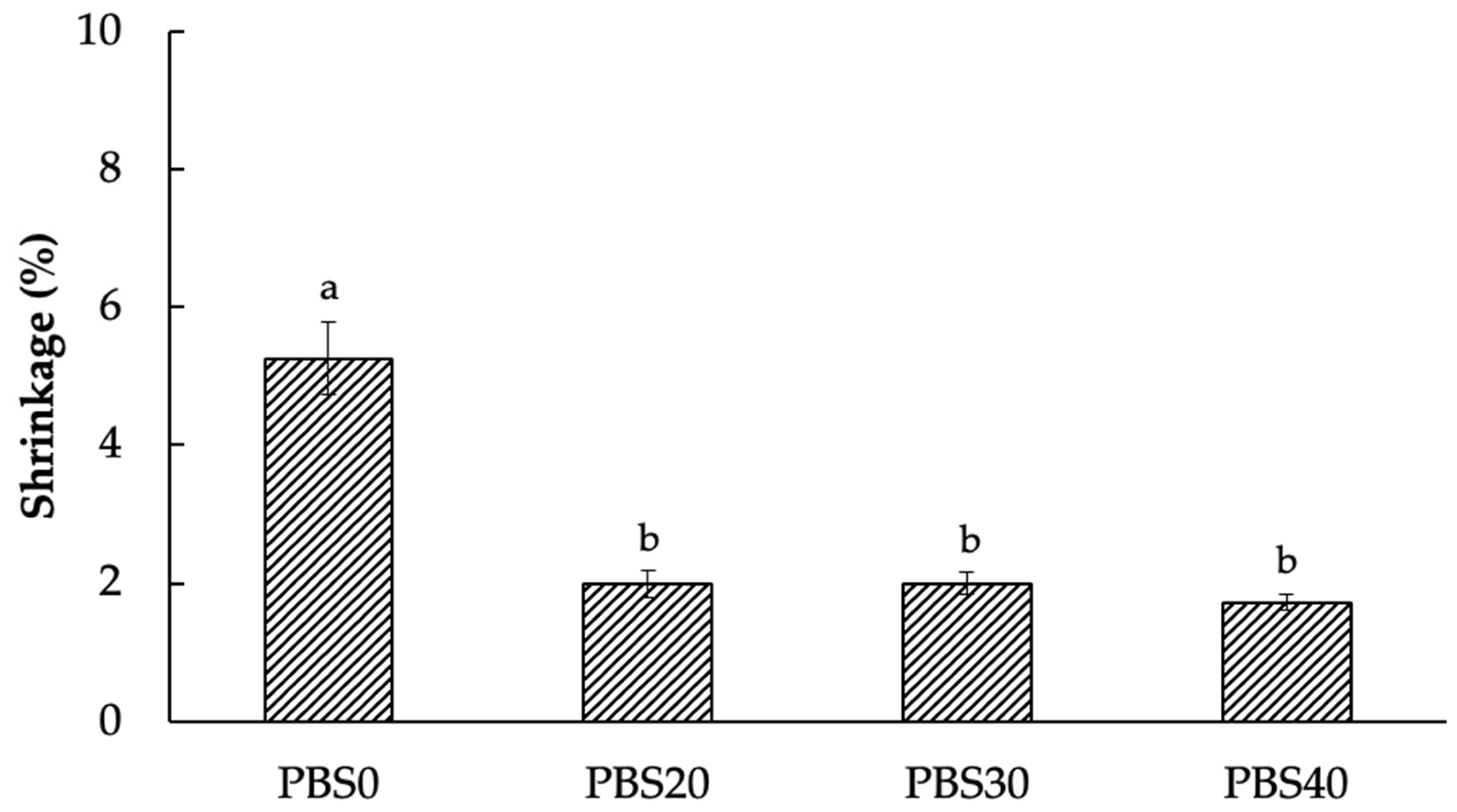
3.2. Shrinkage
3.3. Biocomposites Solubility in Water
3.4. Tensile Strength, Elongation, and Young’s Modulus
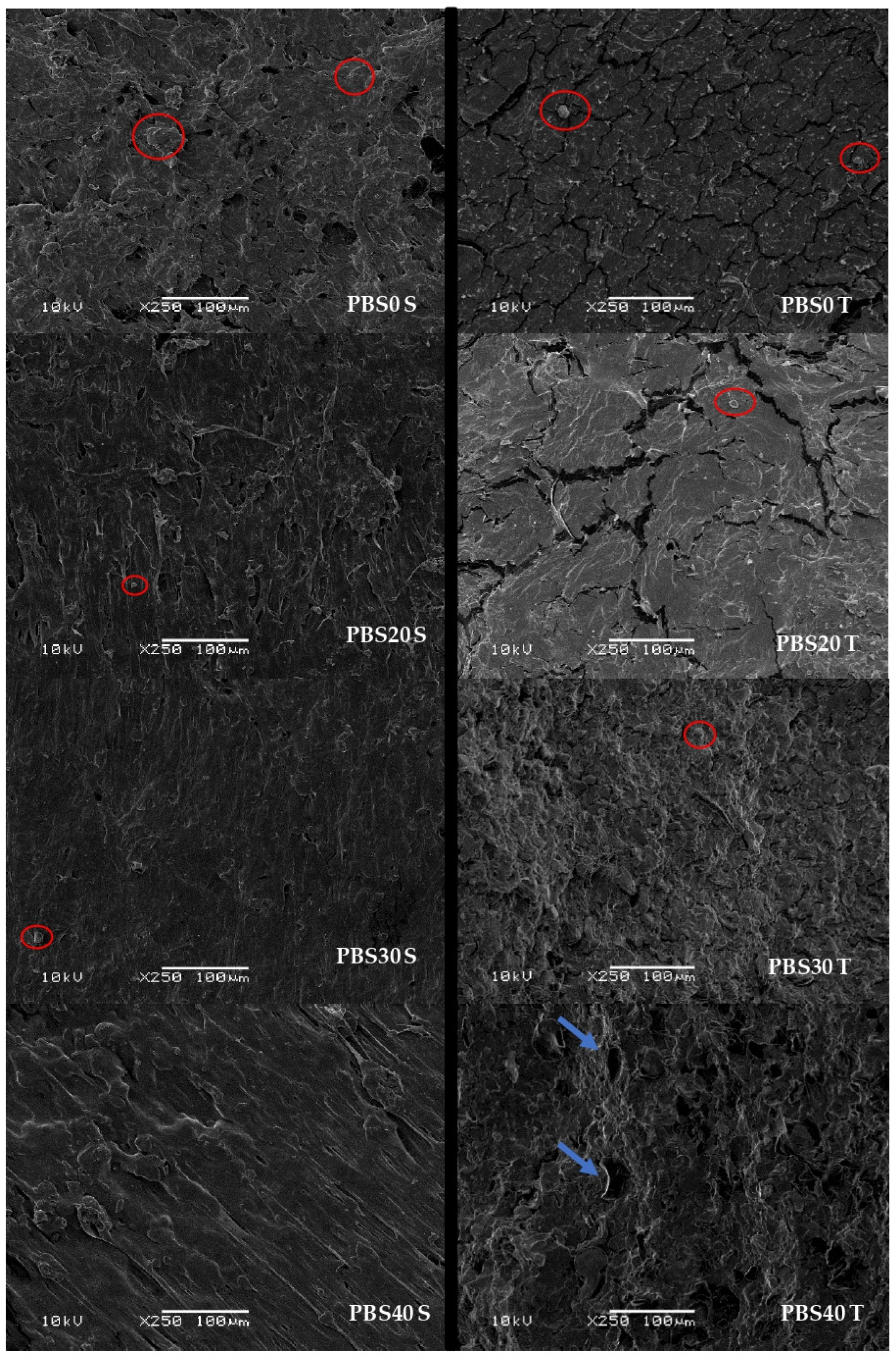
3.5. SEM Analysis
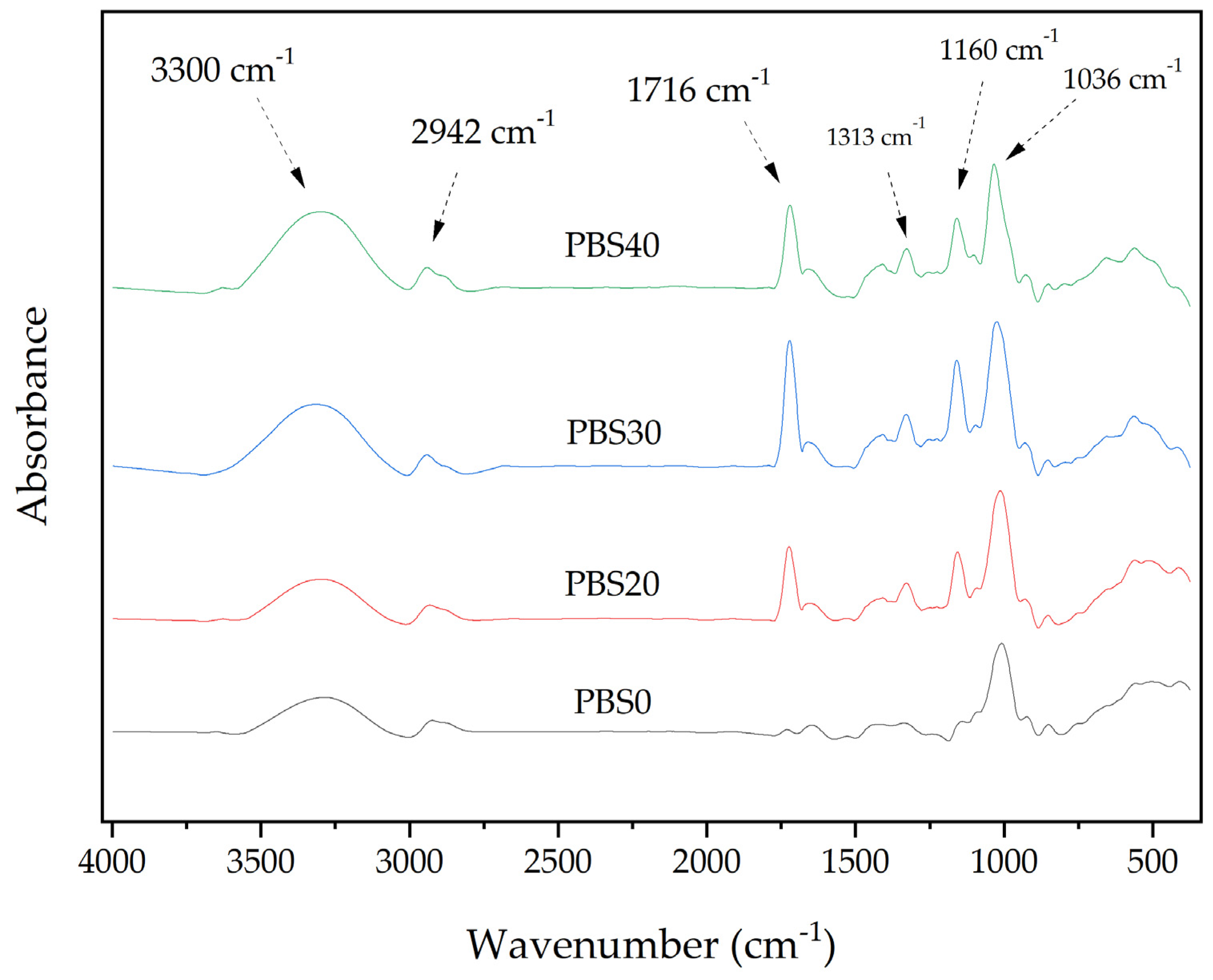
3.6. FTIR (Fourier Transform Infrared) Analysis
3.7. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe Plastics Europe—The Fast Facts 2024. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2024/ (accessed on 5 March 2025).
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N.; Mohd Nurazzi, N.; Atiqah, A.; Ansari, M.N.M.; et al. Effect of Sugar Palm Nanofibrillated Cellulose Concentrations on Morphological, Mechanical and Physical Properties of Biodegradable Films Based on Agro-Waste Sugar Palm (Arenga Pinnata (Wurmb.) Merr) Starch. J. Mater. Res. Technol. 2019, 8, 4819–4830. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.H.; Kwon, E.E. Prospects of Biopolymer Technology as an Alternative Option for Non-Degradable Plastics and Sustainable Management of Plastic Wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Drummond, F.R.; Cardoso, P.H.M.; Anaya-Mancipe, J.M.; Thiré, R.M.d.S.M. Biocomposites of Starch Industry Residues from Cassava and Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) for Food Packaging. Processes 2025, 13, 719. [Google Scholar] [CrossRef]
- Gupta, P.; Toksha, B.; Rahaman, M. A Review on Biodegradable Packaging Films from Vegetative and Food Waste. Chem. Rec. 2022, 22, e202100326. [Google Scholar] [CrossRef]
- Bulatović, V.O.; Mandić, V.; Kučić Grgić, D.; Ivančić, A. Biodegradable Polymer Blends Based on Thermoplastic Starch. J. Polym. Environ. 2021, 29, 492–508. [Google Scholar] [CrossRef]
- USDA—United States Department of Agriculture World Agricultural Production. Available online: https://www.fas.usda.gov/sites/default/files/2025-02/production.pdf (accessed on 5 March 2025).
- Clément, H.; Prost, C.; Chiron, H.; Ducasse, M.B.; Della Valle, G.; Courcoux, P.; Onno, B. The Effect of Organic Wheat Flour By-Products on Sourdough Performances Assessed by a Multi-Criteria Approach. Food Res. Int. 2018, 106, 974–981. [Google Scholar] [CrossRef]
- Frantz, S.C.; Paludo, L.C.; Stutz, H.; Spier, M.R. Production of Amylases from Coprinus Comatus Under Submerged Culture Using Wheat-Milling by-Products: Optimization, Kinetic Parameters, Partial Purification and Characterization. Biocatal. Agric. Biotechnol. 2019, 17, 82–92. [Google Scholar] [CrossRef]
- Peron-Schlosser, B.; Carpiné, D.; Matos Jorge, R.M.; Rigon Spier, M. Optimization of Wheat Flour by Product Films: A Technological and Sustainable Approach for Bio-Based Packaging Material. J. Food Sci. 2021, 86, 4522–4538. [Google Scholar] [CrossRef]
- Peron-Schlosser, B.; Beux, M.R.; Heemann, A.C.W.; Jorge, R.M.M.; Spier, M.R. Development and Characterization of Wheat Mill By-Product Films Enriched with Commercial Rosemary Extract. Acta Scientiarum. Technol. 2024, 46, e66401. [Google Scholar] [CrossRef]
- Chabrat, E.; Abdillahi, H.; Rouilly, A.; Rigal, L. Influence of Citric Acid and Water on Thermoplastic Wheat Flour/Poly(Lactic Acid) Blends. I: Thermal, Mechanical and Morphological Properties. Ind. Crops Prod. 2012, 37, 238–246. [Google Scholar] [CrossRef]
- Lounis, F.M.; Benhacine, F.; Hadj-Hamou, A.S. Improving Water Barrier Properties of Starch Based Bioplastics by Lignocellulosic Biomass Addition: Synthesis, Characterization and Antibacterial Properties. Int. J. Biol. Macromol. 2024, 283, 137823. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Zeng, J.B.; Jiao, L.; Li, Y.D.; Srinivasan, M.; Li, T.; Wang, Y.Z. Bio-Based Blends of Starch and Poly(Butylene Succinate) with Improved Miscibility, Mechanical Properties, and Reduced Water Absorption. Carbohydr. Polym. 2011, 83, 762–768. [Google Scholar] [CrossRef]
- Palai, B.; Mohanty, S.; Nayak, S.K. A Comparison on Biodegradation Behaviour of Polylactic Acid (PLA) Based Blown Films by Incorporating Thermoplasticized Starch (TPS) and Poly(Butylene Succinate-Co-Adipate) (PBSA) Biopolymer in Soil. J. Polym. Environ. 2021, 29, 2772–2788. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, H.; Xu, H.; An, S.; Li, C.; Huang, C.; Wang, S.; Liu, Y.; Chen, J. Study of 4,4′-Methylene Diisocyanate Phenyl Ester-Modified Cassava Residues/Polybutylene Succinate Biodegradable Composites: Preparation and Performance Research. Processes 2019, 7, 588. [Google Scholar] [CrossRef]
- Yin, Q.J.; Chen, F.P.; Zhang, H.; Liu, C.S. Mechanical Properties and Thermal Behavior of TPS/PBS Blends with Maleated PBS as a Compatibilizer. Adv. Mat. Res. 2015, 1119, 306–309. [Google Scholar] [CrossRef]
- Liu, D.; Qi, Z.; Zhang, Y.; Xu, J.; Guo, B. Poly(Butylene Succinate) (PBS)/Ionic Liquid Plasticized Starch Blends: Preparation, Characterization, and Properties. Starch Stärke 2015, 67, 802–809. [Google Scholar] [CrossRef]
- da Silva, S.C.; de Carvalho, F.A.; Yamashita, F. Potential Biodegradable Materials Containing Oat Hulls, TPS, and PBS by Thermoplastic Injection. Polimeros 2024, 34, e20240026. [Google Scholar] [CrossRef]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Jawaid, M.; Lee, C.H. Effect of Modified Tapioca Starch on Mechanical, Thermal, and Morphological Properties of PBS Blends for Food Packaging. Polymers 2018, 10, 1187. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Analysis of Selected Properties of Injection Moulded Sustainable Biocomposites from Poly(Butylene Succinate) and Wheat Bran. Materials 2021, 14, 7049. [Google Scholar] [CrossRef] [PubMed]
- Sasimowski, E.; Grochowicz, M.; Szajnecki, Ł. Preparation and Spectroscopic, Thermal, and Mechanical Characterization of Biocomposites of Poly(Butylene Succinate) and Onion Peels or Durum Wheat Bran. Materials 2023, 16, 6799. [Google Scholar] [CrossRef] [PubMed]
- Abdillahi, H.; Chabrat, E.; Rouilly, A.; Rigal, L. Influence of Citric Acid on Thermoplastic Wheat Flour/Poly(Lactic Acid) Blends. II. Barrier Properties and Water Vapor Sorption Isotherms. Ind. Crops Prod. 2013, 50, 104–111. [Google Scholar] [CrossRef]
- Balan, G.C.; Paulo, A.F.S.; Correa, L.G.; Alvim, I.D.; Ueno, C.T.; Coelho, A.R.; Ströher, G.R.; Yamashita, F.; Sakanaka, L.S.; Shirai, M.A. Production of Wheat Flour/PBAT Active Films Incorporated with Oregano Oil Microparticles and Its Application in Fresh Pastry Conservation. Food Bioprocess Tech. 2021, 14, 1587–1599. [Google Scholar] [CrossRef]
- Schlosser, B.P. Desenvolvimento de Bioplásticos a Partir de Subproduto Da Moagem Do Trigo Por Casting e Extrusão. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2023. [Google Scholar]
- ASTM D638-10; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D955-00; Standard Test Method for Measuring Shrinkage from Mold Dimensions of Thermoplastics. ASTM International: West Conshohocken, PA, USA, 2001.
- Carvalho, F.A. de Biocompósitos de Poli (Ácido Lático) e Bagaço de Mandioca Produzidos Por Injeção Termoplástica. Ph.D. Thesis, Universidade Estadual de Londrina, Londrina, Brazil, 2019. [Google Scholar]
- Kowalska, B. Injection Moulding Contraction and the Pressure-Volume-Time Relation. Int. Polym. Sci. Technol. 2007, 34, 41–48. [Google Scholar] [CrossRef]
- Mougin, G.; Magnani, M.; Eikelenberg, N. Natural-Fibres Composites for the Automotive Industry: Challenges, Solutions and Applications. Int. J. Mater. Product. Technol. 2009, 36, 176–188. [Google Scholar] [CrossRef]
- Manrich, S. Processamento de Termoplásticos: Rosca Única, Extrusão e Matrizes, Injeção e Moldes; Artliber Editora: São Paulo, Brazil, 2005. [Google Scholar]
- Araújo, C.; Pereira, D.; Dias, D.; Marques, R.; Cruz, S. In-Cavity Pressure Measurements for Failure Diagnosis in the Injection Moulding Process and Correlation with Numerical Simulation. Int. J. Adv. Manuf. Technol. 2023, 126, 291–300. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Sheng, S.; Li, Y.; Zhong, J.R.; Tan, J.; Zhang, Y.F. Preparation and Properties of Rapidly Plasticized Poly (Butylene Succinate)/Mechanically Activated Cassava Starch Biocomposite. Polym. Bull. 2024, 81, 6495–6511. [Google Scholar] [CrossRef]
- Sankri, A.; Arhaliass, A.; Dez, I.; Gaumont, A.C.; Grohens, Y.; Lourdin, D.; Pillin, I.; Rolland-Sabaté, A.; Leroy, E. Thermoplastic Starch Plasticized by an Ionic Liquid. Carbohydr. Polym. 2010, 82, 256–263. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Injection Molded Sustainable Biocomposites from Poly(Butylene Succinate) Bioplastic and Perennial Grass. ACS Sustain. Chem. Eng. 2015, 3, 2767–2776. [Google Scholar] [CrossRef]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-Poly(Butylene Succinate) and Its Composites with Grape Pomace: Mechanical Performance and Thermal Properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Bilal Khan Niazi, M.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material—A Review. J. Food Process Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- da Silva, S.C.; Simões, B.M.; Yamashita, F.; de Carvalho, F.A. Compatibilizers for Biodegradable Starch and Poly (Lactic Acid) Materials Produced by Thermoplastic Injection. Res. Soc. Dev. 2022, 11, e476111436521. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Lin, X.; Zhou, Y. Comparative Study on the Blends of PBS/Thermoplastic Starch Prepared from Waxy and Normal Corn Starches. Starch/Staerke 2013, 65, 831–839. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, A.R. Introdução à Espectroscopia, 4th ed.; Cengage Learning: São Paulo, Brazil, 2010. [Google Scholar]
- Beluci, N.d.C.L.; dos Santos, J.; de Carvalho, F.A.; Yamashita, F. Reactive Biodegradable Extruded Blends of Thermoplastic Starch and Polyesters. Carbohydr. Polym. Technol. Appl. 2023, 5, 100274. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Hou, H. Effects of High Starch Content on the Physicochemical Properties of Starch/PBAT Nanocomposite Films Prepared by Extrusion Blowing. Carbohydr. Polym. 2020, 239, 116231. [Google Scholar] [CrossRef]
- Aziman, N.; Kian, L.K.; Jawaid, M.; Sanny, M.; Alamery, S. Morphological, Structural, Thermal, Permeability, and Antimicrobial Activity of PBS and PBS/TPS Films Incorporated with Biomaster-Silver for Food Packaging Application. Polymers 2021, 13, 391. [Google Scholar] [CrossRef]
- Singh, P.; Wani, A.A.; Langowski, H.-C. Food Packaging Materials: Testing & Quality Assurance; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]

| Biocomposites | L* | a* | b* |
|---|---|---|---|
| PBS0 | 38.98 ± 2.04 d | 8.94 ± 1.11 a | 19.15 ± 1.41 b |
| PBS20 | 48.37 ± 1.13 c | 4.97 ± 0.30 b | 31.00 ± 3.17 a |
| PBS30 | 50.86 ± 2.48 b | 4.75 ± 0.35 b | 31.15 ± 1.05 a |
| PBS40 | 53.65 ± 3.33 a | 3.56 ± 0.20 c | 29.03 ± 2.66 a |
| Biocomposites | Solubility (%) |
|---|---|
| PBS0 | 37.03 ± 0.91 a |
| PBS20 | 26.98 ± 1.81 ab |
| PBS30 | 21.99 ± 1.80 bc |
| PBS40 | 16.08 ± 1.19 c |
| Biocomposites | Tensile Strength (MPa) | Elongation (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| PBS0 | 1.36 ± 0.03 d | 21.03 ± 0.50 b | 0.12 ± 0.01 d |
| PBS20 | 6.24 ± 0.22 c | 18.09 ± 0.61 c | 0.94 ± 0.03 c |
| PBS30 | 9.00 ± 0.48 b | 16.06 ± 1.00 d | 1.18 ± 0.04 b |
| PBS40 | 12.23 ± 0.16 a | 24.77 ± 2.08 a | 1.54 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peron-Schlosser, B.; Ramos, R.M.B.; Paludo, L.C.; Monteiro, P.I.; de Carvalho, F.A.; da Silva, S.C.; Cordova, B.A.B.; Carvalho, B.d.M.; Yamashita, F.; Spier, M.R. Injection-Molded Poly(butylene succinate)/Wheat Flour By-Product Biocomposites: Mechanical, Thermal, and Structural Characterization. Processes 2025, 13, 1044. https://doi.org/10.3390/pr13041044
Peron-Schlosser B, Ramos RMB, Paludo LC, Monteiro PI, de Carvalho FA, da Silva SC, Cordova BAB, Carvalho BdM, Yamashita F, Spier MR. Injection-Molded Poly(butylene succinate)/Wheat Flour By-Product Biocomposites: Mechanical, Thermal, and Structural Characterization. Processes. 2025; 13(4):1044. https://doi.org/10.3390/pr13041044
Chicago/Turabian StylePeron-Schlosser, Bianca, Rúbia Martins Bernardes Ramos, Luana Cristina Paludo, Pablo Inocêncio Monteiro, Fabíola Azanha de Carvalho, Samuel Camilo da Silva, Bruno Alexandro Bewzenko Cordova, Benjamim de Melo Carvalho, Fabio Yamashita, and Michele Rigon Spier. 2025. "Injection-Molded Poly(butylene succinate)/Wheat Flour By-Product Biocomposites: Mechanical, Thermal, and Structural Characterization" Processes 13, no. 4: 1044. https://doi.org/10.3390/pr13041044
APA StylePeron-Schlosser, B., Ramos, R. M. B., Paludo, L. C., Monteiro, P. I., de Carvalho, F. A., da Silva, S. C., Cordova, B. A. B., Carvalho, B. d. M., Yamashita, F., & Spier, M. R. (2025). Injection-Molded Poly(butylene succinate)/Wheat Flour By-Product Biocomposites: Mechanical, Thermal, and Structural Characterization. Processes, 13(4), 1044. https://doi.org/10.3390/pr13041044







