The Valorization of Marble Waste to Synthesize a Novel Calcium Niobate–Magnesium Niobate Composite and an Investigation of Its Thermophysical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Synthesis of Calcium Niobate–Magnesium Niobate Composites
2.3. Characterization of Marble Waste and Composites
2.4. Determination of Thermophysical Properties
3. Results and Discussion
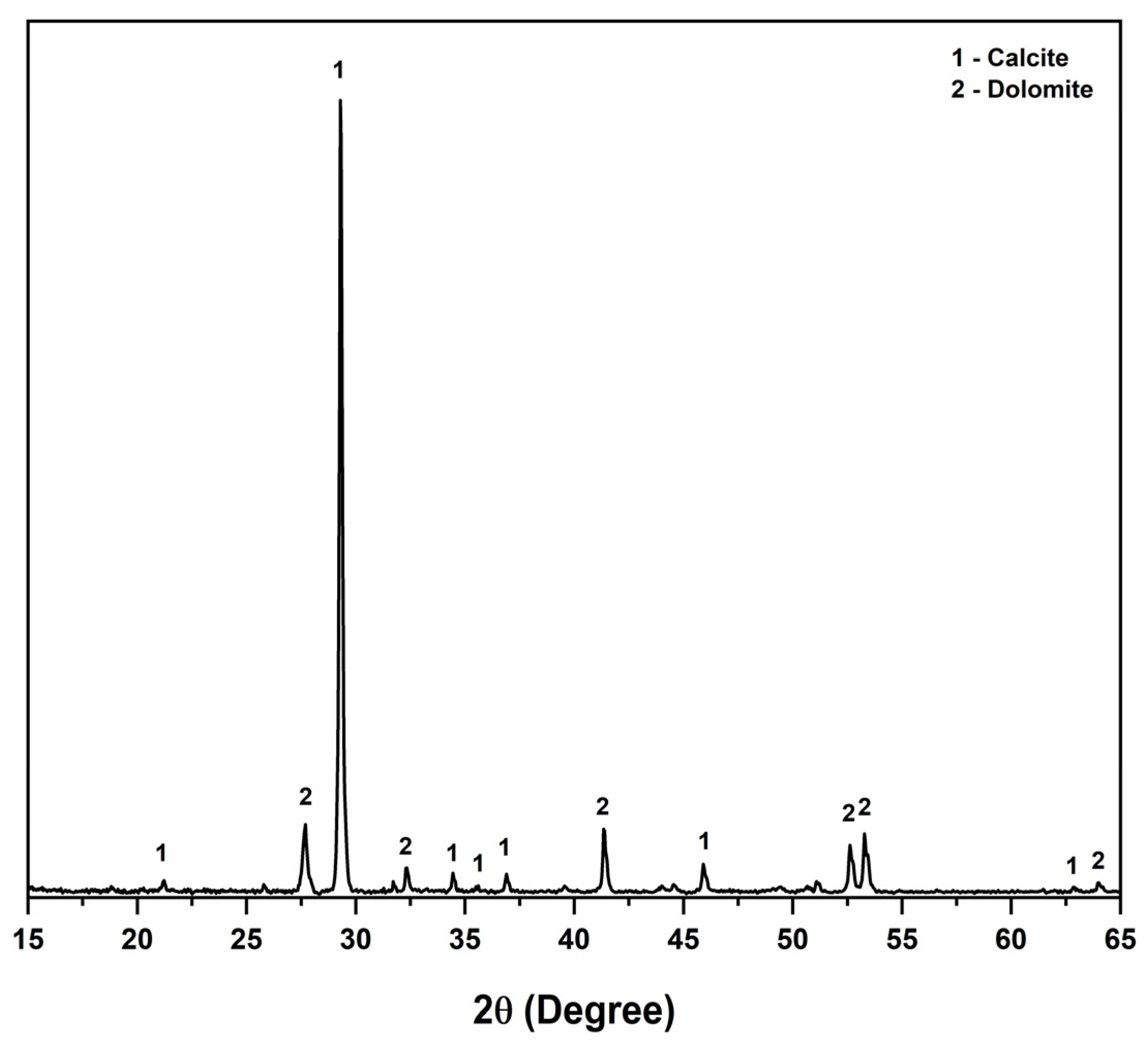
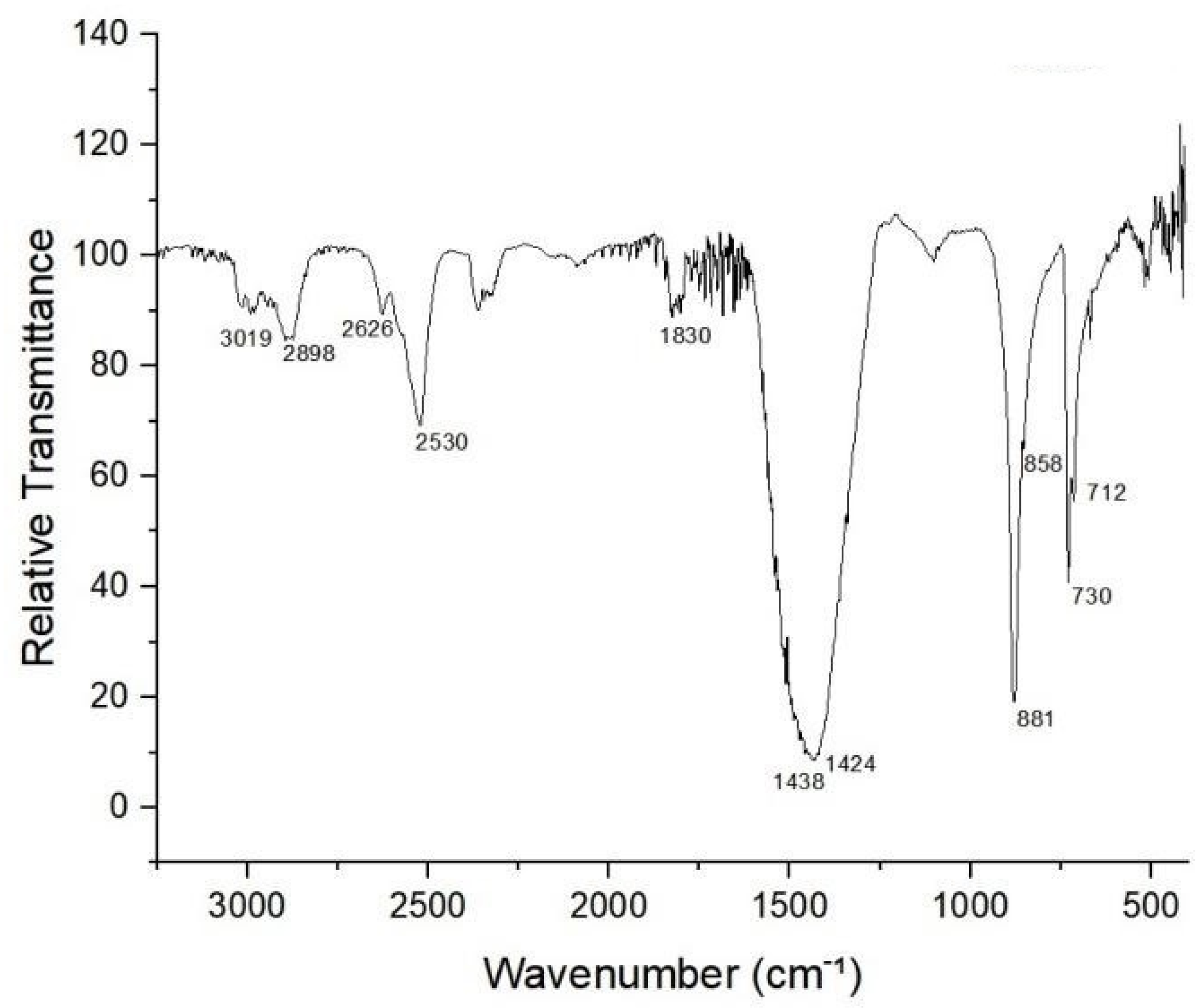
3.1. Characterization of Marble Waste
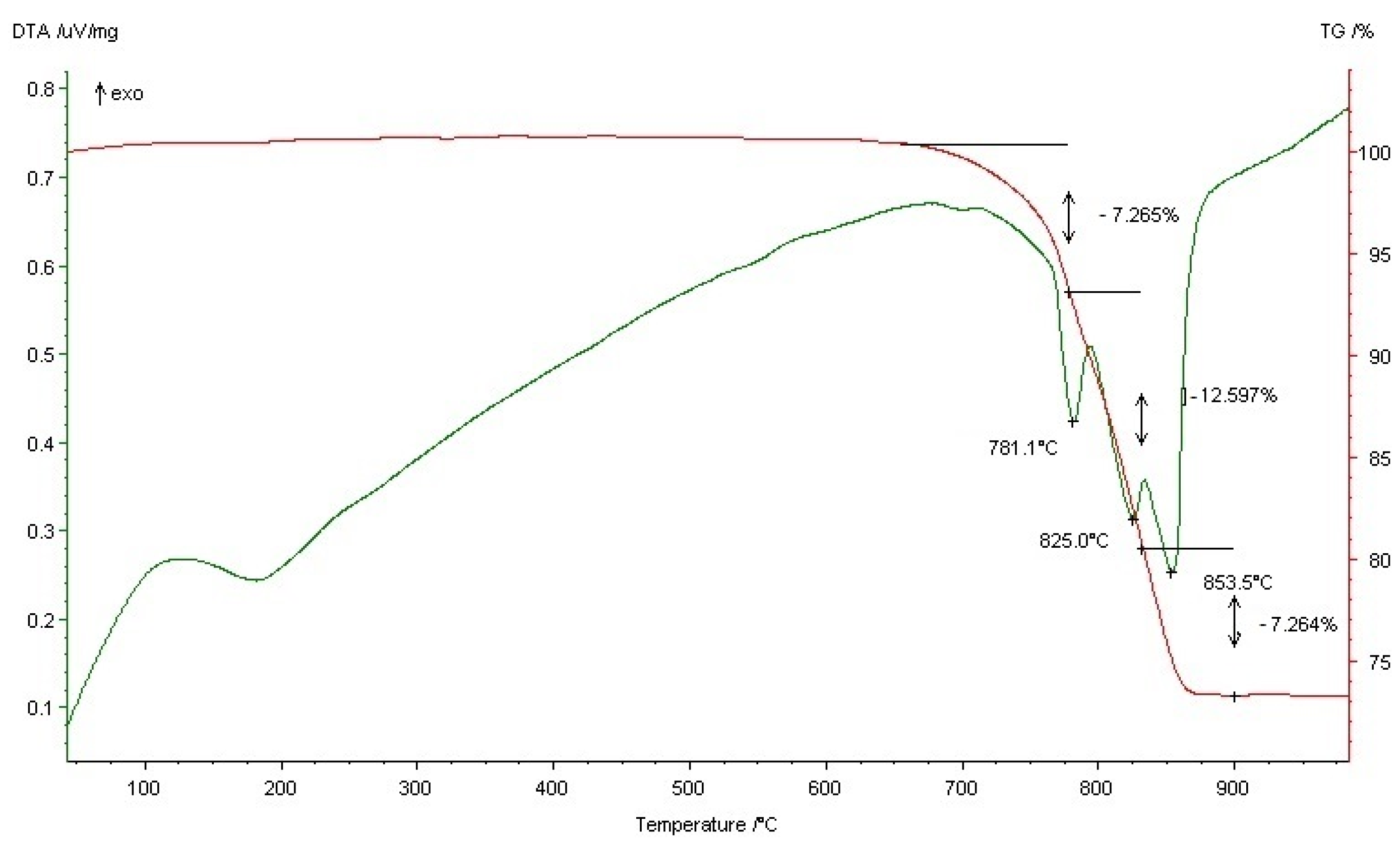
3.2. Thermal Behavior of the Starting Formulation
3.3. Structural Characterization of Synthesized Composites
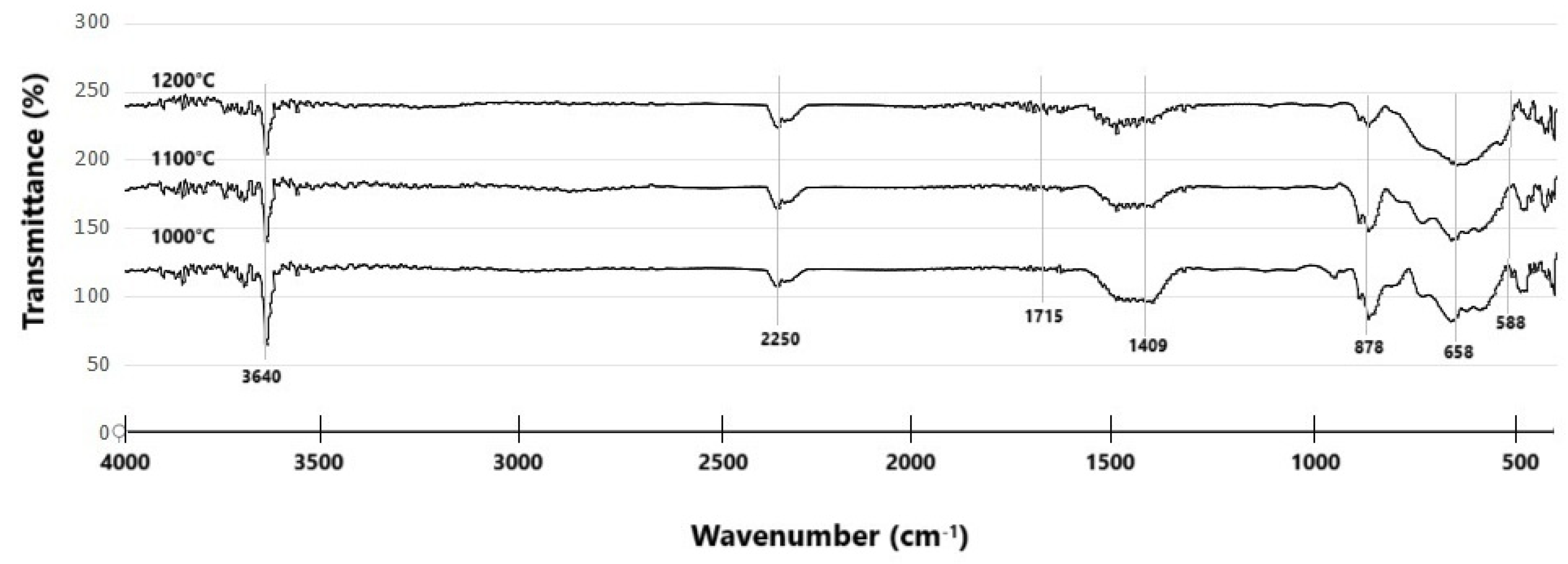
3.4. FTIR Analysis of Synthesized Composites
3.5. Morphological Analysis of Synthesized Composites
3.6. Evaluation of Thermophysical Properties of Synthesized Composites
4. Conclusions
- Marble waste demonstrated excellent chemical compatibility and serves as a sustainable and cost-effective source of calcium and magnesium for the synthesis of dielectric perovskite ceramics.
- The synthesized powders consisted of a composite phase mixture of CaNb2O6, Ca2Nb2O7, MgNb2O6, and CaMg0.33Nb0.67O3, with crystallite sizes ranging from 34.07 nm (1200 °C) to 40.83 nm (1000 °C).
- The morphological analysis revealed that the powders exhibited a polygon-like particle shape with a high degree of agglomeration.
- The thermophysical characterization indicated that the nano-sized calcium niobate–magnesium niobate composite ceramic possesses low thermal conductivity, making it a promising candidate for thermal insulation applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sivagnanapalani, P.; Sahoo, B.; Panda, P.K. Calcium niobate based piezo-resistive materials for high temperature sensor application. Ceram. Int. 2018, 44, 20348–20353. [Google Scholar] [CrossRef]
- Pilarek, B.; Pelczarska, A.J.; Szczygiet, I. Phase equilibria in the CaO–Nb2O5 system up to 1600 °C: New experimental results. Ceram. Int. 2019, 45, 1562–1568. [Google Scholar] [CrossRef]
- Rahman, M.W. Synthetic approach of ternary magnesium niobate (Mg–Nb–O) compounds. Sci. Rep. 2021, 11, 16065. [Google Scholar] [CrossRef]
- Sarkar, K.; Kumar, V.; Das, S.B.; Kumar, M.; Srivastava, R. Investigation of opto-electronic properties and morphological characterization of magnesium niobate ceramics synthesized by two-stage process. Mater. Today Proc. 2022, 49, 446–452. [Google Scholar] [CrossRef]
- Khatun, A.A.; Takei, T.; Permana, M.D.; Saito, N.; Kumada, N. Effect of silver hybridization to magnesium and calcium niobate columbite on their photocatalytic and antibacterial activity. J. Soc. Inorg. Mater. 2024, 31, 119–126. [Google Scholar] [CrossRef]
- Lu, W.; Azough, F.; Free, R. Microstructure and microwave dielectric properties of calcium magnesium niobate. Key Eng. Mater. 2004, 264–268, 1301–1304. [Google Scholar] [CrossRef]
- Paiva, D.V.M.; Silva, M.A.S.; Ribeiro, T.S.; Vasconcelos, I.F.; Sombra, A.S.B.; Góes, J.C.; Fechine, P.B.A. Novel magnetic–dielectric composite ceramic obtained from Y3Fe5O12 and CaTiO3. J. Alloys Compd. 2015, 644, 763–769. [Google Scholar] [CrossRef]
- Huang, C.E.; Lu, X.R.; Lu, M.Y.; Huan, Y. Effect of CaO/SnO2 additives on the microstructure and microwave dielectric properties of SrTiO3–LaAlO3 ceramics. Ceram. Int. 2017, 43, 10624–10627. [Google Scholar] [CrossRef]
- Fu, Z.F.; Ma, J.L.; Liu, P.; Liu, Y. Novel temperature stable Li2Mg3TiO6–SrTiO3 composite ceramics with high Q for LTCC applications. Mater. Chem. Phys. 2017, 200, 264–269. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Sun, W.K.; Liu, H.F.; Xie, G.H.; Chen, X.B.; Zeng, X.H. Synthesis of Zr2WP2O12/ZrO2 composites with adjustable thermal expansion. Front. Chem. 2017, 5, 105. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Guo, J.; Wei, S.; Wang, H.; Tang, X.; Jiao, Y.; Chao, M.; Tian, D.; Liang, E. The preparation and dielectric properties of dielectric ceramic composites with controllable thermal expansion: SrTiO3/ZrMgMo3O12. J. Mater. Sci. Mater. Electron. 2020, 31, 347–353. [Google Scholar] [CrossRef]
- Ouhassan, Y.; Bri, S.; El Boubakraoui, M.C.; Habibi, M. Dielectric properties of ceramic composite materials based on alumina reinforced by titanium carbide. FME Transac. 2020, 48, 908–913. [Google Scholar] [CrossRef]
- Shanmugasundram, H.P.P.V.; Jayamani, E.; Soon, K.H. A comprehensive review on dielectric composites: Classification of dielectric composites. Renew. Sustain. Energy Rev. 2022, 157, 112075. [Google Scholar] [CrossRef]
- Baeza, V.M.; Ortiz, F.; Garcia, S.H.; Lagunas, E. Enhanced communications on satellite-based IoT systems to support maritime transportation services. Sensors 2022, 22, 6450. [Google Scholar] [CrossRef]
- Nkordeh, N.; Popoola, S.; Jefia, A.; Okeoghene, M.; Atayero, A.; Awomoyi, M.; Bobmanuel, I. The impact of satellite communications on environmental hazard control: Tool for the realization of African Union agenda 2063 aspirations. Int. J. Commun. Netw. Syst. Sci. 2023, 16, 191–216. [Google Scholar] [CrossRef]
- Shayea, I.; El-Saleh, A.A.; Ergen, M.; Saoud, B.; Hartani, R.; Turan, D.; Kabbani, A. Integration of 5G, 6G and IoT with low earth orbit (LEO) networks: Opportunity, challenges and future trends. Results Eng. 2024, 23, 102409. [Google Scholar] [CrossRef]
- Sebastian, M.; Solaiappan, A.; Ganesanpotti, S.; Juuti, J.A.; Teirikangas, M.; Jantunen, H. Composite electroceramics. In Wiley Encyclopedia of Composites, 2nd ed.; Nicolais, L., Borzacchiello, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–17. [Google Scholar]
- Shvydyuk, K.O.; Rodrigues, F.F.; Nunes-Pereira, J.; Páscoa, J.C.; Lanceros-Mendez, S.; Silva, A.P. Long-lasting ceramic composites for surface dielectric barrier discharge plasma actuators. J. Eur. Ceram. Soc. 2023, 43, 6112–6121. [Google Scholar] [CrossRef]
- Kamkum, P.; Atiwongsangthong, N.; Muanghlua, R.; Vittayakorn, N. Application of chicken eggshell waste as a starting material for synthesizing calcium niobate (Ca4Nb2O9) powder. Ceram. Int. 2015, 41, S69–S75. [Google Scholar] [CrossRef]
- Kamkum, P.; Vittayakorn, W.; Seeharaj, P.; Woramongkolchai, S.; Muanghlua, R.; Vittayakorn, N. Utilization of eggshell as a low-cost precursor for synthesizing calcium niobate ceramic. Green Mater. 2018, 6, 108–116. [Google Scholar] [CrossRef]
- Alves, A.E.; Bernardo, P.L.; Holanda, J.N.F. Preliminary investigation on the feasibility of obtaining nano-sized calcium niobate derived of seashell bio-waste. Mater. Lett. 2023, 353, 135295. [Google Scholar] [CrossRef]
- Ferrari, V.Z.; Tavares, A.C.; Martins, V.; Malfatti, C.F. Bibliographic review of pure niobium machining. Braz. J. Develop. 2021, 7, 1475–1494. [Google Scholar] [CrossRef]
- Alves, A.R.; Coutinho, A.R. The evolution of the Niobium production in Brazil. Mater. Res. 2015, 18, 106–112. [Google Scholar] [CrossRef]
- Montani, C. XXXII World Marble and Stones Report 2021; Aldus Casa di Edizioni: Carrara, Italy, 2021. [Google Scholar]
- Zichella, L.; Bellopede, R.; Marini, P. Ornamental stone cutting processing and sludge production evaluation with the goal of ending waste. Mater. Proc. 2021, 5, 57. [Google Scholar] [CrossRef]
- Novaes, R.C.; Zagôto, J.T.; Sant’Ana, M.A.K.; Vale, D.; Meyer, A.P.; Faria, T.G. Industrial carbonate production with ornamental stone quarry waste: From the waste pile to final product, Rev. Gest. Soc. Ambient. 2024, 18, e010188. [Google Scholar]
- Mulk, S.; Korai, A.L.; Azizullah, A.; Shahi, L.; Khattak, M.N.K. Marble industry effluents cause an increased bioaccumulation of heavy metals in Mahaseer (Tor putitora) in Barandu River, district Buner, Pakistan. Environ. Sci. Pollut. Res. 2017, 24, 23039–23056. [Google Scholar] [CrossRef]
- Iqbal, Q.; Musarat, M.A.; Ullah, N.; Alaloul, W.S.; Rabbani, M.B.A.; Al Madhoun, W.; Iqbal, S. Marble dust effect on the air quality: An environmental assessment approach. Sustainability 2022, 14, 3831. [Google Scholar] [CrossRef]
- Thakur, A.K.; Pappu, A.; Thakur, V.K. Resource efficiency impact on marble waste recycling towards sustainable green construction materials. Curr. Opin. Green Sustain. Chem. 2018, 13, 91–101. [Google Scholar] [CrossRef]
- Mota, L.; Toledo, R.; Machado, F.A.L.; Holanda, J.N.F.; Vargas, H.; Faria, R.T., Jr. Thermal characterization of red clay from the northern region of Rio de Janeiro state, Brazil using an open photoacoustic cell in relation to structural changes on firing. Appl. Clay Sci. 2008, 42, 168–174. [Google Scholar] [CrossRef]
- Leite, F.H.G.; Almeida, T.F.; Faria, R.T., Jr.; Holanda, J.N.F. Synthesis and characterization of calcium silicate insulating material using avian eggshell waste. Ceram Int. 2017, 43, 4674–4679. [Google Scholar] [CrossRef]
- Mota, L.; Toledo, R.; Bastos Filho, R.P.; Vargas, H.; Faria, R.T., Jr. Photothermal survey of red clay: Measurements of thermal and structural properties. Cerâmica 2010, 56, 1–8. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Farag, A.B.; Kandeel, A.M.; Younes, A.A.; Yousef, M.M. Characteristics of the marble processing powder waste at Shaq El-Thoaban industrial area, Egypt, and its suitability for cement manufacture. HBRC J. 2018, 14, 171–179. [Google Scholar] [CrossRef]
- Kaushal, M.V.N.; Sharma, A. Waste marble powder/dust. Our Herit. J. 2019, 67, 181–205. [Google Scholar]
- Ji, J.; Ge, Y.; Balsam, W.; Damuth, J.E.; Chen, J. Rapid identification of dolomite using a Fourier Transform Infrared Spectrophotometer (FTIR): A fast method for identifying Heinrich events in IODP Site U1308. Marine Geol. 2009, 258, 60–68. [Google Scholar] [CrossRef]
- Petkova, V.; Stoyanov, V.; Kostova, B.; Kostov-Kytin, V.; Kalinkin, A.; Zvereva, I.; Tzvetanova, Y. Crystal-chemical and thermal properties of decorative cement composites. Materials 2021, 14, 4793. [Google Scholar] [CrossRef]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef]
- Santos, L.P.S.; Camargo, E.R.; Fabbro, M.T.; Longo, E.; Leite, E.R. Wet-chemical synthesis of magnesium niobate nanoparticles powders. Ceram. Int. 2007, 33, 1205–1209. [Google Scholar] [CrossRef]
- Lanfredi, S.; Nobre, M.A.L.; Besse, M.R.; Lima, A.R.F.; Palacio, G. Spectral deconvolution as a tool to understanding Curie-temperature shifting and niobium off-centering phenomenon in ferroelectrics of type niobates. Appl. Mathem. Sci. 2015, 9, 5839–5869. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A review of recent advances in thermophysical properties at the nanoscale: From solid state to colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Yang, J.; Qian, X.; Pan, W.; Yang, R.; Li, Z.; Han, Y.; Zhao, M.; Huang, M.; Wan, C. Diffused lattice vibration and ultralow thermal conductivity in the binary Ln–Nb–O oxide system. Adv. Mater. 2019, 31, 1808222. [Google Scholar] [CrossRef]
- Cockayne, E.; Burton, B.P. Phonons and static dielectric constant in CaTiO3 from first principles. Phys. Rev. B 2000, 62, 3735–3743. [Google Scholar]
- Su, Y.; Peng, L.; Du, C.; Wang, X. Controllable Synthesis of undoped and doped calcium niobate nanocrystals for tailored structural, electronic, and luminescent properties. J. Phys. Chem. C 2012, 116, 15–21. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, M.; Ma, X.; Zhao, S.; Jiang, T.; Wen, W. Synthesis and thermal behavior of rare-earth-niobate ceramics with fluorite structure. Ceram. Int. 2024, 50, 18723–18730. [Google Scholar] [CrossRef]
- Yan, X.; Constantin, L.; Lu, Y.; Silvain, J.F.; Nastasi, M.; Cui, B. (Hf0.2Zr0.2 Ta0.2Nb0.2Ti0.2)C high-entropy ceramics with low thermal conductivity. J. Am. Ceram. Soc. 2018, 101, 4486–4491. [Google Scholar] [CrossRef]




| CaO | MgO | Al2O3 | SiO2 | Na2O | P2O5 | * LoI |
|---|---|---|---|---|---|---|
| 42.749 | 15.371 | 0.140 | 0.400 | 0.120 | 0.06 | 41.160 |
| Temperature (°C) | Thermal Diffusivity (×10−7 m2/s) | Specific Heat Capacity (×105 J/Km3) | Thermal Conductivity (W/mK) | Thermal Effusivity (kW√s/Km2) |
|---|---|---|---|---|
| 1000 | 2.15 ± 0.02 | 7.00 ± 0.15 | 0.15 ± 0.01 | 1.02 ± 0.01 |
| 1100 | 1.73 ± 0.03 | 6.65 ± 0.18 | 0.12 ± 0.05 | 0.90 ± 0.05 |
| 1200 | 1.88 ± 0.02 | 8.50 ± 0.21 | 0.16 ± 0.02 | 1.17 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passalini, P.G.S.; Alves, A.E.; Cordeiro, T.C.; Faria, R.d.T., Jr.; Holanda, J.N.F. The Valorization of Marble Waste to Synthesize a Novel Calcium Niobate–Magnesium Niobate Composite and an Investigation of Its Thermophysical Properties. Processes 2025, 13, 1014. https://doi.org/10.3390/pr13041014
Passalini PGS, Alves AE, Cordeiro TC, Faria RdT Jr., Holanda JNF. The Valorization of Marble Waste to Synthesize a Novel Calcium Niobate–Magnesium Niobate Composite and an Investigation of Its Thermophysical Properties. Processes. 2025; 13(4):1014. https://doi.org/10.3390/pr13041014
Chicago/Turabian StylePassalini, Pedro Guilherme Sousa, Andrey Escala Alves, Thallis Custódia Cordeiro, Roberto da Trindade Faria, Jr., and José Nilson França Holanda. 2025. "The Valorization of Marble Waste to Synthesize a Novel Calcium Niobate–Magnesium Niobate Composite and an Investigation of Its Thermophysical Properties" Processes 13, no. 4: 1014. https://doi.org/10.3390/pr13041014
APA StylePassalini, P. G. S., Alves, A. E., Cordeiro, T. C., Faria, R. d. T., Jr., & Holanda, J. N. F. (2025). The Valorization of Marble Waste to Synthesize a Novel Calcium Niobate–Magnesium Niobate Composite and an Investigation of Its Thermophysical Properties. Processes, 13(4), 1014. https://doi.org/10.3390/pr13041014








